Submitted:
31 October 2023
Posted:
01 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. P. vivax Malaria and Exposure to the Non-Malaria vector Aedes Aegypti
| Infection Status | All age years (range) | Female (range) | Male (Range) |
|---|---|---|---|
| Malaria | 33.4 (1 – 67), n=49 | 36.0 (16 – 53), n=14 | 32.4 (1 – 67), n=35 |
| Dengue | 15.3 (1 – 76), n=124 | 17.6 (1 – 76), n=70 | 12.4 (1 – 69), n=54 |
| Healthy | 28.9 (2 – 79), n=103 | 27.0 (2 – 79), n=65 | 32.3 (2 – 72), n=38 |
| Peptide | Pvs25 | Pvs230 | Parasite count |
|---|---|---|---|
| All | |||
| Peroxi-P1 | 0.0121 (p=0.9415) |
-0.2842 (p=0.0795) |
-0.0989 (p=0.5492) |
| Trans-1 | 0.0715 (p=0.6655) |
-0.1355 (p=0.4107) |
-0.1987 (p=0.2254) |
| Trans-2 | -0.1937 (p=0.2373) |
-0.0644 (p=0.6970) |
-0.0984 (p=0.5511) |
| An. albimanus SGE | -0.0497 (p=0.7638) |
-0.3072 (p=0.0571) |
-0.0538 (p=0.7451) |
| gSG6-P1 | -0.0901 (p=0.5855) |
-0.2913 (p=0.0720) |
-0.1123 (p=0.4960) |
| Nterm-34kDa | -0.0109 (p=0.9473) |
-0.1553 (p=0.3453) |
-0.0314 (p=0.8495) |
| Females | |||
| Peroxi-P1 | -0.0824 (p=0.7890) |
-0.5440 (p=0.0546) |
-0.2418 (p=0.4262) |
| Trans-1 | -0.3989 (p=0.1770) |
-0.7510 (p=0.0031) |
-0.1761 (p=0.5650) |
| Trans-2 | 0.1978 (p=0.5171) |
-0.3187 (p=0.2886) |
-0.0165 (p=0.9574) |
| An. albimanus SGE | -0.2802 (p=0.3538) |
-0.6099 (p=0.0269) |
-0.1593 (p=0.6031) |
| gSG6-P1 | -0.3132 (p=0.2974) |
-0.6648 (p=0.0132) |
-0.1593 (p=0.6031) |
| Nterm-34kDa | -0.5000 (p=0.0819) |
-0.7253 (p=0.0050) |
-0.3077 (p=0.3064) |
| Males | |||
| Peroxi-P1 | 0.0510 (p=0.8047) |
-0.1772 (p=0.3866) |
0.0395 (p=0.8480) |
| Trans-1 | 0.2113 (p=0.3001) |
0.1217 (p=0.5356) |
-0.1498 (p=0.4651) |
| Trans-2 | 0.2205 (p=0.2790) |
0.1022 (p=0.6193) |
-0.0728 (p=0.7236) |
| An. albimanus SGE | 0.0168 (p=0.9353) |
-0.1733 (p=0.3971) |
-0.0147 (p=0.9432) |
| gSG6-P1 | -0.0544 (p=0.7919) |
-0.1433 (p=0.4850) |
-0.0332 (p=0.8722) |
| Nterm-34kDa | 0.1829 (p=0.3711) |
0.0715 (p=0.7287) |
0.2151 (p=0.2913) |
| Peptide | Red blood cell count | White blood cell count | Platelet count | Haemoglobin | Haematocrit |
|---|---|---|---|---|---|
| All | |||||
| Peroxi-P1 | -0.2660 (p=0.0847) |
-0.0269 (p=0.8642) |
-0.3167 (p=0.0385) |
-0.1691 (p=0.2782) |
-0.2145 (p=0.1671) |
| Trans-1 | -0.2462 (p=0.1115) |
0.2950 (p=0.0548) |
-0.3288 (p=0.0314) |
-0.1691 (p=0.2782) |
-0.2445 (p=0.1141) |
| Trans-2 | -0.2651 (p=0.0858) |
0.1180 (p=0.4510) |
-0.3651 (p=0.0161) |
-0.2651 (p=0.0974) |
-0.3740 (p=0.0135) |
| An. albimanus SGE | -0.1076 (p=0.4923) |
0.1589 (p=0.3088) |
-0.311 (p=0.0420) |
-0.1158 (p=0.4597) |
-0.1609 (p=0.3028) |
| gSG6-P1 | -2769 (p=0.0723) |
0.3205 (p=0.0361) |
-0.2636 (p=0.0877) |
-0.1189 (p=0.4477) |
-0.1300 (p=0.4061) |
| Nterm-34kDa |
-0.3288 (p=0.0313) |
0.2363 (p=0.1271) |
-0.2078 (p=0.1812) |
-0.1402 (p=0.3699) |
-0.1374 (p=0.3797) |
| Females | |||||
| Peroxi-P1 | -0.3714 (p=0.1910) |
0.6497 (p=0.0119) |
-0.4330 (p=1220) |
-0.1454 (p=0.62000 |
-0.2571 (p=0. 3748) |
| Trans-1 | -0.1958 (p=0.5023) |
0.7522 (p=0.0019) |
-0.5391 (p=0.0467) |
0.1235 (p=0.6741) |
-0.0726 (p=0.8052) |
| Trans-2 | -0.3099 (p=0.2809) |
0.6144 (p=0.0194) |
-0.2308 (p=0.4273) |
-0.1410 (p=0.6307) |
-0.3099 (p=0.2809) |
| An. albimanus SGE | -0.0330 (p=0.9109) |
0.6763 (p=0.0079) |
-0.4857 (p=0.0783) |
0.1828 (p=0.5316) |
0.0857 (p=0.7708) |
| gSG6-P1 | -0.0857 (p=0.7708) |
0.6188 (p=0.0153) |
-0.3626 (p=0.2026) |
0.1564 (p=0.5834) |
0.1121 (p=0.7028) |
| Nterm-34kDa | -01560 (p=0.5942) |
0.5923 (p=0.0256) |
-0.5560 (p=0.0389) |
-0.0683 (p=0.8166) |
-0.0989 (p=0.7366) |
| Males | |||||
| Peroxi-P1 | -0.3224 (p=0.0880) |
-0.2376 (p=0.2145) |
-0.2266 (p=0.2371) |
-0.2902 (p=0.1268) |
-0.2572 (p=0.1780) |
| Trans-1 | -0.2645 (p=0.1656) |
0.1199 (p=0.5356) |
-0.02303 (p=0.2294) |
-0.2740 (p=0.1504) |
-0.3069 (p=0.1053) |
| Trans-2 | -0.2334 (p=0.2230) |
-0.0712 (p=0.7135) |
-0.3548 (p=0.0589) |
-0.2930 9p=0.1229) |
-0.3972 (p=0.0329) |
| An. albimanus SGE | -0.1429 (p=0.4595) |
0.0094 (p=0.9615) |
-0.1947 (p=0.3116) |
-0.2169 (p=0.2584) |
-0.2341 (p=0.2216) |
| gSG6-P1 | -0.3300 (p=0.0804) |
0.2192 (p=0.2534) |
-0.1062 (p=0.5834) |
-0.1468 (p=0.4443) |
-0.1417 (p=0.4634) |
| Nterm-34kDa |
-0.4171 (p=0.0244) |
0.0561 (p=0.7727) |
-0.0017 (p=0.9929) |
-0.1594 (p=0.4090) |
-0.1264 (p=0.5135) |
| Peptide | Age |
|---|---|
| All | |
| Peroxi-P1 | -0.0207 (p=0.8880) |
| Trans-1 |
0.3270 (p=0.0218) |
| Trans-2 | 0.1596 (p=2733) |
| An. albimanus SGE | 0.0471 (p=0.07478) |
| gSG6-P1 | 0.2064 (p=0.1547) |
| Nterm-34kDa | 0.0828 (p=0.5719) |
| Females | |
| Peroxi-P1 | 0.3645 (p=0.2001) |
| Trans-1 |
0.6196 (p=0.0181) |
| Trans-2 | 0.3934 (p=0.1641) |
| An. albimanus SGE |
0.5934 (p=0.0253) |
| gSG6-P1 |
0.5334 (p=0.0495) |
| Nterm-34kDa | 0.2356 (p=0.4175) |
| Males | |
| Peroxi-P1 | -0.1172 (p=0.5027) |
| Trans-1 | 0.2603 (p=0.1310) |
| Trans-2 | 0.0793 (p=0.6507) |
| An. albimanus SGE | -0.0771 (p=0.6599) |
| gSG6-P1 | 0.1113 (p=0.5243) |
| Nterm-34kDa | 0.0417 (p=0.8121) |
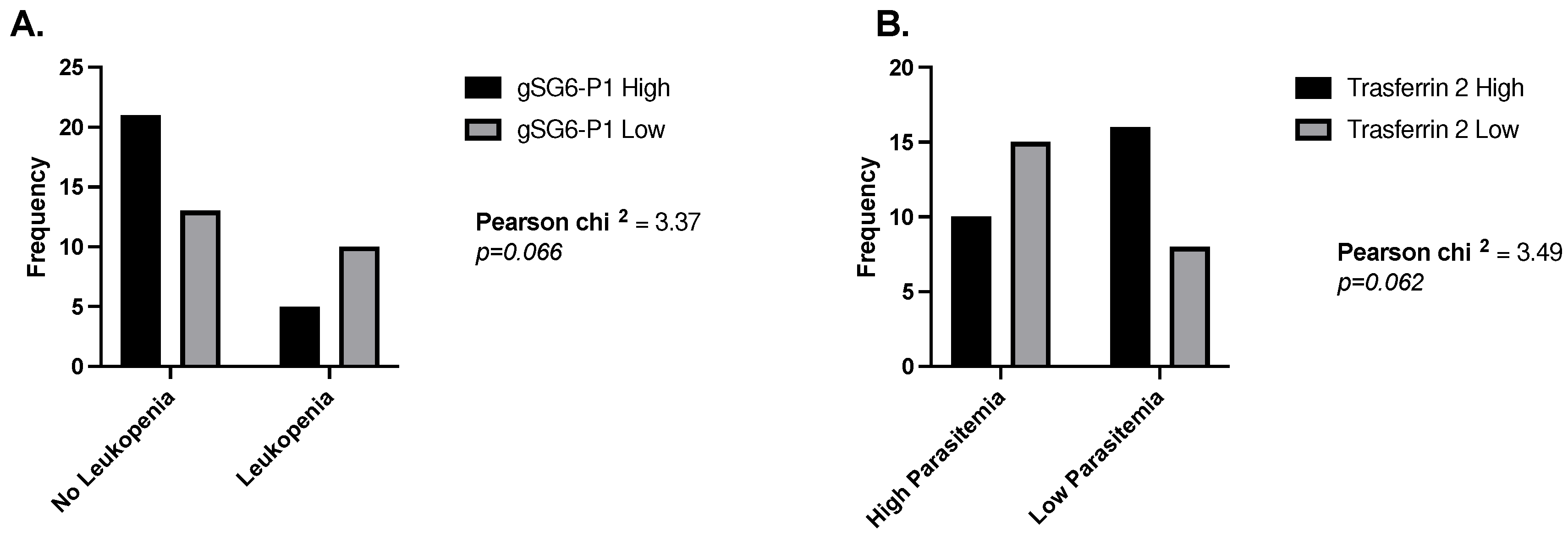
2.2. DENV and Exposure to the Non-DENV vector Anopheles Albimanus
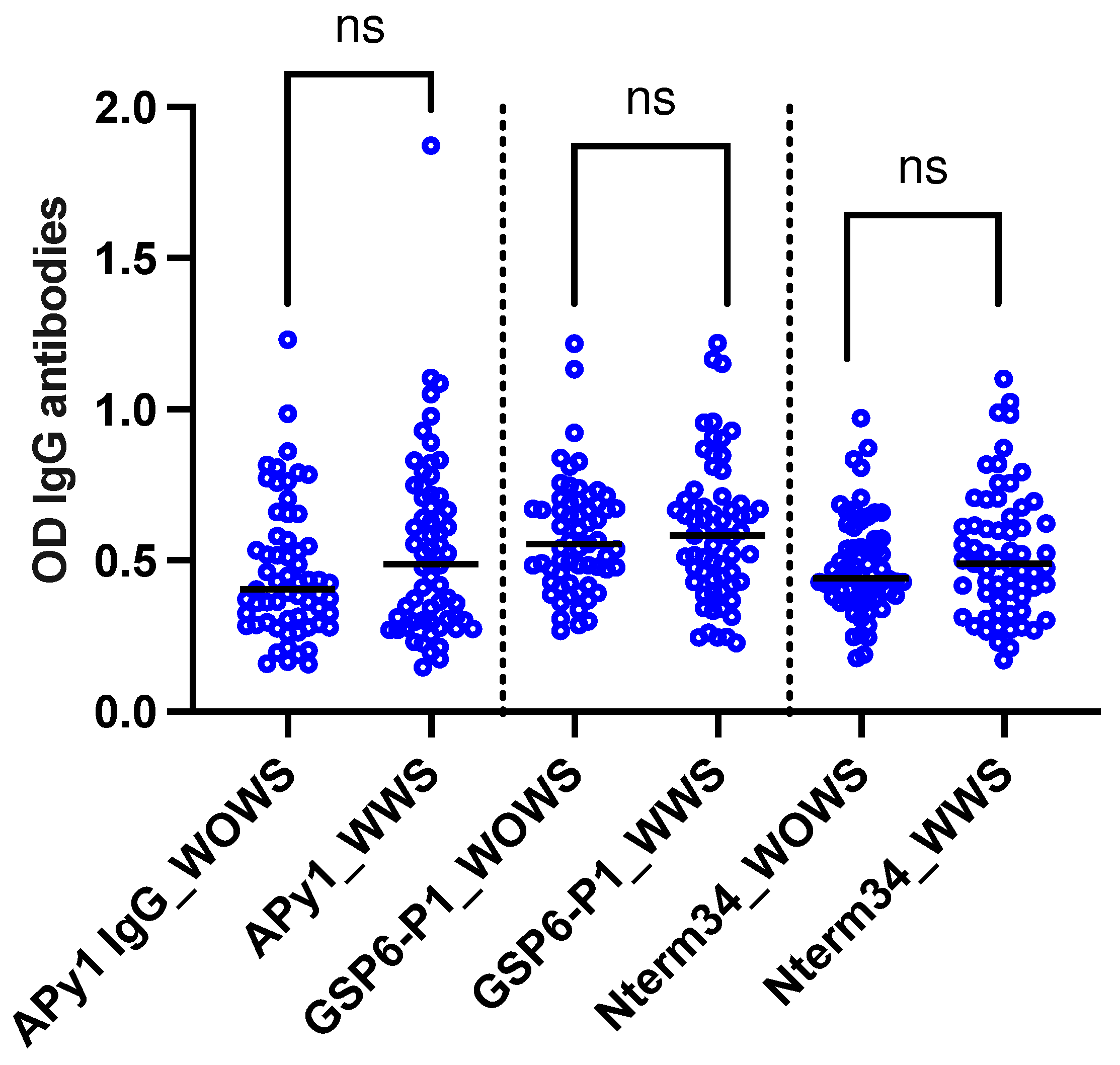
2.3. Healthy Individuals from Endemic and Nonendemic Areas
| Peptide | Red blood cell count | White blood cell count | Platelet count | Haemoglobin | Haematocrit |
|---|---|---|---|---|---|
| All | |||||
| AnDarApy-1 | 0.0955 (p=0.2934) |
0.0277 (p=0.7607) |
-0.0831 (p=0.3609) |
0.0999 (p=0.2715) |
0.1092 (p=0.2294) |
| gSG6-P1 |
0.1807 (p=0.0455) |
-0.0829 (p=0.3617) |
-0.0484 (p=0.5949) |
0.0921 (p=0.3112) |
-0.1097 (p=0.2269) |
| Nterm-34kDa |
0.2107 (p=0.0193) |
-0.0429 (p=0.6375) |
-0.0649 (p=0.4754) |
0.1543 (p=0.0885) |
0.1587 (p=0.0797) |
| Females | |||||
| AnDarApy-1 | -0.0571 (p=0.6390) |
-0.0641 (p=0.5980) |
0.0739 (p=0.5434) |
0.0847 (p=0.4858) |
0.0520 (p=0. 6689) |
| gSG6-P1 | 0.0216 (p=0.8591) |
-0.1233 (p=0.3092) |
0.0338 (p=0.7815) |
0.0015 (p=0.9899) |
-0.0156 (p=0.8981) |
| Nterm-34kDa | 0.0741 (p=0.5422) |
0.0480 (p=0.6932) |
-0.0153 (p=0.9001) |
0.1431 (p=0.2373) |
0.01078 (p=0.3474) |
| Males | |||||
| AnDarApy-1 |
0.2797 (p=0.0425) |
0.2063 (p=0.1382) |
-0.3052 (p=0.0263) |
0.1138 (p=0.4173) |
0.1681 (p=0.2290) |
| gSG6-P1 |
0.4099 (p=0.0023) |
0.0559 (p=0.6908) |
-0.2038 (p=0.1433) |
0.2074 (p=0.1362) |
0.2408 (p=0.0824) |
| Nterm-34kDa |
0.3904 (p=0.0039) |
0.0304 (p=0.8289) |
-0.1611 (p=0.2493) |
0.1922 (p=0.1680) |
0.2288 (p=0.0994) |
| Peptide | Age |
|---|---|
| All | |
| gSG6-P1 |
-0.3533 (p=0.0003) |
| Nterm-34kDa |
-0.4182 (p=0.0000) |
| Correlations by gender | |
| Females | |
| gSG6-P1 |
-0.3575 (p=0.0035) |
| Nterm-34kDa |
-0.4087 (p=0.0007) |
| Males | |
| gSG6-P1 |
-0.3702 (p=0.0370) |
| Nterm-34kDa |
-0.4105 (p=0.0196) |
| Correlations by location | |
| Colombia | |
| gSG6-P1 |
0.2702 (p=0.0481) |
| Nterm-34kDa | 0.0796 (p=0.5675) |
| US | |
| gSG6-P1 |
-0.4772 (p=0.0005) |
| Nterm-34kDa |
-0.4049 (p=0.0039) |
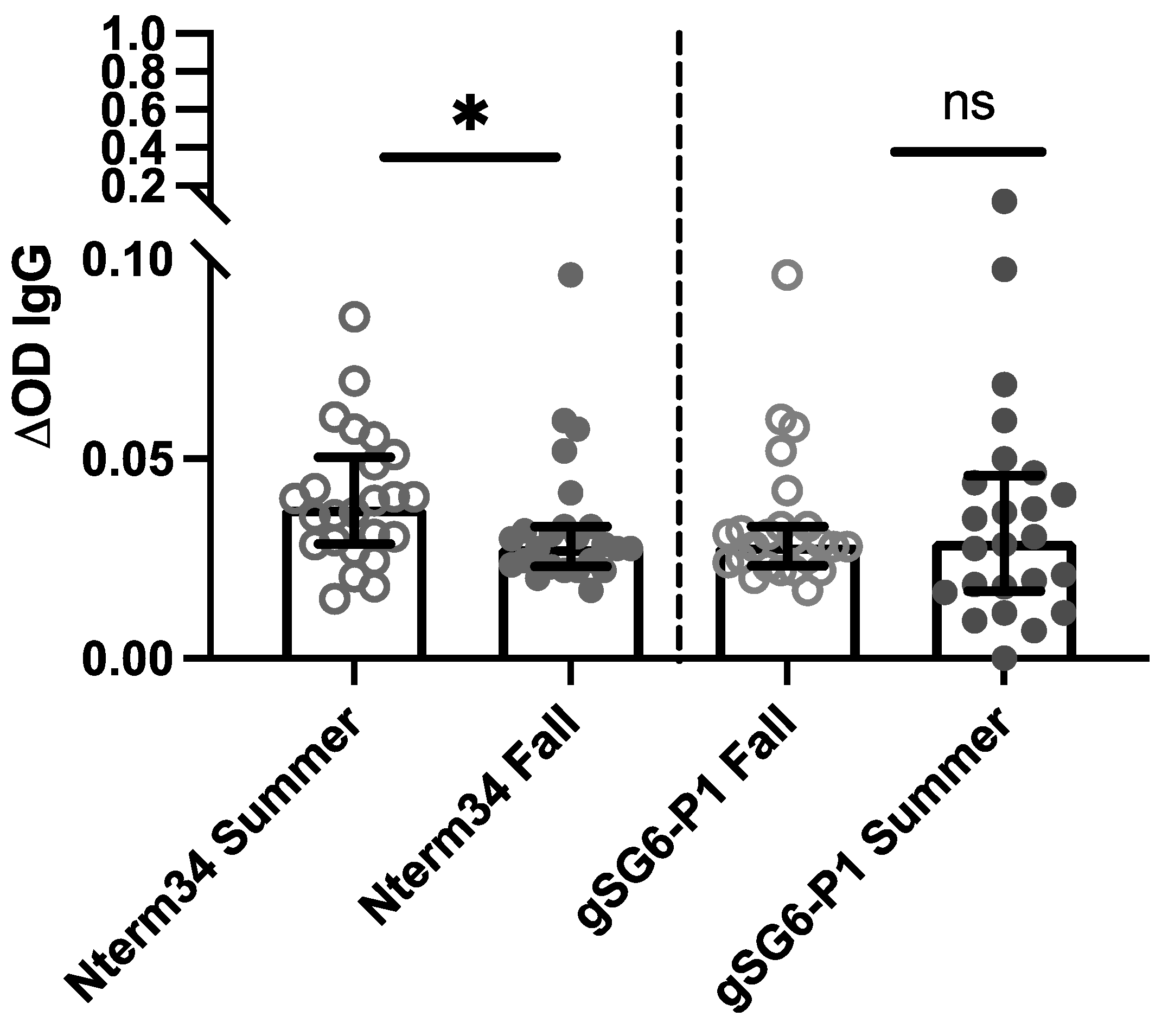
3. Discussion
4. Materials and Methods
4.1. Human Sample Collection and Diagnosis
4.2. Salivary Peptides
4.3. Human IgG Antibody Detection by ELISA
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.F.; Mead, P.S.; Paz-Bailey, G.; Waterman, S.H.; Drexler, N.A.; Kersh, G.J.; et al. Vital Signs: Trends in Reported Vectorborne Disease Cases - United States and Territories, 2004-2016. MMWR Morb Mortal Wkly Rep 2018, 67, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; Adams, L.E.; Durbin, A.P.; Muñoz-Jordán, J.L.; Poehling, K.A.; Sánchez-González, L.M.; Volkman, H.R.; Paz-Bailey, G. Dengue: A Growing Problem With New Interventions. Pediatrics 2022, 149. [Google Scholar] [CrossRef] [PubMed]
- Brathwaite Dick, O.; San Martín, J.L.; Montoya, R.H.; del Diego, J.; Zambrano, B.; Dayan, G.H. The history of dengue outbreaks in the Americas. Am J Trop Med Hyg 2012, 87, 584–593. [Google Scholar] [CrossRef]
- Hernandez-Romieu, A.C.; Adams, L.E.; Paz-Bailey, G. Opportunities for Improved Dengue Control in the US Territories. Jama 2023, 330, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Bagcchi, S. Locally acquired malaria cases in the USA. Lancet Infect Dis 2023, 23, e401. [Google Scholar] [CrossRef] [PubMed]
- Agudelo Higuita, N.I.; Franco-Paredes, C.; Henao-Martínez, A.F.; Mendez Rojas, B.; Suarez, J.A.; Naranjo, L.; Alger, J. Migrants in transit across Central America and the potential spread of chloroquine resistant malaria-a call for action. Lancet Reg Health Am 2023, 22, 100505. [Google Scholar] [CrossRef] [PubMed]
- Coutinho-Abreu, I.V.; Guimaraes-Costa, A.B.; Valenzuela, J.G. Impact of insect salivary proteins in blood feeding, host immunity, disease, and in the development of biomarkers for vector exposure. Curr Opin Insect Sci 2015, 10, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Arca, B.; Ribeiro, J.M. Saliva of hematophagous insects: a multifaceted toolkit. Curr Opin Insect Sci 2018, 29, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.B.; Lahon, A.; Arya, R.P.; Kneubehl, A.R.; Spencer Clinton, J.L.; Paust, S.; Rico-Hesse, R. Mosquito saliva alone has profound effects on the human immune system. PLoS Negl Trop Dis 2018, 12, e0006439. [Google Scholar] [CrossRef]
- Londoño-Rentería, B.; Cárdenas, J.C.; Giovanni, J.E.; Cárdenas, L.; Villamizar, P.; Rolón, J.; Chisenhall, D.M.; Christofferson, R.C.; Carvajal, D.J.; Pérez, O.G.; et al. Aedes aegypti anti-salivary gland antibody concentration and dengue virus exposure history in healthy individuals living in an endemic area in Colombia. Biomedica 2015, 35, 572–581. [Google Scholar] [CrossRef]
- Londono-Renteria, B.L.; Shakeri, H.; Rozo-Lopez, P.; Conway, M.J.; Duggan, N.; Jaberi-Douraki, M.; Colpitts, T.M. Serosurvey of Human Antibodies Recognizing Aedes aegypti D7 Salivary Proteins in Colombia. Front Public Health 2018, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Londono-Renteria, B.; Drame, P.M.; Weitzel, T.; Rosas, R.; Gripping, C.; Cardenas, J.C.; Alvares, M.; Wesson, D.M.; Poinsignon, A.; Remoue, F.; et al. gambiae gSG6-P1 evaluation as a proxy for human-vector contact in the Americas: a pilot study. Parasit Vectors 2015, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Londono-Renteria, B.; Cardenas, J.C.; Cardenas, L.D.; Christofferson, R.C.; Chisenhall, D.M.; Wesson, D.M.; McCracken, M.K.; Carvajal, D.; Mores, C.N. Use of anti-Aedes aegypti salivary extract antibody concentration to correlate risk of vector exposure and dengue transmission risk in Colombia. PLoS One 2013, 8, e81211. [Google Scholar] [CrossRef] [PubMed]
- Traore, D.F.; Sagna, A.B.; Adja, A.M.; Zoh, D.D.; Adou, K.A.; Lingue, K.N.; Coulibaly, I.; Tchiekoi, N.B.; Assi, S.B.; Poinsignon, A.; et al. Exploring the heterogeneity of human exposure to malaria vectors in an urban setting, Bouake, Cote d'Ivoire, using an immuno-epidemiological biomarker. Malar J 2019, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Ndille, E.E.; Dubot-Pérès, A.; Doucoure, S.; Mouchet, F.; Cornelie, S.; Sidavong, B.; Fournet, F.; Remoue, F. Human IgG antibody response to Aedes aegypti Nterm-34 kDa salivary peptide as an indicator to identify areas at high risk for dengue transmission: a retrospective study in urban settings of Vientiane city, Lao PDR. Trop Med Int Health 2014, 19, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Lombardo, F.; Ronca, R.; Mangano, V.; Sirima, S.B.; Nèbiè, I.; Fiorentino, G.; Modiano, D.; Arcà, B. Differential antibody response to the Anopheles gambiae gSG6 and cE5 salivary proteins in individuals naturally exposed to bites of malaria vectors. Parasit Vectors 2014, 7, 549. [Google Scholar] [CrossRef] [PubMed]
- Conway, M.J.; Londono-Renteria, B.; Troupin, A.; Watson, A.M.; Klimstra, W.B.; Fikrig, E.; Colpitts, T.M. Aedes aegypti D7 Saliva Protein Inhibits Dengue Virus Infection. PLoS Negl Trop Dis 2016, 10, e0004941. [Google Scholar] [CrossRef]
- Olajiga, O.M.; Marin-Lopez, A.; Cardenas, J.C.; Gutierrez-Silva, L.Y.; Gonzalez-Pabon, M.U.; Maldonado-Ruiz, L.P.; Worges, M.; Fikrig, E.; Park, Y.; Londono-Renteria, B. Aedes aegypti anti-salivary proteins IgG levels in a cohort of DENV-like symptoms subjects from a dengue-endemic region in Colombia. Frontiers in Epidemiology 2022, 2. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Ho, M.K.; Li, C.; Simons, F.E. Evidence for natural desensitization to mosquito salivary allergens: mosquito saliva specific IgE and IgG levels in children. Ann Allergy Asthma Immunol 2004, 93, 553–556. [Google Scholar] [CrossRef]
- Guerra-Silveira, F.; Abad-Franch, F. Sex bias in infectious disease epidemiology: patterns and processes. PLoS One 2013, 8, e62390. [Google Scholar] [CrossRef]
- Pathak, S.; Rege, M.; Gogtay, N.J.; Aigal, U.; Sharma, S.K.; Valecha, N.; Bhanot, G.; Kshirsagar, N.A.; Sharma, S. Age-dependent sex bias in clinical malarial disease in hypoendemic regions. PLoS One 2012, 7, e35592. [Google Scholar] [CrossRef]
- Bardach, A.E.; Garcia-Perdomo, H.A.; Alcaraz, A.; Lopez, E.T.; Gandara, R.A.R.; Ruvinsky, S.; Ciapponi, A. Interventions for the control of Aedes aegypti in Latin America and the Caribbean: Systematic Review and Meta-Analysis. Trop Med Int Health, 1111. [Google Scholar] [CrossRef]
- Dhiman, S. Are malaria elimination efforts on right track? An analysis of gains achieved and challenges ahead. Infect Dis Poverty 2019, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, Y.; Li, H.; Zhu, J.; Song, W.; Chen, K.; Zhang, Y.; Lou, Y. Vaccine development for mosquito-borne viral diseases. Front Immunol 2023, 14, 1161149. [Google Scholar] [CrossRef]
- Qian, X.; Qi, Z. Mosquito-Borne Flaviviruses and Current Therapeutic Advances. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Elanga Ndille, E.; Doucoure, S.; Poinsignon, A.; Mouchet, F.; Cornelie, S.; D'Ortenzio, E.; DeHecq, J.S.; Remoue, F. Human IgG Antibody Response to Aedes Nterm-34kDa Salivary Peptide, an Epidemiological Tool to Assess Vector Control in Chikungunya and Dengue Transmission Area. PLoS Negl Trop Dis 2016, 10, e0005109. [Google Scholar] [CrossRef]
- Elanga Ndille, E.; Doucoure, S.; Damien, G.; Mouchet, F.; Drame, P.M.; Cornelie, S.; Noukpo, H.; Yamadjako, S.; Djenontin, A.; Moiroux, N.; et al. First attempt to validate human IgG antibody response to Nterm-34kDa salivary peptide as biomarker for evaluating exposure to Aedes aegypti bites. PLoS Negl Trop Dis 2012, 6, e1905. [Google Scholar] [CrossRef]
- Poinsignon, A.; Cornelie, S.; Mestres-Simon, M.; Lanfrancotti, A.; Rossignol, M.; Boulanger, D.; Cisse, B.; Sokhna, C.; Arcà, B.; Simondon, F.; et al. Novel peptide marker corresponding to salivary protein gSG6 potentially identifies exposure to Anopheles bites. PLoS One 2008, 3, e2472. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, F.; Ronca, R.; Rizzo, C.; Mestres-Simòn, M.; Lanfrancotti, A.; Currà, C.; Fiorentino, G.; Bourgouin, C.; Ribeiro, J.M.; Petrarca, V.; et al. The Anopheles gambiae salivary protein gSG6: an anopheline-specific protein with a blood-feeding role. Insect Biochem Mol Biol 2009, 39, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Drame, P.M.; Poinsignon, A.; Besnard, P.; Cornelie, S.; Le Mire, J.; Toto, J.C.; Foumane, V.; Dos-Santos, M.A.; Sembène, M.; Fortes, F.; et al. Human antibody responses to the Anopheles salivary gSG6-P1 peptide: a novel tool for evaluating the efficacy of ITNs in malaria vector control. PLoS One 2010, 5, e15596. [Google Scholar] [CrossRef]
- Sagna, A.B.; Yobo, M.C.; Elanga Ndille, E.; Remoue, F. New Immuno-Epidemiological Biomarker of Human Exposure to Aedes Vector Bites: From Concept to Applications. Trop Med Infect Dis 2018, 3. [Google Scholar] [CrossRef]
- Bellone, R.; Failloux, A.B. The Role of Temperature in Shaping Mosquito-Borne Viruses Transmission. Front Microbiol 2020, 11, 584846. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, P.D.; Widen, S.G.; Huang, J.; Wood, T.G.; Thangamani, S. Discovery of mosquito saliva microRNAs during CHIKV infection. PLoS Negl Trop Dis 2015, 9, e0003386. [Google Scholar] [CrossRef] [PubMed]
- Visser, I.; Koenraadt, C.J.M.; Koopmans, M.P.G.; Rockx, B. The significance of mosquito saliva in arbovirus transmission and pathogenesis in the vertebrate host. One Health 2023, 16, 100506. [Google Scholar] [CrossRef] [PubMed]
- Sulesco, T.M.; Toderas, L.G.; Uspenskaia, I.G.; Toderas, I.K. Larval Habitats Diversity and Distribution of the Mosquito (Diptera: Culicidae) Species in the Republic of Moldova. J Med Entomol 2015, 52, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Tazeen, A.; Abdullah, M.; Hisamuddin, M.; Ali, S.; Naqvi, I.H.; Verma, H.N.; Ahmed, A.; Parveen, S. Concurrent Infection with Plasmodium vivax and the Dengue and Chikungunya Viruses in a Paediatric Patient from New Delhi, India in 2016. Intervirology 2017, 60, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Cortes, F.; Teixeira de Siqueira Filha, N.; Araujo de Franca, G.V.; Degroote, S.; Braga, C.; Ridde, V.; Turchi Martelli, C.M. Scoping review on vector-borne diseases in urban areas: transmission dynamics, vectorial capacity and co-infection. Infect Dis Poverty 2018, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Doucoure, S.; Mouchet, F.; Cournil, A.; Le Goff, G.; Cornelie, S.; Roca, Y.; Giraldez, M.G.; Simon, Z.B.; Loayza, R.; Misse, D.; et al. Human antibody response to Aedes aegypti saliva in an urban population in Bolivia: a new biomarker of exposure to Dengue vector bites. Am J Trop Med Hyg 2012, 87, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Debebe, Y.; Hill, S.R.; Birgersson, G.; Tekie, H.; Ignell, R. Plasmodium falciparum gametocyte-induced volatiles enhance attraction of Anopheles mosquitoes in the field. Malar J 2020, 19, 327. [Google Scholar] [CrossRef]
- Lacroix, R.; Mukabana, W.R.; Gouagna, L.C.; Koella, J.C. Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol 2005, 3, e298. [Google Scholar] [CrossRef]
- Robinson, A.; Busula, A.O.; Voets, M.A.; Beshir, K.B.; Caulfield, J.C.; Powers, S.J.; Verhulst, N.O.; Winskill, P.; Muwanguzi, J.; Birkett, M.A.; et al. Plasmodium-associated changes in human odor attract mosquitoes. Proc Natl Acad Sci U S A 2018, 115, E4209–e4218. [Google Scholar] [CrossRef]
- Busula, A.O.; Bousema, T.; Mweresa, C.K.; Masiga, D.; Logan, J.G.; Sauerwein, R.W.; Verhulst, N.O.; Takken, W.; de Boer, J.G. Gametocytemia and Attractiveness of Plasmodium falciparum-Infected Kenyan Children to Anopheles gambiae Mosquitoes. J Infect Dis 2017, 216, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Wampfler, R.; Mwingira, F.; Javati, S.; Robinson, L.; Betuela, I.; Siba, P.; Beck, H.P.; Mueller, I.; Felger, I. Strategies for detection of Plasmodium species gametocytes. PLoS One 2013, 8, e76316. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, C.; Roobsoong, W.; Kangwanrangsan, N.; Bardelli, M.; Rawlinson, T.A.; Dambrauskas, N.; Trakhimets, O.; Parthiban, C.; Goswami, D.; Reynolds, L.M.; et al. A Humanized Mouse Model for Plasmodium vivax to Test Interventions that Block Liver Stage to Blood Stage Transition and Blood Stage Infection. iScience 2020, 23, 101381. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, M.A.; Cardenas, R.; Yañez, J.; Petzold, M.; Kroeger, A. Risk of dengue, Zika, and chikungunya transmission in the metropolitan area of Cucuta, Colombia: cross-sectional analysis, baseline for a cluster-randomised controlled trial of a novel vector tool for water containers. BMC Public Health 2023, 23, 1000. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Lerma, J.; Solarte, Y.A.; Giraldo-Calderón, G.I.; Quiñones, M.L.; Ruiz-López, F.; Wilkerson, R.C.; González, R. Malaria vector species in Colombia: a review. Mem Inst Oswaldo Cruz 2011, 106 Suppl 1, 223–238. [Google Scholar] [CrossRef]
- Poinsignon, A.; Cornelie, S.; Ba, F.; Boulanger, D.; Sow, C.; Rossignol, M.; Sokhna, C.; Cisse, B.; Simondon, F.; Remoue, F. Human IgG response to a salivary peptide, gSG6-P1, as a new immuno-epidemiological tool for evaluating low-level exposure to Anopheles bites. Malaria Journal 2009, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Sagna, A.B.; Sarr, J.B.; Gaayeb, L.; Drame, P.M.; Ndiath, M.O.; Senghor, S.; Sow, C.S.; Poinsignon, A.; Seck, M.; Hermann, E.; et al. gSG6-P1 salivary biomarker discriminates micro-geographical heterogeneity of human exposure to Anopheles bites in low and seasonal malaria areas. Parasit Vectors 2013, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Ndo, C.; Elanga-Ndille, E.; Cheteug, G.; Metitsi, R.D.; Wanji, S.; Moukoko, C.E.E. IgG antibody responses to Anopheles gambiae gSG6-P1 salivary peptide are induced in human populations exposed to secondary malaria vectors in forest areas in Cameroon. PLoS One 2022, 17, e0276991. [Google Scholar] [CrossRef]
- Shieh, J.N.; Rossingnol, P.A. Opposite influences of host anaemia on blood feeding rate and fecundity of mosquitoes. Parasitology 1992, 105 ( Pt 2) Pt 2, 159–163. [Google Scholar] [CrossRef]
- Zhu, Y.; Tong, L.; Nie, K.; Wiwatanaratanabutr, I.; Sun, P.; Li, Q.; Yu, X.; Wu, P.; Wu, T.; Yu, C.; et al. Host serum iron modulates dengue virus acquisition by mosquitoes. Nat Microbiol 2019, 4, 2405–2415. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Liu, Z.; Peng, Y.; Peng, W.; Tong, L.; Wang, J.; Liu, Q.; Wang, P.; Cheng, G. A volatile from the skin microbiota of flavivirus-infected hosts promotes mosquito attractiveness. Cell 2022, 185, 2510–2522. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, N.; Sonye, G.; Mogi, M.; Githeko, A.; Yan, G. The effects of climatic factors on the distribution and abundance of malaria vectors in Kenya. J Med Entomol 2002, 39, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Asgarian, T.S.; Moosa-Kazemi, S.H.; Sedaghat, M.M. Impact of meteorological parameters on mosquito population abundance and distribution in a former malaria endemic area, central Iran. Heliyon 2021, 7, e08477. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.J.; Kim, H.C.; Klein, T.A.; Chong, S.T.; Sim, K.; Chung, Y.; Cheong, H.K. Comparison of climatic factors on mosquito abundance at US Army Garrison Humphreys, Republic of Korea. PLoS One 2020, 15, e0240363. [Google Scholar] [CrossRef] [PubMed]
- Reisen, W.K.; Cayan, D.; Tyree, M.; Barker, C.M.; Eldridge, B.; Dettinger, M. Impact of climate variation on mosquito abundance in California. J Vector Ecol 2008, 33, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.W.; Ionides, E.L.; Knepper, R.G.; Stanuszek, W.W.; Walker, E.D.; Wilson, M.L. Cross-correlation map analyses show weather variation influences on mosquito abundance patterns in Saginaw County, Michigan, 1989-2005. J Med Entomol 2012, 49, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Ruiz, L.P.; Montenegro-Cadena, L.; Blattner, B.; Menghwar, S.; Zurek, L.; Londono-Renteria, B. Differential Tick Salivary Protein Profiles and Human Immune Responses to Lone Star Ticks (Amblyomma americanum) From the Wild vs. a Laboratory Colony. Front Immunol 2019, 10, 1996. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.C.; Prior, S.; Apperson, C.S.; Irby, W.S. Bionomics of Anopheles quadrimaculatus and Culex erraticus (Diptera: Culicidae) in the Falls Lake basin, North Carolina: seasonal changes in abundance and gonotrophic status, and host-feeding patterns. J Med Entomol 1993, 30, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Dantur Juri, M.J.; Claps, G.L.; Santana, M.; Zaidenberg, M.; Almirón, W.R. Abundance patterns of Anopheles pseudopunctipennis and Anopheles argyritarsis in northwestern Argentina. Acta Trop 2010, 115, 234–241. [Google Scholar] [CrossRef]
- Olajiga, O.M. , Cardenas, J. C., Paulina, L., Worges, M., Fikrig, E., & Park, Y. Aedes aegypti anti-salivary proteins IgG levels in a cohort of DENV-like symptoms subjects from a dengue-endemic region in Colombia. Frontiers in Immunology.
- Peng, Z.; Rasic, N.; Liu, Y.; Simons, F.E. Mosquito saliva-specific IgE and IgG antibodies in 1059 blood donors. J Allergy Clin Immunol 2002, 110, 816–817. [Google Scholar] [CrossRef]
- Anker, M.; Arima, Y. Male-female differences in the number of reported incident dengue fever cases in six Asian countries. Western Pac Surveill Response J 2011, 2, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.E.; Eliason, D.A.; Moore, M.; Sather, G.E.; Schonberger, L.B.; Cabrera-Coello, L.; Fernandez de Castro, J. Epidemiologic investigations of dengue infection in Mexico, 1980. Am J Epidemiol 1983, 117, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Trravassos da Rosa, A.P.; Vasconcelos, P.F.; Travassos Da Rosa, E.S.; Rodrigues, S.G.; Mondet, B.; Cruz, A.C.; Sousa, M.R.; Travassos Da Rosa, J.F. Dengue epidemic in Belém, Pará, Brazil, 1996-97. Emerg Infect Dis 2000, 6, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, J.C.; Giraldo-Parra, S.Y.; Gonzalez, M.U.; Gutierrez-Silva, L.Y.; Jaimes-Villamizar, L.; Roa-Parra, A.L.; Carvajal, D.J.; Valdivia, H.O.; Sanchez, J.F.; Colpitts, T.M.; et al. Laboratory Findings in Patients with Probable Dengue Diagnosis from an Endemic Area in Colombia in 2018. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Londono-Renteria, B.L.; Shakeri, H.; Rozo-Lopez, P.; Conway, M.J.; Duggan, N.; Jaberi-Douraki, M.; Colpitts, T.M. Serosurvey of Human Antibodies Recognizing. Front Public Health 2018, 6, 111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





