Submitted:
31 October 2023
Posted:
01 November 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
- IVT utilization rate
- MT utilization rate
- DTN time
- DTG time
- The Joint Commission (TJC) stroke quality measures: STK1 (venous thromboembolism [VTE] prophylaxis), STK2 (discharge on antithrombotic therapy), STK3 (anticoagulation therapy for atrial fibrillation/flutter), STK4 (thrombolytic therapy), STK5 (antithrombotic therapy by end of hospital day 2), STK6 (discharged on statin medication), STK8 (stroke education), and STK10 (rehabilitation assessment).
- Completion of TIA workup: (1) brain magnetic resonance imaging (MRI), (2) vascular imaging of the head and neck with magnetic resonance angiography (MRA) or computed tomographic angiography (CTA), (3) echocardiogram transthoracic and/or transesophageal, (4) electrocardiogram, (5) lipid panel, (6) hemoglobin A1C. A scale from 0 to 6 was developed and calculated for each patient. A score of 6 out of 6 indicates performance of all 6 TIA tests.
- TJC quality measures: STK1, STK8, and STK10.
- Performance of vascular imaging (CTA, MRA)
- TJC quality measures: STK1, STK8, and STK10.
Results
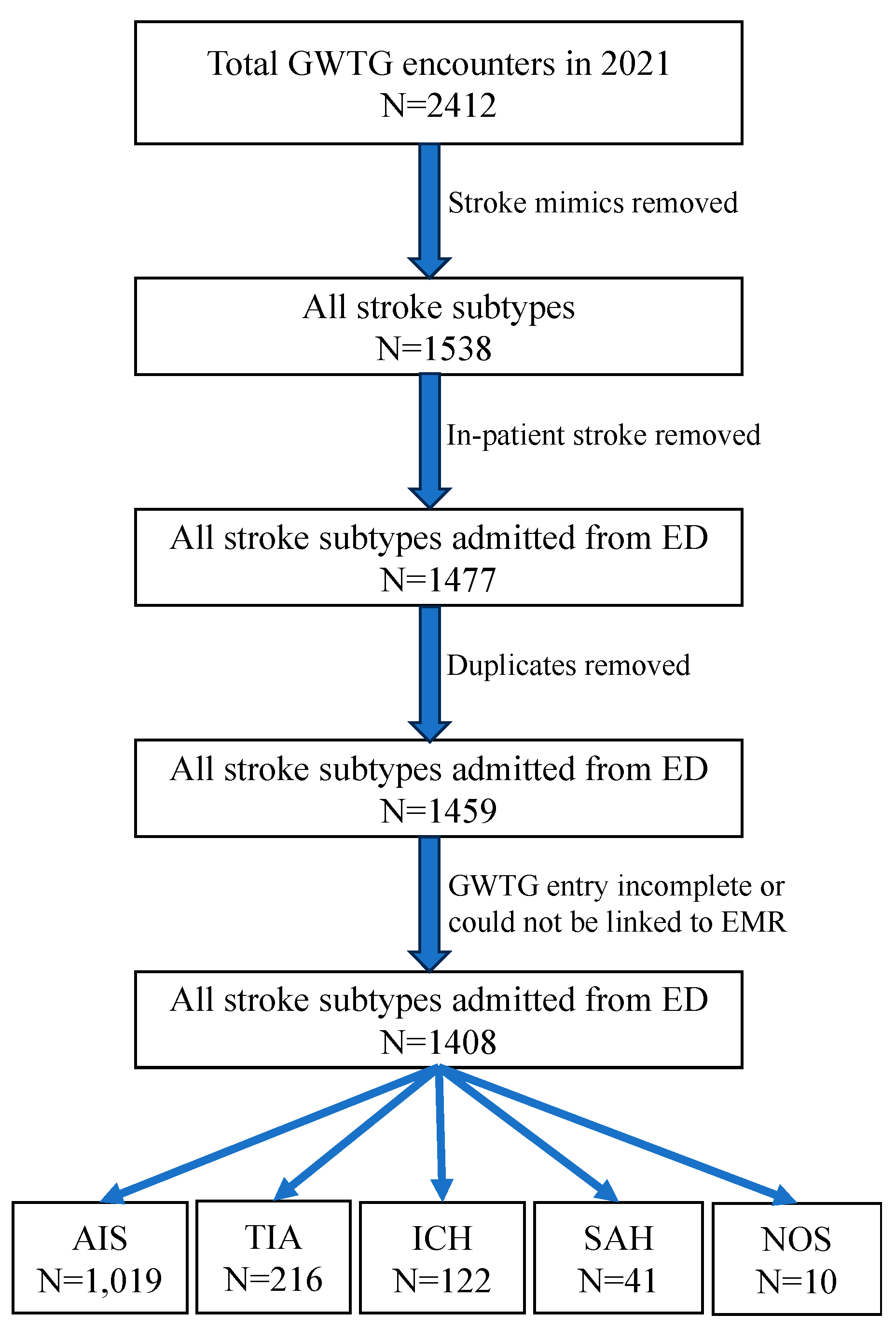
| White | Non-white | p value | |
|---|---|---|---|
| Sample size | 91 | 27 | |
| Age in years, median (IQR) | 74 (64-82) | 57 (43-63) | <0.001 |
| Women, frequency (%) | 41 (45%) | 12 (43%) | 1 |
| Number of concomitant medical diagnoses, median (IQR) | 4 (3-5) | 2 (1-4) | 0.003 |
| Hypertension, frequency (%) | 70 (77%) | 17 (61%) | 0.148 |
| Diabetes mellitus, frequency (%) | 20 (22%) | 6 (21%) | 1 |
| Dyslipidemia, frequency (%) | 53 (58%) | 8 (29%) | 0.011 |
| Atrial fibrillation/flutter, frequency (%) | 16 (18%) | 0 | 0.012 |
| Coronary artery disease, frequency (%) | 18 (20%) | 2 (7%) | 0.154 |
| Heart failure, frequency (%) | 6 (7%) | 1 (4%) | 1 |
| Current smoker, frequency (%) | 6 (7%) | 2 (7%) | 1 |
| Alcohol or illicit drug abuse, frequency (%) | 11 (12%) | 3 (11%) | 1 |
| Sleep apnea, frequency (%) | 6 (7%) | 1 (4%) | 1 |
| Overweight/obesity, frequency (%) | 42 (46%) | 17 (61%) | 0.258 |
| Prior stroke, frequency (%) | 19 (21%) | 7 (25%) | 0.842 |
| Family history of stroke, frequency (%) | 17 (19%) | 1 (4%) | 0.069 |
| Hemoglobin A1c (%), median (IQR) | 7.3 (6.1-9.9) | 8.2 (6.8-9.0) | 1 |
| Ambulatory status prior to index stroke, frequency (%) | 0.531 |
||
| ● Independent | ● 75 (95%0 | ● 24 (100%) | |
| ● With assist | ● 2 (3%) | ● 0 | |
| ● Unable | ● 2 (3%) | ● 0 | |
| Mode of arrival to the ED, frequency (%) | 0.444 | ||
| ● EMS | ● 51 (57%) | ● 13 (50%) | |
| ● Private | ● 17 (19%) | ● 8 (31%) | |
| ● Hospital transfer | ● 21 (24%) | ● 5 (19%) | |
| Time from last known well to ED arrival in minutes, median (IQR) | 281 (83.5-838) | 570 (72.5-792) | 0.942 |
| Time from ED arrival to CT performance in minutes, median (IQR) | 27 (19-69.5) | 41 (20-98.5) | 0.3 |
| SBP in mm Hg, median (IQR) | 162 (138-190) | 172 (140-202) | 0.303 |
| DBP in mm Hg, median (IQR) | 90 (78-106) | 85 (68-112) | 0.412 |
| Blood glucose in ED in mg/dL, median (IQR) | 125 (106-162) | 134 (128-174) | 0.052 |
| GCS score done on admission, frequency (%) | 78 (86%) | 21 (75%) | 0.246 |
| GCS score on admission, median (IQR) | 15 (11-15) | 12 (7-15) | 0.308 |
| IV antihypertensive use in the ED, frequency (%) | 55 (65%) | 19 (68%) | 0.94 |
| Intubation in the ED | 10 (12%) | 6 (22%) | 0.222 |
| External ventricular drain placement, frequency (%) | 5 (6%) | 4 (14%) | 0.224 |
| Decompressive craniectomy, frequency (%) | 1 (1%) | 1 (4%) | 0.439 |
| Hematoma evacuation, frequency (%) | 5 (6%) | 4 (14%) | 0.224 |
| Length of stay in hours, median (IQR) | 84.4 (6.9-143.2) | 41.3 (5.7-73.9) | 0.069 |
| In-hospital mortality, frequency (%) | 17 (19%) | 5 (18%) | 1 |
| Discharge destination, frequency (%) | 0.830 | ||
| ● Home | ● 15 (20%) | ● 7 (30%) | |
| ● Rehab/nursing home | ● 29 (39%) | ● 7 (30%) | |
| ● Acute care hospital | ● 23 (31%) | ● 7 (30%) | |
| ● Hospice | ● 7 (10%) | ● 2 (9%) | |
| ● Against medical advice | ● 0 | ● 0 | |
| Ambulatory status at discharge, frequency (%) | 0.834 | ||
| ● Independent | ● 21 (42%) | ● 5 (33%) | |
| ● With assist | ● 26 (52%) | ● 9 (60%) | |
| ● Unable | ● 3 (6%) | ● 1 (7%) | |
| STK1: VTE prophylaxis | 100% | 92% | 0.25 |
| STK8: stroke education | 91% | 80% | 1 |
| STK10: rehabilitation assessment | 100% | 100% | 1 |
| IQR: interquartile range; EMS: emergency medical services; ED: emergency department; CT: computed tomography; SBP: systolic blood pressure; DBP: diastolic blood pressure; GCS: Glasgow Coma Scale; IV: intravenous; STK: The Joint Commission (TJC) stroke quality metrics (note that we only selected the STKs relevant to ICH); VTE: venous thromboembolism; | |||
| White | Non-White | p value | |
|---|---|---|---|
| Sample size | 212 | 19 | |
| Age in years, median (IQR) | 77 (64-84) | 59 (51-77) | 0.003 |
| Women, frequency (%) | 117 (55%) | 11 (58%) | 1 |
| Number of concomitant medical diagnoses, median (IQR) | 3 (2-5) | 3 (3-4) | 0.579 |
| Hypertension, frequency (%) | 128 (65%) | 13 (68%) | 0.984 |
| Diabetes mellitus, frequency (%) | 40 (20%) | 6 (32%) | 0.252 |
| Dyslipidemia, frequency (%) | 120 (61%) | 9 (47%) | 0.351 |
| Atrial fibrillation/flutter, frequency (%) | 35 (18%) | 1 (5%) | 0.210 |
| Coronary artery disease, frequency (%) | 42 (21%) | 2 (11%) | 0.376 |
| Heart failure, frequency (%) | 16 (8%) | 0 | 0.371 |
| Current smoker, frequency (%) | 9 (5%) | 2 (11%) | 0.252 |
| Alcohol or illicit drug abuse, frequency (%) | 6 (3%) | 1 (5%) | 0.482 |
| Sleep apnea, frequency (%) | 23 (12%) | 1 (5%) | 0.703 |
| Overweight/obesity, frequency (%) | 107 (55%) | 9 (47%) | 0.717 |
| Prior stroke, frequency (%) | 31 (16%) | 3 (16%) | 1.000 |
| Family history of stroke, frequency (%) | 21 (11%) | 0 | 0.228 |
| Hemoglobin A1c (%), median (IQR) | 5.5 (5.3-5.9) | 6.1 (5.9-6.2) | 0.002 |
| Hemoglobin A1c (%) in patients with known diabetes mellitus diagnosis, median (IQR) | 7.0 (5.8-7.8) | 6.6 (6.1-7.5) | 0.918 |
| LDL (mg/dL), median (IQR) | 87 (64-115) | 103 (77-126) | 0.357 |
| LDL (mg/dL) in patients with known dyslipidemia, median (IQR) | 79 (58-113) | 113 (68-141) | 0.300 |
| HDL (mg/dL), median (IQR) | 49 (42-62) | 42 (35-68) | 0.175 |
| HDL (mg/dL) in patients with known dyslipidemia, median (IQR) | 49 (44-59) | 43 (39-64) | 0.491 |
| Triglycerides (mg/dL), median (IQR) | 117 (84-164) | 138 (90-180) | 0.539 |
| Triglycerides (mg/dL) in patients with known dyslipidemia, median (IQR) | 119 (89-163) | 127 (98-158) | 0.942 |
| Total cholesterol (mg/dL), median (IQR)+A34:A43 | 164 (139-201) | 180 (162-211) | 0.272 |
| Total cholesterol (mg/dL) in patients with known dyslipidemia, median (IQR) | 158 (135-200) | 178 (151-212) | 0.348 |
| Ambulatory status prior to index stroke, frequency (%) | 0.108 |
||
| ● Independent | ● 188 (99%) | ● 17 (94%) | |
| ● With assist | ● 1 (1%) | ● 0 | |
| ● Unable | ● 1 (1%) | ● 1 (6%) | |
| Mode of arrival, frequency (%) | 0.016 | ||
| ● EMS | ● 73 (38%) | ● 2 (11%) | |
| ● Private | ● 105 (54%) | ● 16 (89%) | |
| ● Hospital transfer | ● 16 (8%) | ● 0 | |
| Time from last known well to ED arrival in minutes, median (IQR) | 132 (64-560) | 165 (105-368) | 0.203 |
| Time from ED arrival to CT performance in minutes, median (IQR) | 31 (17-64) | 32 (29-39) | 0.828 |
| SBP in mm Hg, median (IQR) | 156 (138-179) | 152 (139-176) | 0.587 |
| DBP in mm Hg, median (IQR) | 83 (75-94) | 82 (70-100) | 0.914 |
| Blood glucose in ED in mg/dL, median (IQR) | 108 (96-133) | 117 (102-137) | 0.421 |
| Initial NIHSS documentation, frequency (%) | 137 (70%) | 13 (68%) | 1.0 |
| Initial NIHSS score, median (IRQ) | 0 (0-1) | 0 (0-1) | 0.586 |
| IV antihypertensive use in the ED, frequency (%) | 8 (4%) | 0 | 1.000 |
| Intubation in the ED | 0 | 0 | 1.000 |
| TIA workup completeness score, median (IQR) | 5 (3-6) | 5 (2-6) | 1.0 |
| TIA workup | 0.93 | ||
| ● Complete | ● 67 (34%) | ● 7 (37%) | |
| ● Partial | ● 128 (65%) | ● 12 (63%) | |
| ● Not done | ● 1 (1%) | ● 0 | |
| Length of stay in hours, median (IQR) | 22.8 (5.3-46) | 26.75 (23.6-46.1) | 0.548 |
| In-hospital mortality, frequency (%) | 0 | 0 | 1 |
| Discharge destination, frequency (%) | 0.484 | ||
| ● Home | ● 178 (91%) | ● 18 (95%) | |
| ● Rehab/nursing home | ● 12 (6%) | ● 0 | |
| ● Acute care hospital | ● 6 (3%) | ● 1 (5%) | |
| ● Hospice | ● 0 | ● 0 | |
| ● Against medical advice | ● 0 | ● 0 | |
| Ambulatory status at discharge, frequency (%) | 0.011 |
||
| ● Independent | ● 86 (87%) | ● 13 (93%) | |
| ● With assist | ● 13 (13%) | ● 0 | |
| ● Unable | ● 0 | ● 1 (7%) | |
| IQR: interquartile range; HDL: high density lipoprotein; LDL: low density lipoprotein; EMS: emergency medical services; ED: emergency department; CT: computed tomography; SBP: systolic blood pressure; DBP: diastolic blood pressure; NIHSS: National Institutes of Health Stroke Scale; IV: intravenous; TIA: transient ischemic attack | |||
| White | Non-White | p value | |
|---|---|---|---|
| Sample size | 31 | 8 | |
| Age in years, median (IQR) | 70 (57-84) | 60 (48-75) | 0.244 |
| Women, frequency (%) | 21 (68%) | 6 (75%) | 1 |
| Number of concomitant medical diagnoses, median (IQR) | 2 (1-3) | 2 (1-3) | 0.901 |
| Hypertension, frequency (%) | 14 (45%) | 4 (50%) | 1 |
| Diabetes mellitus, frequency (%) | 1 (3%) | 2 (25%) | 0.101 |
| Dyslipidemia, frequency (%) | 9 (29%) | 4 (50%) | 0.402 |
| Atrial fibrillation/flutter, frequency (%) | 3 (10%) | 1 (13%) | 1 |
| Coronary artery disease, frequency (%) | 3 (10%) | 0 | 1 |
| Heart failure, frequency (%) | 1 (3%) | 0 | 1 |
| Current smoker, frequency (%) | 4 (13%) | 1 (13%) | 1 |
| Alcohol or illicit drug abuse, frequency (%) | 1 (3%) | 0 | 1 |
| Sleep apnea, frequency (%) | 3 (10%) | 1 (13%) | 1 |
| Overweight/obesity, frequency (%) | 13 (42%) | 3 (38%) | 1 |
| Prior stroke, frequency (%) | 4 (13%) | 0 | 0.563 |
| Family history of stroke, frequency (%) | 3 (10%) | 1 (13%) | 1 |
| Hemoglobin A1c (%), median (IQR) | 5.6 (5.3-6.05) | 5.4 (5.3-6.92) | 0.98 |
| Ambulatory status prior to index stroke, frequency (%) | 1 | ||
| ● Independent | ● 28 (97%) | ● 7 (100%) | |
| ● With assist | ● 1 (3%) | ● 0 | |
| ● Unable | ● 0 | ● 0 | |
| Mode of arrival to the ED, frequency (%) | 0.184 | ||
| ● EMS | ● 15 (52%) | ● 7 (88%) | |
| ● Private | ● 12 (41%) | ● 1 (13%) | |
| ● Hospital transfer | ● 2 (7%) | ● 0 | |
| Time from last known well to hospital arrival in minutes, median (IQR) | 808 (166-1749.5) | 72 (59-1769.5) | 0.862 |
| Time from hospital arrival to CT performance in minutes, median (IQR) | 64 (35.25-98.25) | 33 (28-47.5) | 0.112 |
| SBP in mm Hg, median (IQR) | 154 (131-168) | 118 (103-192) | 0.379 |
| DBP in mm Hg, median (IQR) | 81 (74-106) | 114 (91-133) | 0.038 |
| Blood glucose in ED in mg/dL, median (IQR) | 115 (94-157) | 139 (123-150) | 0.325 |
| GCS on admission, median (IQR) | 15 (12-15) | 15 (9-15) | 0.813 |
| GCS documented on admission, frequency (%) | 29 (94%) | 3 (38%) | 0.002 |
| IV antihypertensive use in the ED, frequency (%) | 8 (4%) | 0 | 1 |
| Intubation in the ED | 6 (19%) | 4 (57%) | 0.063 |
| External ventricular drain placement, frequency (%) | 8 (26%) | 2 (29%) | 1 |
| Performance of CTA or MRA | 25 (81%) | 6 (75.0%) | 0.658 |
| Aneurysm coiling, frequency (%) | 7 (23%) | 2 (29%) | 1 |
| Aneurysm clipping, frequency (%) | 3 (10%) | 0 | 1 |
| Length of stay in hours, median (IQR) | 27.6 (5.7-71.1) | 3 (1.9-5.4) | 0.035 |
| In-hospital mortality, frequency (%) | 2 (6%) | 1 (13%) | 0.508 |
| Discharge destination, frequency (%) | 0.481 | ||
| ● Home | ● 8 (28%) | ● 1 (14%) | |
| ● Rehab/nursing home | ● 4 (14%) | ● 0 | |
| ● Acute care hospital | ● 16 (55%) | ● 6 (86%) | |
| ● Hospice | ● 9 (31%) | ● 0 | |
| ● Against medical advice | ● 0 | ● 0 | |
| IQR: interquartile range; EMS: emergency medical services; ED: emergency department; CT: computed tomography; SBP: systolic blood pressure; DBP: diastolic blood pressure; GCS: Glasgow Coma Scale; IV: intravenous; CTA: computed tomographic angiogram; MRA: magnetic resonance angiogram | |||
Discussion
Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed consent
Data availability
Financial Conflict of Interest
References
- de Havenon, A.; Zhou, L.W.; Johnston, K.C.; Dangayach, N.S.; Ney, J.; Yaghi, S.; Sharma, R.; Abbasi, M.; Delic, A.; Majersik, J.J.; et al. Twenty-Year Disparity Trends in United States Stroke Death Rate by Age, Race/Ethnicity, Geography, and Socioeconomic Status. Neurology 2023, 101, e464–e474. [Google Scholar] [CrossRef] [PubMed]
- Ariss, R.W.; Minhas, A.M.K.; Lang, J.; Ramanathan, P.K.; Khan, S.U.; Kassi, M.; Warraich, H.J.; Kolte, D.; Alkhouli, M.; Nazir, S. Demographic and Regional Trends in Stroke-Related Mortality in Young Adults in the United States, 1999 to 2019. J. Am. Heart Assoc. 2022, 11, e025903. [Google Scholar] [CrossRef] [PubMed]
- Reshetnyak, E.; Ntamatungiro, M.; Pinheiro, L.C.; Howard, V.J.; Carson, A.P.; Martin, K.D.; Safford, M.M. Impact of Multiple Social Determinants of Health on Incident Stroke. Stroke 2020, 51, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Acquah, I.; Javed, Z.; Valero-Elizondo, J.; Yahya, T.; Blankstein, R.; Virani, S.S.; Blaha, M.J.; Hyder, A.A.; Dubey, P.; et al. Social Determinants of Health Among Non-Elderly Adults With Stroke in the United States. Mayo Clin. Proc. 2022, 97, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.A.; Duncan, P.W.; Nguyen-Huynh, M.N.; Ogedegbe, O.G. Interventions Targeting Racial/Ethnic Disparities in Stroke Prevention and Treatment. Stroke 2020, 51, 3425–3432. [Google Scholar] [CrossRef] [PubMed]
- Gu, A.; Yue, Y.; Desai, R.P.; Argulian, E. Racial and Ethnic Differences in Antihypertensive Medication Use and Blood Pressure Control Among US Adults With Hypertension: The National Health and Nutrition Examination Survey, 2003 to 2012. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003166. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.M.; West, B.T.; Yaker, Z.; Lauinger, B.; McCullers, D.; Haubert, J.; Tahboub, M.A.; Everett, G.J. Disparities in Diabetes-Related Multiple Chronic Conditions and Mortality: The Influence of Race. Diabetes Res. Clin. Pract. 2020, 159, 107984. [Google Scholar] [CrossRef] [PubMed]
- Bekelis, K.; Missios, S.; MacKenzie, T.A. Access Disparities to Magnet Hospitals for Ischemic Stroke Patients. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2017, 43, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Suolang, D.; Chen, B.J.; Wang, N.-Y.; Gottesman, R.F.; Faigle, R. Geographic and Regional Variability in Racial and Ethnic Disparities in Stroke Thrombolysis in the United States. Stroke 2021, 52, e782–e787. [Google Scholar] [CrossRef]
- Otite, F.O.; Saini, V.; Sur, N.B.; Patel, S.; Sharma, R.; Akano, E.O.; Anikpezie, N.; Albright, K.; Schmidt, E.; Hoffman, H.; et al. Ten-Year Trend in Age, Sex, and Racial Disparity in tPA (Alteplase) and Thrombectomy Use Following Stroke in the United States. Stroke 2021, 52, 2562–2570. [Google Scholar] [CrossRef]
- Kim, Y.; Sharrief, A.; Kwak, M.J.; Khose, S.; Abdelkhaleq, R.; Salazar-Marioni, S.; Zhang, G.-Q.; Sheth, S.A. Underutilization of Endovascular Therapy in Black Patients With Ischemic Stroke: An Analysis of State and Nationwide Cohorts. Stroke 2022, 53, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, F.; Xu, H.; Maud, A.; Gupta, V.; Vellipuram, A.; Fonarow, G.C.; Matsouaka, R.A.; Xian, Y.; Reeves, M.; Smith, E.E.; et al. Temporal Trends in Racial and Ethnic Disparities in Endovascular Therapy in Acute Ischemic Stroke. J. Am. Heart Assoc. 2022, 11, e023212. [Google Scholar] [CrossRef] [PubMed]
- Ajinkya, S.; Almallouhi, E.; Turner, N.; Al Kasab, S.; Holmstedt, C.A. Racial/Ethnic Disparities in Acute Ischemic Stroke Treatment Within a Telestroke Network. Telemed. J. E-Health Off. J. Am. Telemed. Assoc. 2020, 26, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, S.A.; Wang, K.; Dong, C.; Ciliberti-Vargas, M.A.; Gutierrez, C.M.; Yi, L.; Romano, J.G.; Perez, E.; Tyson, B.A.; Ayodele, M.; et al. Disparities and Trends in Door-to-Needle Time. Stroke 2017, 48, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Siegler, J.E.; Ortega-Gutierrez, S.; Hester, T.; Haussen, D.C.; Nogueira, R.G.; Liebeskind, D.S.; Zaidat, O.O.; Vora, N.; Desai, S.; Jadhav, A.P.; et al. Interaction of Ethnicity and Arrival Method on Thrombectomy Delay: The Society of Vascular and Interventional Neurology Collaboration. Stroke Vasc. Interv. Neurol. 2022, 2, e000217. [Google Scholar] [CrossRef]
- Schwamm, L.H.; Reeves, M.J.; Pan, W.; Smith, E.E.; Frankel, M.R.; Olson, D.; Zhao, X.; Peterson, E.; Fonarow, G.C. Race/Ethnicity, Quality of Care, and Outcomes in Ischemic Stroke. Circulation 2010, 121, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Banks, Evan; Rasner, C. ; Leslie, A.; Hussein, H.M. The Devastating Effects Of Racial Disparities In Acute Stroke Management: Where We Stand Now. Minn. Med. Mag. 2023, 4, 10–14. [Google Scholar]
- U.S. Census Bureau QuickFacts: St. Paul City, Minnesota. Available online: https://www.census.gov/quickfacts/fact/table/stpaulcityminnesota/PST045222 (accessed on 5 October 2023).
- Hussein, H.M.; Kashyap, B.; O’Keefe, L.; Droegemueller, C.; Othman, S.I.; Yang, M.K.; Hanson, L.R. Stroke Characteristics in a Cohort of Hmong American Patients. J. Am. Heart Assoc. 2023, 12, e026763. [Google Scholar] [CrossRef]
- Suzuki, K.; Izumi, M. The Incidence of Hemorrhagic Stroke in Japan Is Twice Compared with Western Countries: The Akita Stroke Registry. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2015, 36, 155–160. [Google Scholar] [CrossRef]
- Tsai, C.-F.; Anderson, N.; Thomas, B.; Sudlow, C.L.M. Comparing Risk Factor Profiles between Intracerebral Hemorrhage and Ischemic Stroke in Chinese and White Populations: Systematic Review and Meta-Analysis. PloS One 2016, 11, e0151743. [Google Scholar] [CrossRef]
- Klatsky, A.L.; Friedman, G.D.; Sidney, S.; Kipp, H.; Kubo, A.; Armstrong, M.A. Risk of Hemorrhagic Stroke in Asian American Ethnic Groups. Neuroepidemiology 2005, 25, 26–31. [Google Scholar] [CrossRef] [PubMed]
- van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; van der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, Case Fatality, and Functional Outcome of Intracerebral Haemorrhage over Time, According to Age, Sex, and Ethnic Origin: A Systematic Review and Meta-Analysis. Lancet Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Jolink, W.M.T.; Klijn, C.J.M.; Brouwers, P.J.A.M.; Kappelle, L.J.; Vaartjes, I. Time Trends in Incidence, Case Fatality, and Mortality of Intracerebral Hemorrhage. Neurology 2015, 85, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C.; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar] [CrossRef] [PubMed]
- Puolakka, T.; Strbian, D.; Harve, H.; Kuisma, M.; Lindsberg, P.J. Prehospital Phase of the Stroke Chain of Survival: A Prospective Observational Study. J. Am. Heart Assoc. 2016, 5, e002808. [Google Scholar] [CrossRef] [PubMed]
- Lioutas, V.-A.; Ivan, C.S.; Himali, J.J.; Aparicio, H.J.; Leveille, T.; Romero, J.R.; Beiser, A.S.; Seshadri, S. Incidence of Transient Ischemic Attack and Association With Long-Term Risk of Stroke. JAMA 2021, 325, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.P.; Madsen, T.E.; Bravata, D.M.; Wira, C.R.; Johnston, S.C.; Ashcraft, S.; Burrus, T.M.; Panagos, P.D.; Wintermark, M.; Esenwa, C.; et al. Diagnosis, Workup, Risk Reduction of Transient Ischemic Attack in the Emergency Department Setting: A Scientific Statement From the American Heart Association. Stroke 2023, 54, e109–e121. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L.; Fonarow, G.C.; Smith, E.E.; Reeves, M.J.; Grau-Sepulveda, M.V.; Pan, W.; Olson, D.M.; Hernandez, A.F.; Peterson, E.D.; Schwamm, L.H. Time to Treatment With Intravenous Tissue Plasminogen Activator and Outcome From Acute Ischemic Stroke. JAMA 2013, 309, 2480–2488. [Google Scholar] [CrossRef]
- Mochari-Greenberger, H.; Xian, Y.; Hellkamp, A.S.; Schulte, P.J.; Bhatt, D.L.; Fonarow, G.C.; Saver, J.L.; Reeves, M.J.; Schwamm, L.H.; Smith, E.E. Racial/Ethnic and Sex Differences in Emergency Medical Services Transport Among Hospitalized US Stroke Patients: Analysis of the National Get With The Guidelines-Stroke Registry. J. Am. Heart Assoc. 2015, 4, e002099. [Google Scholar] [CrossRef]
- Springer, M.V.; Labovitz, D.L.; Hochheiser, E.C. Race-Ethnic Disparities in Hospital Arrival Time after Ischemic Stroke. Ethn. Dis. 27, 125–132. [CrossRef]
- Hussein, H.M.; Droegemueller, C.; Xiong, P.; Xiong, Z.; Dyke, S.V.; Mueller-Hussein, J. Hmong Stroke Knowledge Survey. J. Health Care Poor Underserved 2022, 33, 2052–2059. [Google Scholar] [CrossRef]
- Birmingham, L.E.; Arens, A.; Longinaker, N.; Kummet, C. Trends in Ambulance Transports and Costs among Medicare Beneficiaries, 2007-2018. Am. J. Emerg. Med. 2021, 47, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Asaithambi, G.; Tong, X.; Coleman King, S.M.; George, M.G. Contemporary Trends in the Treatment of Mild Ischemic Stroke with Intravenous Thrombolysis: Paul Coverdell National Acute Stroke Program. Cerebrovasc. Dis. Basel Switz. 2022, 51, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liang, L.; Fonarow, G.C.; Smith, E.E.; Bhatt, D.L.; Matsouaka, R.A.; Xian, Y.; Schwamm, L.H.; Saver, J.L. Comparison of Clinical Care and In-Hospital Outcomes of Asian American and White Patients With Acute Ischemic Stroke. JAMA Neurol. 2019, 76, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, S.J.; Aggarwal, N.T.; Richards, C.; O’Neill, K.; Holl, J.L.; Prabhakaran, S. Racial Disparities in Refusal of Stroke Thrombolysis in Chicago. Neurology 2018, 90, e359–e364. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, S.J.; Zhang, S.; Matsouaka, R.; Xian, Y.; Shah, S.; Lytle, B.L.; Solomon, N.; Schwamm, L.H.; Smith, E.E.; Saver, J.L.; et al. Race-Ethnic Disparities in Rates of Declination of Thrombolysis for Stroke. Neurology 2022, 98, e1596–e1604. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; Ballard, D.J.; Matchar, D.B.; Whisnant, J.P.; Samsa, G.P. Racial Variation in Treatment for Transient Ischemic Attacks: Impact of Participation by Neurologists. Health Serv. Res. 2000, 34, 1413–1428. [Google Scholar] [PubMed]
- Canedo, J.R.; Miller, S.T.; Schlundt, D.; Fadden, M.K.; Sanderson, M. Racial/Ethnic Disparities in Diabetes Quality of Care: The Role of Healthcare Access and Socioeconomic Status. J. Racial Ethn. Health Disparities 2018, 5, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Elhussein, A.; Anderson, A.; Bancks, M.P.; Coday, M.; Knowler, W.C.; Peters, A.; Vaughan, E.M.; Maruthur, N.M.; Clark, J.M.; Pilla, S. Racial/Ethnic and Socioeconomic Disparities in the Use of Newer Diabetes Medications in the Look AHEAD Study. Lancet Reg. Health - Am. 2021, 6, 100111. [Google Scholar] [CrossRef]
- Kenik, J.; Jean-Jacques, M.; Feinglass, J. Explaining Racial and Ethnic Disparities in Cholesterol Screening. Prev. Med. 2014, 65, 65–69. [Google Scholar] [CrossRef]
- Aggarwal, R.; Bhatt, D.L.; Rodriguez, F.; Yeh, R.W.; Wadhera, R.K. Trends in Lipid Concentrations and Lipid Control Among US Adults, 2007-2018. JAMA 2022, 328, 737–745. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Bundy, J.D.; Geng, S.; Tian, L.; He, H.; Li, X.; Ferdinand, K.C.; Anderson, A.H.; Dorans, K.S.; Vasan, R.S.; et al. Social, Behavioral, and Metabolic Risk Factors and Racial Disparities in Cardiovascular Disease Mortality in U.S. Adults. Ann. Intern. Med. 2023, 176, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Chiu, N.; Wadhera, R.K.; Moran, A.E.; Raber, I.; Shen, C.; Yeh, R.W.; Kazi, D.S. Racial/Ethnic Disparities in Hypertension Prevalence, Awareness, Treatment, and Control in the United States, 2013 to 2018. Hypertens. Dallas Tex 1979 2021, 78, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.; Oldroyd, J.; Islam, Md.M.; Tarazona-Meza, C.E.; Islam, R.M. Prevalence of Hypertension and Controlled Hypertension among United States Adults: Evidence from NHANES 2017-18 Survey. Int. J. Cardiol. Hypertens. 2020, 7, 100061. [Google Scholar] [CrossRef]
- Hussein, H.M.; Chrenka, E.; Yang, M.K.; Margolis, K.L.; Kottke, T.E. Describing Racial Disparity in Hypertension Control in a Large Minnesota Outpatient Practice. Health Serv. Res. Manag. Epidemiol. 2023, 10, 23333928231192830. [Google Scholar] [CrossRef]
- Flanagin, A.; Frey, T.; Christiansen, S.L. ; AMA Manual of Style Committee Updated Guidance on the Reporting of Race and Ethnicity in Medical and Science Journals. JAMA 2021, 326, 621–627. [Google Scholar] [CrossRef]
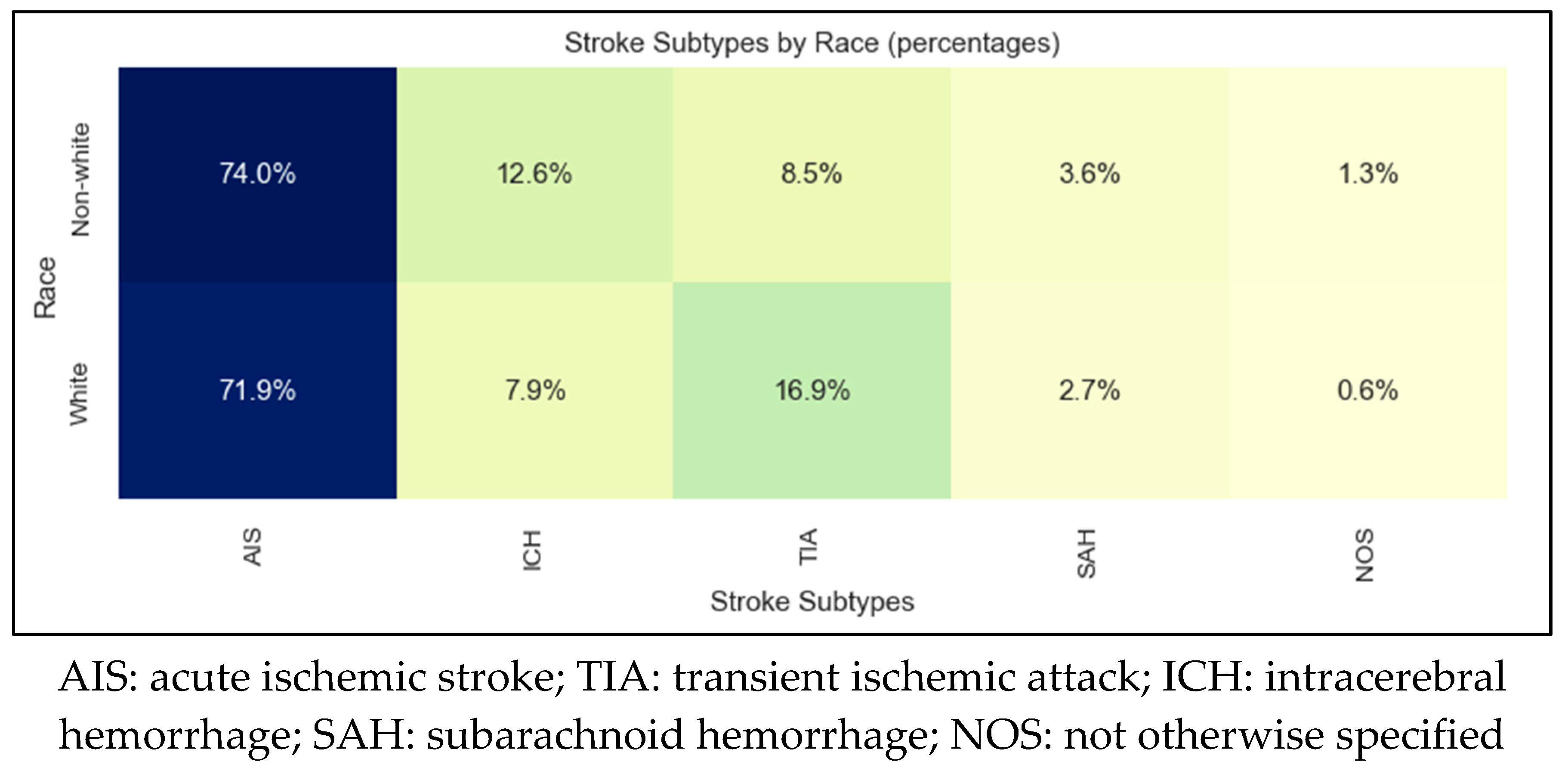
| AIS | TIA | ICH | SAH | |
|---|---|---|---|---|
| Sample size | 1019 | 216 | 122 | 41 |
| Mean age | 72±15 | 72±15 | 68±17 | 67±16 |
| Female sex | 51% | 57% | 44% | 70% |
| Non-White | 15% | 7% | 22% | 20% |
| Countries of origin | United States 87% Laos 6% Russia 1% Myanmar 1% Vietnam 1% Cambodia 1% |
United States 92% Laos 2% United Kingdom 1% Cambodia 1% |
United States 82% Laos 4% Somalia 3% Cambodia 2% Philippines 2% Myanmar 2% Mexico 2% |
United States 85%, Vietnam 10% Cambodia 3% Laos 3% |
| Non-English primary language | 11% | 5% | 13% | 15% |
| Need for interpretation | 7% | 2% | 7% | 5% |
| AIS: acute ischemic stroke; TIA: transient ischemic attack; ICH: intracerebral hemorrhage; SAH: subarachnoid hemorrhage | ||||
| White | Non-White | p value | |
|---|---|---|---|
| Sample size | 860 | 173 | |
| Age in years, median (IQR) | 75 (64-85) | 66 (57-73) | <0.001 |
| Women, median (IQR) | 424 (51%) | 81 (48%) | 0.713 |
| Number of concomitant medical diagnoses, median (IQR) | 4 (3-5) | 4 (2-5) | 0.042 |
| Hypertension, frequency (%) | 600 (72%) | 119 (72%) | 1.000 |
| Diabetes mellitus, frequency (%) | 224 (27%) | 78 (45%) | <0.001 |
| Dyslipidemia, frequency (%) | 502 (60%) | 79 (48%) | 0.004 |
| Atrial fibrillation/flutter, frequency (%) | 175 (21%) | 18 (11%) | 0.004 |
| Coronary artery disease, frequency (%) | 186 (22%) | 17 (10%) | 0.001 |
| Heart failure, frequency (%) | 84 (10%) | 11 (7%) | 0.220 |
| Current smoker, frequency (%) | 111 (13%) | 19 (12%) | 0.610 |
| Alcohol or illicit drug abuse, frequency (%) | 66 (8%) | 9 (5%) | 0.347 |
| Sleep apnea, frequency (%) | 96 (12%) | 11 (7%) | 0.087 |
| Overweight/obesity, frequency (%) | 451 (54%) | 84 (51%) | 0.490 |
| Prior stroke, frequency (%) | 211 (25%) | 58 (35%) | 0.006 |
| Family history of stroke, frequency (%) | 87 (10%) | 13 (8%) | 0.387 |
| Hemoglobin A1c (%), median (IQR) | 5.7 (5.4-6.3) | 6.3 (5.6-8.9) | <0.001 |
| Hemoglobin A1c (%) in patients with known diabetes mellitus diagnosis, median (IQR) | 7.1 (6.3-8.1) | 8.5 (7.0-10.0) | <0.001 |
| LDL (mg/dL), median (IQR) | 84 (61-116) | 99 (75-133) | <0.001 |
| LDL (mg/dL) in patients with known dyslipidemia, median (IQR) | 78 (55-111) | 99 (75-134) | <0.001 |
| HDL (mg/dL), median (IQR) | 47 (38-58) | 44 (37-54) | 0.066 |
| HDL (mg/dL) in patients with known dyslipidemia, median (IQR) | 45 (38-58) | 45 (40-54) | 0.932 |
| Triglycerides (mg/dL), median (IQR) | 112 (81-161) | 125 (85-184) | 0.033 |
| Triglycerides (mg/dL) in patients with known dyslipidemia, median (IQR) | 114 (82-166) | 132 (91-186) | 0.047 |
| Total cholesterol (mg/dL), median (IQR) | 160 (132-194) | 176 (144-210) | <0.001 |
| Total cholesterol (mg/dL) in patients with known dyslipidemia, median (IQR) | 154 (126-188) | 180 (150-211) | <0.001 |
| Ambulatory status prior to index stroke, frequency (%) | 0.841 | ||
| ● Independent | ● 722 (91%) | ● 146 (92%) | |
| ● With assist | ● 47 (6%) | ● 9 (6%) | |
| ● Unable | ● 27 (3%) | ● 4 (3%) | |
| Mode of arrival, frequency (%) | 0.009 | ||
| ● EMS | ● 342 (44%) | ● 50 (32%) | |
| ● Private | ● 279 (39%) | ● 79 (51%) | |
| ● Hospital transfer | ● 131 (17%) | ● 26 (17%) | |
| Time from last known well to ED arrival in minutes, median (IQR) | 393 (93-1188) | 856 (263-1921) | 0.001 |
| Time from ED arrival to CT performance in minutes, median (IQR) | 20 (15-28) | 17 (14-20) | 0.163 |
| Door-to-needle time in minutes, median (IQR) | 65 (46-88) | 56 (41-132) | 0.832 |
| Door-to-groin time in minutes, median (IQR) | 112 (101-123) | 134 (101-190) | 0.342 |
| SBP in mm Hg, median (IQR) | 155 (137-173) | 158 (137-182) | 0.114 |
| DBP in mm Hg, median (IQR) | 84 (74-96) | 87 (78-99) | 0.033 |
| Blood glucose in ED in mg/dL, median (IQR) | 115 (100-143) | 131 (109-188) | <0.001 |
| Initial NIHSS documentation, frequency (%) | 625 (75%) | 119 (72%) | 0.477 |
| Initial NIHSS score, median (IRQ) | 2 (0-6) | 3 (1-6) | 0.023 |
| Initial NIHSS score for IVT patients, median (IRQ) | 6 (3-13) | 7.5 (5.5-14) | 0.546 |
| 24-hour NIHSS score, median (IRQ) | 1 (0-4) | 3 (1-6) | <0.001 |
| 24-hour NIHSS score for IVT patients, median (IRQ) | 3 (1-8) | 2 (1-6) | 0.804 |
| IV antihypertensive use in the ED, frequency (%) | 76 (10%) | 18 (12%) | 0.554 |
| Intubation in the ED | 21 (3%) | 1 (1%) | 0.235 |
| IV thrombolysis utilization rate, frequency (%) | 111 (13%) | 12 (7%) | 0.042 |
| IV thrombolysis utilization rate in patients presenting within 3.5 hours, frequency (%) | 102 (42%) | 8 (32%) | 0.433 |
| IV thrombolysis administered at a different facility before transfer, frequency (%) | 25 (5%) | 3 (4%) | 0.784 |
| Mechanical thrombectomy utilization rate, frequency (%) | 42 (5%) | 5 (3%) | 0.36 |
| Length of stay in hours, median (IQR) | 66.3 (41.7-112.6) | 64 (45.1-113) | 0.716 |
| In-hospital mortality, frequency (%) | 38 (5%) | 1 (1%) | 0.029 |
| Discharge destination, frequency (%) | 0.071 | ||
| ● Home | ● 459 (58%) | ● 113 (69%) | |
| ● Rehab/nursing home | ● 242 (30%) | ● 35 (21%) | |
| ● Acute care hospital | ● 55 (7%) | ● 12 (7%) | |
| ● Hospice | ● 28 (4%) | ● 4 (2%) | |
| ● Against medical advice | ● 10 (1%) | ● 0 | |
| Ambulatory status at discharge, frequency (%) | 0.97 | ||
| ● Independent | ● 440 (61%) | ● 93 (62%) | |
| ● With assist | ● 250 (35%) | ● 51 (34%) | |
| ● Unable | ● 31 (4%) | ● 6 (4%) | |
| STK1: VTE prophylaxis | 95% | 94% | 0.727 |
| STK2: discharge on antithrombotic therapy | 100% | 100% | 1 |
| STK3: anticoagulation therapy for atrial fibrillation/flutter | 99% | 100% | 1 |
| STK4: thrombolytic therapy | 92% | 100% | 1 |
| STK5: antithrombotic therapy by end of hospital day 2 | 99% | 98% | 0.348 |
| STK6: discharged on statin medication | 99% | 100% | 1 |
| STK8: stroke education | 89% | 91% | 0.797 |
| STK10: rehabilitation assessment | 100% | 100% | 1 |
| IQR: interquartile range; HDL: high density lipoprotein; LDL: low density lipoprotein; EMS: emergency medical services; ED: emergency department; CT: computed tomography; SBP: systolic blood pressure; DBP: diastolic blood pressure; NIHSS: National Institutes of Health Stroke Scale; IV: intravenous; VTE: venous thromboembolism; STK: The Joint Commission (TJC) stroke quality metrics (note that STK7 and STK9 have been retired by TJC) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





