1. Introduction
Visceral leishmaniasis (VL), also called kala-azar, is a serious parasitic infection caused by the protozoa
Leishmania donovani or
L. infantum. These parasites are transmitted from host to host by blood-feeding phlebotomine female sandflies (
Diptera:
Psychodidae). Patients with VL present with chronic fever, abdominal swelling and weight loss, and progressively deteriorate. If left untreated, VL will eventually be fatal in 95% of cases [
1]. The eastern African region has the highest VL case load in the world, where several countries are endemic transmission of
L. donovani [
2]. VL is mainly found in rural areas with impoverished populations suffering from poor health and high rates of malnutrition. Consequently, the burden of VL causes a vicious circle of disease and poverty in these communities.
In Kenya, a major VL focus is located in West Pokot County, situated in the northwest on the border with Uganda. The population of West Pokot are mainly pastoralists of the Pokot tribe, and VL (locally referred to as
termes) mostly affects children and adolescents below the age of 15 years [
3]. VL is generally considered to be anthroponotic in West Pokot, but due to a paucity of research into
L. donovani infections in wild or domestic animals, it is unknown whether a zoonotic reservoir exists. The semi-arid climate in this lowland area, with seasonal rains and sparse vegetation with
Acacia trees, creates a favorable environment for
Phlebotomus martini, the local sandfly vector transmitting
L. donovani [
4,
5].
P. martini is known to breed in the ventilation shafts of termite mounds [
6,
7]. Besides this breeding site preference, many characteristics of
P. martini, such as habitat aspects and blood feeding behavior, are still poorly defined, complicating the design of effective vector control strategies. Management of VL in West Pokot is further hindered by the limited availability and accessibility of diagnostic testing and treatment facilities, and the erratic resourcing of these centers [
8]. The current passive case detection is insufficient to reduce the disease burden, as demonstrated by the significant increase in annual VL incidence in the area in recent years: in 2022, the VL treatment center at Kacheliba Sub-County Hospital registered 600 confirmed VL cases, compared to 245 cases in 2019 (unpublished data, patient records from Kacheliba Sub-County Hospital, West Pokot County, Kenya).
Effective interventions to combat VL transmission warrant better characterization of associated risk factors. Apart from the nutritional, immunological and genetic status of the human host, the risk of acquiring VL is dependent on the presence of and exposure to its sandfly vector [
3,
9,
10]. Although multiple studies have investigated behavioral and environmental VL determinants in endemic regions in Ethiopia and Sudan, where
P. orientalis is responsible for disease transmission, these results may not be translatable to the context of West Pokot due to different vector biology [
11,
12]. However, reliable data on VL determinants in West Pokot are scarce. A study from Kolaczinski
et al. described a number of VL-associated factors, including increased risk of infection due to low socio-economic status and protective effects of mosquito net ownership and sleeping near livestock [
13]. To date, these findings have not been confirmed by other studies, nor have they been addressed in more detail. Therefore, this case-control study aimed to further explore potential behavioral, environmental and household factors that could increase the risk of developing VL in West Pokot, Kenya.
2. Materials and Methods
2.1. Ethics
Ethics clearance was obtained from the Amref Health Africa Ethics and Scientific Review Committee (ESRC – ref. ESRC P1196/2022) and a research license was obtained from the National Commission for Science, Technology and Innovation (NACOSTI – ref. 791964). All participants, or the parents or legal guardians of minors, provided written informed consent before enrolment into the study. Collected data were anonymized.
2.2. Study setting
This study was performed in West Pokot County, Kenya, which lies on a plateau at 1000 to 1500 m above sea level, bordered by the Eastern Rift Valley in the east. The area experiences a long rainy season from April until June, and short rains in November and December. West Pokot has a population of 621,000 (census 2019), of which 94.9% live in rural areas with limited infrastructure and health facilities [
14]. About half the West Pokot population is below 15 years of age; 36% of the total population has not received any formal education [
14]. Pastoralism is the most frequent occupation, practiced mostly by adolescent and adult males, while females are generally responsible for taking care of the household and children. Pastoralist villages consist of several separated compounds of houses, called
manyattas, surrounded by a fence of thorny acacia branches; each
manyatta is inhabited by several family households.
West Pokot is the major focus of endemic VL transmission in Kenya [
8]. The most important regional reference center for VL diagnosis and treatment is the Kacheliba Sub-County Hospital, founded by Médecins Sans Frontières in 2006 and later handed over to the Ministry of Health (MoH), with support from the Drugs for Neglected Diseases initiative (DNDi) [
3].
2.3. Participant enrollment
This study had a matched case-control design. Following a pragmatic sampling approach, participants were enrolled from November 8, 2022, to January 8, 2023. Cases were VL patients not previously treated for this disease, recruited at the VL treatment center at Kacheliba Sub-County Hospital. Patients with clinical symptoms of VL, including hepatosplenomegaly, chronic fever and wasting, were tested with the IT LEISH (Bio-Rad, Hercules, USA) rK39 rapid diagnostic test (RDT) for the presence of anti-Leishmania antibodies in finger prick blood, following the hospital’s standard diagnostic practice. In case of a positive rK39 RDT, the patient was asked to participate in the study through an informed consent procedure.
To ensure sufficient analytical power in case of a potentially low recruitment number of VL cases, this study aimed to recruit 2 to 4 healthy controls per enrolled VL case [
15]. Where possible, controls were matched by sex, age and village. A study team traveled to villages in West Pokot from which a VL case originated, where a house was randomly selected by spinning a pen at a central point in the village. At the selected house, an eligible household member was chosen to match the sex and age (within a range of ±2 years) of the VL case from that village. A matched candidate control participant had to be free of VL-associated symptoms, and have no history of VL and no history of malaria in the preceding 2 weeks. After providing informed consent, a finger prick blood sample was collected from the participant and tested with rK39 RDT. In case of a negative test result, the control participant was interviewed using a structured questionnaire (see below). If the rK39 RDT was positive, the participant was referred for care at the Kacheliba Hospital and another household member was randomly selected for participation in the study. After the interview, a pen was spun in front of the house to select the next house for recruiting a healthy volunteer. These household visits were repeated until at least two healthy controls per VL case from each selected village were recruited.
2.4. Questionnaire
A structured questionnaire was administered to each study participant by a trained interviewer (Supplementary File S1). The survey had an exploratory design and included potential determinants for VL based on available literature. Questions related to housing characteristics, day- and night-time activities, sleeping habits, and the house yard and its immediate surroundings, including the presence of domestic animals. When the answer to a question was expected to vary depending on the season, data were obtained separately for the dry and rainy season. For questions about the presence of tree species in or surrounding the house yard, the vernacular Pokot tree names reported by the participant were recorded. During data analysis, the scientific tree species names corresponding to these Pokot names were retrieved from a previous publication [
16].
All interviewers were experienced in administering health and socioeconomic surveys and received explanations and instructions for each individual question in the study’s questionnaire before the start of recruitment. The questionnaire was in English and was first piloted by the interviewers on VL patients at the Kacheliba Hospital before the study start. The interviews were conducted in Swahili or the local Pokot language through the assistance of a local interpreter. For participants below age 15 years, the parent or legal guardian was allowed to assist or answer on behalf of the child. Answers provided by the participants were entered directly into the smartphone application KoboCollect (version v2022.2.3, KoboToolBox, Cambridge, MA, USA).
2.5. Data analysis
Questionnaire data were exported from the KoboCollect online database into Microsoft Excel 2016. The Excel file was next imported into the statistical software IBM SPSS Statistics (version: 28.0, Armonk, NY, USA) for data processing and analysis. Associations between determinants and VL were studied using univariate logistic regression and expressed as odds ratios (ORs). If a statistically significant difference in age and/or sex was found between cases and controls, adjustment for these variables was applied. P-values of the found associations were obtained using the Wald test. Associations with a p-value of <0.05 were considered statistically significant. In case of missing data for a particular variable, the participant was excluded from the analysis of this variable.
3. Results
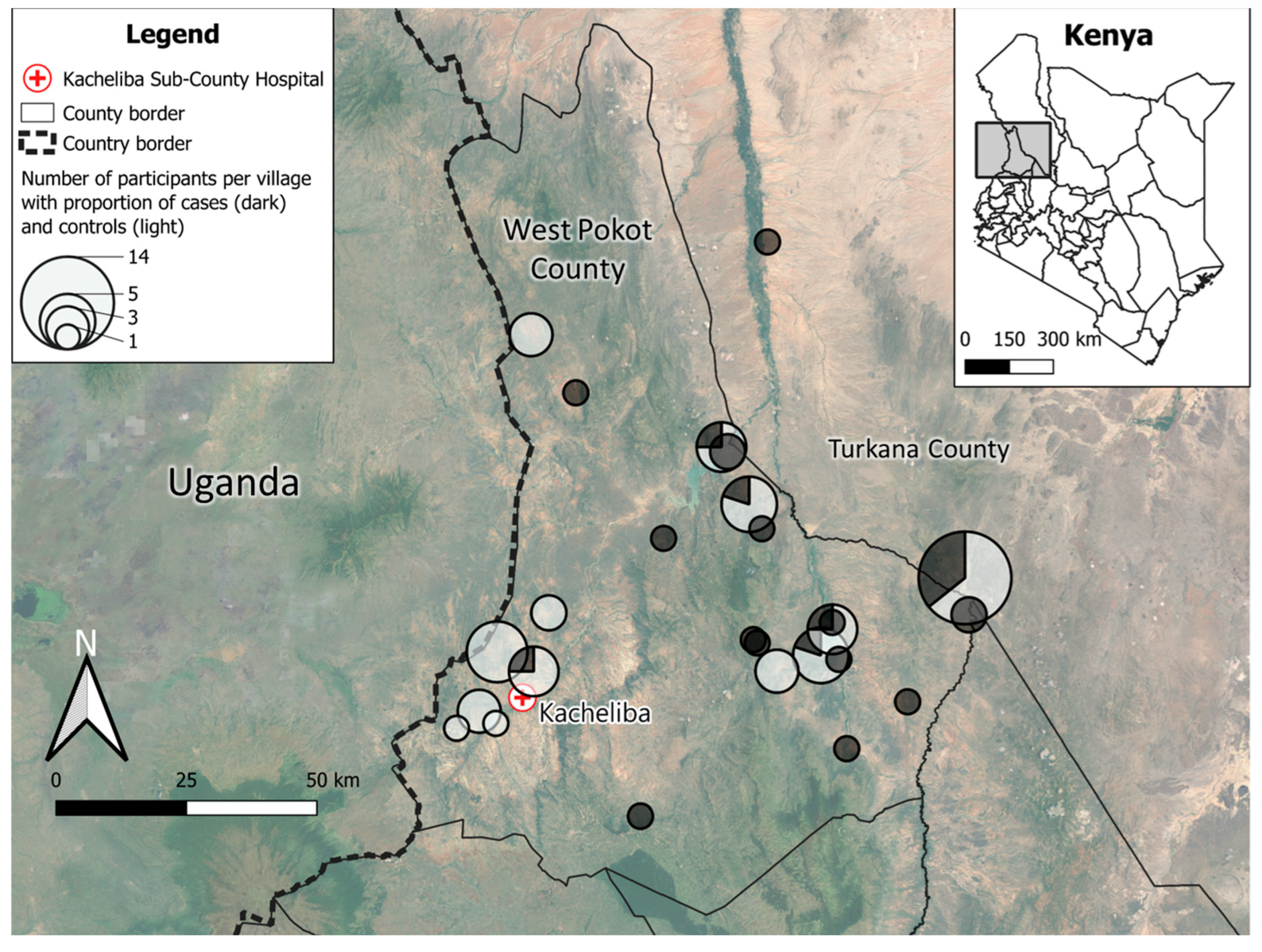
Between November 2022 and January 2023, a total of 36 rK39 RDT-confirmed VL patients were recruited at the Kacheliba Sub-County Hospital. 50 healthy controls were recruited from villages in West Pokot (see
Figure 1). The majority of enrolled VL cases were from the northern and eastern parts of West Pokot, which were difficult to reach due to the distance from the Kacheliba Hospital, as well as security concerns. As a result, a number of healthy controls were not village-matched with cases but recruited in villages less than half a day’s travel from Kacheliba. All recruited cases and controls (N=86) were included in the analysis.
Participants came from a total of 39 villages, of which 37 were situated in West Pokot County, 1 in Turkana County and 1 in Trans-Nzoia County (
Table 1). Cases most frequently lived in Akulo (14%), Akorikwang, Amoler and Turkwel villages (all 6%), while 46% of controls were recruited in Akulo (18%), Asilong (12%), Dungdung and Nasolot villages (both 8%). Cases had a median age of 11 years (interquartile range [IQR]: 5−20 years), while controls (median 6 years, IQR: 2−9 years) were significantly younger (p=0.001). The proportion of females was similar (50% and 47%, resp.,
Table 1).
Due to the unmatched age distribution of cases and controls, associations of all other variables with VL were studied after adjustment for age. All statistically significant associations (p<0.05) are shown in
Table 2. A complete overview of the univariate analysis with age correction is shown in
Supplementary Table S1.
The household size of cases and controls was similar (mean 7.27 and 7.64 household members, resp.). Among those of school-going age and above, cases were more frequently illiterate than controls (41% vs. 16%, resp.), of which the majority (76%) were still attending primary school. However, education level was not significantly associated with VL risk after age adjustment. After excluding children that were reported to be too young to have an occupation, cases were more frequently involved in animal herding than controls, for whom taking care of the house was most often reported. Nevertheless, the overall main occupation was not significantly associated with VL.
Traveling outside the village of residence for more than 2 weeks in the past year was significantly associated with increased risk of VL. Subjects mainly traveled to other villages in West Pokot, except for one case that traveled to the neighboring Baringo County (data not shown).
When reviewing nighttime and sleeping habits, VL cases more frequently went to bed after sunset (6.30 PM). However, this association was likely biased by the younger mean age of the controls, and was not statistically significant after correction for age. Both cases and controls mostly slept outdoors in their house yard during the dry season and indoors during the rainy season. While all interviewed controls normally slept in their own house or yard, 31% (dry season) and 19% (rainy season) of the VL cases frequently slept somewhere else in the village, in a neighboring village or in a field. An OR could not be calculated for this variable as there were no observations of controls sleeping away from their house yard. For those sleeping indoors, the average number of people sleeping in the same room was 4 to 5 for both cases and controls. At night, the most common toilet location for both cases and controls was in a field; this was not associated with VL infection. A little less than half the VL cases and controls used to frequently go outside around sunrise (6.30 AM) during the dry season. This was less common in both groups during the rainy season.
When comparing the housing characteristics of VL cases and controls, there was no statistically significant difference in wall material, which was most often strong mud without outer sheeting. Cracked walls were equally present in cases and controls, and most participants had roofs made of grass and wood. Controls most frequently had floors of sandy soil inside their homes, while VL cases more often reported a mixture based on cattle dung as flooring. As such, house floor type was significantly associated with increased VL risk (p=0.002). Windows were significantly more common (p=0.042) in the houses of VL cases. Indoor residual spraying with insecticides in the last year by either the MoH or the household itself had been performed in the houses of only 1 control and 3 cases. House yard soil type and having compost or decaying material in the house yard were not significantly associated with VL, while having open water bodies in the yard during the rainy season (either puddles of rain water or in buckets) was found to be associated with decreased VL odds (p=0.008).
Distance of the house to a river was significantly associated with VL risk (p=0.013), with cases more frequently living within 100 m from a river than controls. No associations with VL were found for house proximity to a pond, well or irrigated agriculture. Similarly, having termite mounds within visual range of the house was not associated with VL. The majority of participants reported living close to trees. In particular,
Acacia seyal,
Terminalia brownii, Acacia senegal, Acacia mellifera and
panyirit (Pokot vernacular name that can refer to
Acacia mellifera, A. recifiens or
A. etbaica [
16]) trees close to the house were associated with increased odds of VL.
Having domestic animals inside the house at night was uncommon, but the majority of participants had animals in their house yard at night. Goats were most frequently reported by both VL cases and healthy controls, while cattle, sheep and dogs were all significantly associated with having VL (p<0.001, p=0.002 and p=0.003, resp.).
When considering outdoor activities during the day, activities in forests or woodlands during the rainy season was significantly associated with VL (p=0.007). The most commonly reported forest activities included herding animals and collecting firewood (data not shown). Activities close to water bodies were not associated with VL, while activities close to termite mounds significantly increased the risk of VL infection (p<0.001).
Mosquito net use was equally reported by 64% of the cases and controls, with no difference with regard to the season. No statistically significant difference was found between VL cases and controls in the frequency of net use, net impregnation with insecticides, the period of net usage, the net condition or washing the net. Use of skin insect repellents was only reported by one single (VL case) participant.
4. Discussion
This explorative study identified several household, behavioral and environmental factors that could contribute to increased risk of VL infection for the West Pokot VL focus, including some that have not been described before for this region. Most striking were associations with the presence of dogs in the house yard and multiple tree-related factors, such as forest activities, felling trees in the house yard and house proximity to various tree species.
VL patients in this study were mostly children and teenagers, with similar numbers of both sexes. This sex distribution differs from the general epidemiology of the disease in West Pokot, which affects males more commonly than females [
3,
13]. Potential bias of this unrepresentative sex distribution in the study sample was minimized by sex-matching of controls. Although the association with VL risk was not statistically significant for main occupation, the results strongly suggest that the disease mostly affects animal-herding pastoralists, as has been reported before [
13]. Their outdoor and migratory lifestyle is likely to increase the probability of contact with
L. donovani-infected sandflies. This was also reflected by the risk-increasing behavior of traveling outside the village of residence for more than 2 weeks. High rates of illiteracy among cases may have negatively affected their knowledge of VL disease and transmission, although this was not formally assessed here.
This study investigated participants’ nighttime and sleeping behavior, as
P. martini sandflies are considered to be most active between dusk and midnight [
6,
17,
18]. Whether these VL vectors are generally endophagous or exophagous in this period was not clear from our results, as staying or sleeping outdoors after sunset was not more frequently reported by VL cases compared to controls. However, sleeping outside their own house (yard) was only observed for VL cases. This behavior could be associated with more outdoor activities at night, and thereby increased likelihood of encountering sandflies and/or their breeding sites in the surroundings of the household’s
manyatta. For those sleeping inside, using a mosquito net was not associated with decreased risk of VL. This is in contrast with previous studies in the Pokot territory, Sudan and Ethiopia, which found that owning a (insecticide-treated) mosquito net was protective for VL, suggesting indoor biting by sandflies [13,19-21]. This hypothesis was supported by our observation that having windows increases the risk of acquiring VL. The absence of an association between VL and mosquito net use in the current study could be the result of the small sample size; however, it cannot be excluded that other factors are also involved, such as net mesh size or indoor sandfly biting before bedtime.
The role of animals in
L. donovani transmission in eastern Africa, either through attraction of sandflies or as parasite reservoir (zoonotic transmission), is still a matter of debate. A study in Sudan found increased VL risk due to the presence of cattle around the house, while the same was associated with decreased odds of the disease in the Pokot territory [
13,
22]. The latter study also found that applying insecticide to cattle reversely led to increased VL risk, all in all suggesting that
P. martini is mainly zoophilic but will shift to feeding on humans when its preferred source of blood meal is inaccessible. The findings of the current study are in contradiction with the previous report from the Pokot area, as we found that having cattle and sheep around the house increased the risk of VL. As such, the exact feeding preference of
P. martini remains unclear. Initial investigations in Kenya pointed towards anthropophilic behavior, but a later study demonstrated that goats were the most common blood meal source for
P. martini [23-25]. Considering these previous works and the results of this study, we hypothesize that
P. martini feeds on both humans and livestock, and that the latter might attract the sandflies towards residencies, thereby exposing the livestock owners to increased sandfly biting as well.
Besides livestock, the association between dogs in the yard and increased VL risk was particularly noteworthy. Canine animals are an important reservoir for
L. infantum in the Mediterranean and South America.
L. donovani in eastern Africa is mostly believed to be anthroponotic, but earlier studies in Sudan and Ethiopia found evidence of
L. donovani infection in dogs and sleeping near dogs has been associated with increased risk of VL in Ethiopia as well [26-31]. For Kenya, studies from the 1970s and 80s identified one
L. donovani -infected dog in West Pokot and two more in Machakos District [
32,
33]. Given these low numbers, the authors assumed that dogs were an accidental parasite host. Nevertheless, the current study demonstrates for the first time an association between dog ownership and VL risk in West Pokot. The role of dogs in VL transmission in this area should therefore be investigated further, ideally by screening domestic dogs for (past)
L. donovani infection using highly sensitive serological and molecular diagnostic methods. Furthermore, xenodiagnosis to infected animals should be performed to assess their parasite transmission potential.
The use of a cattle dung mixture as flooring inside the house increased the risk of VL. It is possible that this association was confounded by the risk factor of cattle presence in the yard; on the other hand, the moist, organic composition of dung could also be an attractant for sandflies, as has been shown for other
Phlebotomus vectors of
Leishmania [
34,
35].
Multiple studies have demonstrated termite mounds to be one of the major resting and breeding sites of
P. martini [
6,
7]. Paradoxically, no association was found between VL and the proximity of termite mounds to the household. This was also reported by Kolaczinski
et al. and is possibly due to omnipresence of these structures throughout West Pokot [
13]. However, the current study demonstrated that having activities close to these mounds, which is likely to disturb resting and breeding sandflies inside, did result in increased VL risk. Outdoor daytime transmission of
L. donovani parasites by sandflies was also implied by the risk-increasing activities in forests during the rainy season, when
P. martini is believed to be most abundant [
7,
11]. Here, trees may play a particular role in the attraction of sandflies, since cutting down trees in the house yard and household proximity to a number of different
Acacia tree species were associated with increased VL risk as well.
A. seyal and
Balanites aegyptiaca trees are known to be an essential habitat component for
P. orientalis [
12] and in Baringo County, Kenya,
P. martini was demonstrated to forage on
Acacia trees [
36]. Our results further underline the relevance of trees in
P. martini ecology: for the first time, we associated specific tree species with VL infection in West Pokot. These results advocate for further investigations into
P. martini ecology in this area, where tree barks or root systems may provide an alternative breeding site beside termite mounds.
Considering the risk-increasing effects of outdoor activities, personal protection against sandfly bites, such as applying skin repellent, may be an effective way to prevent VL infection and disrupt its transmission [37-39]. However, virtually none of the participants in this study reported use of insect skin repellents. There could be various reasons for this behavior: the local population may not be accustomed to it, and chemical repellents may not be affordable.
Due to the low number of interviewed cases and controls, the findings of this research should be interpreted with reservations. For some variables, it was not possible to make a precise estimation of the OR and there might have been insufficient power to identify more subtle differences between cases and controls. The limited resources and harsh conditions in the rural areas of West Pokot also posed a challenge for the recruitment of uninfected volunteers in the villages and their matching to cases. Although age correction was applied during statistical analysis, it cannot be excluded that the differences in age and residential villages between the groups may have confounded some of the study findings. Furthermore, the English questionnaire was not translated to Swahili or Pokot. Even though the interviewers and interpreter were instructed about the meaning of each question and its answer options, it is possible that some bias occurred in their personal translation of the questions. Lastly, answers provided by participants were not independently verified by the researchers, which might have introduced reporting bias.
Notwithstanding these limitations, this study has provided more insight into environmental and behavioral risk factors for VL in West Pokot. Our results pave the way for more in-depth investigations into P. martini biology, such as its attraction by livestock animals and association with Acacia tree species. Confirmation of alternative sandfly breeding sites, for instance by placing sticky emergence traps, is warranted, as daytime activities in forests appear to contribute significantly to the risk of acquiring VL. Once these sites have been identified, vector control measures can be more efficiently deployed. Moreover, screening of domestic and wild animals (canines in particular) for Leishmania infection is imperative to halt potential zoonotic parasite transmission. Finally, future studies should also address relevant social factors beyond parasite and vector biology, such as the current knowledge, attitude and practices of the local population towards VL.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, File S1: Household Questionnaire; Table S1: Univariate analysis of all determinants with correction for age.
Author Contributions
Conceptualization, NvD, HS, PM and JC; methodology, NvD, HS, JC and PM; formal analysis, NvD; investigation, NvD and DK.; resources, NvD and DK; data curation, NvD; writing—original draft preparation, NvD; writing—review and editing, HS, PM and JC; visualization, NvD; supervision, NvD, HS and PM; project administration, NvD; funding acquisition, NvD. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a Work Visit Grant from the Amsterdam institute for Infection and Immunity.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Amref Health Africa Ethics and Scientific Review Committee (ESRC P1196/2022, May 26th 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Acknowledgments
The authors would like to thank the interviewers Tyson Onyango, Mercy Chepkemoi and Mary Machuma for their essential role in the data acquisition for this study. Our gratitude goes to Isaac Nyeris, community health worker in West Pokot who introduced the study team to the local communities and acted as Pokot interpreter during the interviews. We want to acknowledge Wyckliff Omondi (Head) and Daniel Mwiti (Leishmaniasis Focal Point Person) of the Division of Vector Borne and Neglected Tropical Diseases, Kenya Ministry of Health, for their support in the preparation and implementation of this study. We want to thank DNDi Eastern Africa for facilitating the work of the of the study team in West Pokot during patient enrolment and data collection. Our thanks also go to the Statistics Helpdesk of the Amsterdam University Medical Centres for their support during the data analysis. Last but not least, we want to thank all the study participants for their voluntary participation in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leishmaniasis. Available online: https://www.who.int/news-room/factsheets/
detail/leishmaniasis#:~:text=Visceral%20leishmaniasis%20(VL)%2C%20also,East%20Africa%20and
%20in%20India. (accessed on 12-04-2023).
- Alvar, J.; den Boer, M.; Dagne, D.A. Towards the elimination of visceral leishmaniasis as a public health problem in east Africa: reflections on an enhanced control strategy and a call for action. Lancet Glob Health 2021, 9, e1763–e1769. [Google Scholar] [CrossRef]
- Mueller, Y.K.; Kolaczinski, J.H.; Koech, T.; Lokwang, P.; Riongoita, M.; Velilla, E.; Brooker, S.J.; Chappuis, F. Clinical epidemiology, diagnosis and treatment of visceral leishmaniasis in the Pokot endemic area of Uganda and Kenya. Am J Trop Med Hyg 2014, 90, 33–39. [Google Scholar] [CrossRef]
- Heisch, R.B.; Wijers, D.J.; Minter, D.M. In pursuit of the vector of kala-azar in Kenya. Br Med J 1962, 1, 1456–1458. [Google Scholar] [CrossRef]
- Perkins, P.V.; Githure, J.I.; Mebrahtu, Y.; Kiilu, G.; Anjili, C.; Ngumbi, P.S.; Nzovu, J.; Oster, C.N.; Whitmire, R.E.; Leeuwenburg, J.; et al. Isolation of Leishmania donovani from Phlebotomus martini in Baringo district, Kenya. Trans R Soc Trop Med Hyg 1988, 82, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Ngumbi, P.M.; Lrungu, L.W.; Robert, L.I.; Gordon, D.M.; Githure, J.L. Abundances and nocturnal activities of phlebotomine sandflies (Diptera: Psychodidae) in termite hills and animal burrows in Baringo District, Kenya. Afr J Health Sci 1998, 5, 28–34. [Google Scholar]
- Basimike, M.; Mutinga, M.J.; Kumar, R. Habitat Preference and Seasonal Variations of Phlebotomine Sandflies (Diptera, Psychodidae) in Marigat Area, Baringo District, Kenya. International Journal of Tropical Insect Science 1992, 13, 307–314. [Google Scholar] [CrossRef]
-
The Kenya Strategic Plan for Control of Leishmaniasis; Kenya Ministry of Health: Nairobi, Kenya, 2021.
- Blackwell, J.M.; Fakiola, M.; Castellucci, L.C. Human genetics of leishmania infections. Hum Genet 2020, 139, 813–819. [Google Scholar] [CrossRef]
- Diro, E.; Lynen, L.; Ritmeijer, K.; Boelaert, M.; Hailu, A.; van Griensven, J. Visceral Leishmaniasis and HIV Coinfection in East Africa. PLOS Neglected Tropical Diseases 2014, 8, e2869. [Google Scholar] [CrossRef]
- Gebre-Michael, T.; Malone, J.B.; Balkew, M.; Ali, A.; Berhe, N.; Hailu, A.; Herzi, A.A. Mapping the potential distribution of Phlebotomus martini and P. orientalis (Diptera: Psychodidae), vectors of kala-azar in East Africa by use of geographic information systems. Acta Tropica 2004, 90, 73–86. [Google Scholar] [CrossRef]
- Elnaiem, D.-E.A. Ecology and control of the sand fly vectors of Leishmania donovani in East Africa, with special emphasis on Phlebotomus orientalis. Journal of Vector Ecology 2011, 36, S23–S31. [Google Scholar] [CrossRef]
- Kolaczinski, J.H.; Reithinger, R.; Worku, D.T.; Ocheng, A.; Kasimiro, J.; Kabatereine, N.; Brooker, S. Risk factors of visceral leishmaniasis in East Africa: a case-control study in Pokot territory of Kenya and Uganda. Int J Epidemiol 2008, 37, 344–352. [Google Scholar] [CrossRef] [PubMed]
- 2019 Kenya Population and Housing Census. Population by County and Sub-County 2019.
- Hennessy, S.; Bilker, W.B.; Berlin, J.A.; Strom, B.L. Factors Influencing the Optimal Control-to-Case Ratio in Matched Case-Control Studies. American Journal of Epidemiology 1999, 149, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Timberlake, J. Vernacular names and uses of plants in Northern Kenya. Journal of East African Natural History 1994, 83, 31–69. [Google Scholar] [CrossRef]
- Aklilu, E.; Gebresilassie, A.; Yared, S.; Kindu, M.; Tekie, H.; Balkew, M.; Warburg, A.; Hailu, A.; Gebre-Michael, T. Comparative study on the nocturnal activity of phlebotomine sand flies in a highland and lowland foci of visceral leishmaniasis in north-western Ethiopia with special reference to Phlebotomus orientalis. Parasites & Vectors 2017, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- GEBRE-MICHAEL, T.; LANE, R.P. The roles of Phlebotomus martini and P. celiae (Diptera: Phlebotominae) as vectors of visceral leishmaniasis in the Aba Roba focus, southern Ethiopia. Medical and Veterinary Entomology 1996, 10, 53–62. [Google Scholar] [CrossRef]
- Elnaiem, D.A.; Elnahas, A.M.; Aboud, M.A. Protective efficacy of lambdacyhalothrin-impregnated bednets against Phlebotomus orientalis, the vector of visceral leishmaniasis in Sudan. Medical and Veterinary Entomology 1999, 13, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Ritmeijer, K.; Davies, C.; van Zorge, R.; Wang, S.J.; Schorscher, J.; Dongu'du, S.I.; Davidson, R.N. Evaluation of a mass distribution programme for fine-mesh impregnated bednets against visceral leishmaniasis in eastern Sudan. Trop Med Int Health 2007, 12, 404–414. [Google Scholar] [CrossRef]
- Yared, S.; Deribe, K.; Gebreselassie, A.; Lemma, W.; Akililu, E.; Kirstein, O.D.; Balkew, M.; Warburg, A.; Gebre-Michael, T.; Hailu, A. Risk factors of visceral leishmaniasis: a case control study in north-western Ethiopia. Parasit Vectors 2014, 7, 470. [Google Scholar] [CrossRef] [PubMed]
- Bucheton, B.; Kheir, M.M.; El-Safi, S.H.; Hammad, A.; Mergani, A.; Mary, C.; Abel, L.; Dessein, A. The interplay between environmental and host factors during an outbreak of visceral leishmaniasis in eastern Sudan. Microbes Infect 2002, 4, 1449–1457. [Google Scholar] [CrossRef]
- Ngumbi, P.M.; Lawyer, P.G.; Johnson, R.N.; Kiilu, G.; Asiago, C. Identification of phlebotomine sandfly bloodmeals from Baringo District, Kenya, by direct enzyme-linked immunosorbent assay (ELISA). Med Vet Entomol 1992, 6, 385–388. [Google Scholar] [CrossRef]
- Heisch, R.B.; Guggisberg, C.A.; Teesdale, C. Studies in leishmaniasis in East Africa. II. The sandflies of the Kitui kalaazar area in Kenya, with descriptions of six new species. Trans R Soc Trop Med Hyg 1956, 50, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Heisch, R.B.; Guggisberg, C.A. Notes on the sandflies (Phlebotomus) of Kenya. Ann Trop Med Parasitol 1953, 47, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.a.M.; Osman, O.F.; El-Raba'a, F.M.A.; Schallig, H.D.F.H.; Elnaiem, D.-E.A. Role of the domestic dog as a reservoir host of Leishmania donovani in eastern Sudan. Parasites & Vectors 2009, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Dereure, J.; El-Safi, S.H.; Bucheton, B.; Boni, M.; Kheir, M.M.; Davoust, B.; Pratlong, F.; Feugier, E.; Lambert, M.; Dessein, A.; et al. Visceral leishmaniasis in eastern Sudan: parasite identification in humans and dogs; host-parasite relationships. Microbes and Infection 2003, 5, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Bashaye, S.; Nombela, N.; Argaw, D.; Mulugeta, A.; Herrero, M.; Nieto, J.; Chicharro, C.; Canavate, C.; Aparicio, P.; Velez, I.D.; et al. Risk factors for visceral leishmaniasis in a new epidemic site in Amhara Region, Ethiopia. Am J Trop Med Hyg 2009, 81, 34–39. [Google Scholar] [CrossRef]
- Kalayou, S.; Tadelle, H.; Bsrat, A.; Abebe, N.; Haileselassie, M.; Schallig, H.D. Serological evidence of Leishmania donovani infection in apparently healthy dogs using direct agglutination test (DAT) and rk39 dipstick tests in Kafta Humera, north-west Ethiopia. Transbound Emerg Dis 2011, 58, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kenubih, A.; Dagnachew, S.; Almaw, G.; Abebe, T.; Takele, Y.; Hailu, A.; Lemma, W. Preliminary survey of domestic animal visceral leishmaniasis and risk factors in north-west Ethiopia. Trop Med Int Health 2015, 20, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Argaw, D.; Mulugeta, A.; Herrero, M.; Nombela, N.; Teklu, T.; Tefera, T.; Belew, Z.; Alvar, J.; Bern, C. Risk factors for visceral Leishmaniasis among residents and migrants in Kafta-Humera, Ethiopia. PLoS Negl Trop Dis 2013, 7, e2543. [Google Scholar] [CrossRef]
- Mutinga, M.J.; Ngoka, J.M.; Schnur, L.F.; Chance, M.L. The isolation and identification of leishmanial parasites from domestic dogs in the Machakos District of Kenya, and the possible role of dogs as reservoirs of kala-azar in East Africa. Ann Trop Med Parasitol 1980, 74, 139–144. [Google Scholar] [CrossRef]
- Ngoka, J.M.M., M. J. Visceral leishmaniasis animal reservoirs in Kenya. E Afr Med J 1978, 55, 332–336. [Google Scholar]
- Malaviya, P.; Hasker, E.; Picado, A.; Mishra, M.; Van Geertruyden, J.P.; Das, M.L.; Boelaert, M.; Sundar, S. Exposure to Phlebotomus argentipes (Diptera, Psychodidae, Phlebotominae) sand flies in rural areas of Bihar, India: the role of housing conditions. PLoS One 2014, 9, e106771. [Google Scholar] [CrossRef] [PubMed]
- Schlein, Y.; Yuval, B.; Jacobson, R.L. Leishmaniasis in the Jordan Valley: differential attraction of dispersing and breeding site populations of Phlebotomus papatasi (Diptera: Psychodidae) to manure and water. J Med Entomol 1989, 26, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Hassaballa, I.B.; Sole, C.L.; Cheseto, X.; Torto, B.; Tchouassi, D.P. Afrotropical sand fly-host plant relationships in a leishmaniasis endemic area, Kenya. PLOS Neglected Tropical Diseases 2021, 15, e0009041. [Google Scholar] [CrossRef] [PubMed]
- Weeks, E.N.I.; Wasserberg, G.; Logan, J.L.; Agneessens, J.; Stewart, S.A.; Dewhirst, S. Efficacy of the insect repellent IR3535 on the sand fly Phlebotomus papatasi in human volunteers. Journal of Vector Ecology 2019, 44, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Klun, J.A.; Khrimian, A.; Debboun, M. Repellent and Deterrent Effects of SS220, Picaridin, and Deet Suppress Human Blood Feeding by Aedes aegypti, Anopheles stephensi, and Phlebotomus papatasi. Journal of Medical Entomology 2006, 43, 34–39. [Google Scholar] [CrossRef]
- Naucke, T.J.; Lorentz, S.; Grünewald, H.W. Laboratory testing of the insect repellents IR3535 and DEET against Phlebotomus mascittii and P. duboscqi (Diptera: Psychodidae). Int J Med Microbiol 2006, 296 Suppl 40, 230–232. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).