Submitted:
26 October 2023
Posted:
01 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Preparation of Honey Bee Drone Sperm
- -
- start at 3 °C;
- -
- from 3 °C to –5 °C at a speed of 3 °C/min;
- -
- hold at –5 °C for 1 min;
- -
- from –5 to –12 °C at a speed of 1 °C/min;
- -
- hold at –12 °С for 9 min;
- -
- from –12 °С to –50 °С at a speed of 3 °C/min;
- -
- after –50 °C, drop the free temperature to –196 °С.
2.3. Instrumental Insemination of Queen Bees
2.4. Morphometric Analysis
2.5. Genetic Analysis
3. Results
3.1. Assessment of Morphometric Parameters
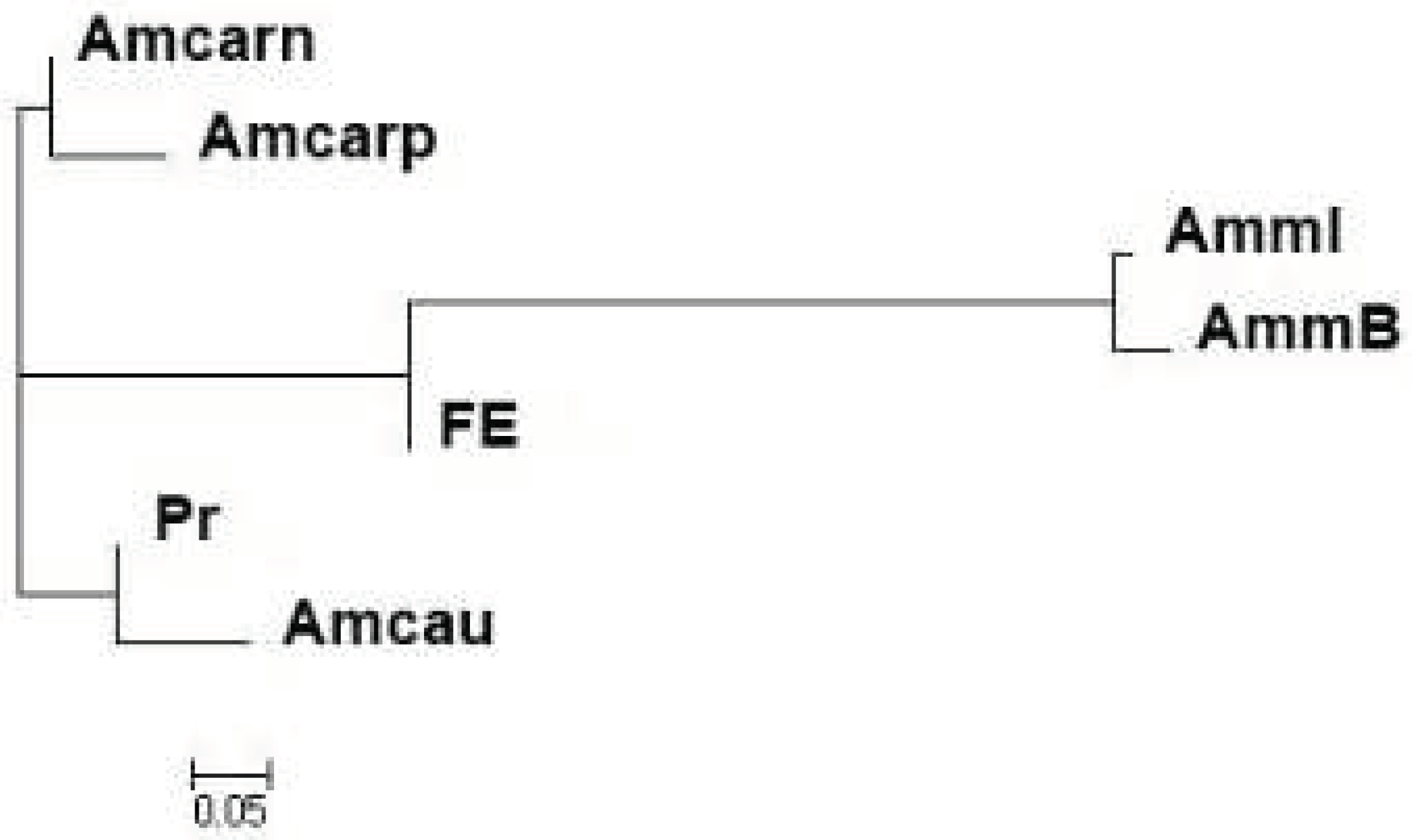
3.2. Assessment of the Genetic Structure of the Apis Mellifera Samples
3.3. Evaluation of Cryopreserved Sperm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Edesi, J.; Tolonen, J.; Ruotsalainen, A.L.; Aspi, J.; Häggman, H. Cryopreservation enables long-term conservation of critically endangered species Rubus humulifolius. Biodivers Conserv 2020, 29, 303–314. [Google Scholar] [CrossRef]
- Kaviani, B.; Kulus, D. Cryopreservation of Endangered Ornamental Plants and Fruit Crops from Tropical and Subtropical Regions. Biology 2022, 11, 847. [Google Scholar] [CrossRef]
- Leroy, G.; Boettcher, P.; Besbes, B.; Danchin-Burge, C.; Baumung, R.; Hiemstra, S.J. Cryoconservation of Animal Genetic Resources in Europe and Two African Countries: A Gap Analysis. Diversity 2019, 11, 240. [Google Scholar] [CrossRef]
- Paillard, M.; Rousseau, A.; Giovenazzo, P.; Bailey, J.L. Preservation of Domesticated Honey Bee (Hymenoptera: Apidae) Drone Semen. J Econ Entomol 2017, 110, 1412–1418. [Google Scholar] [CrossRef]
- Harbo, J.R. Survival of honey bee (Hymenoptera, Apidae) spermatozoa after 2 years in liquid-nitrogen (-196°C). Ann. Entomol. Soc. Am. 1983, 76, 890–891. [Google Scholar] [CrossRef]
- Hopkins, B. K.; Herr, C.; Sheppard, W.S. Sequential generations of honey bee (Apis mellifera) queens produced using cryopreserved semen. Reproduction, Fertility and Development 2012, 24, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Wegener, J.; May, T.; Kamp, G.; Bienefeld, K. New methods and mediafor the centrifugation of honey bee (Hymenoptera: Apidae) drone semen. Journal of Economic Entomology 2014, 107, 47–53. [Google Scholar] [CrossRef]
- Dadkhah, F.; Nehzati-Paghaleh, G.; Zhandi, M.; Hopkins, B.K. Preservation of honey bee spermatozoa using egg yolk and soybean lecithin-based semen extenders and a modified cryopreservation protocol. Journal of Apicultural Research 2016, 55, 279–283. [Google Scholar] [CrossRef]
- Gul, А.; Nuray, S.; Onal, A.G.; Hopkins, B.K.; Sheppard, W.S. Effects of diluents and plasma on honey bee (Apis mellifera L.) drone frozen-thawed semen fertility. Theriogenology 2017, 101, 109–113. [Google Scholar] [CrossRef]
- Alcay, S.; Cakmak, S.; Cakmak, I.; Mulkpinar, E.; Gokce, E.; Ustuner, B.; Sen, H.; Nur, Z. Successful cryopreservation of honey bee drone spermatozoa with royal jelly supplemented extenders. Cryobiology 2019, 87, 28–31. [Google Scholar] [CrossRef]
- Melnichenko, A.N.; Vavilov, Y.L. Long term storage of drone semen by freezing in liquid nitrogen. SP S Kennan, Soviet Agric. Sci 1976, 1, 34–36. (In Russian) [Google Scholar]
- Kakpakov, V.T. Center for instrumental (artificial) insemination of honey bees (CIIHB). Veterinary pathology, 2007; 1, 28–30. (In Russian) [Google Scholar]
- Gulov, A.N. Problems of honeybee conservation of genetic resources. Bee Journal 2018, 6, 22–25. (In Russian) [Google Scholar]
- Gulov, A.N.; Bragina, E.E. Cryopreservation effects on drone swarm Apis mellifera L. morphometric parameters and infrastructure. Veterinary medicine, animal science, biotechnology 2022, 1, 75–86. [Google Scholar] [CrossRef]
- Hinting, A.; Comhaire, F.; Vermeulen, L.; Dhort, M.; Vermeulen, A.; Vanderberhove, D. Value of sperm characteristics and the result of in vitro fertilization for predicting the outcome of assisted reproduction. Int J Androl. 1990, 13, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Duncan, W.W.; Glew, M.J.; Wang, X.; Flaherty, S.P.; Matthews, C.D. Prediction of fertilization rates from semen variables. Fertil Steril 1993, 59, 1233–8. [Google Scholar] [CrossRef]
- Gulov, A.N. , Laskin, A. S. Honey diluent for cryopreservation of honey bee drone sperm. Genetics and animal breeding 2020, 4, 27–36. (In Russian) [Google Scholar] [CrossRef]
- Ruttner, F. Biogeography and Taxonomy of Honeybees. Springer, Berlin, 1988; 291 p. [CrossRef]
- Cridland, J.M.; Tsutsui, N.D.; Ramírez, S.R. The complex demographic history and evolutionary origin of the western honey bee, Apis mellifera. Genome Biol. Evol. 2017, 9, 457–472. [Google Scholar] [CrossRef]
- Harbo, J.R.; Harris, J.W. Selecting honey bees for resistance to Varroa jacobsoni. Apidologie 1999, 183–196. [Google Scholar] [CrossRef]
- Hoppe, A.; Du, M.; Bernstein, R.; Tiesler, F.-K.; Kärcher, M.; Bienefeld, K. Substantial Genetic Progress in the International Apis mellifera carnica Population Since the Implementation of Genetic Evaluation. Insects 2020, 11, 768. [Google Scholar] [CrossRef]
- Maucourt, S.; Fortin, F.; Robert, C.; Giovenazzo, P. Genetic Progress Achieved during 10 Years of Selective Breeding for Honeybee Traits of Interest to the Beekeeping Industry. Agriculture 2021, 11, 535. [Google Scholar] [CrossRef]
- Sprau, L.; Traynor, K.; Rosenkranz, P. Honey bees (Apis mellifera) preselected for Varroa sensitive hygiene discriminate between live and dead Varroa destructor and inanimate objects. Sci Rep 2023, 13, 10340. [Google Scholar] [CrossRef]
- Jensen, A.B.; Palmer, K.A.; Boomsma, J.J.; Pedersen, B.V. Varying degrees of Apis mellifera ligustica introgression in protected populations of the black honeybee, Apis mellifera mellifera, in northwest Europe. Mol. Ecol. 2005, 14, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Oleksa, A.; Chybicki, I.; Tofilski, A.; Burczyk, J. Nuclear and mitochondrial patterns of introgression into native dark bees (Apis mellifera mellifera) in Poland. J Apicult Res. 2011, 50, 116–129. [Google Scholar] [CrossRef]
- Alpatov, W.W. Biometrical studies on variation and races of the honey bee (Apis mellifera L.). Quarterly Review of Biology 1929, 4, 1–58. [Google Scholar] [CrossRef]
- Meixner, M.D.; Pinto, M.A.; Bouga, M.; Kryger, P.; Ivanova, E.; Fuchs, S. Standard methods for characterising subspecies and ecotypes of Apis mellifera. Journal of Apicultural Research 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Garnery, L.; Solignac, M.; Celebrano, G.; Cornuet, J.-M. A simple test using restricted PCR-amplified mitochondrial DNA to study the genetic structure of Apis mellifera L. Experientia 1993, 49, 1016–1021. [Google Scholar] [CrossRef]
- Bertrand, B.; Alburaki, M.; Legout, H.; Moulin, S.; Mougel, F.; Garnery, L. MtDNA COI-COII marker and drone congregation area: an efficient method to establish and monitor honeybee (Apis mellifera L.) conservation centers. Mol Ecol Resour. 2015, 15, 673–683. [Google Scholar] [CrossRef]
- Solignac, M.; Vautrin, D.; Loiseau, A.; Mougel, F.; Baudry, E.; Estoup, A.; Garnery, L.; Haberl, M. ; Cornuet, J-M. Five hundred and fifty microsatellite markers for the study of the honeybee (Apis mellifera L.) genome. Molecular Ecology Notes 2003, 3, 307–311. [Google Scholar] [CrossRef]
- Parejo, M.; Wragg, D.; Gauthier, L.; Vignal, A.; Neumann, P.; Neuditschko, M. Using Whole-Genome Sequence Information to Foster Conservation Efforts for the European Dark Honey Bee, Apis mellifera mellifera. Frontiers in Ecology and Evolution 2016, 4, 583. [Google Scholar] [CrossRef]
- Chapman, N.C.; Harpur, B.A.; Lim, J.; Rinderer, T.E.; Allsopp, M.H.; Zayed, A.; Oldroyd, B.P. A SNP test to identify Africanized honeybees via proportion of “African” ancestry. Mol. Ecol. Resour. 2015, 15, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Momeni, J.; Parejo, M.; Nielsen, R.O.; Langa, J.; Montes, I.; Papoutsis, L.; Farajzadeh, L.; Bendixen, C.; Căuia, E.; et al. Authoritative subspecies diagnosis tool for European honey bees based on ancestry informative SNPs. BMC Genomics 2021, 22, 101. [Google Scholar] [CrossRef]
- Petrov, E. M. Bashkirskaya bortevaya pchela. Ufa, Bashkirskoe knizhnoe izdatel'stvo, 1983. 200 p. (In Russian).
- Krivcov, N.I.; Sokol'skij, S.S.; Lyubimov, E.M. Serye gornye kavkazskie pchyoly. Nauchnoe izdanie, Sochi, 2009; 192 p. (In Russian).
- Johnson, R.M.; Dahlgren, L.; Siegfried, B.D.; Ellis, M.D. Effect of in-hive miticides on drone honey bee survival and sperm viability. Journal of Apicultural Research 2013, 52, 88–95. [Google Scholar] [CrossRef]
- 37. Bienkowska, М; Panasiuk, B.; Loc, K. Influence of the age of honey bee queens and dose of semen on condition of instrumentally inseminated queens kept in cages with 25 worker bees in the colonies. Journal of Apicultural Science 2008, 52, 23–33.
- Ruttner, F. Instrumental Insemination of the Queen Bee. Bucharest, Apimondia, 1975; 127 p.
- Bilash, G. D., Krivcov N. I. Selekciya pchyol. Moscow, Agropromizdat, 1991; 304 p. (In Russian).
- Bouga, M.; Alaux, C.; Bienkowska, M.; Büchler, R.; Carreck, N.L.; Cauia, E.; Chlebo, R.; Dahle, B.; Dall'Olio, R.; De la Rúa, P.; Gregorc, A.; Ivanova, E.; Kence, A.; Kence, M.; Kezic, N.; Kiprijanovska, H.; Kozmus, P.; Kryger, P.; Le Conte, Y.; Lodesani, M.; Manuel, A.; Siceanu, A.; Soland, G.; Uzunov, A.; Wilde, J. A review of methods for discrimination of honey bee populations as applied to European beekeeping. Journal of Apicultural Research 2011, 50, 51–84. [Google Scholar] [CrossRef]
- Frunze, O.; Brandorf, A.; Kang, E.-J.; Choi, Y.-S. Beekeeping Genetic Resources and Retrieval of Honey Bee Apis mellifera L. Stock in the Russian Federation: A Review. Insects 2021, 12, 684. [Google Scholar] [CrossRef] [PubMed]
- Cobey, S.W. Comparison studies of instrumentally inseminated and naturally mated honey bee queens and factors affecting their performance. Apidologie 2007, 38, 390–410. [Google Scholar] [CrossRef]
- Wegener, J.; Bienefeld, K. Toxicity of cryoprotectants to honey bee semen and queens. Theriogenology 2012, 77, 600–607. [Google Scholar] [CrossRef]
- Woyke, J.; Jasinski, Z. Influence of the number of attendant workers on the number of spermatozoa entering the spermatheca of instrumentally inseminated queens kept outdoors in mating nuclei. J. Apicultural Science 1982, 21, 129–133. [Google Scholar] [CrossRef]
- Mannapov, A.G.; Lyakhov, V.V.; Brovarsky, V.D. Evaluation of instrumental insemination technologies. Bee Journal 2013, 6, 21. (In Russian) [Google Scholar]
- Savushkina, L.N.; Borodachev, A.V. Biological signs of Prioksky bees. Bee Journal 2014, 10, 10–12. (In Russian) [Google Scholar]
- Oleksa, A.; Tofilski, A. Wing geometric morphometrics and microsatellite analysis provide similar discrimination of honey bee subspecies. Apidologie 2015, 46, 49–60. [Google Scholar] [CrossRef]
- Bicudo de Almeida-Muradian, L.; Barth, O.M.; Dietemann, V.; Eyer, M.; da Silva de Freitas, A.; Martel, A.-C.; Marcazzan, G.L.; Marchese, C.M.; Mucignat-Caretta, C.; Pascual-Maté, A.; Reybroeck, W.; Sancho, M.T.; Gasparotto Sattler, J.A. Standard methods for Apis mellifera honey research. Journal of Apicultural Research 2020, 59, 1–62. [Google Scholar] [CrossRef]
- El-Sheshtawy, R.I.; El-Badry, D.A. Natural honey as a cryoprotectant to improve Arab stallion post-thawing sperm parameters. Asian Pacific Journal of Reproduction 2016, 5, 331–334. [Google Scholar] [CrossRef]
- Malik, A.; Fauzi, R.; Zakir, M.I. Subtitusi Madu Asli Pengganti Gliserol dalam Pembekuan pada Kualitas Pasca-thawing Spermatozoa Sapi Bali. Acta veterinaria indonesiana 2017, 5, 98–104. [Google Scholar] [CrossRef]
- Kandiel, M.M.M.; El-Khawagah, A.R.M.; Hussein, M.N.A.; Caoet, X. Quantitative Ultrastructure Evaluation of Egyptian Buffalo Bull Frozen-Thawed Spermatozoa under the Effect of Honey. Scholars Journal of Agriculture and Veterinary Sciences 2019, 6, 92–98. [Google Scholar] [CrossRef]
- Shikh Maidin, M.; Padlan, M.H.; Azuan, S.A.N.; Jonit, R.; Mohammed, N.H.; Abdullah, R. Supplementation of Nigella sativa Oil and Honey Prolong the Survival Rate of Fresh and Post-Thawed Goat Sperms. Tropical Animal Science Journal 2018, 41, 94–99. [Google Scholar] [CrossRef]
- Zaghloul, A.A. Relevance of Honey Bee in Semen Extender on the Quality of Chilled-Stored Ram Semen. J. Animal and Poultry Prod. 2017, 8, 1–5. [Google Scholar] [CrossRef]
- Syazana, N.S.; Hashida, N.H.; Majid, A.M.; Durriyah Sharifah, H.A.; Kamaruddin, M.Y. Effects of Gelam Honey on Sperm Quality and Testis of Rat. Sains Malaysiana 2011, 40, 1243–1246. [Google Scholar]
- Zoheir, K.M.A.; Harisa, G.I.; Abo-Salem, O.M.; Ahmad, S.F. Honey bee is a potential antioxidant against cyclophosphamide induced genotoxicity in albino male mice. Pak. J. Pharm. Sci. 2015, 28, 973–981. [Google Scholar]
- Fanni, N.A.; Santanumurti, M.B.; Suprayogi, T.W.; Bendryman, S.S. Quality enhancement of cryopreserved spermatozoa of sutchi catfish (Pangasianodon hypophthalmus) with honey addition. Iraqi Journal of Veterinary Sciences 2018, 32, 231–236. [Google Scholar] [CrossRef]
- Ogretmen, F.; İnanan, B.E. Evaluation of cryoprotective effect of turkish pine honey on common carp (Cyprinus Carpio) spermatozoa. CryoLetters 2014, 35, 427–437. [Google Scholar] [PubMed]
- Fakhrildin, M.B.; Alsaadi, R.A. Honey Supplementation to Semen-Freezing Medium Improves Human Sperm Parameters Post-Thawing. Journal of Family and Reproductive Health 2014, 8, 27–31. [Google Scholar]
| Sampling region | Sample name | N | Subspecies |
|---|---|---|---|
| The Republic of Bashkortostan, Iglinsky district | AmmI | 10 | A. m. mellifera |
| The Republic of Adygea, Maykop | Amcarn | 8 | A. m. carnica |
| The Republic of Adygea, Maykop | Amcarp | 10 | A. m. carpatica |
| Far East, Primorsky Krai (Kondratenovka and Tichoreshnoe villages) | FE | 10 | Hybrid of A. m. mellifera, A. m. carpatica, and A. m. caucasica |
| The Republic of Bashkortostan, Burzyansky district, Shulgan-Tash Nature Reserve | AmmB | 9 | A. m. mellifera |
| Ryazan region, Rybnoye, The Federal Beekeeping Research Centre apiary | Pr | 10 | Prioksky bees, hybrid of A. m. mellifera and A. m. caucasica |
| Krasnodar Krai, Adler, Krasnopolyansk experimental beekeeping station | Amcau | 19 | A. m. caucasica |
| Sample | N | CI, % | Lx, mm | Wt3, mm |
| Pr | 10 | 50.5 | 6.88 | 4.84 |
| Standard values for Prioksky bees | 55–60 | 6.6–6.9 | 4.6–5.0 | |
| AmmI | 10 | 52.9 | 6.17 | 4.92 |
| AmmB | 9 | 57.0 | 6.21 | 4.91 |
| Standard values for A. m. mellifera | 60–65 | 6.0–6.4 | 4.8–5.2 | |
| Amcarp | 10 | 41.9 | 6.63 | 4.81 |
| Standard values for A. m. carpatica | 33–43 | 6.3–7.0 | 4.4–5.1 | |
| Amcarn | 8 | 39.4 | 6.71 | 4.90 |
| Standard values for A. m. carnica | <40.0 | 6.4–6.8 | 4.7–5.1 | |
| FE | 10 | 43.9 | 6.53 | 4.97 |
| Standard values for Far Eastern bees | 28–60 | 6.1–6.8 | 4.6–5.4 | |
| Amcau | 15 | 52.4 | 7.01 | 4.80 |
| Standard values for A. m. caucasica | 50–55 | 6.7–7.2 | 4.4–5.0 | |
| A. m. mellifera* | 61.4 | 6115 | - | |
| A. m. carnica* | 51.2 | 6458 | - | |
| A. m. caucasica* | 54.7 | 6976 | - |
| Sample | N | FL, mm |
FW, mm |
TI, % | Ls3, mm | Lwm, mm | Lwm, mm | Lwmd, mm | Lt3, mm |
| Pr | 10 | 9.43 | 3.16 | 56.5 | 2.86 | 1.39 | 2.48 | 0.29 | 2.29 |
| AmmI | 10 | 9.34 | 3.09 | 54.6 | 2.92 | 1.50 | 2.55 | 0.22 | 2.36 |
| AmmB | 9 | 9.37 | 3.09 | 55.6 | 2.91 | 1.50 | 2.53 | 0.21 | 2.33 |
| Amcarp | 10 | 9.30 | 3.12 | 55.7 | 2.82 | 1.39 | 2.46 | 0.29 | 2.25 |
| Amcarn | 8 | 9.36 | 3.14 | 55.1 | 2.85 | 1.40 | 2.50 | 0.29 | 2.27 |
| FE | 10 | 9.37 | 3.16 | 55.5 | 2.93 | 1.44 | 2.53 | 0.27 | 2.34 |
| Amcau | 15 | 9.40 | 3.13 | 56.8 | 2.85 | 1.39 | 2.47 | 0.31 | 2.27 |
| Mean | 9.37 | 3.13 | 55.7 | 2.88 | 1.43 | 2.50 | 0.27 | 2.30 | |
| Min | 9.30 | 3.09 | 54.6 | 2.82 | 1.39 | 2.46 | 0.21 | 2.25 | |
| Max | 9.43 | 3.16 | 56.8 | 2.93 | 1.50 | 2.55 | 0.31 | 2.36 |
| Sample | Allelic variant of tRNAleu-COII | Gene pool of С/О | Gene pool of М |
|---|---|---|---|
| M lineage | 98 PQQ, 38 PQQQ | 0.016 | 0.984 |
| C/O lineage | 120 Q | 0.993 | 0.007 |
| AmmI | 10 PQQ | 0.184 | 0.816 |
| Amcarn | 7 Q, 1 PQQ | 0.946 | 0.054 |
| Amcarp | 10 Q | 0.967 | 0.033 |
| FE | 10 Q | 0.737 | 0.263 |
| AmmB | 9 PQQ | 0.080 | 0.920 |
| Pr | 10 Q | 0.986 | 0.014 |
| Amcau | 19 Q | 0.988 | 0.012 |
| AmmI | Amcarn | Amcarp | FE | AmmB | Pr | Amcau | |
|---|---|---|---|---|---|---|---|
| AmmI | 0.0000 | * | * | * | NS | * | * |
| Amcarn | 0.4174 | 0.0000 | NS | * | * | NS | * |
| Amcarp | 0.4602 | 0.0389 | 0.0000 | * | * | * | * |
| FE | 0.2546 | 0.1140 | 0.1959 | 0.0000 | * | * | * |
| AmmB | 0.0133 | 0.4682 | 0.5177 | 0.3030 | 0.0000 | * | * |
| Pr | 0.4414 | 0.0944 | 0.1606 | 0.2014 | 0.4677 | 0.0000 | * |
| Amcau | 0.5736 | 0.2466 | 0.3267 | 0.4059 | 0.5843 | 0.1311 | 0.0000 |
| Indicator | Frozen–thawed sperm (n = 27) | Fresh sperm (n = 100) | ||||
| M ± m (min – max) | σ | Cv, % | M ± m (min – max) | σ | Cv, % | |
| Motility, % | 2.2 ± 0.6 (0–11.5) | 3.1 | 141.04 | 55.0 ± 2.6 (0–99.8) | 26.5 | 48.3 |
| Viability, % | 64.0 ± 1.8 (41.5–83.7) | 9.6 | 14.87 | 84.3 ± 1.2 (40–99.9) | 12.2 | 14.5 |
| No. of AI queens | Concentration of sperm in the spermatic receptacle, millions/μL | Presence of sperm in paired oviducts |
| 1 | 2.4 ± 0.25 (2.2–2.7) | absent |
| 2 | 0.9 ± 0.3 (0.6–1.2) | absent |
| 3 | 4.4 ± 0.3 (4.1–4.7) | absent |
| 4 | 0.22 ± 0.02 (0.2–0.25) | sperm traces |
| 5 | 0 | large amount of sperm |
| 6 | 0 | large amount of sperm |
| 7 | 0 | large amount of sperm |
| 8 | 0 | absent |
| 9 | 0 | absent |
| 10 | 0 | absent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





