1. Introduction
Infective endocarditis (IE) is an infectious disease that requires a multidisciplinary management team. Endocarditis can affect native valves and intracardiac prosthetic materials (prosthetic valves, annuloplasty, cardiac devices, intracardiac patches, and shunts) [
1,
2]. Intravenous drug use, prosthetic valves, degenerative valve disease, and intracardiac devices are the main risk factors for IE in developed countries. Additionally, rheumatic heart disease and congenital heart diseases contribute to the incidence of IE in low- and middle-income countries [
4].
Many factors affect the outcome of IE, including virulence of the causative microorganism, patient characteristics, presence of underlying disease, surgical indications, timing of surgery, and delays in diagnosis and treatment. Infective endocarditis results from microbial infection of the endothelial surface of the heart and frequently causes debilitating morbidities, such as heart failure, stroke, renal failure and sepsis [
3,
4]. The characteristic vegetative lesion consists of a mass of platelets and fibrin to which circulating microorganisms adhere and enmesh in a structured biofilm matrix [
3].
The incidence of IE has increased worldwide over the last few decades, and the highest incidence rates have been reported in tropical and southern Latin America [
4]. The global incidence of IE is between 2 and 12 cases per 100,000 people per year [
5]. In addition, predisposing factors may vary among countries; for instance, rheumatic heart disease is the principal factor in the epidemiology of IE in developing countries, while intravenous drug use is more frequent in developed countries [
6,
7].
Before the advent of antimicrobials, the lethality of IE was 94% when the treatment was exclusively clinical [
8]. With the enormous advances in antimicrobial therapy associated with surgical procedures, the mortality rate has improved over the last century. However, despite the large number of antibiotics available, therapeutic success is still low, considering mortality rates of 36% in high-income countries and greater than 40% in low- and middle-income countries [
7,
9,
10].
We believe that understanding the local epidemiology and risk factors for in-hospital mortality in patients with IE is important for improving the outcomes of this fatal heart infection. This study aimed to report the demographic, epidemiological, etiologic, and risk factors for in-hospital mortality in a retrospective cohort of 240 patients diagnosed with IE over a period of 12 years at two teaching hospitals located in Rio de Janeiro, Brazil.
2. Materials and Methods
Location of the observational study of infective endocarditis
Only two teaching hospitals in Rio de Janeiro have a cardiovascular surgery unit and an IE team. The Hospital Universitário Pedro Ernesto (HUPE) and Hospital Universitário Clementino Fraga Filho (HUCFF) are public medical hospitals that serve as both a tertiary care referral center and a primary and secondary care institution for our public assistance security (Sistema Único de Saúde-SUS). The HUPE and HUCFF provide care for all medical and surgical specialties and sub-specialties. The HUPE and HUCFF have 560 and 350 beds, respectively. This retrospective cohort study analyzed information from medical records of all hospitalized patients with a diagnosis of IE in both hospitals from January 2009 and June 2021. Sociodemographic variables such as age, sex (based on the biological characteristics reported in the medical records), and preexisting conditions were analyzed. The STROBE checklist for reporting observational studies was used.
Diagnostic criteria of infective endocarditis (IE) according to the Modified Duke Criteria
The major criteria included typical microorganisms consistent with IE from two separate blood cultures: community-acquired enterococci, in the absence of a primary focus, or microorganisms consistent with IE from persistently positive blood cultures or positive blood culture for an organism known to cause IE or for
Coxiella burnetii antiphase I IgG antibody titer > 1:800 and evidence of endocardial involvement provided by echocardiography. The minor criteria included predisposing factors for IE, temperature > 38.0 °C, vascular and immunological phenomena, and microbiologic evidence of an active infection with a known pathogen causing IE. The following pathological criteria were used for a definite or possible diagnosis of infective endocarditis or rejection of IE cases: 1) Microorganisms demonstrated by culture or histological examination of vegetation, a vegetation that had embolized, or an intracardiac abscess specimen; and 2) Pathologic lesions: vegetation or intracardiac abscess confirmed by histological examination showing active endocarditis [
11]. Healthcare-associated infective endocarditis (HAIE) was considered as either IE-manifested > 48 h after admission to the hospital or IE-acquired in association with a significant invasive procedure performed in the 6 months preceding the following situations: (a) stay and/or treatment in a hospital setting (nosocomial health-associated IE); or (b) extensive outpatient contact with healthcare interventions. Demographic, epidemiological, and clinical data from the patient’s first medical visit to the hospital, blood culture results, echocardiographic findings, cardiac surgical interventions, and outcomes were collected.
Echocardiographic data
Transthoracic and/or transesophageal echocardiography was performed by the echocardiography service at HUPE and HUCFF. An associate professor of echocardiography reviewed all examinations in which IE was suspected in our associated departments (Internal Medicine, Infectious Diseases, Cardiologic and Cardiovascular).
Microbiological data
Clinical samples were processed in the microbiology laboratories of the University Hospitals. Blood cultures were processed using the BD BACTECTM FX system (BD Diagnostics) for at least two aerobic bottles incubated for 5 days (14 days in exceptional cases), and VITEK® 2 system (BioMérieux®) identification cards (ID) and antibiotic susceptibility testing (AST) cards were used according to the manufacturer’s instructions. When possible, the minimal inhibitory concentration (MIC) of vancomycin was determined by the E-test and microdilution for
Enterococcus spp. and methicillin-resistant
Staphylococcus aureus (MRSA), respectively. In relation to IE due to
Bartonella spp. and
Coxiella burnetii, serum samples were evaluated using an indirect immunofluorescence assay for IgG antibodies against anti-
Bartonella spp. and anti-
C. burnetii (phase I and II, PANBIO®, Brisbane, AU), with a titer of ≥ 800 dilution as the cut-off for positive samples. Blood and heart valve tissue samples were analyzed using polymerase chain reaction (PCR) for the
gltA,
ftsZ,
groEL sequences of
Bartonella strains, and the
IS1111 sequence for
Coxiella burnetii as previously described [
9]. The diagnosis of IE was confirmed for
Bartonella henselae and
C. burnetii by the presence of IgG antibodies ≥ 800 and/or a positive result of molecular tests in blood samples and heart valves.
Statistical analysis
In-hospital mortality was defined as all in-hospital deaths due to IE. Data concerning age, sex, pre-existing conditions, vegetation, type of valve, source, surgery, and microorganism information were tested against survivor status using the Pearson chi-square test or Mann-Whitney U test, as appropriate. Absolute and relative frequencies were estimated. Normality of continuous variables was tested. Poisson regression analysis was performed for trend tests. Univariate and then stepwise logistic regression analyses were performed to determine independent predictors of in-hospital mortality. The multivariate Cox proportional hazards model was used to estimate the hazard ratio (HR) of predictors of in-hospital mortality. The proportional hazard assumption was tested and found to be valid by inspection of log-log plots, scaled Schoenfeld residuals, and tests of the nonzero slope. The Kaplan-Meier method was used to generate survival curves, and survival rates between groups were compared using the log-rank test. All analyses were performed using STATA® version 15.0 (StataCorp LP, College Station, TX, USA), and statistical significance was defined as p < 0.05.
Ethics statement
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Research Ethics Committees of HUPE (CAAE- 01247512.3.0000.5259), HUCCF (CEP- No 517/01), and FIOCRUZ (CAAE- 39056120.6.0000.5248).
3. Results
3.1. Baseline characteristics of patients with infective endocarditis
Between January 2009 and June 2021, a total of 251 patients with IE were hospitalized in both institutions, and 11 patients were excluded from the analysis because of transfer to another hospital or missing data. The median age of the cohort was 55 years (IQR: 39-66 years), 57% (136/240) were male, and 41% of IE patients had a Charlson comorbidity index (CCI) score > 3. In order of frequency, the pre-existing conditions were chronic kidney disease (32%), valvular heart disease (32%), history of dialysis (25%), diabetes (21%), congenital heart disease (10%), previous IE (8%), cancer (7%), HIV infection (5%), and intracardiac device (5%); no patients had a history of intravenous drug use.
Infective endocarditis most often affected the native valve (77.5%) and occurred on the left side in 75% of cases. However, IE involving a prosthetic valve occurred more frequently in patients aged ≥ 60 years with an increasing trend in the proportion of prosthetic valves affected by IE per year. The vegetation was most frequently located on the mitral valve (43%), followed by the aortic (22%), tricuspid (11%), and pulmonary (1%) valves. In addition, involvement of more than one valve was identified in 13% of patients, mainly aortic/mitral valves.
Healthcare-associated infective endocarditis was the most frequent (54%) reported with classification into dialysis-associated HAIE (44%) and non-dialysis-associated HAIE (56%). Poisson regression analysis did not show any trend in the annual proportion of HAIE. During the study period, the surgery rate was 29%, and surgical treatment was significantly more frequent in left-sided IE (91.3%, p < 0.001). Patients who underwent surgical treatment had significantly longer hospital stays than those who received medical treatment (60 days, IQR: 40-82 days vs. 41 days, IQR: 23-71 days; p = 0.002).
Table 1.
Baseline characteristics of patients with infective endocarditis in Rio de Janeiro, January 2009 to June 2021.
Table 1.
Baseline characteristics of patients with infective endocarditis in Rio de Janeiro, January 2009 to June 2021.
| |
Total n = 240 |
Survivor n = 130 |
Dead n = 110 |
p |
|
Age (years)* |
55 |
(39-66) |
50 |
(32-60) |
61 |
(48-71) |
< 0.001‡ |
| ≥ 60 years |
97 |
41% |
36 |
28% |
61 |
55% |
< 0.001¶ |
| Sex |
|
|
|
|
|
|
|
| Female |
104 |
43% |
55 |
42% |
49 |
45% |
0.727¶
|
| Male |
136 |
57% |
75 |
58% |
61 |
55% |
|
| Pre-existing conditions |
|
|
|
|
|
|
|
| Charlson comorbidity index ≥ 3 |
99 |
41% |
40 |
31% |
59 |
54% |
< 0.001¶ |
| Chronic kidney disease |
77 |
32% |
36 |
28% |
41 |
37% |
0.113¶
|
| Valvular heart disease |
76 |
32% |
40 |
31% |
36 |
33% |
0.745¶
|
| History of dialysis |
60 |
25% |
28 |
22% |
32 |
29% |
0.178¶
|
| Diabetes |
50 |
21% |
26 |
20% |
24 |
22% |
0.730¶
|
| Congenital heart disease |
25 |
10% |
18 |
14% |
7 |
6% |
0.059¶
|
| Previous infective endocarditis |
18 |
8% |
6 |
5% |
12 |
11% |
0.065¶
|
| Cancer |
17 |
7% |
9 |
7% |
8 |
7% |
0.916¶
|
| HIV infection |
13 |
5% |
7 |
5% |
6 |
5% |
0.981¶
|
| Intracardiac device |
12 |
5% |
5 |
4% |
7 |
6% |
0.373¶
|
| Vegetation† |
|
|
|
|
|
|
|
| mitral |
102 |
43% |
50 |
38% |
52 |
47% |
0.169¶
|
| aortic |
52 |
22% |
25 |
19% |
27 |
25% |
0.319¶
|
| tricuspid |
27 |
11% |
20 |
15% |
7 |
6% |
0.028¶ |
| other location |
14 |
6% |
7 |
5% |
7 |
6% |
0.734¶
|
| left-sided |
180 |
75% |
88 |
68% |
92 |
84% |
0.004¶ |
| Type of valve |
|
|
|
|
|
|
|
| native |
186 |
77.5% |
106 |
82% |
80 |
73% |
0.103¶
|
| prosthetic |
54 |
22.5% |
24 |
18% |
30 |
27% |
|
| Source |
|
|
|
|
|
|
|
| Community-associated IE (CAIE) |
111 |
46% |
69 |
53% |
42 |
38% |
|
| Healthcare-associated IE (HAIE) |
129 |
54% |
61 |
47% |
68 |
62% |
0.021¶
|
| Surgery |
|
|
|
|
|
|
|
| Surgical treatment |
69 |
29% |
36 |
28% |
33 |
30% |
0.694¶
|
| Number of days of hospitalization* |
45 |
(28-78) |
54 |
(36-84) |
34 |
(18-61) |
< 0.001§ |
| Number of days until surgery* |
21 |
[8–35] |
17 |
[6–35] |
25 |
[10–35] |
0.314§
|
3.2. Etiology
The main microorganisms identified in blood samples were
Staphylococcus aureus (26%),
Streptococcus spp. (21%), and
Enterococcus spp. (18%). Fastidious growing bacteria were identified using immunological and molecular methods in 3% (
Bartonella spp.,
C. burnetii, HACEK group,
Abiotrofia defective,
Gemella morbillorum and
Microbacterium testaceum). Finally, blood cultures were negative in 19% of cases (
Table 2).
Staphylococcus aureus (35%) and
Enterococcus spp. (25%) were significantly more frequently identified in HAIE, whereas the viridans group streptococci (26%) and
Streptococcus bovis group (10%) were significantly more frequently identified in CAIE (
Table 3).
3.3. Mortality analysis
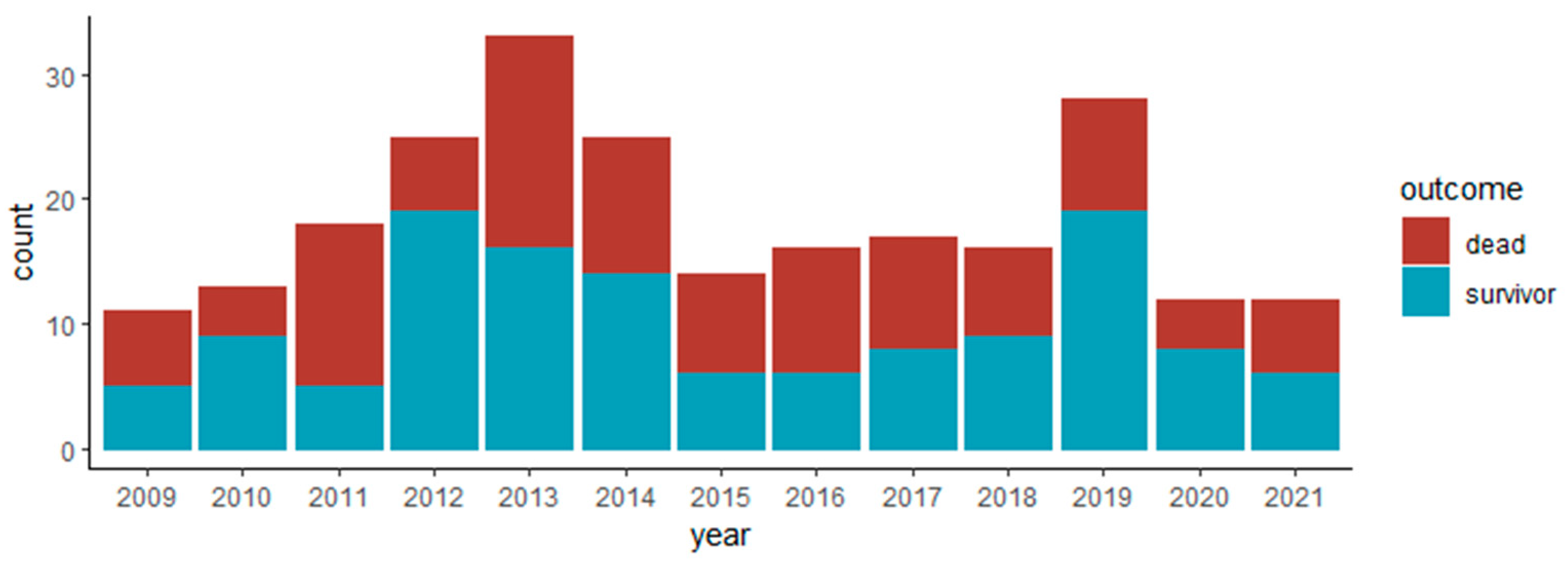
Overall in-hospital mortality was 45.8%. The mortality was significantly higher in patients aged ≥ 60 years (53%), CCI score ≥ 3 (60%), HAIE (53%), left-sided IE (51%), and enterococcal IE (67%). Poisson regression analysis revealed no trend in hospital mortality per year (
Figure 1).
In the univariate analysis, age ≥ 60 years, CCI score ≥ 3, left-side IE, HAIE, and enterococcal IE were significantly and positively associated with in-hospital mortality (
Table 1). The variables that were included in the univariate and multivariate Cox regression models for in-hospital mortality are shown in
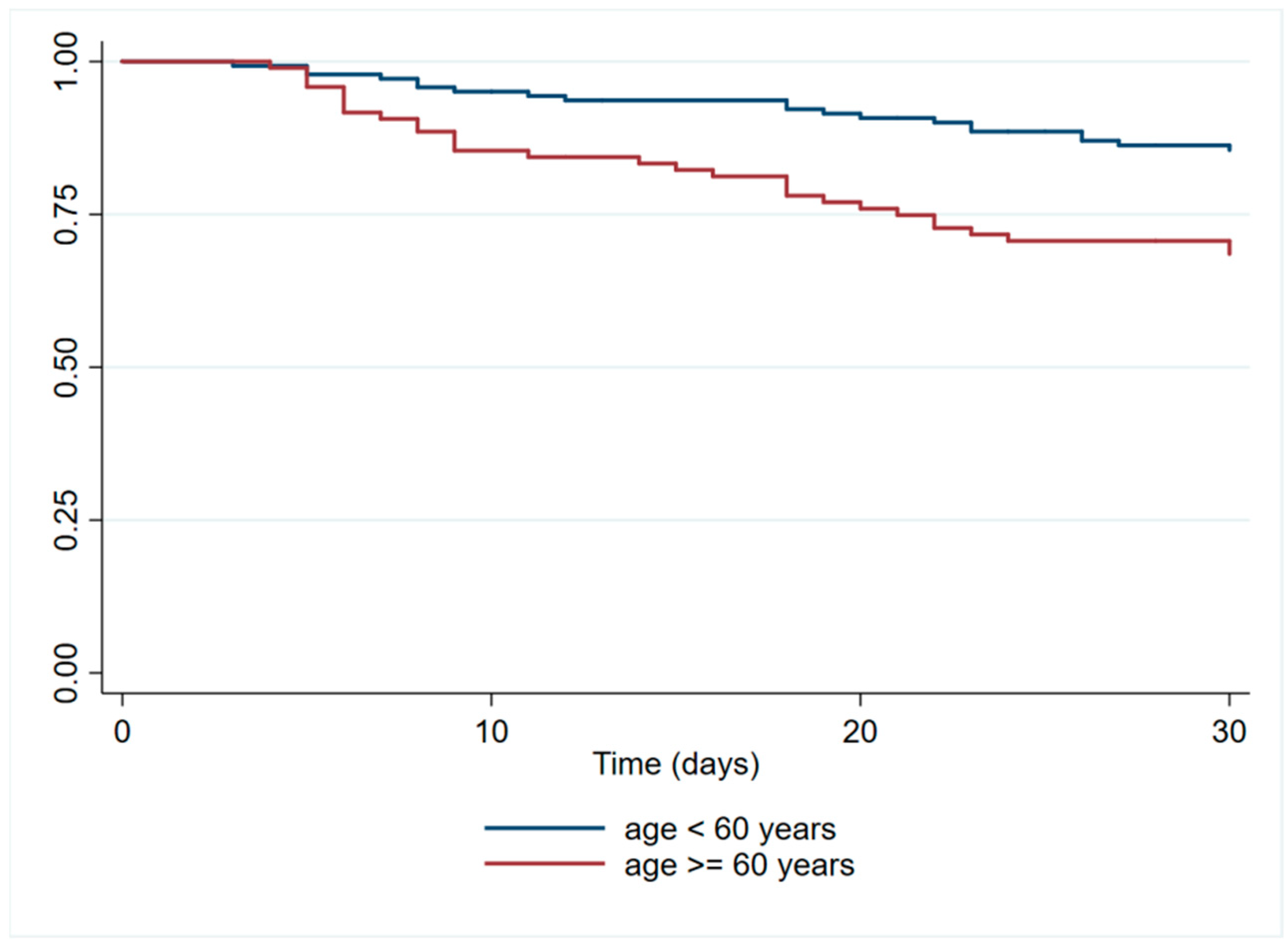
Table 4. Finally, it was determined that only age ≥ 60 years was an independent risk factor for mortality in patients with IE in the adjusted model (HR = 1.9; 95% CI 1.2-3.1; p = 0.008,
Figure 2).
4. Discussion
Although substantial advances have been made in the diagnosis and treatment of patients with IE, morbidity and mortality rates remain very high, especially in developing countries. The present retrospective cohort study assessed the clinical and laboratory characteristics of patients with IE in two tertiary centers in Rio de Janeiro between January 2009 and June 2021. The higher mortality rate in patients over 60 years of age is in accordance with the findings in a study published by Chen et al. on the worldwide burden and trends of IE from 1990 to 2019. The results showed an increasing trend in almost all countries during the study period, mainly affecting men ≥ 50 years of age, demonstrating the potential influence of age on IE morbidity. Among them, the estimated annual incidence increased 1.2% per year, whereas the mortality was 0.71%. Thus, the incidence and mortality from IE in patients aged > 50 years increased from 35% and 63% in 1990 to 60% and 79% in 2019, respectively [
12]. The increasing trend in the incidence of IE in older people is associated with an aging population and the increase in the proportion of older people [
13]. In addition, studies have shown an increase in the incidence of prosthetic valve endocarditis in recent years, mainly in older adults, as evidenced in this study [
14] .
Invasive interventions are often performed in older adults. These interventions including electronic cardiovascular devices and invasive diagnostic or therapeutic procedures are independent predictors of mortality [
13,
15].
In our retrospective cohort of definite cases of IE in two university hospitals in Rio de Janeiro, patients aged ≥ 60 years had a more frequent history of diabetes and use of intracardiac devices and prosthetic valves than younger patients. In our previous investigations [
7,
16] in which IE in low- and middle- income countries was reviewed, we had access to only 12 studies that included data from multivariate analyses of in-hospital mortality rates in IE patients. These studies showed statistically significant relationships between age > 45 years and chronic renal failure, septic shock, heart failure, prosthetic dysfunction, nosocomial IE, neoplasia, mobile vegetation, mental alterations, central nervous system emboli, coronary artery disease, aortic vegetation, and large vegetation. The in-hospital mortality in South America ranges from 24.0 % to 46.4% [
4,
6,
9]. Furthermore, it is necessary to consider an accelerated aging rate in the Brazilian population [
17], which could suggest the increasing comorbidities and, consequently, risk of IE.
There is evidence of an increase in the incidence of HAIE worldwide [
7,
16]. This study did not show an increase in HAIE, but the proportion continues to be similar to that reported in a previous study (56.3%) [
18]. This leads to an academic reflection on the need for greater efforts to prevent HAIE and reduce the prevalence of endocarditis. Although evidence suggests that early surgery can improve survival in patients with complicated IE, this remains controversial [
19]. In this study, despite a significant increase in the rate of surgery, no improvement in survival was observed, similar to that in other cohort studies [
16,
20].
In this Rio de Janeiro cohort infective endocarditis, the vegetation was most frequently located on the mitral valve (43%) and other locations were aortic (22%), tricuspid (11%) and pulmonary (1%). In addition, involvement of more than one valve was identified in 13% of patients, mainly aortic/mitral valves. According to the literature, more than 50% of vegetation generally affects the left side of the heart and is most frequently associated with mitral valve prolapse and mitral and aortic degenerative lesions [
11]. Infective endocarditis often represents a condition that is difficult to diagnose, especially in the initial phase of the illness, due to its indistinct clinical manifestations. Thus, patients with fever and predisposing factors must undergo echocardiography, which is an essential method for the diagnostic evaluation of patients suspected of having IE.
Regarding the etiology of IE, no significant variation has been observed in the frequency of microorganisms since 2009 [
18]. Thus,
S. aureus (26%),
Streptococcus spp. (21%) and
Enterococcus spp. (18%) continue to be the most frequently identified microorganisms. Recent studies worldwide coincide with
S. aureus and
Streptococcus spp. as the most frequent etiologies of IE. However, an increase in the incidence of IE due to
Enterococcus spp. has been reported, and the factors involved must be studied [
21,
22]. In addition, the highest in-hospital mortality was found in cases of IE due to
Enterococcus spp. (11.7%) and
S. aureus (10.4%), similar to what has been shown in recent studies [
23,
24]. The
Enterobacteriaceae family is a rare cause of IE, and it was identified in 9% of patients in this cohort. In a previous publication, it was shown that the molecular characteristics of
Escherichia coli and
Klebsiella pneumoniae isolated from the blood of patients with IE were similar to those isolated from urine; therefore, urinary tract infections should be monitored as a source of bacteremia and IE [
25].
Blood cultures were negative in 19% of cases, corresponding the highest proportion of group of patients with CAIE (24%) compared to HAIE (14%). The percentage of patients with IE with negative blood cultures may vary depending on the availability of immunological and molecular methods that enable the identification of infectious agents such as
C. burnetii, a major criterion for diagnosis, or the genus
Bartonella, a minor criterion in the diagnosis of IE
11. In this study,
B. henselae and
C. burnetii were identified in 0.8% and 0.4% of the cases, respectively. In our first report, we did not have the opportunity to identify these etiologies [
26]. However, in recent years, our research team has been able to advance the investigation of patients with negative blood cultures due to the cooperation of the Fundação Oswaldo Cruz, Rio de Janeiro, Brazil. Therefore, the reference laboratory performed diagnostic analyses using indirect immunofluorescence assays and molecular tests. Nevertheless, it is also necessary to consider the previous use of antibiotics prior to blood culture is the main cause of blood culture-negative infective endocarditis [
4]
Regarding the in-hospital mortality of IE, the study showed that overall in-hospital mortality was 45.8%, similar to that of another study conducted in Brazil [
28]. Independent predictors of mortality were age > 60 years, left-sided IE, and HAIE. Despite significant advances in recent decades, IE is still associated with high mortality, i.e., 25 to 30% in six months [
27], and elderly patients are at a higher risk of in-hospital mortality and complications after surgery than younger patients [
29]. Nevertheless, due to the lack of large multicenter cohorts and the heterogeneity of this population, there is insufficient evidence to support specific recommendations in management guidelines [
30]. In this study, age > 60 years increased the risk of death almost twofold.
This study has some limitations. First, due to its retrospective design, there is information bias regarding medical and surgical treatments, which limits its analysis. Second, there is the possibility of unrecorded confounding factors such as socioeconomic variables and living conditions, which may have affected the results. Third, there was no information on long-term survival or the occurrence of additional complications after hospital discharge, which may have resulted in an underestimation of mortality. Fourth, this was not a population-based study, and mortality rates could not be calculated for comparison to other studies. Finally, our study was conducted exclusively in Rio de Janeiro, and generalization to other regions of the country is not guaranteed. However, an advantage could be that two important tertiary hospitals were included in which both had a team that evaluated these patients during the study period. The findings of this study also allowed the identification of other infectious bacterial agents, mainly the HACEK group of fastidious bacteria, in addition to fungi such as Candida spp. and the emerging opportunistic fungal pathogen Rhodotorula mucilaginosa.
5. Conclusions
This study presents a mortality analysis of a 12-year cohort of patients with IE in the metropolitan area of Rio de Janeiro, Brazil. Overall in-hospital mortality was 45.8%, and mortality was significantly higher in the following patients: age ≥ 60 years (53%), CCI score ≥ 3 (60%), HAIE (53%), left-sided IE (51%), and enterococcal IE (67%). There was no evidence of any significant trend in mortality from IE, and age ≥ 60 years was an independent risk factor for in-hospital mortality. Thus, a consensus is required regarding the management of IE in this group of patients. Finally, we highlight the need to include immunological and molecular methods for the etiological diagnosis of fastidious agents that require special techniques for their isolation.
Author Contributions
Conceptualization, PVD, ERSL and CQF; methodology, PVD, VEFS and MAPH; formal analysis, PVD, VEFS, MAPH, ERSL, CQF; investigation, PVD, VEFS, NRQF, DXBS, AGF, MCAP, JCJ, JGO, MAPH, ERSL, CQF; resources, PVD, ERSL, CQF; data curation, PVD, VEFS, NRQF, DXBS, AGF, MCAP, JCJ, JGO, MAPH, ERSL, CQF; writing—original draft preparation, PVD, VEFS, ERSL, CQF; writing—review and editing, PVD, VEFS, NRQF, DXBS, AGF, MCAP, JCJ, JGO, MAPH, ERSL, CQF; visualization, PVD, VEFS, JGO, MAPH, ERSL, CQF; supervision, PVD, ERSL, CQF; project administration, PVD, ERSL, CQF.; funding acquisition, ERSL.
Funding
Conselho Nacional para o Desenvolvimento Científico e Tecnológico (CNPq), grant number 303024/2019-4 and the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) grant number E-26/202.575/2019..
Institutional Review Board Statement
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Research Ethics Committees of HUPE (CAAE- 01247512.3.0000.5259), HUCCF (CEP- No 517/01), and FIOCRUZ (CAAE- 39056120.6.0000.5248).
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge the patients for their willingness to participate in the study and the teams from the Pedro Ernesto University Hospital of the State of Rio de Janeiro, the Clemente Fraga Filho University Hospital, and the National Reference Laboratory for Rickettsioses at the Oswaldo Cruz Institute.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-128.
- Mestres CA, Paré JC, Miró JM. Organization and Functioning of a Multidisciplinary Team for the Diagnosis and Treatment of Infective Endocarditis: A 30-year Perspective (1985-2014). Rev Esp Cardiol (Engl Ed), 2015;68(5):363-8.
- Que YA, Moreillon P. Infective endocarditis. Nat Rev Cardiol. 2011;8(6):322-36.
- Urina-Jassir M, Jaimes-Reyes MA, Martinez-Vernaza S, Quiroga-Vergara C, Urina-Triana M. Clinical, Microbiological, and Imaging Characteristics of Infective Endocarditis in Latin America: A Systematic Review. Int J Infect Dis. 2022;117:312-21.
- Bin Abdulhak AA, Baddour LM, Erwin PJ, Hoen B, Chu VH, Mensah GA, et al. Global and regional burden of infective endocarditis, 1990-2010: a systematic review of the literature. Glob Heart, 2014;9(1):131-43.
- Mutagaywa RK, Vroon JC, Fundikira L, Wind AM, Kunambi P, Manyahi J, et al. Infective endocarditis in developing countries: An update. Front Cardiovasc Med. 2022;9:1007118.
- Talha KM, Dayer MJ, Thornhill MH, Tariq W, Arshad V, Tleyjeh IM, et al. Temporal Trends of Infective Endocarditis in North America From 2000 to 2017-A Systematic Review. Open Forum Infect Dis. 2021;8(11):ofab479.
- Spellberg B, Chambers HF, Musher DM, Walsh TL, Bayer AS. Evaluation of a Paradigm Shift From Intravenous Antibiotics to Oral Step-Down Therapy for the Treatment of Infective Endocarditis: A Narrative Review. JAMA Intern Med. 2020;180(5):769-77.
- Damasco, PV; Correal, JCD, Cruz-Campos ACD, Wajsbrot BR, Cunha RGD, Fonseca AGD, et al. Epidemiological and clinical profile of infective endocarditis at a Brazilian tertiary care center: an eight-year prospective study. Rev Soc Bras Med Trop. 2019;52:e2018375.
- Ambrosioni J, Hernandez-Meneses M, Téllez A, Pericàs J, Falces C, Tolosana JM, et al. The Changing Epidemiology of Infective Endocarditis in the Twenty-First Century. Curr Infect Dis Rep. 2017;19(5):21.
- Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr., Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633-8.
- Chen H, Zhan Y, Zhang K, Gao Y, Chen L, Zhan J, et al. The Global, Regional, and National Burden and Trends of Infective Endocarditis From 1990 to 2019: Results From the Global Burden of Disease Study 2019. Front Med (Lausanne), 2022;9:774224.
- Armiñanzas C, Fariñas-Alvarez C, Zarauza J, Muñoz P, González Ramallo V, Martínez Sellés M, et al. Role of age and comorbidities in mortality of patients with infective endocarditis. Eur J Intern Med. 2019;64:63-71.
- Hadji-Turdeghal K Jensen AD Bruun NE, Iversen KK, Bundgaard H Smerup M, et al. Temporal trends in the incidence of infective endocarditis in patients with a prosthetic heart valve. Open Heart, 2023;10(1).
- Ursi MP, Durante Mangoni E, Rajani R, Hancock J, Chambers JB, Prendergast B. Infective Endocarditis in the Elderly: Diagnostic and Treatment Options. Drugs Aging, 2019;36(2):115-24.
- Cresti A, Chiavarelli M, Scalese M, Nencioni C, Valentini S, Guerrini F, et al. Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study. Cardiovasc Diagn Ther. 2017;7(1):27-35.
- Tramujas Vasconcellos Neumann L, Albert SM. Aging in Brazil. Gerontologist, 2018;58(4):611-7.
- Damasco PV, Ramos JN, Correal JC, Potsch MV, Vieira VV, Camello TC, et al. Infective endocarditis in Rio de Janeiro, Brazil: a 5-year experience at two teaching hospitals. Infection, 2014;42(5):835-42.
- Cahill TJ, Baddour LM, Habib G, Hoen B, Salaun E, Pettersson GB, et al. Challenges in Infective Endocarditis. J Am Coll Cardiol. 2017;69(3):325-44.
- Slipczuk L, Codolosa JN, Davila CD, Romero-Corral A, Yun J, Pressman GS, et al. Infective endocarditis epidemiology over five decades: a systematic review. PLoS One, 2013;8(12):e82665.
- Ferraris L, Milazzo L, Rimoldi SG, Mazzali C, Barosi A, Gismondo MR, et al. Epidemiological trends of infective endocarditis in a single center in Italy between 2003-2015. Infect Dis (Lond), 2018;50(10):749-56.
- Escolà-Vergé L, Fernández-Hidalgo N, Larrosa MN, Fernandez-Galera R, Almirante B. Secular trends in the epidemiology and clinical characteristics of Enterococcus faecalis infective endocarditis at a referral center (2007-2018). Eur J Clin Microbiol Infect Dis. 2021;40(6):1137-48.
- Østergaard L, Voldstedlund M, Bruun NE, Bundgaard H, Iversen K, Køber N, et al. Temporal Changes, Patient Characteristics, and Mortality, According to Microbiological Cause of Infective Endocarditis: A Nationwide Study. J Am Heart Assoc. 2022;11(16):e025801.
- Shah ASV, McAllister DA, Gallacher P, Astengo F, Rodríguez Pérez JA, Hall J, et al. Incidence, Microbiology, and Outcomes in Patients Hospitalized With Infective Endocarditis. Circulation, 2020;141(25):2067-77.
- Andrade NL, da Cruz Campos AC, Cabral AM, Damasco PH, Lo-Ten-Foe J, Rosa ACP, et al. Infective endocarditis caused by Enterobacteriaceae: phenotypic and molecular characterization of Escherichia coli and Klebsiella pneumoniae in Rio de Janeiro, Brazil. Braz J Microbiol. 2021;52(4):1887-96.
- Ramos JN, dos Santos LS, Vidal LM, Pereira PM, Salgado AA, Fortes CQ, et al. A case report and literature overview: Abiotrophia defectiva aortic valve endocarditis in developing countries. Infection, 2014;42(3):579-84.
- Cahill TJ, Prendergast BD. Infective endocarditis. Lancet, 2016;387(10021):882-93.
- Tagliari AP, Steckert GV, da Silveira LMV, Kochi AN, Wender OCB. Infective endocarditis profile, prognostic factors and in-hospital mortality: 6-year trends from a tertiary university center in South America. J Card Surg. 2020;35(8):1905-11.
- Bea C, Vela S, García-Blas S, Perez-Rivera JA, Díez-Villanueva P, de Gracia AI, et al. Infective Endocarditis in the Elderly: Challenges and Strategies. J Cardiovasc Dev Dis. 2022;9(6).
- Forestier E, Fraisse T, Roubaud-Baudron C, Selton-Suty C, Pagani L. Managing infective endocarditis in the elderly: new issues for an old disease. Clin Interv Aging, 2016;11:1199-206.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).