1. Introduction
1.1. Thermodynamic perspectives on life’s origins
The concept of non-equilibrium thermodynamics provides a framework to understand how life may have originated from abiotic chemical processes [
1,
2,
3,
4]. Sidney Fox’s groundbreaking research showcased the spontaneous formation of proteinoids and protocells in thermal environments, providing valuable evidence for the dissipative models of biogenesis [
5]. Using thermal polymerization of amino acids to produce protein-like microspheres capable of catalysis, signalling, and self-assembly, Fox’s proteinoids mimic characteristics of living cells [
5]. According to the theory of non-equilibrium thermodynamics, life originated as a dissipative structure that was far from equilibrium and was driven by the absorption of solar photons [
6]. Proteinoids are in line with this perspective because their synthesis involves the use of thermal energy to produce organised biomolecular systems. The protocells that have been proposed function as photon absorbers, thereby increasing the production of entropy. Proteinoids offer a thermodynamically feasible pathway to cellular structures that are compatible with life, taking advantage of non-equilibrium conditions. Although there are still unanswered questions regarding the environmental conditions necessary for protocell development, Fox’s proteinoids provide a conceptual demonstration of how life-like entities can spontaneously self-organize under thermodynamic forces. The thermal production of proto-biopolymers and the subsequent assembly of protocells demonstrate possible mechanisms that connect equilibrium and living states.
1.2. Requirements for early biomolecular systems
Our understanding of the transition from prebiotic chemistry to organised, functional biomolecular systems capable of evolution are rapidly advancing [
7,
8,
9,
10] but still limited. There are likely several key requirements that need to be fulfilled. First, the ability to encode information into reproducible polymer sequences would have been essential [
11]. The RNA world hypothesis suggests that RNA was the initial informational polymer [
12,
13,
14]. However, there are still uncertainties surrounding the abiotic synthesis of RNA. Furthermore, the presence of membranes would have facilitated compartmentalisation, enabling reactions to occur in specific locations and allowing for selective permeability [
15]. Lipids, which are amphiphilic molecules, have the ability to self-assemble and form encapsulating vesicles. Moreover, it is likely that thermodynamic forces played a crucial role in driving the self-organisation of networks that became progressively more complex. Energy could have been supplied by geological and chemical gradients, while molecular assembly may have been facilitated by inorganic mineral surfaces. According to Pross and Pascal [
16], the presence of primitive molecular recognition, catalysis, and feedback mechanisms would have allowed for the development of basic metabolism, responsiveness, and growth dynamics. The thermal proteinoids concept developed by Sidney Fox effectively meets several of these criteria [
5]. The process of polymerization from amino acids, followed by self-assembly into membrane-bound protocells, and the resulting lifelike chemical properties provide valuable insights into the formation and functioning of the earliest peptide-based biomolecular systems [
11,
16,
17,
18]. The upcoming sections will discuss proteinoids as models for the abiotic polypeptides that could have formed the informational and compartmental infrastructure of early life.
2. Proteinoids as Primitive Biopolymers
2.1. Thermal polycondensation of amino acids
The potential role of thermal conditions in driving abiotic polypeptide synthesis has been investigated. The process of heating amino acids or their precursors can cause them to undergo polymerization, resulting in the formation of protenoid-like polymers. An example of a reaction is the condensation of glycine and alanine into oligopeptides, which occurs when they are heated with montmorillonite clay. This reaction has the ability to generate peptides containing a maximum of 10 amino acid residues [
19,
20]. Thermal polycondensation has also been successfully used for the direct polymerization of amino acids. When amino acids are subjected to dry heating, they produce mixtures of polypeptides that are diverse in terms of their composition and polymerization parameters [
21]. Clay minerals potentially played a role in this process on early Earth by adsorbing and concentrating amino acids, as suggested by Paecht-Horowitz in 1970: activation energy could have been supplied by thermal gradients in hydrothermal systems or drying lagoons [
22]. These studies offer mechanistic models that explain how polypeptides can be formed abiotically by polymerizing prebiotic amino acids that are readily available. Thermal polycondensation is an example of a thermodynamically favourable process that allows for an increase in molecular complexity. The proteinoid-like polymers that are formed exhibit characteristics similar to those of primordial biopolymers.
2.2. Fox’s proteinoids: characteristics and capabilities
2.2.1. Catalytic activity
Since their initial discovery in the 1960s [
23], Fox’s proteinoids synthesised by thermal polycondensation of amino acids have been extensively characterised. In addition to their abiotic origin, proteinoids exhibit a number of intriguing properties that shed light on the functions of early biopolymers. Catalytic activity, indicating proto-metabolic potential, is a feature of numerous protenoid preparations that is particularly intriguing. According to Fox [
23], proteinoids can have different properties by altering the amino acid composition and adjusting the heating parameters. Proteinoids have the ability to self-assemble into organised microspheres that closely resemble protocells [
24].
The proteinoids produced by Fox and colleagues [
24] exhibit a range of fascinating qualities that closely resemble the behaviours observed in biological proteins and cells. The ability of these structures to spontaneously form membranes has similarities of the early stages of cellularization. Proteinoids also demonstrate basic metabolic functions such as catalysis, energy utilisation, and, albeit limited, growth dynamics [
25].
The catalytic activity observed in many proteinoid preparations is especially intriguing. According to Wolman et al. [
26], proteinoids have been discovered to catalyse a range of reactions, such as ATP synthesis, RNA polymerization, and phosphate ester hydrolysis. The microspheres seem to enhance the concentration of the catalytic effects [
27].
Among the observed catalytic effects are the synthesis of complex biomolecules such as ATP and RNA [
28], and the formation of peptide bonds. According to [
29], proteinoids catalyse their own template-directed reproduction. It appears that the microsphere morphology augments these catalytic effects [
30].
The origins of the biomimetic capabilities of proteinoids, including catalysis, remain inadequately understood. Insights into the properties of early abiotic polypeptides can be gleaned from their ability to self-assemble into organised structures with life-like behaviours. The catalytic activity suggests how the emergence of primitive protometabolism within primitive protocells may have occurred [
31,
32].
2.2.2. Microspheres formation
One of the defining characteristics of Fox’s proteinoids is their remarkable ability to spontaneously self-organize into spherical microstructures that closely resemble primitive cell-like vesicles. After thermal polymerization, proteinoid aggregates undergo a systematic self-assembly process facilitated by non-covalent interactions [
33,
34,
35]. The use of electron microscopy has revealed that the microspheres exhibit a double-layered morphology. This morphology is characterised by an inner particulate core that is surrounded by an outer organised film [
36]. The compartmentalised architecture presented here offers a plausible model for protocells. The size of the microspheres can be adjusted by varying the heating time and the composition of amino acids used, ranging from less than 100 nm to more than 30
m [
37].The morphology of microspheres enhances the biomimetic capabilities of proteinoids in many ways. Enzyme-like activities are localised within the vesicles, resulting in proto-metabolic effects. The organised membrane is crucial for the absorption of nutrients and energy substrates [
38]. Experimental evidence suggests that cooperative interactions between proteinoids play a crucial role in regulating the assembly of microspheres [
32]. A study has shown that the size of the microsphere is primarily influenced by the concentration of salt when the ionic strength is high. On the other hand, when the ionic strength is low, the main factor affecting the size is the pH of the system. Furthermore, it has been observed that the change in solubility is remarkably similar to the change in diameter when considering both ionic strength and pH [
32]. Various studies have been conducted to investigate the formation of microspheres, focusing on the effects of different monovalent anions and polymers with varying amino acid compositions. These studies have revealed that both charge neutralisation and hydrophobic bonding play significant roles in the process of microsphere formation. Particles can also be formed in sea water, particularly when it is heated or slightly alkaline. The microspheres are distinct from those made from acidic proteinoid, but they do share certain similarities with coacervate droplets. These similarities include isothermal formation, limited stability, stabilisation by quinone, and the ability to uptake dyes [
39]. The compositional effects mentioned above serve to emphasise the potential pathways through which protocell membrane properties can be regulated. The ability of proteinoids synthesised abiotically to spontaneously form organised microspheres offers valuable insights into the origins of cellular compartmentalization. The membranous vesicles have selectively permeable boundaries, internal structure, and localised biopolymer functions. The use of protenoid self-assembly provides opportunities for the engineering of synthetic protocells.
2.2.3. Information transfer
As per the findings of Fox et al. [
40], it was noticed that proteinoid microspheres exhibited growth and division into smaller microspheres when subjected to a solution comprising newly introduced amino acids. It is worth noting that the daughter microspheres exhibit some characteristics inherited from the parent microspheres, including size, shape, colour, and chemical composition. Fox et al. [
40] proposed that this particular process possesses the potential to serve as a paradigm for elucidating the genesis of life. This assertion is based on the observation that it showcases the ability of uncomplicated organic compounds to assemble intricate structures capable of self-replication and information transmission [
31].
The proteinoids can catalyse the production of new copies with the same polymeric sequences as the original molecules via template–directed synthesis. This procedure indicates proteinoids’ ability to convey information in a primitive form of inheritance, giving credibility to Fox’s argument that they have real–world properties [
41]. Specifically, the proteinoids can catalyze the formation of new proteinoids that are copies of themselves. This self–replication allows information in the form of molecular sequences to be passed on. The process is analogous to DNA replication in living cells.The process of information transfer takes place as a result of the presence of particular sequences inside the original proteinoids, which function as templates. Proteinoids are synthesised through the use of templates, leading to the production of replicas that possess identical sequence information. This process facilitates the preservation and replication of information across subsequent generations. Fox experimentally demonstrated the cloning process. Under the proper conditions, he demonstrated that when proteinoids were incubated with activated amino acids, new proteinoids formed using the originals as templates [
42,
43,
44,
45]. Electrophoresis demonstrated that the novel molecules had identical sequences [
46]. The ability to transmit information is important since it is a basic property of life. Fox finds that his proteinoids reflect primitive life system characteristics. This viewpoint is supported by information transfer via self-replication, which implies that such molecular activities could have been antecedents to life [
42,
47,
48,
49,
50].
2.2.4. Evolutionary potential
We hypothesize that proteinoids could potentially evolve if they are exposed to suitable environmental conditions for an extended period of time. In appropriate contexts, they have the potential to evolve into more complex organisms. Proteinoids are likely to exhibit varying levels of stability in different environments. Under these conditions, variants that are more stable would persist for a longer duration. Subsequently, they have the ability to catalyse the creation of additional copies of themselves, resulting in a more successful reproduction process. Through multiple generations, the proteinoids that exhibit greater stability and fitness would gradually become dominant as a result of their repetitive replication. Random copying errors could also introduce new variations that may be better adapted to survive. This process bears resemblance to Darwinian evolution. Experimental evidence suggests that when mixtures of proteinoids are exposed to conditions that break down the less stable variants, the more robust ones are able to survive and replicate themselves. The selective persistence of certain sequences imitates the process of natural selection. Although speculative, these ideas demonstrate the potential of simple informational polymers to facilitate open-ended evolution in the presence of environmental selection. This supports theories suggesting the existence of early evolving proto-life before cellular life [
51,
52,
53,
54,
55].
3. Proteinoids and Cellular Emergence
3.1. Protocells from proteinoids
Proteinoids, as demonstrated in the seminal work by Sidney Fox [
23,
51,
56,
57], have the ability to interact and form structures that resemble droplets similar to primitive cells. Proteinoid microspheres are structures where important proto-metabolic reactions occur, including the synthesis of peptides, which are relevant to the development of early cells. This satisfies the necessary conditions for the development of protocells through prebiotic chemistry.
In addition to encapsulation, proteinoids also display other behaviours that are characteristic of cellular life. The populations exhibit growth and division dynamics that are influenced by environmental factors. Electrical measurements indicate the presence of spiking, similar to signalling, in irritable cells. One of the most remarkable observations is that proteinoid microspheres are capable of transferring internal particles between volumes, which can be considered a form of inheritance.
Proteinoids provide valuable insights into potential pathways to the development of cells. However, it is important to note that their thermal synthesis has certain limitations when it comes to accurately modelling prebiotic environments. However, the wide range of realistic characteristics exhibited by basic thermal proteinoids confirms their usefulness as protocell imitations. The field of engineering has made significant progress in developing highly advanced protocells based on proteinoids. These protocells possess remarkable capabilities such as phototrophy and the ability to communicate with other cells.
In addition to investigations into their origins, bioengineered proteinoids exhibit considerable potential as platforms for nanomedicine, bioelectronic components, and smart biomaterials. This is attributed to their biocompatibility, ability to undergo structural reconfiguration, and membrane activity. Proteinoids demonstrate the capacity for the spontaneous emergence of biological characteristics through the self-assembly of polymeric subunits. The investigation of the fundamental principles behind biomimetic behaviours continues to be a prominent field of study in the disciplines of origins of life and materials science.
One notable observation was the discovery that the connections formed between proteinoid microspheres facilitated the transport of internalised particles conveying molecular information across different volumes. This method offers a means of inheritance and evolution that precedes the emergence of nucleic acids, so addressing a significant conceptual barrier in theories of abiogenesis.
The proteinoids theory, which provides insights into possible pathways for cellularization, has faced criticism regarding the issue of thermal synthesis [
31,
58]. Subjecting amino acid mixtures to prolonged heating until they are completely dried may not provide an accurate representation of prebiotic environments. However, the wide range of realistic characteristics exhibited by even basic thermal proteinoids confirms their usefulness as protocell mimics. The development of advanced compositional control and assembly techniques has allowed for the engineering of proteinoid–based protocells with greater sophistication [
59,
60]. Integrating light-harvesting proteinoids, for example, can introduce a basic phototrophic metabolism. Spatial patterning facilitates the formation of interconnected networks that exhibit cell-like compartmentalization and enable effective communication.
Beyond the study of the origins of life, bioengineered proteinoids have the potential to be smart materials for nanomedicine, bioelectronics, and synthetic biology. Their biocompatibility, dynamic structural reconfigurability, and membrane activity make them particularly appealing for drug delivery, biosensing, and modifying cellular activities. Proteinoids, while not literal protocells, exhibit a surprising ability for primitive biological behaviours to evolve spontaneously from the self-organization of simple polymeric subunits. The physicochemical basis for proteinoids’ realistic properties is still being researched in both origins of life and biomaterials research [
53,
57,
61,
62,
63].
3.2. Membrane assembly and composition
Upon hydration, Fox’s proteinoids exhibit a remarkable ability to self-assemble into spherical vesicles. These microspherical membranes serve as a model for the formation of semi-permeable protocell structures from simple abiotic polypeptides [
64,
65,
66,
67,
68,
69].
The morphology of microspheres is influenced by various factors, such as the composition of amino acids, temperature, and duration of heating during proteinoid synthesis. By adjusting these parameters, it is possible to customise the size of the vesicles, ranging from tens of nanometers to tens of micrometres. The membranous boundary controls the movement of solutes into the internalised aqueous core [
70,
71,
72,
73,
74].
The use of electron microscopy has facilitated the observation that the microspheres possess a complex structure consisting of many layers. Specifically, these layers consist of an internal matrix composed of particles, which is enveloped by an external film that exhibits a high degree of organisation. The architectural design of this structure facilitates the formation of localised compartments inside the protocell. The microenvironment facilitates the accumulation of solutes and supports the functioning of the cell membrane [
75,
76,
77,
78].
The interface that is well-structured demonstrates a characteristic of selectivity, allowing for the absorption of essential nutrients while preventing the entry of harmful substances. The findings from the conducted experiments indicate that the process of self-assembly is regulated by the collaborative interplay of hydrophobic and ionic contacts among proteinoid chains. The introduction of amino acids with a positive charge effectively inhibits the process of vesiculation by causing disruption to the coacervation phenomenon [
79,
80,
81].
The proteinoid protocells possess a membrane-bound structure that facilitates the connection between external energy sources and internal chemical processes via transverse gradients. A scenario of light-induced ion transport across the interface of a microsphere facilitates the process of charging an interior matrix resembling a battery [
82,
83,
84].
Furthermore, proteinoid vesicles have the capability to undergo growth through membrane replication via budding, which serves as a mechanism for the propagation and evolution of protocells. The process of thermal cycling elicits a periodic pattern of budding activity, suggesting the presence of intricate dynamic phenomena [
51,
80].
In general, the inherent tendency of proteinoids to form membranes sheds light on potential mechanisms by which compartmentalization and spatial organisation could have arisen in primitive protocellular systems constructed from basic non-living peptides. The implications of the obtained boundary features would have significant effects on the advancement of coordinated protometabolism and the transmission of information [
49,
85,
86,
87].
Although the exact origins of proteinoid membrane assembly and its relationship with structure-function are not fully understood, the fact that they possess biomimetic capabilities suggests potential mechanisms for the formation of organised protocells through unconstrained chemical processes on the prebiotic Earth. The exploration of proteinoid membrane dynamics has the potential to reveal the universal physicochemical driving forces that govern the self-structuring of primitive life-like soft matter.
3.3. Homeostasis mechanisms
It is probable that proto–organisms evolved out of disordered networks of chemical processes [
88,
89,
90,
91]. Kauffman employed a modelling approach wherein genes were represented as binary switches inside extensive random networks, with the aim of investigating the potential emergence of order from a state of randomness [
92]. The models propose that networks of genes, characterised by random connections between a limited number of genes, exhibit significant levels of organisation and stability. These organisms experience cognitive cycles, the duration of which can be utilised for predicting the replication times of cells, based on the amount of genes present. The network possesses multiple behavioural modes that enable the prediction of cell types inside an organism, relying on the quantity of genes present. When subjected to noise, individual cells have the ability to distinguish between various behavioural modes. This can be explained by conceptualising cellular differentiation as a series of transitions occurring inside the behaviour modes of a genetic network.
Biomimetic protocell models, such as the ones mentioned, possess significant value beyond their heuristic function, as they also offer practical applications [
93,
94,
95]. Research on reconstituted biological materials precedes the appearance of entirely synthetic analogues [
96,
97]. It is a well observed phenomenon that living organisms, even non-animal organisations, frequently exhibit the characteristic of irritability or excitability [
98]. Approximately one hour after reassembly, plant protoplast droplets exhibited excitability similar to that of membranes and action potentials [
99,
100]. This observation showcased the propensity for artificial cells to exhibit excitability [
101], which subsequently informed and directed subsequent research endeavours [
102,
103]. Excitability was also shown in synthesised proteinoid microspheres in the year 1973 [
104]. Subsequent investigations have delved into the concepts of selective permeability, osmotic characteristics, and proteinoid bilayer membranes [
70,
105,
106]. This study offers a comprehensive understanding of the underlying physical mechanisms responsible for the emergence of excitability [
107].
Artificial proteinoid cells exhibit fundamental phenomena such as selective permeability, osmotic characteristics, the creation of bilayer membranes, and excitability. The protocells, which have been constructed using basic proteins, have characteristics commonly observed in living cells. This experimental approach provides valuable insights into the potential natural origins of these protocells. The biomimetic method involves the study of simplified model systems in order to elucidate fundamental principles. The systematic assembly and examination of synthetic cells using their fundamental biomolecular components provides valuable insights into the evolutionary progression from chemical systems to biological entities.
The emergent order and stability observed in simple chemical networks, such as Kauffman’s random binary gene models, bear resemblance to cellular systems. The phenomenon of spontaneous self-organization observed in these systems provides insights into the potential mechanisms underlying the emergence of order in early living forms. The introduction of noise results in the emergence of diverse modes, analogous to the differentiation of cell types observed in the process of evolution. These abstract models aim to capture the fundamental characteristics of the collective behaviour of biomolecules during the early stages of life.
The examination of the bottom-up designing of artificial cells and cell-like structures provides insights into the physical origins of complex phenomena like as irritability and excitability [
80,
108]. Synthetic protocells of a basic nature exhibit certain behaviours typical of living entities, such as life like electrodes, metabolic processes, and the ability to reproduce. The use of biomolecule analogues for piecewise reconstruction of cellular processes provides a valuable addition to the study of extant biology’s intricate complexity. The combination of these two perspectives enhances our understanding of the fundamental aspects of existence.
3.3.1. Ion gradients
Thermal proteinoids display some variation in their molecular characteristics, although not as much as what is expected based on theoretical models [
23,
109,
110]. When chromatography and amino acid analysis methods are applied to proteinoids produced through wet-dry cycles, interesting variations in their chemical structure are revealed. Based on this observation, it appears that the self-assembly process of amino acid structures involves a degree of randomness. Proteinoids consistently form structures that closely resemble cells, suggesting that they possess a sufficient level of uniformity that allows them to function in a coordinated manner. Many scientists believe that the interaction between balanced randomness and order was crucial in helping cellular evolution progress during its early stages [
111,
112,
113,
114].
3.3.2. Permeability regulation
According to traditional models on the origins of life, it is believed that the initial step involved the emergence of polymer replication systems [
115]. These systems were then followed by the development of membranes for encapsulation. There is another model for protocells that suggests the formation of vesicular membranes through the self-assembly of prebiotic amphiphiles before the formation of polymers [
7,
116,
117].
Proteinoids are hypothesised to have fulfilled several functions within primitive cellular structures throughout the early stages of life [
118,
119]. The amphiphilic amino acids possessed the ability to spontaneously form boundary membranes by self-assembly. Proteinoids also demonstrate a form of selective permeability that is similar to that observed in contemporary transport proteins [
25,
27]. The vesicle protocells were facilitated to accumulate nutrients, chemicals, and ions. The inherent adaptability of proteinoids renders them highly suitable for serving as fundamental constituents of cellular structures and processes.
The presence of ion gradients across protocell membranes is believed to have played a vital role as energy sources [
120]. The use of an asymmetric orientation of primordial pigments has the potential of efficiently using light energy for the purpose of ion pumping. The synthesis of membrane components may be facilitated by the resulting electrochemical gradients. The process of anabolic metabolism is closely linked to the replication of cellular membranes, hence facilitating the propagation of early cells. The potential development of protocells, which possess both metabolic capabilities and a protective vessel, may have surpassed that of bare polymer replicators that lack membranes.
Proteinoids demonstrate the simplicity with which cellular structures can self-assemble from prebiotic compounds. Their spontaneous encapsulation into vesicles demonstrates an innate organising principle. The binding of amino acids to polymers allowed for more complex functions, such as selective transport. Proteinoids facilitated the transition from nonliving chemistry to the first primitive cells through a series of incremental steps. Bottom-up construction of biomimetic protocells continues to shed light on the origins of life.
4. Proteinoids as Molecular Assemblies
4.1. Aggregation states and dynamics
Proteinoids display intricate patterns of aggregation and dynamics due to their amphiphilic polymeric nature [
71,
85,
121,
122]. Proteinoids exhibit diverse molecular assemblages in response to environmental factors such as temperature, pH, and ionic strength.
The varied morphologies arise from the equilibrium of hydrophilic and hydrophobic interactions among proteinoids. For example, the addition of salt can elicit the creation of spherical structures by effectively neutralising charges. Proteinoids undergo continuous processes of assembly and disassembly in response to fluctuations in the environment. The collective inclinations of these entities exhibit similarities to those observed in naturally occurring proteins [
55,
85,
123,
124].
Proteinoids in assemblies such as microspheres exhibit great molecular mobility, according to kinetic studies [
77,
125]. They exhibit swift exchange and diffusion characteristics while remaining fluid. This dynamic enables conformational changes, substrate transport, and particle communication. Such motion may allow for the evolution of order and complexity.
The complexities of proteinoid aggregation reveal information about early proto-life. The spontaneously crafting structures create organised microenvironments that can be further chemically manipulated. The assemblies operate as a bridge for interactions that could lead to metabolic pathways and information transmission. Proteinoids provide evidence for possible steps along the path from prebiotic chemistry to real life systems.
Proteinoids self-assemble into a variety of forms from the bottom up. Because of their sensitivity to external variables, they exhibit dynamic behaviour. These intricate molecular processes most likely occurred before the first primitive cells appeared. Proteinoids remain an important model for researching the origins of life-like molecular organisation [
126].
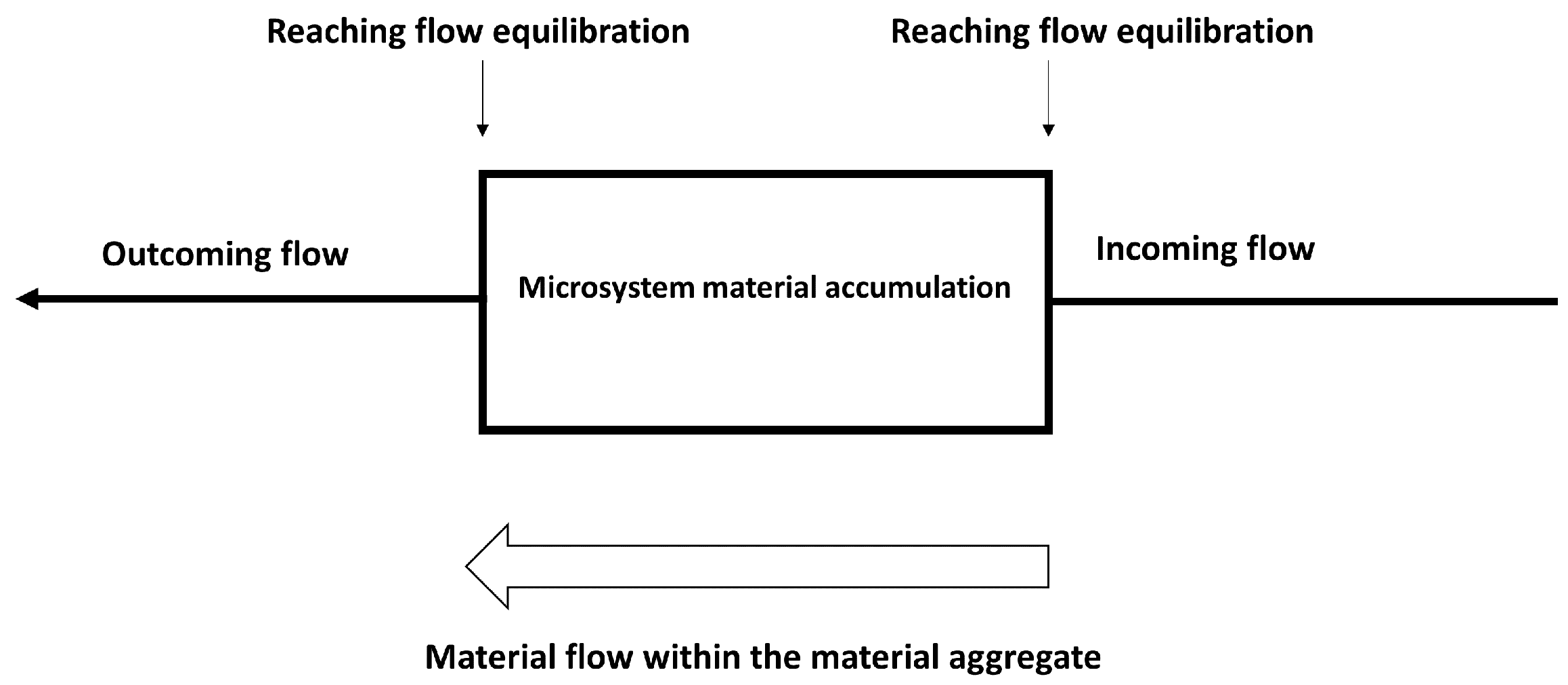
Figure 1 depicts the dynamic process underlying proteinoids aggregation. As depicted, as aggregates form and dissipate, there is a constant interplay between incoming, outgoing, and internal flows. If incoming fluxes alter, internal reorganisations adapt to preserve equilibrium. This propagates additional changes in outgoing flows and external interactions. As flows perpetually re-equilibrate, an unending cycle develops. The key insight is that changes cannot propagate instantly within the aggregate matrix. Instead, finite velocities result in non-concurrent equilibration of incoming, internal, and outgoing flows. Adaptation to the propagating interaction organises the fluid, yet organised, nature of proteinoids aggregates.
In his formalisation of the cell theory, Rudolf Virchow formalised the notion that cells arise from pre-existing cells [
127,
128,
129]. He did not, however, discuss the origins of the first cells. The first living cells emerged continuously from a pre-living “protocell" while retaining their fundamental physical properties, according to an expanded viewpoint. Self-organized, cell-shaped macromolecules with the potential for life comprised the protocell. The interior biophase of the protocell was identified by layers of adsorbed water surrounding precursor biomolecules such as intrinsically disordered peptides. Examining protein solutions, coacervates, and ion-exchange polymers reveals similarities between their physical properties and those of living cells. This supports a model in which the properties of the protocell progressively transformed into those of the first cells via incremental enrichment. As protocell models, proteinoids are bridging the gap between nonliving and living matter [
67].
4.2. Environmental interactions
4.2.1. Mineral templating
It is highly probable that amino acids were synthesised abiotically during the early stages of Earth’s existence, facilitated by energy–driven reactions involving reduced gases such as methane, ammonia, and hydrogen. Amino acids are obtained through experimental exposure of these gases to sparks or shocks, with greater yields observed in atmospheres that exhibit higher levels of reduction. While the precise composition of the early atmosphere remains uncertain, it is postulated that the release of gases from volcanic activity might have sustained a sufficient amount of hydrogen to for significant synthesis of amino acids [
130,
131,
132].
In addition to atmospheric synthesis, mineral surface templating represents an alternative abiotic mechanism for the production of amino acids. Clays and metal sulphides possess the ability to arrange basic precursors, such as formaldehyde, into structured polymers. The promotion of peptide bond formation is facilitated by mineral lattices through the productive alignment of molecules. Experimental findings indicate that peptides with a length of up to 55 units can be shown to assemble on clay templates by the activation of amino acids [
133,
134,
135].
After their formation, amino acids and short peptides could potentially undergo assembly into spherical proto–cells, which would be stabilised by membranes composed of fatty acids. Despite the absence of programmed activities, the many peptide contents of the protocell facilitate intricate dynamics. The phenomenon of entropy plays a crucial role in the emergence of transitory peptide networks that include rudimentary sensory, signalling, and response capabilities.
The protocellular peptide networks demonstrate characteristics such as oscillations, excitability, plasticity, and collective electrical spiking that bear resemblance to neurons [
136]. Peptide–derived membraneless protocells exhibit emergent proto–neural phenomena [
137]. The basic polypeptides exhibit self–organization capabilities in order to effectively process stimuli, similar to the functioning of natural sensory systems. This concept establishes a connection between inanimate chemical processes and the initial emergence of cognitive abilities.
Amino acids, which were abundant during the early stages of Earth’s existence, might have easily formed membrane–bound proto–cells that contained basic peptide networks. The microenvironments supplied in this context are conducive to the development of proto-neural dynamics, enhancing the ability to react to the environment and adapt accordingly. The process of templating synthesis and self–organization of polypeptides establishes the foundation for the more intricate and organised functions observed in living organisms [
138].
In addition to the natural production of amino acids through atmospheric and geological processes, it is plausible that the influx of extraterrestrial materials played a role in the delivery of amino acids to the early Earth [
139,
140]. The examination of comet dust has yielded substantial quantities of organic matter, notably comprising chemicals such as hydrogen cyanide (HCN) and formaldehyde, which has the capacity to quickly generate amino acids. Amino acid precursors such as ammonia, methane, and hydrogen cyanide have been directly discovered in observations of comet comae [
51,
138].
Proteinoids, being amphiphilic polymers, exhibit a tendency for self–assembly into microscale spheres upon precipitation from supersaturated solutions, as depicted in
Figure 2a,b. The proteinoids have the ability to undergo reconfiguration, resulting in various morphologies, such as the cubic crystals featuring central cavities as illustrated in
Figure 2c. The use of electrical stimulation induces the reorganisation of proteinoids [
141,
142,
143,
143]. Moreover, proteinoids exhibit interactions with inorganic crystal surfaces, displaying a preference for the formation of nanoscale arrays on cubic substrates (see
Figure 2d). The presented SEM photos serve as visual evidence of the capacity of proteinoid assembly to generate a wide range of supramolecular structures that exhibit responsiveness to changes in environmental variables. The inherent tendency of proteinoids to exhibit spontaneous organisation provides them with certain benefits, making them suitable models for investigating the fundamental physical mechanisms involved in the formation of early protocells [
144].
The concept of thermodynamic inversion suggests that the shift from disorganised protocell assembly to integrated life systems was driven by oscillating hydrothermal stresses. Periodic fluctuations in physical and chemical conditions exerted selective pressure on microsystems, favouring those capable of mounting amplified and deliberate reactions. Only such adaptive coordination enabled persistence and propagation.
Proteinoids were probably crucial in facilitating this shift. Their ability to self–assemble and exhibit signalling, oscillations, and neural–like excitation makes them well–suited to connect non–living and living matter in response to environmental changes. The gradual enhancement of proteinoids towards cellular functions provides valuable insights into the development of life’s intricate and self–sustaining activities [
85,
145].
4.2.2. Adsorption phenomena
The distinctive photochemical characteristics of Protoporphyrin IX [
146,
147,
148] may have had a role in the early development of photosynthesis. Protoporphyrin IX exhibits modified absorption, fluorescence, and enhanced photoreactivity when it forms complexes with proteins such as serum albumin or basic proteinoids. The protein microenvironments provide benefits by enhancing and altering the pigment’s ability to absorb light.
The adsorption capabilities of proteinoids may have facilitated the creation of protocells without membranes, in addition to their role in organising pigments. Biophases, which are aqueous phases, can form around proteins as a result of water layers being adsorbed. Biophases exhibit a discerning permeability similar to membranes, which allows them to compartmentalise their contents [
148].
The development of small peptides driven by entropy leads to the formation of temporary networks of peptides within protocells in the biophase. These exhibit intricate dynamics such as electrical excitability and spiking, which are characteristic features of brain activity. The basic non–living polypeptides spontaneously arrange themselves to display primitive brain activities.
Membraneless protein–based protocells serve as credible representations of early life, incorporating the ability to sense, signal, and respond. The study of the adsorption biophysics that drives the organelle–like evolution of proteinoids sheds light on the physical properties of cytoplasm and nucleoplasm.
Utilising the adsorption processes and emergent networked activities of proteinoids holds the potential to significantly enhance our understanding of the origins of life and the development of novel bioinspired materials through engineering. Their remarkable adaptability endures as a connection between primordial chemistry and the initial operational systems [
149].
The biophase framework expands upon Rudolf Virchow’s cellular theory [
150,
151,
152], which states that cells originate from pre–existing cells. His principles probably governed the emergence of the first live cells from pre-living protocells during early life. The key lies in maintaining the invariance of fundamental physical features throughout this shift. Protocells formed spontaneously, creating peptide networks that resembled cells and had the ability to support life [
67].
Protocell models, such as protein microspheres, elucidate the biophysical foundation of early cellularity. Their interior phases display resemblances to contemporary cytoplasm and nucleoplasm. An informative exploration of potential processes in the genesis of life is achieved through the comparative study of model systems and real cells. To do this, a protophysiology approach is necessary to evaluate the common physical characteristics that underlie both. The gradual enhancement of the organisational complexity of proteinoids remains a viable approach to understanding the beginnings of life [
153].
4.3. Self-organization tendencies
Ecosystems demonstrate intricate self–organizing networks, characterised by indirect interactions and feedback loops that produce system–wide coordination. The interdependent web homogenizes and amplifies flows, increasing order and utility for constituent organisms. Proteinoids also have the ability to easily come together and form microenvironments that exhibit cooperative effects [
154].
Protocells emerged by the spontaneous self–assembly of several amphiphilic chemicals that existed on the primordial Earth. Enclosures are formed by synthesising components such as fatty acids, while proteins have a role in organising metabolism and signalling. The integration of these rudimentary biopolymers into protocells was driven by entropic processes [
155,
156].
Proteinoids exhibit intricate self–organizing properties, forming a wide array of structures and networks. Their interactions result in realistic behaviours such as signalling, oscillations, and neural–like excitation. Ordered complexity arises spontaneously from the lower levels without any external programming [
157].
Natural selection exerts evolutionary pressure to enhance the spontaneous coordination of self-organization in systems such as protocells [
158]. The gradual enhancement of organisational complexity continued as a means to achieve genuine living systems [
159]. Proteinoids, which endure as models of protocells, serve as a connection between non–living chemistry and the initial emergence of life.
The combined interaction of self–assembly, complexification, and selection provides a viable paradigm for the emergence of cellular life [
160]. Proteinoids are still shedding light on the possible stages involved in the shift from ambient chemistry to early life [
161].
5. Implications of Proteinoids for Thermodynamic Inversion
Trifonov’s concept of life as self-reproduction with variation serves as an excellent guide for determining the point at which non-living systems become living systems [
162,
163,
164,
165]. This has important implications for prebiotic chemistry and artificial cell research, since the distinction between protocells and genuine cells is still hazy. Evaluating proteinoids and other model systems against self–replication and evolvability criteria can show whether they exhibit these essential aspects of life.
Beyond precise definitions, thinking about essential biological traits like energy capture, adaptive responsiveness, regulated dynamics, and self–renewal sheds light on the origins of life. Proteinoids exhibit some of these traits, such as the ability to create dissipative structures, exhibit complicated assembly behaviours, and integrate rudimentary sensitivity and signalling. Examining how proto–life models gradually gain lifelike characteristics aids in identifying important transitions towards cellularity.
The mechanism by which proteinoids and other protocell components give rise to the coordinated thermodynamic and kinetic processes that are characteristic of animals is of great interest. The spontaneous inversion of chaotic chemistry into highly organised life represents a fundamental physical change. Proteinoids serve as a link between the two, revealing glimpses of life’s intricate, interrelated, and self–sustaining mechanisms [
166].
Advancements in prebiotic chemistry have significantly progressed the development of more intricate protocell models, such as vesicles containing nucleic acids and lipids [
7,
167,
168,
169]. Some display the characteristics of self–replicating polymers and proto–metabolism, which resemble those found in actual cells. However, essential characteristics of life such as intricate coordinated actions, swift reactions, and population dynamics are still not fully understood [
170].
5.1. Dissipative proteinoid systems
Proteinoids have a high tendency to spontaneously form microspheres and other structures that display dynamic behaviour outside of equilibrium. Proteinoid aggregates are dissipative structures that function as open systems, maintaining steady states that are far from thermodynamic equilibrium. They consistently assimilate substances from their surroundings to counteract internal degradation.
The dissipation of simpler, unstable molecules within proteinoids microspheres results in the preferential preservation of more complex and stable proteinoid polymers. This inherent process of spontaneous filtering results in a gradual enhancement of order and organisation. Dissipative self-assembly allows for the process of evolutionary self–complexification in proteinoid systems.
Dissipativity theory offers a paradigm for examining the energy dynamics and stability of driven, non-linear systems such as proteinoids. Storage functions describe how the internal state of a system is influenced by the past inputs and outputs. Passivity quantifies the extent to which the system absorbs or releases input energy.
The proteinoids’ complex behaviour demonstrates how dissipative structures can manifest lifelike characteristics. Their dynamic and unbalanced state likely played a crucial role in the development of cellular life. Exploiting the dissipative features of proteinoids is shedding light on possible routes from inorganic matter to the intricate organisation of biological.
Continuing to clarify the non-equilibrium thermodynamics that underlie the assembly and activity of proteinoids is still a significant area of research. Their spontaneous self-complexification suggests the underlying mechanisms that influenced the early stages of prebiotic evolution. Examining proteinoids and modern cells within the framework of dissipative structures theory shows great potential for further research [
31].
The proteinoid microspheres produced by Fox exhibit fundamental catalytic properties, such as the conversion of ATP, which can be linked to the build-up of complexes. Nevertheless, the process of self-reproduction remains ambiguous. According to the dissipative structural perspective [
31,
171,
172], the process of selectively retaining proteinoids with greater catalytic efficiency can result in the formation of “activated" microspheres.
The process of molecule filtration through dissipation generates microenvironments that are favourable for the synthesis of nucleotides and oligomers [
173]. RNA potentially organised the formation of early proteins with template-like properties, while proteinoids likely enhanced the process of catalysis. This has the potential to establish a connection between nucleic acids and proteins before the existence of life. Proteinoids probably provided a framework for the development of biopolymer networks, where the movement and behaviour of catalytic complexes were controlled by the gradual buildup of energy through self–organization. Exploring these interrelated steps further advances credible explanations for the transition from primordial chemistry to biology.
Willems first introduced the concept of dissipativity in systems theory [
174] to describe dynamical systems by input–output properties. Considering a dynamical system described by its state
, its input
and its output
, the input–output correlation is given a supply rate
. A system is said to be dissipative with respect to a supply rate if there exists a continuously differentiable storage function
such that
,
and
As a special case of dissipativity, a system is said to be passive if the above dissipativity inequality holds with respect to the passivity supply rate
.
The physical interpretation is that
is the energy stored in the system, whereas
is the energy that is supplied to the system. This notion connects to Lyapunov stability [
175], where storage functions play the role of Lyapunov functions. Dissipativity theory aids feedback control design for proteinoid systems and other complex dynamics. It continues being an active research area in systems analysis and control due to broad applicability.
5.2. Proteinoid–mediated energy flow
It is probable that basic protein-like molecules had important functions in early processes of converting energy that happened before the development of contemporary metabolic pathways. Inorganic ions and minerals likely had a role in the first formation of basic phosphorylation events within proteinoid microspheres. Porphyrins complexed with proteinoids could potentially enhance the photochemical capabilities by serving as organic cofactors.
Self-assembled proteinoid membranes, with pigments with asymmetric orientations, could have facilitated the simultaneous processes of light harvesting and photophosphorylation. The potential energy contained in ion gradients has the ability to facilitate the production of extra membrane components in a basic photosynthesis process. Proteinoid vesicles, which have the ability to selectively allow certain substances to pass through, create suitable conditions for the integration of proto-metabolic networks.
The adsorption features of proteinoids promoted the emergence of cell-like biophase enclosures that included networked peptides and proto-cofactors. These dissipative systems displayed intricate dynamics that facilitated the transformation and use of various environmental inputs into valuable energy outputs. Energy flows were naturally directed towards the functioning of proto–cells [
141].
Proteinoids, with their diverse skills in assembly, compartmentalization, stimulation, and catalysis, facilitate the gradual integration of proto-bioenergetic systems. Their emerging activities were reasonable steps towards building interconnected reactions involving both autotrophs and heterotrophs. Studying how proteinoids facilitate the transfer of energy helps uncover the fundamental principles that underlie the origins of metabolism.
Proteinoids are useful models for studying the possible connections between primordial chemistry and the thermodynamics of living organisms. Their wide array of biomimetic characteristics created a non-living foundation ready to be covered by emerging proto-biopolymers [
105,
176,
177].
5.3. Drivers of complexity in proteinoid networks
The transition from primordial chemistry to primitive biology presumably necessitated the collaboration of peptides and oligonucleotides. The process of non-ribosomal peptide bond emergence predates ribosomal synthesis. The dual functions of ribose, as a component of nucleic acids and as an activator of peptides, may have played a crucial role in early linkages. Phosphorylated amino acids may facilitate the linkage between proto-protein and nucleic acid production.
To promote efficient peptide synthesis in water under prebiotic conditions, activation is required to overcome hydrolysis. The oscillation of this was likely caused by either sunlight or geothermal gradients on the early Earth. Mineral-assisted primordial photosynthesis models have recently become more popular than deep sea vent models. Terrestrial environments that contain a plentiful supply of phosphorus and potassium may have been more favourable for the initiation of life.
UV–resistant base–paired nucleic acids and hydrogen-bonded peptides could have been obtained through photochemical selection [
178]. Short photo-stable sequences like stem–loops or
–helices withstand irradiation. Peptide–binding RNAs were used to enhance the stability of mixed complexes [
179]. The presence of hydrophobic interactions among nonpolar amino acids enhanced the ability of peptides to form structured arrangements, facilitating the formation of membrane–like assemblies that served as templates for additional organisation.
Proteinoids, such as Sidney Fox’s microspheres, probably served a similar function by creating organised microenvironments where complex proto–biopolymers might accumulate. The combination of diverse surface chemistries in proteinoids resulted in cooperative effects and system–wide repercussions similar to those observed in current proteins. Their intricate properties emerged from assembly driven by entropy, without the need of templates. Studying the self–organization of proteinoids can provide valuable insights into the fundamental forces that drove early evolution.
While genetics–first theories presently dominate beginnings of life research, concepts like Oparin’s coacervates and Fox’s proteinoids better reflect the graded transition from prebiotic chemistry to life’s sophisticated, hierarchical organizations [
63]. The dynamic behaviour of proteinoids continues to uncover potential connections between inanimate and living substances [
178].
The transition from prebiotic chemistry to cells probably involved several significant evolutionary stages. Biomolecules formed intricate aggregates such as chromosomes and ribosomes, creating novel quasi–species. The divergence into distinct lineages was driven by competition between quasi–species of different compositions. The integration of quasi–species resulted in the development of more intricate systems over time.
As an illustration, ribozymes, proteinoids, and lipids may have worked together to create ribosomes. Additionally, it is possible that proteinoids and other biopolymers may have developed into chromosomes. Chromosomes and ribosomes were integrated into prokaryotic cells, marking the emergence of the first fossilizable lifeforms. It is highly probable that the proteinoids’ remarkable adaptability played a significant role in numerous transitions during early evolution. The understanding of the physical basis behind their spontaneous self–assembly and complexification offers ongoing insights into the development of life’s hierarchical organisations.
The dynamic nature of proteinoids serves as a bridge between non–living and living matter. Their gradual development towards cellular structures offers insights into the origins of life that cannot be obtained from biological fossil records. Studying proteinoids’ capabilities allows us to piece together potential stages in the evolution from primordial chemistry to the existence of even the most basic forms of life [
180].
6. Unconventional Computing with Proteinoids
6.1. Memristive and memcapacitive behaviors
The memfractance properties [
181] exhibited by proteinoids and proteinoid–mineral composites emphasise their potential as bioelectronic materials.
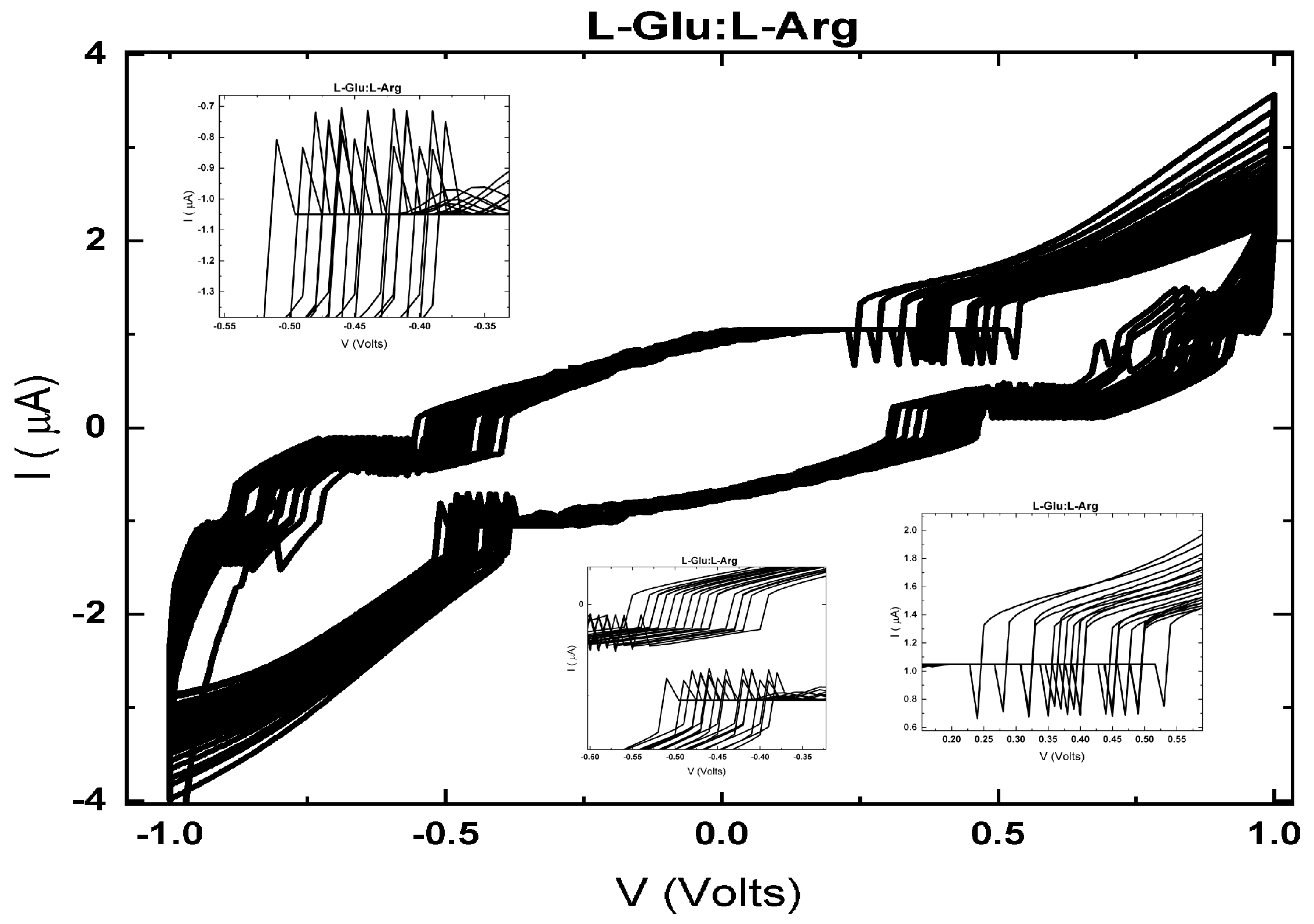
Figure 3 demonstrates that pure L-Glu:L-Arg proteinoids display non–linear I–V characteristics with asymmetrical hysteresis near 0 V, suggesting the presence of memristive–like memfractance. However, when HAP biomimetic minerals are added, the memfractance properties of the proteinoids undergo considerable changes.
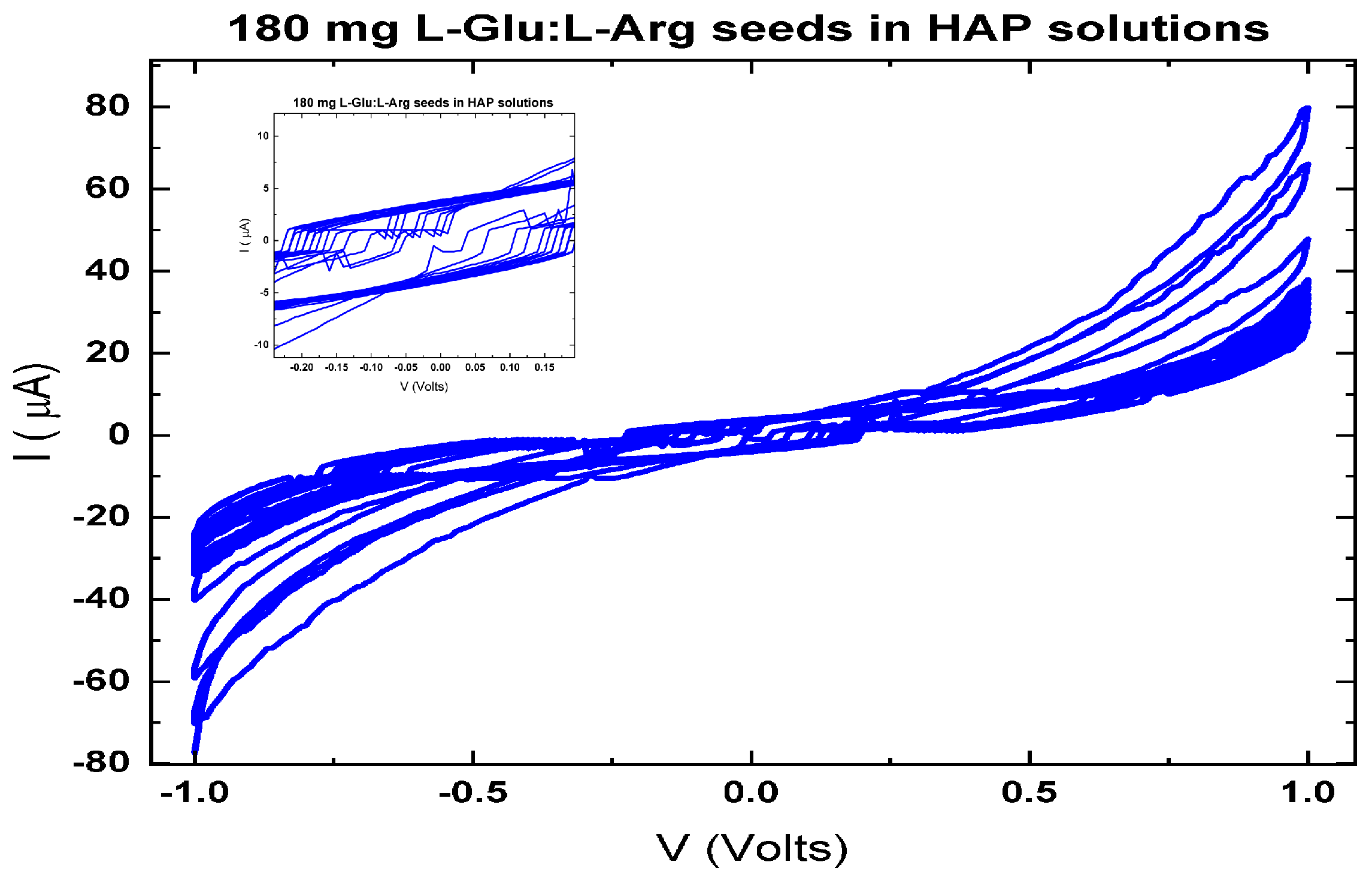
Figure 4 illustrates that the HAP growing environment significantly improves the memfractance, resulting in a more than 20-fold increase in the ±1 V currents and almost complete elimination of hysteresis.
The findings demonstrate that the electrical characteristics of proteinoids can be specifically adjusted through the process of templating and through interactions with mineral substrates. The versatile memfractance allows for the manipulation of input signals and the incorporation of memory effects into proteinoid networks. Continued research on understanding the relationships between memfractance variables has the possibility for further development of specialised bioelectronic devices using proteinoids. Proteinoids possess a distinctive suitability as active materials for integrated biological–electronic systems due to their lifelike excitability and tunable electronic response. Exploring various assembly and compositional alterations offers promising prospects for creating adaptable, versatile bioelectronic structures.
6.1.1. Electrical excitability
The electrical signalling capacity of proteinoids [
27,
105,
136,
182,
183,
184,
185] is partially attributable to their synthetic pathway, as illustrated in
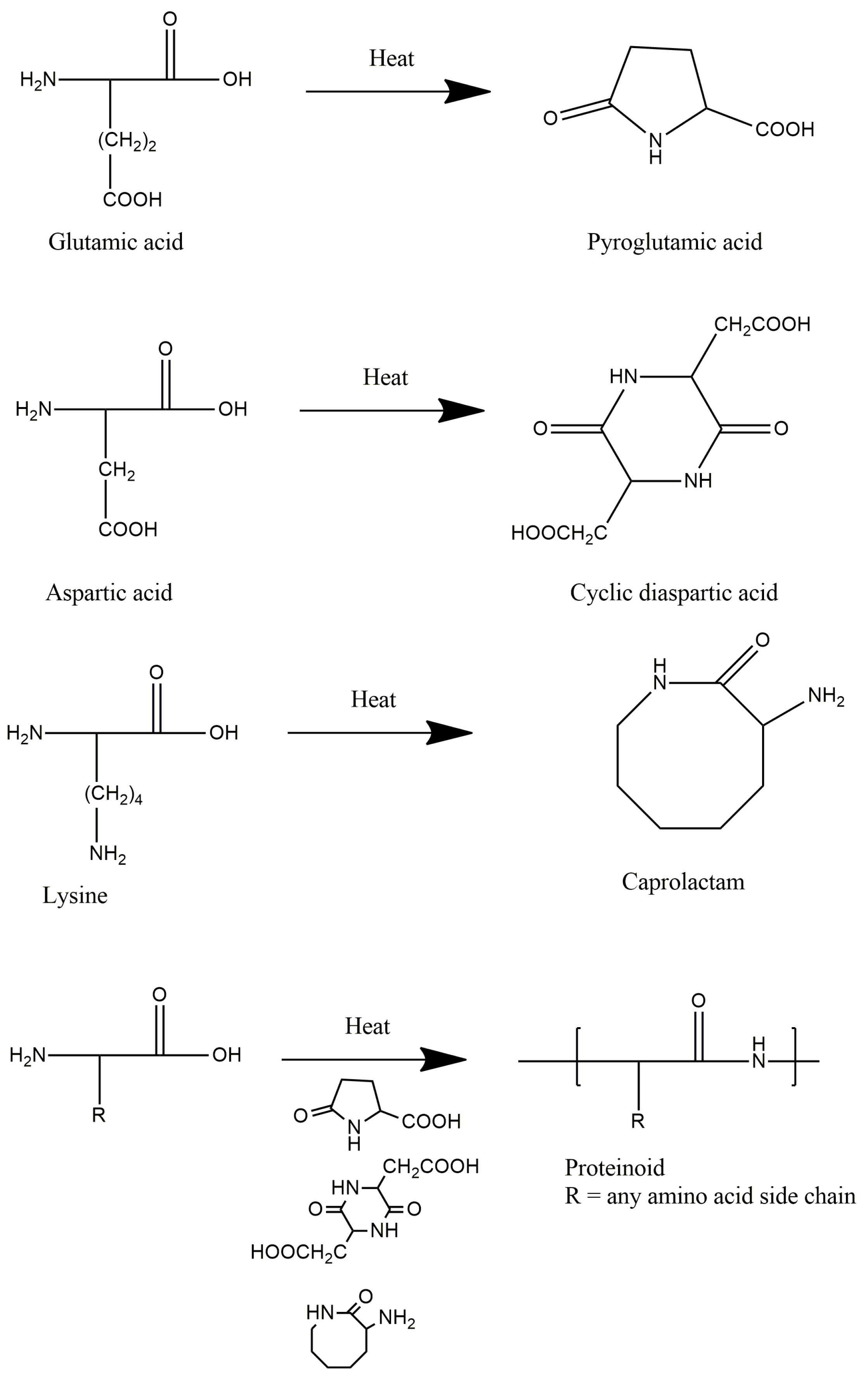
Figure 5. Through intramolecular condensation reactions, cyclic monomers such as pyroglutamic acid are produced when amino acids are heated. Subsequently, these monomers undergo polymerization to form polypeptide chains, which coalesce into proteinoid microspheres. Pyroglutamic acid and other cyclized amino acid derivatives provide the proteinoid backbone with rigidity and stability. In addition, when organised into dense proteinoid aggregates, they generate electrostatic dipoles that are capable of excitation and signal propagation. By adjusting the composition and rigidity of the polymer during synthesis, the emergent excitability of proteinoid systems is thus directly influenced. Determining the structure-function relationships underlying the electrical properties of proteinoids will facilitate the practical development of customised bioelectronic materials.
One proposed mechanism for the proteinoids’ intrinsic electrical excitability was developed by Matsuno et al. [
107].
The inherent capacity of proteinoids to demonstrate electrical signalling patterns is demonstrated by extended recordings of microspheres activity.
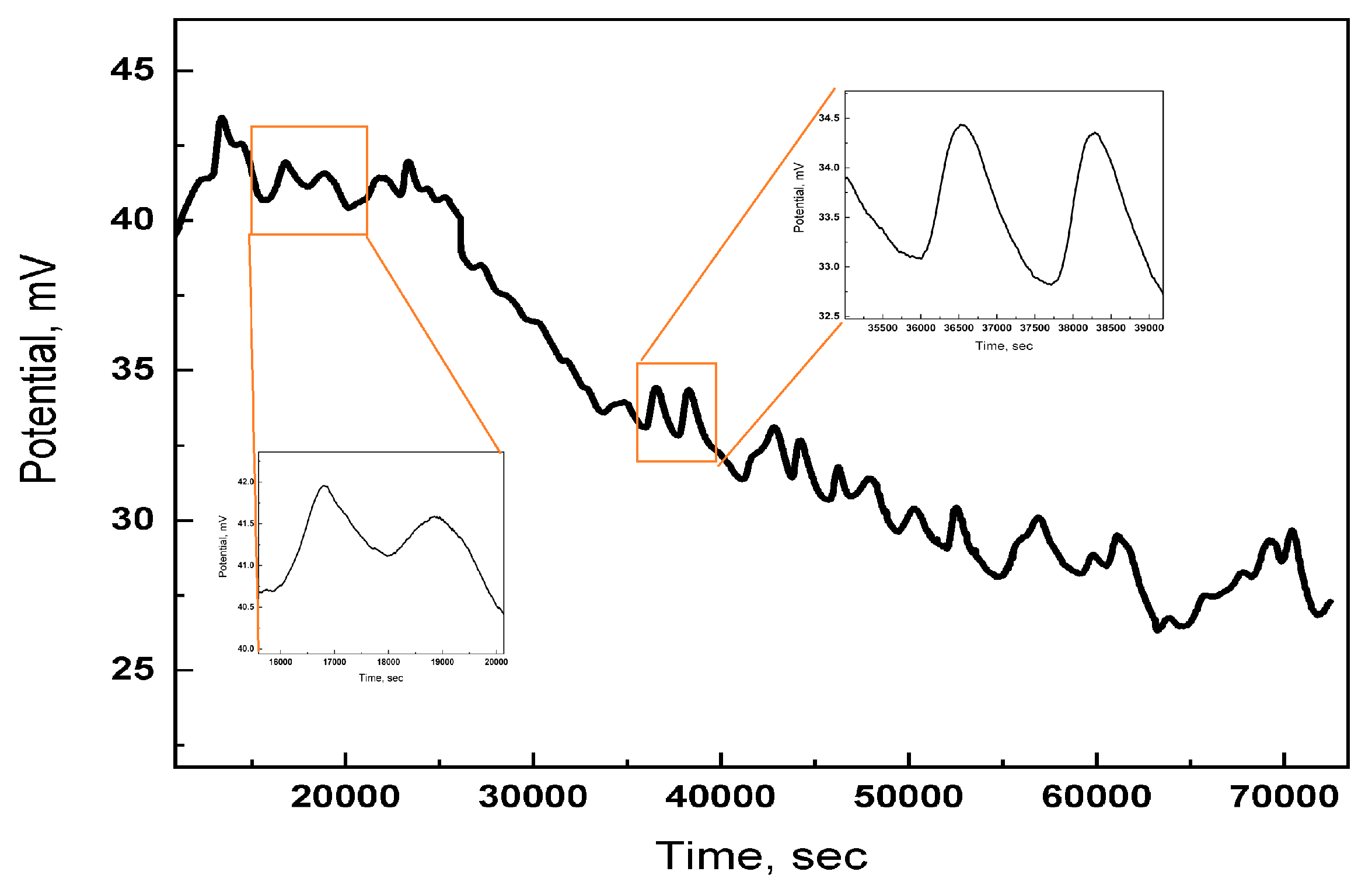
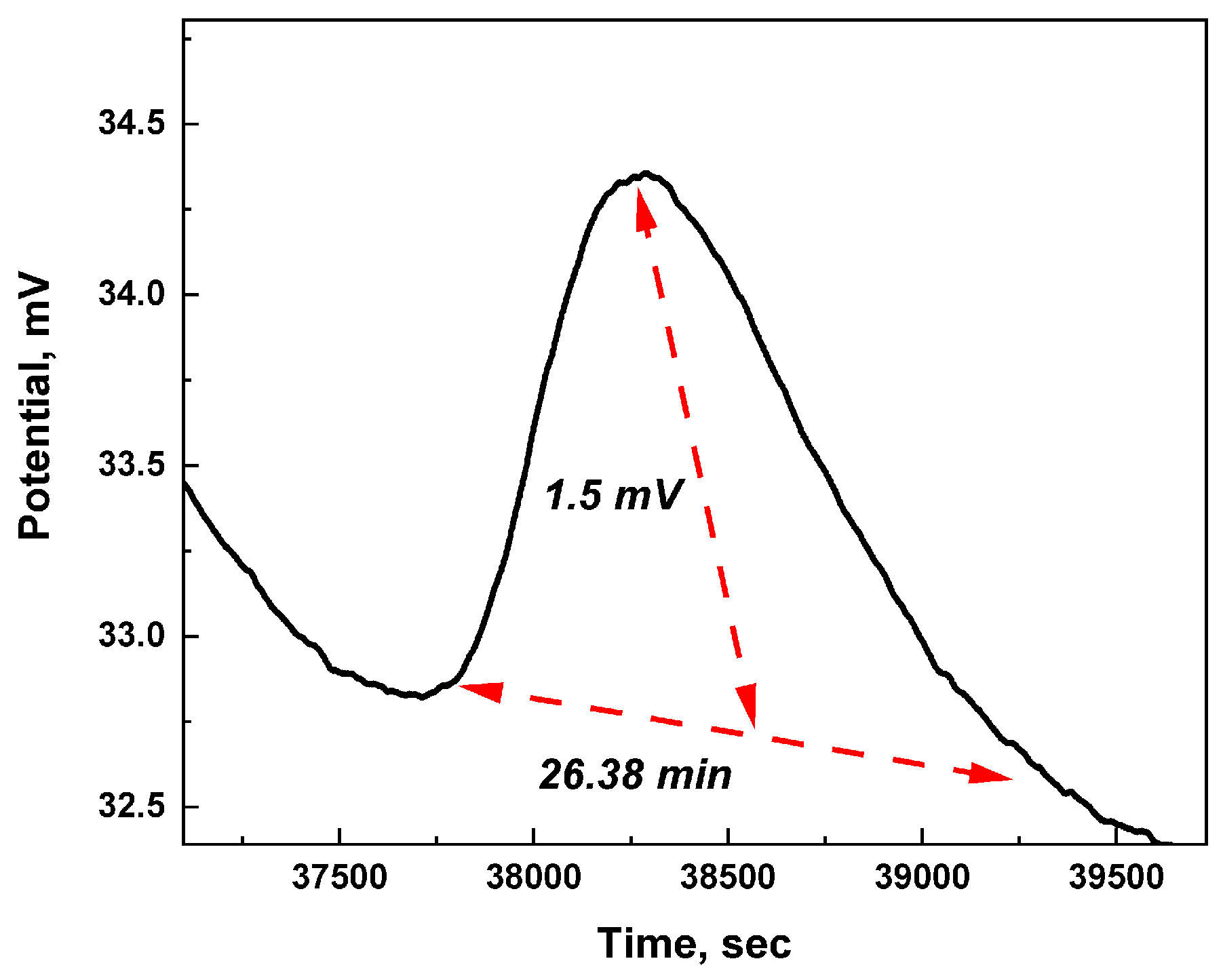
Figure 6 displays voltage measurements taken over a period of 21 hours. The results demonstrate a continuous pattern of distinct spikes, with enlarged sections emphasising their repetition throughout time intervals ranging from minutes to hours. The consistent and repetitive surges in electrical activity illustrate the capacity of proteinoids to produce enduring electrical stimulation by self–arrangement.
The spikes have characteristics that resemble neuronal action potentials, as depicted in the typical occurrence shown in
Figure 7. The swift change in electrical charge and subsequent restoration indicate the synchronised activation and recuperation of the microspheres, facilitated by temporary electrostatic contacts among proteinoid subunits. The combination of persistent spiking patterns throughout time and distinctive spike shape demonstrate how the emergent dynamics of proteinoids can imitate crucial signalling behaviours observed in biological brain networks [
184].
A modelled interaction between basic proteinoid
and acidic proteinoid
inside a microsphere
S can be written as [
107]:
Where all
are inside
S and
can enter and leave. The intake flow
of
is:
The outflow of
is:
Where
and
are interaction rate coefficients. Maintaining continuity:
With accumulation rate
. As
accumulates, electrostatic attraction decreases, reducing
. When
, the difference widens due to instability around
. But as
decreases, electrostatic attraction increases
again until it overcompensates, starting the cycle again.
This sawtooth-like spiking arises from:
The intrinsic instability and singularities in the dynamics lead to sharp, repetitive spiking behaviors. The proteinoids’ electrical excitability emerges from the nonlinear kinetics and nonequilibrium nature of the system.
The linear stability around the stationary state
can be examined by considering small perturbations
,
,
:
The linear increment
is:
This positive
indicates intrinsic instability. The dynamics satisfy:
The nonzero accumulation rate combined with the singularities in the kinetics leads to oscillatory, spiking behaviors. The nonequilibrium thermodynamics underlying the unstable dynamics and excitability could be further analyzed using systems approaches like dissipative structures theory.
The accumulation rate
can be written in terms of changes in the interaction rates:
The changes propagate through the medium over time interval
:
Imposing flow continuity:
In the limit as
:
Where
and
are denoted as
and
in the limit. The material flow equilibration maintains continuity:
The change propagation and continuity constraints lead to the dynamic instabilities and spiking.
6.1.2. Ionic and protonic conduction
The excitable dynamics of proteinoids are supported by their ability to conduct both ionic and protonic electrical currents [
184,
187].
We can hypothesize that organised proteinoid assemblies utilise the inherent redox properties of amino acids to regulate transmembrane ion gradients. The presence of water layers surrounding hydrated proteinoids can create distinct boundaries, effectively separating the distribution of ions inside and outside.
The chemical structures of proteinoids give rise to their selective binding and permeation properties. Their amphiphilic nature facilitates the formation of selective interactions and gradients of cations such as , and . The proteinoid gels effectively concentrate over in a manner similar to living cells, despite the absence of a membrane. The conduction mechanism involves the movement of protons between closely packed proteinoids.
The charging dynamics are influenced by the composition, morphology, and permeability of proteinoids. Conduction is influenced by environmental factors such as pH and ionic strength, which have the ability to alter surface charge and solvation. The versatility of proteinoid systems enables the customization of ionic and protonic currents that underlie intricate excitation patterns and information transmission. The molecular properties of proteinoids and their impact on macroscale electrochemical gradients and electrical activities offer valuable insights into bioinspired engineering and the origins of cellular bioenergetics. The remarkable capabilities of proteinoids in ion selection, pumping, and signalling underscore their potential as protocell materials that can bridge the gap between non–living and living electrophysiology.
6.2. Configurable analog logic operations
Unconventional computing systems inspired by biology are feasible due to the spiking dynamics and modifiable interactions exhibited by proteinoid networks. As an example of analogue logic operations that can be implemented in collections of signalling proteinoids microspheres are amplification, integration, and thresholding. These result from the modulatable connections and intrinsic excitability of proteinoids.
Proteinoid interaction patterns are generated from applied inputs such as voltages, sounds, and photons via nonlinear transfer functions. By observing alterations in molecular diffusion or electrical activity, output signals are deduced. By adjusting environmental conditions, proteinoid composition, and morphology, it is possible to configure the functions.
Proteogs organised in networks with predetermined connectivity are capable of processing multiple inputs and outputs. Cascading logic operations facilitate the manipulation of complex signals. Hybrid combinations with other materials facilitate digital system and real–world input interfacing. Proteinoid computing leverages adaptive, collective behaviours in order to process information at an advanced level.
Systematically delineating the computational capabilities of proteinoids and implementing designed functions as opposed to emergent ones remains an unresolved challenge. Proteinoid computing architectures that are robust and generalizable necessitate traversing multiple scales, beginning with molecular interactions and progressing to collective ensemble behaviours. Nevertheless, the abundance of proteinoids continues to unveil appealing prospects for analogue computing inspired by biology.
The combination of programmability and lifelike dynamics in proteinoids makes them highly promising materials for unconventional computational paradigms. The intriguing parallels between primordial cognition and the configurable, collective information processing of synthetic protocells are highlighted. Proteinoids contribute to the development of unconventional bioelectronic systems and illuminate potential pathways from prebiotic chemistry to proto–life [
58,
172,
188,
189].
6.3. Spiking neural network dynamics
Proteinoid network emergent spiking behaviours are strikingly similar to the excitation dynamics observed in biological neurons: proteinoids exhibit propagating spikes, refractory periods, and explosive patterns that are characteristic of neural activity through spontaneous self–organization [
27,
105,
136,
182,
183,
184,
185]. In the absence of genetic encoding, this presents a compelling model system for investigating the origins of neural–like signalling.
Proteinoids exhibit analogue spiking responses; however, for precise signal propagation, optimising network connectivity via synaptic–like junctions could facilitate digital, all–or–nothing spikes. Incorporating plasticity into proteinoid networks could enable them to be tuned similarly to artificial neural networks in order to acquire associations and recognise patterns. Incorporating actuators for outputs and sensing elements for external inputs would complete the essential components of closed–loop neural control.
Scaling the intricacy of proteinoid networks to match the enormous dimensionality of biological brains is a significant obstacle. This necessitates hierarchical architectures as opposed to haphazardly interconnected spheres. Compositional diversity and morphological control innovations will be crucial. However, cascades of emergent complexity can be observed in even the most basic proteinoid networks due to their collective interactions.
The investigation of natural neural dynamics and proteinoid signalling in comparison provides advantages for both engineering and biology. Proteinoids serve as scaffolds to reveal fundamental mechanisms, whereas neural networks propose design principles for the development of sophisticated proteinoid computation. This dialogue underscores the profound compatibility that exists between the investigation of the origins of life and the progression of biological intelligence.
The biomimetic parallels that proteinoid networks continue to reveal now extend to neural systems. Ongoing collaboration between bottom–up synthetic biology and neurobiology holds great promise for groundbreaking advancements in understanding the origins of cognition and creating artificial replicas of such capabilities. Proteinoids continue to serve as an intermediary between inanimate and organic complexity [
105,
187,
190].
6.4. Evolutionary learning capacities
Proteinoids display several biomimetic characteristics that offer insights into the early cognitive and evolutionary abilities of proto–life. The heat condensation process allows for the preferential buildup of stable, functional sequences over less viable polymers. Proto–catalytic proteinoids have the ability to proliferate in a way that favours their own existence, contributing to the process of molecular evolution towards increased complexity [
191,
192,
193,
193].
Protocells, when organised, exhibit emergent responses to stimuli that result in basic adaptive behaviours [
185]. In conjunction with reproduction, heritable variations in composition could facilitate the process of natural selection, leading to the survival of more advantageous populations. Proteinoid protocells that have the ability to bind resources, detect gradients, and actively migrate towards favourable conditions would have a competitive advantage over other protocells [
45].
The combination of self–organization, learning, and selection forms a basic evolutionary loop. Iterating over generations enables a step–by–step adjustment of responsiveness, signalling, and collective dynamics. Current proteinoid assemblages already demonstrate rudimentary excitation, oscillations, and adaptability similar to brain systems.
Utilising the biomimetic characteristics of proteinoids could facilitate the development of lifelike materials that possess the ability to adapt autonomously. Bioinspired evolutionary algorithms propose methods for encoding adaptable characteristics and behaviours. Dynamic proteinoid composites provide favourable conditions for flexible investigation of various arrangements and functionalities.
The bridging of non–living and living materials by proteinoids offers insight into the genesis of evolvable systems. The combination of their ability to self–organize, exhibit proto–cognition, and engage in selective processes provides potential stages in the emergence of life. Investigating the evolutionary learning of proteinoids enhances our comprehension of the origins of biology and the ambitions of biological intelligence [
118,
192,
194].
6.5. Hybrid proteinoid–inorganic systems
The combination of proteinoids with inorganic materials provides opportunities to improve their assembly, dynamics, and functioning. Specifically, when combined with calcium phosphates such as hydroxyapatite (HAP), it results in the formation of bioinspired organic–inorganic composites [
75]. The process of thermally condensing proteinoids in the presence of HAP results in the formation of heterogeneous nucleation on proteinoid chains, which is driven by interactions with
ions.
The process of templating creates structures that resemble layers of an onion, with proteinoids creating many layers around a core made of HAP
Figure 8. The morphology can be adjusted from particles at the microscale to hybrids at the nanoscale. In addition to providing mechanical reinforcement, the introduction of HAP modifies the electrical, optical, and chemical properties of proteinoids.
There are multiple proteinoid–inorganic composites that can be created by taking advantage of various mineral interactions [
195]. Silicates facilitate the process of polymerization and the creation of membranes, whilst clays assist in the organisation of molecules. Introducing magnetic nanoparticles or quantum dots into proteinoids enhances their capabilities for sensing, actuation, and manipulation. Photoactive minerals enable the collection of energy in proteinoid photoanodes [
60,
196,
197].
The investigation of proteinoid–inorganic hybrids propels the progress of bioinspired materials chemistry and engineering. The composites incorporate features ranging from semiconductivity to magnetism, while still maintaining essential proteinoid capabilities. This broadens the range of possible uses in the fields of catalysis, photonics, medication delivery, and electronic devices. The self–organized integration also offers models for studying biomineralization and proto–cellular development.
Proteinoids consistently demonstrate remarkable adaptability when combined with functional nanomaterials, resulting in synergistic effects. Their receptiveness to hybridization underscores the significance of dynamic biomolecules as captivating constituents for future bioinspired systems. Proteinoid composites represent advancements in the development of self–regulating systems that bridge the gap between life and non-living substances [
45].
6.6. Potential for low-power, adaptive biocomputing devices
6.6.1. Memory and switching applications
Proteinoids are highly suitable for memory and switching applications, including biologically integrated devices, due to their versatile electrical properties. Proteinoids are capable of neuromorphic computation and nonvolatile data storage due to their memristive properties. Their modifiable conduction states emulate synaptic plasticity in order to facilitate pattern recognition and learning.
CMOS logic integrated with proteinoid memristors can produce dense, low–power adaptive biocomputing architectures. In–memory computation enables an on–chip data processing that is efficient. In addition, biocompatibility permits biosensing applications in which proteinoid networks are reconfigured in response to stimuli to improve signal detection and amplification.
The lifelike signalling dynamics of proteinoids have the potential to enhance implantable devices such as brain–computer interfaces. In situ learning and stimulation modulation facilitate biological system integration. Distributed proteinoid logic prevents implant heat accumulation. Cell–composites containing composite polymers enable electrical or pharmacological manipulation of cultures.
Scalability and ensuring stable encapsulation for physiological operation are obstacles. The rich potential for adaptable, low–power bioelectronics, nevertheless, inspires sustained effort. The convergence of top–down microfabrication and bottom–up synthetic biology holds great potential for groundbreaking scientific and practical developments at the intersection of electronic computing and biology.
Proteinoids continue to serve as model systems that unveil potential avenues for enhancing electronics with autonomous and realistic capabilities. Leveraging proteinoids for unconventional biocomputing underscores the potential for synergies between the advancement of biological intelligence and the comprehension of the origins of biology. Their biocompatibility, switching capabilities, and memory all stimulate bioinspired technological innovation [
198,
199,
200].
6.6.2. Neuromorphic circuits
Because of their emergent electrical excitability, signalling behaviours, and dynamic assembly, proteinoids provide novel prospects for bio-inspired neuromorphic engineering. Crossbar arrays of proteinoid memristors with changeable conductivities imitating plasticity could provide vast synaptic connections for in-memory computing. Distributed Boolean operations and reconfigurable bio–logic would be possible with modular logic gates made from spiking proteinoids.
Furthermore, because proteinoid hydrogels are biocompatible, they can be used as extracellular matrix scaffolds for interacting with biological neuronal cells. This allows for the development of hybrid biological–synthetic networks for neuroscience research and medicinal devices. Composite materials that mix proteinoids and organic electronics provide the benefits of both biofunctionality and established device integration [
201].
Beyond solid–state electronics, leveraging proteinoids’ signalling, diffusion, and response capabilities offers up novel modalities for neuromorphic processors. Using diffusing chemicals to programme proteinoids to interact creates reaction–diffusion patterns for synthetic chemical computation. Impedance–modulating biosensors inspired by sensory organs are created by including enzymes or receptors.
In conclusion, proteinoids provide a rich platform for simulating brain structures and signal processing with dynamic biomaterials. Proteinoids’ versatility in electronics and chemistry position them as intriguing building blocks for future neuromorphic systems with lifelike adaptability, autonomy, and intelligence. Using proteinoids to realise bio–inspired computing highlights their potential for uncovering paths from primordial chemistry to primitive cognition [
52].
6.6.3. Elucidating Synaptic–Like Linkages in Proteinoid Systems
Complex behaviours in even the most basic biological systems depend heavily on networks of molecular interactions and signalling channels [
202,
203,
204]. Although contemporary cells employ complex protein machinery to transmit information, the beginnings of biological communication probably emerged in simpler chemical processes. Remarkably, amino acids can spontaneously create abiotic condensates that display remarkably complex realistic behaviours. This section investigates the potential of higher-order assembly and microscopic fluidity in proteinoids, which are non-living polypeptide complexes, to create synaptic-like connections capable of transmitting information in a basic way. Understanding these systems offers important insights into how the adaptable signalling structures commonly found in existing life may have initially developed.
The depicted
Figure 9 showcases an artificial neural network model that offers a conceptual computational depiction of the dynamic signalling behaviours observed in interconnected networks of proteinoid microspheres [
136]. The individual proteinoid vesicles are represented as artificial neural network (ANN) nodes, with weighted connections that can be compared to synaptic strengths. The weights correspond to postulated diffusive or electrical connection between neighbouring microspheres, facilitating the transmission of spikes. However, the specific physical mechanisms are still being investigated.
Let be a vector of time values in seconds, be a matrix of potential values in volts for 12 samples, be the number of neurons in the ANN, be the time window for temporal coding in seconds, be the threshold for spike detection in volts, be a matrix of temporal codes for each neuron over time, and be a matrix of synaptic weights between neurons.
Then, for each sample
, we have:
where
is an indicator function that returns 1 if
is greater than
, and 0 otherwise. The temporal coding represents spikes as discrete events based on measured potential traces.
The input data comprises measurements of electrical potentials in proteinoid samples, which exhibit spiking patterns similar to those observed in neurons. Temporal coding converts the spikes into distinct signalling occurrences for every virtual node. Unsupervised learning algorithms subsequently modify the simulated synaptic weights in order to accurately represent the observed co–activation correlations, replicating the self–organized structure of the experimental proteinoid networks.
The ANN model offers a conceptual framework to investigate the emergence of collective excitation and transmission in proteinoid systems, however it requires a more comprehensive understanding of the underlying biophysics. The computer recreation of emergent signalling strengthens the functional similarities between these model abiogenetic assemblies and integrated biological brain networks. The combination of in silico modelling and in vitro research is further enhancing our understanding of how lifelike behaviours emerge in proteinoid protocell communities [
205,
206].
6.6.4. Biosensing and bioelectronics
Because of their biocompatibility, adjustable construction, and emergent electrical properties, proteinoids provide interesting possibilities for biosensing and bioelectronic systems. Spiking dynamics have been observed in networks of proteinoid microspheres, which could enable neural–mimetic signal processing and logic operations. Proteinoids also have configurable memfractance behaviours that allow them to convert stimuli into readable outputs.
They can detect biomarkers and biological substances by functionalizing amino acids with ligands or enzymes. Coupled to electrical or optical signals, point–of–care diagnostics can be quantified quickly and affordably. Proteinoids’ stiffness and stability enable for strong performance in complicated media. The incorporation of microfluidics and nanomaterials can improve sensitivity.
Proteinoids’ flexible electrical properties further position them as bioelectronic interface components. Their dynamic construction allows for conformal sensor connections for better in vivo recordings. Proteinoids also hold potential for regulated drug release, therapeutic stimulation, and cell behaviour modulation.
Ongoing research is aimed at unravelling the mechanisms underlying proteinoids’ sensing capacities in order to build responsive materials. Using proteinoids for applications ranging from real–time monitoring to biocomputing necessitates an interdisciplinary approach encompassing chemistry, biology, physics, and engineering [
207].
Proteinoids, due to their adjustable design, emergent dynamics, and biocompatibility, offer a wide range of options for biosensing and bioelectronic technology. Based on their composition and self–assembled nanostructure, peptide–based hydrogels provide a wide range of biological, pharmacological, and technological uses, as summarised in
Table 1. Proteinoids, as synthetic polypeptides, have similar customizability and biofunctionality advantages. Proteinoid hydrogels could be developed for scaffolding cells, controlled drug delivery, shear–thinning injectables, and other applications by optimising proteinoid monomers and polymerization. Taking advantage of proteinoids’ peptide–like properties will allow them to be used in more versatile bioinspired materials and technologies. Proteinoids’ programmable assembly, excitation dynamics, and biointegration make them an appealing platform for improving bioelectronics, biosensing, and biocomputing.
7. Conclusion
Proteinoids are still proving to be a valuable system for studying the origin of life because they can be easily synthesised in prebiotic conditions, they have features that resemble living organisms, and they possess basic cognitive capacities. Amino acid blends can be heated to produce polypeptide assemblies that display essential features of primitive life, such as catalytic activity, microtubule production, protocell structures enclosed by membranes, and self–replication behaviour. Proteinoids possess inherent electrical excitability and adjustable signal processing, which suggest the presence of early problem–solving abilities. While far from modern organisms, proteinoids reveal plausible pathways by which the first polypeptides could have acquired lifelike behaviors like signaling, learning, and control. The potential of proteinoids as primitive cognitive systems is demonstrated by experiments that integrate them into synthetic proto–neural networks and biocomputing architectures. While there are still many unanswered concerns about the beginnings of coding and developing life, proteinoids provide valuable insights into the potential connections between prebiotic chemistry and the early development of collective dynamics, memory, and intelligence that preceded the birth of life on Earth. Their strong origin and extensive capacities continue to illuminate the gradual transition from non–living matter to conscious life forms.
Author Contributions
“Conceptualization, A.A. and P.M.; methodology, A.A and P.M; software, P.M.; validation, A.A.; formal analysis, P.M.; investigation, A.A and P.M.; resources, A.A.; data curation, P.M; writing—original draft preparation, P.M.; writing—review and editing, A.A.; visualization, P.M.; supervision, A.A.; project administration, A.A.; funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.”, please turn to the
CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
The research was supported by EPSRC Grant EP/W010887/1 “Computing with proteinoids”.
Data Availability Statement
The data is uploaded in the supplementary material.
Acknowledgments
Authors are grateful to David Paton for helping with SEM imaging and to Neil Phillips for helping with instruments.
Conflicts of Interest
We, the authors Panagiotis Mougkogiannis and Andy Adamatzky, declare that we don’t have conflicts of interest associated with the article.
References
- Pascal, R.; Pross, A.; Sutherland, J.D. Towards an evolutionary theory of the origin of life based on kinetics and thermodynamics. Open biology 2013, 3, 130156. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, S.J.; Beckett, P. Probing complexity: Thermodynamics and computational mechanics approaches to origins studies. Interface focus 2019, 9, 20190058. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; De Los Rios, P.; Busiello, D.M. Non-Equilibrium Chemical Reactions Under Non-Isothermal Conditions: Kinetic Stabilization, Selection and Thermophoresis.
- Kleidon, A.; Lorenz, R.D. Non-equilibrium thermodynamics and the production of entropy: life, earth, and beyond; Springer Science & Business Media, 2004.
- Fox, S.W. Thermodynamic perspectives and the origin of life. In Quantum Statistical Mechanics in the Natural Sciences: A Volume Dedicated to Lars Onsager on the Occasion of his Seventieth Birthday; Springer, 1974; pp. 119–142.
- Michaelian, K. Non-equilibrium thermodynamic foundations of the origin of life. Foundations 2022, 2, 308–337. [Google Scholar] [CrossRef]
- Ruiz-Mirazo, K.; Briones, C.; de la Escosura, A. Prebiotic systems chemistry: new perspectives for the origins of life. Chemical reviews 2014, 114, 285–366. [Google Scholar] [CrossRef]
- Ruiz-Mirazo, K.; Briones, C.; de la Escosura, A. Chemical roots of biological evolution: the origins of life as a process of development of autonomous functional systems. Open biology 2017, 7, 170050. [Google Scholar] [CrossRef] [PubMed]
- Frenkel-Pinter, M.; Samanta, M.; Ashkenasy, G.; Leman, L.J. Prebiotic peptides: Molecular hubs in the origin of life. Chemical reviews 2020, 120, 4707–4765. [Google Scholar] [CrossRef] [PubMed]
- Mann, S. Systems of creation: the emergence of life from nonliving matter. Accounts of chemical research 2012, 45, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, H.S. The RNA world hypothesis: the worst theory of the early evolution of life (except for all the others) a. Biology direct 2012, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.P.; Joyce, G.F. The origins of the RNA world. Cold Spring Harbor perspectives in biology 2012, 4, a003608. [Google Scholar] [CrossRef]
- Joyce, G.F.; Orgel, L.E. Prospects for understanding the origin of the RNA world. Cold Spring Harbor Monograph Series 1993, 24, 1–1. [Google Scholar]
- Orgel, L.E. Some consequences of the RNA world hypothesis. Origins of Life and Evolution of the Biosphere 2003, 33, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, M.; Szostak, J. Semipermeable lipid bilayers exhibit diastereoselectivity favoring ribose. Proceedings of the National Academy of Sciences 2005, 102, 6004–6008. [Google Scholar] [CrossRef] [PubMed]
- Pross, A.; Pascal, R. The origin of life: what we know, what we can know and what we will never know. Open biology 2013, 3, 120190. [Google Scholar] [CrossRef]
- Deamer, D. The role of lipid membranes in life’s origin. Life 2017, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Barge, L.; Branscomb, E.; Brucato, J.; Cardoso, S.; Cartwright, J.; Danielache, S.; Galante, D.; Kee, T.; Miguel, Y.; Mojzsis, S.; others. Thermodynamics, disequilibrium, evolution: Far-from-equilibrium geological and chemical considerations for origin-of-life research. Origins of Life and Evolution of Biospheres 2017, 47, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Hill Jr, A.R.; Liu, R.; Orgel, L.E. Synthesis of long prebiotic oligomers on mineral surfaces. Nature 1996, 381, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Mishra, V.; Mukherjee, M.D.; Saini, P.; Ranjan, K.R. Amino acid derived biopolymers: Recent advances and biomedical applications. International Journal of Biological Macromolecules 2021, 188, 542–567. [Google Scholar] [CrossRef]
- Harada, K.; Matsuyama, M. Polycondensation of thermal precursors of amino acids and characterization of constituent amino acids. BioSystems 1979, 11, 47–53. [Google Scholar] [CrossRef]
- Paecht-Horowitz, M.; Berger, J.; Katchalsky, A. Prebiotic synthesis of polypeptides by heterogeneous polycondensation of amino-acid adenylates. Nature 1970, 228, 636–639. [Google Scholar] [CrossRef]
- Fox, S.W.; Harada, K. The thermal copolymerization of amino acids common to protein1. Journal of the American Chemical Society 1960, 82, 3745–3751. [Google Scholar] [CrossRef]
- Fox, S.W. Thermal proteins in the first life and in the “mind-body” problem. In Evolution of Information Processing Systems: An Interdisciplinary Approach for a New Understanding of Nature and Society; Springer, 1992; pp. 203–228.
- Przybylski, A.T.; Fox, S.W. Excitable artificial cells of proteinoid. Applied Biochemistry and Biotechnology 1984, 10, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Wolman, Y.; Haverland, W.J.; Miller, S.L. Nonprotein amino acids from spark discharges and their comparison with the Murchison meteorite amino acids. Proceedings of the National Academy of Sciences 1972, 69, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, A.T.; Stratten, W.P.; Syren, R.M.; Fox, S.W. Membrane, action, and oscillatory potentials in simulated protocells. Naturwissenschaften 1982, 69, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Egel, R. Origins and emergent evolution of life: The colloid microsphere hypothesis revisited. Origins of Life and Evolution of Biospheres 2014, 44, 87–110. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T. Metabolism of proteinoid microspheres. Organic Geo-and Cosmochemistry, 2005, pp. 57–81.
- Schwartz, A.W.; Visscher, J.; Bakker, C.; Niessen, J. Nucleic acid-like structures II. Polynucleotide analogues as possible primitive precursors of nucleic acids. Origins of life and evolution of the biosphere 1987, 17, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z. On the origin of life: a possible way from Fox’s microspheres into primitive life. SOJ Biochem 2018, 4, 1–7. [Google Scholar] [CrossRef]
- Kokufuta, E.; Sakai, H.; Harada, K. Factors controlling the size of proteinoid microspheres. BioSystems 1983, 16, 175–181. [Google Scholar] [CrossRef]
- Fox, S.W.; Bahn, P.R.; Dose, K.; Harada, K.; Hsu, L.; Ishima, Y.; Jungck, J.; Kendrick, J.; Krampitz, G.; Lacey Jr, J.C.; others. Experimental retracement of the origins of a protocell: it was also a protoneuron. Journal of biological physics 1995, 20, 17–36. [Google Scholar] [CrossRef]
- Ignatov, I.; Mosin, O. The Formation of Thermal Proteinoids in Hot Water. Journal of Medicine, Physiology and Biophysics 2016, 26, 15–27. [Google Scholar]
- Ignatov, I.; Mosin, O. S. Miller’s Experiments in Modelling of Non-Equilibrium Conditions with Gas Electric Discharge Simulating Primary Atmosphere. Journal of Medicine, Physiology and Biophysics 2015, 15, 61–76. [Google Scholar]
- Steinman, G.; Cole, M.N. Synthesis of biologically pertinent peptides under possible primordial conditions. Proceedings of the National Academy of Sciences 1967, 58, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W. A theory of macromolecular and cellular origins. Nature 1965, 205, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W.; Harada, K.; Kendrick, J. Production of spherules from synthetic proteinoid and hot water. Science 1959, 129, 1221–1223. [Google Scholar] [CrossRef] [PubMed]
- Rohlfing, D.L. Coacervate-like microspheres from lysine-rich proteinoid. Origins of Life 1975, 6, 203–209. [Google Scholar] [CrossRef]
- Fox, S.W.; Dose, K. Molecular evolution and the origin of life. (No Title), 1977.
- Fox, S.W. Metabolic microspheres: origins and evolution. Naturwissenschaften 1980, 67, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Villanueva, D.; Ciaccia, M.; Iadevaia, G.; Sanna, E.; Hunter, C.A. Sequence information transfer using covalent template-directed synthesis. Chemical Science 2019, 10, 5258–5266. [Google Scholar] [CrossRef]
- Hansen, B.N.; Larsen, K.S.; Merkle, D.; Mihalchuk, A. DNA-templated synthesis optimization. Natural Computing 2018, 17, 693–707. [Google Scholar] [CrossRef]
- Sharma, S.; Mougoyannis, P.; Tarabella, G.; Adamatzky, A. A review on the protocols for the synthesis of proteinoids. arXiv preprint arXiv:2212.02261 2022. [Google Scholar]
- Matsuno, K. Molecular evolution and protobiology; Springer Science & Business Media, 2012.
- Fox, S.W. Physical principles and proteinoid experiments in the emergence of life. In Information Processing in Biological Systems; Springer, 1985; pp. 69–91.
- Adamala, K.; Szostak, J.W. Nonenzymatic template-directed RNA synthesis inside model protocells. Science 2013, 342, 1098–1100. [Google Scholar] [CrossRef] [PubMed]
- Ertem, G.; Ferris, J.P. Synthesis of RNA oligomers on heterogeneous templates. Nature 1996, 379, 238–240. [Google Scholar] [CrossRef]
- Fox, S.W. Origins of the protein synthesis cycle. International Journal of Quantum Chemistry 1981, 20, 441–454. [Google Scholar] [CrossRef]
- Ratner, V.A.; Zharkikh, A.A.; Kolchanov, N.; Rodin, S.N.; Solovyov, V.V.; Antonov, A.S.; Ratner, V.A.; Zharkikh, A.A.; Kolchanov, N.; Rodin, S.N. others. The Origin and Evolution of the Genetic Coding-System. Molecular Evolution 1996, pp. 39–70.
- Fox, S.W. Molecular evolution of the first cells. Pure and Applied Chemistry 1973, 34, 641–670. [Google Scholar] [CrossRef] [PubMed]
- Snyder, W.; Fox, S.W. A model for the origin of stable protocells in a primitive alkaline ocean. BioSystems 1975, 7, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W.; Jungck, J.R.; Nakashima, T. From proteinoid microsphere to contemporary cell: formation of internucleotide and peptide bonds by proteinoid particles. Origins of Life 1974, 5, 227–237. [Google Scholar] [CrossRef]
- Bion, I.J. Electromagnetic origin of life. Electro-and Magnetobiology 1998, 17, 401–413. [Google Scholar] [CrossRef]
- Deamer, D.W. Prebiotic amphiphilic compounds: self-assembly and properties of early membrane structures. Origins: genesis, evolution and diversity of life, 2004, pp. 75–89.
- Fox, S.W.; Harada, K. Thermal copolymerization of amino acids to a product resembling protein. Science 1958, 128, 1214–1214. [Google Scholar] [CrossRef]
- Fox, S.W. Bioorganic chemistry and the emergence of the first cell 1977.
- Adamatzky, A. Towards proteinoid computers. arXiv preprint arXiv:2106.00883 2021. [Google Scholar]
- Itzhaki, E.; Elias, Y.; Moskovits, N.; Stemmer, S.M.; Margel, S. Proteinoid Polymers and Nanocapsules for Cancer Diagnostics, Therapy and Theranostics: In Vitro and In Vivo Studies. Journal of Functional Biomaterials 2023, 14, 215. [Google Scholar] [CrossRef]
- Kolitz-Domb, M.; Margel, S. Engineering of novel proteinoids and PLLA-proteinoid polymers of narrow size distribution and uniform nano/micro-hollow particles for biomedical applications. In Advances in Bioengineering; IntechOpen, 2015.
- Hsu, L.L.; Brooke, S.; Fox, S.W. Conjugation of proteinoid microspheres: a model of primordial communication. Biosystems 1971, 4, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Grimes, P.J.; Galanti, A.; Gobbo, P. Bioinspired networks of communicating synthetic protocells. Frontiers in Molecular Biosciences 2021, 8, 804717. [Google Scholar] [CrossRef]
- Fox, S.W. Coacervate droplets, proteinoid microspheres, and the genetic apparatus. In The origin of life and evolutionary biochemistry; Springer, 1974; pp. 119–132.
- Cannelli, S.M.; Gupta, R.; Nguyen, T.; Poddar, A.; Sharma, S.; Vithole, P.V.; Jia, T.Z. A compositional view comparing modern biological condensates and primitive phase-separated compartment. Peptide Science, 2023, p. e24331. [CrossRef]
- Cannelli, S.M.; Gupta, R.; Nguyen, T.; Poddar, A.; Sharma, S.; Vithole, P.V.; Jia, T.Z. Bridging the Gap Between Primitive and Modern Phase Separation 2023.
- Ikehara, K. How Did Life Emerge in Chemically Complex Messy Environments? Life 2022, 12, 1319. [Google Scholar] [CrossRef]
- Matveev, V.V. Cell theory, intrinsically disordered proteins, and the physics of the origin of life. Progress in Biophysics and Molecular Biology 2019, 149, 114–130. [Google Scholar] [CrossRef]
- Dalai, P.; Sahai, N. Protocell emergence and evolution. Handbook of Astrobiology 2018, pp. 491–520.
- Ayoubi-Joshaghani, M.H.; Dianat-Moghadam, H.; Seidi, K.; Jahanban-Esfahalan, A.; Zare, P.; Jahanban-Esfahlan, R. Cell-free protein synthesis: The transition from batch reactions to minimal cells and microfluidic devices. Biotechnology and Bioengineering 2020, 117, 1204–1229. [Google Scholar] [CrossRef] [PubMed]
- Ishima, Y.; Przybylski, A.T.; Fox, S.W. Electrical membrane phenomena in spherules from proteinoid and lecithin. BioSystems 1981, 13, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Yang, S.R.; Cho, Y.W.; Park, K. 18 Fast Responsive Nanoparticles of Hydrophobically Modified Poly (Amino Acid) s and Proteinoids. Reflexive Polymers and Hydrogels: Understanding and Designing Fast Responsive Polymeric Systems, 2004, p. 373.
- Lugasi, L.; Grinberg, I.; Rudnick-Glick, S.; Okun, E.; Einat, H.; Margel, S. Designed proteinoid polymers and nanoparticles encapsulating risperidone for enhanced antipsychotic activity. Journal of Nanobiotechnology 2020, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bahn, P.R.; Fox, S.W. Models for protocellular photophosphorylation. BioSystems 1981, 14, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Hadad, E.; Rudnick-Glick, S.; Grinberg, I.; Kolitz-Domb, M.; Chill, J.H.; Margel, S. Synthesis and characterization of Poly (RGD) proteinoid polymers and NIR fluorescent nanoparticles of optimal D, L-configuration for drug-delivery applications—in vitro study. ACS omega 2020, 5, 23568–23577. [Google Scholar] [CrossRef]
- Tallawi, M. Proteinoid/hydroxyapatite hybrid microsphere composites. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2011, 96, 261–266. [Google Scholar] [CrossRef]
- Ma, X.; Santiago, N.; Chen, Y.S.; Chaudhary, K.; Milstein, S.J.; Baughman, R.A. Stability study of drug-loaded proteinoid microsphere formulations during freeze-drying. Journal of Drug Targeting 1994, 2, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Quirk, S. Triggered release of small molecules from proteinoid microspheres. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2009, 91, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.M.; Rao, K.P. Preparation and characterization of pH-sensitive proteinoid microspheres for the oral delivery of methotrexate. Biomaterials 1998, 19, 725–732. [Google Scholar] [CrossRef]
- Correa, P.N.; Correa, A.N. The PA & SAPA Bion Experiments and Proto-Prokaryotic Biopoiesis. Journal of Biophysics, Hematology and Oncology 2010, 1, 1–49. [Google Scholar]
- Monnard, P.A.; Deamer, D.W. Membrane self-assembly processes: Steps toward the first cellular life. The Anatomical Record: An Official Publication of the American Association of Anatomists 2002, 268, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Bailey, J.; Boncella, J.; Chen, L.; Collis, G.; Colgate, S.; DeClue, M.; Fellermann, H.; Goranovic, G.; Jiang, Y. others. Assembly of a minimal protocell. Protocells: Bridging Nonliving and Living Matter; Rasmussen, S., Bedau, M., Chen, L., Deamer, D., Krakauer, D., Packard, N., Stadler, P., Eds, 2008, pp. 125–156.
- Peterson, I. Proteinoids: Clues to Cellular Origins? BioScience 1985, 35, 74–76. [Google Scholar] [CrossRef]
- Ottenbrite, R.M.; Fadeeva, N. Polymer systems for biomedical applications: An overview 1994.
- LING, G.N. THE FUNCTIONS OF POLARIZED WATER AND MEMBRANE LIPIDS: A REBUTI’AL 1977.
- Monnard, P.A.; Walde, P. Current ideas about prebiological compartmentalization. Life 2015, 5, 1239–1263. [Google Scholar] [CrossRef]
- Wicken, J.S. An organismic critique of molecular Darwinism. Journal of theoretical biology 1985, 117, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W. Stereomolecular interactions and microsystems in experimental protobiogenesis. BioSystems 1975, 7, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, E.; Lafusa, A.; Pantano, P. Life-like self-reproducers. Complexity 2003, 9, 38–55. [Google Scholar] [CrossRef]
- Kauffman, S.A. Metabolic stability and epigenesis in randomly constructed genetic nets. Journal of theoretical biology 1969, 22, 437–467. [Google Scholar] [CrossRef]
- Bilotta, E.; Pantano, P. Biological traits in artificial self-reproducing systems. In Reflexing Interfaces: The Complex Coevolution of Information Technology Ecosystems; IGI global, 2008; pp. 109–123.
- Solé, R. Synthetic transitions: towards a new synthesis. Philosophical Transactions of the Royal Society B: Biological Sciences 2016, 371, 20150438. [Google Scholar] [CrossRef]
- Kauffman, S.A. Cellular homeostasis, epigenesis and replication in randomly aggregated macromolecular systems 1971.
- Abbas, M.; Lipiński, W.P.; Wang, J.; Spruijt, E. Peptide-based coacervates as biomimetic protocells. Chemical Society Reviews 2021, 50, 3690–3705. [Google Scholar] [CrossRef]
- Wu, H.; Qiao, Y. Engineering coacervate droplets towards the building of multiplex biomimetic protocells. Supramolecular Materials, 2022, p. 100019. [CrossRef]
- Wei, M.; Lin, Y.; Qiao, Y. Engineered Colloidosomes as Biomimetic Cellular Models. Giant, 2023, p. 100143. [CrossRef]
- Elbourne, A.; Crawford, R.J.; Ivanova, E.P. Nano-structured antimicrobial surfaces: From nature to synthetic analogues. Journal of Colloid and Interface Science 2017, 508, 603–616. [Google Scholar] [CrossRef]
- Laursen, J.B.; Nielsen, J. Phenazine natural products: biosynthesis, synthetic analogues, and biological activity. Chemical reviews 2004, 104, 1663–1686. [Google Scholar] [CrossRef] [PubMed]
- Kilgour, O. Irritability. In Mastering Biology; Springer, 1987; pp. 270–303.
- Koppenhöfer, E. Electrical responses of isolated protoplasm from Nitella. Pflügers Archiv 1975, 358, 179–187. [Google Scholar] [CrossRef]
- Bentrup, F.W. Cell electrophysiology and membrane transport. In Progress in botany; Springer, 1989; pp. 70–79.
- Dennis, R.G.; Kosnik, P.E.; Gilbert, M.E.; Faulkner, J.A. Excitability and contractility of skeletal muscle engineered from primary cultures and cell lines. American Journal of Physiology-Cell Physiology 2001, 280, C288–C295. [Google Scholar] [CrossRef] [PubMed]
- De Biase, L.M.; Nishiyama, A.; Bergles, D.E. Excitability and synaptic communication within the oligodendrocyte lineage. Journal of Neuroscience 2010, 30, 3600–3611. [Google Scholar] [CrossRef] [PubMed]
- Teorell, T. Excitability phenomena in artificial membranes. Biophysical Journal 1962, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, K., Proteinoid Microsphere. In Encyclopedia of Astrobiology; Gargaud, M.; Irvine, W.M.; Amils, R.; Cleaves, H.J.J.; Pinti, D.L.; Quintanilla, J.C.; Rouan, D.; Spohn, T.; Tirard, S.; Viso, M., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2015; pp. 2033–2034. [CrossRef]
- Stratten, W.P. Protocell action potentials: a new perspective of bio-excitation. In Molecular evolution and protobiology; Springer, 1984; pp. 233–251.
- Przybylski, A.T.; Fox, S.W. Electrical phenomena in proteinoid cells. In Modern Bioelectrochemistry; Springer, 1986; pp. 377–396.
- Matsuno, K. Electrical excitability of proteinoid microspheres composed of basic and acidic proteinoids. BioSystems 1984, 17, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Takinoue, M. Creation of artificial cell-like structures promoted by microfluidics technologies. Micromachines 2019, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Dose, K. Chemical and catalytical properties of thermal polymers of amino acids (proteinoids). Origins of Life 1974, 5, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W.; Nakashima, T. Fractionation and characterization of an amidated thermal 1: 1: 1-proteinoid. Biochimica et Biophysica Acta (BBA)-Protein Structure 1967, 140, 155–167. [Google Scholar] [CrossRef]
- Fox, S.W. The emergence of life: Darwinian evolution from the inside. (No Title), 1988.
- Fox, S.W. The Origins of Prebiological Systems and of Their Molecular Matrices: Proceedings of a Conference Conducted at Wakulla Springs, Florida, on 27-30 October 1963 under the Auspices of the Institute for Space Biosciences, the Florida State University and the National Aeronautics and Space Administration; Elsevier, 2013.
- Fox, S.W. Molecular selection and natural selection. The Quarterly Review of Biology 1986, 61, 375–386. [Google Scholar] [CrossRef]
- Gish, D.T. Speculations and experiments related to theories on the origin of life: A critique; Institute for Creation Research, 1972.
- Tkachenko, A.V.; Maslov, S. Spontaneous emergence of autocatalytic information-coding polymers. The Journal of Chemical Physics 2015, 143. [Google Scholar] [CrossRef] [PubMed]
- Black, R.A.; Blosser, M.C. A self-assembled aggregate composed of a fatty acid membrane and the building blocks of biological polymers provides a first step in the emergence of protocells. Life 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Monnard, P.A.; Apel, C.L.; Kanavarioti, A.; Deamer, D.W. Influence of ionic inorganic solutes on self-assembly and polymerization processes related to early forms of life: Implications for a prebiotic aqueous medium. Astrobiology 2002, 2, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W.; Nakashima, T. The assembly and properties of protobiological structures: The beginnings of cellular peptide synthesis. BioSystems 1980, 12, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Ubrich, N.; Maincent, P. Recent advances in heparin delivery. DRUGS AND THE PHARMACEUTICAL SCIENCES 2006, 158, 481. [Google Scholar]
- Xu, J.; Vanderlick, T.K.; LaVan, D.A.; others. Energy conversion in protocells with natural nanoconductors. International Journal of Photoenergy 2011, 2012. [Google Scholar] [CrossRef]
- Bae, S.K.; Kim, J.D. Aggregation behaviors and their pH sensitivity of cholesterol-conjugated proteinoids composed of glutamic acid and aspartic acid matrix. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2003, 64, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Haratake, M.; Ottenbrite, R.M. Oligopeptides as an oral delivery system: I. Aggregation characteristics and drug encapsulation. Journal of bioactive and compatible polymers 1997, 12, 112–126. [Google Scholar] [CrossRef]
- Jia, T.Z.; Wang, P.H.; Niwa, T.; Mamajanov, I. Connecting primitive phase separation to biotechnology, synthetic biology, and engineering. Journal of biosciences 2021, 46, 79. [Google Scholar] [CrossRef]
- Freire, M.Á. Nontemplate-driven polymers: Clues to a minimal form of organization closure at the early stages of living systems. Theory in Biosciences 2015, 134, 47–64. [Google Scholar] [CrossRef]
- DOSE, K. OF THERMAL POLYMERS OF AMINO ACIDS (PROTEINOIDS). Cosmochemical Evolution and the Origins of Life: Proceedings of the Fourth International Conference on the Origin of Life and the First Meeting of the International Society for the Study of the Origin of Life, Barcelona, June 25–28, 1973, Volume I: Invited Papers and Volume II: Contributed Papers. Springer Science & Business Media, 2013, p. 239.
- Matsuno, K. A theoretical construction of protobiological synthesis: from amino acids to functional protocells. International Journal of Quantum Chemistry 1982, 22, 181–193. [Google Scholar] [CrossRef]
- Brochier-Armanet, C. Minimal cell: the biologist’s point of view. Origins and Evolution of Life: An Astrobiological Perspective 2011, 6, 26. [Google Scholar]
- Korn, R.W. Biological organization—a new look at an old problem. BioScience 1999, 49, 51–57. [Google Scholar] [CrossRef]
- Silverstein, A.M. A history of immunology; Academic Press, 2009.
- Shaw, G.H. Evidence and arguments for methane and ammonia in Earth’s earliest atmosphere and an organic compound–rich early ocean. Earth’s Early Atmosphere and Surface Environment. Geological Society of America Special Paper 2014, 504, 1–10. [Google Scholar]
- Miller, S.L.; Urey, H.C.; Oró, J. Origin of organic compounds on the primitive earth and in meteorites. Journal of Molecular Evolution 1976, 9, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Baross, J.A. The rocky road to biomolecules, 2018.
- Ferrazzano, L.; Catani, M.; Cavazzini, A.; Martelli, G.; Corbisiero, D.; Cantelmi, P.; Fantoni, T.; Mattellone, A.; De Luca, C.; Felletti, S.; others. Sustainability in peptide chemistry: Current synthesis and purification technologies and future challenges. Green Chemistry 2022, 24, 975–1020. [Google Scholar] [CrossRef]
- Jensen, K.J.; Shelton, P.T.; Pedersen, S.L. Peptide synthesis and applications; Springer, 2013.
- Nagendrappa, G.; Chowreddy, R.R. Organic reactions using clay and clay-supported catalysts: a survey of recent literature. Catalysis Surveys from Asia 2021, 25, 231–278. [Google Scholar] [CrossRef]
- Mougkogiannis, P.; Adamatzky, A. Proteinoid microspheres as protoneural networks. ACS Omega 2023. [Google Scholar] [CrossRef] [PubMed]
- Mougkogiannis, P.; Adamatzky, A. Logical gates in ensembles of proteinoid microspheres. Plos one 2023, 18, e0289433. [Google Scholar] [CrossRef]
- Brack, A. From interstellar amino acids to prebiotic catalytic peptides: a review. Chemistry & biodiversity 2007, 4, 665–679. [Google Scholar] [CrossRef]
- Furukawa, Y.; Nakazawa, H.; Sekine, T.; Kobayashi, T.; Kakegawa, T. Nucleobase and amino acid formation through impacts of meteorites on the early ocean. Earth and Planetary Science Letters 2015, 429, 216–222. [Google Scholar] [CrossRef]
- Pasek, M.; Lauretta, D. Extraterrestrial flux of potentially prebiotic C, N, and P to the early Earth. Origins of Life and Evolution of Biospheres 2008, 38, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Mougkogiannis, P.; Adamatzky, A. Learning in ensembles of proteinoid microspheres. arXiv preprint arXiv:2306.14362. 2023.
- Manouras, T.; Vamvakaki, M. Field responsive materials: photo-, electro-, magnetic-and ultrasound-sensitive polymers. Polymer Chemistry 2017, 8, 74–96. [Google Scholar] [CrossRef]
- Manoj, K.M.; Tamagawa, H. Revisiting the mechanisms for cellular homeostasis and electrophysiological responses: Classical membrane theory, association-induction hypothesis and murburn concept 2020.
- Kompanichenko, V.; Kotsyurbenko, O. Role of Stress in the Origin of Life. Life 2022, 12, 1930. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.R. A proposal concerning the origin of life on the planet earth. Journal of Molecular Evolution 1979, 13, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Sachar, M.; Anderson, K.E.; Ma, X. Protoporphyrin IX: the good, the bad, and the ugly. Journal of Pharmacology and Experimental Therapeutics 2016, 356, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Suo, Z.; Avci, R.; Schweitzer, M.H.; Deliorman, M. Porphyrin as an ideal biomarker in the search for extraterrestrial life. Astrobiology 2007, 7, 605–615. [Google Scholar] [CrossRef]
- Lozovaya, G.; Masinovsky, Z.; Sivash, A. Protoporphyrin IX as a possible ancient photosensitizer: spectral and photochemical studies. Origins of life and evolution of the biosphere 1990, 20, 321–330. [Google Scholar] [CrossRef]
- Matveev, V.V. Membraneless physiology of the living cell. The past and the present. 4open 2022, 5, 15. [Google Scholar] [CrossRef]
- Wilson, J.W. Virchow’s contribution to the cell theory. Journal of the History of Medicine and Allied Sciences 1947, 2, 163–178. [Google Scholar] [CrossRef]
- DeWalt, D.A.; Pincus, T. The legacies of Rudolf Virchow: cellular medicine in the 20th century and social medicine in the 21st century. IMAJ-RAMAT GAN- 2003, 5, 395–397. [Google Scholar]
- Maulitz, R.C. Rudolf Virchow, Julius Cohnheim and the program of pathology. Bulletin of the History of Medicine 1978, 52, 162–182. [Google Scholar] [PubMed]
- Matveev, V. Comparison of fundamental physical properties of the model cells (protocells) and the living cells reveals the need in protophysiology. International Journal of Astrobiology 2017, 16, 97–104. [Google Scholar] [CrossRef]
- Patten, B.C. Network orientors: steps toward a cosmography of ecosystems: orientors for directional development, self-organization, and autoevolution. In Eco Targets, Goal Functions, and Orientors; Springer, 1998; pp. 137–160.
- Patten, B.C. 2.8 Network Orientors: Steps Toward a Cosmogra-phy of Ecosystems: Orientors for Directional Develop-ment, Self-Organization, and Autoevolution. Eco Targets, Goal Functions, and Orientors, 1998, p. 137.
- Weber, B.; others. What does natural selection have to be like in order to work with self-organization? Cybernetics & Human Knowing 1998, 5, 18–31. [Google Scholar]
- Yockey, H.P. Self organization origin of life scenarios and information theory. Journal of Theoretical Biology 1981, 91, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Abel, D.L. The Birth of Protocells. The First Gene: The Birth of Programming, Messaging and Formal Control. New York, NY: LongView Press–Academic, Biol. Res 2011, 504, 189–230. [Google Scholar]
- Mitleton-Kelly, E. Ten principles of complexity and enabling infrastructures. Complex systems and evolutionary perspectives on organisations: The application of complexity theory to organisations 2003, 1, 23–50. [Google Scholar]
- Spitzer, J.; Pielak, G.J.; Poolman, B. Emergence of life: Physical chemistry changes the paradigm. Biology direct 2015, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pross, A. On the emergence of biological complexity: life as a kinetic state of matter. Origins of Life and Evolution of Biospheres 2005, 35, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Trifonov, E.N. Tracing life back to elements. Physics of Life Reviews 2008, 5, 121–132. [Google Scholar] [CrossRef]
- Pino, S.; Trifonov, E.N.; Di Mauro, E. On the observable transition to living matter. Genomics, proteomics & bioinformatics 2011, 9, 7–14. [Google Scholar] [CrossRef]
- Di Mauro, E.; Dunker, A.K.; Trifonov, E.N. Disorder to order, nonlife to life: In the beginning there was a mistake. In Genesis-In The Beginning: Precursors of Life, Chemical Models and Early Biological Evolution; Springer, 2012; pp. 415–435.
- Trifonov, E.N.; Gabdank, I.; Barash, D.; Sobolevsky, Y. Primordia vita. Deconvolution from modern sequences. Origins of Life and Evolution of Biospheres 2006, 36, 559–565. [Google Scholar] [CrossRef]
- Kompanichenko, V. Thermodynamic inversion and self-reproduction with variations: integrated view on the life-nonlife border. Journal of Biomolecular Structure and Dynamics 2012, 29, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Walde, P.; Boiteau, L. Prebiotic chemistry: from simple amphiphiles to protocell models; Vol. 259, Springer Science & Business Media, 2005.
- Imai, M.; Sakuma, Y.; Kurisu, M.; Walde, P. From vesicles toward protocells and minimal cells. Soft Matter 2022, 18, 4823–4849. [Google Scholar] [CrossRef] [PubMed]
- Kahana, A.; Lancet, D. Self-reproducing catalytic micelles as nanoscopic protocell precursors. Nature Reviews Chemistry 2021, 5, 870–878. [Google Scholar] [CrossRef]
- Kompanichenko, V. Origin of life by thermodynamic inversion: a universal process. In Genesis-In The Beginning: Precursors of Life, Chemical Models and Early Biological Evolution; Springer, 2012; pp. 305–320.
- Hua, Z. From Fox’s microspheres into primitive life: An inferencing hypothesis on the origin of life 2018.
- Berger, G. Deterministic hypotheses on the origin of life and of its reproduction. Medical hypotheses 2003, 61, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Michaelian, K.; Simeonov, A. Fundamental molecules of life are pigments which arose and co-evolved as a response to the thermodynamic imperative of dissipating the prevailing solar spectrum. Biogeosciences 2015, 12, 4913–4937. [Google Scholar] [CrossRef]
- Willems, J.C. Dissipative dynamical systems part I: General theory. Archive for rational mechanics and analysis 1972, 45, 321–351. [Google Scholar] [CrossRef]
- Lyapunov, A.M. The general problem of the stability of motion. International journal of control 1992, 55, 531–534. [Google Scholar] [CrossRef]
- Kritsky, M.; Telegina, T.; Buglak, A.; Kolesnikov, M.; Lyudnikova, T.; Vechtomova, Y.L. Modeling of abiotic ATP synthesis in the context of problems of early biosphere evolution. Geochemistry International 2014, 52, 1227–1238. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Zekiy, A.; Benedicenti, S.; Pasquale, C. A narrative review on oral and periodontal bacteria Microbiota photobiomodulation, through visible and near-infrared light: from the origins to modern therapies. International Journal of Molecular Sciences 2022, 23, 1372. [Google Scholar] [CrossRef] [PubMed]
- Egel, R. Life’s order, complexity, organization, and its thermodynamic–holistic imperatives. Life 2012, 2, 323–363. [Google Scholar] [CrossRef]
- Tan, R.; Frankel, A.D. Structural variety of arginine-rich RNA-binding peptides. Proceedings of the National Academy of Sciences 1995, 92, 5282–5286. [Google Scholar] [CrossRef]
- Kikvidze, Z.; Callaway, R.M. Ecological facilitation may drive major evolutionary transitions. BioScience 2009, 59, 399–404. [Google Scholar] [CrossRef]
- Abdelouahab, M.S.; Lozi, R.; Chua, L. Memfractance: a mathematical paradigm for circuit elements with memory. International Journal of Bifurcation and Chaos 2014, 24, 1430023. [Google Scholar] [CrossRef]
- Przybylski, A.T. Excitable cell made of thermal proteinoids. BioSystems 1985, 17, 281–288. [Google Scholar] [CrossRef]
- Müller-Herold, U.; Nickel, G. The stability of proteinoid microspheres. Biosystems 1994, 33, 215–220. [Google Scholar] [CrossRef]
- Mougkogiannis, P.; Adamatzky, A. Low frequency electrical waves in ensembles of proteinoid microspheres. Scientific Reports 2023, 13, 1992. [Google Scholar] [CrossRef] [PubMed]
- Mougkogiannis, P.; Adamatzky, A. Light induced spiking of proteinoids. arXiv preprint arXiv:2303.17563 2023. [Google Scholar]
- Kolitz-Domb, M.; Margel, S. Recent advances of novel proteinoids and proteinoid nanoparticles and their applications in biomedicine and industrial uses. Israel Journal of Chemistry 2018, 58, 1277–1285. [Google Scholar] [CrossRef]
- Manoj, K.M.; Tamagawa, H. Critical analysis of explanations for cellular homeostasis and electrophysiology from murburn perspective. Journal of Cellular Physiology 2022, 237, 421–435. [Google Scholar] [CrossRef]
- Manoj, K.M.; Bazhin, N.; Tamagawa, H. The murburn precepts for cellular ionic homeostasis and electrophysiology. Journal of Cellular Physiology 2022, 237, 804–814. [Google Scholar] [CrossRef]
- Andrade, E. The processing of information (analog/digital) is the causal factor of the emergence of natural hierarchies. Ludus Vitalis 2003, 11, 85–106. [Google Scholar]
- Browner, D.; Sareh, S.; Anderson, P. Additive manufacture of polymeric organometallic ferroelectric diodes (POMFeDs) for structural neuromorphic hardware. Proceedings of the 2023 Annual Neuro-Inspired Computational Elements Conference, 2023, pp. 92–99.
- Dose, K. Self-instructed condensation of amino acids and the origin of biological information. International Journal of Quantum Chemistry 1984, 26, 91–101. [Google Scholar] [CrossRef]
- Mamajanov, I.; Caudan, M.; Jia, T.Z. Protoenzymes: The case of hyperbranched polymer-scaffolded ZnS nanocrystals. Life 2020, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, S.A. Autocatalytic sets of proteins. Journal of theoretical biology 1986, 119, 1–24. [Google Scholar] [CrossRef]
- Fox, S.W.; Nakashima, T.; Przybylski, A.; Syren, R.M. The updated experimental proteinoid model. International Journal of Quantum Chemistry 1982, 22, 195–204. [Google Scholar] [CrossRef]
- Mann, S. Biomineralization: principles and concepts in bioinorganic materials chemistry 2001.
- Babu, A.K.; Raja, M.M.M.; Zehravi, M.; Mohammad, B.D.; Anees, M.I.; Prasad, C.; Yahya, B.A.; Sultana, R.; Sharma, R.; Singh, J.; others. An overview of polymer surface coated synthetic quantum dots as therapeutics and sensors applications. Progress in biophysics and molecular biology 2023. [Google Scholar] [CrossRef] [PubMed]
- Kolitz-Domb, M.; Corem-Salkmon, E.; Grinberg, I.; Margel, S. Synthesis and characterization of bioactive conjugated near-infrared fluorescent proteinoid-poly (L-lactic acid) hollow nanoparticles for optical detection of colon cancer. International Journal of Nanomedicine, 2014, pp. 5041–5053.
- Basu, A.; Acharya, J.; Karnik, T.; Liu, H.; Li, H.; Seo, J.S.; Song, C. Low-power, adaptive neuromorphic systems: Recent progress and future directions. IEEE Journal on Emerging and Selected Topics in Circuits and Systems 2018, 8, 6–27. [Google Scholar] [CrossRef]
- ávan Doremaele, E.R.; de Burgt, Y.; others. Towards organic neuromorphic devices for adaptive sensing and novel computing paradigms in bioelectronics. Journal of Materials Chemistry C 2019, 7, 12754–12760. [Google Scholar] [CrossRef]
- Abedin, M.I. RRAM Device Optimization and Circuit Design for Low Power Low Latency Domain Specific Edge Applications. PhD thesis, 2023.
- Liu, X.; Wang, F.; Su, J.; Zhou, Y.; Ramakrishna, S. Bio-Inspired 3D Artificial Neuromorphic Circuits. Advanced Functional Materials 2022, 32, 2113050. [Google Scholar] [CrossRef]
- Sorkin, A.; Von Zastrow, M. Endocytosis and signalling: intertwining molecular networks. Nature reviews Molecular cell biology 2009, 10, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Marks, F.; Klingmüller, U.; Müller-Decker, K. Cellular signal processing: an introduction to the molecular mechanisms of signal transduction; Garland Science, 2017.
- Mu, Q.; Jiang, G.; Chen, L.; Zhou, H.; Fourches, D.; Tropsha, A.; Yan, B. Chemical basis of interactions between engineered nanoparticles and biological systems. Chemical reviews 2014, 114, 7740–7781. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W. The origins of behavior in macromolecules and protocells. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 1980, 67, 423–436. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Huang, X. Bioinspired Protein-Based Assembling: Toward Advanced Life-Like Behaviors. Advanced Materials 2020, 32, 2001436. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Singh, M.; Pareek, D.; Wasnik, K.; Gupta, P.S.; Paik, P. Advances in the Development of Biodegradable Polymeric Materials for Biomedical Applications 2022.
Figure 1.
The mechanism underlying the aggregation of proteinoids. Proteinoids have the inherent ability to undergo self-assembly and disintegration processes, resulting in the formation of complex molecular structures like microspheres. This process is facilitated through the presence of hydrophobic interactions and hydrogen bonding between proteinoid branches, which has resemblance to the biological processes of protein folding and aggregation. Proteinoid aggregates exhibit a perpetual influx and efflux of material, hence sustaining an internal state characterised by constant change. Various environmental conditions, including as temperature, pH, and ionic strength, have the ability to influence the equilibrium towards specific aggregated states [
126].
Figure 1.
The mechanism underlying the aggregation of proteinoids. Proteinoids have the inherent ability to undergo self-assembly and disintegration processes, resulting in the formation of complex molecular structures like microspheres. This process is facilitated through the presence of hydrophobic interactions and hydrogen bonding between proteinoid branches, which has resemblance to the biological processes of protein folding and aggregation. Proteinoid aggregates exhibit a perpetual influx and efflux of material, hence sustaining an internal state characterised by constant change. Various environmental conditions, including as temperature, pH, and ionic strength, have the ability to influence the equilibrium towards specific aggregated states [
126].
Figure 2.
a,b) Perfect proteinoid microspheres self–assembled from a supersaturated precursor solution. Microspheres have a diameter of 1.2 microns. Magnification 60000x, scale bar 500 nm. c) Cubic crystal with a central cavity formed after applying an electrical voltage to proteinoids. The cubic morphology suggests reorganization of proteinoids under electrical stimuli. Magnification 8000x, scale bar 5 m. d) Nanoscale proteinoid spheres arranged on the surface of a cubic crystal substrate. This highlights preferential interactions between proteinoids and crystal surfaces. Magnification 40000x, scale bar 1 m.
Figure 2.
a,b) Perfect proteinoid microspheres self–assembled from a supersaturated precursor solution. Microspheres have a diameter of 1.2 microns. Magnification 60000x, scale bar 500 nm. c) Cubic crystal with a central cavity formed after applying an electrical voltage to proteinoids. The cubic morphology suggests reorganization of proteinoids under electrical stimuli. Magnification 8000x, scale bar 5 m. d) Nanoscale proteinoid spheres arranged on the surface of a cubic crystal substrate. This highlights preferential interactions between proteinoids and crystal surfaces. Magnification 40000x, scale bar 1 m.
Figure 3.
Memfractance current–voltage characteristics of L-Glu:L-Arg proteinoids. The I–V curve shows the nonlinear memfractance behavior, with currents of -3.95 A at -1 V and 3.57 A at +1 V. Hysteresis is observed around 0 V, with currents of -0.6898 A when sweeping from high to low voltages and 0.9043 A when sweeping low to high. The asymmetric I–V response demonstrates proteinoids can exhibit memristive–like electrical properties that may be harnessed for bioelectronic applications. Further tuning the composition and assembly conditions enables engineering proteinoids as adaptive, multifunctional electronic materials.
Figure 3.
Memfractance current–voltage characteristics of L-Glu:L-Arg proteinoids. The I–V curve shows the nonlinear memfractance behavior, with currents of -3.95 A at -1 V and 3.57 A at +1 V. Hysteresis is observed around 0 V, with currents of -0.6898 A when sweeping from high to low voltages and 0.9043 A when sweeping low to high. The asymmetric I–V response demonstrates proteinoids can exhibit memristive–like electrical properties that may be harnessed for bioelectronic applications. Further tuning the composition and assembly conditions enables engineering proteinoids as adaptive, multifunctional electronic materials.
Figure 4.
Memfractance of L-Glu:L-Arg proteinoid gel in HAP. The I–V characteristics were measured with the proteinoid gel immersed in 200 ml HAP solution at pH 7.4, 0.15 M ionic strength, and 37°C. The HAP environment enhances memfractance, with currents of -77.4 A at -1 V and 79.788 A at +1 V. The near-zero current of -0.494 A at 0 V indicates reduced hysteresis. Incorporating biomimetic minerals thus tunes proteinoids’ memfractance performance.
Figure 4.
Memfractance of L-Glu:L-Arg proteinoid gel in HAP. The I–V characteristics were measured with the proteinoid gel immersed in 200 ml HAP solution at pH 7.4, 0.15 M ionic strength, and 37°C. The HAP environment enhances memfractance, with currents of -77.4 A at -1 V and 79.788 A at +1 V. The near-zero current of -0.494 A at 0 V indicates reduced hysteresis. Incorporating biomimetic minerals thus tunes proteinoids’ memfractance performance.
Figure 5.
Amino acids undergo thermal polymerization resulting in proteinoids. By means of intramolecular cyclization and condensation reactions, heating glutamic acid, aspartic acid, and lysine produces pyroglutamic acid, cyclic diaspartic acid, and caprolactam, respectively (top). Cyclic amino acid derivatives have the ability to undergo additional polymerization, resulting in the formation of proteinoid microsphere chains (see bottom). The figure depicts the standard chemical reactions that occur during the synthesis of proteinoids from amino acid precursors. By manipulating the monomer composition and heating conditions, it is possible to produce proteinoids with specific properties under control [
186].
Figure 5.
Amino acids undergo thermal polymerization resulting in proteinoids. By means of intramolecular cyclization and condensation reactions, heating glutamic acid, aspartic acid, and lysine produces pyroglutamic acid, cyclic diaspartic acid, and caprolactam, respectively (top). Cyclic amino acid derivatives have the ability to undergo additional polymerization, resulting in the formation of proteinoid microsphere chains (see bottom). The figure depicts the standard chemical reactions that occur during the synthesis of proteinoids from amino acid precursors. By manipulating the monomer composition and heating conditions, it is possible to produce proteinoids with specific properties under control [
186].
Figure 6.
Long–term electrical activity in proteinoids microspheres. Voltage recording over 21 hours exhibits characteristic spiking patterns, with magnified inserts showing details of spikes over time. The continued signaling demonstrates sustained excitability arising from the proteinoids’ self–assembly.
Figure 6.
Long–term electrical activity in proteinoids microspheres. Voltage recording over 21 hours exhibits characteristic spiking patterns, with magnified inserts showing details of spikes over time. The continued signaling demonstrates sustained excitability arising from the proteinoids’ self–assembly.
Figure 7.
A typical spike in proteinoids electrical potential. The spike displays rapid depolarization and repolarization phases. This transient electrical event results from electrostatic interactions between proteinoid dipoles, which produce propagation of excitation through the microsphere network. The spike shape shows proteinoids can mimic key features of neural action potentials.
Figure 7.
A typical spike in proteinoids electrical potential. The spike displays rapid depolarization and repolarization phases. This transient electrical event results from electrostatic interactions between proteinoid dipoles, which produce propagation of excitation through the microsphere network. The spike shape shows proteinoids can mimic key features of neural action potentials.
Figure 8.
Onion–like proteinoid–HAP nanostructures. This is a scanning electron micrograph that displays proteinoids arranged in many layers around a hydroxyapatite (HAP) core. The proteinoids are templated on HAP substrates. The onion–like structure is formed due to the selective aggregation of proteinoids around the mineral particles during nucleation. The scale bar is 500 nanometers. The magnification is 60,000 times. The spot size is 2.0 and the accelerating voltage is 2.0 kilovolts.
Figure 8.
Onion–like proteinoid–HAP nanostructures. This is a scanning electron micrograph that displays proteinoids arranged in many layers around a hydroxyapatite (HAP) core. The proteinoids are templated on HAP substrates. The onion–like structure is formed due to the selective aggregation of proteinoids around the mineral particles during nucleation. The scale bar is 500 nanometers. The magnification is 60,000 times. The spot size is 2.0 and the accelerating voltage is 2.0 kilovolts.
Figure 9.
The PSI and PPI values of several proteinoids are shown in the colour map. The postsynaptic index, or PSI, measures the strength of interneuronal connections in a network, either chemically or functionally. For post–postsynaptic index, see PPI. It measures how effective interneuronal connections are within a certain network. Lighter shades of green imply higher PPI values, while darker shades of blue suggest higher PSI values. The relationship between postsynaptic and presynaptic neurons and how they affect proteinoid function is depicted in the map.
Figure 9.
The PSI and PPI values of several proteinoids are shown in the colour map. The postsynaptic index, or PSI, measures the strength of interneuronal connections in a network, either chemically or functionally. For post–postsynaptic index, see PPI. It measures how effective interneuronal connections are within a certain network. Lighter shades of green imply higher PPI values, while darker shades of blue suggest higher PSI values. The relationship between postsynaptic and presynaptic neurons and how they affect proteinoid function is depicted in the map.
Table 1.
Applications of proteinoid–based hydrogels [
207].
Table 1.
Applications of proteinoid–based hydrogels [
207].
| Type of proteinoid |
Amino acid used |
Uses |
| Dipeptide |
Diphenylalanine, Glyoxylamide, Tryptophan |
Cell scaffolding, drug delivery, tissue engineering |
| Tripeptide |
Cysteine, Phenylalanine |
Biomedical and sensing application |
| Tetrapeptide |
Glycine, Phenylalanine, Tyrosine, lysine |
Ophthalmic drug delivery |
| Pentapeptide |
Histidine, Proline, Lysine, Valine |
Self-healing, carrier for cell transplantation, shear thinning |
| Hexapeptide |
Alanine, Valine, Glycine, Proline |
Reduce distant metastasis and measurement of accurate and reversible pH |
| Oligo–peptide |
Phenylalanine, Tryptophan |
Vaccine nodule, catalyst, carrier, biosensor |
| Polypeptide |
Arginine, Glycine, Aspartic acid, Glutamic acid |
Delivery of antisteroidal non–inflammatory drugs, cell culture, tissue engineering |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).