1. Introduction
Water, compared to other natural polymolecular structures, has more than 35 „anomalies“. The term „anomaly“ (under quotation marks) is used because compared to, for instance, 100 structures exhibiting one type of behavior, water behaves differently. One of such examples is that all materials contract in cold, and expand in heat. Water behaves differently; liquid water has greater density than ice, thus ice floats. Water expands at lower temperatures, and contracts at higher temperatures. Without this „anomaly“, biological form of life probably would not exist. Water effected a breakthrough and created biological life, aided by external factors, primarily light, heat, and gravitation. Water within cell makes up for about 70% of the cell’s contents, the remainders are other chemicals, mostly proteins (15%). The organism as a whole contains approximately 72% water, the rest being dry matter. Lungs, blood and the brain are most abundant with water. With ageing, the water percentage decreases in every organ, mostly within skin (from 70% to 60%). The interaction between water and biomolecules assists in molecular recognition, which makes water an active rather than a passive factor for biomolecular function [
1].
The water molecule is composed of two hydrogen atoms and one oxygen atom, connected by covalent hydrogen bonds. The strength of the covalent bond O-H is 460 kJ/ mol (4.77 eV), while the strength of the noncovalent bond O...H ranges from 10 to 120 kJ/mol. (0.09–1.24 eV) depending on the pKw, temperature and the organization of water molecules; dimer, trimer - open chains, clusters, crystalline linear form (liquid crystals), self-similar quasicrystals or Penrose tiles [
2,
3,
4]. The organization of water molecules depends primarily on the angle between the hydrogen atoms covalently bound to oxygen in the water molecule. It was shown that both, bond lengths and bond angels are related to icosahedral symmetry element Ф (
[
5]. Moreover, it was shown that the fine structure of matter, which was defined by Sommerfeld
(α = 2π e2/hc) = 0.007297…, where
e is the elementary charge
, h- Plank constant and c- speed of light), can also be defined by Ф (α=Ф
2/(360-2/Ф)= 0.007297…, accuracy to six decimal places). Since, fine structure constant is considered a fundamental physical constant (inverse α
−1 = (360 – 2/Ф)/Ф
2 = 137.0359…), which quantifies the strength of the electromagnetic interaction between elementary charged particles, it indicates that there is a connection between the distribution of charges of valence electrons in molecule and distance proportion between atoms in molecule defined by Ф [
6].
Two water molecules (dimer) or more (trimer or longer water chains) engage in interaction via noncovalent hydrogen bonds. The basic reaction is 2H
2O ↔[H
3O]
++[OH]
+, where K
w = [H
3O]
+ × [OH]-, and pK
w = -log
10Kw. The rate of water molecules interaction, primarily of noncovalent hydrogen bonds and of the reorganization of dipole moments, is within limits 1–100
ps at nano scale, the microscopic dynamics and the conformation changes of biomolecules occur within microseconds (μs) while on macroscopic level (large clusters) can be in range from milli seconds (
ms) to a few seconds (
s). It was demonstrated that charge distribution (donor/acceptor) and the length of the hydrogen bond in water and biomolecules are determined by Ф [
7,
8]. This new evidence is in line with Felix Franks’ claim (1972) that “...of all known liquids, water is probably the most studied and least understood”.
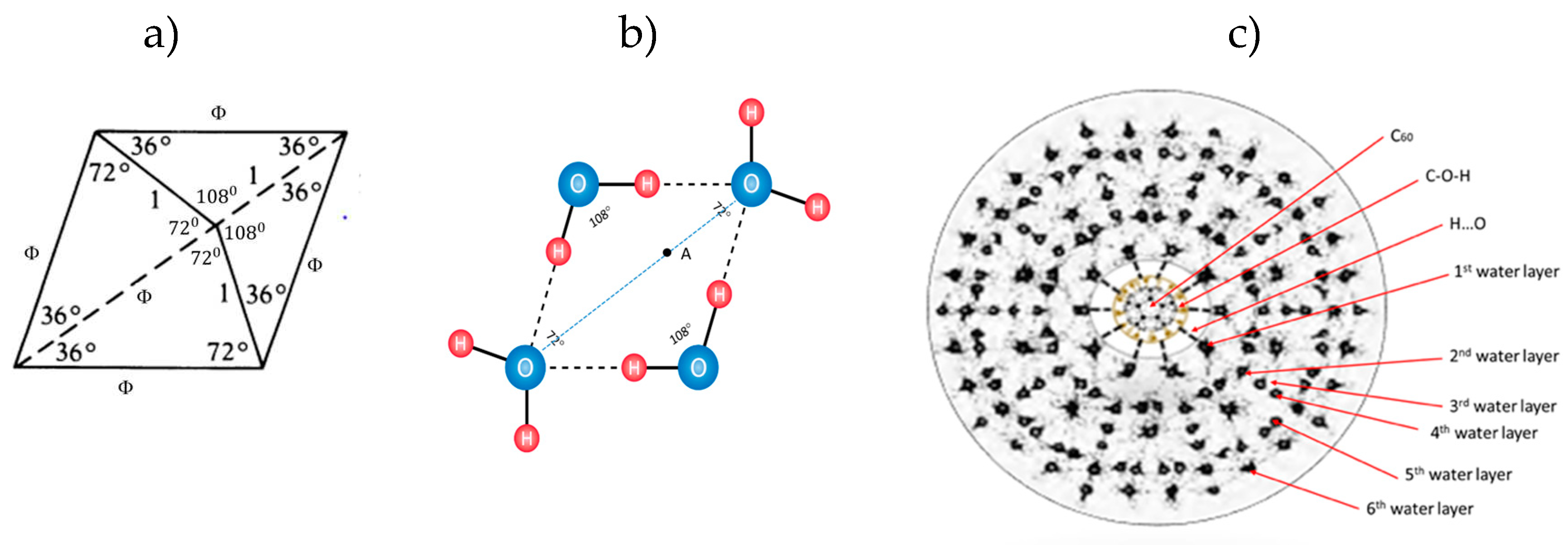
The organization of water molecules in chains, clusters and multi-shells, possesses an element of icosahedral symmetry (
= Ф = 1.61803…, with particular solutions of Ф, as set of Fibonacci sequences: 3/2 =1.5, 5/3 = 1.66, 8/5=1.60, 13/8=1.625, 21/13=1.615, 34/21=1.619, 55/34=1.617, 89/55=1.618, 144/89= 1.617……
Suppl. Figure S1 and S2, and
Suppl. Table S1). There is theoretical and experimental evidence that cold water may be organized as Penrose aperiodic tiles [
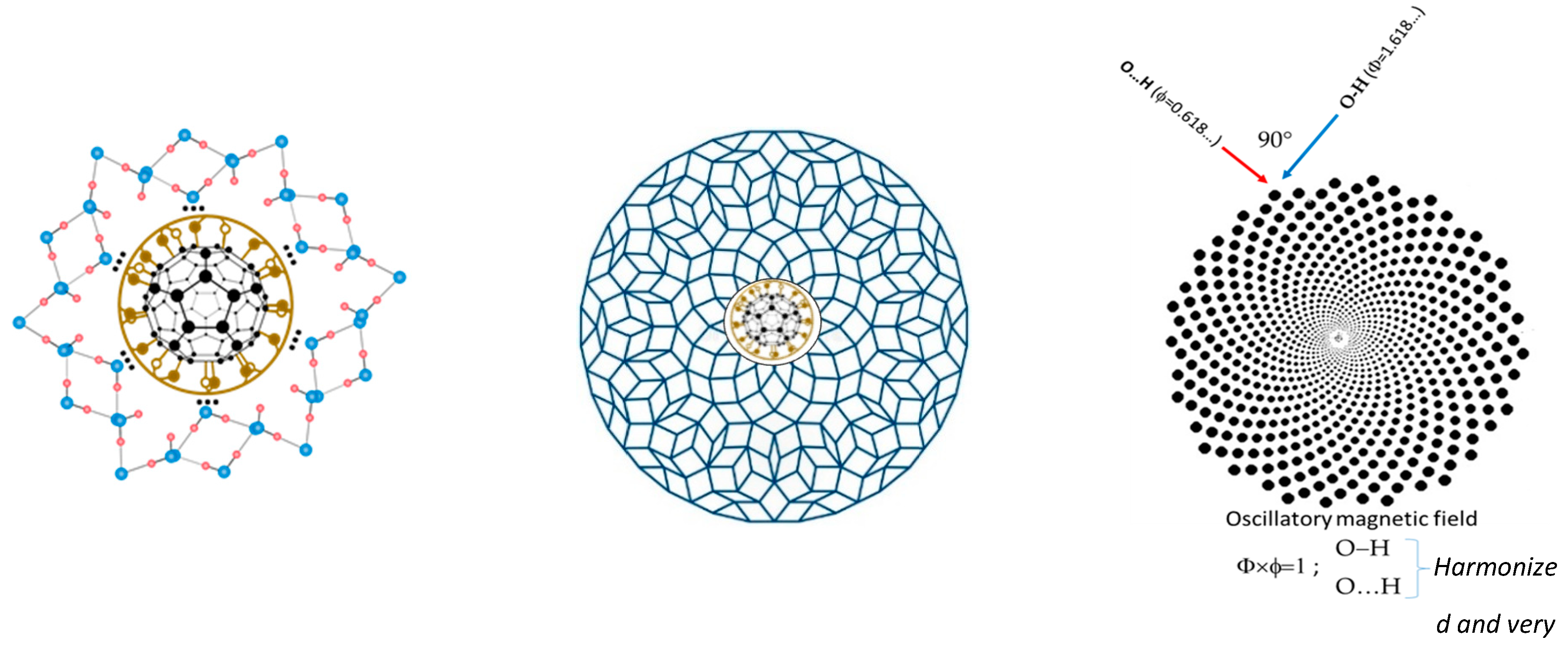
9]. Water organization in quasicrystal is possible because the angle between hydrogen atoms in molecules can be from 104°30′ (liquid) to 109°17′ (ice) and may have one of possible particular solution of Ф. However, Penrose ideal tiles have angle of 108° (Figure 1,
left) and it is one of the reason for icosahedral symmetry formation. On other hand, de Boissieu [
9] said that Penrose tiling is not completely reliable to represent quasicrystal in 3-dimensional space. In spite of this observation de Boissieu wrote that three-dimensional Penrose tiling (3DPT) can be generated by the six-dimensional cut method (
→
where
, rhombohedra with four edges perpendicular to a fixed face of the dodecahedron) and that such “parallel” components have been experimentally observed for the first time [
9]. However, icosahedral aperiodic tiling are well described as solid quasicrystal (alloys of three or more elements: Al, Mn, Fe, and Cr) by Dan Shechtman, who received the Nobel prize for chemistry in 2011
.[
10,
11,
12]. Based on theoretical and experimental results it is shown that soft-matter quasicrystals with icosahedral symmetry may exists in biological systems [
13]. There are huge differences of mechanical properties between solid quasicrystals and soft-matter quasicrystals. “For example, under action of impact tension with stress amplitude σ
0 = 5MPa, the variation of mass density δρ/ρ
0 is 10
−14 for solid quasicrystal, while under action of impact tension with stress amplitude σ
0 =0.01MPa, the variation of mass density δρ/ρ
0 is 10
−3 for soft-matter quasicrystals” [
13]. In this paper, we experimentally demonstrated by TEM and AFM/MFM that a very stable soft quasi-crystalline water shells (dry state) around C
60(OH)
36, in a form of icosahedral 3-dimensional structure, exists. Water layers composed of elastic soft-matter quasicrystal (deformable, similar to peptide planes of proteins) can be obtained, using set of 9 or 16 different Fibonacci numbers from its sequence, which determines the water icosahedral structure (different angles between hydrogen atoms of water molecule according to 3x3 and 4x4 Fibonacci determinants,
Suppl. Figure S6).
In order to stabilize the water layers’ icosahedral structure of a soft substance, water molecules have to be organized around a hard quasi-crystal that will generate icosahedral vibrations that will be resonantly transmitted to the water layers. This will amplify the vibration amplitudes depending on the number of layers, and transmit them to the surrounding water and to the biomolecules. Of all the existing molecular crystals with icosahedral symmetry, the C60 molecule turned out to be the best candidate as a vibrational nano generator of icosahedral symmetry.
From physics point of view, the C
60 is spherical molecular crystal composed of 60 carbon atoms arranged in 12 pentagons and 20 hexagons. The C
60 possesses high symmetry with 120 symmetrical transformations (higher than diamond which has 48), having high speed rotation, “twisting” movement (a billion per second), and it possesses wave-particle duality (quantum/classical properties) [
14]. It also, possess self-harmonized attractive-repulsion forces through vibration of carbon atoms of pentagons (diamagnetic) and hexagons (paramagnetic).
From chemistry point of view, the C60 molecule has both advantages and disadvantages. The advantages are the existence of icosahedral symmetry (as some biomolecules and complex biological structures), ability to be functionalized as well as to form small or large molecules, it can be a carrier of drugs, and it can be used as a marker in diagnostics. The disadvantages are that it is not soluble in water, it is reactive (double bonds of hexagons can be open) and can be toxic, which depends on its concentration. The advantages are the existence of icosahedral symmetry (as some biomolecules and complex biological structures), ability to be functionalized as well as to form small or large molecules, it can be a carrier of drugs, and it can be used as marker in diagnostics.
In order to overcome some of the above mentioned disadvantages, the C
60 molecule is funcionalized with OH groups (the first C
60 derivative, FD-C
60). Fullerene hydroxylation (FD-C
60) increases water solubility and affects these nano particles interaction with biological systems. It is demonstrated that by increasing fullerene water solubility through C
60 surface modification leads to significantly decreased toxicity [
15]. Specifically, in this study decreased toxicity of FD-C
60 compared to cytotoxic effect of fullerene aggregates to human skin (HDP) and liver carcinoma (HepG2) cells was observed [
15]. Similarly, it was observed that hydroxylation decreases toxic potential of fullerene on mouse L929 fibrosarcoma, rat C6 glioma, and U251 human glioma cell lines [
16]. Additionally, FD-C
60 induced apoptotic changes on investigated cells lines, while fullerene C
60 induced necrotic cell death [
16]. Distinct effects of pristine and modified fullerene originate from the different nanoparticles’ interaction with the intracellular metabolic pathways [
16,
17].
However, FD-C
60 did not provide the satisfactory absence of the toxicity on living systems [
15]. In order to reduce toxicity and even completely eliminate it, as well as to improve the transfer of vibrational signals of C
60 molecules to biological water and biomolecules, the second derivative of the C
60 molecule, SD-C
60 (commercial name: 3HFWC - hyperharmonized hydroxylated fullerene water complex) was designed and made by creating water layers (shells) around FD-C
60 (fullerol). The size of SD-C
60 (3HFWC) varies from 3-30 nm depending on the number of water layers [
18,
19].
Bearing in mind that biochemically FD-C60 showed beneficial effects in the treatment of cancer, the design and synthesis of SD-C60 was done, which reduce or even completely eliminated toxicity of original molecule (FD-C60). Since interactions with biomolecules are based on a biophysical approach this will produce different effects than a biochemical approach.
Our goal is to develop a method that will reprogram malignant cells, rather than causing tumor cell death that might have serious consequences like triggering compensatory proliferation and tumor repopulation upon applied treatment in advanced tumor stages.
2. Materials and Methods
2.1 Water: Tap water, from Belgrade city water supply system, is used (Ca2+-71,1 mg/l, Mg2+- 14.7 mg/l, Na+ - 11.4, mg/l, K+- 1,0 mg/l, Fe2+/3+- 0.05 mg/l, NH+4 – 0.03, mg/l,Cl--14.1 mg/l and NO-3-6.1 mg/l.), conductivity 85 mS/cm. Then, water is treated by reverse osmose (Ca2+-0,60 mg/l, Mg2+- 0.53 mg/l, Na+ - 0,32 mg/l, K+- 0,04 mg/l, Fe2+/3+- < 0.005 mg/l, NH+4 – <0.03, Cl- - 0.18 mg/l and NO-3-0.12. mg/l ), conductivity in tank 0.05 mS/cm, and it is used for production SD-C60.
It is well known that under normal conditions, free water is organized into dimers, trimers, tetramers and slightly more complex water structures with lifetimes from 50
fs to 100
ps. However, in a limited space at the nano and micro level, water can be organized into water chains according to the laws in the elements of icosahedral symmetry (
Suppl. Table 1), because the values 3/2, 5/3, 8/5, 13/8 .... respectively are: 1.500, 1.666, 1.600, 1.625, which “oscillatory” converge to the value Ф = 1.618...(
Supplement Figure S2), i.e., to the element of icosahedral symmetry
with eigenvalues T
1g and T
2g (
Suppl. Table S1).
2.2 Manufacturing of SD-C60. The FD-C
60 (fullerol, purity 99.5%) is ordered from Solaris, Canada, while SD-C
60 is synthesized at two laboratories the NanoWorld, Belgrade and the TFT Nano Center, Belgrade. Before mixing with water, fullerol is pre-treated with UVC sterilizer. Ultra-pure water is obtained by purifying water from water supply system by the reverse osmosis process. Certain amount of 0.150 g fullerol is mixed with 1 L ultra-pure water for initial experiment (NanoWorld lab) and 3g fullerol (TFT NanoCenter) is mixed with 20
L of ultra-pure water for commercial use. This mixture passed through the process of steering and was heated at 38 °C for 40 min and then pumped to magnetic vessel by filtration through the membrane filter of 220 nm. Under external action of +250/-92
mT oscillatory magnetic field, according to eigenvalues of icosahedral symmetry T1g, T1u, T2g and T2u, which for symmetry elements
C5,
, S
10 and
give solutions ±½(1+ √5) and ±½(1-√5) (
Suppl., Table S1). Under the internal action of the vibrations of the C
60 molecules and the vibrations induced by the external magnetic field according to the same law (principle: “between a rock and a hard place”) at 37
0C in the reactor, water layers (shells) are formed around fullerol. Depending on the production conditions, two basic types of SD-C
60 can be synthesized, light yellow solution (with a small presence of free fullerol in the solution) and white (without free fullerol in solution), with different numbers of water layers.
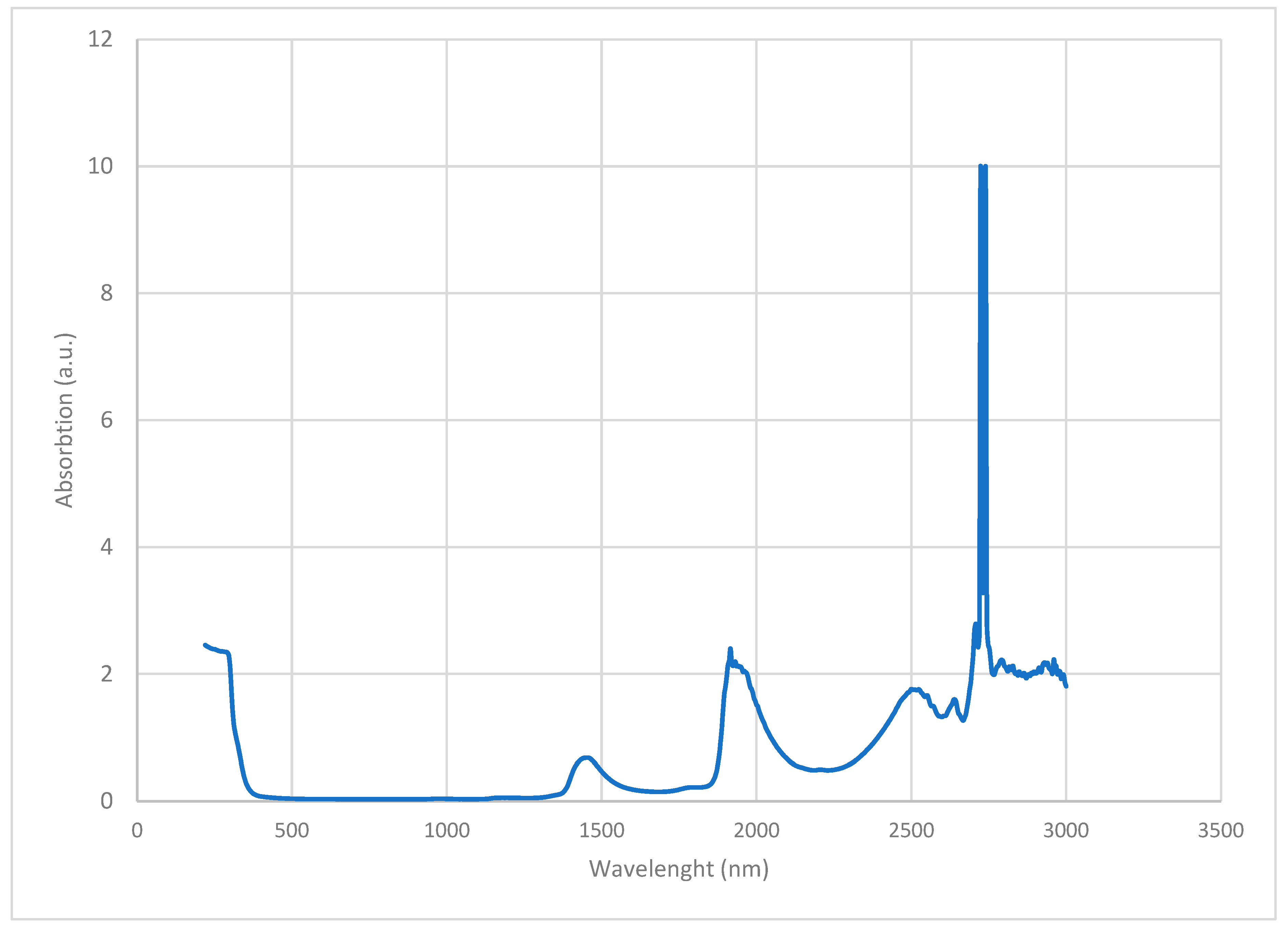
2.3 UV-VIS-NIR: Water, FD-C60 (fullerol) and SD-C60 (3HFWC), both yellow and white are characterized by Lambda 500 spectrometer, Perkin-Elmer, USA (the NanoLab, Faculty of Mechanical Engineering, University of Belgrade) in the range of 250 – 3000 nm.
2.4 FTIR: Water, FD-C60 (fullerol) and SD-C60 (3HFWC, both yellow and white) are characterized by the Spectrum Spotlight 400 FTIR Imaging System, Perkin-Elmer, USA (the NanoLab, Faculty of Mechanical Engineering, University of Belgrade) in the range of 2500 - 14000 nm.
2.5 TEM: The samples in liquid state ware applied to the copper mash coated with carbon. After draying the samples, we analyzed and recorded on the CM12 Philips/FEI Transmission Electron Microscopy, Eindhoven, The Netherlands, magnification x45,000 and x60,000, installed at the Faculty of Biology, University of Belgrade (the sample was prepared 6 months ago) and on TEM, JEM 1400, JEOL, Japan, magnification x 120,000 up to x 200,000, installed at the Faculty of Agriculture, University of Belgrade (one prepared 8 months ago, the other 3 years ago).
2.6 AFM/MFM: The materials in liquid state, FD-C60 (fullerol) and SD-C60 (3HFWC), each in bottles of 100 mL, were prepared in TFT Nano Center, Belgrade. The concentration of fullerol in the first derivative of C60 was 0.15 mg/mL, and in 3HFWC it was also 0.15 mg/mL of fullerol (as a precursor), around which water layers were formed. The color of fullerol in solution was light brown, and 3HFWC samples in solution were very light yellow to white.
Solutions of 2 mL of each were applied to diamagnetic foil in each of the 5 samples. First, the sample was dried with SD-C60 (3HFWC) with a gradual increase in temperature, from 25 - 1100C over the next two hours. During the drying time, a visual control was performed every 15 min and the temperature was measured. After two hours, samples with dry SD-C60 (3HFWC) were removed and samples with fullerol were placed to dry. The procedure was repeated as in the previous case.
After drying, the samples with SD-C60 (3HFWC) had two characteristic areas: one was white-grayish (transparent scrum) and the other was medium brown, while the fullerol samples were dark brown. After drying, the samples were stored in dark closed containers and kept at room temperature until use.
Characterization of dry samples was done by JSPM-5200, Scanning Probe Microscopy, JEOL, Japan, (installed at the NanoLab, Faculty of Mechanical Engineering, University of Belgrade). Two methods are used: (1) AFM (Atomic Force Microscopy), and (2) MFM (Magnetic Force Microscopy). Both techniques are non-invasive. AFM method is based on van der Waals forces and London-type dispersive forces between tip and sample, while MFM, in the non-contact imaging mode, is based on magnetic dipole-dipole interaction between tip and sample (measuring ± deflection of tip “ϕ” in deg.). For the purpose of magnetic gradient investigation, specialized cantilevers, type HQ NSC18/Co-Cr Al BS (MikroMasch, Estonia) with force constant in the range between 1.2 and 5.5 N/m and with the resonant frequency range between 60 to 90 kHz were used. Prior to experiments, the cantilevers were placed in the external magnetic field of 0.4 T in order to induce magnetic field themselves. Scanning size of sample depends on the object size and its number, which were of interest. Bering in mind that a fullerol is 1.2 nm in size (as single molecule) and could be up to 2-3 nm (as aggregates) the optimal scan size is between 30 nm and 100 nm. Expected size of SD-C60 (3HFWC), based on theory and initial experiment by TEM, is between 5 nm and 30 nm, so the optimal scan size should be between 100 nm and 300 nm.
2.7 Biological study in vitro
Materials: Culture media DMEM (Dulbecco′s Modified Eagle′s Medium) and RPMI-1640 (Roswell Park Memorial Institute-1640) supplemented with 20 mM HEPES, 2 nM L-glutamine and 0.01% sodium pyruvate were bought from Capricorn Scientific GmbH (Ebsdorfergrund, Germany). Penicillin/Streptomycin solution was from Biological Industries (Cromwell, CT, USA). Fetal bovine serum (FBS), phosphate-buffered saline (PBS), dimethyl sulfoxide (DMSO), trypsin, crystal violet (CV), propidium iodide (PI), and carboxyfluorescein diacetate succinimidyl ester (CFSE), were obtained from Sigma-Aldrich (St. Louis, MO, USA). 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was from AppliChem (St. Louis, MO, USA). Paraformaldehyde (PFA) was purchased from Serva (Heidelberg, Germany). Annexin V-FITC (AnnV-FITC) was bought from BD Pharmingen (San Diego, CA, USA), Apostat was from R&D Systems (Minneapolis, MN, USA), and acridine orange (AO) was obtained from LaboModerne (Paris, France).
The tested substance SD-C
60 (3HFWC-W) [
19] was obtained from TFT Nano Center (Belgrade, Serbia). The initial concentration of the substance was 0.15
g/L, while the working solutions for
in vitro treatment were made in a culture medium, immediately before use. According to the manufacturer’s instructions, the substance was stored at room temperature (RT), and protected from light due to photosensitivity, while the working solutions were made far from light sources.
This research was performed on two melanoma cell lines of murine origin and different level of aggressiveness - B16F1 and B16F10, and a mouse embryonic fibroblast cell line – NIH/3T3. Cell lines are commercially available and originate from the American Type Culture Collection (ATCC). Both melanoma cell lines were kept in HEPES-buffered RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine, 0.01% sodium pyruvate, penicillin (100 units/mL), and streptomycin (100 μg/mL). Cells were grown at 37°C in a humidified atmosphere with 5% CO2. Mouse embryonic fibroblast cells were kept in the same conditions in the DMEM medium.
4. Conclusion
A Penrose rhombic tiling, based on Ф (icosahedral symmetry), has two characteristic angles between hydrogen atoms in water molecule; 108° and 72° (hydrogen covalently connected to oxygen and non-covalent connected, respectively). Angle of 108° is also angle of pentagonal water order. In this paper it was founded out that there are 9 or 16 types of stable particular solutions of Ф ({9,16}Ф) and that they synergistically fit the 3DPT (three-dimensional icosahedral Penrose tilings) much better than only one type of rhombic tiling with 108° angle.
On the basis of 3DPT{9,16}Ф water icosahedral structure around the first derivative of C60 molecules (FD-C60) and thus the second derivative of the C60 (SD-C60), are explained. Water layers in SD-C60 were experimentally identified in solution (NIR and FTIR spectroscopy) and in the dry state in the form of soft-matter (TEM and AFM/MFM).
Two forms of SD-C60 with different number of water (shells) layers: from 1 to 5 (SD-C60-Y, commercial name 3HFWC) sizes up to 10 nm, and from 6 to 9 (SD-C60-W, commercial name 3HFWC-W) with size 10-30 nm, were synthesized and characterized by UV-Vis-NIR spectroscopy. Their difference in color, number of water layers, and effects on biological structures were shown.
The existence of water layers, for the concentration of SD-C60 in the range from 1 to 10 mg/mL, eliminated toxicity, extended viability and improved the effects on biological water and biomolecules (with hydrogen bonds; proteins, DNA, etc.).
The obtained results show that the main mechanism emphasizing the suppression of melanoma cell growth in vitro was the same for SD-C60 –Y(3HFWC) and SD C60-W. Upgraded formula of 3HFWC- 3HFWC-W demonstrated greater potential to diminish cell growth in comparison to the previously tested one, while the induction of senescence might be considered as more desirable mechanism of antitumor action than conventional nonselective chemotherapy in advanced melanoma treatment.
Bearing in mind beneficial effects of SD-C
60 on murine cell lines, presented in this paper, as well as previously demonstrated effects on melanoma models
in vitro and
in vivo [
40,
41], animal models of Alzheimer disease [
42], memory and pain [
43] it can be speculated that nano SD-C
60 substance can open a new era for treatment of different pathologies, called “water-based nanomedicine”.
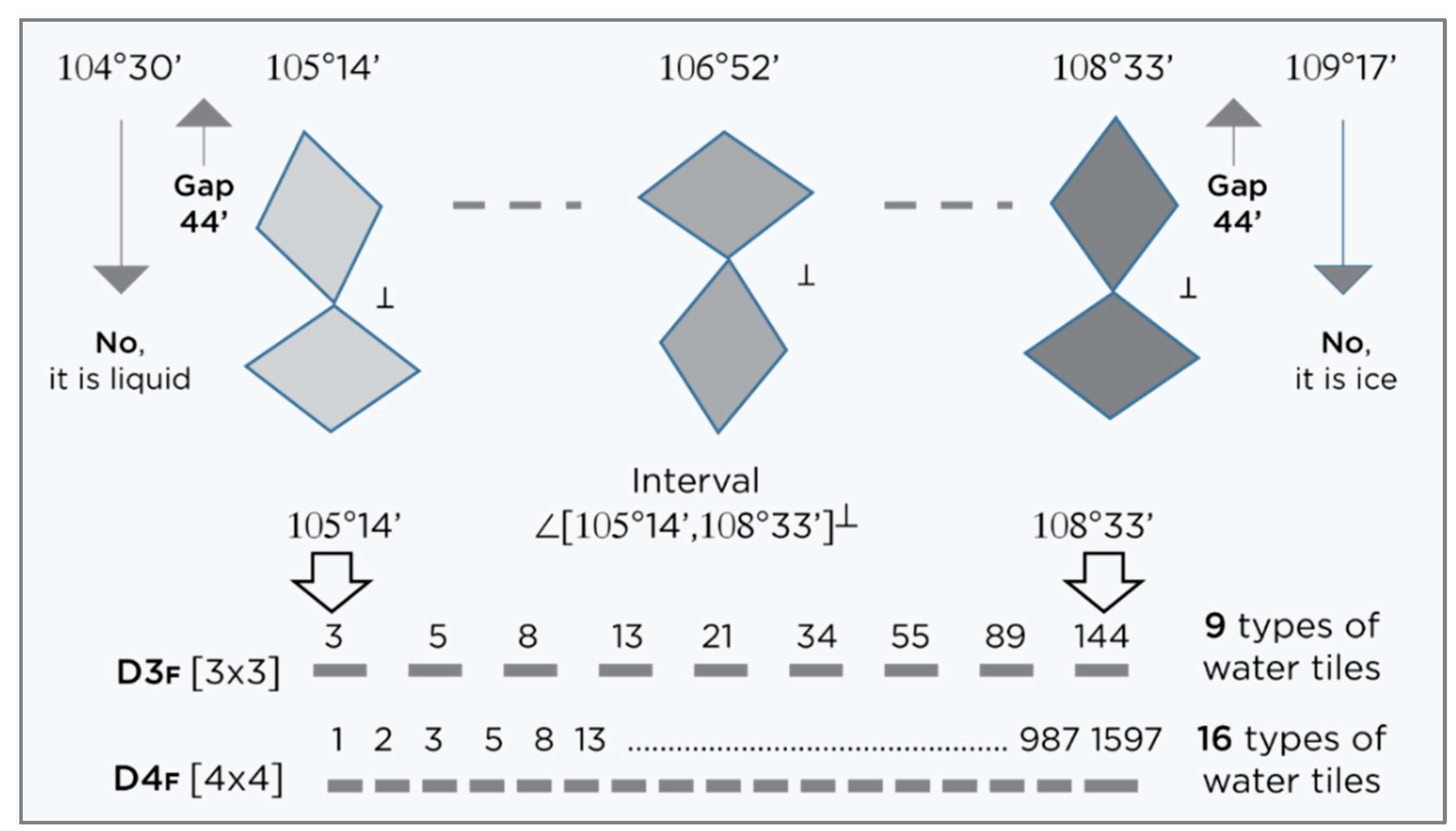
Figure 2.
Bearing in mind that extreme states (liquid water and ice) are not desirable in the water layer around FD-C
60, the states of water molecules with angle of hydrogen atoms 104°30′ and 109°17′ are excluded. The distance between these two extreme states and “water tiles” should be at least 0°44′, so that the soft quasicrystal state of water with icosahedron symmetry starts at 105°14′ and ends at 108°33′ values (⊥ - system is perpendicular, D3
F = det
, determinant 3 x 3 of the Fibonacci sequence 3,5,8,13,21,34,55,89,144 and D4
F=det
, determinant 4x4 of the Fibonacci sequence 1,2,3,5,8,13,21,34,55,89,144,233,377,610,987, 1597, are set of {9,16}
Ф,
Suppl., Figure S6).
Figure 2.
Bearing in mind that extreme states (liquid water and ice) are not desirable in the water layer around FD-C
60, the states of water molecules with angle of hydrogen atoms 104°30′ and 109°17′ are excluded. The distance between these two extreme states and “water tiles” should be at least 0°44′, so that the soft quasicrystal state of water with icosahedron symmetry starts at 105°14′ and ends at 108°33′ values (⊥ - system is perpendicular, D3
F = det
, determinant 3 x 3 of the Fibonacci sequence 3,5,8,13,21,34,55,89,144 and D4
F=det
, determinant 4x4 of the Fibonacci sequence 1,2,3,5,8,13,21,34,55,89,144,233,377,610,987, 1597, are set of {9,16}
Ф,
Suppl., Figure S6).
Figure 3.
The first water layer of “icosahedar water base-tiles (only 6 are visible in cros section) interacting with FD-C
60 (fullerol) (
left). Order of 9 or 16 different “water icosahedral base-tiles” of Ф in the system of water layers (adapt from [
4]) (
middle) and the harmony of the oscillatory process of hydrogen bonds in 3DPT system as attractor of O-H and O..H hydrogen bonds (
right), where Ф×ϕ = 1.
Figure 3.
The first water layer of “icosahedar water base-tiles (only 6 are visible in cros section) interacting with FD-C
60 (fullerol) (
left). Order of 9 or 16 different “water icosahedral base-tiles” of Ф in the system of water layers (adapt from [
4]) (
middle) and the harmony of the oscillatory process of hydrogen bonds in 3DPT system as attractor of O-H and O..H hydrogen bonds (
right), where Ф×ϕ = 1.
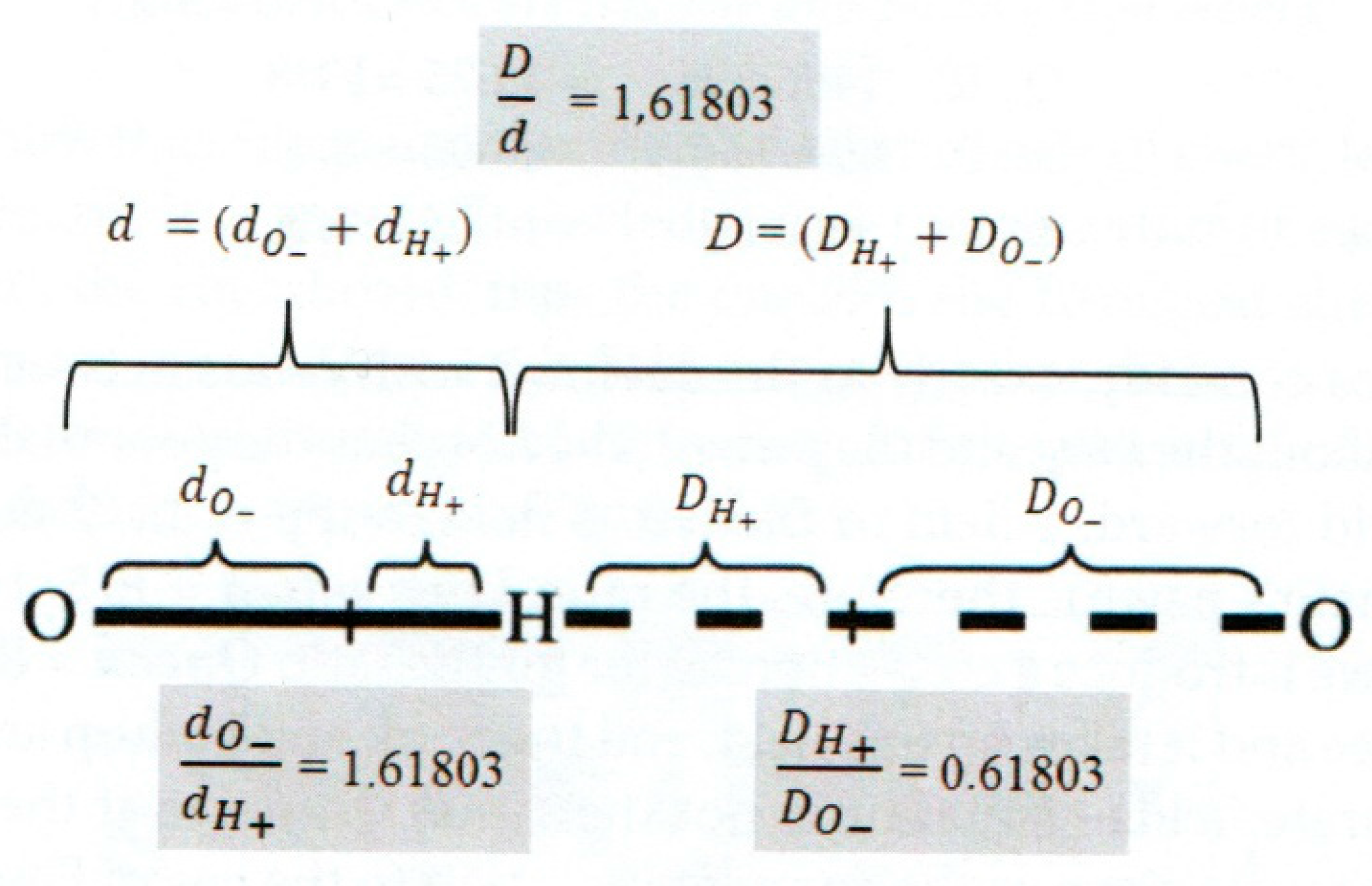
Figure 4.
Nano machinery of charge distribution in the 3DPT system of covalent and non-covalent hydrogen bonds of water molecules. The hydrogen bonds are, actually, one of the best examples of the Yin-Yang concept, the concept of an ordered quadruple: big Yin (d
o-), small yin (d
H+), big Yang (D
O-), small yang (D
H+). Adapt from [
6].
Figure 4.
Nano machinery of charge distribution in the 3DPT system of covalent and non-covalent hydrogen bonds of water molecules. The hydrogen bonds are, actually, one of the best examples of the Yin-Yang concept, the concept of an ordered quadruple: big Yin (d
o-), small yin (d
H+), big Yang (D
O-), small yang (D
H+). Adapt from [
6].
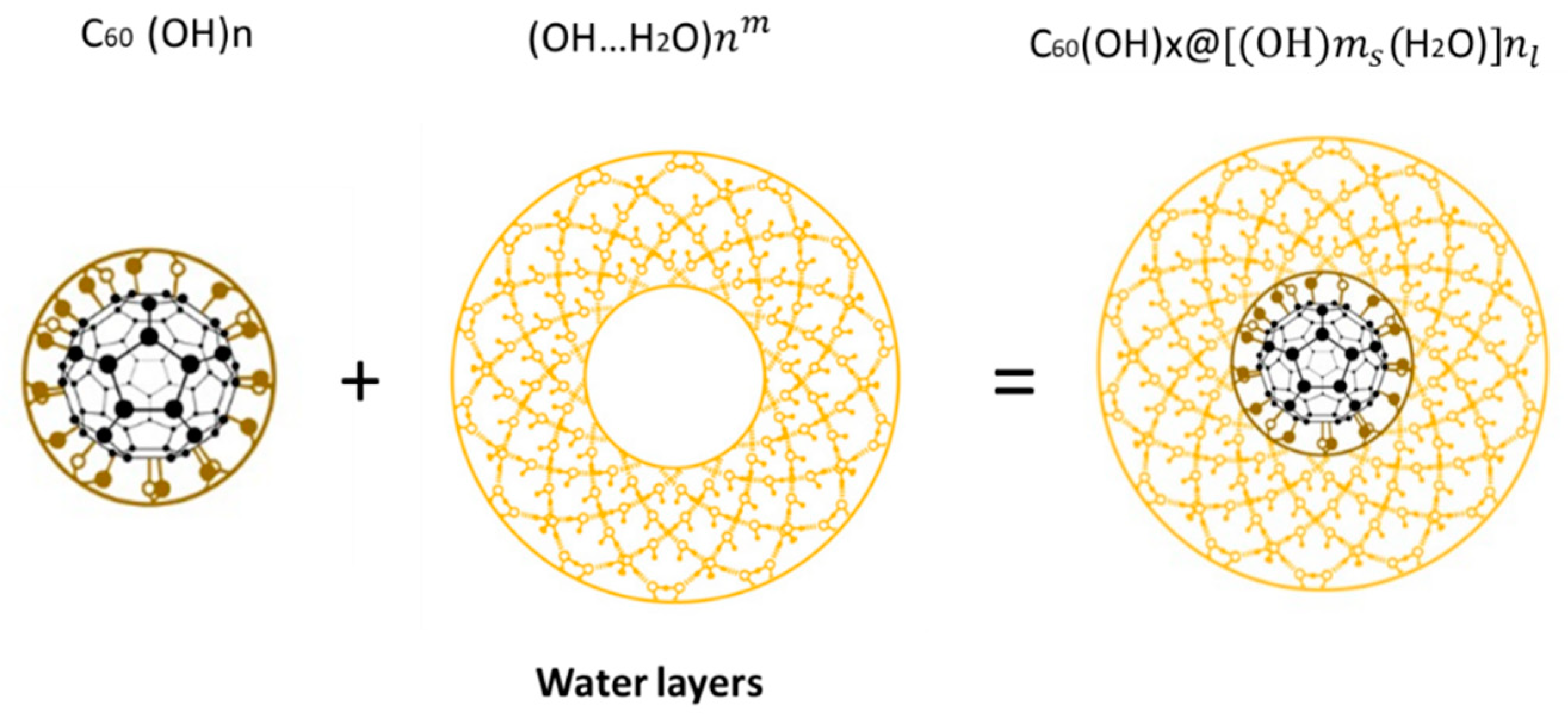
Figure 5.
FD-C60 (fullerol, C60 (OH)24-48) is composed of molecule C60 and OH24-48 groups (average 36) (left), water layers as shells of 28, 60, 100, 280, 320 nm … surrounding FD-C60 (middle), and SD-C60 as soft-matter structure, size 3-30 nm depends of shells number (right).
Figure 5.
FD-C60 (fullerol, C60 (OH)24-48) is composed of molecule C60 and OH24-48 groups (average 36) (left), water layers as shells of 28, 60, 100, 280, 320 nm … surrounding FD-C60 (middle), and SD-C60 as soft-matter structure, size 3-30 nm depends of shells number (right).
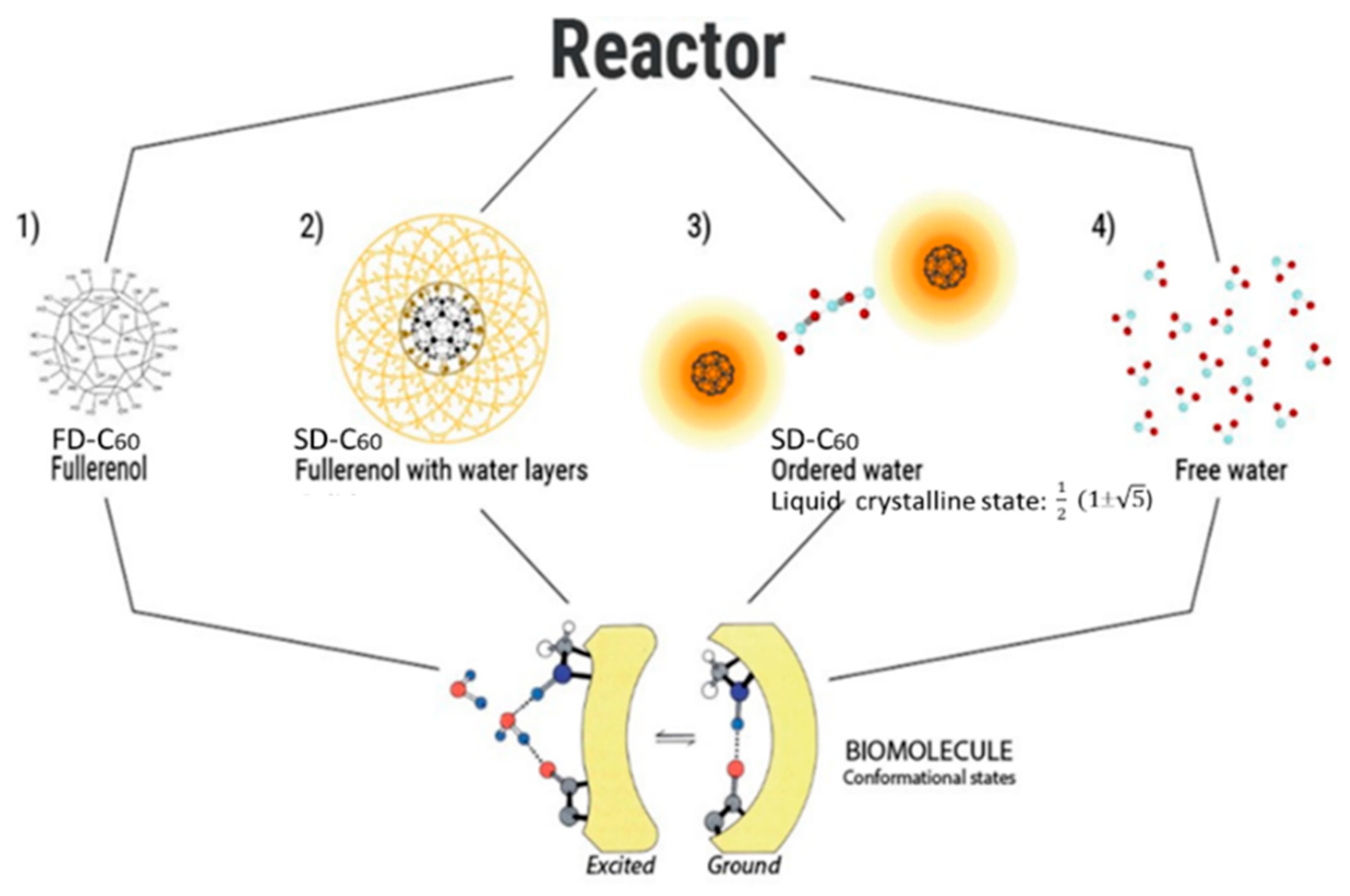
Figure 6.
The input substances in the magnetic reactor are FD-C60 (fullerol or fullerenol) and highly pure water, while the output substances are SD-C60 in solid state (2.5-3%), ordered water in chains between solid SD-C60 is about 58-60%, some small percentage (about 0.05%) of FD-C60 (around which water layers failed to form), and free water 36 - 38%.
Figure 6.
The input substances in the magnetic reactor are FD-C60 (fullerol or fullerenol) and highly pure water, while the output substances are SD-C60 in solid state (2.5-3%), ordered water in chains between solid SD-C60 is about 58-60%, some small percentage (about 0.05%) of FD-C60 (around which water layers failed to form), and free water 36 - 38%.
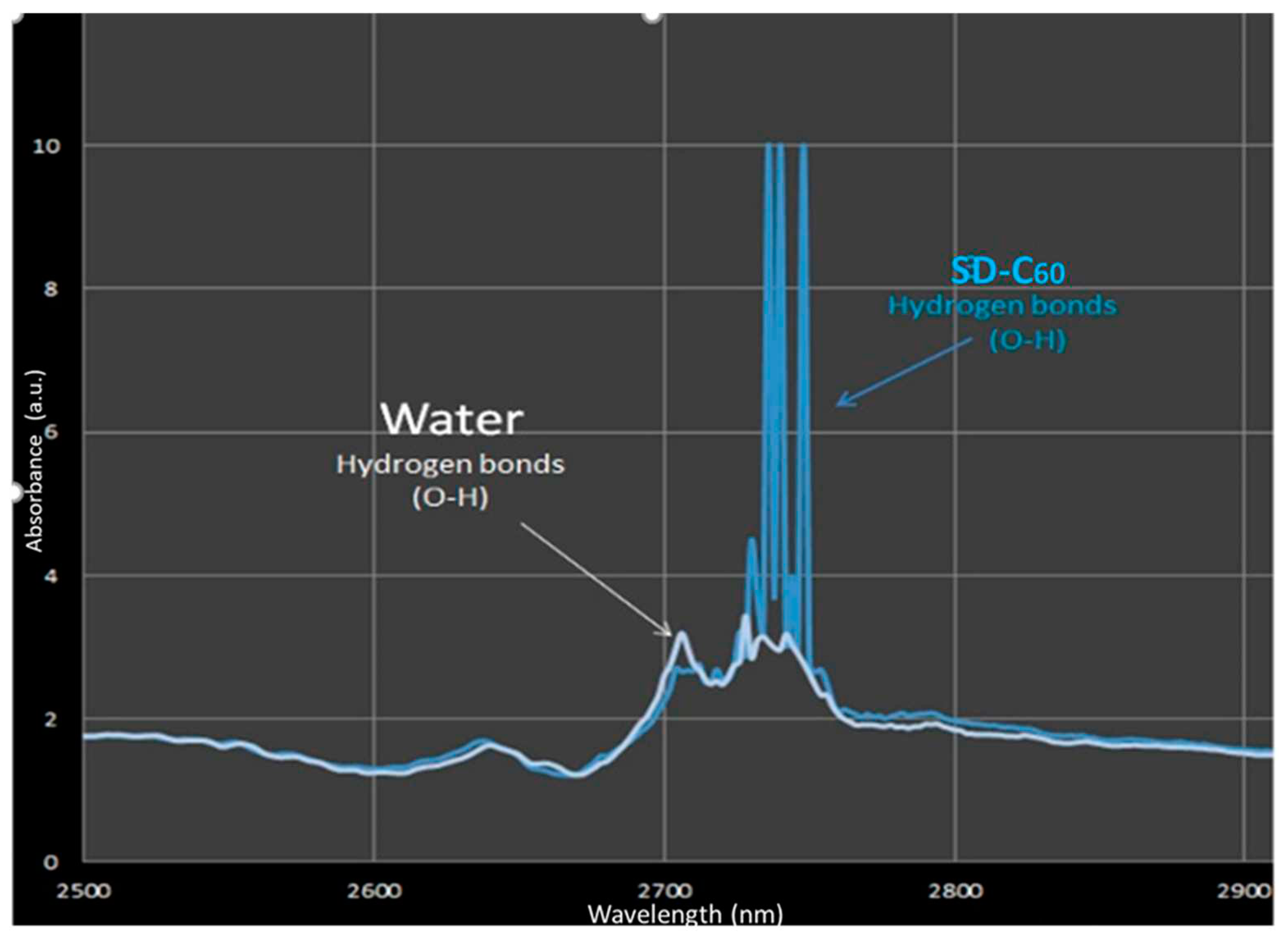
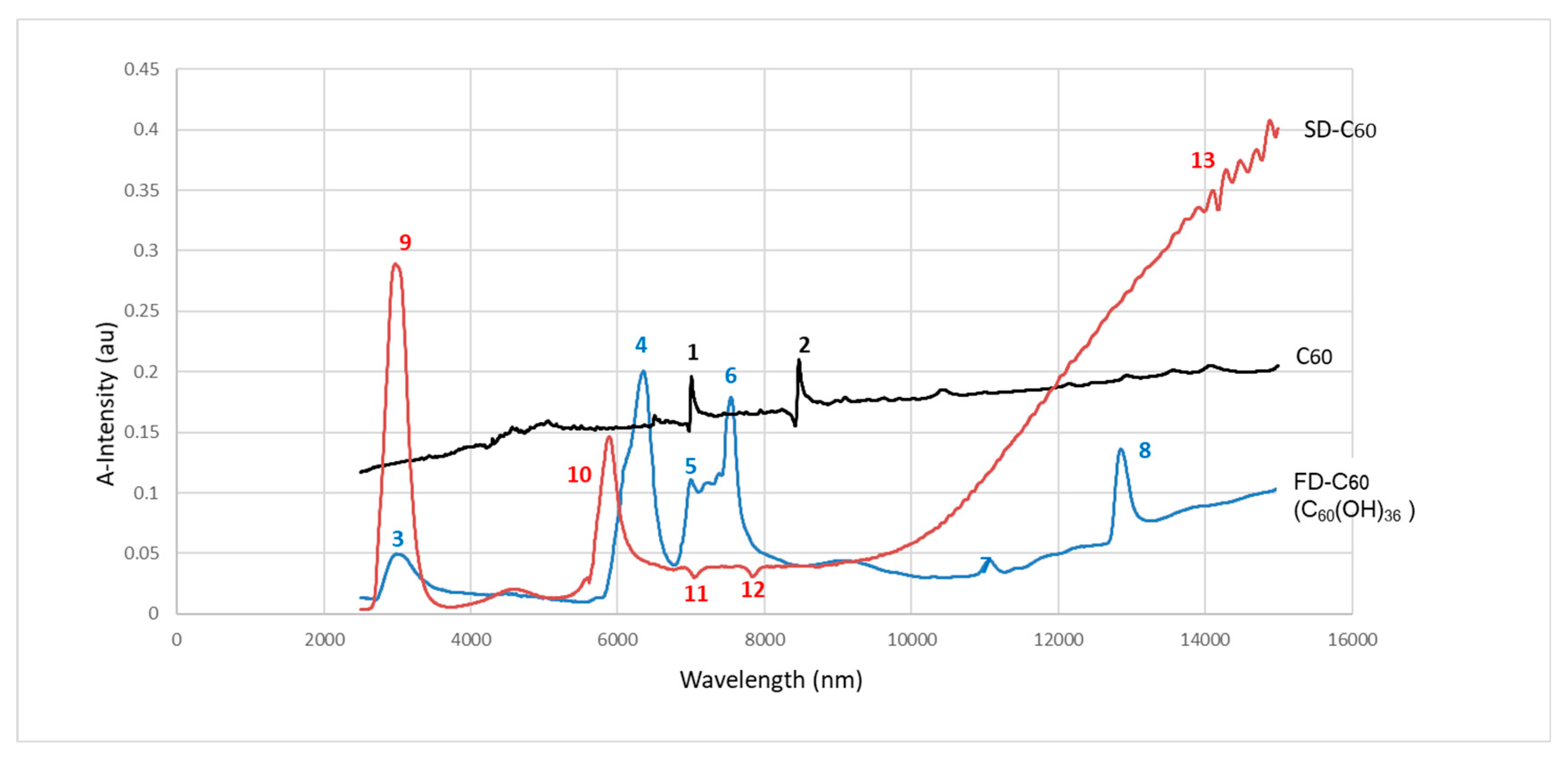
Figure 10.
, FD-C60 ) and SD-C60 () in domain 2500-14000 nm. Peaks 1 and 2 are from C60 molecule, peaks 3 - 8 are from FD-C60, while peaks 9-13 are from SD-C60. Difference of intensity of hydrogen bonds of FD-C60 and SD-C60 is about 5.7 times (peaks 3 and 9).
Figure 10.
, FD-C60 ) and SD-C60 () in domain 2500-14000 nm. Peaks 1 and 2 are from C60 molecule, peaks 3 - 8 are from FD-C60, while peaks 9-13 are from SD-C60. Difference of intensity of hydrogen bonds of FD-C60 and SD-C60 is about 5.7 times (peaks 3 and 9).
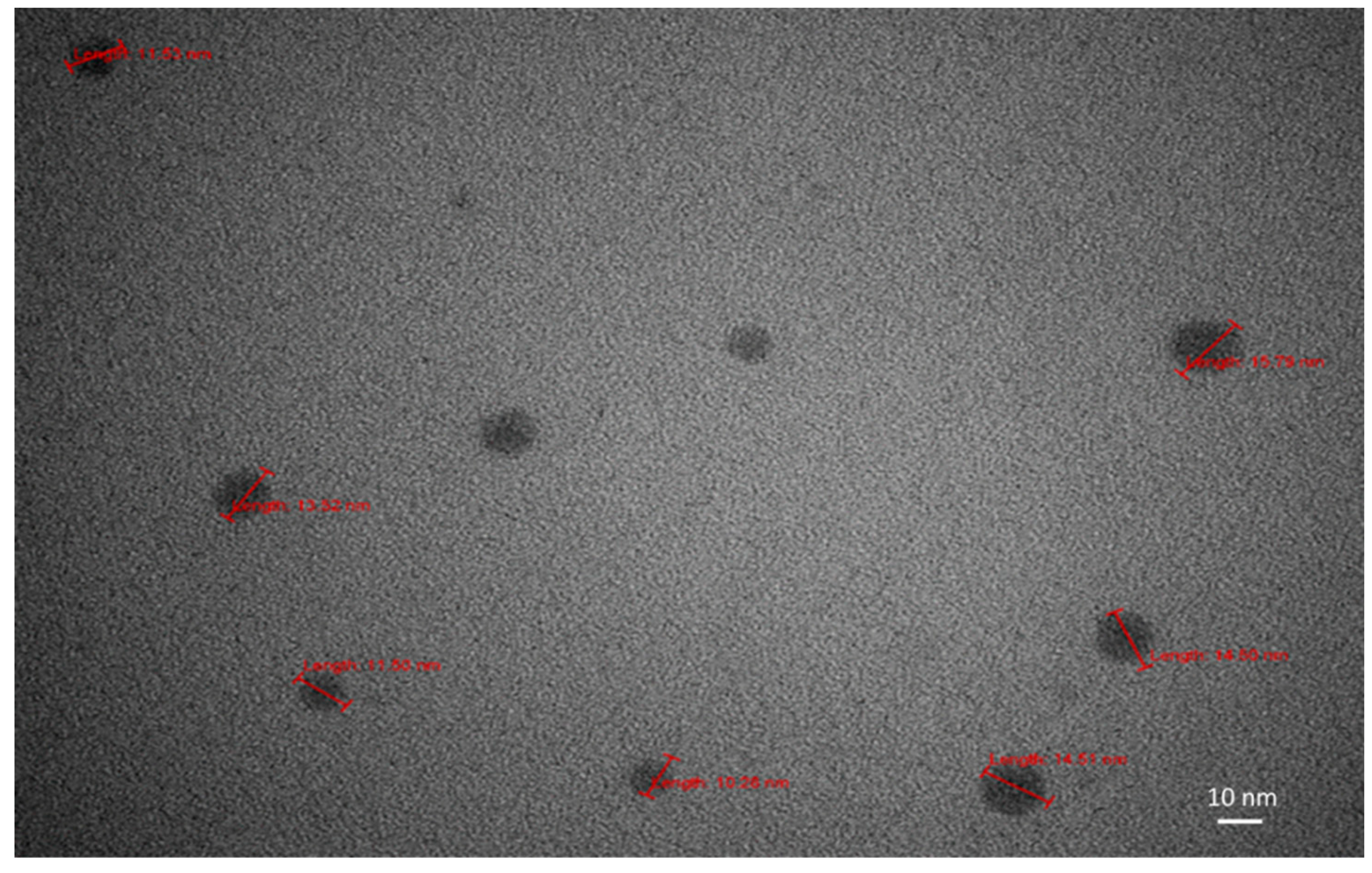
Figure 11.
TEM images of dry SD-C60 (soft-matter), size 10-15 nm. Solution of SD-C60 was three years old (stored at room temperature), what showed that soft-matter of SD-C60 is very stable in solution.
Figure 11.
TEM images of dry SD-C60 (soft-matter), size 10-15 nm. Solution of SD-C60 was three years old (stored at room temperature), what showed that soft-matter of SD-C60 is very stable in solution.
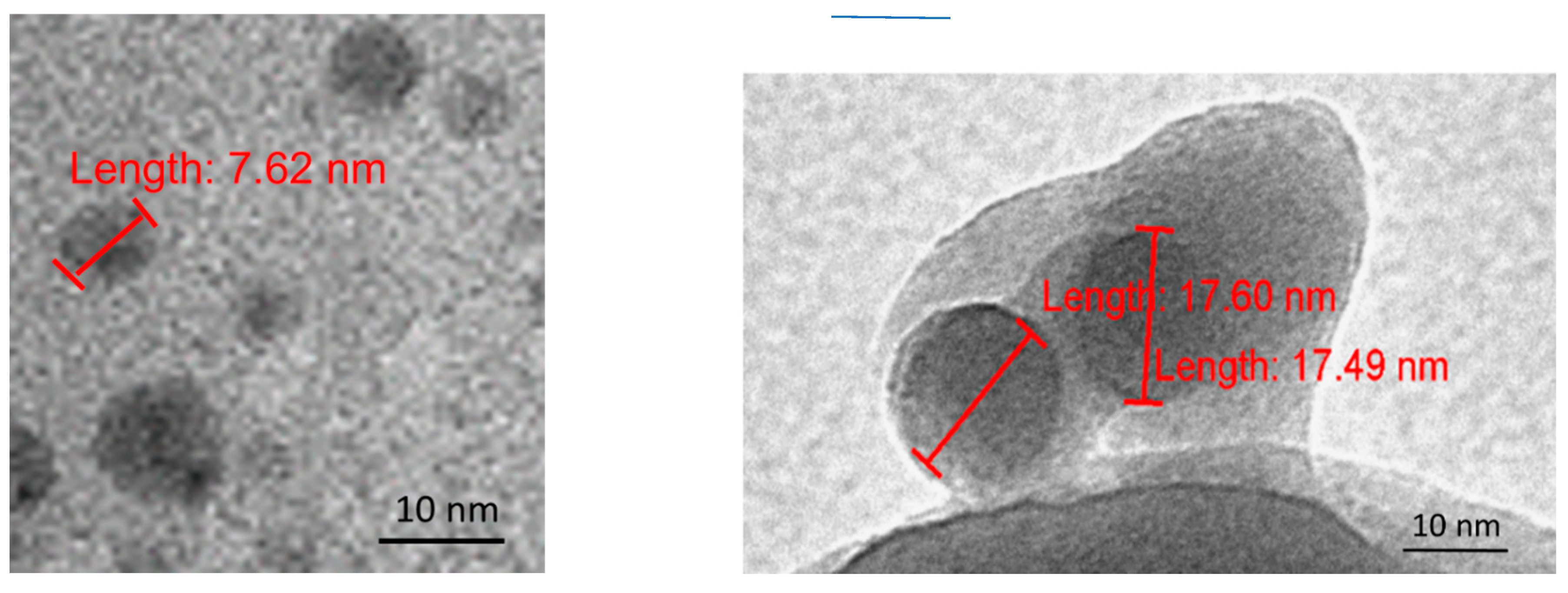
Figure 12.
TEM images of dray SD-C60-Y (3HFWC, as a soft-matter), size 5-10 nm, old 3m years (before drying has been stayed 3 years in solution) (left) and dray SD-C60-W (3HFWC-W, soft-matter), size 17-18 nm, old eight months.
Figure 12.
TEM images of dray SD-C60-Y (3HFWC, as a soft-matter), size 5-10 nm, old 3m years (before drying has been stayed 3 years in solution) (left) and dray SD-C60-W (3HFWC-W, soft-matter), size 17-18 nm, old eight months.
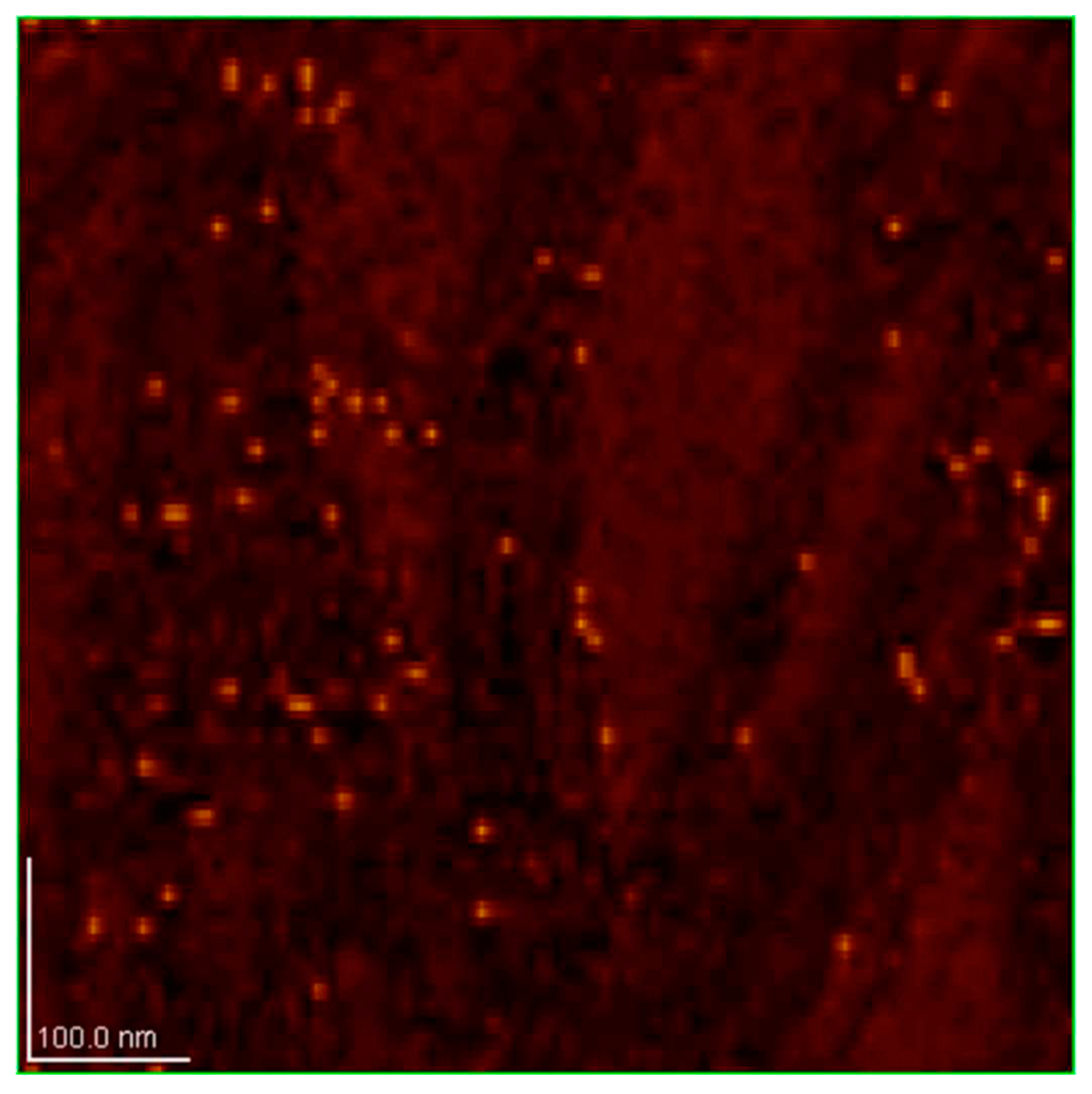
Figure 13.
MFM image of dray SD-C60 (soft-matter), scan size 1.0 × 1.0 μm. On this image SD-C60 are well observed but without precise determination of size.
Figure 13.
MFM image of dray SD-C60 (soft-matter), scan size 1.0 × 1.0 μm. On this image SD-C60 are well observed but without precise determination of size.
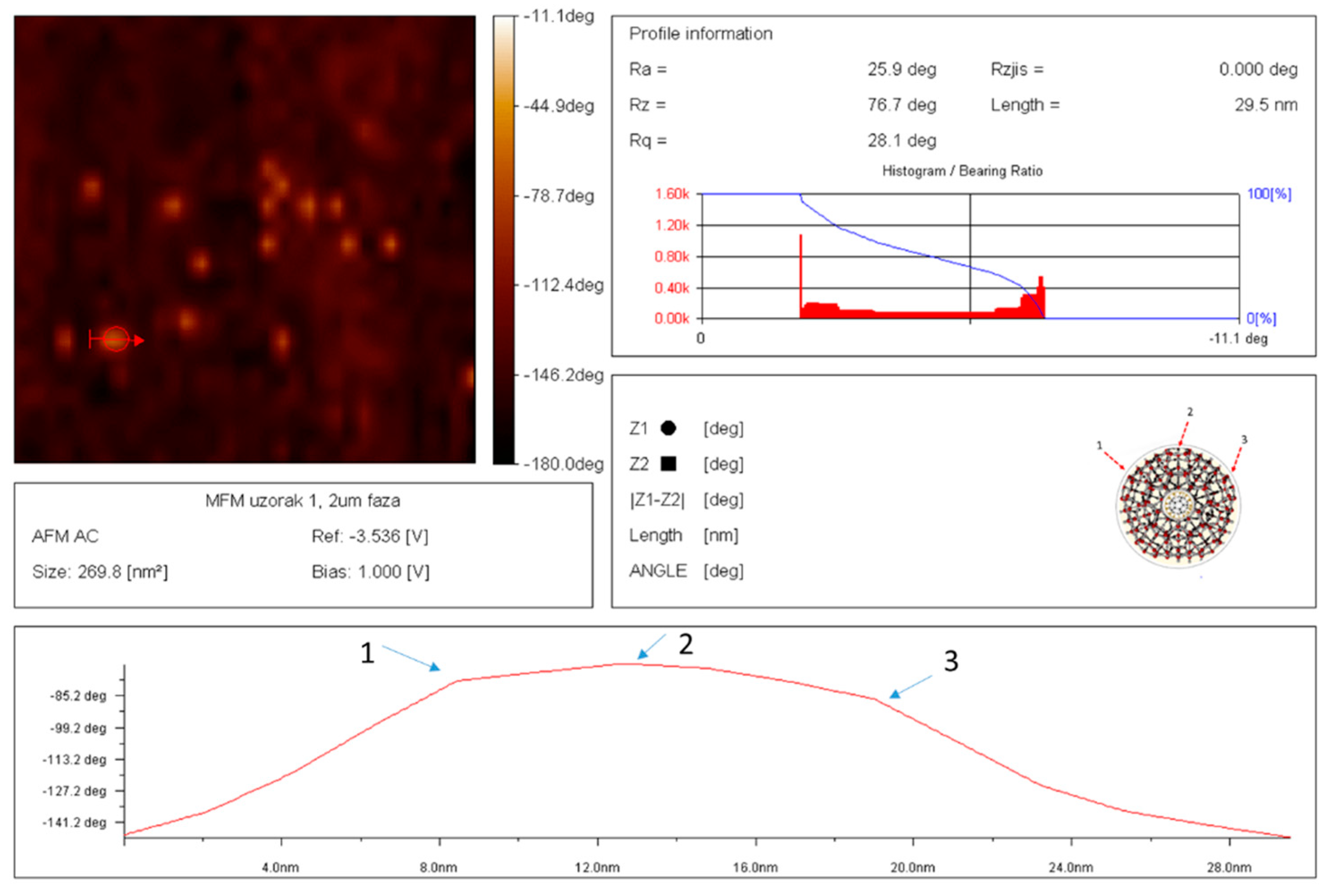
Figure 14.
AFM/MFM image of dray SD-C60-W (3HFWC-W) with precise determination, size 29.5 nm, intensity of dipole-dipole interaction is determinate by angle of declination -80 deg., what means that SD-C60 are rich with dipole interaction (water molecules). It can be seen from the picture that the line that determines the size of the SD-C60 as soft-matter structure is not ideally smooth, but two sudden changes of direction (8 nm and 19 nm) can be observed. The reason for this is that the dry SD-C60 substance is not an ideal sphere but rather an icosahedral symmetry structure that have small “mild breaking points “, position 1,2 and 3.
Figure 14.
AFM/MFM image of dray SD-C60-W (3HFWC-W) with precise determination, size 29.5 nm, intensity of dipole-dipole interaction is determinate by angle of declination -80 deg., what means that SD-C60 are rich with dipole interaction (water molecules). It can be seen from the picture that the line that determines the size of the SD-C60 as soft-matter structure is not ideally smooth, but two sudden changes of direction (8 nm and 19 nm) can be observed. The reason for this is that the dry SD-C60 substance is not an ideal sphere but rather an icosahedral symmetry structure that have small “mild breaking points “, position 1,2 and 3.
Figure 15.
MFM spectra of dray FD-C60 (fullerol). Intensity of paramagnetic spectra is very small because presence of water is very low, only from humidity.
Figure 15.
MFM spectra of dray FD-C60 (fullerol). Intensity of paramagnetic spectra is very small because presence of water is very low, only from humidity.
Figure 16.
MFM spectra of dray SD-C60–W (3HFWC-W). Intensity of paramagnetic spectra is very high which means that 3HFWC-W is very rich in water molecules that interact with MFM tip (dipole-dipole interaction).
Figure 16.
MFM spectra of dray SD-C60–W (3HFWC-W). Intensity of paramagnetic spectra is very high which means that 3HFWC-W is very rich in water molecules that interact with MFM tip (dipole-dipole interaction).
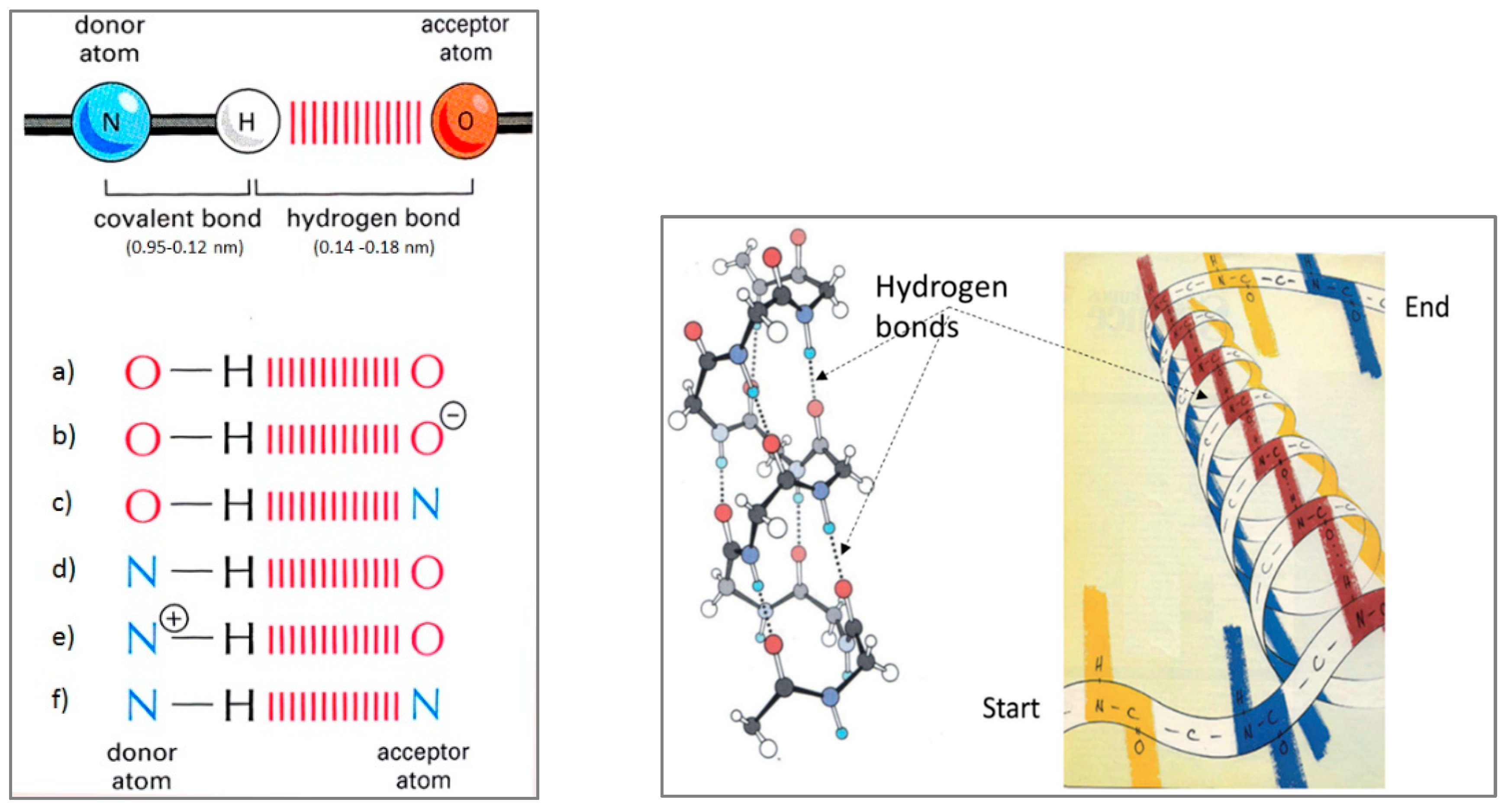
Figure 17.
Six basic types of hydrogen bonds in biological systems (
left) and an example of the arrangement of hydrogen bonds in the collagen structure (
right). It can be seen that collagen (which has 100% an a-helix secondary structure) has three chains of hydrogen bonds. Any disturbance in the vibrations of hydrogen bonds leads to a greater or lesser degree of collagen dysfunction. (Adapted from [
33]).
Figure 17.
Six basic types of hydrogen bonds in biological systems (
left) and an example of the arrangement of hydrogen bonds in the collagen structure (
right). It can be seen that collagen (which has 100% an a-helix secondary structure) has three chains of hydrogen bonds. Any disturbance in the vibrations of hydrogen bonds leads to a greater or lesser degree of collagen dysfunction. (Adapted from [
33]).
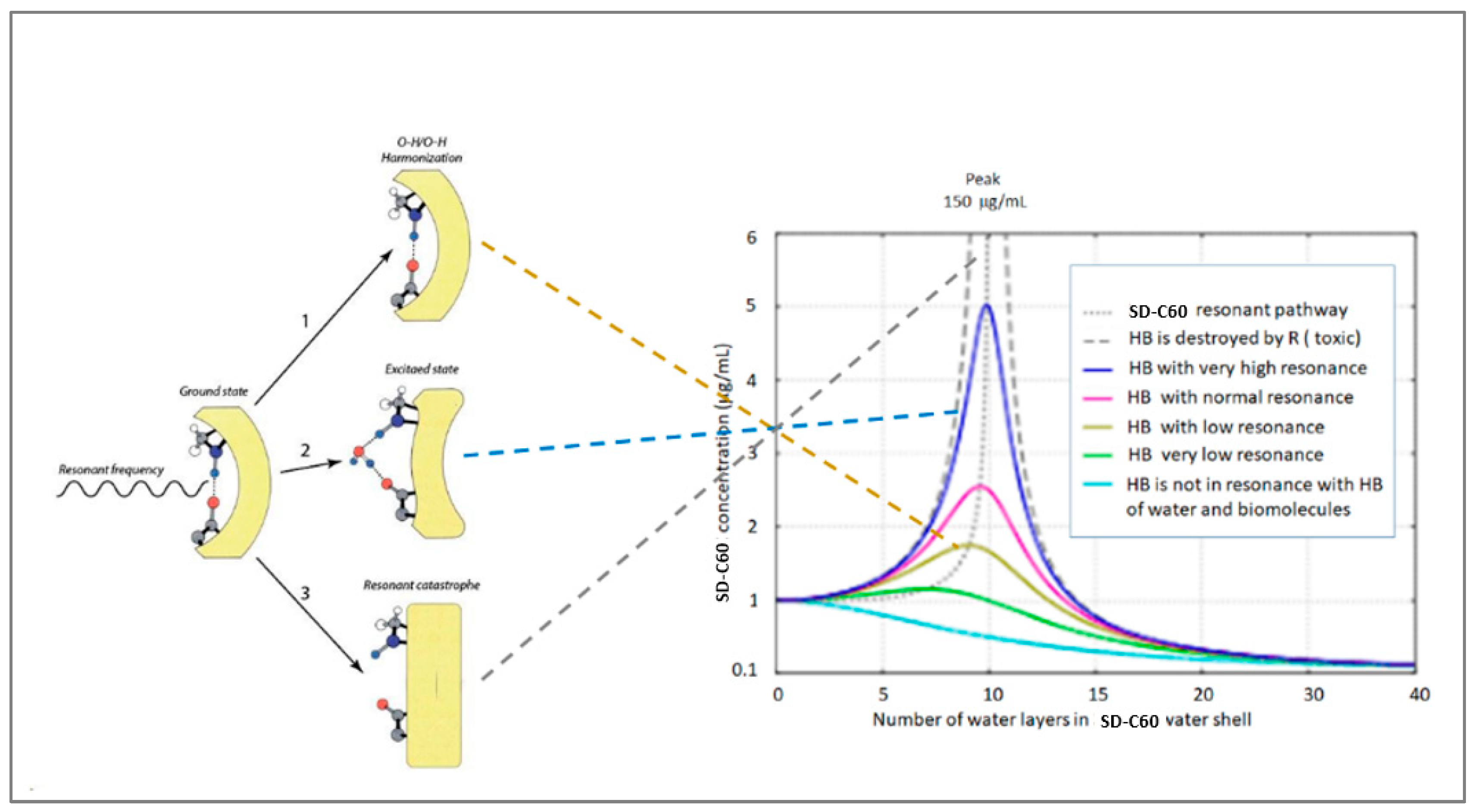
Figure 18.
When the SD-C60 vibrations, transmitted through the hydrogen bonds of water, reach biomolecules (that have hydrogen bonds in their structure), then three basic cases can occur: (1) harmonization of the operation of the hydrogen bond (if the bond is broken, it will be re-established and lead structure to a normal conformational state), (2) excitation of biomolecules by bringing water molecules to the place where the hydrogen bond was (there is a change in the conformational state of the biomolecule at that place), and (3) breaking of the hydrogen bond and dysfunction of the biomolecule. Intensity of action depends of SD-C60 concentration (in soft-matter stae) and number of water layers in icosahedral water onion.
Figure 18.
When the SD-C60 vibrations, transmitted through the hydrogen bonds of water, reach biomolecules (that have hydrogen bonds in their structure), then three basic cases can occur: (1) harmonization of the operation of the hydrogen bond (if the bond is broken, it will be re-established and lead structure to a normal conformational state), (2) excitation of biomolecules by bringing water molecules to the place where the hydrogen bond was (there is a change in the conformational state of the biomolecule at that place), and (3) breaking of the hydrogen bond and dysfunction of the biomolecule. Intensity of action depends of SD-C60 concentration (in soft-matter stae) and number of water layers in icosahedral water onion.
Figure 19.
Antimelanoma action of 3HFWC-W (A) The viability of B16F1, B16F10, and NIH/3T3 cells treated with a wide range of concentrations of 3HFWC-W for 24 h was determined by MTT and CV assays. Cell viability is represented as a percentage of the absorbance value of the control culture grown in the medium, which was arbitrarily assigned a viability value of 100%. Results represent the mean ± SD of one representative out of three independently performed experiments. * p < 0.05 compared to control. (B) The percentage of early (Ann+/PI–) and late (Ann+/PI+) apoptotic cells, (C) morphology of cell nuclei, (D) caspase activity, and (E) proliferation rate in B16F1 and B16F10 melanoma cell cultures treated with the IC50 value of 3HFWC-W for 24 h were determined by Ann V-FITC/PI, PI, ApoStat, and CFSE staining, respectively, and subsequent flow cytometry (B, D, E) and fluorescence microscopy (C, orig. magnification 200×). Representative dot plots, histograms, and micrographs of one out of three independently performed experiments are shown.
Figure 19.
Antimelanoma action of 3HFWC-W (A) The viability of B16F1, B16F10, and NIH/3T3 cells treated with a wide range of concentrations of 3HFWC-W for 24 h was determined by MTT and CV assays. Cell viability is represented as a percentage of the absorbance value of the control culture grown in the medium, which was arbitrarily assigned a viability value of 100%. Results represent the mean ± SD of one representative out of three independently performed experiments. * p < 0.05 compared to control. (B) The percentage of early (Ann+/PI–) and late (Ann+/PI+) apoptotic cells, (C) morphology of cell nuclei, (D) caspase activity, and (E) proliferation rate in B16F1 and B16F10 melanoma cell cultures treated with the IC50 value of 3HFWC-W for 24 h were determined by Ann V-FITC/PI, PI, ApoStat, and CFSE staining, respectively, and subsequent flow cytometry (B, D, E) and fluorescence microscopy (C, orig. magnification 200×). Representative dot plots, histograms, and micrographs of one out of three independently performed experiments are shown.
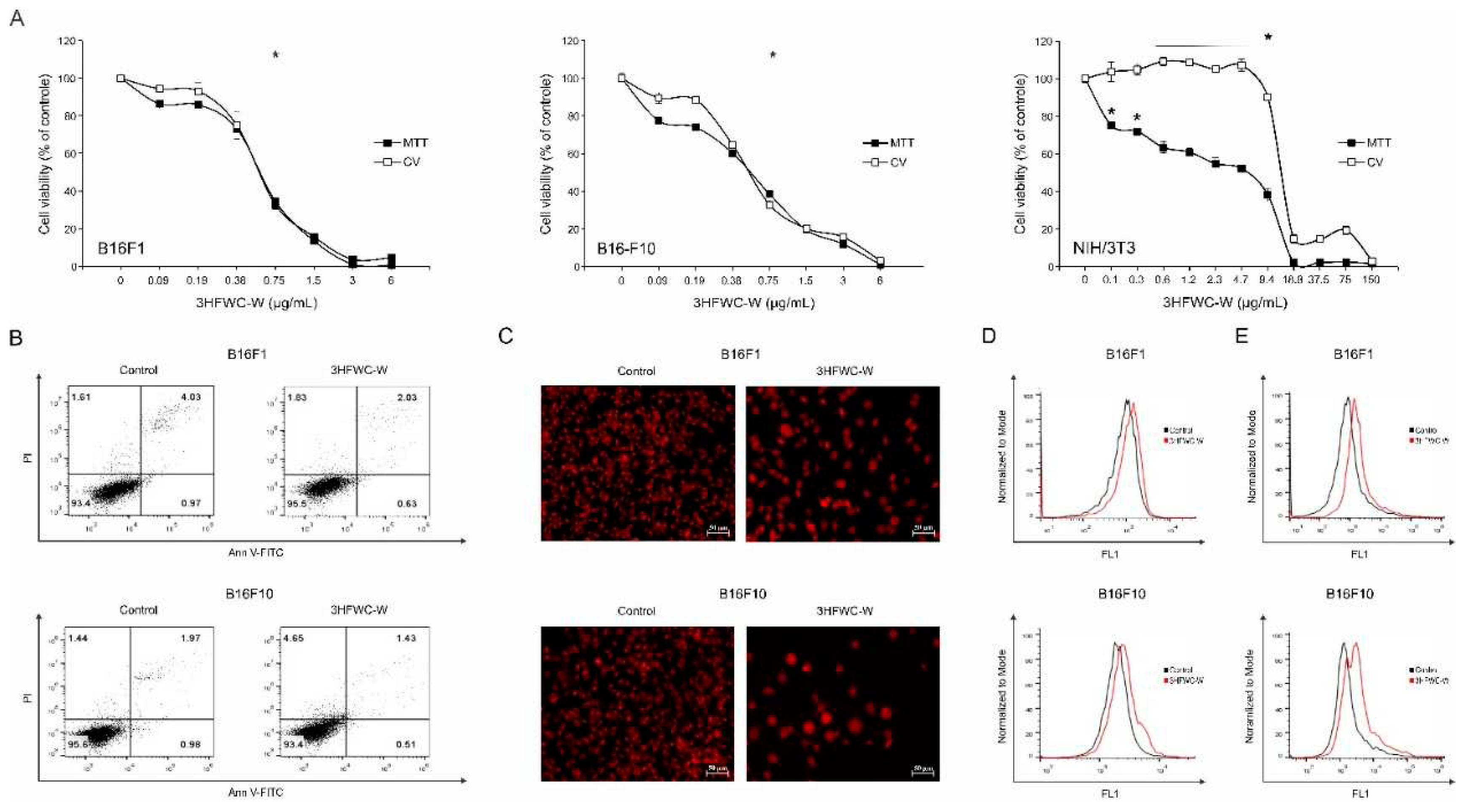
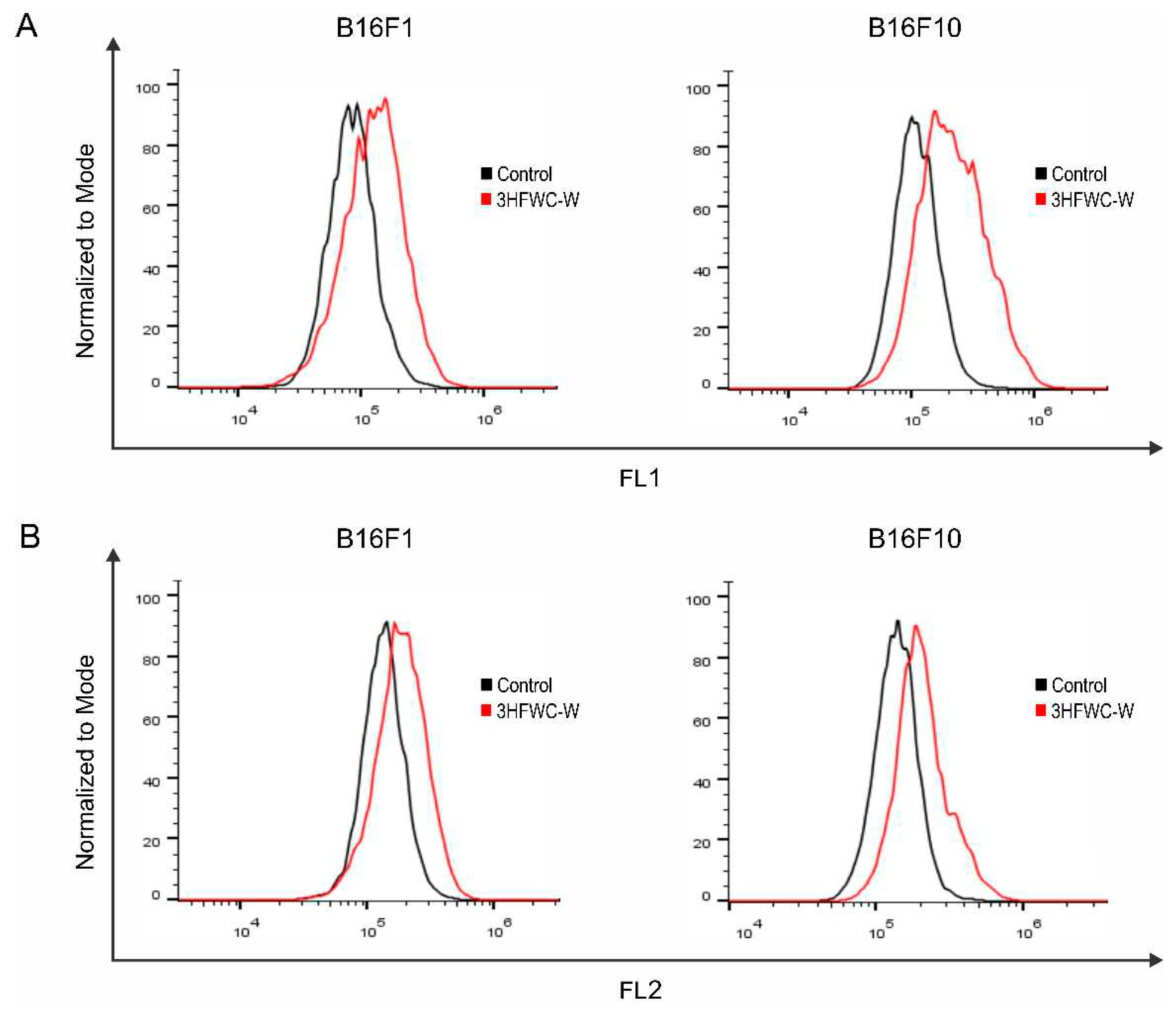
Figure 20.
The effect of 3HFWC-W on intracellular ROS/RNS production in melanoma cells. Fluorescence intensity of B16F1 and B16F10 cells treated with IC50 value of 3HFWC-W for 24 h was determined by (A) DHR 123 and (B) DHE staining and subsequent flow cytometry. Representative histograms of one out of three independently performed experiments are shown.
Figure 20.
The effect of 3HFWC-W on intracellular ROS/RNS production in melanoma cells. Fluorescence intensity of B16F1 and B16F10 cells treated with IC50 value of 3HFWC-W for 24 h was determined by (A) DHR 123 and (B) DHE staining and subsequent flow cytometry. Representative histograms of one out of three independently performed experiments are shown.
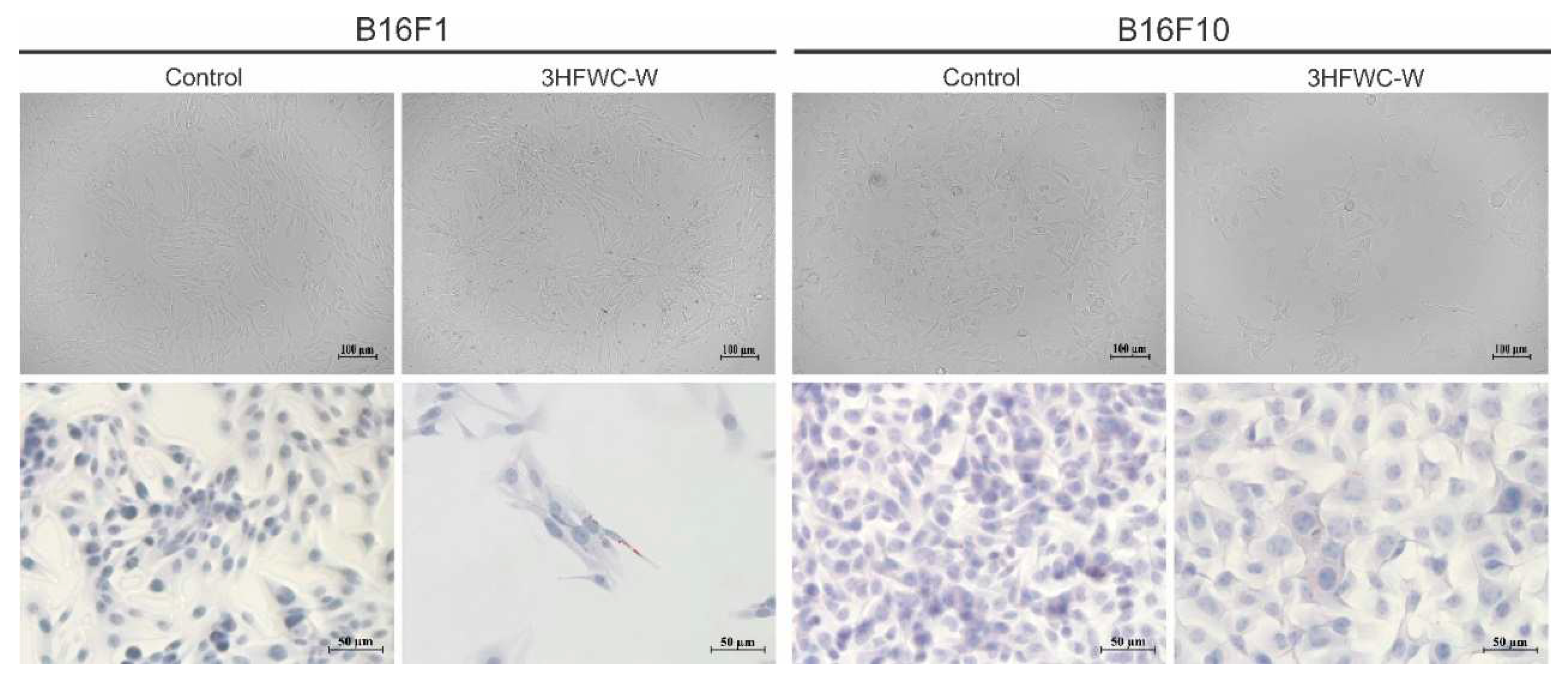
Figure 21.
The effect of 3HFWC-W on the morphology and phenotype of melanoma cells. Morphological and intracellular changes of B16F1 and B16F10 cells treated with the IC50 value of 3HFWC-W for 24 h was determined without staining (upper panel micrographs) and by Oil Red O staining (lower panel micrographs, orig. magnification 200×) and subsequent light microscopy. Representative micrographs are shown.
Figure 21.
The effect of 3HFWC-W on the morphology and phenotype of melanoma cells. Morphological and intracellular changes of B16F1 and B16F10 cells treated with the IC50 value of 3HFWC-W for 24 h was determined without staining (upper panel micrographs) and by Oil Red O staining (lower panel micrographs, orig. magnification 200×) and subsequent light microscopy. Representative micrographs are shown.
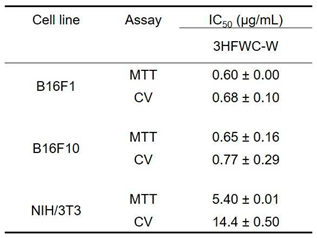
Table 1.
IC50 values of 3HFWC-W in all tested cell lines obtained from MTT and CV assays1.
Table 1.
IC50 values of 3HFWC-W in all tested cell lines obtained from MTT and CV assays1.