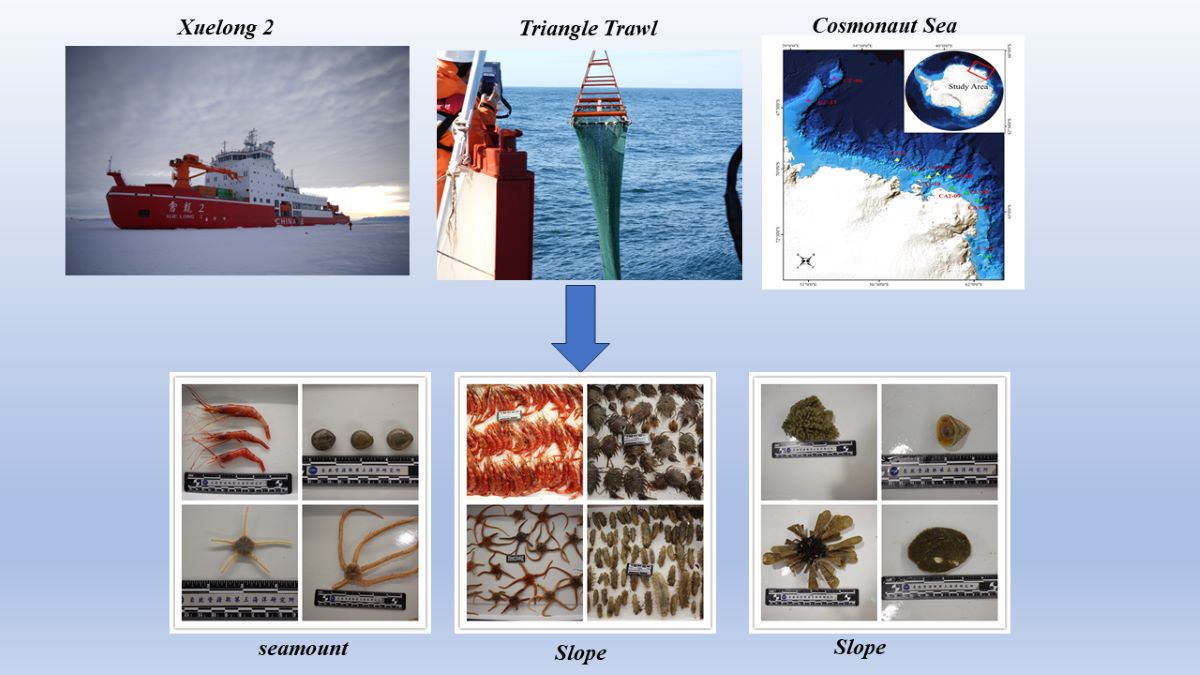
1. Introduction
The Cosmonaut Sea is located between 30 and 60° E in the Indian Ocean sector of the Southern Ocean [
1,
2], which is west of Enderby Land in East Antarctica and borders the Cooperation Sea to the east and the Lisser-Larsen Sea to the west [
3]. It is an important fishery and component of the Southern Ocean ecosystem[
4].
The Cosmonaut Sea is a less explored region of Antarctic waters. The first detailed surveys were carried out during the austral summer of 1972-1973 and the winter of 1973 [
1,
5]. The second large-scale surveys were conducted during the summer of 1984 [
6]. In 2006, a systematic multidisciplinary survey (BROKE-WEST) was carried out to perform a broad-scale stock assessment of marine living organisms in the western zone of the Indian Sector of the Southern Ocean[
7,
8,
9]. The results of these three surveys described the characteristics of geostrophic currents, general circulation and marine chemistry. Protistan community composition, distribution and biomass of phytoplankton, spatial distribution and abundance of larvaceans, mesozooplankton community structure and krill composition and distribution were described in a series of publications. To date, the biodiversity and distribution of macrobenthos in the Cosmonaut Sea are still unstudied.
The composition of communities in Antarctica was dominated by suspension feeders and associated fauna[
10]. To date, we know that macrobenthic communities on the Weddell Sea Shelf have high spatial heterogeneity in biodiversity, species composition and biomass at all spatial scales ranging from meters to hundreds of kilometers[
11]. The benthic communities in the Amundsen Sea were dominated by echinoderms[
12]. The soft-bottom communities in the Ross Sea are dominated by polychaetes and bivalves [
13]. However, there are fewer reports about the benthic communities in the Cosmonaut Sea.
Shallow marine benthic communities around Antarctica show high levels of endemism, gigantism, slow growth, longevity and late maturity, as well as adaptive radiations that have generated considerable biodiversity in some taxa[
14,
15]. The characteristics of macrobenthos render them less vulnerable to interannual variability and fluctuations in productivity[
16,
17]. Antarctic benthic ecosystems can be considered good sentinels for monitoring the effects of climate change[
18]. Therefore, investigating Antarctic macrobenthos can can offer valuable insights into their biodiversity and biodistribution patterns in response to environmental changes.
Systematic macrobenthos collection was carried out in the Cosmonaut Sea from the 38th Chinese National Antarctic Research Expedition (CHINARE). This survey was conducted at the shelf, slope and seamount of the Cosmonaut Sea. This is the first study to describe the species composition and community structure of macrobenthos in different geographical regions and water depths within the Cosmonaut Sea. The results establish a baseline for future research on the macrobenthos community of the region. This study aims to supplement existing research on macrobenthos in the Southern Ocean.
2. Materials and Methods
2.1. Study area
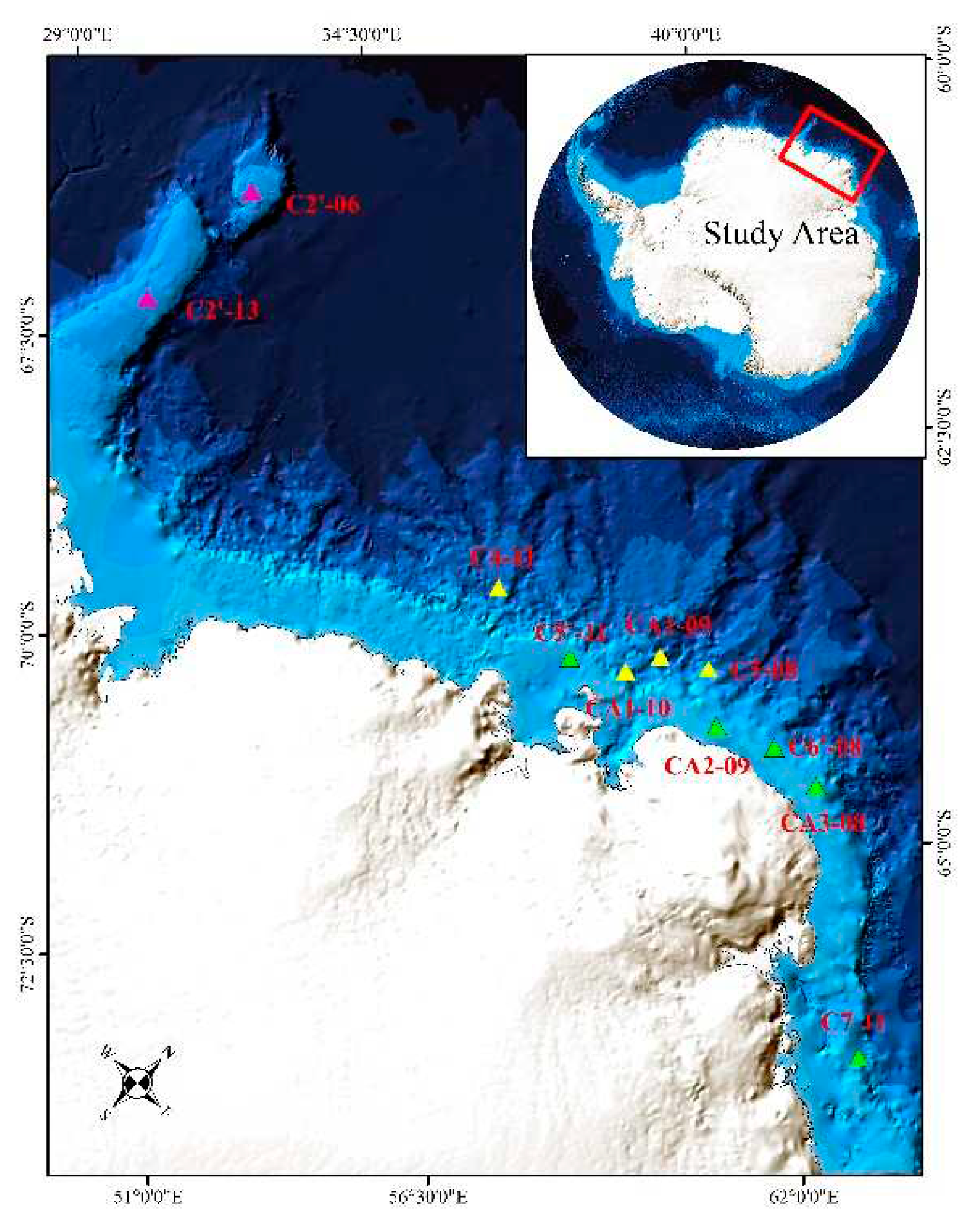
The study area was the shelf, slope and seamount of the Cosmonaut Sea (
Figure 1). The survey was conducted at 11 sites at water depths ranging between 207 m and 1999 m from January 28, 2021, to March 8, 2022 (
Table 1). This is a less explored region in the Southern Ocean. The study area was divided into three parts: 1) Kainan Maru Seamount, including stations C2'-06 and C2'-13. 2) Slope regions (>1000 m), including stations C4-11, CA1-09, CA1-10 and C5-08. 3) Shelf regions (approximately 500 m), including stations C5'-11, C6'-08, CA3-08 and C7-11.
2.2. Sample Processing
A triangle trawl was used to sample macrobenthos during the 38
th Chinese National Antarctic Research Expedition (CHINARE) aboard the R/V
Xuelong2. The width of the triangle trawl was 2.2 m, with a net mesh size of 20 mm. It could capture specimens larger in length, which includes the larger macro- and megafauna, and still sample some smaller animals as well. Once on the seafloor, the net would be trawled at approximately 2 knots with approximately 2-3 times the cable length to the water depth for 15 minutes. The trawling area, which was 2037 m
2, was evaluated to compensate for the qualitative nature of the triangle trawl data. The collected samples were sieved by a 0.5 mm mesh on board and then fixed in 75% ethanol. The numbers of individuals and masses were standardized to 1000 m
2 trawled areas. The macrobenthic number and wet weight (g) were calculated per m
2, and the species number, density (ind.
/1000 m
2), and biomass (g/m²) were determined. For colonial animals, such as sponges, bryozoans and cnidarians, counts were calculated based on one individual[
17,
19].
The water depth (m), temperature (℃), salinity (psu) and dissolved oxygen (DO: mg/L) of the bottom layer were measured using conductivity-temperature-depth (CTD) meter (SBE911).
The grain size of the sediments was determined by a laser particle size analyzer (Mastersizer 2000, Malvern Instruments, Ltd., UK). Approximately 0.5 g of fresh sediment was pretreated using 10 mL of 30% H2O2 to remove the organic matter and then decalcified with 5 ml of diluted hydrochloric acid. The measurement error of the instrument was within 3%. The grain-size fractions were <4 μm for clay, 4-63 μm for silt, and >63 μm for sand.
The sediment was decalcified with 1.2 mol/L HCl, washed 6 times with deionized water and dried at 60°C for analysis. Then, 25-30 mg carbonate-free samples were wrapped in a tin cup and analyzed for TOC.
2.3. Data Processing
The Shannon–Wiener diversity index (
H'log2), Margalef species richness index and Pielou evenness index were calculated for each station using species abundance. The species abundance was summed and converted to abundance values (1000 m²). For multivariate analysis, the species abundance per site was transformed to the fourth root to scale down the scores of the highly abundant species. A cluster analysis was performed using the Bray‒Curtis similarity through group average linking[
20]. Similarity percentage (SIMPER) analysis was performed to identify species that contributed to the similarity and dissimilarity of the clusters. Analysis of similarity (ANOSIM) was performed to determine differences among communities[
21]. A Principal coordinate analysis (PCO) was applied to the Euclidean distance matrix to visualize the differences in macrobenthic communities. Biota-environment matching (BIO-ENV) was used to determine the environmental factors that affect the spatial distribution of macrobenthos. The environmental parameters through morphologic treatment were carried out by principal component analysis (PCA), understanding the environmental gradient changes. All analyses were conducted using Primer 6.0 statistical software.
3. Results
3.1. Environmental Parameters
The average depth of the shelf stations, including C5'-11, C6'-08, CA2-09, CA3-08 and C7-11, was 434 m. The average depth of the deep-water (>1000 m) stations was 1594 m. The average bottom-water temperature for all stations was -0.40℃, the maximum temperature was 0.17℃ at station C2'-06, and the minimum was -0.76℃ at station C5'-11. The average salinity of the bottom layer was 34.53 psu, the maximum salinity was 34.67 psu at station C2'-06, and the minimum salinity was 34.2 psu at station CA2-09. The average DO content was 7.71 mg/L, the maximum DO content was 9.6 at station CA2-09, and the minimum was 6.9 mg/L at station C5-08 (
Table 1). The average DO content was 6.86 mg/L at deep-water stations and 8.75 mg/L at shelf stations.
The grain size of sediments at stations C2'-06, C2'-11, C6'-08 and CA2-09 indicated sandy sediments, but the grain size of sediment at stations C4-11, C5-08, CA1-09 and CA1-10 indicated clay sediments. Other stations had no sediment data. The average TOC of sediments was 0.48% at stations C4-11, C5-08, CA1-09 and CA1-10 and 0.10% at stations C2'-06, C2'-11, C6'-08 and CA2-09 (
Table 1). The TOC of sediment in the area of 40-50°E was higher than that in the other areas in the Cosmonaut Sea.
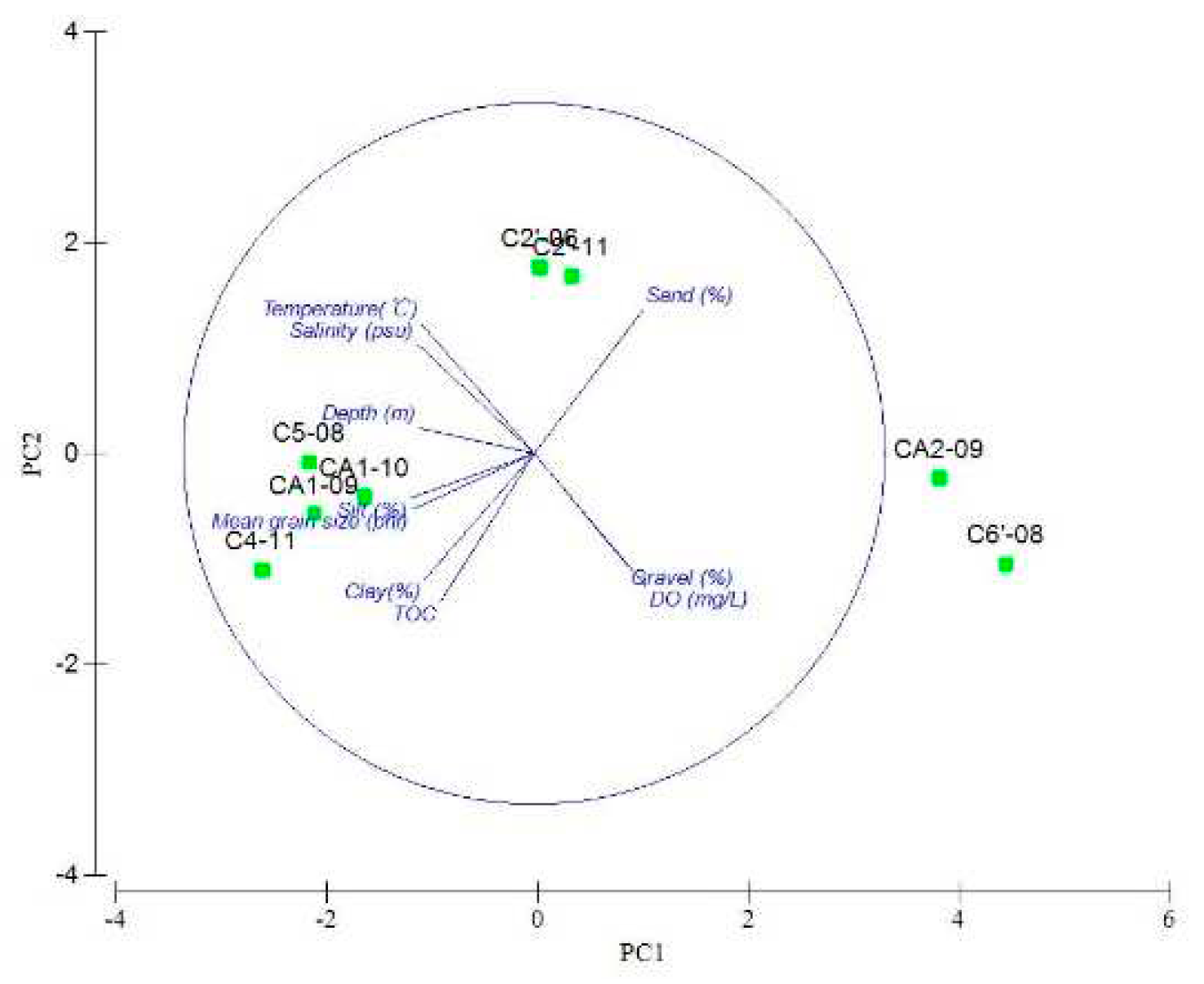
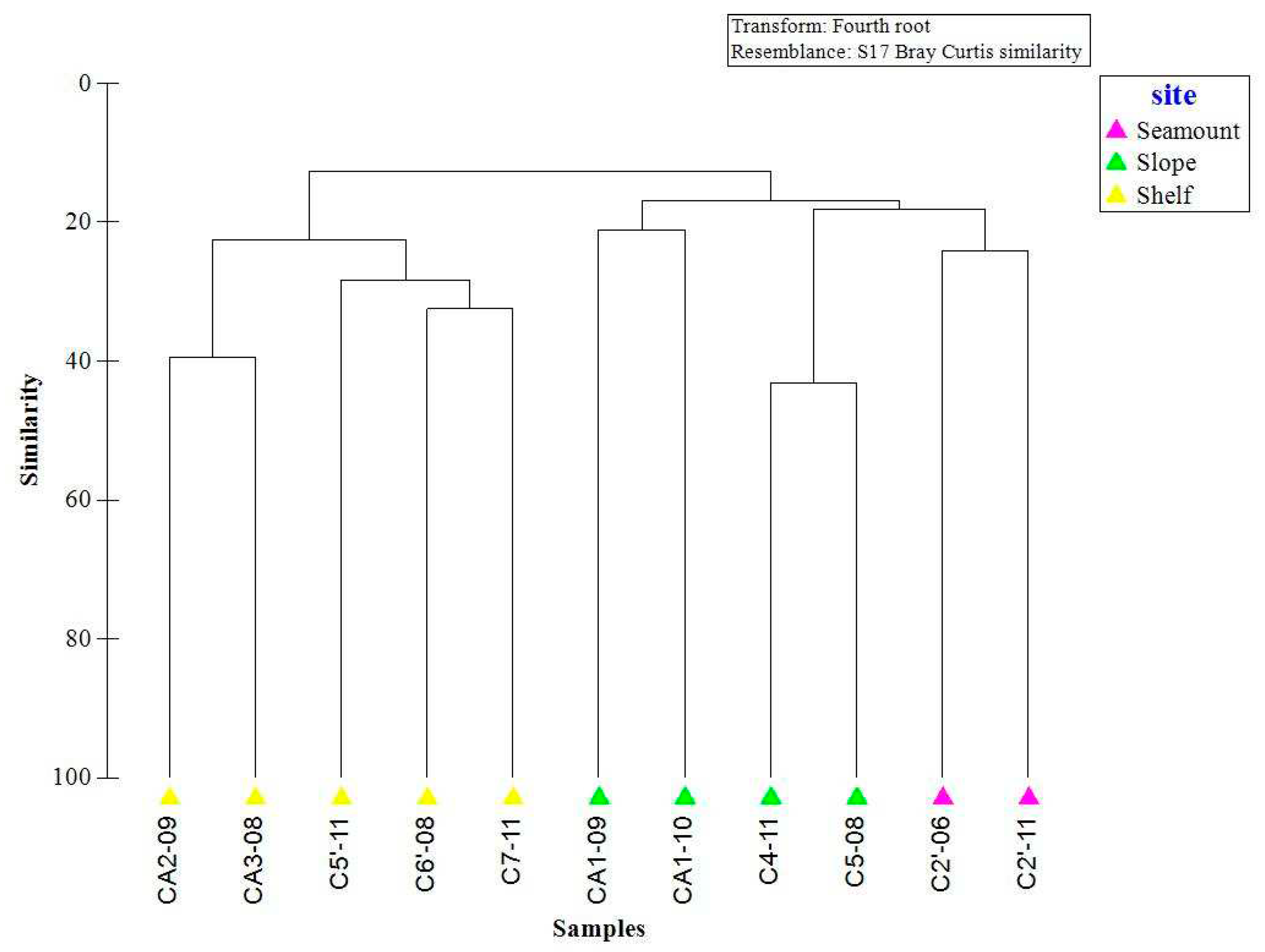
Stations C5'-11, CA3-08 and C7-11 lacked sediment data. The PCA results were from the other stations. PC1 explained 75.9% of the environmental variability. PC2 and PC1 accumulation can explain 88.6% of environmental variability. PC1 exhibited significant discrimination in relation to depth and clay. The average depth was 1705 m at the slope stations and 347 m at the shelf stations. The average clay content of the sediments was 20.07% at the slope stations and 2.58% at the shelf stations. PC2 exhibited significant discrimination in relation to TOC and DO. The average TOC of sediment and DO at seamount stations was the smallest among the three areas. The results of the station clustering analysis are shown in
Figure 2.
3.2. Macrobenthos
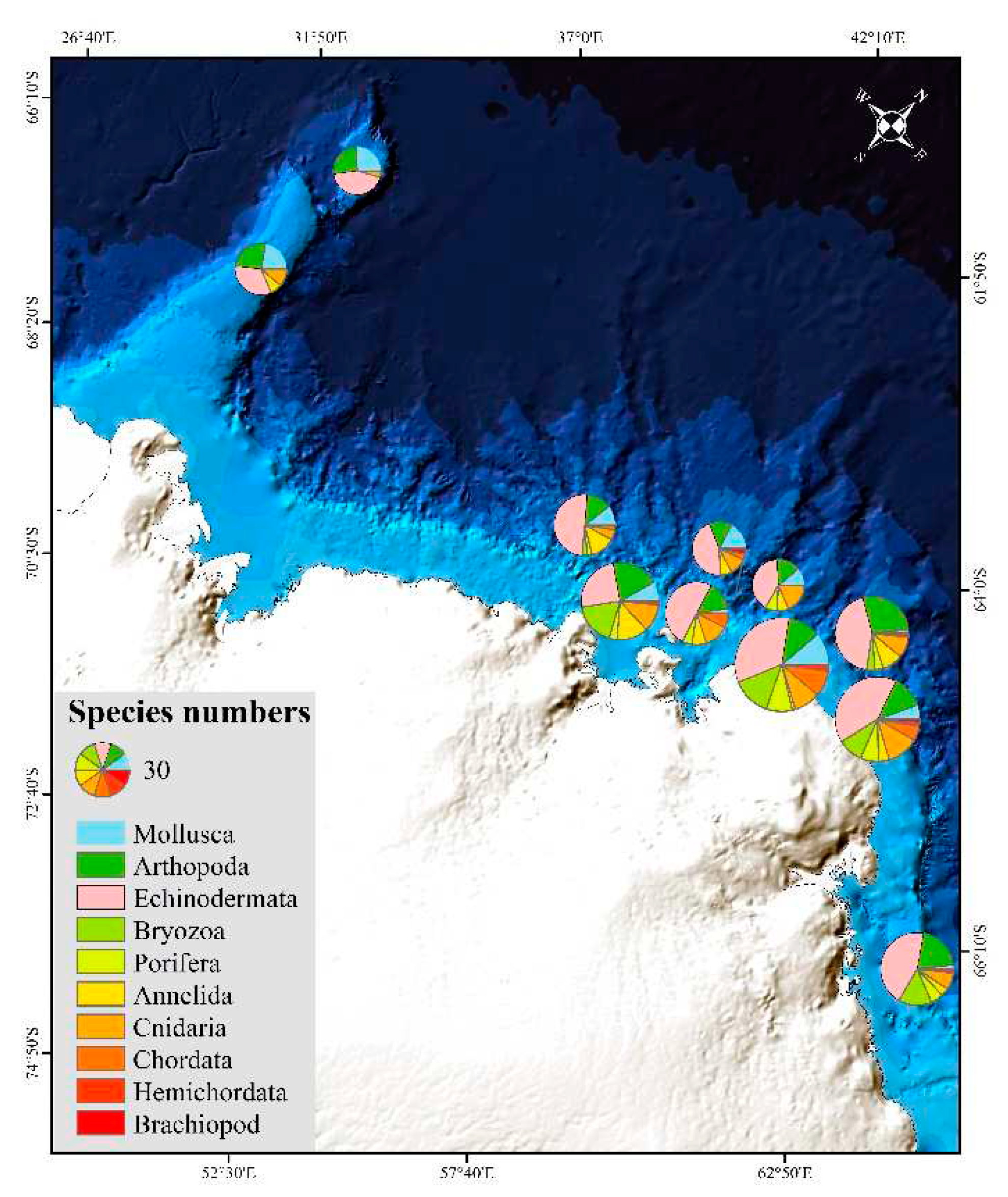
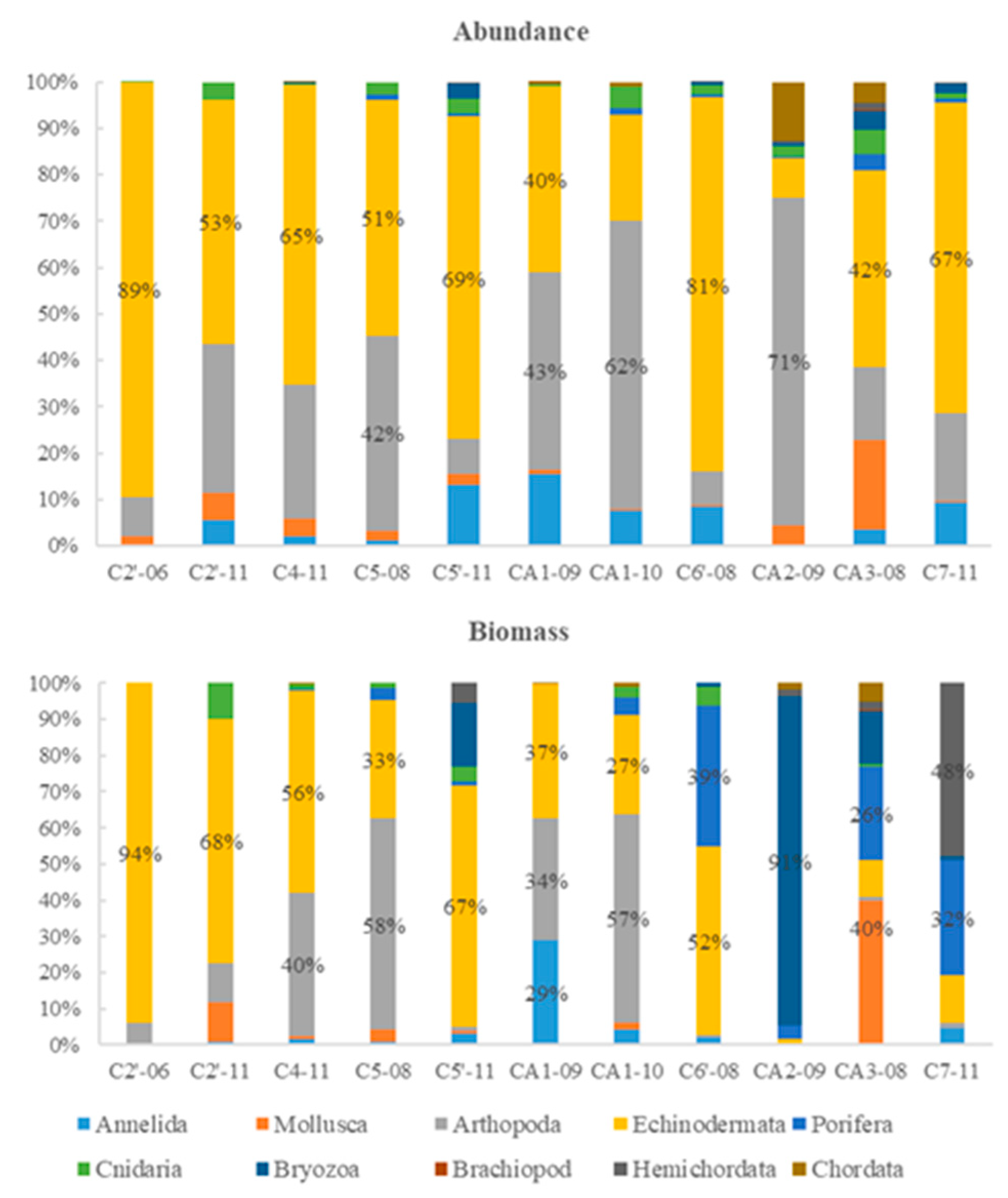
A total of 275 macrobenthic species were found in the Cosmonaut Sea. There were 100 echinoderms (36%), 48 arthropods (18%), 36 mollusks (13%) and 15 annelids (5%). The remaining groups included 15 sponges, 19 bryozoans, 28 cnidarians, 2 brachiopods, 2 hemichordates and 10 chordates. Echinoderms had the highest numbers of species, followed by arthropods. The most common phyla were mollusks, echinoderms, arthropods and cnidarians, occurring at each station, followed by annelids (10 stations). The average number of species at the sites was 47. Taxon richness varied among different stations, with 23-89 per station in the study area (
Figure 3). The highest species numbers were at station CA2-09, followed by station CA3-08 (73). There were 61 species at station C5'-11. The three sites were shelf sites, whose depths were less than 500 m.
The average abundance of macrobenthos was 343 ind./1000 m² overall and was the highest at station CA2-09 (1221 ind./1000 m²), followed by 522 ind./1000 m² at station CA1-10. The average abundance of macrobenthos was higher at shelf stations (400 ind./1000 m²) than at slope stations (300 ind./1000 m²), and the average abundance of macrobenthos was 285 ind./m² at seamount stations. Echinoderms and arthropods predominated (42%), with an average abundance of 144 ind./1000 m2. This group was followed by polychaetes (4%), mollusks (3%) and cnidarians (2%).
The average biomass of macrobenthos was 20.38 g/m
2. The average biomass was 1.43 and 43.13 g/m² at the slope and shelf sites, respectively. The average biomass was 0.59 g/m² at seamount stations. The biomass of major macrobenthic taxa was 84% bryozoans, 5% sponges, 4% echinoderms and 2% arthropods. The biomass of bryozoans was extremely high at station CA2-09, occupying 91% of the total biomass. Echinoderms had the highest relative biomass at stations C2'-06, C2'-11, C4-11, C5'-11, CA1-09 and C6'-08. Arthropods had the highest relative biomass at stations C5-08 and CA1-10. (
Figure 4)
The average species diversity index (
H'log2) was 3.40, the species richness index (
d) was 7.36, and the species evenness index (
J) was 0.62. The averages of the three indices for the shelf stations were 4.05, 10.26 and 0.68, respectively. At slope stations they were 2.82, 5.22 and 0.56, respectively. The average values of the three indices for seamount stations were 2.92, 4.37 and 0.62, respectively. The species diversity index, richness index and evenness index were all higher at shelf stations than in the other two areas. The species diversity index was higher at the seamount stations than at the slope stations (
Figure 5).
3.3. Community structure
The macrobenthic communities of the Cosmonaut Sea were divided into two groups by site type, one consisting of the shallow-water stations and one consisting of the deep-water stations. The SIMPER analysis yielded a dissimilarity between the two groups of 90%. The species that contributed to the division of the community were arthropods (
Nematocarcinus lanceopes and
Ceratoserolis meridionalis), echinoderms (
Ophioplinthus brevirima,
Amphiophiura sp.1
, Amphiophiura sp.,
Ophiacantha sp. and
Thoracaster sp.), polychaetes( Sigalionidae und. and Polynoidae und.), and cnidarians (
Echinisis sp.2) (
Table 2).
ANOSIM based on Bray–Curtis similarity confirmed significant differences between the shelf stations and the deep-water stations (0.0013) based on the factor “depth”. The SIMPER analysis showed an average similarity of 19.76% for the deep-water stations and 26.41% for the shallow-water stations (
Figure 6) and displayed the species most responsible for this similarity (
Table 3).
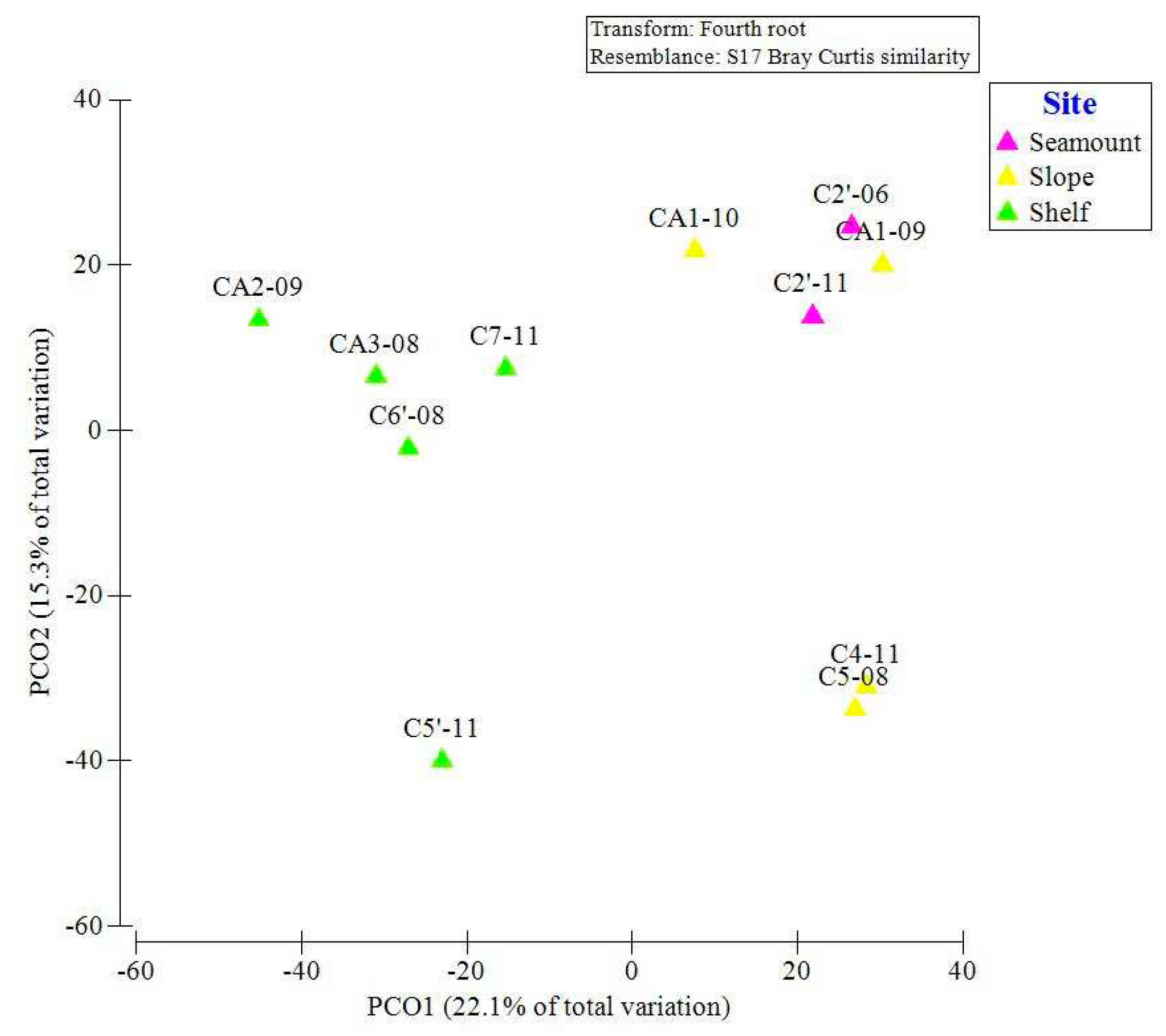
The horizontal axis and vertical axis of the PCO explained 15.3% and 22.1% of the variability in the macrobenthic communities, respectively (
Figure 7). The sampling stations were separated by water depth groups.
3.4. Relationship between the macrobenthic community and environmental factors
The BIO-ENV results showed that the macrobenthos community had the highest correlation with water depth, and the correlation was 0.629. Then, the combination of water depth, DO and clay was 0.615. Third, the correlation of the combination of water depth, DO, salinity and clay was 0.613 (
Table 4). Water depth was a very important environmental factor in macrobenthic communities in the Cosmonaut Sea, and salinity, DO and clay played relatively important roles.
4. Discussion
4.1. Taxon richness
The overall observed species richness (275 species) in the Cosmonaut Sea was similar to that in the Amundsen Sea Embayment and Pine Island (274 species)[
12] and that in Prydz Bay (206 species) [
17] but far lower than the overall species richness in the eastern Weddell Sea (829 species) [
22]. The taxon richness (e.g., phylum, class and order levels) in the Cosmonaut Sea was as high as that of other Antarctic regions. However, the macrobenthos collected in this study are comparatively species rich in echinoderms, arthropods, mollusks, bryozoans and cnidarians in the Cosmonaut Sea. Isopods (21 species), corals (18 species), ophiuroids (26 species), asteroids (28 species) and holothurians (32 species) had higher species diversity.
The number of species per trawl (23-89 species) from the triangle trawl in the Cosmonaut Sea was higher than that reported in the Amundsen Sea (1-55 species) but lower than that in the eastern Weddell Sea (46-306 species) [
23,
24]. A total of 25-112 species were reported from trawls taken at 252-1176 m in the southern Weddell Sea [
25]. The species per trawl numbers in the Cosmonaut Sea were more similar to those observed in the southern Weddell Sea at similar water depths.
Notocrangon antarcticus and
Nematocarcinus lanceopes were common in the Cosmonaut Sea. Basher et al. found that
N. antarcticus was distributed on the continental shelf and upper slope, and
N. lanceopes was distributed on the slopes, seamounts and abyssal plain in the Ross Sea[
26]. The environmental parameters that contributed most to
N. lanceopes were depth, ice concentration, seabed slope and temperature. In this study, more
N. lanceopes was distributed on the slope stations. The results were consistent with previous studies.
N. lanceopes was widespread throughout the Cosmonaut Sea slope and may play an important functional role in the ecosystem.
4.2. Abundance
The abundance patterns in the Cosmonaut Sea were dominated by echinoderms and arthropods. Echinoderms and arthropods dominated in abundance at the Kainan Manu Seamount, and echinoderms, arthropods and polychaetes dominated in abundance at the slope, while Bryozoans, corals, ascidians and sponges stood out on the Cosmonaut Sea Shelf. Griffiths et al. reported that taxon dominance varied with depth, and crustacea dominated at 200 m in abundance[
27]. Holothurians at 500 m and polychaetes were most abundant at 1000 m, and crustaceans and polychaetes dominated at 1500 m. Video surveys of macrobenthos in the George V Shelf revealed dominance of bryozoans, sponges and soft corals from 117 m to 1175 m [
28]. The distribution of macrobenthos on the Cosmonaut Sea Shelf is similar to that on the George V Shelf. Barry et al. noted lower densities with increasing depth and a higher abundance of suspension feeding taxa in shallow waters and deposit-feeders at the deepest sites[
29].
Compared to standardized macrobenthos abundances (2.5-397 ind./1000 m²) from the Amundsen Sea by Agassiz trawls[
12], the Cosmonaut Sea abundances (52-1221 ind./1000 m²) are higher at comparable depths.
4.3. Community structure
Similarity analysis of the Cosmonaut Sea revealed macrobenthic community separation by depth. In this study, the macrobenthic communities were divided into two groups. The deep-water group had a species similarity of 19.76% and was mainly distributed on the slope and Kainan Manu Mountain.
N. lanceopes was the dominant taxon, with a contribution of 30.03%, followed by
Ceratoserolis meridionalis and
Ophiacantha sp. The community type was a mobile deposit feeder community (MIC). The space was monopolized by echinoderms and arthropods. The shelf group had a species similarity of 26.41%. The major taxa were bryozoans, sponges, cnidarians and echinoderms. The community type included sessile suspension feeders and associated fauna dominated by sponges (SSFA-SPO) and sessile suspension feeders and associated fauna dominated by Others than sponges (SSFA-OTH). Sponges, bryozoans, and cold-water corals are vulnerable marine ecosystem (VME) indicator taxa. SSFA-SPO and SSFA-OTH are vulnerable communities. Anthropogenic climate change is causing the retreat of glaciers[
30], and the recovery of communities is predicted to be extremely slow because growth is slow in the cold waters of the Southern Ocean. Therefore, the protection of vulnerable communities as refugia becomes more urgent if their biodiversity is to survive the accelerated rate of glacial carving [
31].
4.4. Species distribution and environmental factors
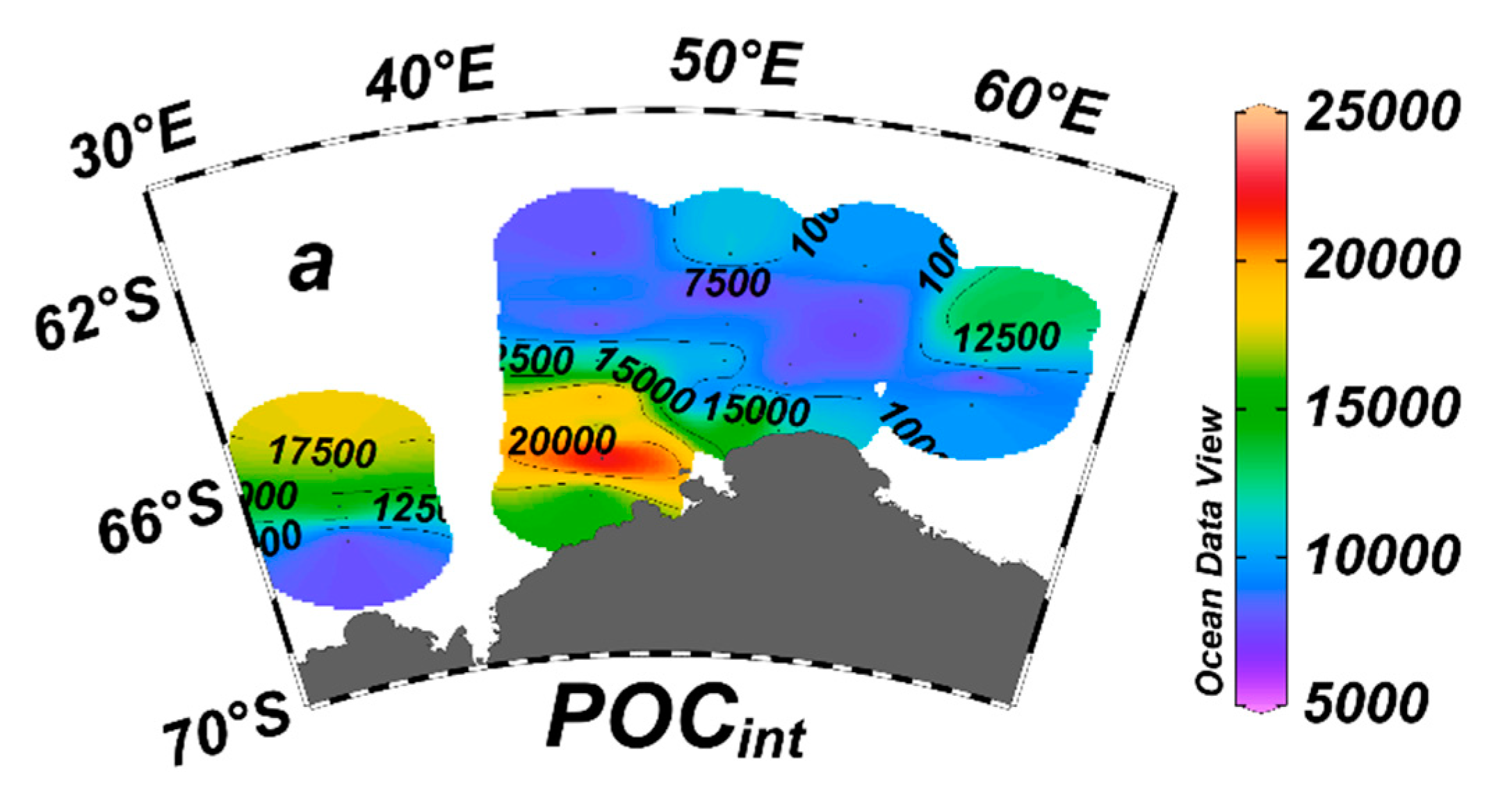
In this study, depth was the major driving force influencing the distribution of macrobenthos. The distribution of macrobenthos at seamount stations is not clearly separated from that at slope stations but is separated from that at shelf stations. In addition, the slope in the southwest of the Cosmonaut Sea is an important area. Li et al., 2023 pointed out that the upwelling of warm deep water (WDW) in the Weddell Gyre (WG) and the influx of nutrients from the Antarctic Slope Current (ASC) together lead to algal blooms, resulting in significantly higher POC concentrations in this area. It exhibits a characteristic pattern with higher concentrations to the southwest of the Cosmonaut Sea and lower values on the northeast side of the Cosmonaut Sea (
Figure 8).
The TOC concentrations in this study were higher at slope stations, and they are food sources for polychaetes, shrimps, ophiurids and holothrians.
Dissostichus mawsoni was observed by photographic survey for the first time in the Cosmonaut Sea (
Figure 9)[
33]. Antarctic toothfish is an important fishery resource in the Southern Ocean and plays an important role in Antarctic ecosystems (Barnes and Clark, 2011). West of Endby Land (40°-50°E) may be an important area in the Cosmonaut Sea. In future studies, the interspecies relationships will be analyzed to help us understand the ecological processes in the slope of the Cosmonaut Sea.
5. Conclusions
In this study, we found 275 macrobenthic species in the Cosmonaut Sea. Echinoderms were the dominant taxon, accounting for 36% of all species, followed by arthropods (18%). The number of species and the species diversity index were highest at the shelf stations. . The macrobenthos community structure differed between the shelf stations and the slope/seamount stations. Water depth was the primary factor influencing community composition. The studies have revealed the distribution of macrobenthic organisms at various water depths and distinct geographic regions. The slope stations area is a unique region where POC concentration was significantly higher. Additionally, the abundance of Nematocarcinus lanceopes was very high, while Dissostichus mawsoni was observed in this area. This study has some limitations, such as the limited number of stations, which do not fully reflect the distribution of macrobenthos in the Cosmonaut Sea. Future studies can further expand the sampling range and increase the number of stations to more comprehensively reveal the distribution characteristics of macrobenthos. Additionally, additional research into the interactions and relationships between different species will aid in a better understanding of the complexity and stability of marine ecosystems.
Author Contributions
Jianfeng Mou: Investigation, Data curation, Writing-original draft preparation, Validation. Kun Liu: Conceptualization, Software, Writing-reviewing and editing. Yaqin Huang: Data curation, Software. Junhui Lin: Data curation, Writing-reviewing and editing.Xuebao He: Data curation, Writing-reviewing and editing. Shuyi Zhang: Data curation. Dong Li: Data curation, Writing-reviewing and editing. Yongcan Zu: Investigation, Writing reviewing and editing. Zhihua Chen: Investigation. Sujing Fu: Investigation, Supervision. Heshan Lin: Supervision, Conceptualization. Wenhua Liu: Supervision, Conceptualization, Resources.
Funding
This work was supported by The National Natural Science Foundation of China under contract (No. 42076237, No. 42106227, No. 42206252), Impact and Response of Antarctic Seas to Climate Change (IRASCC2020-2022-NO.01-02-02B & 02-03) and the National Key Research and Development Program of China (2019YFA0607003) .
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hunt, B.P.V.; Pakhomov EA, Trotsenko, B.G. The macrozooplankton of the Cosmonaut Sea, east Antarctica (30° E–60° E), 1987–1990. Deep Sea Research Part I. 2007, 54, 1042–1069. [CrossRef]
- Ran, Q.; Duan, M.; Wang, P.; Ye, Z.; et al. Predicting the current habitat suitability and future habitat changes of Antarctic jonasfish Notolepis coatsorum in the Southern Ocean. Deep-Sea Research Part II. 2022, 199, 105077. [Google Scholar] [CrossRef]
- Huang, W.H.; Yang, X.F.; Zhao, J.; et al. Dissolved nutrient distributions in the Antarctic Cosmonaut Sea in austral summer 2021. Advances in Polar Science. 2022, 33, 267–290. [Google Scholar] [CrossRef]
- Wright, S.W.; van den Enden, R.L.; Pearce, I.; et al. Phytoplankton community structure and stocks in the Southern Ocean (30–80°E) determined by CHEMTAX analysis of HPLC pigment signatures. Deep-Sea Research Part II. 2010, 57, 758–778. [Google Scholar] [CrossRef]
- Chimitza, V.A. Investigation of geostrophic currents in the Antarctic zone of the Indian Ocean. Oceanology 1976, 16, 234–238. [Google Scholar]
- Lubimova, T.G.; Makarov, R.R.; Maslennikov, V.V.; et al. Interdisciplinary Investigations of Pelagic Ecosystem in the Commonwealth and Cosmonaut Seas. Collected Papers. VNIRO Publishers, Moscow. 1988, 1–241 (in Russian).
- Davidson, A.T.; Scott, F.j.; Nash, G.V.; et al. Physical and biological control of protistan community composition, distribution and abundance in the seasonal ice zone of the Southern Ocean between 30 and 80°E. Deep-Sea Research Part II. 2010, 57, 828–848. [Google Scholar] [CrossRef]
- Swading, K.M.; Kawaguchi, S.; Hosie, G.W. Antarctic mesozooplankton community structure during BROKE-West (30°E–80°E), January–February 2006. Deep-Sea Research Part II. 2010, 57, 887–904. [Google Scholar] [CrossRef]
- Westwood, K.J.; Griffiths, F.B.; Meiners, K.M.; et al. Primary productivity off the Antarctic coast from 30°E–80°E; BROKE-West survey, 2006. Deep-Sea Research Part II. 2010, 57, 794–814. [Google Scholar] [CrossRef]
- Gutt, J.; Griffiths HJ, Jones, C.D. Circumpolar overview and spatial heterogeneity of Antarctic macrobenthic communities. Mar Biodive. 2013, 43, 281–487. [CrossRef]
- Gutt, J.; Griffiths HJ, Jones, C.D. Circum-polar overview and spatial heterogeneity of Antarctic macrobenthic communities. Marine Biodiversity. 2013, 43, 481–487. [CrossRef]
- Linse, K.; Griffiths, H.J.; Barnes, D.K.A.; et al. The macro- and megabenthic fauna on the continental shelf of the eastern Amundsen Sea, Antarctica. Continent. Shelf Res. 2013, 68, 80–90. [Google Scholar] [CrossRef]
- Gambi, M.C. and Bussotti, S. Composition, abundance and stratification of softbottom macrobenthos from selected areas of the Ross Sea shelf (Antarctica). Polar Biology. 1999, 21, 347–354. [Google Scholar] [CrossRef]
- Brandt, A.; Gooday, A.J.; Brandão, S.N.; et al. First insights into the biodiversity and biogeography of the Southern Ocean deep sea. Nature 2007, 447. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A. & Johnston, N.M. Antarctic marine benthic diversity. Oceanography and Marine Biology, 2003, 41, 47–114.
- Barnes, D.K.A. and Clarke, A. Antarctic marine biology. Curr Biol. 2011, 21, 451–457. [PubMed]
- Mou, J.F.; Liu, K.; Huang, Y.Q.; et al. The macro-and megabenthic fauna on the continental shelf of Prydz Bay, east Antarctica. Deep-Sea Research Part II. 2022, 198, 105052. [Google Scholar] [CrossRef]
- Sahade, R.; Lagger, C.; Torre, L.; et al. Climate change and glacier retreat drive shifts in an Antarctic benthic ecosystem. Marine Ecology. 2015, 1, e1500050. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H.J.; Linse, K.; Barnes and, D.K.A. Distribution of macrobenthic taxa across the Scotia Arc, Antarctica. Antarctic Science. 2008, 20, 213–226. [Google Scholar] [CrossRef]
- Chapman, M.G. Relationships between spatial patterns of benthic assemblages in a mangrove forest using different levels of taxonomic resolution. Marine Ecology Progress Series. 1998, 162, 71–78. [Google Scholar] [CrossRef]
- Kim, S.L.; Kang, S.M.; Lee, H.G.; et al. Species Diversity and Community Structure of Macrobenthos in the Ulleung Basin, East Sea, Republic of Korea. Journal of Marine Science and Engineering. 2023, 11, 92. [Google Scholar] [CrossRef]
- Gutt, J.; Sirenko, B.I.; Smirnov, I.S.; et al. How many macrobenthic species might inhabit the Antarctic shelf? Antarct. Sci. 2004, 16, 11–16. [Google Scholar] [CrossRef]
- Arntz, W.E; Thatje, S.; Linse, K.; et al. Sampling the marine fauna of remote Bouvet Island, Southern Ocean. Polar Biology. 2006, 29, 83–96. [Google Scholar] [CrossRef]
- Arntz, W.E.; Thatje, S.; Gerdes, D.; et al. The Antarctic-Magellan connection: macrobenthos ecology on the shelf and upper slope, a progress report. Scientia Marina. 2005, 69, 237–269. [Google Scholar] [CrossRef]
- Voβ, J. Zoogeographie und Gemeinschaftsanalyse des Makrozoobenthos des Weddellmeeres (Antarktis). Berichte zur Polarforschung. 1988, 45, 1–145. [Google Scholar]
- Basher, Z.; Bowden, D.A. and Costello M.J. Diversity and Distribution of Deep-Sea Shrimps in the Ross Sea Region of Antarctica. PLoS ONE 2014, 9, e103195. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H.J.; Linse, K.; Barnes and, D.K.A. Distribution of macrobenthic taxa across the Scotia Arc, Antarctica. Antarctic Science. 2008, 20, 213–226. [Google Scholar] [CrossRef]
- Post, A.L.; Beaman, R.J.; O′Brien, P.E.; et al. Community structure and benthic habitats across the George V Shelf, East Antarctica: trends through space and time. Deep-Sea Research Part II: Topical Studies in Oceanography. 2010, 58, 105–118. [Google Scholar] [CrossRef]
- Barry, J.P.; Grebmeier, J.M.; Smith, J.; et al. Oceanographic versus seafloor-habitat control of benthic megafaunal communities in the S.W. Ross Sea, Antarctica. Antarctic Research Series. 2003, 78, 327–354. [Google Scholar] [CrossRef]
- Siegert, M.; Atkinson, A.; Banwell, A.; et al. The Antarctic Peninsula under a 1.5 ℃global warming scenario. Front. Environ. Sci. 2019, 7, 102. [Google Scholar] [CrossRef]
- Lockhart, S.J. and Hocevar J. Combined Abundance of AllVulnerable Marine Ecosystem Indicator Taxa Inadequate as Sole Determiner of Vulnerability, Antarctic Peninsula. Front. Mar. Sci. 2021, 8, 577761. [CrossRef]
- Li, Y.H.; Li D.; ZHAO, J.; et al. Factors controlling the phytoplankton crops, taxonomic composition and particulate organic carbon stocks in the Cosmonaut Sea, East Antarctica. Unpublished Data.
- Mou, J.F.; He, X.B.; Liu, K.; et al. Benthic biodiversity by baited camera observations on the Cosmonaut Sea shelf of East Antarctica. Polar Biology, Unpublished Data. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).