Submitted:
31 October 2023
Posted:
01 November 2023
You are already at the latest version
Abstract
Keywords:
1. Sarcopenia: Age-Related Loss of Muscle Mass and Function
1.1. Aging
1.2. Definition and Primary Characteristics of Sarcopenia
1.3. Sarcopenia Management
1.4. Pathophysiology of Sarcopenia
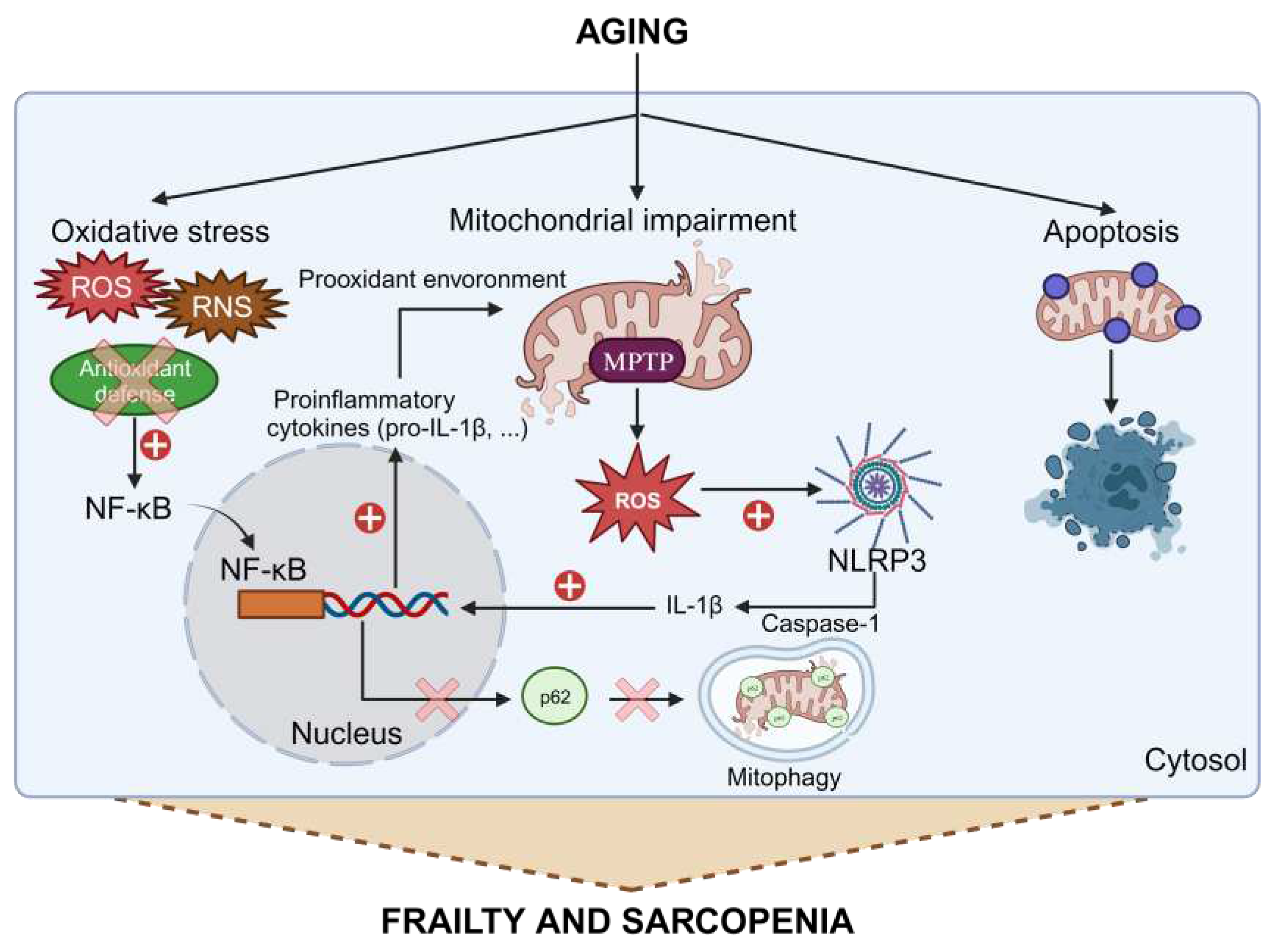
1.5. Connection between Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis in Aging
2. Role of Clock Genes in Skeletal Muscle
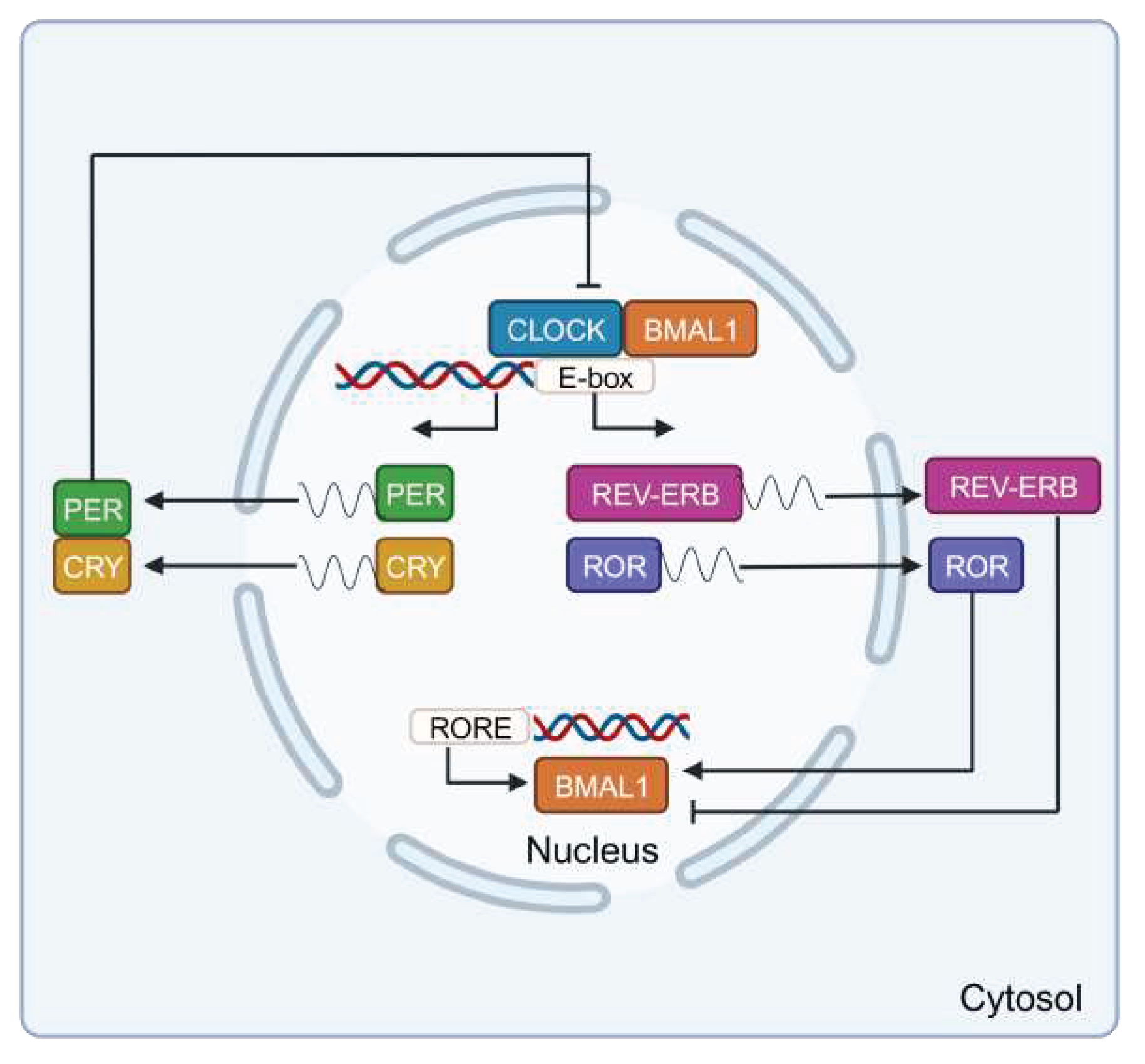
2.1. Circadian System Organization
2.2. Connection between Clock Genes and Inflammation in Aging
2.3. Clock Genes in Skeletal Muscle
3. Melatonin as a Potential Therapeutic Approach in Sarcopenia
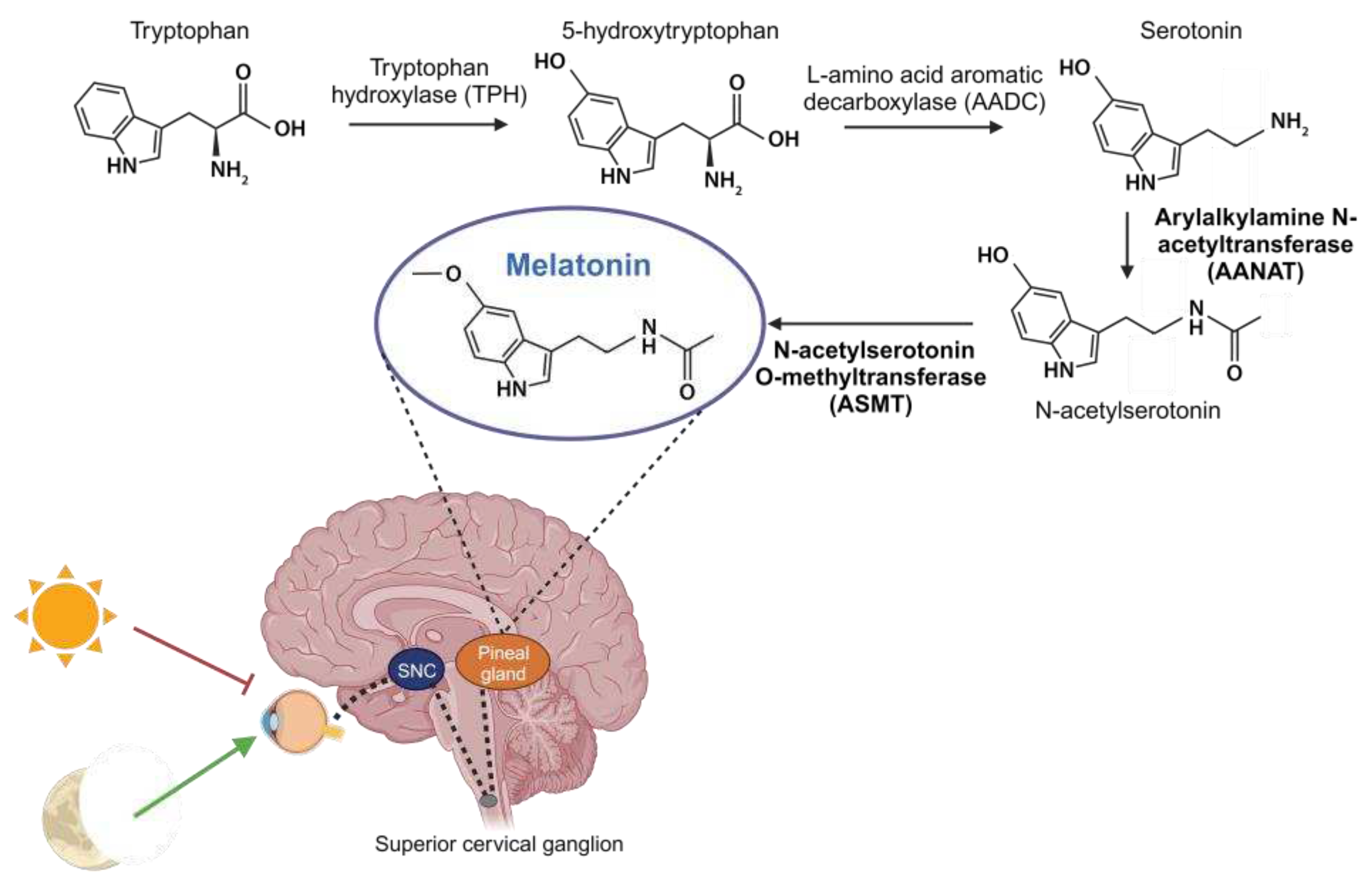
3.1. Synthesis, Metabolism, and Targets of Melatonin
3.2. Actions of Pineal and Extrapineal Melatonin
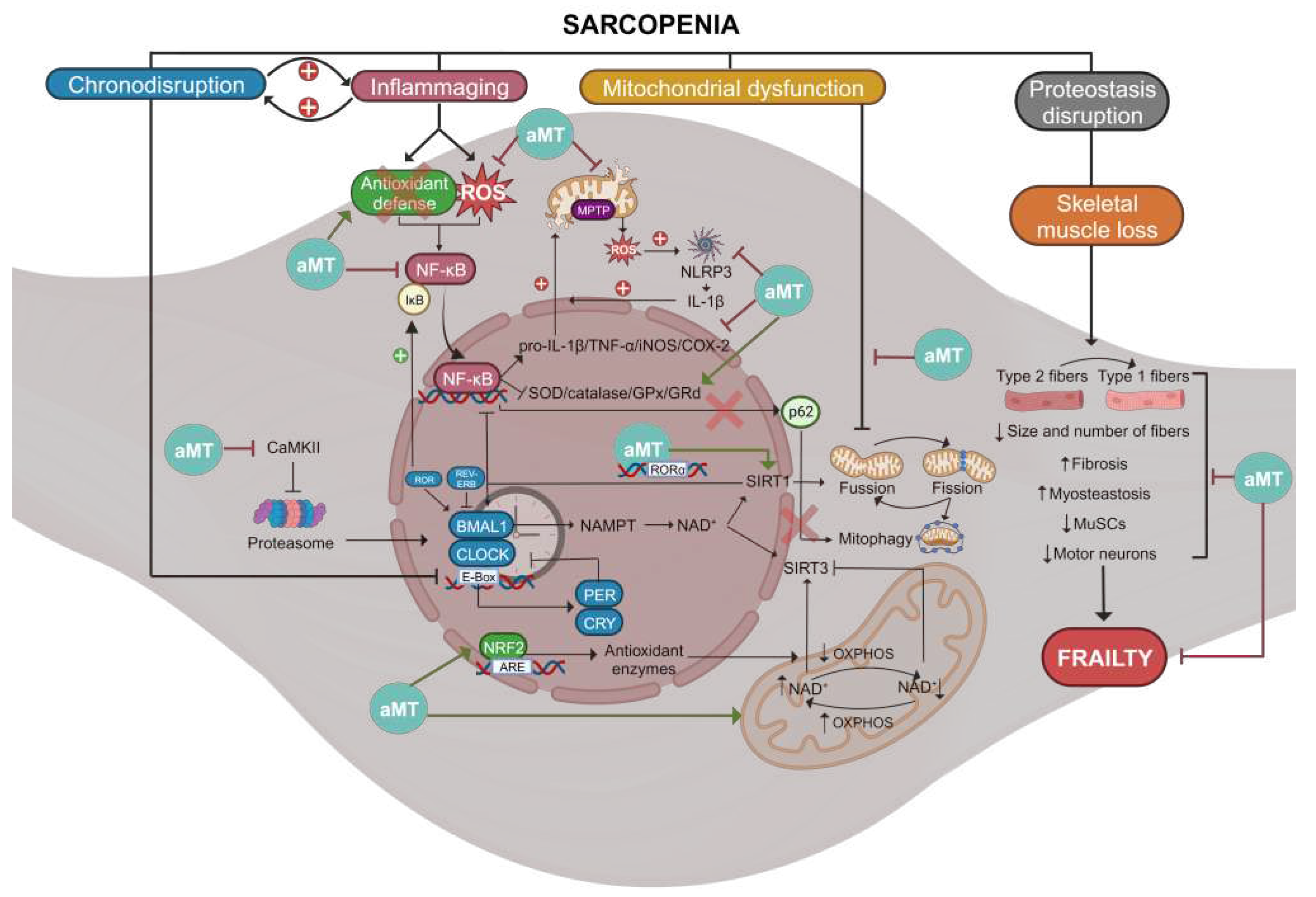
3.3. Melatonin as a Link between Clock Genes and Mitochondria in Sarcopenia
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Otín, C.; Blasco, M. A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153 (6), 1194-217.
- López-Otín, C.; Blasco, M. A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186 (2), 243-278. [CrossRef]
- Angulo, J.; El Assar, M.; Rodríguez-Mañas, L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol. Asp. Med. 2016, 50, 1-32. [CrossRef]
- Rosenberg, I. H. Sarcopenia: origins and clinical relevance. J. Nutr. 1997, 127 (5 Suppl), 990s-991s.
- Cruz-Jentoft, A. J.; Sayer, A. A. Sarcopenia. Lancet 2019, 393 (10191), 2636-2646.
- Anker, S. D.; Morley, J. E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7 (5), 512-514. [CrossRef]
- Cruz-Jentoft, A. J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A. A.; Schneider, S. M.; Sieber, C. C.; Topinkova, E.; Vandewoude, M.; Visser, M.; Zamboni, M. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019, 48 (1), 16-31. [CrossRef]
- Fielding, R. A.; Vellas, B.; Evans, W. J.; Bhasin, S.; Morley, J. E.; Newman, A. B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; Cederholm, T.; Chandler, J.; De Meynard, C.; Donini, L.; Harris, T.; Kannt, A.; Keime Guibert, F.; Onder, G.; Papanicolaou, D.; Rolland, Y.; Rooks, D.; Sieber, C.; Souhami, E.; Verlaan, S.; Zamboni, M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12 (4), 249-56. [CrossRef]
- Cruz-Jentoft, A. J. Sarcopenia, the last organ insufficiency. Eur. Geriatr. Med. 2016, 7 (3), 195-196. [CrossRef]
- Ferrucci, L.; de Cabo, R.; Knuth, N. D.; Studenski, S. Of Greek heroes, wiggling worms, mighty mice, and old body builders. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67 (1), 13-6. [CrossRef]
- Morley, J. E.; Abbatecola, A. M.; Argiles, J. M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Coats, A. J.; Cummings, S. R.; Evans, W. J.; Fearon, K.; Ferrucci, L.; Fielding, R. A.; Guralnik, J. M.; Harris, T. B.; Inui, A.; Kalantar-Zadeh, K.; Kirwan, B. A.; Mantovani, G.; Muscaritoli, M.; Newman, A. B.; Rossi-Fanelli, F.; Rosano, G. M.; Roubenoff, R.; Schambelan, M.; Sokol, G. H.; Storer, T. W.; Vellas, B.; von Haehling, S.; Yeh, S. S.; Anker, S. D. Sarcopenia with limited mobility: an international consensus. J. Am. Med. Dir. Assoc. 2011, 12 (6), 403-9. [CrossRef]
- Scott, D.; Sanders, K. M.; Aitken, D.; Hayes, A.; Ebeling, P. R.; Jones, G. Sarcopenic obesity and dynapenic obesity: 5-year associations with falls risk in middle-aged and older adults. Obesity 2014, 22 (6), 1568-74. [CrossRef]
- Thomas, D. R. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin. Nutr. 2007, 26 (4), 389-99. [CrossRef]
- Jeejeebhoy, K. N. Malnutrition, fatigue, frailty, vulnerability, sarcopenia and cachexia: overlap of clinical features. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15 (3), 213-9.
- Cruz-Jentoft, A. J.; Landi, F.; Schneider, S. M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L. K.; Fielding, R. A.; Martin, F. C.; Michel, J. P.; Sieber, C.; Stout, J. R.; Studenski, S. A.; Vellas, B.; Woo, J.; Zamboni, M.; Cederholm, T. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43 (6), 748-59. [CrossRef]
- Peterson, M. D.; Rhea, M. R.; Sen, A.; Gordon, P. M. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res. Rev. 2010, 9 (3), 226-37. [CrossRef]
- Peterson, M. D.; Sen, A.; Gordon, P. M. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med. Sci. Sports Exerc. 2011, 43 (2), 249-58.
- Suetta, C.; Andersen, J. L.; Dalgas, U.; Berget, J.; Koskinen, S.; Aagaard, P.; Magnusson, S. P.; Kjaer, M. Resistance training induces qualitative changes in muscle morphology, muscle architecture, and muscle function in elderly postoperative patients. J. Appl. Physiol. 2008, 105 (1), 180-6. [CrossRef]
- Vlietstra, L.; Hendrickx, W.; Waters, D. L. Exercise interventions in healthy older adults with sarcopenia: A systematic review and meta-analysis. Australas. Ageing 2018, 37 (3), 169-183. [CrossRef]
- Lozano-Montoya, I.; Correa-Pérez, A.; Abraha, I.; Soiza, R. L.; Cherubini, A.; O'Mahony, D.; Cruz-Jentoft, A. J. Nonpharmacological interventions to treat physical frailty and sarcopenia in older patients: a systematic overview - the SENATOR Project ONTOP Series. Clin. Interv. Aging 2017, 12, 721-740. [CrossRef]
- Robinson, S. M.; Reginster, J. Y.; Rizzoli, R.; Shaw, S. C.; Kanis, J. A.; Bautmans, I.; Bischoff-Ferrari, H.; Bruyère, O.; Cesari, M.; Dawson-Hughes, B.; Fielding, R. A.; Kaufman, J. M.; Landi, F.; Malafarina, V.; Rolland, Y.; van Loon, L. J.; Vellas, B.; Visser, M.; Cooper, C. Does nutrition play a role in the prevention and management of sarcopenia? Clin. Nutr. 2018, 37 (4), 1121-1132.
- Cho, M. R.; Lee, S.; Song, S. K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37 (18), e146. [CrossRef]
- Buford, T. W.; Anton, S. D.; Judge, A. R.; Marzetti, E.; Wohlgemuth, S. E.; Carter, C. S.; Leeuwenburgh, C.; Pahor, M.; Manini, T. M. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res. Rev. 2010, 9 (4), 369-83. [CrossRef]
- Grassi, B.; Cerretelli, P.; Narici, M. V.; Marconi, C. Peak anaerobic power in master athletes. Eur. J. Appl. Physiol. Occup. Physiol. 1991, 62 (6), 394-9. [CrossRef]
- Pearson, S. J.; Young, A.; Macaluso, A.; Devito, G.; Nimmo, M. A.; Cobbold, M.; Harridge, S. D. Muscle function in elite master weightlifters. Med. Sci. Sports Exerc. 2002, 34 (7), 1199-206. [CrossRef]
- Brisswalter, J.; Nosaka, K. Neuromuscular factors associated with decline in long-distance running performance in master athletes. Sports Med. 2013, 43 (1), 51-63. [CrossRef]
- Frontera, W. R.; Hughes, V. A.; Fielding, R. A.; Fiatarone, M. A.; Evans, W. J.; Roubenoff, R. Aging of skeletal muscle: a 12-yr longitudinal study. J. Appl. Physiol. 2000, 88 (4), 1321-6. [CrossRef]
- Ciciliot, S.; Rossi, A. C.; Dyar, K. A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45 (10), 2191-9. [CrossRef]
- Murgia, M.; Nogara, L.; Baraldo, M.; Reggiani, C.; Mann, M.; Schiaffino, S. Protein profile of fiber types in human skeletal muscle: a single-fiber proteomics study. Skelet. Muscle 2021, 11 (1), 24. [CrossRef]
- Hastings, R. L.; Massopust, R. T.; Haddix, S. G.; Lee, Y. I.; Thompson, W. J. Exclusive vital labeling of myonuclei for studying myonuclear arrangement in mouse skeletal muscle tissue. Skelet. Muscle 2020, 10 (1), 15. [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 Suppl 1, S4-9. [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15 (9), 505-522. [CrossRef]
- Viña, J.; Tarazona-Santabalbina, F. J.; Pérez-Ros, P.; Martínez-Arnau, F. M.; Borras, C.; Olaso-Gonzalez, G.; Salvador-Pascual, A.; Gomez-Cabrera, M. C. Biology of frailty: Modulation of ageing genes and its importance to prevent age-associated loss of function. Mol. Asp. Med. 2016, 50, 88-108. [CrossRef]
- Acuña-Castroviejo, D.; Rahim, I.; Acuña-Fernández, C.; Fernández-Ortiz, M.; Solera-Marín, J.; Sayed, R. K. A.; Díaz-Casado, M. E.; Rusanova, I.; López, L. C.; Escames, G. Melatonin, clock genes and mitochondria in sepsis. Cell Mol. Life Sci. 2017, 74 (21), 3965-3987. [CrossRef]
- Ristow, M.; Zarse, K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp. Gerontol. 2010, 45 (6), 410-8. [CrossRef]
- García, J. A.; Volt, H.; Venegas, C.; Doerrier, C.; Escames, G.; López, L. C.; Acuña-Castroviejo, D. Disruption of the NF-κB/NLRP3 connection by melatonin requires retinoid-related orphan receptor-α and blocks the septic response in mice. Faseb J. 2015, 29 (9), 3863-75.
- Nakahira, K.; Haspel, J. A.; Rathinam, V. A.; Lee, S. J.; Dolinay, T.; Lam, H. C.; Englert, J. A.; Rabinovitch, M.; Cernadas, M.; Kim, H. P.; Fitzgerald, K. A.; Ryter, S. W.; Choi, A. M. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011, 12 (3), 222-30. [CrossRef]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S. R.; McGeough, M. D.; Ellisman, M. H.; Seki, E.; Gustafsson, A. B.; Hoffman, H. M.; Diaz-Meco, M. T.; Moscat, J.; Karin, M. NF-κB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell 2016, 164 (5), 896-910.
- Marzetti, E.; Calvani, R.; Bernabei, R.; Leeuwenburgh, C. Apoptosis in skeletal myocytes: a potential target for interventions against sarcopenia and physical frailty - a mini-review. Gerontology 2012, 58 (2), 99-106. [CrossRef]
- Buhr, E. D.; Takahashi, J. S. Molecular components of the Mammalian circadian clock. Handb. Exp. Pharmacol. 2013, (217), 3-27. [CrossRef]
- Lowrey, P. L.; Takahashi, J. S. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu. Rev. Genomics Hum. Genet. 2004, 5, 407-41. [CrossRef]
- Reiter, R. J. The melatonin rhythm: both a clock and a calendar. Experientia 1993, 49 (8), 654-64. [CrossRef]
- Arosio, B.; Calvani, R.; Ferri, E.; Coelho-Junior, H. J.; Carandina, A.; Campanelli, F.; Ghiglieri, V.; Marzetti, E.; Picca, A. Sarcopenia and Cognitive Decline in Older Adults: Targeting the Muscle-Brain Axis. Nutrients 2023, 15 (8). [CrossRef]
- Scisciola, L.; Fontanella, R. A.; Surina; Cataldo, V.; Paolisso, G.; Barbieri, M. Sarcopenia and Cognitive Function: Role of Myokines in Muscle Brain Cross-Talk. Life-Basel 2021, 11 (2). [CrossRef]
- Hattar, S.; Liao, H. W.; Takao, M.; Berson, D. M.; Yau, K. W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 2002, 295 (5557), 1065-70. [CrossRef]
- Harrington, M. E. The ventral lateral geniculate nucleus and the intergeniculate leaflet: interrelated structures in the visual and circadian systems. Neurosci. Biobehav. Rev. 1997, 21 (5), 705-27. [CrossRef]
- Rosenwasser, A. M.; Turek, F. W. Neurobiology of Circadian Rhythm Regulation. Sleep Med. Clin. 2015, 10 (4), 403-12. [CrossRef]
- Perreau-Lenz, S.; Pévet, P.; Buijs, R. M.; Kalsbeek, A. The biological clock: the bodyguard of temporal homeostasis. Chronobiol. Int. 2004, 21 (1), 1-25. [CrossRef]
- Kalsbeek, A.; Palm, I. F.; La Fleur, S. E.; Scheer, F. A.; Perreau-Lenz, S.; Ruiter, M.; Kreier, F.; Cailotto, C.; Buijs, R. M. SCN outputs and the hypothalamic balance of life. J. Biol. Rhythms 2006, 21 (6), 458-69. [CrossRef]
- Vriend, J.; Reiter, R. J. Melatonin feedback on clock genes: a theory involving the proteasome. J. Pineal Res. 2015, 58 (1), 1-11. [CrossRef]
- Cox, K. H.; Takahashi, J. S. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol. 2019, 63 (4), R93-r102. [CrossRef]
- Goriki, A.; Hatanaka, F.; Myung, J.; Kim, J. K.; Yoritaka, T.; Tanoue, S.; Abe, T.; Kiyonari, H.; Fujimoto, K.; Kato, Y.; Todo, T.; Matsubara, A.; Forger, D.; Takumi, T. A novel protein, CHRONO, functions as a core component of the mammalian circadian clock. PLoS. Biol. 2014, 12 (4), e1001839. [CrossRef]
- Lefta, M.; Wolff, G.; Esser, K. A. Circadian rhythms, the molecular clock, and skeletal muscle. Curr. Top. Dev. Biol. 2011, 96, 231-71.
- Zehring, W. A.; Wheeler, D. A.; Reddy, P.; Konopka, R. J.; Kyriacou, C. P.; Rosbash, M.; Hall, J. C. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell 1984, 39 (2 Pt 1), 369-76. [CrossRef]
- Bargiello, T. A.; Jackson, F. R.; Young, M. W. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature 1984, 312 (5996), 752-4. [CrossRef]
- Siwicki, K. K.; Eastman, C.; Petersen, G.; Rosbash, M.; Hall, J. C. Antibodies to the period gene product of Drosophila reveal diverse tissue distribution and rhythmic changes in the visual system. Neuron 1988, 1 (2), 141-50. [CrossRef]
- Hardin, P. E.; Hall, J. C.; Rosbash, M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 1990, 343 (6258), 536-40. [CrossRef]
- Liu, X.; Zwiebel, L. J.; Hinton, D.; Benzer, S.; Hall, J. C.; Rosbash, M. The period gene encodes a predominantly nuclear protein in adult Drosophila. J. Neurosci. 1992, 12 (7), 2735-44. [CrossRef]
- Vosshall, L. B.; Price, J. L.; Sehgal, A.; Saez, L.; Young, M. W. Block in nuclear localization of period protein by a second clock mutation, timeless. Science 1994, 263 (5153), 1606-9.
- Price, J. L.; Blau, J.; Rothenfluh, A.; Abodeely, M.; Kloss, B.; Young, M. W. double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell 1998, 94 (1), 83-95. [CrossRef]
- Abbott, S. M.; Malkani, R. G.; Zee, P. C. Circadian disruption and human health: A bidirectional relationship. Eur. J. Neurosci. 2020, 51 (1), 567-583. [CrossRef]
- Erren, T. C.; Reiter, R. J. Defining chronodisruption. J. Pineal Res. 2009, 46 (3), 245-7. [CrossRef]
- Verma, A. K.; Singh, S.; Rizvi, S. I. Aging, circadian disruption and neurodegeneration: Interesting interplay. Exp. Gerontol. 2023, 172, 112076. [CrossRef]
- Wolff, C. A.; Gutierrez-Monreal, M. A.; Meng, L.; Zhang, X.; Douma, L. G.; Costello, H. M.; Douglas, C. M.; Ebrahimi, E.; Pham, A.; Oliveira, A. C.; Fu, C.; Nguyen, A.; Alava, B. R.; Hesketh, S. J.; Morris, A. R.; Endale, M. M.; Crislip, G. R.; Cheng, K. Y.; Schroder, E. A.; Delisle, B. P.; Bryant, A. J.; Gumz, M. L.; Huo, Z.; Liu, A. C.; Esser, K. A. Defining the age-dependent and tissue-specific circadian transcriptome in male mice. Cell Rep. 2023, 42 (1), 111982. [CrossRef]
- Welz, P. S.; Benitah, S. A. Molecular Connections Between Circadian Clocks and Aging. J. Mol. Biol. 2020, 432 (12), 3661-3679. [CrossRef]
- Morena da Silva, F.; Esser, K. A.; Murach, K. A.; Greene, N. P. Inflammation o'clock: interactions of circadian rhythms with inflammation-induced skeletal muscle atrophy. J. Physiol. 2023. [CrossRef]
- Curtis, A. M.; Bellet, M. M.; Sassone-Corsi, P.; O'Neill, L. A. Circadian clock proteins and immunity. Immunity 2014, 40 (2), 178-86. [CrossRef]
- Hood, S.; Amir, S. The aging clock: circadian rhythms and later life. J. Clin. Invest. 2017, 127 (2), 437-446. [CrossRef]
- Spengler, M. L.; Kuropatwinski, K. K.; Comas, M.; Gasparian, A. V.; Fedtsova, N.; Gleiberman, A. S.; Gitlin, II; Artemicheva, N. M.; Deluca, K. A.; Gudkov, A. V.; Antoch, M. P. Core circadian protein CLOCK is a positive regulator of NF-κB-mediated transcription. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (37), E2457-65.
- Nguyen, K. D.; Fentress, S. J.; Qiu, Y.; Yun, K.; Cox, J. S.; Chawla, A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 2013, 341 (6153), 1483-8.
- Yeung, F.; Hoberg, J. E.; Ramsey, C. S.; Keller, M. D.; Jones, D. R.; Frye, R. A.; Mayo, M. W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. Embo J. 2004, 23 (12), 2369-80.
- Peek, C. B.; Affinati, A. H.; Ramsey, K. M.; Kuo, H. Y.; Yu, W.; Sena, L. A.; Ilkayeva, O.; Marcheva, B.; Kobayashi, Y.; Omura, C.; Levine, D. C.; Bacsik, D. J.; Gius, D.; Newgard, C. B.; Goetzman, E.; Chandel, N. S.; Denu, J. M.; Mrksich, M.; Bass, J. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 2013, 342 (6158), 1243417. [CrossRef]
- Volt, H.; García, J. A.; Doerrier, C.; Díaz-Casado, M. E.; Guerra-Librero, A.; López, L. C.; Escames, G.; Tresguerres, J. A.; Acuña-Castroviejo, D. Same molecule but different expression: aging and sepsis trigger NLRP3 inflammasome activation, a target of melatonin. J. Pineal Res. 2016, 60 (2), 193-205. [CrossRef]
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 2009, 324 (5927), 654-7. [CrossRef]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F. W.; Schibler, U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008, 134 (2), 317-28. [CrossRef]
- Delerive, P.; Monté, D.; Dubois, G.; Trottein, F.; Fruchart-Najib, J.; Mariani, J.; Fruchart, J. C.; Staels, B. The orphan nuclear receptor ROR alpha is a negative regulator of the inflammatory response. Embo Rep. 2001, 2 (1), 42-8.
- Gibbs, J. E.; Blaikley, J.; Beesley, S.; Matthews, L.; Simpson, K. D.; Boyce, S. H.; Farrow, S. N.; Else, K. J.; Singh, D.; Ray, D. W.; Loudon, A. S. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (2), 582-7. [CrossRef]
- Liu, J.; Malkani, G.; Shi, X.; Meyer, M.; Cunningham-Runddles, S.; Ma, X.; Sun, Z. S. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect. Immun. 2006, 74 (8), 4750-6. [CrossRef]
- Preitner, N.; Damiola, F.; Lopez-Molina, L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110 (2), 251-60.
- Narasimamurthy, R.; Hatori, M.; Nayak, S. K.; Liu, F.; Panda, S.; Verma, I. M. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (31), 12662-7. [CrossRef]
- Keller, M.; Mazuch, J.; Abraham, U.; Eom, G. D.; Herzog, E. D.; Volk, H. D.; Kramer, A.; Maier, B. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (50), 21407-12. [CrossRef]
- Fernández-Ortiz, M.; Sayed, R. K. A.; Román-Montoya, Y.; de Lama MÁ, R.; Fernández-Martínez, J.; Ramírez-Casas, Y.; Florido-Ruiz, J.; Rusanova, I.; Escames, G.; Acuña-Castroviejo, D. Age and Chronodisruption in Mouse Heart: Effect of the NLRP3 Inflammasome and Melatonin Therapy. Int. J. Mol. Sci. 2022, 23 (12).
- Sayed, R. K.; Fernández-Ortiz, M.; Fernández-Martínez, J.; Aranda Martínez, P.; Guerra-Librero, A.; Rodríguez-Santana, C.; de Haro, T.; Escames, G.; Acuña-Castroviejo, D.; Rusanova, I. The Impact of Melatonin and NLRP3 Inflammasome on the Expression of microRNAs in Aged Muscle. Antioxidants 2021, 10 (4). [CrossRef]
- Zhu, Y.; Liu, Y.; Escames, G.; Yang, Z.; Zhao, H.; Qian, L.; Xue, C.; Xu, D.; Acuña-Castroviejo, D.; Yang, Y. Deciphering clock genes as emerging targets against aging. Ageing Res. Rev. 2022, 81, 101725. [CrossRef]
- Reeds, P. J.; Palmer, R. M.; Hay, S. M.; McMillan, D. N. Protein synthesis in skeletal muscle measured at different times during a 24 hour period. Biosci. Rep. 1986, 6 (2), 209-13. [CrossRef]
- McCarthy, J. J.; Andrews, J. L.; McDearmon, E. L.; Campbell, K. S.; Barber, B. K.; Miller, B. H.; Walker, J. R.; Hogenesch, J. B.; Takahashi, J. S.; Esser, K. A. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol. Genomics 2007, 31 (1), 86-95. [CrossRef]
- Miller, B. H.; McDearmon, E. L.; Panda, S.; Hayes, K. R.; Zhang, J.; Andrews, J. L.; Antoch, M. P.; Walker, J. R.; Esser, K. A.; Hogenesch, J. B.; Takahashi, J. S. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc. Natl. Acad. Sci. U. S. A. 2007, 104 (9), 3342-7. [CrossRef]
- Andrews, J. L.; Zhang, X.; McCarthy, J. J.; McDearmon, E. L.; Hornberger, T. A.; Russell, B.; Campbell, K. S.; Arbogast, S.; Reid, M. B.; Walker, J. R.; Hogenesch, J. B.; Takahashi, J. S.; Esser, K. A. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (44), 19090-5. [CrossRef]
- Pastore, S.; Hood, D. A. Endurance training ameliorates the metabolic and performance characteristics of circadian Clock mutant mice. J. Appl. Physiol. 2013, 114 (8), 1076-84.
- Vitaterna, M. H.; King, D. P.; Chang, A. M.; Kornhauser, J. M.; Lowrey, P. L.; McDonald, J. D.; Dove, W. F.; Pinto, L. H.; Turek, F. W.; Takahashi, J. S. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 1994, 264 (5159), 719-25. [CrossRef]
- DeBruyne, J. P.; Weaver, D. R.; Reppert, S. M. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat. Neurosci. 2007, 10 (5), 543-5. [CrossRef]
- Debruyne, J. P.; Noton, E.; Lambert, C. M.; Maywood, E. S.; Weaver, D. R.; Reppert, S. M. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron 2006, 50 (3), 465-77. [CrossRef]
- Dubrovsky, Y. V.; Samsa, W. E.; Kondratov, R. V. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging 2010, 2 (12), 936-44. [CrossRef]
- van der Horst, G. T.; Muijtjens, M.; Kobayashi, K.; Takano, R.; Kanno, S.; Takao, M.; de Wit, J.; Verkerk, A.; Eker, A. P.; van Leenen, D.; Buijs, R.; Bootsma, D.; Hoeijmakers, J. H.; Yasui, A. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 1999, 398 (6728), 627-30. [CrossRef]
- Zheng, B.; Albrecht, U.; Kaasik, K.; Sage, M.; Lu, W.; Vaishnav, S.; Li, Q.; Sun, Z. S.; Eichele, G.; Bradley, A.; Lee, C. C. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 2001, 105 (5), 683-94. [CrossRef]
- Liu, A. C.; Welsh, D. K.; Ko, C. H.; Tran, H. G.; Zhang, E. E.; Priest, A. A.; Buhr, E. D.; Singer, O.; Meeker, K.; Verma, I. M.; Doyle, F. J. 3rd; Takahashi, J. S.; Kay, S. A. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 2007, 129 (3), 605-16.
- Woldt, E.; Sebti, Y.; Solt, L. A.; Duhem, C.; Lancel, S.; Eeckhoute, J.; Hesselink, M. K.; Paquet, C.; Delhaye, S.; Shin, Y.; Kamenecka, T. M.; Schaart, G.; Lefebvre, P.; Nevière, R.; Burris, T. P.; Schrauwen, P.; Staels, B.; Duez, H. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 2013, 19 (8), 1039-46. [CrossRef]
- Zhu, P.; Hamlish, N. X.; Thakkar, A. V.; Steffeck, A. W. T.; Rendleman, E. J.; Khan, N. H.; Waldeck, N. J.; DeVilbiss, A. W.; Martin-Sandoval, M. S.; Mathews, T. P.; Chandel, N. S.; Peek, C. B. BMAL1 drives muscle repair through control of hypoxic NAD(+) regeneration in satellite cells. Genes Dev. 2022, 36 (3-4), 149-166. [CrossRef]
- Chatterjee, S.; Yin, H.; Nam, D.; Li, Y.; Ma, K. Brain and muscle Arnt-like 1 promotes skeletal muscle regeneration through satellite cell expansion. Exp. Cell Res. 2015, 331 (1), 200-210. [CrossRef]
- Wada, T.; Ichihashi, Y.; Suzuki, E.; Kosuge, Y.; Ishige, K.; Uchiyama, T.; Makishima, M.; Nakao, R.; Oishi, K.; Shimba, S. Deletion of Bmal1 Prevents Diet-Induced Ectopic Fat Accumulation by Controlling Oxidative Capacity in the Skeletal Muscle. Int. J. Mol. Sci. 2018, 19 (9). [CrossRef]
- Kohsaka, A.; Das, P.; Hashimoto, I.; Nakao, T.; Deguchi, Y.; Gouraud, S. S.; Waki, H.; Muragaki, Y.; Maeda, M. The circadian clock maintains cardiac function by regulating mitochondrial metabolism in mice. PLoS One 2014, 9 (11), e112811. [CrossRef]
- de Goede, P.; Wefers, J.; Brombacher, E. C.; Schrauwen, P.; Kalsbeek, A. Circadian rhythms in mitochondrial respiration. J. Mol. Endocrinol. 2018, 60 (3), R115-r130. [CrossRef]
- van Moorsel, D.; Hansen, J.; Havekes, B.; Scheer, F.; Jörgensen, J. A.; Hoeks, J.; Schrauwen-Hinderling, V. B.; Duez, H.; Lefebvre, P.; Schaper, N. C.; Hesselink, M. K. C.; Staels, B.; Schrauwen, P. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol. Metab. 2016, 5 (8), 635-645. [CrossRef]
- Chen, G.; Tang, Q.; Yu, S.; Shen, Y.; Sun, J.; Peng, J.; Yin, Y.; Feng, G.; Lu, X.; Mei, G.; Zhang, Y.; Wan, Q.; Zhang, L.; Chen, L. Developmental growth plate cartilage formation suppressed by artificial light at night via inhibiting BMAL1-driven collagen hydroxylation. Cell Death Differ. 2023, 30 (6), 1503-1516. [CrossRef]
- Schroder, E. A.; Harfmann, B. D.; Zhang, X.; Srikuea, R.; England, J. H.; Hodge, B. A.; Wen, Y.; Riley, L. A.; Yu, Q.; Christie, A.; Smith, J. D.; Seward, T.; Wolf Horrell, E. M.; Mula, J.; Peterson, C. A.; Butterfield, T. A.; Esser, K. A. Intrinsic muscle clock is necessary for musculoskeletal health. J. Physiol. 2015, 593 (24), 5387-404. [CrossRef]
- Bunger, M. K.; Wilsbacher, L. D.; Moran, S. M.; Clendenin, C.; Radcliffe, L. A.; Hogenesch, J. B.; Simon, M. C.; Takahashi, J. S.; Bradfield, C. A. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 2000, 103 (7), 1009-17. [CrossRef]
- Kondratov, R. V.; Kondratova, A. A.; Gorbacheva, V. Y.; Vykhovanets, O. V.; Antoch, M. P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006, 20 (14), 1868-73.
- Rudic, R. D.; McNamara, P.; Curtis, A. M.; Boston, R. C.; Panda, S.; Hogenesch, J. B.; Fitzgerald, G. A. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004, 2 (11), e377. [CrossRef]
- Fernández-Martínez, J.; Ramírez-Casas, Y.; Aranda-Martínez, P.; López-Rodríguez, A.; Sayed, R. K. A.; Escames, G.; Acuña-Castroviejo, D. iMS-Bmal1(-/-) mice show evident signs of sarcopenia that are counteracted by exercise and melatonin therapies. J. Pineal Res. 2023, e12912.
- Hodge, B. A.; Wen, Y.; Riley, L. A.; Zhang, X.; England, J. H.; Harfmann, B. D.; Schroder, E. A.; Esser, K. A. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet. Muscle 2015, 5, 17. [CrossRef]
- Dyar, K. A.; Ciciliot, S.; Wright, L. E.; Biensø, R. S.; Tagliazucchi, G. M.; Patel, V. R.; Forcato, M.; Paz, M. I.; Gudiksen, A.; Solagna, F.; Albiero, M.; Moretti, I.; Eckel-Mahan, K. L.; Baldi, P.; Sassone-Corsi, P.; Rizzuto, R.; Bicciato, S.; Pilegaard, H.; Blaauw, B.; Schiaffino, S. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab. 2014, 3 (1), 29-41. [CrossRef]
- Lerner, A. B.; Case, J. D.; Takahashi, Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J. Biol. Chem. 1960, 235, 1992-7. [CrossRef]
- Hardeland, R.; Pandi-Perumal, S. R.; Cardinali, D. P. Melatonin. Int. J. Biochem. Cell Biol. 2006, 38 (3), 313-6.
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M. E.; Lima-Cabello, E.; López, L. C.; Rosales-Corral, S.; Tan, D. X.; Reiter, R. J. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol. Life Sci. 2014, 71 (16), 2997-3025. [CrossRef]
- Stefulj, J.; Hörtner, M.; Ghosh, M.; Schauenstein, K.; Rinner, I.; Wölfler, A.; Semmler, J.; Liebmann, P. M. Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. J. Pineal Res. 2001, 30 (4), 243-7. [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R. J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [CrossRef]
- Liu, T.; Borjigin, J. N-acetyltransferase is not the rate-limiting enzyme of melatonin synthesis at night. J Pineal Res 2005, 39 (1), 91-6. [CrossRef]
- Venegas, C.; García, J. A.; Escames, G.; Ortiz, F.; López, A.; Doerrier, C.; García-Corzo, L.; López, L. C.; Reiter, R. J.; Acuña-Castroviejo, D. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52 (2), 217-27. [CrossRef]
- Ma, X.; Idle, J. R.; Krausz, K. W.; Gonzalez, F. J. Metabolism of melatonin by human cytochromes p450. Drug Metab. Dispos. 2005, 33 (4), 489-94. [CrossRef]
- Tian, X.; Huo, X.; Dong, P.; Wu, B.; Wang, X.; Wang, C.; Liu, K.; Ma, X. Sulfation of melatonin: enzymatic characterization, differences of organs, species and genders, and bioactivity variation. Biochem. Pharmacol. 2015, 94 (4), 282-96. [CrossRef]
- Tan, D. X.; Manchester, L. C.; Terron, M. P.; Flores, L. J.; Reiter, R. J. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42 (1), 28-42.
- Costa, E. J.; Lopes, R. H.; Lamy-Freund, M. T. Permeability of pure lipid bilayers to melatonin. J Pineal Res 1995, 19 (3), 123-6. [CrossRef]
- Slominski, R. M.; Reiter, R. J.; Schlabritz-Loutsevitch, N.; Ostrom, R. S.; Slominski, A. T. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol. Cell. Endocrinol. 2012, 351 (2), 152-66. [CrossRef]
- Aranda-Martínez, P.; Fernández-Martínez, J.; Ramírez-Casas, Y.; Guerra-Librero, A.; Rodríguez-Santana, C.; Escames, G.; Acuña-Castroviejo, D. The Zebrafish, an Outstanding Model for Biomedical Research in the Field of Melatonin and Human Diseases. Int. J. Mol. Sci. 2022, 23 (13). [CrossRef]
- Dubocovich, M. L. Melatonin receptors: are there multiple subtypes? Trends Pharmacol. Sci. 1995, 16 (2), 50-6.
- Nosjean, O.; Ferro, M.; Coge, F.; Beauverger, P.; Henlin, J. M.; Lefoulon, F.; Fauchere, J. L.; Delagrange, P.; Canet, E.; Boutin, J. A. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J. Biol. Chem. 2000, 275 (40), 31311-7. [CrossRef]
- Benítez-King, G.; Antón-Tay, F. Calmodulin mediates melatonin cytoskeletal effects. Experientia 1993, 49 (8), 635-41. [CrossRef]
- Benítez-King, G.; Ríos, A.; Martínez, A.; Antón-Tay, F. In vitro inhibition of Ca2+/calmodulin-dependent kinase II activity by melatonin. Biochim. Biophys. Acta 1996, 1290 (2), 191-6.
- Macías, M.; Escames, G.; Leon, J.; Coto, A.; Sbihi, Y.; Osuna, A.; Acuña-Castroviejo, D. Calreticulin-melatonin. An unexpected relationship. Eur. J. Biochem. 2003, 270 (5), 832-40.
- Cardinali, D. P.; Freire, F. Melatonin effects on brain. Interaction with microtubule protein, inhibition of fast axoplasmic flow and induction of crystaloid and tubular formations in the hypothalamus. Mol. Cell. Endocrinol. 1975, 2 (5), 317-30. [CrossRef]
- Jetten, A. M. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal. 2009, 7, e003. [CrossRef]
- Menendez-Pelaez, A.; Poeggeler, B.; Reiter, R. J.; Barlow-Walden, L.; Pablos, M. I.; Tan, D. X. Nuclear localization of melatonin in different mammalian tissues: immunocytochemical and radioimmunoassay evidence. J. Cell. Biochem. 1993, 53 (4), 373-82. [CrossRef]
- Acuña-Castroviejo, D.; Pablos, M. I.; Menendez-Pelaez, A.; Reiter, R. J. Melatonin receptors in purified cell nuclei of liver. Res. Commun. Chem. Pathol. Pharmacol. 1993, 82 (2), 253-6.
- Acuña-Castroviejo, D.; Reiter, R. J.; Menéndez-Peláez, A.; Pablos, M. I.; Burgos, A. Characterization of high-affinity melatonin binding sites in purified cell nuclei of rat liver. J. Pineal Res. 1994, 16 (2), 100-12. [CrossRef]
- Becker-André, M.; Wiesenberg, I.; Schaeren-Wiemers, N.; André, E.; Missbach, M.; Saurat, J. H.; Carlberg, C. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 1994, 269 (46), 28531-4. [CrossRef]
- Wiesenberg, I.; Missbach, M.; Kahlen, J. P.; Schräder, M.; Carlberg, C. Transcriptional activation of the nuclear receptor RZR alpha by the pineal gland hormone melatonin and identification of CGP 52608 as a synthetic ligand. Nucleic Acids Res. 1995, 23 (3), 327-33.
- Carlberg, C.; Wiesenberg, I. The orphan receptor family RZR/ROR, melatonin and 5-lipoxygenase: an unexpected relationship. J. Pineal Res. 1995, 18 (4), 171-8.
- Venegas, C.; García, J. A.; Doerrier, C.; Volt, H.; Escames, G.; López, L. C.; Reiter, R. J.; Acuña-Castroviejo, D. Analysis of the daily changes of melatonin receptors in the rat liver. J. Pineal Res. 2013, 54 (3), 313-21. [CrossRef]
- Acuña Castroviejo, D.; López, L. C.; Escames, G.; López, A.; García, J. A.; Reiter, R. J. Melatonin-mitochondria interplay in health and disease. Curr. Top. Med. Chem. 2011, 11 (2), 221-40. [CrossRef]
- Gatfield, D.; Schibler, U. Physiology. Proteasomes keep the circadian clock ticking. Science 2007, 316 (5828), 1135-6. [CrossRef]
- Stojkovic, K.; Wing, S. S.; Cermakian, N. A central role for ubiquitination within a circadian clock protein modification code. Front. Molec. Neurosci. 2014, 7, 69.
- Vriend, J.; Reiter, R. J. Melatonin as a proteasome inhibitor. Is there any clinical evidence? Life Sci. 2014, 115 (1-2), 8-14.
- León, J.; Macías, M.; Escames, G.; Camacho, E.; Khaldy, H.; Martín, M.; Espinosa, A.; Gallo, M. A.; Acuña-Castroviejo, D. Structure-related inhibition of calmodulin-dependent neuronal nitric-oxide synthase activity by melatonin and synthetic kynurenines. Mol. Pharmacol. 2000, 58 (5), 967-75. [CrossRef]
- Fukunaga, K.; Horikawa, K.; Shibata, S.; Takeuchi, Y.; Miyamoto, E. Ca2+/calmodulin-dependent protein kinase II-dependent long-term potentiation in the rat suprachiasmatic nucleus and its inhibition by melatonin. J. Neurosci. Res. 2002, 70 (6), 799-807.
- Jarome, T. J.; Kwapis, J. L.; Ruenzel, W. L.; Helmstetter, F. J. CaMKII, but not protein kinase A, regulates Rpt6 phosphorylation and proteasome activity during the formation of long-term memories. Front. Behav. Neurosci. 2013, 7, 115. [CrossRef]
- Chong, N. W.; Bernard, M.; Klein, D. C. Characterization of the chicken serotonin N-acetyltransferase gene. Activation via clock gene heterodimer/E box interaction. J. Biol. Chem. 2000, 275 (42), 32991-8. [CrossRef]
- Acuna-Castroviejo, D.; Escames, G.; Rodriguez, M. I.; Lopez, L. C. Melatonin role in the mitochondrial function. Front. Biosci. 2007, 12, 947-63. [CrossRef]
- Zhang, H.; Squadrito, G. L.; Uppu, R.; Pryor, W. A. Reaction of peroxynitrite with melatonin: A mechanistic study. Chem. Res. Toxicol. 1999, 12 (6), 526-34. [CrossRef]
- Acuña-Castroviejo, D.; Martín, M.; Macías, M.; Escames, G.; León, J.; Khaldy, H.; Reiter, R. J. Melatonin, mitochondria, and cellular bioenergetics. J. Pineal Res. 2001, 30 (2), 65-74.
- Tomás-Zapico, C.; Coto-Montes, A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J. Pineal Res. 2005, 39 (2), 99-104. [CrossRef]
- Rodriguez, C.; Mayo, J. C.; Sainz, R. M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R. J. Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 2004, 36 (1), 1-9. [CrossRef]
- Escames, G.; López, L. C.; Tapias, V.; Utrilla, P.; Reiter, R. J.; Hitos, A. B.; León, J.; Rodríguez, M. I.; Acuña-Castroviejo, D. Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J. Pineal Res. 2006, 40 (1), 71-8. [CrossRef]
- Martín, M.; Macías, M.; Escames, G.; León, J.; Acuña-Castroviejo, D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. Faseb J. 2000, 14 (12), 1677-9. [CrossRef]
- Urata, Y.; Honma, S.; Goto, S.; Todoroki, S.; Iida, T.; Cho, S.; Honma, K.; Kondo, T. Melatonin induces gamma-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radic. Biol. Med. 1999, 27 (7-8), 838-47.
- Pierrefiche, G.; Laborit, H. Oxygen free radicals, melatonin, and aging. Exp. Gerontol. 1995, 30 (3-4), 213-27.
- Leon, J.; Acuña-Castroviejo, D.; Sainz, R. M.; Mayo, J. C.; Tan, D. X.; Reiter, R. J. Melatonin and mitochondrial function. Life Sci. 2004, 75 (7), 765-90. [CrossRef]
- Martín, M.; Macías, M.; Escames, G.; Reiter, R. J.; Agapito, M. T.; Ortiz, G. G.; Acuña-Castroviejo, D. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J. Pineal Res. 2000, 28 (4), 242-8. [CrossRef]
- Martín, M.; Macías, M.; León, J.; Escames, G.; Khaldy, H.; Acuña-Castroviejo, D. Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int. J. Biochem. Cell Biol. 2002, 34 (4), 348-57. [CrossRef]
- García, J. J.; Piñol-Ripoll, G.; Martínez-Ballarín, E.; Fuentes-Broto, L.; Miana-Mena, F. J.; Venegas, C.; Caballero, B.; Escames, G.; Coto-Montes, A.; Acuña-Castroviejo, D. Melatonin reduces membrane rigidity and oxidative damage in the brain of SAMP8 mice. Neurobiol. Aging 2011, 32 (11), 2045-54. [CrossRef]
- López, A.; García, J. A.; Escames, G.; Venegas, C.; Ortiz, F.; López, L. C.; Acuña-Castroviejo, D. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J. Pineal Res. 2009, 46 (2), 188-98. [CrossRef]
- Hood, D. A.; Memme, J. M.; Oliveira, A. N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19-41. [CrossRef]
- Deng, W. G.; Tang, S. T.; Tseng, H. P.; Wu, K. K. Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood 2006, 108 (2), 518-24. [CrossRef]
- Rahim, I.; Sayed, R. K.; Fernández-Ortiz, M.; Aranda-Martínez, P.; Guerra-Librero, A.; Fernández-Martínez, J.; Rusanova, I.; Escames, G.; Djerdjouri, B.; Acuña-Castroviejo, D. Melatonin alleviates sepsis-induced heart injury through activating the Nrf2 pathway and inhibiting the NLRP3 inflammasome. Naunyn-Schmiedebergs Arch. Pharmacol. 2021, 394 (2), 261-277. [CrossRef]
- Dinkova-Kostova, A. T.; Abramov, A. Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88 (Pt B), 179-188. [CrossRef]
- Mehrzadi, S.; Pourhanifeh, M. H.; Mirzaei, A.; Moradian, F.; Hosseinzadeh, A. An updated review of mechanistic potentials of melatonin against cancer: pivotal roles in angiogenesis, apoptosis, autophagy, endoplasmic reticulum stress and oxidative stress. Cancer Cell Int. 2021, 21 (1), 188. [CrossRef]
- Sanchez-Hidalgo, M.; de la Lastra, C. A.; Carrascosa-Salmoral, M. P.; Naranjo, M. C.; Gomez-Corvera, A.; Caballero, B.; Guerrero, J. M. Age-related changes in melatonin synthesis in rat extrapineal tissues. Exp. Gerontol. 2009, 44 (5), 328-34. [CrossRef]
- Hardeland, R. Melatonin in aging and disease -multiple consequences of reduced secretion, options and limits of treatment. Aging Dis. 2012, 3 (2), 194-225.
- Rahim, I.; Djerdjouri, B.; Sayed, R. K.; Fernández-Ortiz, M.; Fernández-Gil, B.; Hidalgo-Gutiérrez, A.; López, L. C.; Escames, G.; Reiter, R. J.; Acuña-Castroviejo, D. Melatonin administration to wild-type mice and nontreated NLRP3 mutant mice share similar inhibition of the inflammatory response during sepsis. J. Pineal Res. 2017, 63 (1). [CrossRef]
- Sayed, R. K. A.; Fernández-Ortiz, M.; Diaz-Casado, M. E.; Aranda-Martínez, P.; Fernández-Martínez, J.; Guerra-Librero, A.; Escames, G.; López, L. C.; Alsaadawy, R. M.; Acuña-Castroviejo, D. Lack of NLRP3 Inflammasome Activation Reduces Age-Dependent Sarcopenia and Mitochondrial Dysfunction, Favoring the Prophylactic Effect of Melatonin. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74 (11), 1699-1708. [CrossRef]
- Sayed, R. K. A.; Fernández-Ortiz, M.; Diaz-Casado, M. E.; Rusanova, I.; Rahim, I.; Escames, G.; López, L. C.; Mokhtar, D. M.; Acuña-Castroviejo, D. The Protective Effect of Melatonin Against Age-Associated, Sarcopenia-Dependent Tubular Aggregate Formation, Lactate Depletion, and Mitochondrial Changes. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73 (10), 1330-1338. [CrossRef]
- Sayed, R. K. A.; Fernández-Ortiz, M.; Rahim, I.; Fernández-Martínez, J.; Aranda-Martínez, P.; Rusanova, I.; Martínez-Ruiz, L.; Alsaadawy, R. M.; Escames, G.; Acuña-Castroviejo, D. The Impact of Melatonin Supplementation and NLRP3 Inflammasome Deletion on Age-Accompanied Cardiac Damage. Antioxidants 2021, 10 (8). [CrossRef]
- Fernández-Ortiz, M.; Sayed, R. K. A.; Fernández-Martínez, J.; Cionfrini, A.; Aranda-Martínez, P.; Escames, G.; de Haro, T.; Acuña-Castroviejo, D. Melatonin/Nrf2/NLRP3 Connection in Mouse Heart Mitochondria during Aging. Antioxidants 2020, 9 (12).
- Rodríguez-Santana, C.; López-Rodríguez, A.; Martinez-Ruiz, L.; Florido, J.; Cela, O.; Capitanio, N.; Ramírez-Casas, Y.; Acuña-Castroviejo, D.; Escames, G. The Relationship between Clock Genes, Sirtuin 1, and Mitochondrial Activity in Head and Neck Squamous Cell Cancer: Effects of Melatonin Treatment. Int. J. Mol. Sci. 2023, 24 (19). [CrossRef]
- Aranda-Martínez, P.; Fernández-Martínez, J.; Ramírez-Casas, Y.; Rodríguez-Santana, C.; Rusanova, I.; Escames, G.; Acuña-Castroviejo, D. Chronodisruption and Loss of Melatonin Rhythm, Associated with Alterations in Daily Motor Activity and Mitochondrial Dynamics in Parkinsonian Zebrafish, Are Corrected by Melatonin Treatment. Antioxidants 2023, 12 (4). [CrossRef]
- Sayed, R. K.; de Leonardis, E. C.; Guerrero-Martínez, J. A.; Rahim, I.; Mokhtar, D. M.; Saleh, A. M.; Abdalla, K. E.; Pozo, M. J.; Escames, G.; López, L. C.; Acuña-Castroviejo, D. Identification of morphological markers of sarcopenia at early stage of aging in skeletal muscle of mice. Exp. Gerontol. 2016, 83, 22-30. [CrossRef]
- Christian, C. J.; Benian, G. M. Animal models of sarcopenia. Aging Cell 2020, 19 (10), e13223. [CrossRef]
- Andersen, L. P.; Gögenur, I.; Rosenberg, J.; Reiter, R. J. The Safety of Melatonin in Humans. Clin. Drug Invest. 2016, 36 (3), 169-75. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




