Preprint
Review
An Updated Review of Fossil Pollen Evidence for the Study of the Origin, Evolution and Diversification of Caribbean Mangroves
Altmetrics
Downloads
89
Views
25
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
31 October 2023
Posted:
01 November 2023
You are already at the latest version
Alerts
Abstract
Recently, the evolutionary history of the Caribbean mangroves has been reconsidered using partial palynological databases organized by the time intervals of interest, namely Late Cretaceous to Eocene for the origin, Eocene-Oligocene transition for major turnover and Neogene to Quaternary for diversification. These discussions have been published in a set of sequential papers but the raw information remains unknown. This paper reviews all the information available and provides the first comprehensive and updated compilation of the abovementioned partial databases. This compilation is called CARMA-F (CARibbean MAngroves-Fossil) and includes nearly 90 localities from the present and past Caribbean coasts, ranging from the Late Cretaceous to the Pliocene. Details on the Quaternary localities (CARMA-Q) will be published later. CARMA-F lists and illustrates the fossil pollen from past mangrove taxa and their extant representatives, and includes a map of the studied localities and a conventional spreadsheet with the raw data. The compilation is the most complete available for the study of the origin, evolution and diversification of Caribbean mangroves, and is open to modifications for adapting it to the particular interests of each researcher.
Keywords:
Subject: Environmental and Earth Sciences - Paleontology
1. Introduction
Mangroves are intertidal ecosystems that develop a worldwide forested fringe along tropical/subtropical coasts between approximately 25°N and 25°S (Figure 1). Structurally, these ecosystems are organized around a number of tree species from varied orders and families that confer mangrove formations their characteristic physiognomy, which has been considered an example of evolutionary convergence among taxonomically distant clades (Rull 2023c). In addition to their intrinsic value as natural systems (Soulé 1985), mangroves are important for the following reasons: (i) they protect coasts and coastal ecosystems such as corals, seagrasses and salt marshes from erosion, thus favoring seaward progradation; (ii) they play a key role in the maintenance of biodiversity and ecological dynamics across the marine/terrestrial ecotone; (iii) they provide relevant ecological and cultural services (fisheries, cultivation, aquaculture, timber, fuel, aesthetics, ecotourism, etc.); and (iv) they are among the most efficient blue-carbon ecosystems that contribute to alleviating atmospheric CO2 increases by sequestering carbon in their organic-rich sediments (Lugo & Snedaker 1974; Saenger 2002; Nagelkerken et al. 2008; Laegdsgaard & Johnson 2001; Afonso et al. 2021; Nellemann et al. 2009; Mcleod et al. 2011; Fest et al. 2022).
However, mangroves are among the world’s most threatened ecosystems (Worthington et al. 2020). According to the latest estimates, the global mangrove extent was reduced by 3.4% in less than 25 years (1996-2020) due to natural and anthropogenic deforestation (Bunting et al. 2022). If these rates are maintained, these ecosystems will be severely reduced during this century, and their long-term survival is at great risk (Duke et al. 2017), which would imply increasing coastal erosion rates and biodiversity depletion, as well as losses in ecological and cultural services and in the global warming mitigation capacity. This has fostered the launching of numerous worldwide initiatives for mangrove conservation and restoration, which need sound ecological knowledge (Makowski & Finkl 2018; Lacerda et al. 2019; Lester et al. 2020; Mishra & Farooq 2022). Most of these initiatives have been based on present-day ecological studies, but paleoecological research may also be useful, as it provides first-hand empirical evidence on the actual response of mangrove ecosystems to environmental climatic, eustatic and anthropogenic drivers of ecological change. This allows characterization of the main threats and helps define the corresponding response thresholds, thus providing information useful for mangrove conservation management. Evolutionary studies are also valuable, as they furnish straightforward evidence on the evolutionary potential of mangrove species and their capacity to undergo genetic changes in response to environmental shifts (Rull 2023d).
The Caribbean region (Figure 2) has been considered the cradle of Neotropical mangroves and a biodiversity hotspot for these ecosystems (Duke 2017; Bryan-Brown et al. 2020; Goldberg et al. 2020). Current estimates for mangrove loss in the region are similar to global figures and several conservation actions have been proposed specifically for the region (Lacerda et al. 2019). In this context, the Caribbean mangroves were considered direct descendants of former pantropical Cretaceous mangroves that experienced regional differentiation after the closure of the Tethys Sea. However, a detailed quantitative analysis of the evidence strongly suggested that the first Caribbean mangroves did not appear until the Middle Eocene and were ecological and evolutionary innovations that emerged de novo, rather than a consequence of the regional differentiation of former hypothetical Late Cretaceous pantropical mangroves (Rull 2022a).
The Eocene Caribbean mangroves were dominated by the ancestor of the extant Pelliciera, which was replaced by the ancestor of the modern Rhizophora after the Eocene/Oligocene transition (EOT), likely due to the global and intense cooling and sea-level fall that characterized this geological boundary (Rull, 2023c). The Pelliciera mangroves never returned, and their modern representatives remain as subordinate mangrove elements restricted to a small equatorial patch in Central America/NW South America (Rull 2023a). The Rhizophora mangroves diversified during the Neogene and attained their present-like taxonomical composition in the Late Miocene-Pliocene after the emergence of Avicennia and Laguncularia, the other important mangrove-forming trees of extant Caribbean mangroves (Rull 2023b). The Quaternary was a time of spatial and ecological reorganization due to the recurrent Pleistocene climatic/sea-level shifts, and the Holocene was characterized by the consequences of human disturbance, especially during the last 6000 years (Rull 2022b). The last centuries have been characterized by a significant reduction in Caribbean mangrove cover due to natural and anthropogenic deforestation, which calls for urgent conservation/restoration actions (Rull 2023d). A graphical summary of these events is provided in Figure 3.
These conclusions were based on partial datasets organized chronologically according to the time lapse of interest (i.e., Late Cretaceous to Eocene, EOT, Neogene and Quaternary), which are available in the corresponding papers. A first attempt to synthesize all this information led to the development of a compilation called CARMA (CARibbean MAngroves), but only the main features of the existing fossil records were available, and the specific data remain unpublished (Rull 2023d). The CARMA compilation has been further updated and subdivided into two conceptually different parts: a pre-Quaternary fossil section (CARMA-F) and a section containing Quaternary and modern records (CARMA-Q). This paper presents the most updated version of CARMA-F, which constitutes the basis for the study of Eocene origin, EOT evolutionary turnover and Neogene diversification of Caribbean mangroves. The CARMA-Q update, useful for the study of modern mangroves in the face of Quaternary environmental shifts and their anthropization is in progress and will be published later. In addition to providing a comprehensive view of the published information to unravel the origin and evolution of Caribbean mangroves, CARMA-F may be used as a guide for the interested researchers to locate the required data aimed at addressing their own particular interests. The present version of CARMA-F is fairly complete, considering the published data, and its content is consistent with the above evolutionary insights. However, the compilation remains open to new updates from future research and further improvements, modifications and alternative hypotheses regarding the origin and evolution of Caribbean mangroves cannot be disregarded.
The paper is subdivided into three main sections. The first section briefly characterizes the extant Caribbean mangroves in terms of their taxonomic composition, whereas the second section illustrates the pollen of the main taxa, with emphasis on those with fossil representatives. The third section describes the CARMA-F compilation, which is provided as a spreadsheet in the Supplementary Material, the main geographical and chronological features of the localities studied and the types of data provided in the original references, with illustrative examples of all of them. Finally, some comments are provided in reference to…
2. Extant Caribbean mangroves
According to the latest estimates using remote sensing methods (Bunting et al. 2022), Caribbean mangroves occupy a total extent of approximately 14,700 km2, which represents ~10% of the world’s total (Figure 4; Table 1). The countries with more extensive mangrove cover are Cuba, Venezuela, Colombia and Panama (1500-3600 km2); all other countries are below 750 km2, and 15 of them have less than 100 km2 of mangroves, with 9 below 10 km2 (Table 1).
Floristically, there are two main types of mangrove constituents: true (or strict) mangrove elements and mangrove associates (Table 2). The conditions for being considered a true mangrove element are the following (Tomlinson 2016): (i) present only in mangroves, not extending into terrestrial communities; (ii) playing a major role in the structure of the community and able to form pure stands; (iii) having specific morphological adaptations to intertidal environments, typically pneumatophores and viviparous embryos; (iv) bearing physiological mechanisms for salt exclusion, as an adaptation to grow in saline waters; and (v) being systematically isolated from their terrestrial relatives, usually at the generic level, but often at the family/subfamily level.
True mangrove elements are further subdivided into major and minor elements. Major true mangrove elements are mostly trees and are also known as mangrove-forming trees. In the Caribbean, the major true mangrove elements are of the genera Rhizophora (Rhizophoraceae), Avicennia (Acanthaceae) and Laguncularia (Combretaceae) (Figure 5). Minor true mangrove elements have similar traits but are not structurally important for the community (condition ii) and are unable to develop pure stands (iii), usually living in peripheral intertidal habitats. This is the case for Pelliciera (Tetrameristaceae) and Acrostichum (Pteridaceae) species, although the first can locally develop small pure stands under perhumid and shading conditions (Dangremond et al. 2015). Mangrove associates are typical of mangrove environments but are not restricted to them (i), are not structurally important (ii) and lack morphologically and physiologically adaptations to intertidal habitats (iii, iv). These elements also occur in other habitats, such as coastal swamps, back-mangrove wetlands, salt marshes, riverbanks, beach communities and inland rainforests (Tomlinson 2016). The herb Crenea maritima (Lythraceae) is exclusive to mangrove environments (i) and might be treated as a true mangrove element but it fails to meet conditions (ii) and (iii) and is therefore considered a mangrove associate. Conocarpus erectus is able to develop pure stands (ii) and is sometimes considered a true mangrove element, but it lacks morphological adaptations (iii) and does not tolerate flooding and high salinities (iv), thus living in marginal mangrove environments (Lonard et al. 2020b). Some reviews on taxonomic, biogeographical, environmental and ecological features of some of the most important Caribbean true-mangrove elements have recently been published (DeYoe et al. 2020; Lonard et al. 2017; 2020a, b).
In addition to the above true and associate mangrove species, ~120 other accompanying species have been identified in the Neotropical mangroves, defining 30 phytosociological associations, all of which are present in the Caribbean region (García-Fuentes et al. 2020).
3. Modern and fossil pollen types
Fossil pollen/spores are, by large, the main evidence utilized in the evolutionary study of Caribbean mangroves. The pollen morphology of the main Caribbean mangrove components is illustrated in Figure 6, which is based on material from living plants and sedimentary pollen from modern sediments. It should be stressed that pollen morphology is rather homogeneous within each genus, and identification at the species level is not possible in most genera, with a few exceptions. This is why, when dealing with pollen, we will refer to genera, except when some type of morphological differentiation at the species level is possible. The generic names of extant mangrove components are usually extended to the whole Quaternary, assuming that they have been present during the last 2.6 Ma, which is a common procedure in Quaternary paleoecology (Rull 2020). In older sediments, where the occurrence of extant taxa is not guaranteed, artificial (as opposed to natural or living) species have been defined based on pollen morphology (morphospecies) and associated with extant genera, also on the basis of morphological identity. Since pollen morphology is a highly conservative character, from an evolutionary point of view (Erdtman 1986; Traverse 2007), it has traditionally been assumed that these morphospecies represent the ancestors (likely at the generic level) of extant species, having similar ecological requirements. Indeed, paleoecological studies using fossils commonly rely on a reasonable degree of niche constancy over time (niche conservatism), especially at the genus level, in long-lasting communities (Wiens & Graham 2005; Hadly et al. 2009; Wiens et al. 2010; Lososová et al. 2020), which is the case for mangroves.
This procedure, which has long been used in plant evolution, in general, and the Neotropics, in particular (e.g., Flenley 1979; Morley 2000; Graham 2011), has been validated by recent molecular phylogenetic studies demonstrating that the main extant Caribbean mangrove genera were already present in the Paleogene, and their modern species emerged mostly in the Neogene (Lo et al. 2014; Li et al. 2016; Duke 2017). The fossil representatives of the main extant mangrove genera are listed in Table 3; the remaining true and associate mangrove genera (Table 2) have not known Cretaceous, Paleogene or Neogene fossil equivalents and occur only in Quaternary and modern sediments. The palm Nypa fruticans Wurmb, now restricted to the IWP region, is included because it was present in the Caribbean region until the Eocene (Graham 1995; Gee 2001). In this review, the names of extant genera are used as representatives of the corresponding lineages, according to the fossil representatives listed in Table 3.
4. The CARMA-F compilation
The most updated CARMA version contains almost 160 entries/localities, of which 86 correspond to CARMA-F (Figure 7). The details on these localities and their fossil pollen data are provided in the Supplementary Material and are summarized as follows. Geographically, most fossil pollen sites (86%) are in the southern Caribbean coasts, especially in Colombia and Venezuela. This is due to the intensive and extensive exploration/production activities developed in these countries by the oil industry since the early 20th century. In these activities, fossil pollen played a key biostratigraphic role, especially in coastal and shallow-marine environments (Kuyl et al. 1955; Germeraad et al. 1968; González de Juana et al. 1980; Lorente 1986; Muller et al. 1987). Many of the northern South American sites are located far from the present Caribbean coasts, but they were on near-mangrove coastal/shelf environments in the Paleogene and the Neogene. This is due to the highly dynamic paleogeography of the region driven mainly by the migration of the Caribbean plate and the occurrence of extensive marine incursions in NW South America (Hoorn et al. 2010; Jaramillo et al. 2017; Romito & Mann, 2020; Mann 2021). The remaining CARMA-F localities lie in Central America (12%) and the Greater Antilles (2%), while the Lesser Antilles are devoid of fossil pollen records involving mangrove elements. The location of fossil records is approximate in many cases, especially in wells, due to the lack of coordinates, mostly for industrial confidentiality reasons. In these cases, the location of the records in Figure 7 has been placed according to maps and descriptions with the aid of Google Earth.
Chronologically, 6 localities bear Late Cretaceous sediments, 37 include Paleogene rocks and 59 contain Neogene formations (this makes more than 86 items, actually 102, because a number of sections include combinations of these ages). The majority of records (61; 71%) provide quantitative data, usually pollen percentages but also raw counts in a few cases (5), whereas 19 (22%) report only presence and 6 (7%) yield a semiquantitative parameter called re-observation probability (ROP), using the formula: ROP = 1-(1-(a/N))M, where a = number of grains of a species counted in a sample; N = total number of grains of all species in the same sample; and M = total number of grains of all species in a new sample (Germeraad et al., 1968). These data are displayed in several formats in the original references, namely, in-text taxa lists, tables and range charts for qualitative (presence/absence) data and diagrams or tables for percentages. ROP values are provided as range charts using symbols for probability classes. Illustrative examples of range charts, percentage tables/diagrams and ROP charts are provided in Figs. 8 to 11.
Figure 8.
Range chart indicating the present/absence patterns in the Late Eocene-Early Miocene interval of well COT-1X from Venezuela (see Figure 7 for location and the Supplementary Material for details). Mangrove representatives included in CARMA-F are highlighted in pink (see Table 3 for equivalences with extant taxa). Modified from Rull (2003).
Figure 8.
Range chart indicating the present/absence patterns in the Late Eocene-Early Miocene interval of well COT-1X from Venezuela (see Figure 7 for location and the Supplementary Material for details). Mangrove representatives included in CARMA-F are highlighted in pink (see Table 3 for equivalences with extant taxa). Modified from Rull (2003).
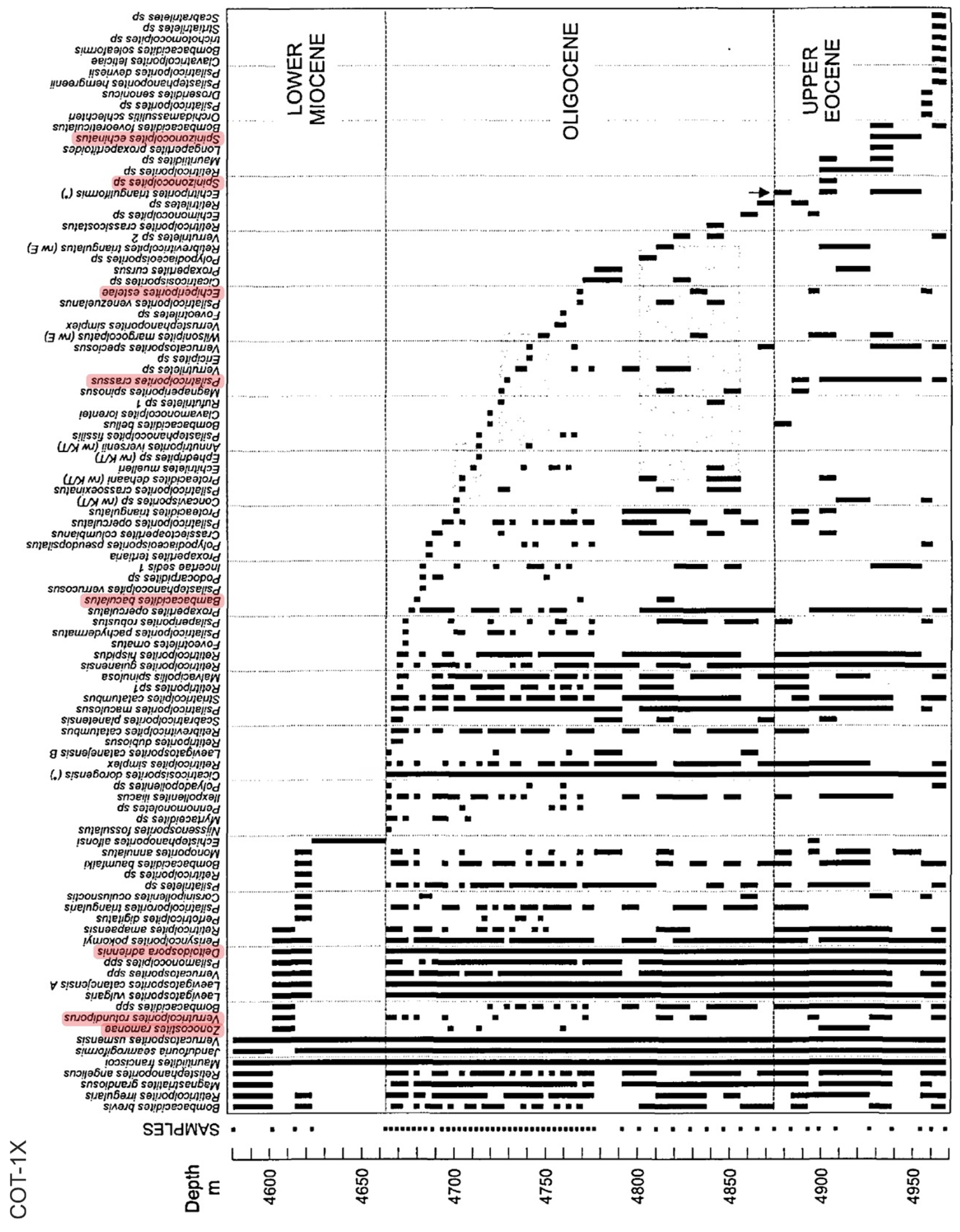
Figure 9.
Percentage diagram of the Early-Middle Miocene section of well Panchita-1X from Venezuela (Figure 7 and Supplementary Material), indicating the mangrove fossil pollen species highlighted in pink (Table 3). Values at the base of the diagram (in red) are the approximate percentage ranges used in the dataset. Modified from Lorente, 1986).
Figure 9.
Percentage diagram of the Early-Middle Miocene section of well Panchita-1X from Venezuela (Figure 7 and Supplementary Material), indicating the mangrove fossil pollen species highlighted in pink (Table 3). Values at the base of the diagram (in red) are the approximate percentage ranges used in the dataset. Modified from Lorente, 1986).
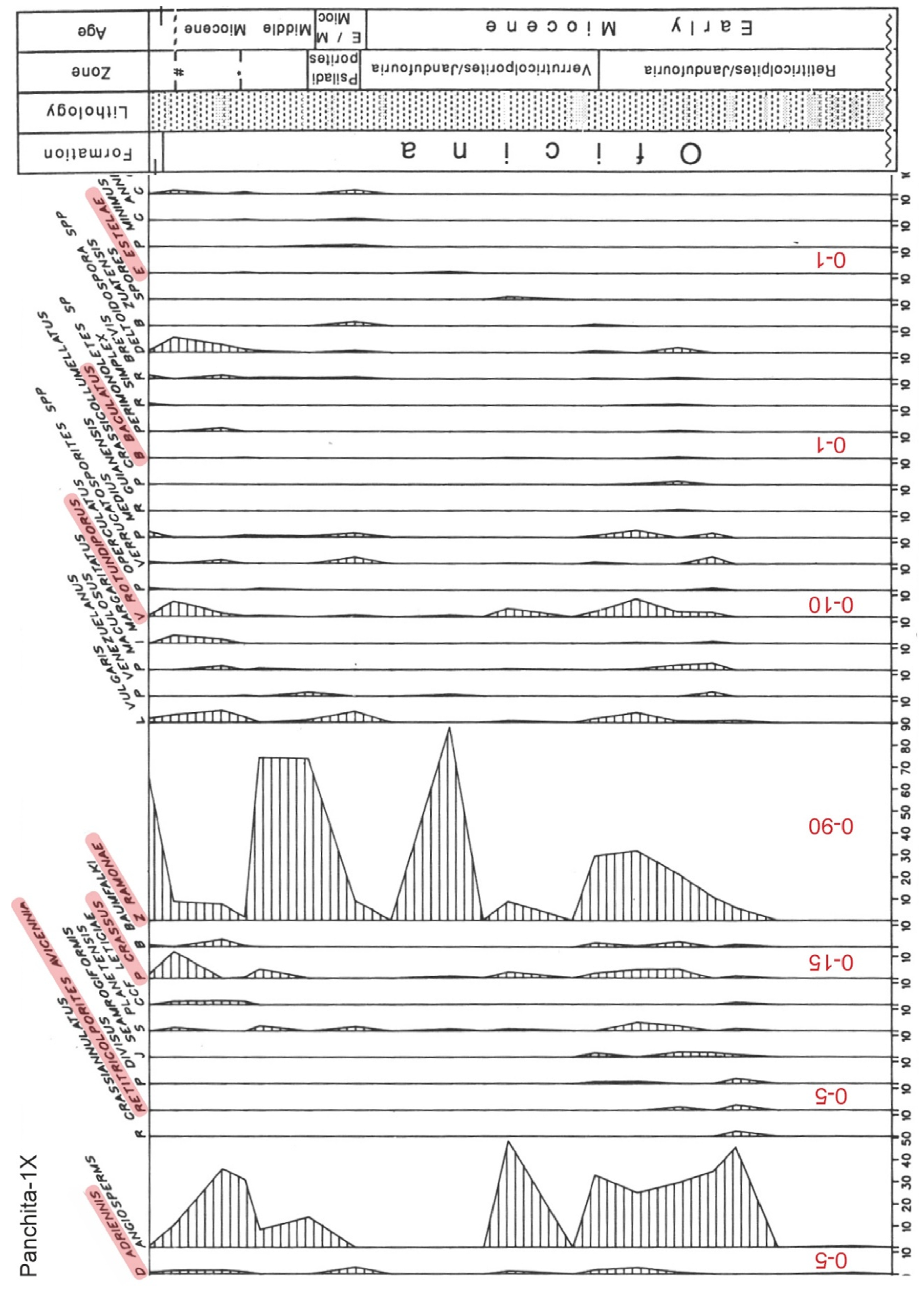
Figure 10.
Raw counts of mangrove fossil pollen taxa (highlighted in pink) for the Middle-Late Miocene interval of the Quebrada Jarana in the Yopal site (Colombia) (Figure 7; Supplementary Material). Modified from Dueñas & Van der Hammen (2007).
Figure 10.
Raw counts of mangrove fossil pollen taxa (highlighted in pink) for the Middle-Late Miocene interval of the Quebrada Jarana in the Yopal site (Colombia) (Figure 7; Supplementary Material). Modified from Dueñas & Van der Hammen (2007).
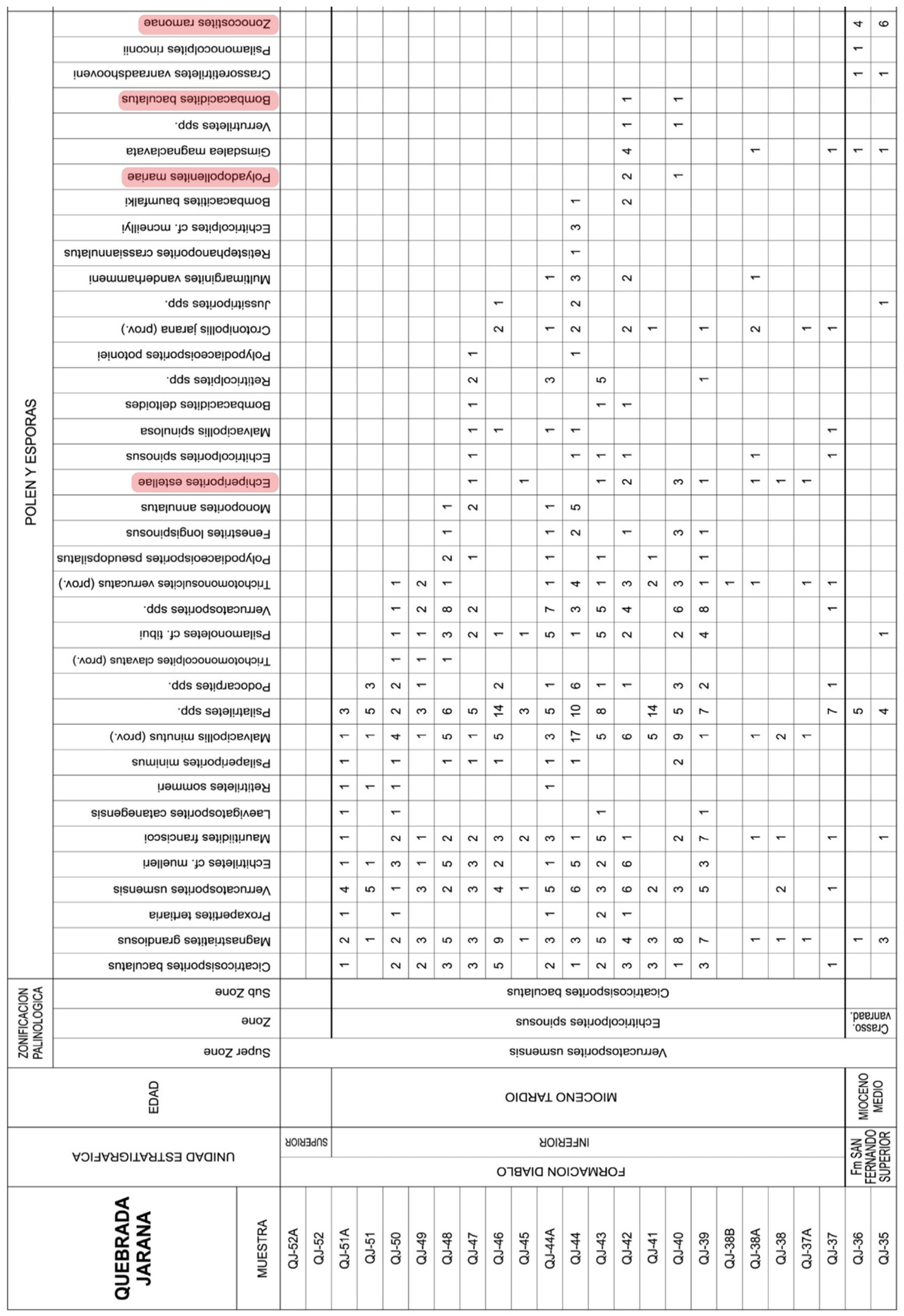
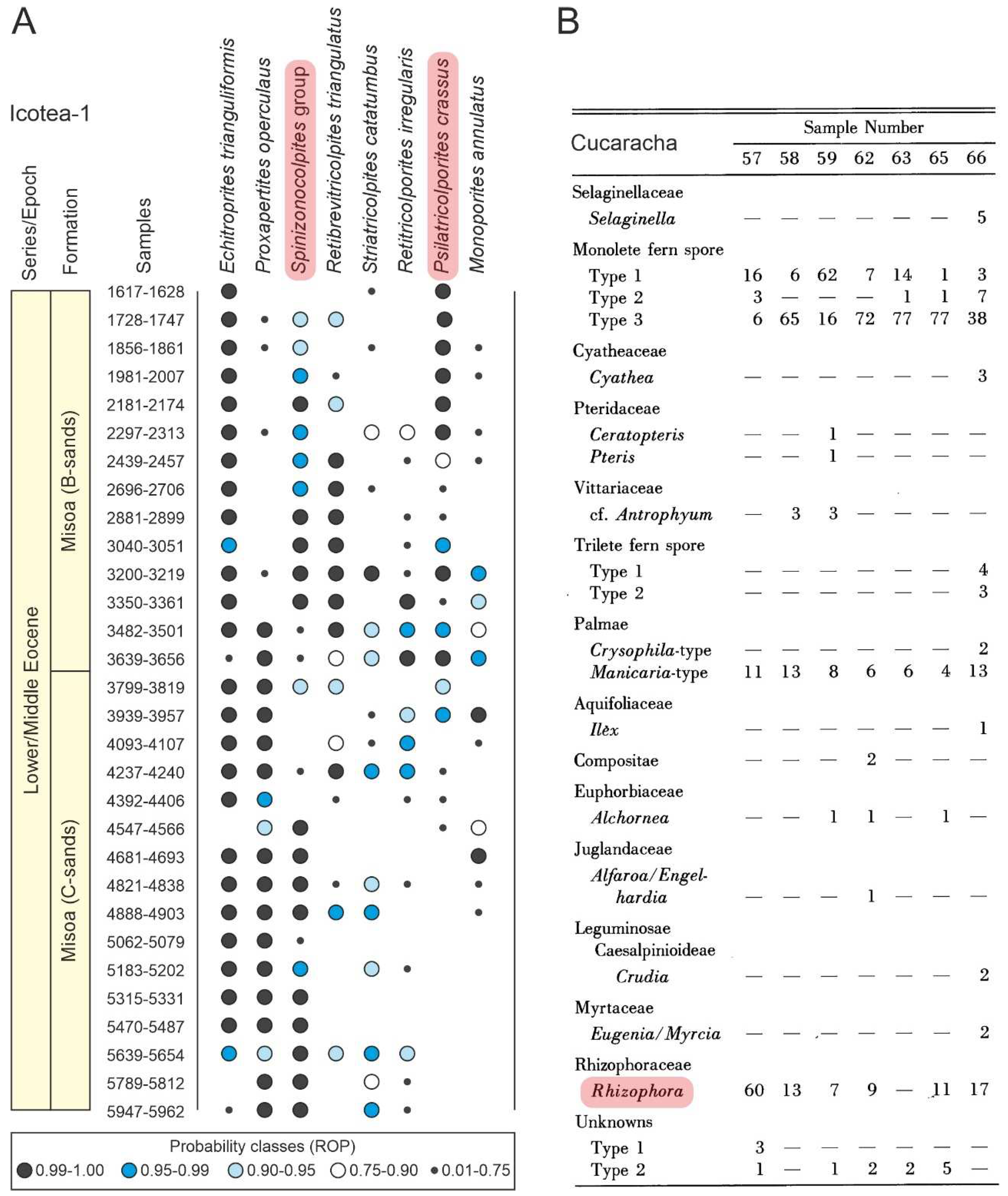
Figure 11.
(A) Semiquantitative range chart of the Middle Eocene section of well Icotea-1 (Venezuela) using the re-observation probability (ROP). Modified from Germeraad et al. (1968). (B) Percentage table of the Early Miocene Cucaracha Formation (Panama). Modified from Graham (1988b). Mangrove taxa are highlighted in pink (see Figure 7, Table 3 and the Supplementary Material for location, botanical affinities and more details).
Figure 11.
(A) Semiquantitative range chart of the Middle Eocene section of well Icotea-1 (Venezuela) using the re-observation probability (ROP). Modified from Germeraad et al. (1968). (B) Percentage table of the Early Miocene Cucaracha Formation (Panama). Modified from Graham (1988b). Mangrove taxa are highlighted in pink (see Figure 7, Table 3 and the Supplementary Material for location, botanical affinities and more details).
5. Final remarks
The CARMA-F version presented here replaces the unpublished partial compilations used in previous papers (Rull 2022a; 2023a, b, c), but the main conclusions in relation to the origin, evolution and diversification of Caribbean mangroves, as summarized in Rull (2023d) and synthesized in Figure 3, do not change. The refinements introduced by the updated dataset are addressed in detail in a book that will be issued next year (Rull, in press). The available version of CARMA-F is open to further additions and improvements and constitutes the most complete available compilation for studying any aspect of the origin and evolution of Caribbean mangroves. The format chosen for making the compilation public is a conventional spreadsheet so that interested researchers can freely use and modify this information according to their particular interests. As a former industry-based biostratigrapher, the author is aware that many palynological datasets potentially useful for the study of mangrove evolution remain unknown in confidential databases from oil companies. Some classical and highly cited papers, such as those by Germeraad et al. (1968) or Lorente (1986), among others, have demonstrated that it is possible to bring these data to light maintaining reasonable confidentiality rules. Continued efforts in this sense for the benefit of evolutionary knowledge would be acknowledged. Further improvements of CARMA-F would include the expansion of the compilation to the Caribbean/Gulf of Mexico region and eventually to the entire Neotropical region.
Author Contributions
Single-authored paper.
Funding
No funding was received specifically for the development of this paper.
Data Availability Statement
Data are provided as Supplementary Material.
Acknowledgments
Reviewers…
Conflicts of Interest
The author declares no competing interest.
References
- Afonso, F.; Félix, P.M.; Chainho, P.; Heumüller, J.A.; de Lima, R.F.; Ribeiro, F.; Brito, A.C. Assessing ecosystem services in mangroves: insights from São Tomé Island (Central Africa). Front. Environ. Sci. 2021, 9, 501673. [CrossRef]
- Bermúdez, M. A., Hoorn, C., Bernet, M., Carrillo, E., van der Beek, P. A., Garver, J. I., Mora, J. L., and K. Mehrkian. 2015. The detrital record of late-Miocene to Pliocene surface uplift and exhumation of the Venezuelan Andes in the Maracaibo and Barinas foreland basins. Basin Research 29: 370–395. [CrossRef]
- Bryan-Brown, Dale N., Connolly, Rod M., Richards, Daniel R., Adame, Fernanda, Friess, Daniel A., and Christopher J. Brown. 2020. Global trends in mangrove forest fragmentation. Scientific Reports 10: 1–8. [CrossRef]
- Bunting, Pete, Rosenqvist, Ake, Hilarides, Lammert, Lucas, Richard M., and Nathan Thomas. 2022. Global Mangrove Watch: Updated 2010 Mangrove Forest Extent (v2.5). Remote Sensing 14: 1034. [CrossRef]
- Celis, Sergio A., Rodríguez-Tovar, Francisco J., Pardo-Trujillo, Andrés, García-García, Fernando, Giraldo-Villegas, Carlos A., Gallego, Fabián, Plata, Ángelo, Trejos-Tamayo, Raúl, Vallejo-Hincapié, Felipe, and Francisco Javier Cardona. 2023. Deciphering influencing processes in a tropical delta system (middle-late Eocene? to Early Miocene, Colombian Caribbean): Signals from a well-core integrative sedimentological, ichnological, and micropaleontological analysis. Journal of South American Earth Sciences, 127. [CrossRef]
- Colmenares, O.A. A palynological study of samples from three wells of the Boscan Field, Venezuela. Rev. Técn. INTEVEP 1988, 8, 83-97.
- Colmenares, Omar A., and Lizaveta Teran. 1993. A biostratigraphic study of Paleogene sequences in southwestern Venezuela. Palynology 17: 67–89. [CrossRef]
- Dangremond, Emily M., Feller, Ilka C., and Wayne P. Sousa. 2015. Environmental tolerances of rare and common mangroves along light and salinity gradients. Oecologia 179: 1187–1198. [CrossRef]
- De La Parra, Felipe, Pinzon, Diego, Mantilla-Duran, Fernando, Rodriguez, Guillermo, and Victor Caballero. 2021. Marine-lacustrine systems during the Eocene in northern South America – Palynological evidence from Colombia. Journal of South American Earth Sciences 108: 103188. [CrossRef]
- DeYoe, Hudson, Lonard, Robert I., Judd, Frank W., Stalter, Richard, and Ilka Feller. 2020. Biological Flora of the Tropical and Subtropical Intertidal Zone: Literature Review for Rhizophora mangle L.. Journal of Coastal Research 36: 857–884. [CrossRef]
- Dueñas, H. Palynology of Oligocene-Miocene strata of borehole Q-E-22, Planeta Rica, northern Colombia. Rev. Palaeobot. Palynol. 1980. 30, 313-328. [CrossRef]
- Dueñas, H.; Van der Hammen, T. Significado geológico y asociaciones palinológicas de las formaciones Diablo Inferior (Mioceno Tardío) y San Fernando Superior (Mioceno Medio), piedemonte cuenca de los Llanos Orientales, Colombia. Rev. Acad. Colomb. Cienc. 2007, 31, 481-498. [CrossRef]
- Duke, N.C. Mangrove floristics and biogeography revisited: further deductions from biodiversity hot spots, ancestral discontinuities, and common evolutionary processes. In Mangrove Ecosystems: A Global Biogeographic Perspective; Rivera-Monroy, V.H.; Lee, S.Y.; Kristensen, E.; Twilley, R.R., Eds.; Springer: Berlin, Germany, 2017; pp. 17-53.
- Duke, N.C.; Meyneccke, J.-O.; Dittman, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D.; et al. A world without mangroves? Science 2017, 317, 41b-42b. [CrossRef]
- Godwin, H., and G. E. Erdtman. 1953. Pollen-Morphology and Plant Taxonomy: Angiosperms.. Journal of Ecology 41: 400. [CrossRef]
- Fest, Benedikt J., Swearer, Stephen E., and Stefan K. Arndt. 2021. A review of sediment carbon sampling methods in mangroves and their broader impacts on stock estimates for blue carbon ecosystems. Science of The Total Environment 816: 151618. [CrossRef]
- Flenley, J.R. The Equatorial Rain Forest: a Geological History. Butterworths: London, UK, 1979.
- Frederiksen, N.O. Review of early Tertiary sporomorph paleoecology. Am. Assoc. Strat. Palynol. Contr. Ser. 1985, 19, 1-92.
- García-Fuentes, Antonio, Lendínez-Barriga, Mª Lucía, Torres-Cordero, Juan Antonio, Ruiz-Valenzuela, Luis, Quesada, Juan, León, Yolanda, and Carlos Salazar-Mendías. 2020. A study on the mangrove formations of the Neotropical-Austroamerican Kingdom. Phytocoenologia 50: 137–162. [CrossRef]
- Garzon, Sandra, Warny, Sophie, and Philip J. Bart. 2012. A palynological and sequence-stratigraphic study of Santonian–Maastrichtian strata from the Upper Magdalena Valley basin in central Colombia. Palynology 36: 112–133. [CrossRef]
- Gee, Carole T.. 2001. The mangrove palm Nypa in the geologic past of the New World. Wetlands Ecology and Management 9: 181–203. [CrossRef]
- Gentry, A.H. Phytogeographic patterns as evidence for a Choco refuge. In Biological Diversification in the Tropics: Prance GT, Ed.; Columbia Univ Press: New York, USA, 1982; pp 112-136.
- Germeraad, J.H., Hopping, C.A., and J. Muller. 1968. Palynology of tertiary sediments from tropical areas. Review of Palaeobotany and Palynology 6: 189–348. [CrossRef]
- Goldberg, Liza, Lagomasino, David, Thomas, Nathan, and Temilola Fatoyinbo. 2020. Global declines in human-driven mangrove loss. Global Change Biology 26: 5844–5855. [CrossRef]
- González de Juana, C.; Iturralde, J.M.; Picard, X. Geología de Venezuela y de sus Cuencas Petrolíferas (I and II). FONINVES: Caracas, Venezuela, 1980.
- González Guzmán, A.E. A Palynological Study of the Upper Los Cuervos and Mirador formations (Lower and Middle Eocene), Tibú Area, Colombia. E.J. Brill: Amsterdam, The Netherlands, 1967.
- Graham, A. Late Cenozoic evolution of tropical lowland vegetation in Veracruz, Mexico. Evolution 1975, 29, 723-735. [CrossRef]
- Graham, Alan. 1976. Studies in Neotropical Paleobotany. II. The Miocene Communities of Veracruz, Mexico. Annals of the Missouri Botanical Garden 63: 787. [CrossRef]
- Graham, A. New records of Pelliciera (Theaceae/Pellicieraceae) in the Tertiary of the Caribbean. Biotropica 1977, 9, 48-52.
- Graham, Alan. 1985. Studies in Neotropical Paleobotany. IV. The Eocene Communities of Panama. Annals of the Missouri Botanical Garden 72: 504. [CrossRef]
- Graham, A. Miocene communities and paleoenviroments of southern Costa Rica. Am. J. Botany 1987, 74, 1501-1518. [CrossRef]
- Graham, Alan. 1988. Studies in Neotropical Paleobotany. V. The Lower Miocene Communities of Panama-The Culebra Formation. Annals of the Missouri Botanical Garden 75: 1440. [CrossRef]
- Graham, Alan. 1988. Studies in Neotropical Paleobotany. VI. The Lower Miocene Communities of Panama-the Cucaracha Formation. Annals of the Missouri Botanical Garden 75: 1467. [CrossRef]
- Graham, Alan. 1989. Studies in Neotropical Paleobotany. VII. The Lower Miocene Communities of Panama-The La Boca Formation. Annals of the Missouri Botanical Garden 76: 50. [CrossRef]
- Graham, A. Late Tertiary microfossil flora from the Republic of Haiti. Am. J. Bot. 1990, 77, 911-926. [CrossRef]
- Graham, A. New angiosperm records from the Caribbean Tertiary. Am. J. Bot. 1990, 77, 897-910. [CrossRef]
- Graham, Alan. 1991. Studies in Neotropical Paleobotany. X. The Pliocene Communities of Panama- Composition, Numerical Representations, and Paleocommunity Paleoenvironmental Reconstructions. Annals of the Missouri Botanical Garden 78: 465. [CrossRef]
- Graham, Alan. 1995. Diversification of Gulf/Caribbean Mangrove Communities Through Cenozoic Time. Biotropica 27: 20. [CrossRef]
- Graham, A. Studies in Neotropical botany. XI. Late Tertiary vegetation and environments of southeastern Guatemala: palynofloras from the Mio-Pliocene Padre Miguel Group and the Pliocene Herrería Formation. Am. J. Bot. 1998, 85, 1409-1425. [CrossRef]
- Graham, A. Studies in Neotropical botany. XIII. An Oligo-Miocene palynoflora from Simojovel (Chiapas, Mexico). Am. J. Botany 1999, 86, 17-31. [CrossRef]
- Graham, A. A Natural History of the New World. The Ecology and Evolution of Plants in the Americas. Univ. Chicago Press, Chicago, USA, 2011.
- Graham, Alan, and David M. Jarzen. 1969. Studies in Neotropical Paleobotany. I. The Oligocene Communities of Puerto Rico. Annals of the Missouri Botanical Garden 56: 308–+. [CrossRef]
- Graham, Alan, and David L. Dilcher. 1998. Studies in Neotropical paleobotany. XII. A palynoflora from the Pliocene Rio Banano Formation of Costa Rica and the Neogene vegetation of Mesoamerica. American Journal of Botany 85: 1426–1438. [CrossRef]
- Graham, A.; Cozadd, D.; Areces-Mallea, A.; Frederiksen, N.O. Studies in Neotropical paleobotany. XIV. A palynoflora from the Middle Eocene Saramaguacán Formation of Cuba. Am. J. Bot. 2000, 87, 1526-1539.
- Graham, Shirley A.. 2013. Fossil Records in the Lythraceae. The Botanical Review 79: 48–145. [CrossRef]
- Hadly, Elizabeth A., Spaeth, Paula A., and Cheng Li. 2009. Niche conservatism above the species level. Proceedings of the National Academy of Sciences of the United States of America 106: 19707–19714. [CrossRef]
- Hambalek, N.; Rull, V.; Di Giacomo, E.; Dàiz de Gamero, M.L. Evolución paleoecológica y paleoambiental de la secuencia del Neógeno en el Surco de Urumaco, Estado Falcón. Estudio palinológico y litológico. Bol. Soc. Venez. Geól. 1994, 1-2, 7-19.
- Helenes, J.; Cabrera, D. Oligo-Miocene palynomorph assemblages from eastern Venezuela. Palynology 2002, 27, 5-25.
- Hoorn, C., Wesselingh, F. P., ter Steege, H., Bermudez, M. A., Mora, A., Sevink, J., Sanmartín, I., Sanchez-Meseguer, A., Anderson, C. L., Figueiredo, J. P., and et al. 2010. Amazonia Through Time: Andean Uplift, Climate Change, Landscape Evolution, and Biodiversity. Science 330: 927–931. [CrossRef]
- Iturralde-Vinent, Manuel A.. 2006. Meso-Cenozoic Caribbean Paleogeography: Implications for the Historical Biogeography of the Region. International Geology Review 48: 791–827. [CrossRef]
- Jaramillo, Carlos A., and David L. Dilcher. 2001. Middle Paleogene palynology of Central Colombia, South America: A study of pollen and spores from tropical latitudes. Palaeontographica Abteilung B 258: 87–259. [CrossRef]
- Jaramillo, C., Bayona, G., Pardo-Trujillo, A.; Rueda, M.; Torres, V.; Harrington, G.J.; Mora, G. The palynology of the Cerrejón Formarion (Upper Paleocene) of northern Colombia. Palynology 2007, 31, 153-189. [CrossRef]
- Jaramillo, Carlos, Romero, Ingrid, D’apolito, Carlos, Bayona, German, Duarte, Edward, Louwye, Stephen, Escobar, Jaime, Luque, Javier, Carrillo-Briceño, Jorge D., Zapata, Vladimir, and et al. 2017. Miocene flooding events of western Amazonia. Science Advances 3: e1601693–1601693. [CrossRef]
- Kuyl, O.S.; Muller, J.; Waterbolk, H.T. The application of palynology to oil geology with reference to western Venezuela. Geol. Mijnb. 1955, 3, 49-76.
- de Lacerda, Luiz Drude, Borges, Rebecca, and Alexander Cesar Ferreira. 2019. Neotropical mangroves: Conservation and sustainable use in a scenario of global climate change. Aquatic Conservation: Marine and Freshwater Ecosystems 29: 1347–1364. [CrossRef]
- Laegdsgaard, P.; Johnson, C. Why do juvenile fish utilize mangrove habitats. J. Exp. Mar. Biol. Ecol. 2001, 257, 229-253. [CrossRef]
- Lamy, A. Plio-Pleistocene palynology and visual kerogen studies, Trinidad, W.I., with emphasis on the Columbus Basin. Geol. Soc. Trinidad Tobago, Geol. Conf. Trans., 1986; pp 114-127.
- Lester, Sarah E., Dubel, Alexandra K., Hernan, Gema, McHenry, Jennifer, and Andrew Rassweiler. 2020. Spatial Planning Principles for Marine Ecosystem Restoration. Frontiers in Marine Science, 7. [CrossRef]
- Li, Xinnian, Duke, Norman C., Yang, Yuchen, Huang, Lishi, Zhu, Yuxiang, Zhang, Zhang, Zhou, Renchao, Zhong, Cairong, Huang, Yelin, and Suhua Shi. 2016. Re-Evaluation of Phylogenetic Relationships among Species of the Mangrove Genus Avicennia from Indo-West Pacific Based on Multilocus Analyses. PLOS ONE 11: e0164453–e0164453. [CrossRef]
- Lo, Eugenia Yy, Duke, Norman C, and Mei Sun. 2014. Phylogeographic pattern of Rhizophora (Rhizophoraceae) reveals the importance of both vicariance and long-distance oceanic dispersal to modern mangrove distribution. BMC Evolutionary Biology 14: 83–83. [CrossRef]
- Lonard, R.I.; Judd, F.W.; DeYoe, H.; Stalter, R. Biology and ecology of the halophyte Laguncularia racemosa (L.) Gaertn. f.: a review. In Handbook of Halophytes; Grigore, M.-N., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 1-6.
- Lonard, Robert I., Judd, Frank W., DeYoe, Hudson R., and Richard Stalter. 2021. Biology of the Mangal Halophyte Conocarpus erectus L.: A Review., 1819–1831. [CrossRef]
- Lonard, Robert I., Judd, Frank W., Summy, K.R., DeYoe, Hudson, and Richard Stalter. 2017. The Biological Flora of Coastal Dunes and Wetlands:Avicennia germinans(L.) L.. Journal of Coastal Research 331: 191–207. [CrossRef]
- Lorente, M.A. Palynology and palynofacies of the Upper Tertiary in Venezuela. Diss. Bot. 1986, 99, 1-222.
- Lososova, Zdenka, Divisek, Jan, Chytry, Milan, Gotzenberger, Lars, Tesitel, Jakub, and Ladislav Mucina. 2021. Macroevolutionary patterns in European vegetation. Journal of Vegetation Science, 32. [CrossRef]
- Lugo, A.E.; Snedaker, S.C. The ecology of mangroves. Annu. Rev. Ecol. Syst. 1974, 5, 39-64.
- Makowski, Christopher, and Charles W. Finkl. 2018. Erratum to: Threats to Mangrove Forests., E1–E1. [CrossRef]
- Mann, P. Gulf of Mexico, Central America, and the Caribbean. In Encyclopedia of Geology; Alderton, D.; Elias, S.A., Eds.; Academic Press: London, UK, 2021; pp 47-67.
- Mcleod, Elizabeth, Chmura, Gail L, Bouillon, Steven, Salm, Rodney, Björk, Mats, Duarte, Carlos M, E Lovelock, Catherine, Schlesinger, William H, and Brian R Silliman. 2011. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Frontiers in Ecology and the Environment 9: 552–560. [CrossRef]
- Miller, Kenneth G., Browning, James V., Schmelz, W. John, Kopp, Robert E., Mountain, Gregory S., and James D. Wright. 2020. Cenozoic sea-level and cryospheric evolution from deep-sea geochemical and continental margin records. Science Advances 6: eaaz1346. [CrossRef]
- Mishra, Amrit Kumar, and Syed Hilal Farooq. 2022. Lack of ecological data hinders management of ecologically important saltmarsh ecosystems: A case study of saltmarsh plant Porterasia coarctata (Roxb.). Journal of Environmental Management 321: 115957. [CrossRef]
- Montaño, P.C.; Nova, G.; Bayona, G.; Mahecha, H.; Ayala, C.; Jaramillo, C.; De la Parra, F. Análisis de secuencias y procedencia en sucesiones sedimentarias de grano fino: un ejemplo de la Formación Umir y base de la Formación Lisama, en el sector de Simacota (Santander, Colombia). Bol. Geol. 2016, 38, 51-72.
- Burnham, Robyn J., and Robert J. Morley. 2000. Origin and Evolution of Tropical Rain Forests. PALAIOS 15: 580–581. [CrossRef]
- Muller, Jan. 1981. Fossil pollen records of extant angiosperms. The Botanical Review 47: 1–142. [CrossRef]
- Muller, J.; Di Giacomo, E.; Van Erve, A.W. A palynological zonation for the Cretaceous, Tertiary and Quaternary of northern South America. Am. Assoc. Strat. Palynol. Contr. Ser. 1987, 19, 7-76.
- Nagelkerken, I., Blaber, S.J.M., Bouillon, S., Green, P., Haywood, M., Kirton, L.G., Meynecke, J.-O., Pawlik, J., Penrose, H.M., Sasekumar, A., and et al. 2008. The habitat function of mangroves for terrestrial and marine fauna: A review. Aquatic Botany 89: 155–185. [CrossRef]
- Nellemann, C.; Corcoran, E.; Duarte, C.; Valdés, L.; De Young, C.; Fonseca, L.; Grimsditch, G. Blue Carbon. A Rapid Response Assessment. UNEP, GRID-Arendal, Norway, 2009.
- Ochoa, D., Hoorn, C., Jaramillo, C., Bayona, G., Parra, M., and F. De la Parra. 2012. The final phase of tropical lowland conditions in the axial zone of the Eastern Cordillera of Colombia: Evidence from three palynological records. Journal of South American Earth Sciences 39: 157–169. [CrossRef]
- Pocknall, David T., and Robert N. Erlich. 2020. Palynostratigraphy and lithostratigraphy of Upper Cretaceous and Paleogene outcrop sections, Merida Andes (Maracaibo Basin), Western Venezuela. Journal of South American Earth Sciences 104: 102830. [CrossRef]
- Pocknall, D.T.; Wood, L.J.; Geen, A.F.; Harry, B.E.; Hedlund, R. Integrated paleontological studies of Pliocene to Pleistocene deposits of the Orinoco Delta, Eastern Venezuela and Trinidad. Proc. IX Int. Palynol. Cong. Houston, USA, 2001; pp. 319-326.
- Pocknall, D.T., Clowes, C.D., Jarzen, D.M. Spinizonocolpites prominatus (McIntyre) Stover & Evans: fossil Nypa pollen, taxonomy, morphology, global distribution, and paleoenvironmental significance. N. Zealand J. Geol. Geophys. 2022, 66, 558-570. [CrossRef]
- Rodríguez-Forero, Guillermo, Oboh-Ikuenobe, Francisca E., Jaramillo-Munoz, Carlos, Rueda-Serrano, Milton J., and Edwin Cadena-Rueda. 2012. Palynology of the Eocene Esmeraldas Formation, Middle Magdalena Valley Basin, Colombia. Palynology 36: 96–111. [CrossRef]
- Romito, S.; Mann, P. Tectonic terrains underlying the present-day Caribbean plate: their tectonic origin, sedimentary thickness, subsidence histories and regional controls on hydrocarbon resources. In The Basins, Orogens, and Evolution of the Southern Gulf of Mexico and Northen Caribbean; Davidson, I.; Hull, J.N.F.; Pindell, J., Eds.; Geol. Soc., London, UK, 2020; pp 343-378.
- Rull, V. Paleoecología y análisis secuencial de una sección deltaica terciaria de la cuenca de Maracaibo. Bol. Soc. Venez. Geól. 1992, 46, 16-26.
- Rull, V. Oligo-Miocene palynology of the Rio Chama sequence (western Venezuela), with comments on fossil algae as paleoenvironemntal indicators. Palynology 1997, 21, 213-229. [CrossRef]
- Rull, Valentí. 1999. Sequence analysis of western Venezuelan cretaceous to Eocene sediments using palynology: Chrono-paleoenvironmental and paleovegetational approaches: Discussion & reply. Palynology 23: 35–36. [CrossRef]
- Rull, V. Biogeographical and evolutionary considerations on Mauritia (Arecaceae), based on palynological evidence. Rev. Palaeobot. Palynol. 1998, 100, 109-122. [CrossRef]
- Rull, Valenti. 1998. Middle Eocene Mangroves and Vegetation Changes in the Maracaibo Basin, Venezuela. PALAIOS 13: 287. [CrossRef]
- Rull, V. 1999. Palaeofloristic and palaeovegetational changes across the Paleocene/Eocene boundary in northern South America. Review of Palaeobotany and Palynology 107: 83–95. [CrossRef]
- Rull, V. Holocene sea level rise in Venezuela: a preliminary curve. Bol. Soc. Ven. Geól. 2000, 25, 32-36.
- Rull, V. A quantitative palynological record from the Early Miocene of western Venezuela, with emphasis on mangroves. Palynology 2001, 25, 109-126. [CrossRef]
- Rull, V. Contribution of quantitative ecological methods to the interpretation of stratigraphically homogeneous pre-Quaternary sequences: a palynological example from the Oligocene of Venezuela. Palynology 2003, 27, 75-98.
- Rull, V. Quaternary Ecology, Evolution and Biogeography. Elsevier/Academic Press, London, UK, 2020.
- Rull, Valentí. 2022. The Caribbean mangroves: An Eocene innovation with no Cretaceous precursors. Earth-Science Reviews, 231. [CrossRef]
- Rull, V. Responses of Caribbean mangroves to Quaternary climatic, eustatic and anthropogenic drivers of ecological change: a review. Plants 2022, 11, 3502. [CrossRef]
- Rull, Valentí. 2023. Taxon Cycles in Neotropical Mangroves. Plants 12: 244. [CrossRef]
- Rull, Valenti. 2023. The Neogene-Quaternary diversification trend in the shaping of modern Caribbean mangroves. Quaternary Science Reviews, 300. [CrossRef]
- Rull, Valentí. 2023. Eocene/Oligocene global disruption and the revolution of Caribbean mangroves. Perspectives in Plant Ecology, Evolution and Systematics, 59. [CrossRef]
- Rull, Valentí. 2023. Rise and fall of Caribbean mangroves. Science of The Total Environment 885: 163851. [CrossRef]
- Rull, V. Origin and Evolution of Caribbean Mangroves. A Time-Continuum Integrative Approach. Springer Nature, Cham, Switzerland (in press).
- Rull, V.; Poumot, C. Eocene to Miocene palynocycles from western Venezuela and correlations with global eustatic cycles. Mem. VII Cong. Geol. Venez. II, 1997; pp 343-349.
- Scarnecchia, David L., and Peter Saenger. 2004. Mangrove Ecology, Silviculture and Conservation. Journal of Range Management 57: 422. [CrossRef]
- Santos, Carlos. 2022. Palynostratigraphy of the Umir Formation, Middle Magdalena Valley Basin (MMVB) Colombia.,. [CrossRef]
- Soulé, M.E. What is conservation biology? BioScience 1985, 35, 727-734. [CrossRef]
- Spalding, M.; Kainuma, M.; Collins, L. World Atlas of Mangroves. Routledge, London, UK, 2010.
- Tomlinson, P.B. The Botany of Mangroves. Cambridge Univ Press, Cambridge, UK, 2016.
- Traverse, A. Paleopalynology. Springer, Dordrecht, The Netherlands, 2007.
- Van der Hammen, T.; Wijmstra, T.A. A palynological study on the Tertiary and Upper Cretaceous of British Guiana. Leidse Geol. Mededel. 1964, 30, 183-241.
- Westerhold, Thomas, Marwan, Norbert, Drury, Anna Joy, Liebrand, Diederik, Agnini, Claudia, Anagnostou, Eleni, Barnet, James S. K., Bohaty, Steven M., De Vleeschouwer, David, Florindo, Fabio, and et al. 2020. An astronomically dated record of Earth’s climate and its predictability over the last 66 million years. Science 369: 1383–1387. [CrossRef]
- Wiens, John J., Ackerly, David D., Allen, Andrew P., Anacker, Brian L., Buckley, Lauren B., Cornell, Howard V., Damschen, Ellen I., Davies, T. Jonathan, Grytnes, John-Arvid, Harrison, Susan P., and et al. 2010. Niche conservatism as an emerging principle in ecology and conservation biology. Ecology Letters 13: 1310–1324. [CrossRef]
- Wiens, John J., and Catherine H. Graham. 2005. Niche Conservatism: Integrating Evolution, Ecology, and Conservation Biology. Annual Review of Ecology, Evolution, and Systematics 36: 519–539. [CrossRef]
- Wijmstra, T. A.. 1968. THE IDENTITY OF PSILATRICOLPORITES AND PELLICIERA. Acta Botanica Neerlandica 17: 114–116. [CrossRef]
- Worthington, T.A.; zu Ermgassen, P.S.E.; Friess, D.A.; Krauss, K.W.; Lovelock, C.E.; Throley, J.; Tingey, R.; Woodroffe, C.E.; Bunting, P.; Cormier, N.; et al. A global biophysical typology of mangroves and its relevance for ecosystem structure and deforestation. Sci. Rep. 2020, 10, 15652. [CrossRef]
Figure 1.
Worldwide distribution of mangroves (green fringes), with the Caribbean region highlighted by a red box. The barrier between the AEP and IWP biogeographical regions is represented as a gray band. AEP, Atlantic-East Pacific region; IWP, Indo-West Pacific region. Base map from Spalding et al. (2010).
Figure 1.
Worldwide distribution of mangroves (green fringes), with the Caribbean region highlighted by a red box. The barrier between the AEP and IWP biogeographical regions is represented as a gray band. AEP, Atlantic-East Pacific region; IWP, Indo-West Pacific region. Base map from Spalding et al. (2010).
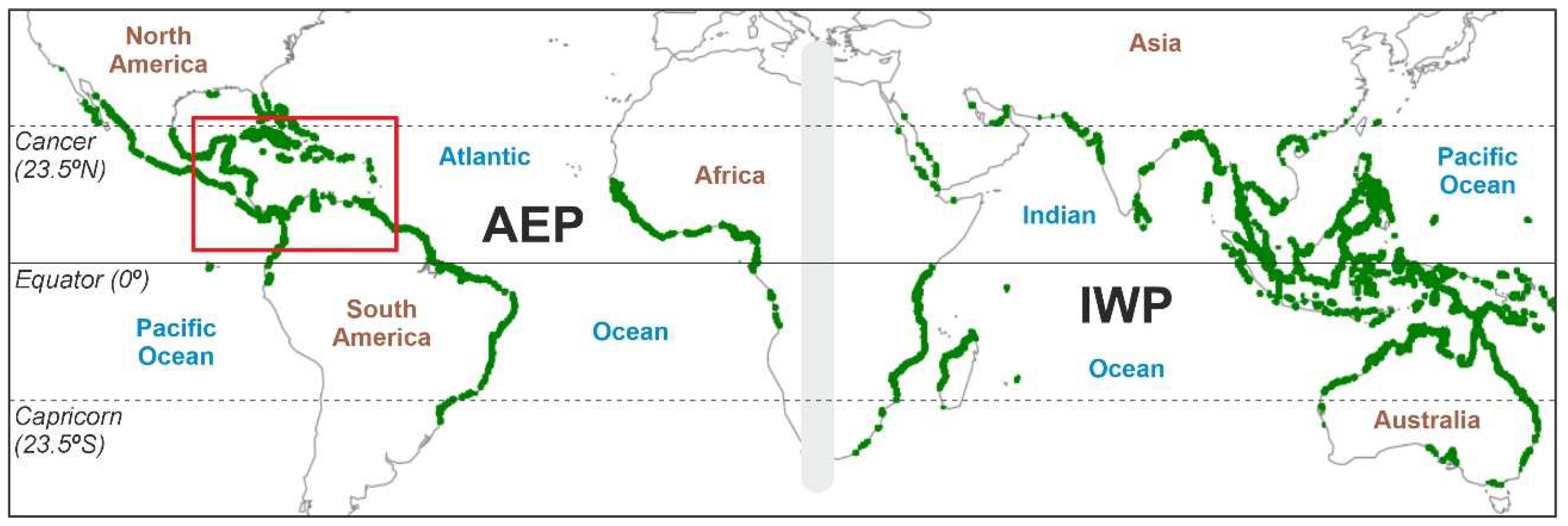
Figure 2.
The Caribbean region, as considered in this paper, and its main physiographical features. Base map from Google Earth.
Figure 2.
The Caribbean region, as considered in this paper, and its main physiographical features. Base map from Google Earth.
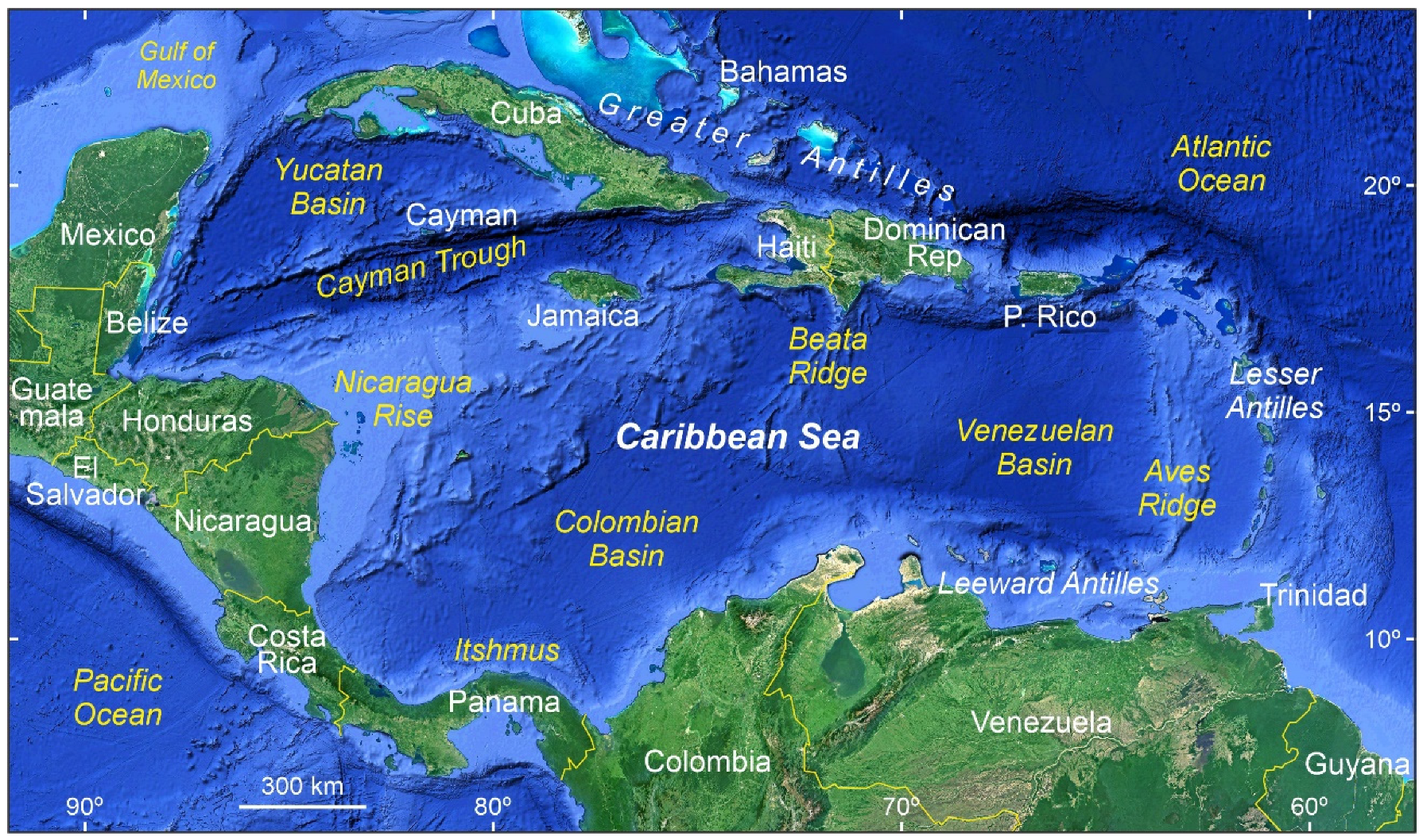
Figure 3.
Summary of the main evolutionary trends of Caribbean mangroves, from their Eocene origin to their Neogene diversification, as analyzed and discussed by Rull (2022a, b; 2023a, b, c, d). Paleogeographic reconstruction according to Iturralde-Vinent (2006) and paleoclimatic/paleoesutatic curves according to Westerhold et al. (2020) and Miller et al. (2020), respectively. Chronology: Quat, Quaternary; Pli, Pliocene; E, Early, M. Middle; L, Late. Paleogeography: PI, Panama Isthmus. Paleoclimates: EECO, Early Eocene Climatic Optimum; MECO, Middle Eocene Climatic Optimum; EOT, Eocene–Oligocene Transition; OMT, Oligocene/Miocene Transition; MCO, Miocene Climatic Optimum; Iceh, Icehouse; NQ, Neogene-Quaternary. Polar Ice Caps (IC): NH, Northern Hemisphere. Richness: NQ, Neogene-Quaternary.
Figure 3.
Summary of the main evolutionary trends of Caribbean mangroves, from their Eocene origin to their Neogene diversification, as analyzed and discussed by Rull (2022a, b; 2023a, b, c, d). Paleogeographic reconstruction according to Iturralde-Vinent (2006) and paleoclimatic/paleoesutatic curves according to Westerhold et al. (2020) and Miller et al. (2020), respectively. Chronology: Quat, Quaternary; Pli, Pliocene; E, Early, M. Middle; L, Late. Paleogeography: PI, Panama Isthmus. Paleoclimates: EECO, Early Eocene Climatic Optimum; MECO, Middle Eocene Climatic Optimum; EOT, Eocene–Oligocene Transition; OMT, Oligocene/Miocene Transition; MCO, Miocene Climatic Optimum; Iceh, Icehouse; NQ, Neogene-Quaternary. Polar Ice Caps (IC): NH, Northern Hemisphere. Richness: NQ, Neogene-Quaternary.
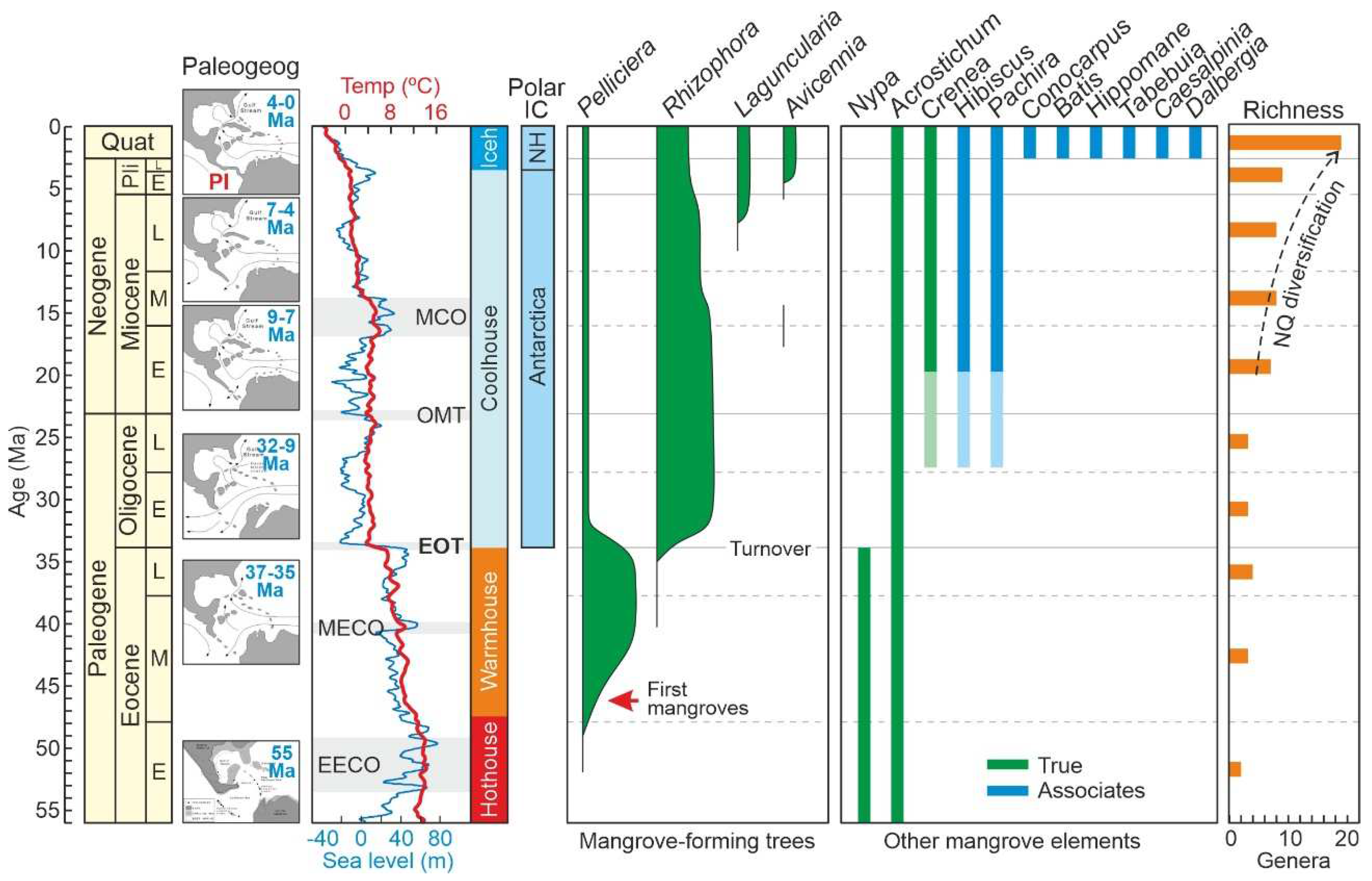
Figure 4.
NASA Landsat 5-TM image of the Caribbean mangrove areas (green patches) using the data of Bunting et al. (2022). Country/island abbreviations as in Table 1. Base map downloaded from https://earthobservatory.nasa.gov/images/47427/mapping-mangroves-by-satellite.
Figure 4.
NASA Landsat 5-TM image of the Caribbean mangrove areas (green patches) using the data of Bunting et al. (2022). Country/island abbreviations as in Table 1. Base map downloaded from https://earthobservatory.nasa.gov/images/47427/mapping-mangroves-by-satellite.
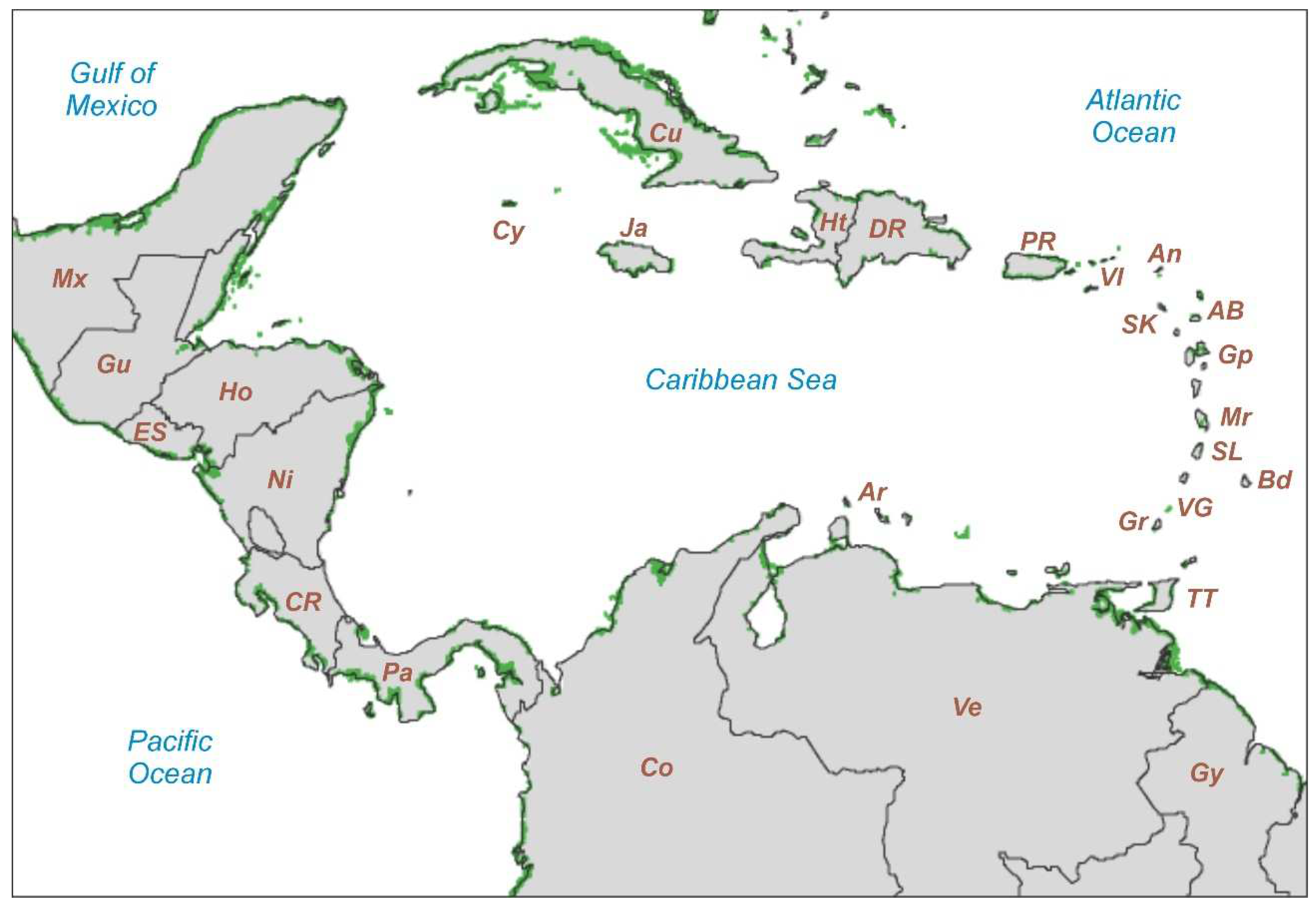
Figure 5.
The main mangrove-forming tree species from the Caribbean region: (A) Rhizophora mangle (red mangrove); (B) Avicennia germinans (black mangrove); (C) Laguncularia racemosa (white mangrove); and (D) Pelliciera rhizophorae (tea mangrove). Modified from Rull (2022b).
Figure 5.
The main mangrove-forming tree species from the Caribbean region: (A) Rhizophora mangle (red mangrove); (B) Avicennia germinans (black mangrove); (C) Laguncularia racemosa (white mangrove); and (D) Pelliciera rhizophorae (tea mangrove). Modified from Rull (2022b).

Figure 6.
Pollen/spores from the main extant Caribbean mangrove species with fossil representatives (Table 3). (A,B), Acrostichum aureum; (C,D), Nypa fruticans; (E–G), Rhizophora mangle; (H,I), Conocarpus erectus; (J,K), Laguncularia racemosa; (L,M), Avicennia germinans; (N–P), Pelliciera rhizophorae; (Q), Hibiscus tiliaceous; (R,S), Crenea patentinervis; (T), Pachira aquatica. The palm Nypa, now restricted to the IWP region (Figure 1), is included because it was part of Caribbean mangroves until the Eocene (Graham 1995; Gee 2001). Vertical bars are measurement scales in μm.
Figure 6.
Pollen/spores from the main extant Caribbean mangrove species with fossil representatives (Table 3). (A,B), Acrostichum aureum; (C,D), Nypa fruticans; (E–G), Rhizophora mangle; (H,I), Conocarpus erectus; (J,K), Laguncularia racemosa; (L,M), Avicennia germinans; (N–P), Pelliciera rhizophorae; (Q), Hibiscus tiliaceous; (R,S), Crenea patentinervis; (T), Pachira aquatica. The palm Nypa, now restricted to the IWP region (Figure 1), is included because it was part of Caribbean mangroves until the Eocene (Graham 1995; Gee 2001). Vertical bars are measurement scales in μm.
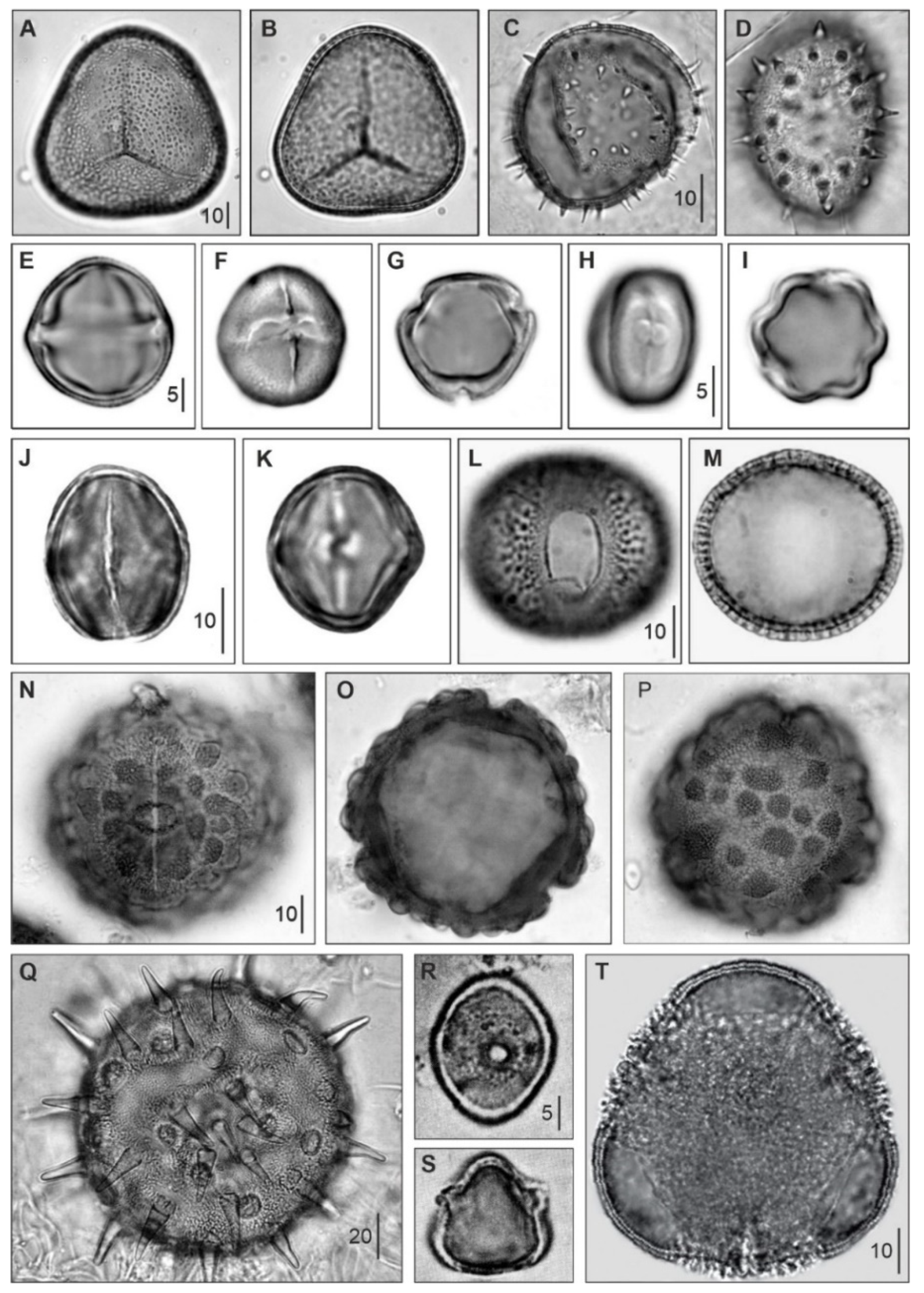
Figure 7.
Localities with pollen records included in the CARMA compilation. Green areas represent the present extent of Caribbean mangroves (Spalding et al. 2010). Red dots mark the sites included in the CARMA-F section reviewed in this paper. Yellow dots (Quaternary records) and blue boxes (modern sediments) correspond to the CARMA-Q section, whose update is in progress. CARMA-F localities compiled after Lamy (1986), Pocknall & Erlich (2020), Pocknall et al. (2001), Graham (1975; 1976; 1985; 1987; 1988a, b; 1989; 1990a, b; 1991; 1998; 1999), Graham & Jarzen (1969), Graham & Dilcher (1998), Graham et al. (2000), Lorente (1986), Bermúdez et al. (2017), Hambalek et al. (1994), Dueñas (1980), Dueñas & Van der Hammen (2007), Rull (1992; 1997a, b; 1998a, b; 1999; 2000; 2001; 2003), Rull & Poumot (1997), Jaramillo & Dilcher (2001), Jaramillo et al. (2007; 2017), Germeraad et al. (1968), Helenes & Cabrera (2002), Van der Hammen & Wijmstra (1964), Ochoa et al. (2012), Colmenares (1988), Colmenares & Terán (1993), Celis et al. (2023), De la Parra et al. (2021), González Guzmán (1967), Rodríguez-Forero et al. (2012), Santos (2012), Montaño et al. (2016), Garzón et al. (2012). See the Supplementary Material for more details.
Figure 7.
Localities with pollen records included in the CARMA compilation. Green areas represent the present extent of Caribbean mangroves (Spalding et al. 2010). Red dots mark the sites included in the CARMA-F section reviewed in this paper. Yellow dots (Quaternary records) and blue boxes (modern sediments) correspond to the CARMA-Q section, whose update is in progress. CARMA-F localities compiled after Lamy (1986), Pocknall & Erlich (2020), Pocknall et al. (2001), Graham (1975; 1976; 1985; 1987; 1988a, b; 1989; 1990a, b; 1991; 1998; 1999), Graham & Jarzen (1969), Graham & Dilcher (1998), Graham et al. (2000), Lorente (1986), Bermúdez et al. (2017), Hambalek et al. (1994), Dueñas (1980), Dueñas & Van der Hammen (2007), Rull (1992; 1997a, b; 1998a, b; 1999; 2000; 2001; 2003), Rull & Poumot (1997), Jaramillo & Dilcher (2001), Jaramillo et al. (2007; 2017), Germeraad et al. (1968), Helenes & Cabrera (2002), Van der Hammen & Wijmstra (1964), Ochoa et al. (2012), Colmenares (1988), Colmenares & Terán (1993), Celis et al. (2023), De la Parra et al. (2021), González Guzmán (1967), Rodríguez-Forero et al. (2012), Santos (2012), Montaño et al. (2016), Garzón et al. (2012). See the Supplementary Material for more details.
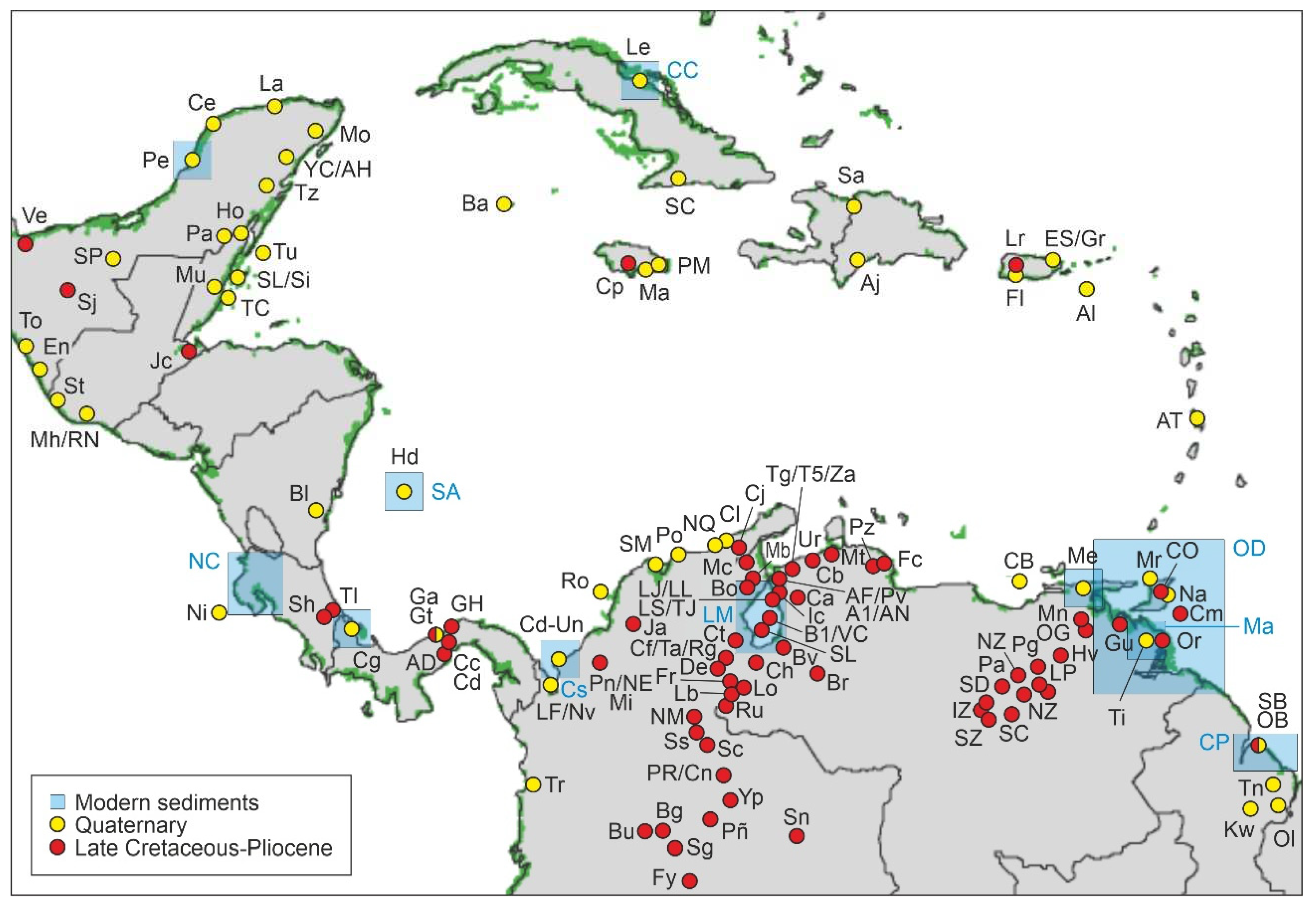
Table 1.
Mangrove cover by country/island in the Caribbean region. Raw data from Bunting et al. (2022), rounded to integers.
Table 1.
Mangrove cover by country/island in the Caribbean region. Raw data from Bunting et al. (2022), rounded to integers.
| Country/island | Map | Mangroves (km2) |
|---|---|---|
| Cuba | Cu | 3597 |
| Venezuela | Ve | 2847 |
| Colombia | Co | 2808 |
| Panama | Pa | 1536 |
| Nicaragua | Ni | 747 |
| Honduras | Ho | 606 |
| Belize | Bz | 529 |
| El Salvador | ES | 373 |
| Costa Rica | CR | 371 |
| Guyana | Gy | 289 |
| Guatemala | Gu | 250 |
| Dominican Republic | DR | 192 |
| Haiti | Ht | 154 |
| Jamaica | Ja | 99 |
| Puerto Rico | PR | 83 |
| Trinidad and Tobago | TT | 82 |
| Cayman Islands (UK) | Cy | 45 |
| Guadeloupe (France) | Gp | 34 |
| Martinique (France) | Mr | 19 |
| Antigua and Barbuda | AB | 9 |
| Virgin Islands (UK/USA) | VI | 4 |
| Grenada | Gr | 2 |
| Saint Lucia | SL | 2 |
| Anguilla (UK) | An | <1 |
| Aruba | Ar | <1 |
| Barbados | Bd | <1 |
| Saint Kitts and Nevis | SK | <1 |
| Saint Vincent and The Grenadines | VG | <1 |
| Total | 14677 |
Table 2.
True (major and minor) and associate mangrove plant elements of the Caribbean region. Based on Gentry (1982), Tomlinson (2016) and Duke (2017). Nomenclature according to the International Plant Names Index (IPNI) (https://www.ipni.org/).
Table 2.
True (major and minor) and associate mangrove plant elements of the Caribbean region. Based on Gentry (1982), Tomlinson (2016) and Duke (2017). Nomenclature according to the International Plant Names Index (IPNI) (https://www.ipni.org/).
| Type | Species | Family | Plant type | |
|---|---|---|---|---|
| True | Major | Avicennia bicolor Standl.* | Acanthaceae | Tree |
| Avicennia germinans (L.) Stearn* | Acanthaceae | Tree | ||
| Avicennia schaueriana Stapf & Leechm. ex Moldenke* | Acanthaceae | Tree | ||
| Laguncularia racemosa C.F.Gaertn.* | Combretaceae | Tree | ||
| Rhizophora mangle L.* | Rhizophoraceae | Tree | ||
| Rhizophora racemosa (G.Mey.) Engl.* | Rhizophoraceae | Tree | ||
| Minor | Acrostichum aureum L. | Pteridaceae | Fern | |
| Acrostichum daneaeifolium Langsd. & Fisch.* | Pteridaceae | Fern | ||
| Pelliciera benthamii (Planch. & Triana) N.C.Duke | Tetrameristaceae | Tree | ||
| Pelliciera rhizophorae Planch. & Triana* | Tetrameristaceae | Tree | ||
| Associate | Amphitecna latifolia (Mill.) A.H.Gentry | Bignoniaceae | Tree | |
| Anemopaegma chrysoleucum (Kunth) Sandwith | Bignoniaceae | Vine | ||
| Batis maritima L. | Batidaceae | Shrub | ||
| Caesalpinia bonduc (L.) Roxb. | Fabaceae | Tree | ||
| Conocarpus erectus L.* | Combretaceae | Tree | ||
| Crenea patentinervis (Koehne) Standl.* | Lythraceae | Herb | ||
| Dalbergia ecastaphyllum Taub. | Fabaceae | Tree/Shrub | ||
| Dalbergia amerimnum Benth. | Fabaceae | Tree/Shrub | ||
| Hibiscus tiliaceus L. | Malvaceae | Tree | ||
| Hippomane mancinella L. | Euphorbiaceae | Tree | ||
| Mora oleifera Duke* | Fabaceae | Tree | ||
| Muellera moniliformis L.f.* | Fabaceae | Tree | ||
| Pachira aquatica Aubl. | Bombacaceae | Tree | ||
| Pavonia rhizophorae Killip* | Malvaceae | Shrub | ||
| Pavonia spicata Cav. | Malvaceae | Shrub | ||
| Phryganocydia phellosperma (Hemsl.) Sandwith | Bignoniaceae | Vine | ||
| Pluchea odorata (L.) Cass. | Asteraceae | Herb | ||
| Rhabdadenia biflora Müll.Arg. | Apocynaceae | Vine | ||
| Rustia occidentalis (Benth.) Hemsl. | Rubiaceae | Tree/Shrub | ||
| Scaevola plumieri (L.) Vahl | Goodeniaceae | Shrub | ||
| Tabebuia palustris Hemsl.* | Bignoniaceae | Tree | ||
| Thespesia populnea (L.) Sol. ex Corrêa | Malvaceae | Tree | ||
| Thespesia populneoides (Roxb.) Kostel. | Malvaceae | Tree | ||
| Tuberostylis axilaris S.F.Blake | Asteraceae | Shrub | ||
| Tuberostylis rhizophorae Steetz | Asteraceae | Epiphyte | ||
* Species used by Duke (2017) to characterize the Atlantic-East Pacific (AEP) mangroves.
Table 3.
Paleogene and Neogene fossil pollen representatives of extant mangrove genera from the Caribbean region. Based on Germeraad et al. (1968), Wijmstra (1968), Graham (1977, 1995, 2013), Muller (1981), Frederiksen (1985), Lorente (1986) and Pocknall et al. (2022).
Table 3.
Paleogene and Neogene fossil pollen representatives of extant mangrove genera from the Caribbean region. Based on Germeraad et al. (1968), Wijmstra (1968), Graham (1977, 1995, 2013), Muller (1981), Frederiksen (1985), Lorente (1986) and Pocknall et al. (2022).
| Genus | Fossil representative (morphospecies) | Range |
|---|---|---|
| Acacia* | Polyadopollenites mariae Dueñas | Paleogene-Neogene |
| Acrostichum | Deltoidospora adriennis (Potonié & Gelletich) Frederiksen | Cretaceous-Neogene |
| Avicennia |
Avicennia Retitricolporites sp. Lorente |
Neogene |
| Crenea | Verrutricoporites rotundiporus Van der Hammen & Wijsmtra | Neogene |
| Hibiscus | Echiperiporites estelae Germeraad, Hopping & Muller | Neogene |
| Laguncularia | Laguncularia | Neogene |
| Nypa |
Spinizocolpites echinatus Muller, S. baculatus Muller S. prominatus (McIntyre) Stover & Evans |
Cretaceous-Paleogene |
| Pachira | Bombacacidites baculatus Muller, Di Giacomo & Van Erve | Neogene |
| Pelliciera |
Psilatricolporites crassus Van der Hammen & Wijsmtra Lanagiopollis crassa (Van der Hammen & Wijmstra) Frederiksen |
Paleogene-Neogene |
| Rhizophora |
Zonocostites ramonae Germeraad, Hopping & Muller Zonocostites spp. |
Paleogene-Neogene |
* Not included in Table 2 but considered to be a past mangrove associate by some authors (Graham, 1995).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated





