Submitted:
31 October 2023
Posted:
31 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Microbial strains
2.2. Preparation of LAB CFS (Cell Free Supernatants)
2.3. Biofilm removal activity
2.4. Statistical analysis
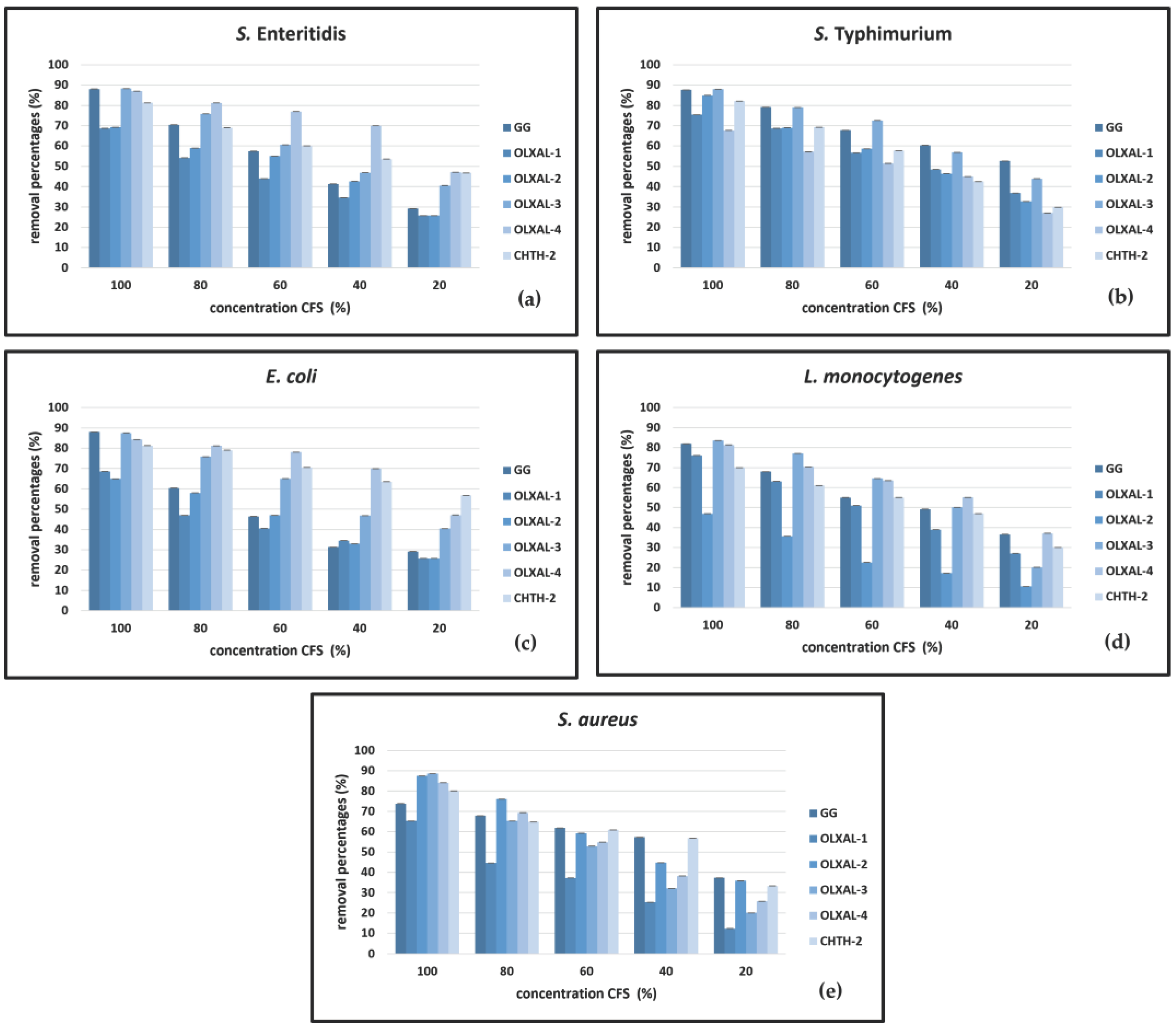
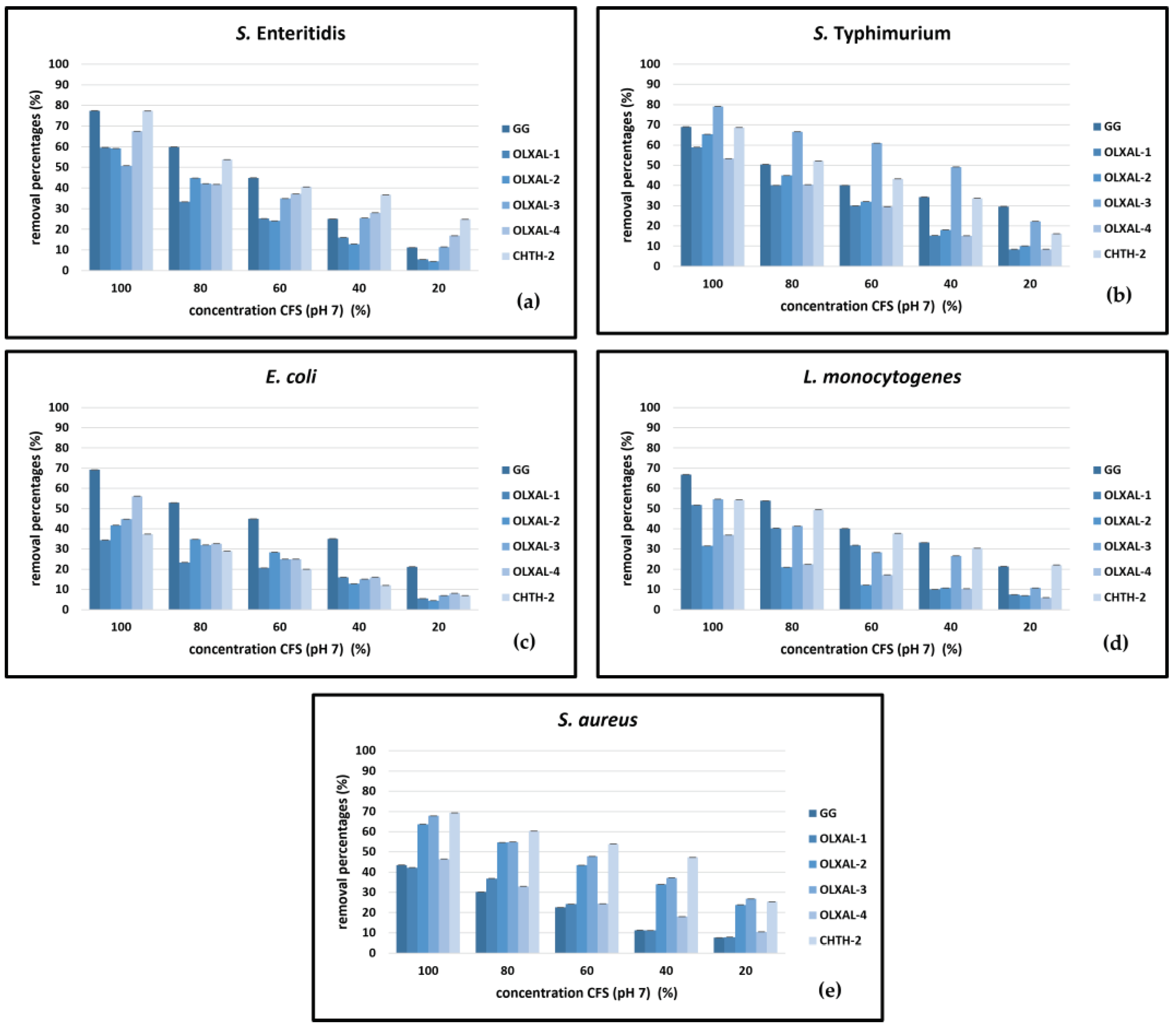
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clinical Microbiology Reviews 2020, 33, 10.1128/cmr.00181-19. [Google Scholar] [CrossRef] [PubMed]
- Šušković, J. , Kos, B., Beganović, J., Pavunc, A., Habjanič, K., & Matošić, S. Antimicrobial Activity – the Most Important Property of Probiotic and Starter Lactic Acid Bacteria. Food Technol Biotechnol 2010, 3, 296–307. [Google Scholar]
- Júnior, W.; Ferrari, Í.; Viana de Souza, J.; Silva, C.; Costa, M.; Dias, F. Characterization and Evaluation of Lactic Acid Bacteria Isolated from Goat Milk. Food Control 2015, 53. [Google Scholar] [CrossRef]
- Tejero-Sariñena, S.; Barlow, J.; Costabile, A.; Gibson, G.R.; Rowland, I. In Vitro Evaluation of the Antimicrobial Activity of a Range of Probiotics against Pathogens: Evidence for the Effects of Organic Acids. Anaerobe 2012, 18, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Ait Ouali, F.; Al Kassaa, I.; Cudennec, B.; Abdallah, M.; Bendali, F.; Sadoun, D.; Chihib, N.-E.; Drider, D. Identification of Lactobacilli with Inhibitory Effect on Biofilm Formation by Pathogenic Bacteria on Stainless Steel Surfaces. Int J Food Microbiol 2014, 191, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Aminnezhad, S.; Kasra-Kermanshahi, R. Antibiofilm Activity of Cell-Free Supernatant from Lactobacillus Casei in Pseudomonas Aeruginosa. Feyz 2014. [Google Scholar]
- Khiralla, G.M.; Mohamed, E.A.H.; Farag, A.G.; Elhariry, H. Antibiofilm Effect of Lactobacillus Pentosus and Lactobacillus Plantarum Cell-Free Supernatants against Some Bacterial Pathogens. J Biotech Res 2015. [Google Scholar]
- Bulgasem, B.Y.; Lani, M.N.; Hassan, Z.; Wan Yusoff, W.M.; Fnaish, S.G. Antifungal Activity of Lactic Acid Bacteria Strains Isolated from Natural Honey against Pathogenic Candida Species. Mycobiology 2016, 44, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Stitt, G.; Son, L.; Enioutina, E.Y. Probiotics and Their Bioproducts: A Promising Approach for Targeting Methicillin-Resistant Staphylococcus Aureus and Vancomycin-Resistant Enterococcus. Microorganisms 2023, 11, 2393. [Google Scholar] [CrossRef]
- Xin, W.-G.; Li, X.-D.; Zhou, H.-Y.; Li, X.; Liu, W.-X.; Lin, L.-B.; Wang, F. Isolation, Antibacterial Characterization, and ATF-Based Preparation of Viable Cells of Lacticaseibacillus Paracasei XLK 401 and Its Potential Application in Milk Preservation. J Dairy Sci 2023, S0022-0302(23)00701-4. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Li, J.; Qin, G. New Strategies for Biocontrol of Bacterial Toxins and Virulence: Focusing on Quorum-Sensing Interference and Biofilm Inhibition. Toxins (Basel) 2023, 15, 570. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg Infect Dis 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Barzegari, A.; Kheyrolahzadeh, K.; Hosseiniyan Khatibi, S.M.; Sharifi, S.; Memar, M.Y.; Zununi Vahed, S. The Battle of Probiotics and Their Derivatives Against Biofilms. Infect Drug Resist 2020, 13, 659–672. [Google Scholar] [CrossRef]
- Srey, S.; Jahid, I.K.; Ha, S.-D. Biofilm Formation in Food Industries: A Food Safety Concern. Food Control 2013, 31, 572–585. [Google Scholar] [CrossRef]
- Shi, X.; Zhu, X. Biofilm Formation and Food Safety in Food Industries. Trends in Food Science & Technology 2009, 20, 407–413. [Google Scholar] [CrossRef]
- Nelios, G.; Santarmaki, V.; Pavlatou, C.; Dimitrellou, D.; Kourkoutas, Y. New Wild-Type Lacticaseibacillus Rhamnosus Strains as Candidates to Manage Type 1 Diabetes. Microorganisms 2022, 10, 272. [Google Scholar] [CrossRef]
- Lebeer, S.; De Keersmaecker, S.C.J.; Verhoeven, T.L.A.; Fadda, A.A.; Marchal, K.; Vanderleyden, J. Functional Analysis of luxS in the Probiotic Strain Lactobacillus Rhamnosus GG Reveals a Central Metabolic Role Important for Growth and Biofilm Formation. J Bacteriol 2007, 189, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Kohestani, M.; Moradi, M.; Tajik, H.; Badali, A. Effects of Cell-Free Supernatant of Lactobacillus Acidophilus LA5 and Lactobacillus Casei 431 against Planktonic Form and Biofilm of Staphylococcus Aureus. Vet Res Forum 2018. [Google Scholar] [CrossRef] [PubMed]
- Divyashree, S.; Anjali, P.G.; Somashekaraiah, R.; Sreenivasa, M.Y. Probiotic Properties of Lactobacillus Casei - MYSRD 108 and Lactobacillus Plantarum-MYSRD 71 with Potential Antimicrobial Activity against Salmonella Paratyphi. Biotechnol Rep (Amst) 2021, 32, e00672. [Google Scholar] [CrossRef]
- Pelyuntha, W.; Chaiyasut, C.; Kantachote, D.; Sirilun, S. Cell-Free Supernatants from Cultures of Lactic Acid Bacteria Isolated from Fermented Grape as Biocontrol against Salmonella Typhi and Salmonella Typhimurium Virulence via Autoinducer-2 and Biofilm Interference. PeerJ 2019, 7, e7555. [Google Scholar] [CrossRef]
- Tazehabadi, M.H.; Algburi, A.; Popov, I.V.; Ermakov, A.M.; Chistyakov, V.A.; Prazdnova, E.V.; Weeks, R.; Chikindas, M.L. Probiotic Bacilli Inhibit Salmonella Biofilm Formation Without Killing Planktonic Cells. Front Microbiol 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.G.; Esaam, A.; Hazaa, M.M. Cell Free Preparations of Probiotics Exerted Antibacterial and Antibiofilm Activities against Multidrug Resistant E. Coli. Saudi Pharm J 2018, 26, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Apiwatsiri, P.; Pupa, P.; Yindee, J.; Niyomtham, W.; Sirichokchatchawan, W.; Lugsomya, K.; Shah, A.A.; Prapasarakul, N. Anticonjugation and Antibiofilm Evaluation of Probiotic Strains Lactobacillus Plantarum 22F, 25F, and Pediococcus Acidilactici 72N Against Escherichia Coli Harboring Mcr-1 Gene. Front Vet Sci 2021, 8, 614439. [Google Scholar] [CrossRef]
- Shao, X.; Fang, K.; Medina, D.; Wan, J.; Lee, J.; Hong, S.H. The Probiotic, Leuconostoc mesenteroides, Inhibits Listeria monocytogenes Biofilm Formation. Journal of Food Safety 2020, 40, e12750. [Google Scholar] [CrossRef]
- Stiles, M.E. Bacteriocins Produced by Leuconostoc Species. J Dairy Sci 1994, 77, 2718–2724. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Mardani, K.; Tajik, H. Characterization and Application of Postbiotics of Lactobacillus Spp. on Listeria Monocytogenes in Vitro and in Food Models. LWT 2019, 111, 457–464. [Google Scholar] [CrossRef]
- Mahasneh, A.M.; Hamdan, S.; Mahasneh, S.A. Probiotic Properties of Lactobacillus Species Isolated from Local Traditional Fermented Products. JJBS 2015, 8, 81–87. [Google Scholar] [CrossRef]
| Isolate code | Bacterial Species | Source of isolation |
|---|---|---|
| GG (ATCC 53103) | Lacticaseibacillus rhamnosus | Human intestines |
| OLXAL-1 | Lacticaseibacillus rhamnosus | Olive (fruit) |
| OLXAL-2 | Lacticaseibacillus rhamnosus | Olive (fruit) |
| OLXAL-3 | Lacticaseibacillus rhamnosus | Olive (fruit) |
| OLXAL-4 | Lacticaseibacillus rhamnosus | Olive (fruit) |
| CHTH-2 | Lacticaseibacillus rhamnosus | Feta-type cheese |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





