Submitted:
31 October 2023
Posted:
31 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Raw fish and fish processing
2.2. Lipid oxidation assessment
2.3. Assessment of fluorescent compounds
2.4. Determination of free fatty acid (FFA) and phospholipid (PL) content
2.5. Analysis of FA composition
2.6. Other physico-chemical determinations
2.7. Statistical analysis
3. Results and discussion
3.1. Lipid oxidation development
3.2. Lipid hydrolysis development
3.3. Fatty acid analysis
3.4. Determination of colour changes
3.5. TMA content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horner, W. Canning fish and fish products. In Fish Processing Technology; Hall, G., Ed.; 2nd edition. Blackie Academic and Professional, Chapman and Hall: London, UK, 1997; pp. 119–159. [Google Scholar]
- Veiga, A.; Martínez, E.; Ojea, G.; Caride, A. Principles of thermal processing in canned seafood. In Quality Parameters in Canned Seafoods; Cabado, A.G., Vieites, J.M., Eds.; Nova Science Publishers, Inc., New York, USA, 2008; pp. 83–103.
- Lukoshkina, M.; Odoeva, G. Kinetics of chemical reactions for prediction of quality of canned fish during storage. App. Biochem. Microb. 2003, 39, 321–327. [Google Scholar] [CrossRef]
- Ling, B.; Tang, J.; Kong, F.; Mitcham, E.J.; Wang, S. Kinetics of food quality changes during thermal processing: A review. Food Bioprocess Technol. 2015, 8, 343–358. [Google Scholar] [CrossRef]
- Pitarch, J.L.; Vilas, C.; de Prada, C.; Palacín, C.G.; Alonso, A.A. Optimal operation of thermal processing of canned tuna under product variability. J. Food Eng. 2021, 304, 110594. [Google Scholar] [CrossRef]
- Tokur, B.; Korkmaz, K. Novel thermal sterilisation technologies in seafood processing. In Innovative Technologies in Seafood Processing; Özoğul, Y., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 303–322. [Google Scholar]
- James, C.; Purnell, G.; James, S.J. A review of novel and innovative food freezing technologies. Food Bioprocess Technol. 2015, 8, 1616–1634. [Google Scholar] [CrossRef]
- Wu, X.F.; Zhang, M.; Adhikari, B.; Sun, J.C. Recent developments in novel freezing and thawing technologies applied to foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3620–3631. [Google Scholar] [CrossRef] [PubMed]
- Sista, R.; Erickson, M.; Shewfelt, R. Quality deterioration in frozen foods associated with hydrolytic enzyme activities. In Quality in Frozen Food; Erickson, M., Hung, Y.C., Eds.; Chapman and Hall: New York, NY, USA, 1997; pp. 101–110. [Google Scholar]
- Sikorski, Z.; Kolakowski, E. Endogenous enzyme activity and seafood quality: Influence of chilling, freezing, and other environmental factors. In Seafood Enzymes; Haard, N., Simpson, B., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 451–487. [Google Scholar]
- Aubourg, S.P.; Medina, I. Quality differences assessment in canned sardine (Sardina pilchardus) by fluorescence detection. J. Agric. Food Chem. 1997, 45, 3617–3621. [Google Scholar] [CrossRef]
- Prego, R.; Fidalgo, L.G.; Saraiva, J.A.; Vázquez, M.; Aubourg, S.P. Impact of prior high-pressure processing on lipid damage and volatile amines formation in mackerel muscle subjected to frozen storage and canning. LWT-Food Sci. Technol. 2021, 135, 109957. [Google Scholar] [CrossRef]
- Little, A. Effect of pre- and post-mortem handling on reflectance characteristics of skipjack tuna. J. Food Sci. 1972, 37, 502. [Google Scholar] [CrossRef]
- Bandarra, N.M.; Batista, I.; Nunes, M.L.; Empis, J.M. Seasonal variation in the chemical composition of horse mackerel (Trachurus trachurus). Eur. Food Res. Technol. 2001, 212, 535–539. [Google Scholar] [CrossRef]
- El Mghazli, H.; Znari, M.; Mounir, A. Stock discrimination in the horse mackerel Trachurus trachurus (Telostei: Carangidae) off the Moroccan Atlantic Coastal Waters using a morphometric-meristic analysis. Thalassas: An Int. J. Mar. Sci. 2022, 38, 171–181. [Google Scholar] [CrossRef]
- Aubourg, S.P. Damage detection in horse mackerel (Trachurus trachurus) during chilled storage. J. Amer. Oil Chem. Soc. 2001, 78, 857–862. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Piñeiro, C.; González, M.J. Quality loss related to rancidity development during frozen storage of horse mackerel (Trachurus trachurus). J. Amer. Oil Chem. Soc. 2004, 81, 671–678. [Google Scholar] [CrossRef]
- Farvin, K.H.S.; Grejsen, H.D.; Jacobsen, C. Potato peel extract as a natural antioxidant in chilled storage of minced horse mackerel (Trachurus trachurus): Effect on lipid and protein oxidation. Food Chem. 2012, 131, 843–851. [Google Scholar] [CrossRef]
- Albertos, I.; Martín-Diana, A.B.; Jaime, I.; Avena-Bustillos, R.J.; McHugh, T.H.; Takeoka, G.R.; Dao, L.; Rico, D. Antioxidant effect of olive leaf powder on fresh Atlantic horse mackerel (Trachurus trachurus) minced muscle. J. Food Process Presev. 2018, 42, e13397. [Google Scholar] [CrossRef]
- Zarandona, I.; López-Caballero, M.E.; Montero, M.P.; Guerrero, P.; de la Caba, K.; Gómez-Guillén, M.C. Horse mackerel (Trachurus trachurus) fillets biopreservation by using gallic acid and chiotosan coatings. Food Cont. 2021, 120, 107511. [Google Scholar] [CrossRef]
- Alves Silva, H.; Mendes, R.; Nunes, M.L.; Empis, J. Protein changes after irradiation and ice storage of horse mackerel (Trachurus trachurus). Eur. Food Res. Technol. 2006, 224, 83–90. [Google Scholar] [CrossRef]
- Erkan, N.; Üretener, G.; Alpas, H.; Selçuk, A.; Özden, Ö.; Buzrul, S. Effect of high hydrostatic pressure (HHP) treatment on physicochemical properties of horse mackerel (Trachurus trachurus). Food Bioprocess Technol. 2011, 4, 1322–1329. [Google Scholar] [CrossRef]
- Méndez, L.; Trigo, M.; Zhang, B.; Aubourg, S.P. Antioxidant effect of octopus by-products in canned horse mackerel (Trachurus trachurus) previously subjected to different frozen storage times. Antioxidants 2022, 11, 2091. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roso, B.; Cuesta, I.; Pérez, M.; Borrego, E.; Pérez-Olleros, L.; Varela, G. Lipid composition and palatability of canned sardines. Influence of the canning process and storage in olive oil for five years. J. Sci. Food Agric. 1998, 77, 244–250. [Google Scholar] [CrossRef]
- Bligh, E.; Dyer, W. A rapid method of total extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Chapman, R.; McKay, J. The estimation of peroxides in fats and oils by the ferric thiocyanate method. J. Am. Oil Chem. Soc. 1949, 26, 360–363. [Google Scholar] [CrossRef]
- Vyncke, W. Direct determination of the thiobarbituric acid value in trichloracetic acid extracts of fish as a measure of oxidative rancidity. Fette, Seifen, Anstrichm. 1970, 72, 1084–1087. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Medina, I.; Pérez-Martín, R.I. A comparison between conventional and fluorescence detection methods of cooking-induced damage to tuna fish lipids. Z. Lebensm. Unters. Forsch. 1995, 200, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Lowry, R.; Tinsley, I. Rapid colorimetric determination of free fatty acids. J. Am. Oil Chem. Soc. 1976, 53, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Raheja, R.; Kaur, C.; Singh, A.; Bhatia, A. New colorimetric method for the quantitative determination of phospholipids without acid digestion. J. Lipid Res. 1973, 14, 695–697. [Google Scholar] [CrossRef] [PubMed]
- Prego, R.; Trigo, M.; Martínez, B.; Aubourg, S.P. Effect of previous frozen storage, packing medium and sterilisation on fatty acid composition of canned mackerel (Scomber scombrus). Marine Drugs 2022, 20, 666. [Google Scholar] [CrossRef] [PubMed]
- Tozawa, H.; Erokibara, K.; Amano, K. Proposed modification of Dyer’s method for trimethylamine determination in codfish. In Fish Inspection and Quality Control; Kreuzer, R., Ed.; Fishing News Books Ltd: London, UK, 1971; pp. 187–190. [Google Scholar]
- Rustad, T. Lipid oxidation. In Handbook of Seafood and Seafood Products Analysis; Nollet, L.M., Toldrá, F., Eds.; CRC Press, Francis and Taylor Group: Boca Raton, FL, USA, 2010; pp. 87–95. [Google Scholar]
- Özoğul, Y. Methods for freshness quality and deterioration. In Handbook of Seafood and Seafood Products Analysis; Nollet, L.M., Toldrá, F., Eds.; CRC Press, Francis and Taylor Group: Boca Raton, FL, USA, 2010; pp. 189–214. [Google Scholar]
- Hassoun, A. Exploring the Potential of Fluorescence Spectroscopy for the Discrimination between Fresh and Frozen-Thawed Muscle Foods. Photochem. 2021, 1, 247–263. [Google Scholar] [CrossRef]
- Hassoun, A.; Sahar, A.; Lakhal, L.; Aït-Kaddour, A. Fluorescence spectroscopy as a rapid and non-destructive method for monitoring quality and authenticity of fish and meat products: Impact of different preservation conditions. LWT-Food Sci. Technol. 2019, 103, 279–292. [Google Scholar] [CrossRef]
- Selmi, S.; Monser, L.; Sadok, S. The influence of local canning process and storage on pelagic fish from Tunisia: Fatty acids profile and quality indicators. J. Food Proc. Preserv. 2008, 32, 443–457. [Google Scholar] [CrossRef]
- Gómez-Limia, L.; Sanmartín, N.M.; Carballo, J.; Domínguez, R.; Lorenzo, J.M.; Martínez, S. Oxidative stability and antioxidant activity in canned eels: Effect of processing and filling medium. Foods 2021, 10, 790. [Google Scholar] [CrossRef] [PubMed]
- Malga, J.M.; Trigo, M.; Martínez, B.; Aubourg, S.P. Preservative effect on canned mackerel (Scomber colias) lipids by addition of octopus (Octopus vulgaris) cooking liquor in the packaging medium. Molecules 2022, 27, 739. [Google Scholar] [CrossRef] [PubMed]
- Tironi, V.; Tomás, M.; Añón, M.C. Structural and functional changes in myofibrillar proteins of sea salmon (Pseudopercis semifasciata) by interaction with malondialdehyde (RI). J. Food Sci. 2002, 67, 930–935. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Trigo, M.; Martínez, B.; Rodríguez, A. Effect of prior chilling period and alga-extract packaging on the quality of a canned underutilised fish species. Foods 2020, 9, 1333. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.O.; Remya, S.; Murthy, L.N.; Ravishankar, C.N.; Kumar, K.A. Effect of filling medium on cooking time and quality of canned yellowfin tuna (Thunnus albacares). Food Cont. 2015, 50, 320–327. [Google Scholar] [CrossRef]
- Naseri, M.; Rezaei, M.; Moieni, S.; Hosseini, H.; Eskandari, S. Effects of different filling media on the oxidation and lipid quality of canned silver carp (Hypophthalmichthys molitrix). Int. J. Food Sci. 2011, 46, 1149–1156. [Google Scholar] [CrossRef]
- Barbosa, R.G.; Trigo, M.; Campos, C.A.; Aubourg, S.P. Preservative effect of algae extracts on lipid composition and rancidity development in brine–canned Atlantic chub mackerel (Scomber colias). Eur. J. Lipid Sci. Technol. 2019, 121, 1900129. [Google Scholar] [CrossRef]
- Barbosa, R.G.; Trigo, M.; Fett, R.; Aubourg, S.P. Impact of a packing medium with alga Bifurcaria bifurcata extract on canned Atlantic mackerel (Scomber scombrus) quality. J. Sci. Food Agric. 2018, 98, 3462–3467. [Google Scholar] [CrossRef] [PubMed]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, Article 3. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Medina, I.; Pérez-Martín, R. Polyunsaturated fatty acids in tuna phospholipids: Distribution in the sn-2 location and changes during cooking. J. Agric. Food Chem. 1996, 44, 585–589. [Google Scholar] [CrossRef]
- Takahashi, K.; Inoue, Y. Marine by-product phospholipids as booster of medicinal compounds. Adv. Food Nutr. Res. 2012, 65, 31–46. [Google Scholar]
- Naseri, M.; Rezaei, M. Lipid changes during long-term storage of canned sprat. J. Aquat. Food Prod. Technol. 2012, 21, 48–58. [Google Scholar] [CrossRef]
- Magalhães, J.P.; Müller, M.; Rainger, G.; Steegenga, W. Fish oil supplements, longevity and aging. Aging 2016, 8, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Devassy, J.G.; Leng, S.; Gabbs, M.; Monirujjaman, M.; Aukema, H.M. Omega-3 polyunsaturated fatty acids and oxylipins in neuroinflammation and management of Alzheimer disease. Adv. Nutr. 2016, 7, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Swanson, S.; Block, R.; Mousa, S. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, F.K.; Daliri, E.B.M.; Lee, B.H.; Yu, X. Current trends and future perspectives on omega-3 fatty acids. Res. J. Biol. 2017, 5, 11–20. [Google Scholar]
- Uauy, R.; Valenzuela, A. Marine oils: The health benefits of n-3 fatty acids. Nutrition 2000, 16, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Komprda, T. Eicosapentaenoic and docosahexaenoic acids as inflammation-modulating and lipid homeostasis influencing nutraceuticals: A review. J. Funct. Foods 2012, 4, 25–38. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharm. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Limia, L.; Cobas, N.; Franco, I.; Martínez-Suárez, S. Fatty acid profiles and lipid quality indices in canned European eels: Effects of processing steps, filling medium and storage. Food Res. Int. 2020, 136, 109601. [Google Scholar] [CrossRef] [PubMed]
- Domiszewski, Z. Effect of sterilization on true retention rate of eicosapentaenoic and docosahexaenoic acid content in mackerel (Scomber scombrus), herring (Clupea harengus), and sprat (Sprattus sprattus) canned products. J. Food Proc. Preserv. 2021, 45, e15461. [Google Scholar] [CrossRef]
- Targueta Barreira, C.F.; Sales de Oliveira, V.; Hidalgo Chávez, D.W.; Domingues Gamallo, O.; Castro, R.N.; Côrrea Damasceno Júnior, P.; Frankland Sawaya, A.C.H.; da Silva Ferreira, M.; Rodrigues Sampaio, G.; Ferraz da Silva Torres, E.A.; Saldanha, T. The impacts of pink pepper (Schinus terebinthifolius Raddi) on fatty acids and cholesterol oxides formation in canned sardines during thermal processing. Food Chem. 2023, 403, 134347. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Joo, S.; Alderton, A.; Hill, D.; Faustman, C. Oxymioglobin and lipid oxidation in yellowfin tuna (Thunnus albacares) loins. J. Food Sci. 2003, 68, 1664–1668. [Google Scholar] [CrossRef]
- Wetterskog, D.; Undeland, I. Loss of redness (a*) as a tool to follow hemoglobin-mediated lipid oxidation in washed cod mince. J. Agric. Food Chem. 2004, 52, 7214–7221. [Google Scholar] [CrossRef] [PubMed]
- Undeland, I.; Hultin, H.; Richards, M. Aqueous extracts from some muscles inhibit hemoglobin-mediated oxidation of cod muscle membrane lipids. J. Agric. Food Chem. 2003, 51, 3111–3119. [Google Scholar] [CrossRef] [PubMed]
- Schubring, R. Comparative study of the DSC pattern, color, texture and water-binding capacity of rainbow trout muscle during heating. J. Food Process. Preserv. 2008, 32, 190–218. [Google Scholar] [CrossRef]
- Mohan, C.O.; Remya, S.; Ravishankar, C.N.; Vijayan, P.K.; Srinivasa Gopal, T.K. Effect of filling ingredient on the quality of canned yellowfin tuna (Thunnus albacares). Int. J. Food Sci. Technol. 2014, 49, 1557–1564. [Google Scholar] [CrossRef]
- Gómez-Limia, L.; Carballo, J.; Rodríguez-González, M.; Martínez, S. Impact of the filling medium on the colour and sensory characteristics of canned European eels (Anguilla anguilla L.). Foods, 2022, 11, 1115. [Google Scholar] [CrossRef] [PubMed]
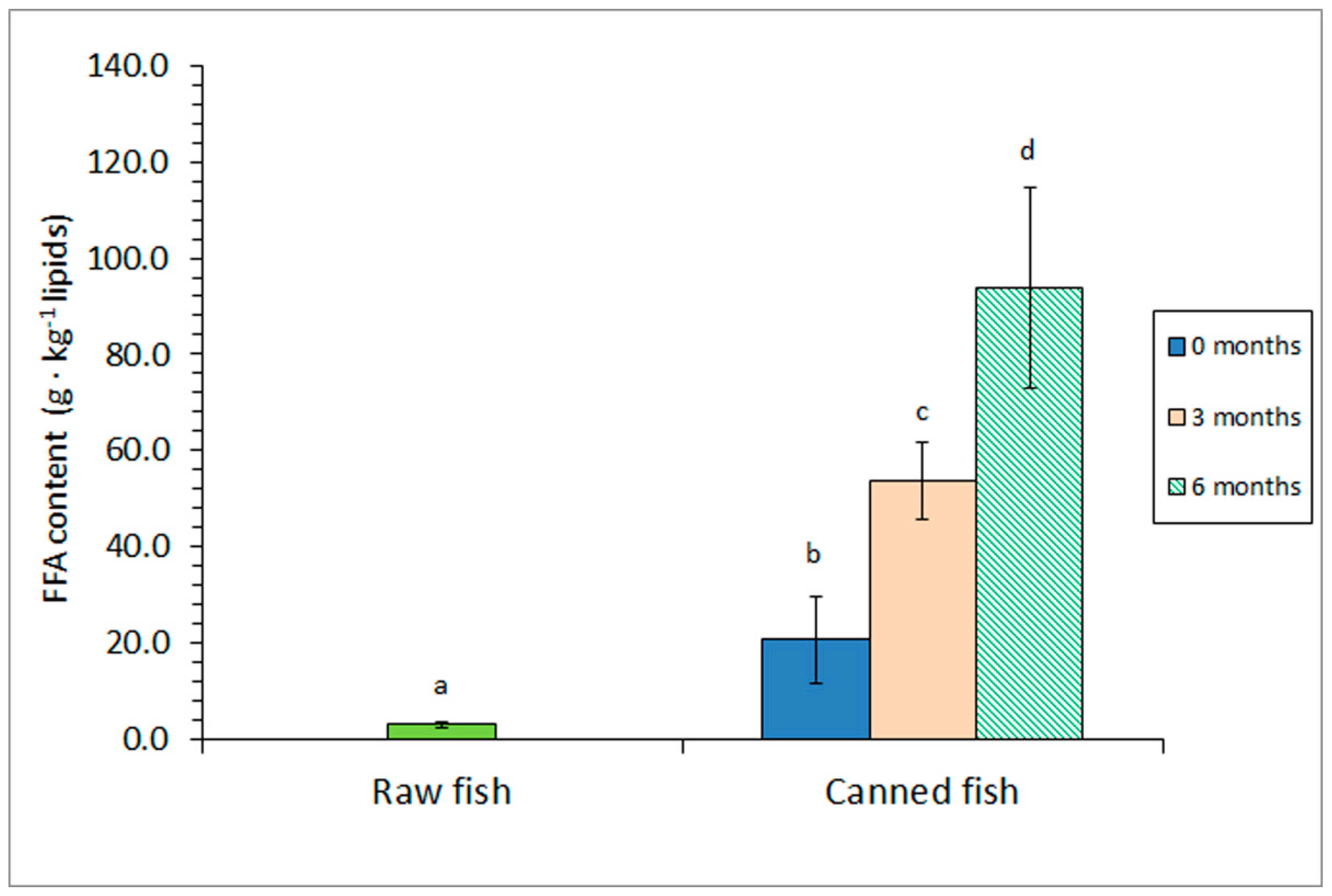
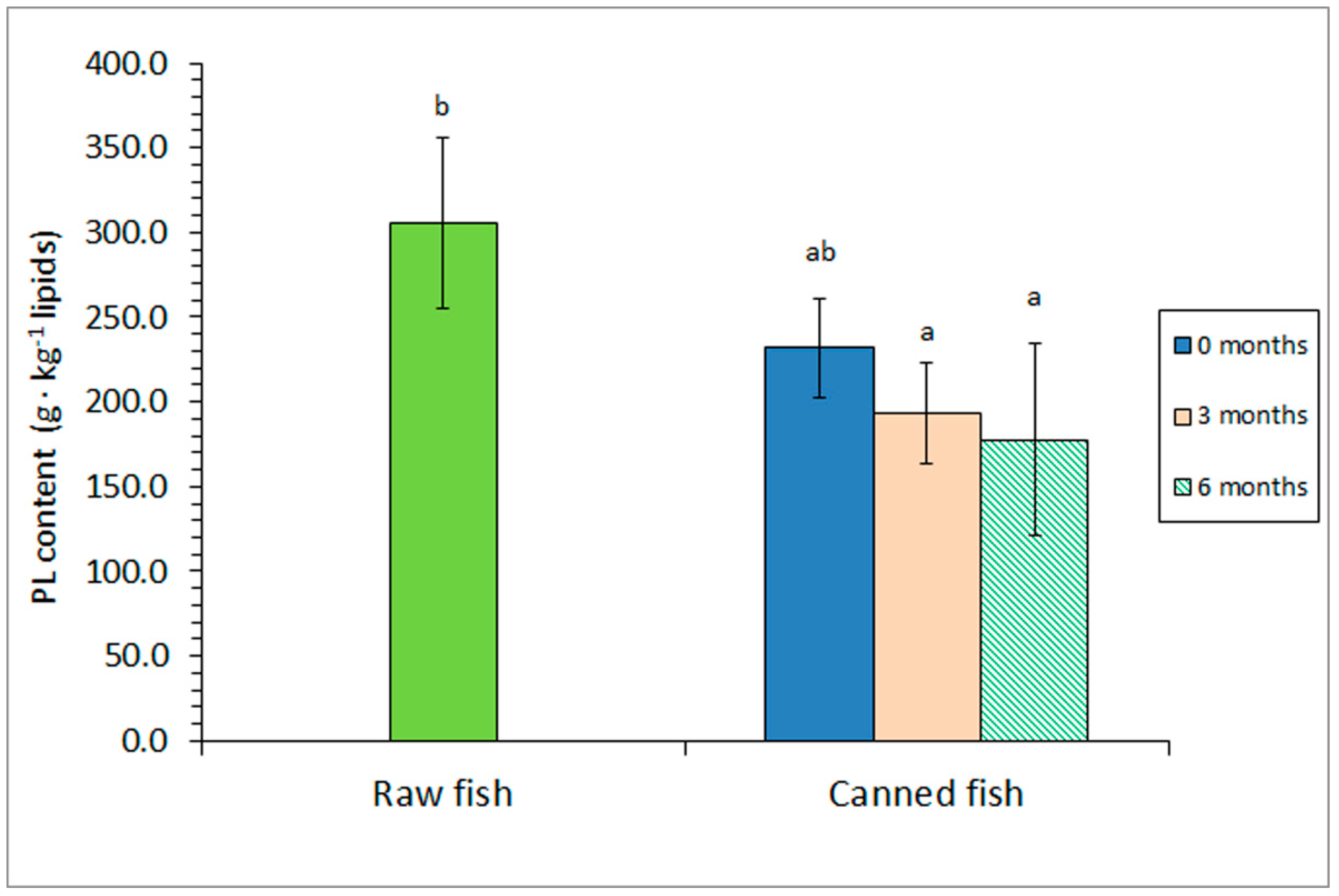
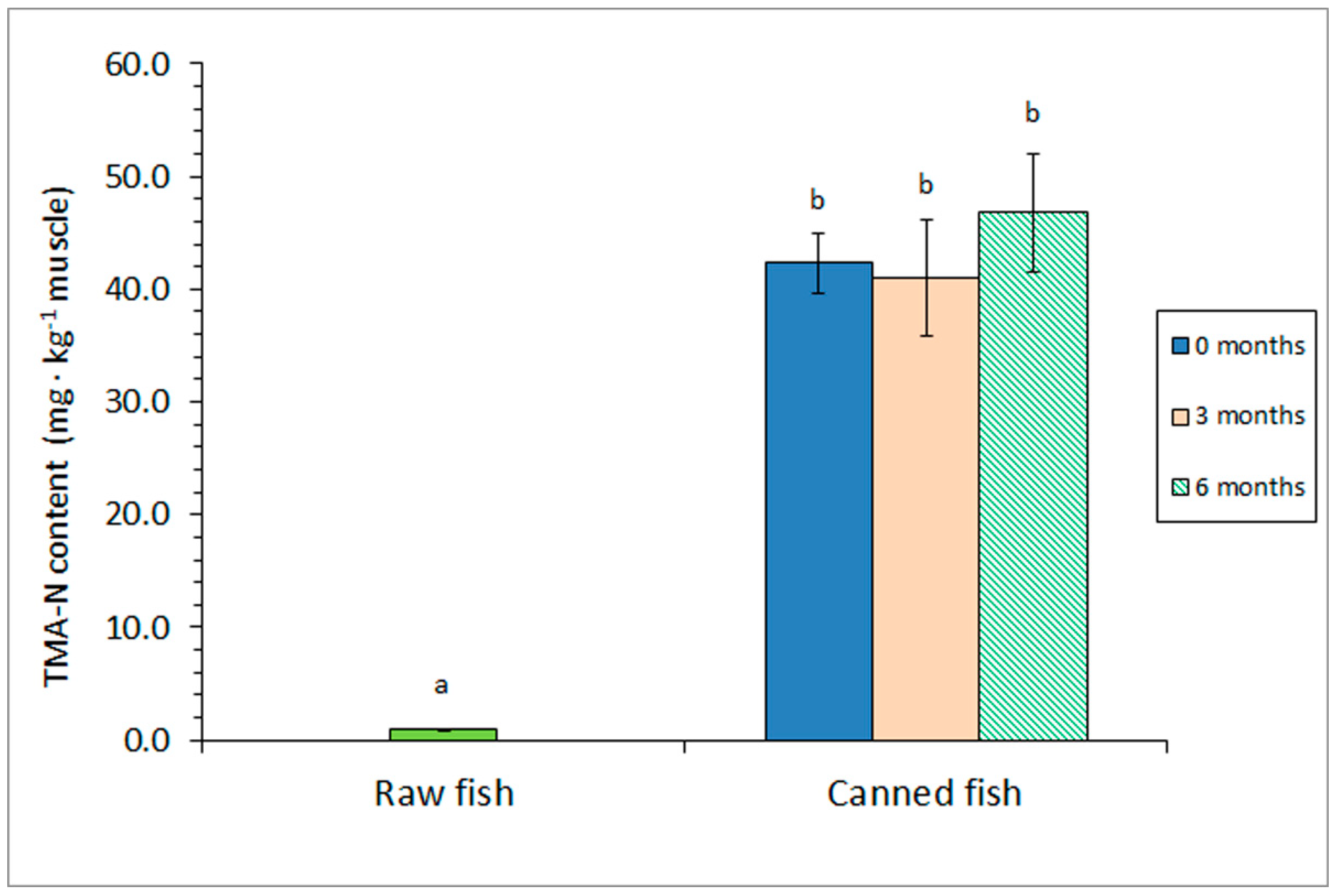
| Lipid quality index | Raw fish | Canned fish | ||
|---|---|---|---|---|
| Prior frozen storage time (months) | ||||
| 0 | 3 | 6 | ||
| Peroxide value (meq active oxygen·kg-1 lipids) | 1.36 a (0.51) |
1.45 a (0.32) |
4.21 b (1.07) |
5.91 b (1.23) |
| Thiobarbituric acid index (mg malondialdehyde·kg-1 muscle) | 0.04 a (0.02) |
0.12 a (0.07) |
0.30 b (0.02) |
0.32 b (0.03) |
| Fluorescence ratio | 1.31 a (0.40) |
3.43 b (0.54) |
4.32 c (0.17) |
4.89 c (0.81) |
| FA parameter§§ | Raw fish | Canned fish | ||
|---|---|---|---|---|
| Prior frozen storage time (months) | ||||
| 0 | 3 | 6 | ||
| EPA (g·100 g-1 total FAs) | 7.67 a (0.04) |
8.26 ab (1.29) |
8.79 b (0.41) |
8.03 ab (0.40) |
| DHA (g·100 g-1 total FAs) | 23.89 b (2.82) |
19.39 ab (2.87) |
17.04 a (1.71) |
15.91 a (3.13) |
| ω3 (g·100 g-1 total FAs) | 34.45 b (2.87) |
30.50 ab (2.64) |
28.74 a (1.51) |
26.32 a (2.71) |
| ω3/ω6 Ratio | 9.38 b (0.70) |
7.67 a (0.79) |
8.08 ab (0.71) |
7.55 a (0.52) |
| Polyene index | 1.42 c (0.12) |
1.21 bc (0.09) |
1.06 ab (0.07) |
0.92 a (0.11) |
| Colour parameter | Raw fish | Canned fish | ||
|---|---|---|---|---|
| Prior frozen storage time (months) | ||||
| 0 | 3 | 6 | ||
| L* | 42.14 a (3.19) |
64.86 b (1.15) |
64.03 b (0.86) |
67.43 c (0.62) |
| a* | 4.90 b (0.73) |
0.77 a (0.34) |
1.46 a (0.51) |
0.93 a (0.25) |
| b* | 3.13 a (0.07) |
12.81 b (1.63) |
14.25 bc (2.33) |
16.47 c (1.32) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





