1. Introduction
Non-antibiotic bactericidal methods, such as blue light from 405 nm to 500 nm, are of great interest due to their inherent bactericidal effect without additional external photosensitive substances [
1,
2,
3,
4]. The bactericidal mechanism of blue light is thought to be able to stimulate intrinsic photosensitizer, iron-free porphyrins, and thus generate reactive oxygen species (ROS) that are toxic to bacterial cells. Recent studies show that blue light at 460 nm wavelength (BL460 nm) can influence the cytotoxicity of
S. aureus by disrupting the pigment staphyloxanthin (STX) structure located on the bacterial cell wall. The STX is believed to be the virulence component that aids bacteria in their defense against oxidizing agents such as singlet oxygen (
1O2), hydrogen peroxide (H
2O
2), peroxide anion radical, and hydroxyl radical (OH•) [
5]. These reactive cytotoxic species can cause irreversible damages to molecular cell constituents or even its destruction. Staphyloxanthin might also have an important implication in antibiotic resistant as the pigment involves in structuring a cell wall platform for localization and functioning of Penicillin binding protein 2a (PBP2a) encoded by mecA gene [
6]. The BL460nm is believed to be safer for human cells than blue lights at other wavelengths [
7]. Therefore, the BL460nm is being studied and has a high potential for practical application.
Previous studies have shown that using BL460nm light in continuous and pulsed manners can impart
S. aureus to oxidizing agents to varying degrees [
6,
8]. The pulsed BL460nm displayed a better inhibitory effect on
S. aureus better than the continuous light source did [
6]. Both BL460nm irradiance exhibit a strong photolysis toward the STX and that can be utilized to effectively eradicate the planktonic
S. aureus and infected skin in murine models [
6,
9]. The use of pulsed light, however, might restrict its application as a home device that patients can use it by themselves due to the higher cost and complication of device assembly [
10]. Numerous studies have demonstrated that using non-antibiotic therapies can have unfavorable effects that change the target bacterial population's biology and pathogenicity [
11,
12,
13]. Therefore, in attempt for the BL460nm therapy to be used in practice, a thorough of its safety must be conducted.
The objective of the present study is to determine the optimal combination of BL 460nm light condition and a minimal H2O2 concentration for eradicating S. aureus effectively and safely without affecting bacterial pathogenicity or host cells. By utilizing 460nm light sources with varying light intensities and irradiating durations, we aim to determine the parameters for obtaining effective in vitro bacterial treatment results. In addition, the efficacy and safety of therapy are evaluated to treat S. aureus-infected skin wounds. The results of this study will contribute to developing of a novel non-antibiotic therapy for treating S. aureus skin infection.
2. Materials and Methods
2.1. Blue light 460nm source, bacterial strains, and growth condition
In our previous study, we successfully built a light-emitting diode (LED) source of 460nm wavelength based on a COB LED LE B P2W chip (Osram Licht AG Company, Germany) [
14]. The LED was calibrated to light at 460nm wavelength with a narrow spectral peak and low heat emission. The BL460nm emitted from the LED system was adjusted to the desired light intensity using a built-in Pulse-width modulation (PWM) compartment and calibrated by a spectroradiometer (Apogee Instruments, USA). PWM controls the average power delivered by the electrical signal of the P2W chip, allowing the increase and decrease of light intensity through an adjustable dimmer switch. The exact light intensity (50, 100, 200, and 400 mW/cm2) was adjusted and calibrated with the help of a spectroradiometer (Apogee Instruments, USA). All irradiation treatments were performed at room temperature of 25°C.
The strains of S. aureus used in the study were methicillin-resistant Staphylococcus aureus (MRSA) strain ATCC® 33592TM and methicillin-susceptible Staphylococcus aureus (MSSA) strain ATCC® 29213™ (American Type Culture Collection – USA). The bacterial cells were grown and stocked in Trypticase soy broth (TSB) medium (Oxoid, Thermo-Fisher Scientific, United Kingdom).
2.2. The growth of the S. aureus under the BL460nm irradiance.
The MSSA and MRSA cells were grown in Tryptic Soy Broth (TSB) medium at 37°C to a suspension of approximately 108 CFU/mL. The suspension was then inoculated into 150 mL of fresh TSB medium and cultured to 105 - 106 CFU/mL density. The entire volume of culture was subsequently transferred to a cylindrical glass flask with a bottom diameter of 5 cm; the mouth of the flask was covered with a round piece of quartz to prevent contamination during the light treatment process. The flask was incubated at 37°C under the BL460 irradiating conditions as follows. The LED light positioned eight centimeters above the flask was operated to emit the BL460nm directly into the bacterial suspension, as the focal point of light on the LED system was designed to be approximately eight cm from the light source. To minimize the effects of focal length variation on light intensity, all irradiated samples were positioned at this constant distance from the LED light. The OD600 values of culturing suspension were measured every two hours for 24h. The control sample was similarly arranged, except the 460 nm light source was replaced with a white LED light.
2.3. Virulance and antibiotic susceptibility testing
The modifications in the production of caseinase for casein hydrolysis virulence [
15], gelatinase for hydrolysis of the host extracellular matrix [
16], lipase for lipid hydrolysis [
17], lecithinase for cytotoxicity of animal tissue [
18], and haemolysin for lysis of red blood cells [
19] were tested in
S. aureus following BL460nm irradiance. Bacterial inoculum was prepared by resuspending overnight-grown
S. aureus cells in PBS to a 10
6 CFU/mL density. A 5 mL cell suspension volume was transferred to cylindrical glass flasks and treated with different intensities of BL460nm (50 mW/cm
2, 100 mW/cm
2, 200 mW/cm
2, and 400 mW/cm
2). Bacterial samples were collected 5 min, 10 min, 20 min, and 40 min after irradiance for testing of extracellular virulence enzymes. A volume of 10 µL of bacterial suspension was dropped on the surface of Skim milk agar (for caseinase test), Gelatin agar (for gelatinase test), Trybutirin agar (for lipase test), Tryptic soy agar supplied with egg yolk emulsion (for lecithinase test), and Sheep blood agar (for haemolysin test). The material and substrate medium were obtained from Oxoid company (UK). The agar plates were then incubated at 37°C for 24 - 48 h. After incubation, the enzymatic hydrolysis zones surrounding the bacterial colony spot were measured (in millimeters). The activity of each virulence factor was evaluated by calculating the Enzyme activity Index (EAI) = (Diameter of hydrolysis zone)/ (Diameter of bacterial colony spot). The EAI = 1 was considered as no change in that tested virulence factor [
20].
The changes in MRSA antibiotic susceptibility were evaluated by using the antibiotic paper disc diffusion method (Himedia - India; Oxoid, United Kingdom) in accordance with the guideline of the European Committee on Antimicrobial Susceptibility Testing – EUCAST [
21]. The MRSA colonies were resuspended in Mueller-Hinton Broth (MHB) medium to prepare a bacterial suspension with density of approximately 10
6 CFU/mL. In cylindrical glass flasks, a 5 mL volume of bacterial suspension was illuminated with BL460 nm for 10 min, 20 min, and 40 min. After lightning treatment, the cell suspension was spread on a 4mm thick Mueller-Hinton Agar (MHA) plate. Subsequently, Antimicrobial Susceptibility discs (Himedia - India) were placed on the surface of the agar. The MHA plates were then incubated at 37°C, and the diameter of inhibitory zone (in millimeter) was measured after 18-24 h. The changes in antibiotic susceptibility of MRSA were calculated by the Zone of Inhibition change Index = (Zone of Inhibition of light treated MRSA)/ (Zone of Inhibition of negative control MRSA). Zone of Inhibition change Indexes of all 21 antibiotics used in this study were illustrated in a heatmap by GraphPad PRISM 9.5.2 (San Diego, California USA,
www.graphpad.com).
2.4. Anti-MRSA assay by 460nm light in combination with H2O2
Similar to the previous tests, a series of cylindrical glass flasks containing 107 CFU/mL MRSA suspension were illuminated by BL460 nm at 50 mW/cm2, 100 mW/cm2, 200 mW/cm2, and 400 mW/cm2 for 5 min, 10 min, 20 min, and 40 min. A volume of 100 µl of each light-treated cell suspension was added to the wells of a 96-well plate which were pre-filled with 100 µl of a two-fold serial dilution of H2O2, ranging from 3% to 0.08%. After 5 minutes of exposure to H2O2, each bacterial mixture was withdrawn from the well and spread on the TSA plate so that the surviving bacterial cells could grow and form colonies. The Minimum Inhibitory Concentration of H2O2 (MICH2O2) was determined to be the concentration of H2O2 at which all MRSA cells in the treatment were killed. The number of colonies counted on the plate after 24 hours of incubation were also used to calculate the bacterial density (CFU/mL).
2.5. Safety of 460nm light and H2O2 on bare skin
A mouse skin model was tested for the safety of using 460nm and H2O2 treatment. Ten mice (Mus musculus Swiss Albino) had their back hair shaved to expose a 2 x 2 cm skin area. The light beam of BL460nm was adjusted to cover the skin with the 100mW/cm2 intensity for 5 min, followed by the application of 0.75% H2O2 solution once daily for 10 days. Daily observation and photograph are taken of the treated skin in order to detect signs of skin irritation, such as discoloration, dermatitis, or redness.
2.6. MRSA-infected wound model
Seven- to eight-week-old mice were housed for one week prior to use in the experiment. Each individual was housed in a separate cage and fed rice bran pellets. The MRSA infection mouse model was induced by injecting Cyclophosphamide, as described in previous studies [
11,
12], and creating an excisional infection wound following procedures described previously elsewhere [
11,
12,
13]. Before the surgery to create an infected wound, each of the ten mice (five males and five females) was injected with Cyclophosphamide (Sigma-Aldrich – C0768) at doses of 150 mg/kg, 250 mg/kg, and 350 mg/kg. Each dose of Cyclophosphamide was divided into two smaller doses and administered intravenously four and one day before the surgery.
2.7. A mouse skin abrasion model infected with S. aureus
The cyclophosphamide-treated mice were anesthetized by intraperitoneal injection with a mixed dose of 5 mg/kg Zoletil and 4 mg/kg Xylaxin (Virbac, France). Subsequently, the back of each mouse was shaved with a razor to expose a 3 x 3 cm skin area. Then, a 1cm-diameter circular portion of the dorsal skin was removed using sterile surgical scissors and forceps. The cutting must remove the epidermis, dermis, and subcutaneous layers to expose the underlying muscle [
24]. The open muscle part in the wound was then lightly abraded with a scalpel to induce a light abrasion. Immediately after the abrasion, one drop of 10 μL (10
8 CFU/mL) MRSA bacterial suspension was pipetted and evenly applied to the wound. The wound was immediately covered with a 2x2 cm of Tegaderm™ tape (3M, Vietnam) and a sterile medical bandage wrapped around the mouse’s waist. Mice were returned to their cage to recover for one day before the bandages were removed. The mortality of mice from each Cyclophosphamide treated group was monitored for 10 days. On the basis of the survival rate and infection efficiency, the optimal Cyclophosphamide dose for the mouse infection model was determined.
2.8. Treatment of MRSA infection wound model using 460nm light and H2O2 combination
Mice with successfully infected wounds were separated into five groups of 20 individuals each. Each group received a unique treatment for the injured back skin. Group 1 mice were untreated serving as negative control. Group 2 mice were given 0.75% H2O2. Group 3 mice were exposed to 460 nm light (100 mW/cm2 for 5 min). Group 4 mice (positive control) were topically treated with Fucidin H® cream (containing the antibiotic Fusidic acid and Hydrocortisone specifically for skin infections caused by Staphylococcus bacteria). Group 5 mice were treated with a combination of BL460nm and H2O2. Individual mice in groups 3 and 5 were placed in transparent polyethylene (PE) plastic boxes to restrict the movement of the aminals during treatment. The BL460nm LED source was positioned 10 cm away from the mouse wound and vertically emitted light to the wound. After the prescribed duration of light treatment, 0.75% H2O2 solution was additionally applied to the wounds of mice in Group 5. Every mouse in each group received wound treatment once per day for fifteen consecutive days.
The infected wound was photographed daily throughout the course of treatment. ImageJ software (NIH, USA) was used to measure and quantify the wound's surface area. To compare the rate of wound healing among treatment groups, the wound area was converted to the percentage of wound healing (%WHL) value which equals to (1-W1/W0) x 100 [
13]. W1 is the wound area of the individual mouse on the date of recording, and W0 is the initial wound area on the first day after bandage removal.
2.9. Statistics
Statistical analysis was performed using GraphPad PRISM 9.5.2 in which normality of the data being analyzed by the integrated Shapiro-Wilk test. All replicated data set were presented as the mean ± SEM or SD, with differences between means being compared for significance by either student t-test or one-way ANOVA following appropriate post hoc test for pairwise comparisons. P values of less than 0.05 were considered significant.
2.10. Study approval
All animal procedures were approved by the Institutional Animal Care and Use Committees of Vietnam National University (ACUCUS), Ho Chi Minh City, Vietnam with ethics approval code 930/KHTN-ACUCUS.
3. Results and Discussion
3.1. Blue light 460nm delayed the lag prowing phase of the bacteria
We designed and assembled a 460nm wavelength-emitting LED system as previously described [
14]. Using this LED setup, we demonstrated that the BL460nm effectively photolyzed the carotenoid pigment STX isolated from S. aureus. The BL460 additionally showed the capacity to directly photolyze the color of the STX pigment on the MSSA and MRSA cells but did not elicit antibacterial effects [
14]. In this study, we evaluated the effect of BL460 nm on the biological and physiological properties of S. aureus.
The MSSA and MRSA cells were grown under BL460nm at four light intensities (50, 100, 200, and 400 mW/cm
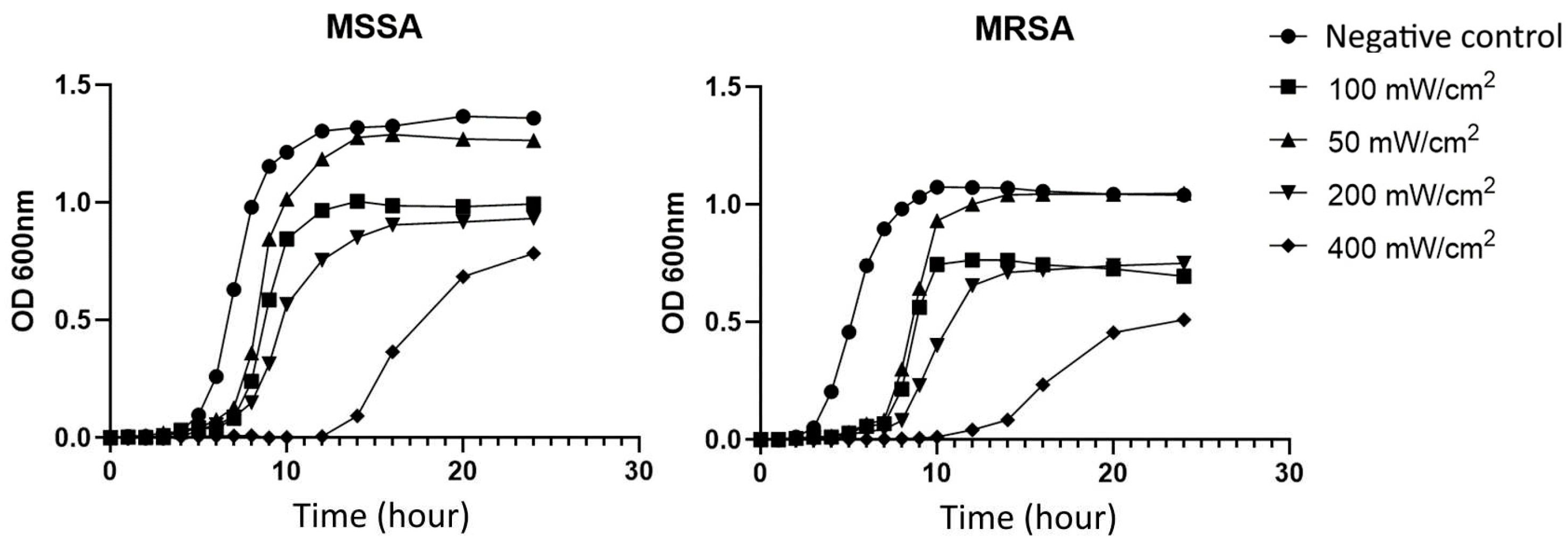
2). The growth curves clearly showed that the BL460nm light significantly hindered the growth of the bacteria. Specifically, S. aureus cells considerably prolonged the lag phase (
Figure 1). Under 400 mW/cm
2, the lag phase of the studied MSSA and MRSA strains lasted up to 10 hours. In contrast, the untreated cells completed the lag phase in four hours, and the cells entered exponential growth. It is interesting to note that the delay for MRSA was more profound than for MSSA. Compared to untreated cells and 50 mW/cm
2 light-treated cells, cells growing at 100, 200, and 400 mW/cm
2 achieved the plateau at lower OD600 values. These results indicated that BL460nm at a higher intensity strongly affected the biology of S. aureus during the lag phase, which may lead to intrinsic changes in the biological properties of the bacterial cells. The fact that light-treated cells had lower OD600 values for plateau implied that the light might also have a deleterious effect on S. aureus during the lag growth phase and/or exponential phase.
3.2. BL460nm does not modulate the pathophysiological adverse properties of the tested S. aureus
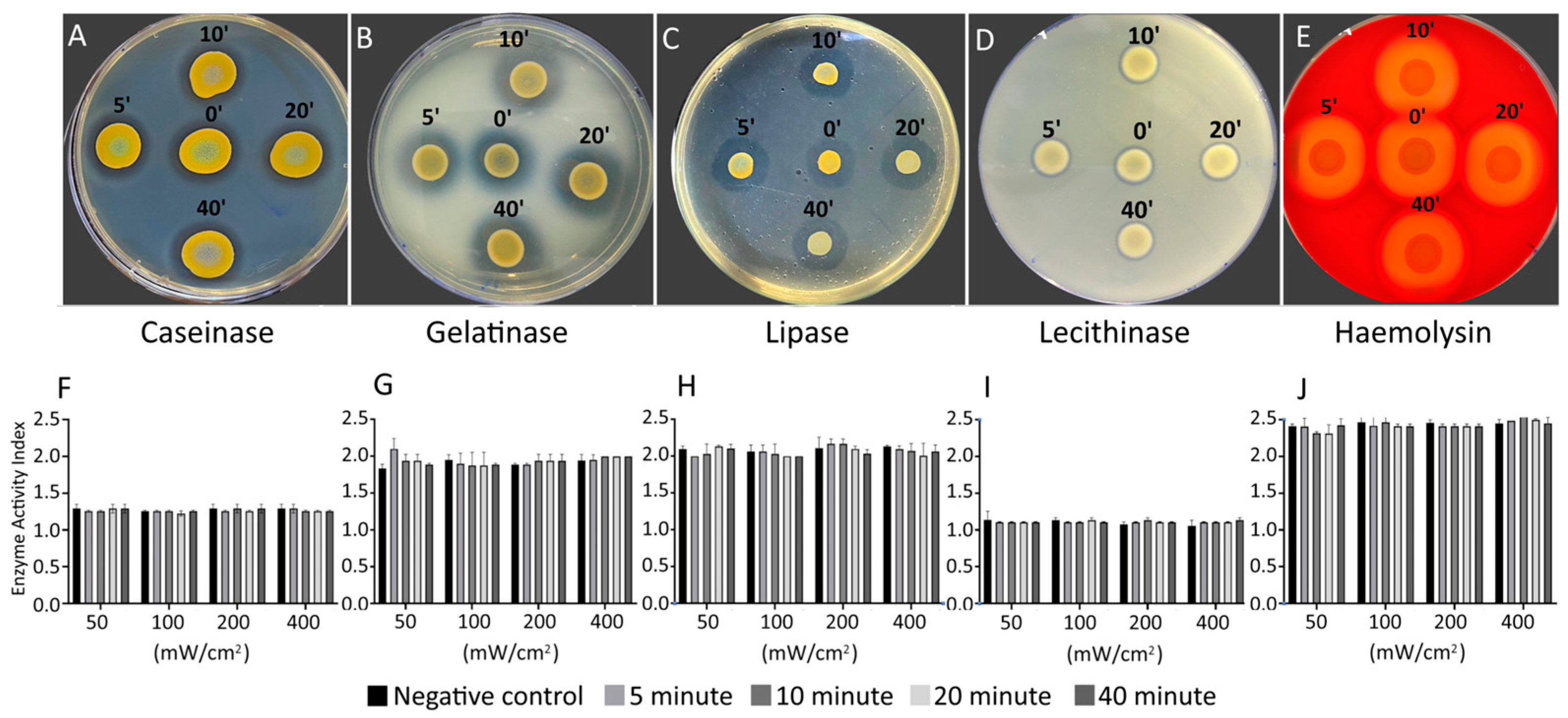
Next, we seek to know to what extent the BL460nm influences the pathophysiological characteristics of S. aureus, including its virulence and sensitivity to antibiotics. The changes in virulence factors were evaluated in the bacterial cells after being treated with various BL460nm doses, which can be tracked by the duration and intensity of irradiance. The cells were collected at four different irradiating durations (5, 10, 20, and 40 min) for each of the four light intensities used (50, 100, 200, and 400 mW/cm2).
The data demonstrated that the MRSA strain exposed to BL460nm did not differ from untreated cells in any of the virulence factors evaluated, including casein hydrolysis virulence, gelatinase, lipid hydrolysis, lecithinase, and haemolysis (
Figure 2). This finding suggested that the BL460nm did not alter MRSA's capacity to manufacture any of the assessed virulence factors. Therefore, we concluded that the BL460nm may be employed without endangering S. aureus or resulting in a predisposed increase in bacterial toxicity. We obtained similar results with the MSSA strain (
Supplemental Figure 1).
3.3. Changes in the antibiotic susceptibility of S. aureus under the BL460nm irradiance
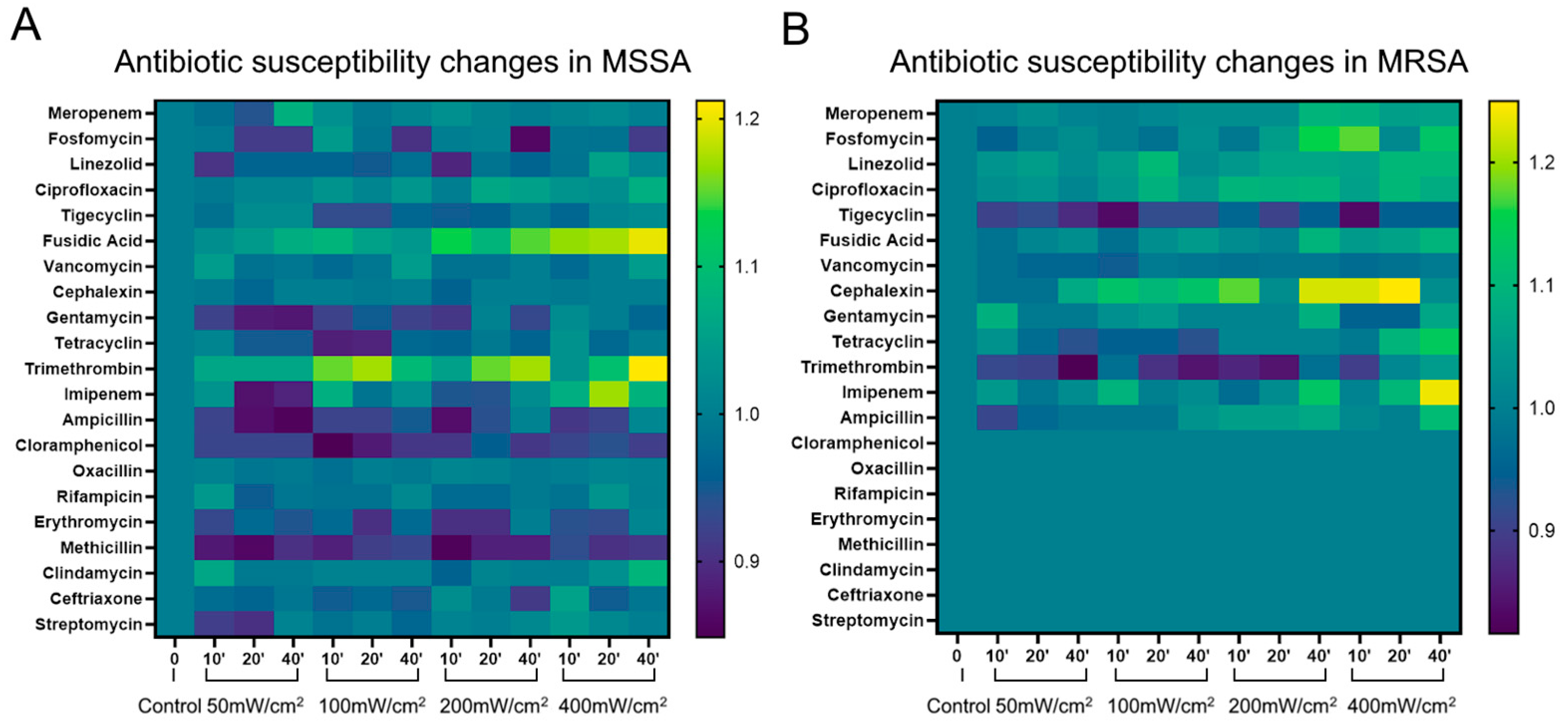
When bacteria are exposed to non-antibiotic antimicrobial agents, new antibiotic resistance can evolve or become more pronounced in some strains of bacteria. These unanticipated adjustments may restrict the use of novel antimicrobial therapies. Therefore, in this study, we examined how the BL460nm irradiance affects the drug susceptibility of
S. aureus. We found that, among the 21 antibiotics used, the light-treated MSSA strain tended to be more sensitive to the antibiotics Fusidic Acid and Trimethrompin. This increased sensitivity was clearly shown when the cells were exposed to 400 mW/cm
2 of BL460nm for 40 min (
Figure 3A).
The BL460nm exposure also showed enhanced resistance of the MSSA strain to Chloramphenicol, Gentamycin, and Methicillin. However, these changes were statistically insignificant, with the diameter of the sterile ring decreasing or increasing slightly from 1 to 2 mm. The resistance to the remaining antibiotics varies unevenly (
Figure 3A).
The light-treated MRSA strain showed increased sensitivity to the antibiotics Fosfomycin, Cefalexin, and Imipenem (the antibacterial ring diameter increased from 3 to 5 mm). This increase in antibiotic sensitivity was clearly shown in the cells treated with 400 mW/cm
2 of light intensity for 40 minutes (
Figure 3B). Other antibiotics, including Meropenem, Linezolid, Ciprofloxacin, Fusidic acid, Tetracycline, and Ampicillin, exhibited a mild increase in antimicrobial effectiveness. Similar to the MSSA strain, the 460 nm light-treated MRSA strain did not show a noticeable enhancement in antibiotic resistance. Overall, the aforementioned findings demonstrated that BL460nm did not increase the antibiotic resistance of the bacteria to most antibiotics. Therefore, the light can be used safely with most antibiotics without causing adverse effects.
3.4. BL460nm decreases Minimum Inhibitor Concentration value of H2O2.
Our work and other studies showed that S. aureus cells could not be killed by BL460nm through the photolyzing of STX pigment. However, the light attenuated the S. aureus to a low concentration of H2O2 in culturing conditions and an infected skin lesion in a murine model. In this study, we employed a similar approach to identify a minimum concentration of H2O2 that can be combined with our LED 460nm system to kill S. aureus cells. To find the ideal lighting setup that may be used in practical and clinical settings, we screened for various BL460nm doses in terms of light intensity and irradiating duration.
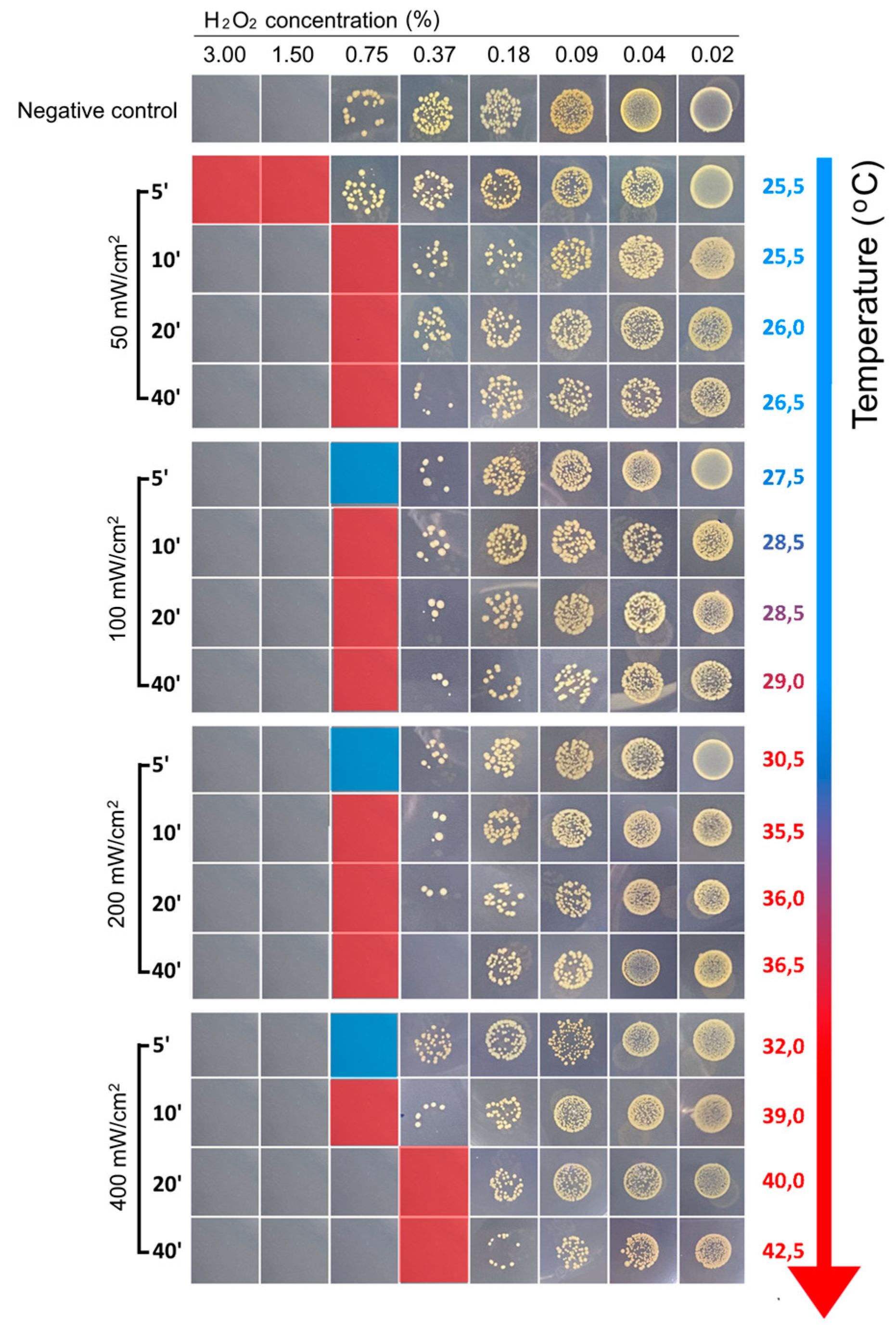
We first determined the MIC value of H
2O
2 (MIC
H2O2) for the MRSA 33592TM strain under BL460nm treatment. The results showed that with an intensity of 50 mW/cm
2, it took 10 minutes of lightning to obtain MIC
H2O2 of 0.75% compared to MIC
H2O2 of 1.5% in the untreated bacterial cells (
Figure 4). Meanwhile, it only required 5 min to elicit the same impact of lowering the MIC
H2O2 when 100 mW/cm
2 and higher lighting intensities were employed to treat the bacterial cells (
Figure 4).
We noticed that the MICH2O2 for the MRSA did not alter significantly at three light intensities of 100, 200, and 400 mW/cm2. When the bacteria were illuminated for 20 min at an intensity of 400 mW/cm2, the MICH2O2 could decrease to 0.375%. However, under 200 and 400 mW/cm2 lighting, the temperature measured in the 20 min-illuminated samples increased over 30°C and 40°C, respectively. Due to the high temperature of the illuminated sample, using intensities of 200 and 400 mW/cm2in animal models and clinical settings is impractical. In light of this heating concern, we deduced that the ideal condition for the upcoming in vivo test is the combination of 100 mW/cm2 light intensity for 5 minutes and an H2O2 concentration of 0.75%.
3.5. Combination of BL460nm and hydrogen peroxide is safe for the skin
The antibacterial effects of combined therapy (BL460nm at 100 mW/cm
2 for 5 min and 0.75% H
2O
2) was next investigated on an MRSA-infected wound in mice. We first examined the combined therapy for its safety on mouse skin. A 2x2 cm area of open dorsal skin was exposed to 100 mW/cm
2 of BL460nm for 5 minutes, after which the skin was swiped with 0.75% H
2O
2 solution. The procedure was carried out on the targeted skin area every day for 15 days.
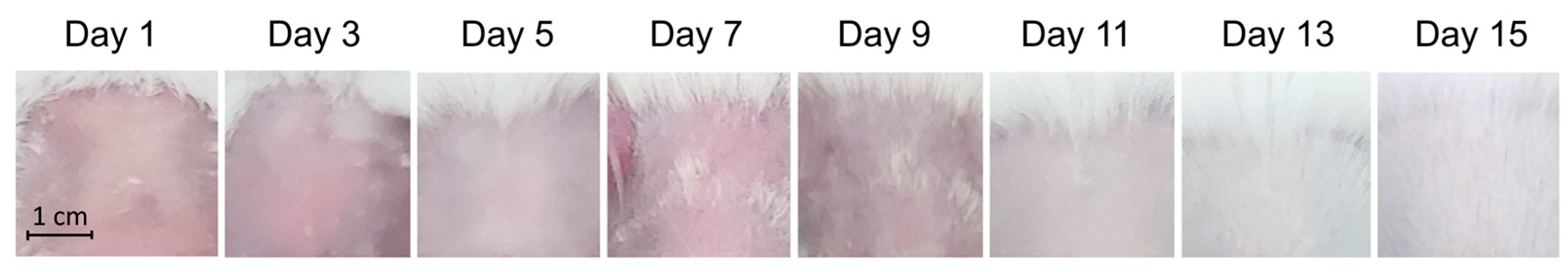
Figure 5 demonstrated no visible rashes or other adverse effects on the skin. Therefore, we concluded that the combined therapy is safe to be applied on mouse skin.
3.6. Combined therapy showed the skin abrasion healing comparable to Fucidin
We then examined the therapeutic efficacy of BL460nm and H
2O
2 on a mouse skin abrasion model infected by
S. aureus. The mice were first subjected to immuno-supression by IP injection of Cyclophosphamide to ameliorate the infection. The appropriate amount of the drug was determined by testing with three concentrations of Cyclophosphamide (50, 100 và 250 mg/kg of body weight). The 50mg/kg Cyclohosphamide was insufficient to induce the infection caused by MRSA, as indicated by self-healing of the infected skin (
Figure 6B and E).
The mice injected with 250 mg/kg Cyclophosphamide survived 70% on day four and lasted until the end of 10 days of the trial (
Figure 6A). The wounds in this group showed signs of spreading compared to the original, accompanied by yellow pus appearance and swollen wound edges on the first day, demonstrating that the infectious process of MRSA bacteria in mice was successful (
Figure 6C and F). The mice that survived to the 10
th day showed recovery, and the wound began to close. The mice injected with 350 mg/kg cyclophosphamide had the lowest survival rate, with 40% of the mice dying after three days of the first injection (200 mg/kg) and only 50% of the mice surviving after the second injection (
Figure 6A, blue line). The infected wounds of the animals in this group that survived until day 5 showed severe infection. The infection began to spread beneath the skin and ran along the trunk and body (
Figure 6D and E). We, therefore, concluded that the dose of 250 mg/kg is most suitable to induce skin abrasion with MRSA infection in the Swiss albino mice.
3.7. Low concentration of H2O2 and BL460nm combination effectively heal the skin
Next, we sought to evaluate the combined therapy of BL460nm and H2O2 based on the above results in healing the skin-infected mouse model. The mice with abrasion skin infected by MRSA were administered five different therapies labeled from Group 1 to Group 5, as described in the method section.
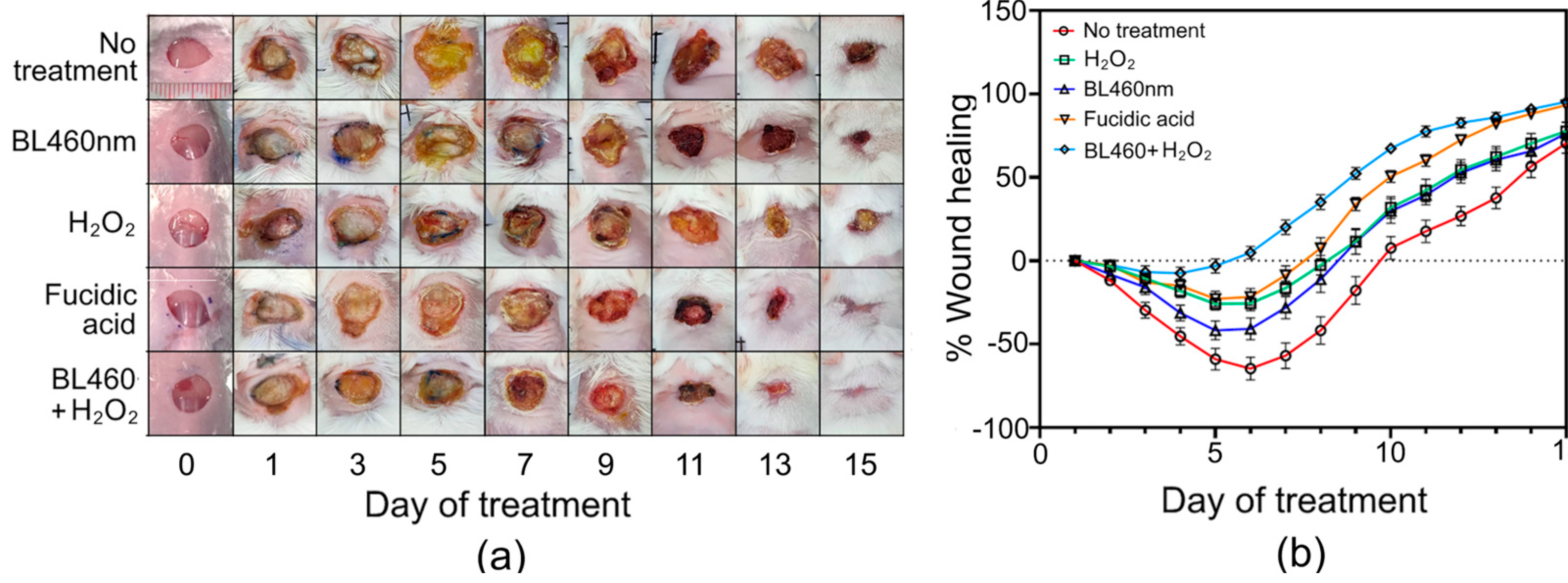
Group 1 (untreated group) exhibited the worst wound healing progression among the five tested groups. In the first three days, the wound area of mice in group 1 grew by up to 30% (±4.72%) of the initial wound area, and the infection peaked on day six when the wound area grew by more than 64.5% (±6.9%). On day seven, the healing process of Group 1 was observed when the wound began to dry and narrow (
Figure 7A and B). On the last day of the experiment (day 15), only 3 of these 20 untreated mice had completely healed wounds, while the remaining 17 individuals had wounds that had not yet recovered (the mean WHL value was 70.47% (±6.04%).
Group 2 mice that were illuminated with BL460nm for 5 minutes per day had comparable wound healing to Group 1 mice for the first three days (p > 0.05) (
Figure 8a). On the fifth day, however, the wound healing process of group 2 mice differed from that of group 1 mice, as the wound area of group 2 mice only expanded by 41.76% (± 5.68%) compared to 58.94% (±6.54%) in Group 1. On the fifth day, the wounds of the Group 2 animals also began to exhibit signs of healing. Statistical analysis showed that there was a significant difference in the %WHL of mice between Groups 1 and 2 on day 5 (p<0.0021) and day 7 (p<0.0002) (
Figure 7B). Notably, when the therapies reached their ninth day, the rate of wound healing in group 1 was lower than in Group 2. (the average percent WHL value of Group 1 on this day was -17% (± 8.32%), compared to 11% (±7.25%) in Group 2). Statistical analysis, however, revealed no significant difference between these two groups (
Figure 8a).
In Groups 3 and 4, mice were treated with H
2O
2 and Fucidin, respectively. In comparison to group 1 and 2, the wounds treated with the two bactericidal agents had a striking effect on the first day. Groups 3 and 4 healed at comparable rates during the first six days, but the Fucidin-treated group reduced wound size more rapidly than the H
2O
2-treated group beginning on day 7 of treatment. On the final day of treatment (15 days), seven of the 20 mice in the H
2O
2-treated group were completely cured. In the Fucidin-treated group, 11 of the 20 mice were completely healed, with three individuals recovering entirely by day 13. (
Figure 7). Statistical analysis, however, displayed no significant difference between groups 3 and 4 during the 15-day experiment (
p>0.05) (
Figure 8a).
Group 5, which utilized BL460nm and H
2O
2 to treat the wounds, demonstrated outstanding outcomes to Groups 1, 2, 3, and 4 (
p<0.0001), particularly during the first week of treatment (
Figure 8a). The mice in group 5 had the quickest wound healing rates. Even though the infected wound still grew during the first five days of therapy, the mean percent WHL value of group 5 was as low as -7.47% (±3.57%), indicating that the wound of this group grew by no more than 7.47% (±3.57%) of its initial wounded size (
Figure 7b and
Figure 8a). The results indicated that the combination of BL460nm and H
2O
2 potentially prevented the wound from expanding, thus shortening the wound-healing process. In addition, group 5 wounds were dry and rarely exhibited yellow pus discharge. In particular, the dry scab on the wound surface appeared very early, on average, within 6-7 days of treatment (
Figure 7a). Thus, combining BL460nm with an intensity of 100 mW/cm
2 for 5 minutes and 0.75% H
2O
2 solution was as effective as using Fucidin in treating MRSA-infected wounds. The combination of these two factors contributes to inhibiting MRSA bacteria and restricting the spread of wounds, thereby accelerating the healing of wounds.
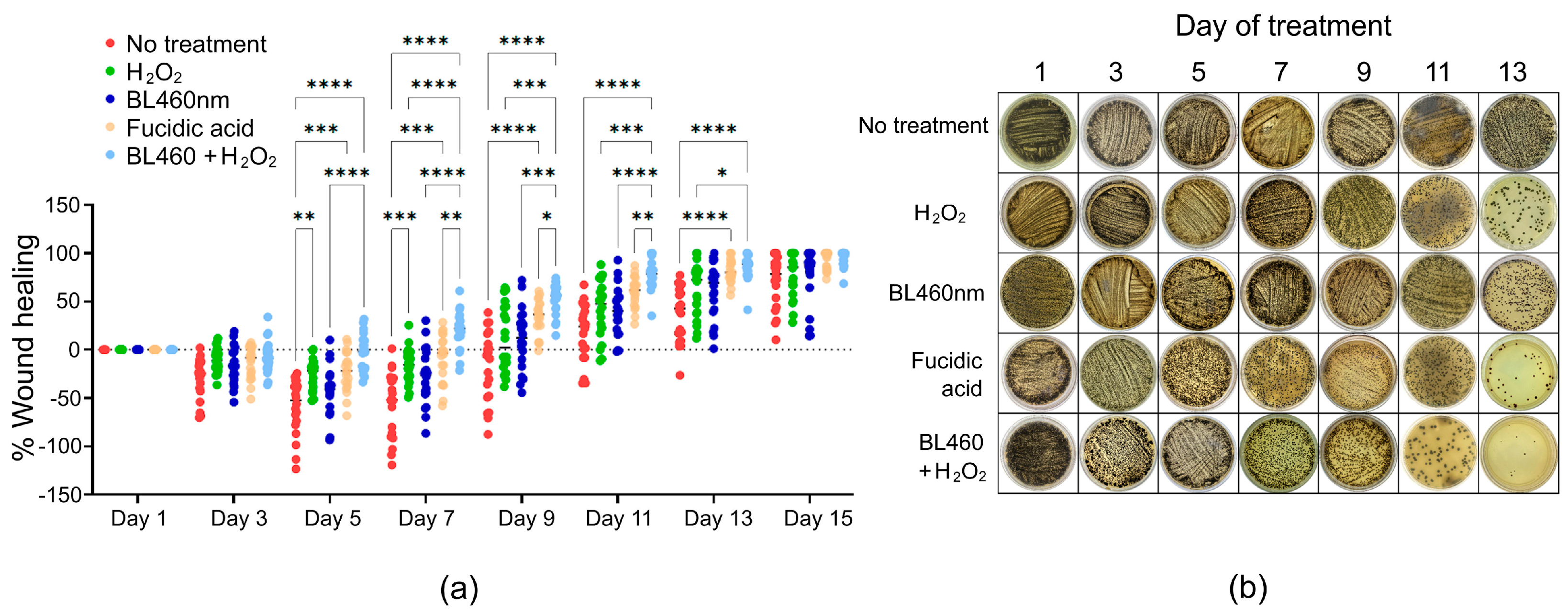
3.8. Combined therapy might erradicate S. aureus more effectively than Fucidin
To track the amount of bacterial number during the couse of treatments among the five groups, the smears of lesion skin area was collected and cultured on Baird-Paker agar. The formation of colonies was observed and recorded. The data indicated that the least number of colonies were found from the group 5, indicating that the combined method was more successful than Fusidic acid at eliminating the bacteria from the skin lesion (
Figure 8b and Supplemental
Figure 3).
4. Discussion
In this study, we illustrated that using BL460nm and a low concentration of H2O2 (0.75%) was safe and effective to eradicate S. aureus from a skin abrasion in mice. We demonstrated the safety of BL460 in the context of virulence toxicity and antibiotic resistance. Upon BL460nm treatment, neither MRSA nor MSSA in this study were affected in terms of virulence production. The BL460nm has a negligible effect on the development of antibiotic resistance in the examined MRSA and MSSA strains. The majority of antibiotics tested did not exhibit increased S. aureus resistance after treatment with BL460nm. In addition, a 10-day evaluation of the combined therapy on the skin of mice revealed no adverse effects. The results above indicate that BL460nm and H2O2 are safe for the skin. We also demonstrated the therapy's efficacy in treating S. aureus-infected skin wounds in mice. The effect of the combined method on wound healing was comparable to that of the antibiotic Fucidin. Intriguingly, the combination therapy reduced the number of S. aureus bacteria in the wound more effectively than Fucidin.
Using 460 nm to 470 nm blue light is of great interest for developing non-antibiotic bactericidal therapies [
4,
25]. The BL460nm wavelength was selected due to its advantages in host cell safety compared to other wavelengths. Multiple studies have confirmed that BL460nm is insufficient for killing bacteria. However, BL460 can cause detrimental changes in bacteria, allowing them to be destroyed by adjuvant agents (such as magnetic, microwave, and silver nanoparticles) to enhance the killing effect of
S. aureus [
26,
27]. A recent study showed that BL460nm effectively photolyzes STX pigment on the
S. aureus cell wall. This pigment is believed to be a virulence factor that aids bacterial cells in resisting oxidative stresses generated by host cells [
5,
27]. STX is also a crucial component of the domains that facilitate the localization of cell wall proteins, such as penicillin-binding proteins [
6]. Hence, bacterial treatment with BL460nm increases the sensitivity to multiple conventional antibiotics [
6].
Our data is consistent with the study by Dong et al. (2021), in which they used pulse lighting at BL460nm and a lower H
2O
2 concentration of 0.35% to kill
S. aureus [
6]. They also showed that the pulse laser 460nm irradiance effectively damages the membrane microdomain structure, which STX stabilizes. The disorganization of the microdomain was also shown to disrupt the function of Penicillin Binding Protein 2 by STX deregulation, which sensitizes the cell to penicillin and other beta-lactam-based antibiotics. In our study, we employed continuous BL460mm irradiance, which is more conventional and cost-effective to effectively treat the skin when the light is used with a low concentration of H
2O
2. Despite the fact that pulsed light demonstrated superior efficacy over continuous light in eradicating
S. aureus, the complexity of pulsed light generation may limit the potential of the therapy as a home-use device. Future research should therefore concentrate on delivering a home device that utilizes continuous light in a simple and cost-effective way.
Furthermore, we investigated the light dose in detail for the light intensities and the irradiation duration required to treat S. aureus effectively. This information is helpful for those who wish to employ the same strategy to develop their own way of utilizing the BL460nm light in treating the S. aureus infection. We suggest that 100mW/cm2 irradiance in 5 mins should be used for therapeutic effectiveness. The 5 min irradiation is also practical for patients in future research. As for clinical applications, the length of treatment is critical as it is not empirical if the irradiating time is too long, which can cause many problems (i.e., over heating).
Nonetheless, these results must be interpreted with caution, and several limitations should be considered. The concern of resistance development in the bacteria to cope with the deleterious action of BL460nm has been tested previously. In a study, the bacterial cells, after light treatment, were subcultured for 10 generations and then tested for drug resistance [
6]. Their finding indicated that the BL460nm did not cause significant resistance to all tested antibiotics. The explanation was proposed due to the non-specific and broad impacts of the light on multiple targets, including STX molecules and varieties of other targets, which can impair the function of
S. aureus survival.
In our study, the effect of BL460nm on virulence and antibiotic resistance was only evaluated after a single exposure of the bacterial to the light; therefore, it is difficult to determine the safety of phototherapy when being used more frequently. Due to the limited sample size of two
S. aureus ATCC strains, it is difficult to generalize these findings to various strains. Extensive studies on other strains of
S. aureus will demonstrate how well the combination therapy works so that solid evidence can be gathered regarding potential clinical use of the therapy in treating skin lesions infected by
S. aureus. Despite this, numerous studies have indicated that blue light is safe for both bacterial and host cells [
1,
13,
25,
29,
30].
In the antibiotic susceptibility testing, we observed the MSSA become more sensitive to Fucidin. The increased sensitivity of bacteria to Fusidic acid under blue light treatment was also confirmed in another study [
31]. Topical Fucidin is still commonly used to treat skin lesions infected with
S. aureus. Therefore, the combined therapy should be investigated for use with Fusidic acid in order to increase the efficacy of the drug and potentially reduce the MIC of Fusidic acid, thereby limiting the phenomenon of Fusidic acid resistance. The 460nm light does not make
S. aureus bacteria more dangerous; on the contrary, it also weakens this bacterium to certain antibiotics, especially making MRSA bacteria more sensitive to Imipenem and Ampicillin, the two antibiotics less effective against MRSA. The improvement of the bactericidal efficacy of antibiotics by BL460nm should also be interesting to explore in future.
To move forward with the use of BL460nm and H
2O
2 on humans, the therapy must be tested on more subjects, specifically on a wider variety of infected wounds (postoperative, diabetes mellitus). Other
S. aureus strains with distinct antibiotic resistance spectra must also be examined to ensure the combined therapy has a broader antibacterial spectrum of action. The combined method is also needed to test with the Panton-Valentine leucocidin-producing MRSA (PVL) positive strain, as it is highly associated with skin and soft tissue infection diseases [
32,
33]. It would be useful to apply the light and H
2O
2 method to treat PVL + strain, which is highly prevalent in a community acquired manner [
34,
35]. In conclusion, more studies are needed to demonstrate the efficacy of BL460-based therapy for human use.
Author Contributions
Conceptualization, V.N.N, T. T. T and M.T.V.; methodology, N.V.V, N.T.T.T, T. T. T and M.T.V.; formal analysis, V.N.N, T.T.T and M.T.V.; writing—original draft preparation, N.V.N, V.V.V and M.T.V.; writing—review and editing, N.V.N and M.T.V.; visualization V.N.N and T.T.T.; supervision, T.T.H.N, V.V.V, and M.T.V.; funding acquisition, M.T.V. All authors have read and agreed to the published version of the manuscript.
Figure 1.
The 460nm blue light delays the growing of S. aureus in lag phase. Both the MSSA and MRSA were grown under the continuous BL460nm at different powers. Each data point expressed the mean of three technical measurements of OD600.
Figure 1.
The 460nm blue light delays the growing of S. aureus in lag phase. Both the MSSA and MRSA were grown under the continuous BL460nm at different powers. Each data point expressed the mean of three technical measurements of OD600.
Figure 2.
Evaluation of virulance factors in MRSA under BL460nm irradiance. The changes in virulent factors of the bacterial cells were examined in different light intensities (50, 100, 200, and 400 mW/cm2) and different lightning duration (5, 10, 20 and 40 min). A and F: Caseinase, B and G: Gelatinase, C and H: Lipase, D and I: Lecithinase D, E and J: Heamolysin. Graphs in F-J were plotted from data of four replicates. Data are expressed as mean and standard deviation (SD). No statistical significance was found from the results of ANOVA and post hoc Tukey analysis for all the data.
Figure 2.
Evaluation of virulance factors in MRSA under BL460nm irradiance. The changes in virulent factors of the bacterial cells were examined in different light intensities (50, 100, 200, and 400 mW/cm2) and different lightning duration (5, 10, 20 and 40 min). A and F: Caseinase, B and G: Gelatinase, C and H: Lipase, D and I: Lecithinase D, E and J: Heamolysin. Graphs in F-J were plotted from data of four replicates. Data are expressed as mean and standard deviation (SD). No statistical significance was found from the results of ANOVA and post hoc Tukey analysis for all the data.
Figure 3.
Evaluation of antibiotic susceptibility of MSSA and MRSA strains after BL460nm exposure. Heat maps illustrated the ratio values of the Zone of Inhibition change Index. Ratio values > 1 (green to yellow) indicate increased antibiotic resistance. A ratio value < 1 (dark blue to purple-black) indicates a decrease in antibiotic resistance. A ratio value of approximately = 1 (blue) indicates no change in antibiotic resistance. A) Changes in antibiotic susceptibility in MSSA and B) in MRSA. The ratio calculation was presented in the method section.
Figure 3.
Evaluation of antibiotic susceptibility of MSSA and MRSA strains after BL460nm exposure. Heat maps illustrated the ratio values of the Zone of Inhibition change Index. Ratio values > 1 (green to yellow) indicate increased antibiotic resistance. A ratio value < 1 (dark blue to purple-black) indicates a decrease in antibiotic resistance. A ratio value of approximately = 1 (blue) indicates no change in antibiotic resistance. A) Changes in antibiotic susceptibility in MSSA and B) in MRSA. The ratio calculation was presented in the method section.
Figure 4.
The changes in hydrogen peroxide sensitivity of the MRSA after BL460nm irradiance. The bacterial cells were tested for the MICH2O2 value under different BL460nm intensities and treatment duration. The temperature of the irradiated samples was monitored during the treatment. Squares with red labeling indicate the values of MICH2O2 for each experimental condition.
Figure 4.
The changes in hydrogen peroxide sensitivity of the MRSA after BL460nm irradiance. The bacterial cells were tested for the MICH2O2 value under different BL460nm intensities and treatment duration. The temperature of the irradiated samples was monitored during the treatment. Squares with red labeling indicate the values of MICH2O2 for each experimental condition.
Figure 5.
The test of skin sensitivity treated with the combined therapy during 15-day treatment. A skin area of 2x2 cm was exposed to the BL460nm at 100mW/cm2 in 5 min, then was swiped with 0.75% H2O2. The whole procedure was repeated daily for 15 days. The treated skin area was pictured and recorded for rashness, change in color, and skin irritation caused by the combined therapy.
Figure 5.
The test of skin sensitivity treated with the combined therapy during 15-day treatment. A skin area of 2x2 cm was exposed to the BL460nm at 100mW/cm2 in 5 min, then was swiped with 0.75% H2O2. The whole procedure was repeated daily for 15 days. The treated skin area was pictured and recorded for rashness, change in color, and skin irritation caused by the combined therapy.
Figure 6.
Survival analysis of mice underwent different doses of cyclophosphamide injection. A) The symbol a indicates the day of the first treatment, b indicates the second cyclophosphamide treatment, and c indicates the day of S. aureus being infected on the skin. B-D) the open skin sites after the second dose of cyclophosphamide injection and the initial application of MRSA. E-F) The injured skin area after five days of cyclophosphamide treatment and MRSA infection; B and E: 150 mg/kg cyclophosphamide, C and F: 350 mg/kg cyclophosphamide, D and G: 350 mg/kg cyclophosphamide.
Figure 6.
Survival analysis of mice underwent different doses of cyclophosphamide injection. A) The symbol a indicates the day of the first treatment, b indicates the second cyclophosphamide treatment, and c indicates the day of S. aureus being infected on the skin. B-D) the open skin sites after the second dose of cyclophosphamide injection and the initial application of MRSA. E-F) The injured skin area after five days of cyclophosphamide treatment and MRSA infection; B and E: 150 mg/kg cyclophosphamide, C and F: 350 mg/kg cyclophosphamide, D and G: 350 mg/kg cyclophosphamide.
Figure 7.
Wound healing recovery of an abrasion skin infection model. A) The skin healing model in mice was treated by only LBL460nm, H202, fusidic acid. Day 1 indicated the initiation of treatment. B) Wound healing after 15 days of different treatment was recorded on a daily basis. Data were expressed as mean and standard error of the mean (SEM) of at least 10 animals per group.
Figure 7.
Wound healing recovery of an abrasion skin infection model. A) The skin healing model in mice was treated by only LBL460nm, H202, fusidic acid. Day 1 indicated the initiation of treatment. B) Wound healing after 15 days of different treatment was recorded on a daily basis. Data were expressed as mean and standard error of the mean (SEM) of at least 10 animals per group.
Figure 8.
Statistical analysis of the healing efficacy of different therapies in the skin model and its correlation with the number of MRSA colonies on the lesion skin. a) Statistically significant differences among the treated groups of animals. Each circular point represents data from one animal. (b) Image of MRSA colonies collected from the wounds when smeared and cultured on Baird-Parker agar. The one-way ANOVA and Tukey
post hoc test were used to determine the statistically significant comparison between the two groups. Those pairs that show statistical significance were highlighted. *:
p<0,0332; ** :
p <0,0021; *** :
p <0,0002; ****:
p< 0,0001. Each point in the data represented an individual animal. Normality of data was checked by Shapiro-Wilk test in Graphpad Prism (
Supplenmetal Table 1 and Supplemental Figure 2).
Figure 8.
Statistical analysis of the healing efficacy of different therapies in the skin model and its correlation with the number of MRSA colonies on the lesion skin. a) Statistically significant differences among the treated groups of animals. Each circular point represents data from one animal. (b) Image of MRSA colonies collected from the wounds when smeared and cultured on Baird-Parker agar. The one-way ANOVA and Tukey
post hoc test were used to determine the statistically significant comparison between the two groups. Those pairs that show statistical significance were highlighted. *:
p<0,0332; ** :
p <0,0021; *** :
p <0,0002; ****:
p< 0,0001. Each point in the data represented an individual animal. Normality of data was checked by Shapiro-Wilk test in Graphpad Prism (
Supplenmetal Table 1 and Supplemental Figure 2).