1. Introduction
‘Play’ covers a range of behaviors with shared characteristics such as apparent lack of an immediate goal, being accompanied by specific body movements (‘play markers, e.g., Newberry, 1988) and often being expressed in the absence of fitness threats (e.g., Burghardt, 2005). Play behaviors can be expressed solitarily or in a group (Fagen, 1981). Three types are distinguished: locomotor play (running, hopping, leaping etc.), social play (playing with another) and object play (playing with objects). Burghardt (2005) states five characteristics that distinguish a behavior as being ‘play’: “ a) this behavior is incompletely functional in the context expressed, b) play is voluntary or rewarding, c) it is modified developmentally or structurally compared with when it is used in its normal, functional context, d) performed repeatedly, but not necessarily in an invariant form and f) that it starts in healthy, relatively unstressed animals in a relaxed context”.
Some of these characteristics have led researchers to suggest play as a promising welfare indicator, while recognizing that play can generate, as well as reflect, improved or good welfare (Boissy et al., 2007; Held and Spinka, 2011; Ahloy-Dallaire et al, 2018; Keeling et al., 2021). Play behavior is easy to detect and measure in a practical and non-invasive way (Burghardt, 2005; Jones et al., 2021). Another peculiar characteristic of this behavior is its tendency to be contagious, as when initiated by one or two individuals it tends to spread to other individuals in the group (Reimert et al., 2013; Schwing et al., 2017). Therefore, stimulating the occurrence of play behavior in captive animals has the potential to improve their current welfare and spread improved welfare in a group (Held and Spinka, 2011), as has been observed in some species using environmental enrichment (e.g., Tayassu pecari: Nogueira et al., 2011; domestic pig: Godyń et al., 2019). To validate the use of play as a positive welfare indicator, it is first necessary to verify its association with other pre-validated markers of improved welfare, such as the increased expression of affiliative, exploratory behaviors or some specific calls (Boissy et al., 2007).
Affiliative interactions, characterized by close spatial proximity and the exchange of social-positive behaviors, promote group cohesion through the formation of bonds between animals (Borges et al., 2011; Mitani et al., 2000). They can thus reflect positive welfare (Miranda-de la Lama et al., 2012). Vocalizations can also be used as markers of positive welfare. For example, in rats (Rattus norvegicus), 50 kHz calls are associated with positive appetitive behaviors such as play and mating, while 22 kHz calls are associated with negative affective behaviors such as biting at the start of a fight (Burgdorf et al., 2008). Establishing links between play behavior and these other types of welfare indicator can help validate play as a positive welfare marker in species about which little is known and whose domestication process is still in its infancy, such as the spotted paca (Cuniculus paca).
The spotted paca is the second largest rodent in the world (Oliveira and Bonvicino, 2011), after the capybara (Hydrochoerus hydrochaeris). Considered an important seed disperser, this species is frugivorous, feeding on fruit flesh and seeds that fall to the forest floor (Eisenberg and Redford, 1999). The spotted paca is thought to be nocturnal, solitary, territorial, burrowing, and aggressive toward conspecifics, with a monogamous or promiscuous mating system (Smythe and Brown de Guanti, 1995; Emmoms, 2016). However, the presumed solitary habits of this species are questionable. Although an adult spotted paca usually forages alone or with its young at night, small groups may forage together in areas of abundant food (Beck-King et al., 1999). Moreover, according to territory use, the social system in the species appears to be more flexible than originally expected (Harmsen et al. (2018). Spotted paca may have overlapping home ranges, with neighboring individuals sharing food. This depends on the availability of resources such as food, suitable sites for burrows and water pools, and predation/hunting pressure (Harmsen et al., 2018). Furthermore, Lima et al. (2018) described a vocal complexity in spotted paca communication similar to that of group-living species. This finding may explain the relative ease with which farmers can raise spotted pacas in groups (Smythe and Brown de Guanti, 1995).
Spotted paca are one of the most hunted neotropical animals due to widespread appreciation of their meat (Smythe and Brown de Guanti, 1995; Mattos and Silva, 2016; Hosken, et al., 2021). In Brazil, the spotted paca is farmed legally, mainly by small producers (Correia et al., 2016). Although captive breeding of the species is considered relatively simple and promising for farmers, there is very little known about the welfare of this neotropical species in captivity (e.g., Sabatini and Paranhos da Costa, 2001; Nogueira et al., 2021; Lima et al., 2022; Altino et al., 2023). Recently, a study showed that some vocalizations are associated with the animals’ affective state and arousal level (Lima et al., 2022). Specifically, the authors found that during a positively-valenced context (feeding time), pacas emitted many more bark calls with lower mean amplitudes and a shift in the 3rd quartile frequency (Q75) to a lower frequency, compared to those emitted during a negative context (pen cleaning) (Lima et al., 2022). Lima et al. (2022) also verified that the mean amplitude of the bark was higher when spotted pacas were at high arousal levels compared to low arousal levels. Based on these results, these authors suggested that the decrease in both the mean amplitude and energy distribution (Q75) of the bark call are associated with positive emotional valence for the spotted paca, while an increase in the mean amplitude of the bark call is associated with negative conditions and an increase in arousal levels in this species.
Lack of attention to the needs of captive-bred animals can lead to chronic stress and abnormal behaviors, such as stereotypies that compromise their welfare (Newberry, 1995; Mason, 2010) and their productivity (Waiblinger et al., 2006; Zulkifli, 2013). On the other hand, play behavior may reflect positive welfare, although this relationship is not always straightforward (e.g., Spinka and Held 2011; Ahloy-Dallaire et al., 2018). In spotted paca, play has so far been reported only in captive young animals up to two months old (Sabatini and Paranhos da Costa, 2001), and is classified as locomotor play, where the animals run alone in the enclosure and sometimes shake their heads. The aim of this study, therefore, was to evaluate whether play behavior also occurs in adult paca, and whether it might be a useful welfare indicator for farmed spotted paca. This was done by investigating its association with other behaviors that have been previously found to be associated with positive welfare in pacas.
If play behavior indicates positive affective states and welfare as observed by Lawrence (1987), we would predict an increase in pre-validated ‘positive’ behaviors, such as affiliative and exploratory behaviors, as verified in other species (e.g., Tayassu pecari: Nogueira et al., 2011) and reduced aggression, as observed in domestic pigs following announcement of environmental enrichment (Dudink et al., 2006). If an increase in bark calls with lower mean amplitude and a shift in the energy distribution (Q75) toward lower frequency indicates a positive affective state in spotted pacas (Lima et al., 2022), we expect more bark calls with lower mean amplitude and lower frequency of Q75 when the spotted pacas are playing than when they are not.
2. Materials and Methods
Ethical note
This work followed the Brazilian laws and was approved by the Ethics Committee on Animal Use (CEUA) of the State University of Santa Cruz (protocol # 029/18).
Subjects and housing conditions
The experiment was conducted at a farm located near the city of Soledade de Minas, state of Minas Gerais, Brazil. We recorded data from 18 adult spotted pacas (Cuniculus paca) (12 females and six males), born and raised in captivity, aged from one to four years, and kept in six groups (one male and two females per group). The spotted pacas were identified by natural characteristics, such as scars and coat color, without requiring additional marking. The animals were housed in 4 m2 pens, with cement floors, covered with ceramic tiles, surrounded by 0.5 m high brick walls and 2.0 m high wire mesh above the walls. In each pen there was a wooden shelter (1.5 m long x 1.5 m wide x 1.0 m high), a water tank (0.6 m long x 0.3 m wide x 0.3 m high) – that provided water ad libitum – and three feeders (0.4 m long x 0.3 m wide x 0.3 m high; one feeder per animal). The keeper provided food at the three feeders at around 16h00 each day. The meal consisted of corn meal (150 g per animal), seasonal fruits and vegetables. The keeper cleaned the pens daily (7h00), sweeping the floor and shelter, and washing feeders and the water tank.
Data collection
A single observer (SGCL) recorded the behavior of spotted pacas (
Table 1) using a camcorder (Sony HDR-CX240, Manaus, Brazil), which was fixed on a tripod. We set up the camcorder outside the enclosure at about 2.0 m from the animals. Spotted pacas were habituated to the presence of the observer for a period of seven consecutive days prior to data collection. After habituation, the observation of the groups and the order of the animals were drawn daily by lot. Each group was observed for 10 minutes per day for three consecutive days for all phases of the study (described below), for a total of 27 hours of data collection (9 hours per phase, details below). Observation sessions took place between 16h00 and 18h00 (before feeding), using animal focal sampling (Altmann, 1974).
We used the ABA paradigm (Heffner, 2004): phases A1 and A2 corresponded to control phases, in the absence of objects used to motivate spotted pacas to play (boomer balls), while during phase B we introduced objects (boomer balls) to motivate animals to play. The introduction of boomer balls was necessary, because adult pacas do not usually show play behavior spontaneously. Thus, in phase B we introduced three boomer balls (0.15 m in diameter and made of hard plastic material) to each group (one ball per individual, trying to avoid competition) to motivate play behavior. The choice of boomer balls was made because in a previous study, Nogueira et al. (2021) found that spotted paca interacted with them during a novel object temperament test, such as chasing the ball in a way that met all of Burghardt’s (2005) criteria for play behavior. Furthermore, balls have not been shown to be a threat to animals and are one of the most highly valued objects for inducing play behavior in mammals (Fagen, 1981). After collecting the data, another observer (AFL) analyzed the recorded footage using CowLog software version 3.0.2 (Hänninen and Pastell, 2009).
Acoustic parameters of bark calls
The same observer (SGCL) recorded animal calls (
Table 1) using a Sennheiser ME-66 unidirectional microphone (Wedemark, Germany) and a Tascam digital recorder (model: DR-100 MK II, settings: WAV format, mono mode, 48 kHz sampling rate and 16-bit resolution). The observer recorded the bark calls emitted by the animals without interruption, keeping the recorder on until the end of each call, in accordance with the method of Lima et al. (2018; 2022). For acoustic analysis, another researcher, blinded to treatment condition and animal identity, selected only the high-quality bark calls without background noise and/or overlap. Therefore, from a total of 261 recorded bark calls, we selected 257 bark calls of better quality for the analysis of acoustic parameters. Nevertheless, to compare the occurrence of call types between the experimental phases, all emitted vocalizations were considered, regardless of their acoustic quality.
We used Raven Pro version 1.5 software (Cornell Lab of Ornithology, Ithaca NY) to measure the 3rd frequency quartile (Hz) (Q75: frequency value at the upper threshold of the third energy quartile) (Charif et al., 2010) of barks emitted by the pacas in all experimental phases. Additionally, we used Praat software version 5.3.06 (Boersma and Weenink, 2022) to measure the mean amplitude (dB) (Briefer, 2012) of these barks. For this analysis, we applied the following settings: time range: FFT method, window size: 0.01; time range: 1000; frequency range: 250; Hanning window shape; dynamic range: 60 dB.
Data analysis and statistics
We compared the time animals spent in each of the recorded behaviors (see
Table 1) across treatments using mixed linear models (MLMs); one model for each behavior (affiliative, agonistic, and exploratory). Because play behavior only occurred during the ball phase (B), there was no way to compare between phases. In MLMs, we considered as fixed factors the sex (male and female) and the treatments (controls: A1 and A2 and ball phase: B), as well as their possible interactions. When the interaction was significant, we used Tukey’s
post hoc tests. We also used MLMs to compare the acoustic parameters (mean amplitude and Q75) of the barks emitted by the pacas. In the models used to analyze the acoustic parameters, only treatments were included as a fixed factor. In all models, the identity of each individual nested within its group was included in the models as a random factor. This allowed us to control for repeated measures dependencies. We graphically checked the residuals of each model for a normal distribution and homoscedasticity and used logarithmic transformations for all but the mean amplitude parameter to satisfy these assumptions. We used the chi-square goodness-of-fit test to compare the barking of the pacas between treatments. We used Minitab 21.1 software (Minitab Inc., State College, PA) for all analyses, considering P< 0.05.
3. Results
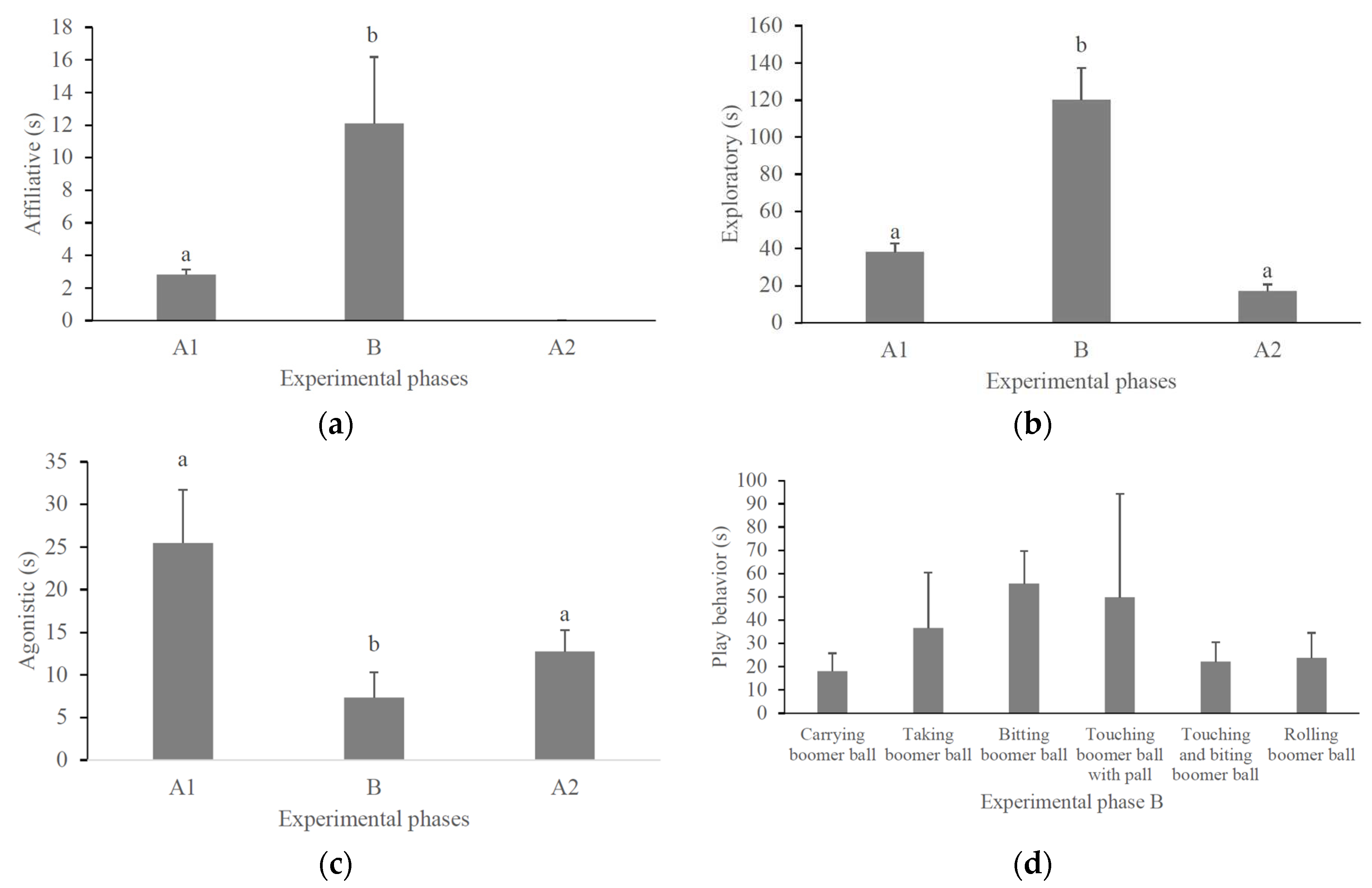
Play behavior occurred only during the environmental ball phase (B) (mean = 35.5-s, standard error (SE) = 6.4;
Figure 1d). Pacas displayed six different subtypes of object play behavior (
Table 1). The most common were biting the ball, rolling the ball, and touching and biting the ball (
Table 2). There was no difference in the amount of time females (mean = 13.3-s, SE = 1.4) and males (mean = 24.0-s, SE = 1.4) played with the balls (Test-t = 1.29, P = 0.21).
We recorded affiliative behaviors only in the first control phase (A
1) and in the ball phase (B). No affiliative behavior occurred in phase A
2. Pacas were observed to spend longer performing affiliative behaviors in phase B (
Table 3,
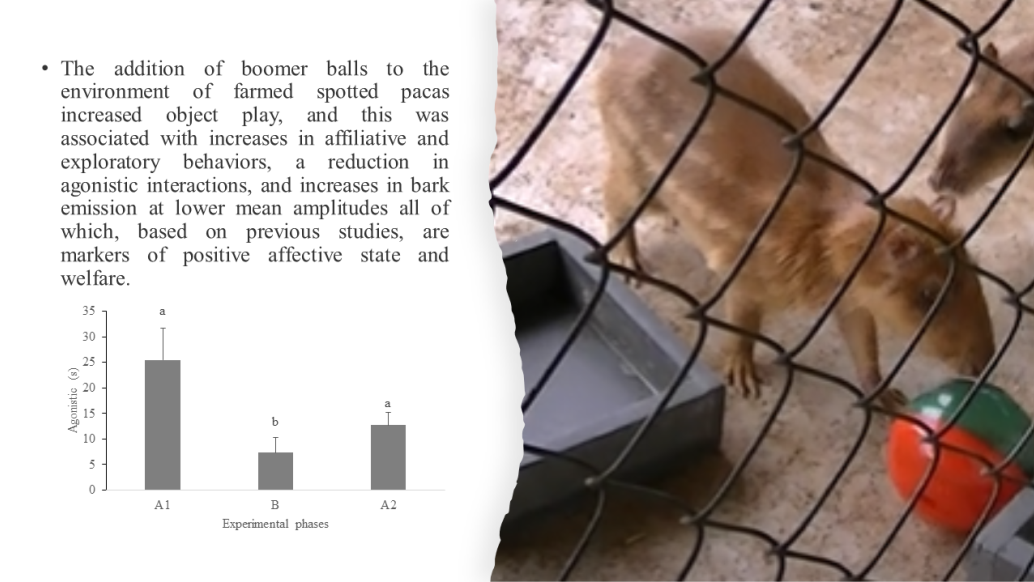
Figure 1a). There was also variation in the expression of exploratory behavior and agonistic interactions depending on the experimental phase (
Table 3). Pacas remained in exploratory behavior longer during the ball phase (B) than in the control phases (A
1 and A
2) (
Figure 1b). On the other hand, pacas interacted agonistically for less time during the ball phase (B) than during the control phases (A
1 and A
2) (
Figure 1c). Among the types of play behaviors of the pacas, biting the ball was the most frequent (
Figure 1d). Sex and the interaction between sex and experimental phase did not affect the time the pacas were observed in the behaviors analyzed (
Table 3).
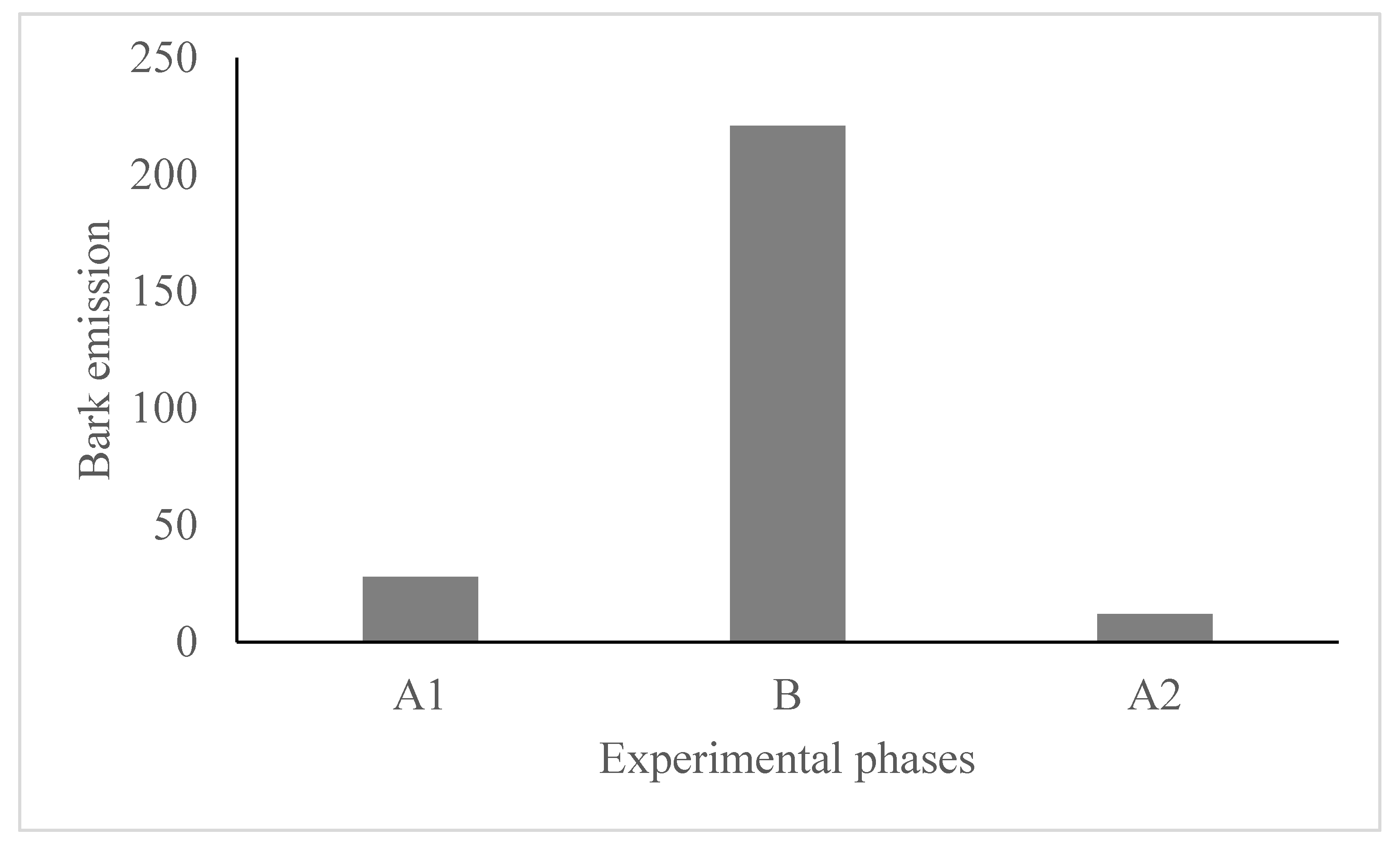
We found an increase in bark emissions during the ball phase (B) (Χ
2 = 311.06, DF = 2, P < 0.001,
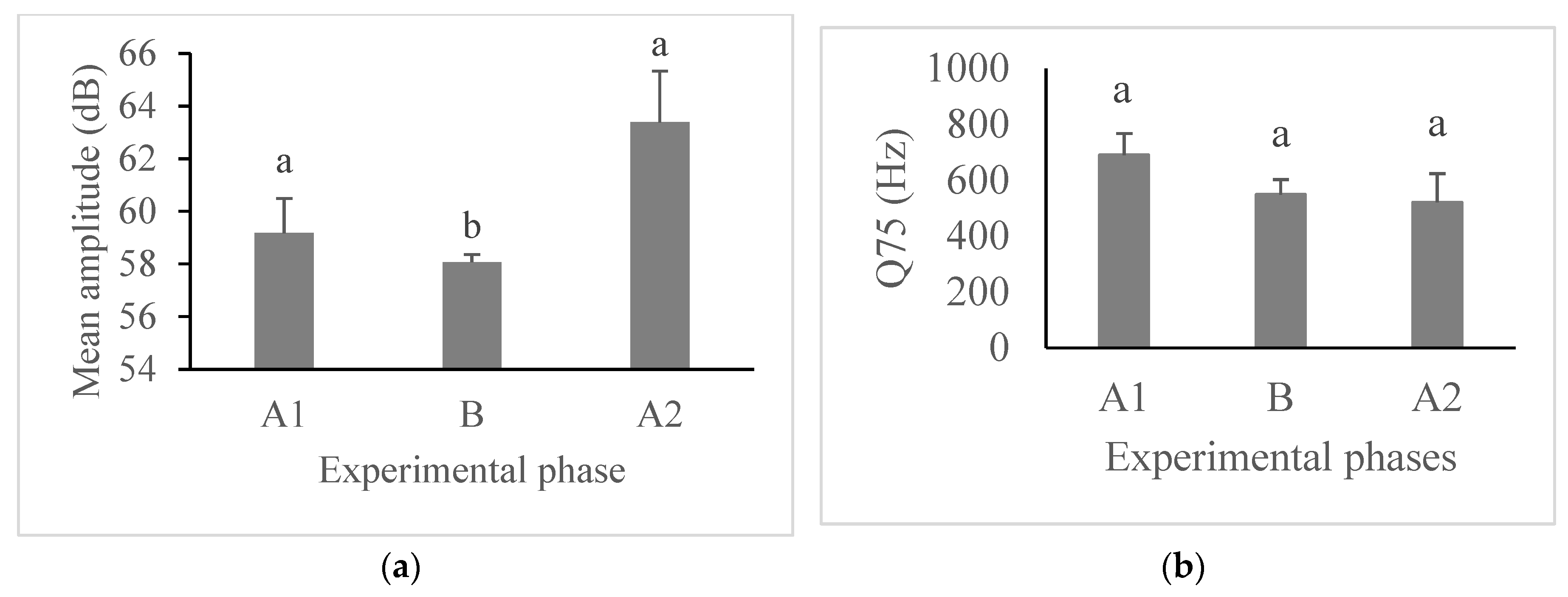
Figure 2). We also found an effect of the experimental phase on the mean amplitude of the barks (F
2, 204.64 = 9.03, P < 0.001). The
post hoc tests showed that during the ball phase (B), pacas emitted barks with a lower mean amplitude than in the two control phases (A
1 and A
2) (
Figure 3a). On the other hand, the frequency in the third quartile (Q75) of barks did not differ between experimental phases (F
2, 156.6 = 2.24, P = 0.110) (
Figure 3b).
4. Discussion
The presence of the boomer balls in the spotted pacas’ enclosures motivated play behavior, increased the time spent on affiliative and exploratory behaviors, and reduced the frequency of agonistic behaviors. The amount of barking with a lower mean amplitude was increased in the presence of play behavior, indicating that playing with balls is a positive event, corroborating our hypothesis. However, the expected lowering of the energy distribution (Q75) of barks towards a lower frequency, also thought to indicate appositive state (Lima et al. 2022), was not observed.
The time the pacas spent interacting with the boomer ball showed that this object was an attractive stimulus for the animals, generating object play involving manipulation with the mouth and/or paws. We considered such interactions of paca with the boomer ball to be consistent with Burghardt’s (2005) five characteristics that distinguish a behavior as being in a “play” state. The interaction with the boomer ball was incompletely functional in the context in which it was expressed. Such interaction was voluntary, performed repeatedly along with some variant forms, started in healthy and relatively unstressed animals in a relaxed context. We observed that individuals sometimes carried the boomer balls into the burrow. This behavior may be related to competition for the object as a limited resource and thus movement away from others to avoid disputes. Solitary play behavior is common in several species, including those that are social, such as the Tayassu pecari (Nogueira et al., 2011). For example, in this species, adding objects to the environment promotes increased possession of the objects by the dominant individual early in the animals’ exposure to the new objects, and later the objects are shared by the dominant individuals (Nogueira et al., 2011). Solitary play facilitates the acquisition of new behavioral patterns, such as in long-tailed Burmese monkeys (Macaca fascicularis aurea), whose juveniles improve their foraging skills through solitary play with objects (Pelletier et al., 2017). Furthermore, solitary play is important in a human-controlled environment because it allows individuals to exercise control over their own activity pattern (Greene et al., 2011).
We found no effect of sex on the expression of object play, affiliative, exploratory, or agonistic behaviors in spotted pacas, suggesting that males and females were equally interested in the boomer ball. Some authors have reported sex differences in object manipulation and play behavior (Maestripieri and Ross, 2004; Koops et al., 2015; Webber and Lee, 2020). Chimpanzee males (Pan troglodytes schweinfurthii) show different preferences for objects and type of play than females, while in Bonobos (Pan paniscus) no differences have been found between sexes (Koops et al. 2015). Maestripieri and Ross (2004) studied sex differences in play behavior of Gorilla gorilla gorilla and reported that these differences can occur when males and females differ in their physical and behavioral characteristics or social preferences. In adult spotted pacas, males and females can be distinguished by the head size (Smythe, 1991). The skull of pacas has an expanded zygomatic arch in males, making their heads larger than those of females (Bonatelli, 2005). This head characteristic can be seen reflected in the differences in some of their vocal parameters (Lima et al., 2018); however, our data do not reveal any association with object play and other behavior evaluated herein.
As we predicted, affiliative behaviors were enhanced in the boomer ball phase, favoring the occurrence of positive social interaction in the group, which denotes a beneficial social condition for the pacas in our study. Increased affiliative interactions are indicative of increased group cohesion (Borges et al., 2011), due to the formation of bonds between animals, which are characterized by physical approaches, and can express positive welfare (Miranda-de la Lama et al., 2012). Thus, the increase in affiliative behaviors indicates that during the period when the boomer balls were present, animals were in a more positive state.
Exploratory behaviors also intensified in the enriched ball phase compared to the control phases. One of the important properties of an enrichment object, such as a boomer ball, is to generate novelty and promote an increase in general activities, including exploration (Gifford et al., 2007). Increasing activities for animals may contribute to reducing inactivity which itself may be associated with a state of distress (Fureix and Meagher, 2015). Exploratory behavior is desirable in captive animals (Young, 2003), suggesting that promoting this behavior contributes to improved animal welfare (Boissy et al., 2007). We can consider, therefore, that in our study there was improvement in the pacas’ welfare because their play and exploratory behaviors increased during the ball phase. On a practical level, our findings indicate that pacas kept in rather barren standard farm environments may benefit from the inclusion of objects to increase investigation and general activity.
Agonistic behavior is very common in spotted pacas (Emmons and Feer, 1997; Lima et al., 2018; Sabatini and Paranhos da Costa, 2001), although it is considered easy to manage in captivity (Smythe and Brown de Guanti, 1995; Hosken et al., 2021). In our study, we observed a reduction in agonistic behavior during the boomer ball phase compared to the control phases, as we predicted. Studies involving other species have also reported a decrease in aggressive behavior during object play behavior (e.g., Pan paniscus and Pan troglodytes: Palagi, 2006; Tursiops truncates: Serres et al., 2020), while social play in adult animals can increase at times of social tension and high aggression risk (e.g., Yamanashi et al., 2018, Cordoni et al., 2022). In our study, we provided one boomer ball per paca to allow access for all animals, thus minimizing potential resource disputes, although sometimes the pacas chased and tried to take the boomer ball from each other. In pigs (Sus scrofa scrofa), the ratio between the number of objects used in environmental enrichment can be a limiting factor for the success of the procedure, as a low ratio can lead to competition and increased group aggression and frustration (Machado et al., 2017). Thus, we infer that the low incidence of agonistic behavior during the ball phase suggests that the number of boomer balls in the pen was sufficient for all pacas, and the overall effect of their provision was positive for the animals.
Calls can indicate positive or negative emotions and provide information about animal welfare (Briefer, 2012). In our study there were more in bark emissions with mean lower amplitude in the ball phase than the control phases, allowing us to infer that playing with boomer balls was associated with positive affective states. Lima et al. (2022), when studying acoustic parameters as indicators of affective states and welfare in spotted pacas submitted to different affective state manipulations (negative, positive, ambiguous, and highly positive), found that barks with lower amplitudes were more prevalent in the assumed positive and highly positive valence treatments. However, they also found a decrease in the third quartile (Q75) frequency of bark calls in the positive situations (Lima et al., 2022), which was not evident in the current study. Given our other findings, this calls into question whether the specific energy distribution and fundamental frequency of bark calls provide reliable indications of positive emotional valence in spotted paca. The observation that introduction of boomer balls into the pens stimulated play behavior without increasing the mean amplitude of bark calls, as appears to happen in negative conditions indicating an elevated arousal state (Lima et al. 2022), can be interpreted as the active play behavior observed being of a relaxed nature (cf. “having fun”, Spinka et al., 2001).
In general, play is more frequent in juveniles and becomes less frequent in adults (Bekoff and Byers, 1998), as in the case of the spotted paca (Sabatini and Paranhos da Costa, 2001). Our study showed that this behavior can be induced, however, by enriching enclosures with objects such as boomer balls. This strategy can be used in farmed spotted pacas to reduce a state of boredom resulting from a predictable environment, as observed by other authors (Meagher and Mason, 2012). In adulthood, captive animals may particularly benefit from play stimulated by additional objects in their enclosures, when social play becomes less common (van der Harst et al. 2003; Nogueira et al., 2011; Lee and Moss, 2014).
5. Conclusions
We conclude that the addition of boomer balls to the environment of farmed spotted pacas increased object play, and this was associated with increases in affiliative and exploratory behaviors, a reduction in agonistic interactions, and increases in bark emission at lower mean amplitudes all of which, based on previous studies, can be considered markers of positive affective state and welfare. The presence of elevated levels of object play alongside these indicators suggests that play behavior is a promising non-invasive indicator of animal welfare in this species. In addition, to achieve the potential welfare benefits of play, this behavior must be stimulated in captivity because adult spotted pacas rarely show play behavior; provision of boomer balls may be one way to stimulate this.
Author Contributions
The study conception and design were made by S. Nogueira, S. Nogueira-Filho, S. Held and M. Mendl. Material preparation, data collection and analysis were performed by S. G. Calazans Lima, A. Lima and S. Nogueira-Filho. The first draft of the manuscript was written by Allison Lima and Stella G. C. Lima, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was financed in part by the Bahia State Research Support Foundation (Fundação de Amparo à Pesquisa do Estado da Bahia, FAPESB #APP074/2016, PI: SSCN), Coordination for the Improvement of Higher Education Personnel (CAPES - Finance Code 001) and National Institute of Science and Technology in Interdisciplinary and Transdisciplinary Studies in Ecology and Evolution (IN-TREE – Process CNPq #465767/2014-1 and CAPES #23038.000776/2017-54), Bahia, Brazil. SSCN and SLGNF received a grant from the Council for Scientific and Technological Development (CNPq) (Processes # 303320/2022-2 and # 304593/2022-2, respectively). We are also grateful for a UK BBSRC Brazil Partnering Award (BB/R021112/1; PI: M. Mendl) for supporting the collaborative work described here.
Acknowledgments
We are very grateful to Mr. Adilson Campos Pimenta for allowing us to study his pacas. We would also like to thank Dr. Liz Paul for commenting on the original manuscript.
Conflicts of Interest
None.
References
- Ahloy-Dallaire, J.; Espinosa, J.; Mason, G. Play and optimal welfare: does play indicate the presence of positive affective states? Behavioural Process 2018, 156, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Altino, V.S.; Rezende, D.C.; Nogueira, S.S.C.; Aldrigui, L.G.; Roldan, M.; Duarte, J.M.; Fureix, C.; Mendel, M.; Nogueira-Filho, S.L. Validation of complementary non-invasive tools for stress assessment in spotted paca (Cuniculus paca). Animal Welfare 2023, 32, e54. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J. Observational study of behavior: sampling methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef]
- Beck–King, H.; Helversen, O.V.; Beck–King, R. Home range, population density, and food resources of Agouti paca (Rodentia: Agoutidae) in Costa Rica: A study using alternative methods 1. Biotropica 1999, 31, 675–685. [Google Scholar] [CrossRef]
- Bekoff, M.; Byers, J.A. Animal play: Evolutionary, comparative and ecological approaches. Cambridge University Press: Nova York, 1998; 262p. [Google Scholar]
- Boersma, P.; Weenink, D. Praat: Doing Phonetics By Computer (Version 5.3.06) [Computer software]. Institute of Phonetic Sciences, Amsterdam. 2022. Available online: http:// www.praat.org.
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Aubert, A. Assessment of positive emotions in animals to improve their welfare. Physiology & Behavior 2007, 92, 375–397. [Google Scholar]
- Bonatelli, M. A subplacenta da paca (Agouti paca, Linnaeus 1766). 2005. Doctoral dissertation, Universidade de São Paulo.
- Borges, M.P.; Byk, J.; Del-Claro, K. Influência de técnicas de enriquecimento ambiental no aumento do bem-estar de Callithrix penicillata (E. Geoffroy, 1812) (Primates: Callitrichidae). [Influence of environmental enrichment techniques on increasing the well-being of Callithrix penicillata (E. Geoffroy, 1812) (Primates: Callitrichidae). Biotemas 2011, 24, 83–94. [Google Scholar]
- Briefer, E.F. Vocal expression of emotions in mammals: mechanisms of production and evidence. Journal of Zoology 2012, 288, 1–20. [Google Scholar] [CrossRef]
- Burgdorf, J.; Kroes, R.A.; Moskal, J.R.; Pfaus, J.G.; Brudzynski, S.M.; Panksepp, J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. Journal of Comparative Psychology 2008, 122, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, G.M. The genesis of animal play: testing the limits. Bradford Books; MIT Press: Cambridge, 2005. [Google Scholar]
- Charif, R.A.; Waack, A.M.; Strickman, L.M. Raven Pro 1.4 user’s manual. Cornell Lab of Ornithology, Ithaca, New York, 25506974. 2010. [Google Scholar]
- Cordoni, G. Social play in captive wolves (Canis lupus): not only an immature affair. Behaviour 2009, 146, 1363–1385. [Google Scholar] [CrossRef]
- Cordoni, G.; Pirarba, L.; Elies, S.; Demuru, E.; Guéry, J.P.; Norscia, I. Adult–adult play in captive lowland gorillas (Gorilla gorilla gorilla). Primates 2022, 63, 225–235. [Google Scholar] [CrossRef]
- Correia, F.C.S.; Silva, F.R.; Souza, V.T.; Ribeiro, V.M.F.; Gomes, F.A. Criação de pacas (Cuniculus paca) como alternativa de diversificação de produção e renda em Rio Branco-Acre. [The captive breeding of Cuniculus paca as an alternative for production diversification] Arquivos de Ciências Veterinárias e Zoologia da UNIPAR, 2016, 19.
- Dudink, S.; Simonse, H.; Marks, I.; de Jonge, F.H.; Spruijt, B.M. Announcing the arrival of enrichment increases play behaviour and reduces weaning-stress-induced behaviours of piglets directly after weaning. Applied Animal Behaviour Science 2006, 101, 86–101. [Google Scholar] [CrossRef]
- Eisenberg, J.F.; Redford, K.H. Mammals of the Neotropics. The Central Neotropics. The University of Chicago Press, Chicago, USA, 1999.
- Emmons, L. Cuniculus paca. The IUCN Red List of Threatened Species 2016: e.T699A22197347. 2016. Available online: https://dx.doi.org/10.2305/IUCN.UK.2016-2.RLTS.T699A22197347.en (accessed on 3 October 2023).
- Emmons, L.; Feer, F. Neotropical Rainforest Mammals: A Field Guide, 2nd ed.; University of Chicago Press: Chicago, IL, USA, 1997. [Google Scholar]
- Fagen, R. Animal Play Behavior Oxford University Press. New York, 1981.
- Fureix, C.; Meagher, R.K. What can inactivity (in its various forms) reveal about affective states in non-human animals? A review. Applied Animal Behaviour Science 2015, 171, 8–24. [Google Scholar] [CrossRef]
- Gifford, A.K.; Cloutier, S.; Newberry, R.C. Objects as enrichment: Effects of object exposure time and delay interval on object recognition memory of the domestic pig. Applied Animal Behaviour Science 2007, 107, 206–206. [Google Scholar] [CrossRef]
- Godyń, D.; Nowicki, J.; Herbut, P. Effects of environmental enrichment on pig welfare—a review. Animals 2019, 9, 383. [Google Scholar] [CrossRef]
- Greene, W.E.; Melillo-Sweeting, K.; Dudzinski, K.M. Comparing object play in captive and wild dolphins. International Journal of Comparative Psychology 2011, 24. [Google Scholar] [CrossRef]
- Hänninen, L.; Pastell, M. CowLog: open-source software for coding behaviors from digital video. Behavior Research Methods 2009, 41, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, B.J.; Wooldridge, R.L.; Gutierrez, S.M.; Doncaster, C.P.; Foster, R.J. Spatial and temporal interactions of free-ranging pacas (Cuniculus paca). Mammal Research 2018, 63, 161–172. [Google Scholar] [CrossRef]
- Heffner, C.L. Research methods for education, psychology and the social sciences. Retrieved October 2004, 17, 2007. [Google Scholar]
- Held, S.D.; Špinka, M. Animal play and animal welfare. Animal behaviour 2011, 81, 891–899. [Google Scholar] [CrossRef]
- Horback, K. Nosing around: Play in pigs. Animal Behavior and Cognition 2022, 2, 186–186. [Google Scholar] [CrossRef]
- Hosken, F.M.; de Oliveira, M.H.V.; Malheiros, J.M.; Martins, E.H.; Ferreira, F.N.A.; Ferreira, W.M.; Lara, L.B. ;Experimental ethology of intensively reared lowland pacas (Cuniculus paca). Tropical Animal Health and Production 2021, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Sherwen, S.; Robbins, R.; McLelland, D.; Whittaker, A. Welfare assessment tools in zoos: from theory to practice. Veterinary Sciences 2021, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Keeling, L.J.; Winckler, C.; Hintze, S.; Forkman, B. Towards a positive welfare protocol for cattle: A critical review of indicators and suggestion of how we might proceed. Frontiers in Animal Science 2021, 2, 70. [Google Scholar] [CrossRef]
- Koops, K.; Furuichi, T.; Hashimoto, C.; Van Schaik, C.P. Sex differences in object manipulation in wild immature chimpanzees (Pan troglodytes schweinfurthii) and bonobos (Pan paniscus): Preparation for tool use? . PLoS One 2015, 10, e0139909. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A. Consumer demand theory and the assessment of animal welfare. Animal Behavior 1987, 35, 293–295. [Google Scholar] [CrossRef]
- Lee, P.C.; Moss, C.J. African elephant play, competence, and social complexity. Animal Behavior and Cognition 2014, 1, 144–156. [Google Scholar] [CrossRef]
- Lima, S.G.; Sousa-Lima, R.S.; Tokumaru, R.S.; Nogueira-Filho, S.L.G.; Nogueira, S.S.C. Vocal complexity and sociality in spotted paca (Cuniculus paca). PloS one 2018, 13, e0190961. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.F.; Lima, S.G.; Nogueira-Filho, S.L.G.; Held, S.; Paul, E.; Mendl, M.; Nogueira, S.S.C. Vocal expression of emotions in farmed spotted paca (Cuniculus paca). Applied Animal Behaviour Science 2022, 256, 105753. [Google Scholar] [CrossRef]
- Machado, S.P.; Caldara, F.R.; Foppa, L.; de Moura, R.; Gonçalves, L.M.P.; Garcia, R.G.; de Oliveira, G.F. Behavior of pigs reared in enriched environment: alternatives to extend pigs attention. PloS one 2017, 12, e0168427. [Google Scholar] [CrossRef]
- Maestripieri, D.; Ross, S.R. Sex differences in play among western lowland gorilla (Gorilla gorilla gorilla) infants: implications for adult behavior and social structure. American Journal of Physical Anthropology 2004, 123, 52–61. [Google Scholar] [CrossRef]
- Martin, J.E.; Ison, S.H.; Baxter, E.M. The influence of neonatal environment on piglet play behaviour and post-weaning social and cognitive development. Applied Animal Behaviour Science 2015, 163, 69–79. [Google Scholar] [CrossRef]
- Mason, G.J. Species differences in responses to captivity: stress, welfare, and the comparative method. Trends in Ecology & Evolution 2010, 25, 713e721. [Google Scholar]
- Mattos, A.; Silva, V.J. Viabilidade econômica da criação de pacas (Cuniculus paca L.) em Presidente Tancredo Neves, Bahia. [Economic viability of breeding pacas (Cuniculus paca L.) in Presidente Tancredo Neves, Bahia]. Revista iPecege 2016, 2, 56–79. [Google Scholar] [CrossRef]
- Meagher, R.K.; Mason, G.J. Environmental enrichment reduces signs of boredom in caged mink. PloS one 2012, 7, e49180. [Google Scholar] [CrossRef]
- Miranda-de La Lama, G.C.; Villarroel, M.; María, G.A. Behavioural and physiological profiles following exposure to novel environment and social mixing in lambs. Small Ruminant Research 2012, 103, 158–163. [Google Scholar] [CrossRef]
- Mitani, J.C.; Merriwether, D.A.; Zhang, C. Male affiliation, cooperation and kinship in wild chimpanzees. Animal Behaviour 2000, 59, 885–893. [Google Scholar] [CrossRef]
- Newberry, R.C. Environmental enrichment: increasing the biological relevance of captive environments. Applied Animal Behaviour Science 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Nogueira, S.S.C.; Soledade, J.P.; Pompéia, S.; Nogueira-Filho, S.L.G. The effect of environmental enrichment on play behaviour in white-lipped peccaries (Tayassu pecari). Animal Welfare-The UFAW Journal 2011, 20, 505. [Google Scholar] [CrossRef]
- Nogueira, S.S.C.; Nogueira-Filho, S.L.G.; Duarte, J.; Mendl, M. Temperament, plasticity, and emotions in defensive behaviour of paca (Mammalia, Hystricognatha). Animals 2021, 11, 293. [Google Scholar] [CrossRef]
- Oliveira, J.A. & Bonvicino, C.R. In: Reis, N.R.; Peracchi, A.L.; Pedro, W.A. & Lima, I.P. (Eds.). Mamíferos do Brasil [Brazilian Mammals]. 2. Ed. Londrina, Nelio R. dos Reis. 2011, p. 358-406.
- Palagi, E. Social play in bonobos (Pan paniscus) and chimpanzees (Pan troglodytes): implications for natural social systems and interindividual relationships. American Journal of Physical Anthropology 2006, 129, 418e4. [Google Scholar] [CrossRef]
- Pelletier, A.N.; Kaufmann, T.; Mohak, S.; Milan, R.; Nahallage, C.A.D.; Agung, I.G. Behavior systems approach to object play: Stone handling repertoire as a measure of propensity for complex foraging and percussive tool use in the genus Macaca. Animal Behavior and Cognition 2017, 4, 455–473. [Google Scholar] [CrossRef]
- Reimert, I.; Bolhuis, J.E.; Kemp, B.; Rodenburg, T.B. Indicators of positive and negative emotions and emotional contagion in pigs. Physiology & Behavior 2013, 109, 42–50. [Google Scholar]
- Sabatini, V.; Paranhos da Costa, M.J.R. Etograma da paca (Agouti paca, Linnaeus, 1766) em cativeiro. [The ethogram of captive paca (Agouti paca, Linnaeus, 1766)]. Revista de Etologia 2001, 3:3-14.
- Schwing, R.; Nelson, X.J.; Wein, A.; Parsons, S. Positive emotional contagion in a New Zealand parrot. Current Biology 2017, 27, R213–R214. [Google Scholar] [CrossRef]
- Serres, A.; Hao, Y.; Wang, D. Body contacts and social interactions in captive odontocetes are influenced by the context: An implication for welfare assessment. Animals 2020, 10, 924. [Google Scholar] [CrossRef]
- Smythe, N.; Brown Guanti, O.D. La domesticación y cría de la paca (Agouti paca) (No. L01/1667). FAO, Rome, 1995.
- Smythe, N.P. Paca. Robinson, J.G.; Redford, K.H. Microlivestock. Little know small animals with promising economic future. Washington: National Academy 1991, 263-269.
- Spinka, M.; Newberry, R.C.; Bekoff, M. Mammalian play: training for the unexpected. The Quarterly Review of Biology 2001, 76, 141–168. [Google Scholar] [CrossRef]
- van der Harst, J.E.; Baars, A.M.; Spruijt, B.M. Standard housed rats are more sensitive to rewards than enriched housed rats as reflected by their anticipatory behaviour. Behavioural Brain Research 2003, 142, 151–156. [Google Scholar] [CrossRef]
- Waiblinger, S.; Boivin, X.; Pedersen, V.; Tosi, M.V.; Janczak, A.M.; Visser, E.K.; Jones, R.B. Assessing the human–animal relationship in farmed species: a critical review. Applied Animal Behaviour Science 2006, 101, 185–242. [Google Scholar] [CrossRef]
- Webber, C.E.; Lee, P.C. Play in elephants: Wellbeing, welfare or distraction? Animals 2020, 10, 305. [Google Scholar] [CrossRef]
- Yamanashi Y, Nogami E, Teramoto M et al () Adult-adult social play in captive chimpanzees: is it indicative of positive animal welfare? Applied Animal Behaviour Science 2018, 199, 75–83. [CrossRef]
- Yang, C.H.; Ko, H.L.; Salazar, L.C.; Llonch, L.; Manteca, X.; Camerlink, I.; Llonch, P. Pre-weaning environmental enrichment increases piglets’ object play behaviour on a large-scale commercial pig farm. Applied Animal Behaviour Science 2018, 202, 7–12. [Google Scholar] [CrossRef]
- Young, R. Environmental Enrichment. Blackwell Science, Oxford. 2003.
- Zulkifli, I. Review of human-animal interactions and their impact on animal productivity and welfare. Journal of Animal Science and Biotechnology 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).