Submitted:
31 October 2023
Posted:
01 November 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Herpes Simplex virus Type 1
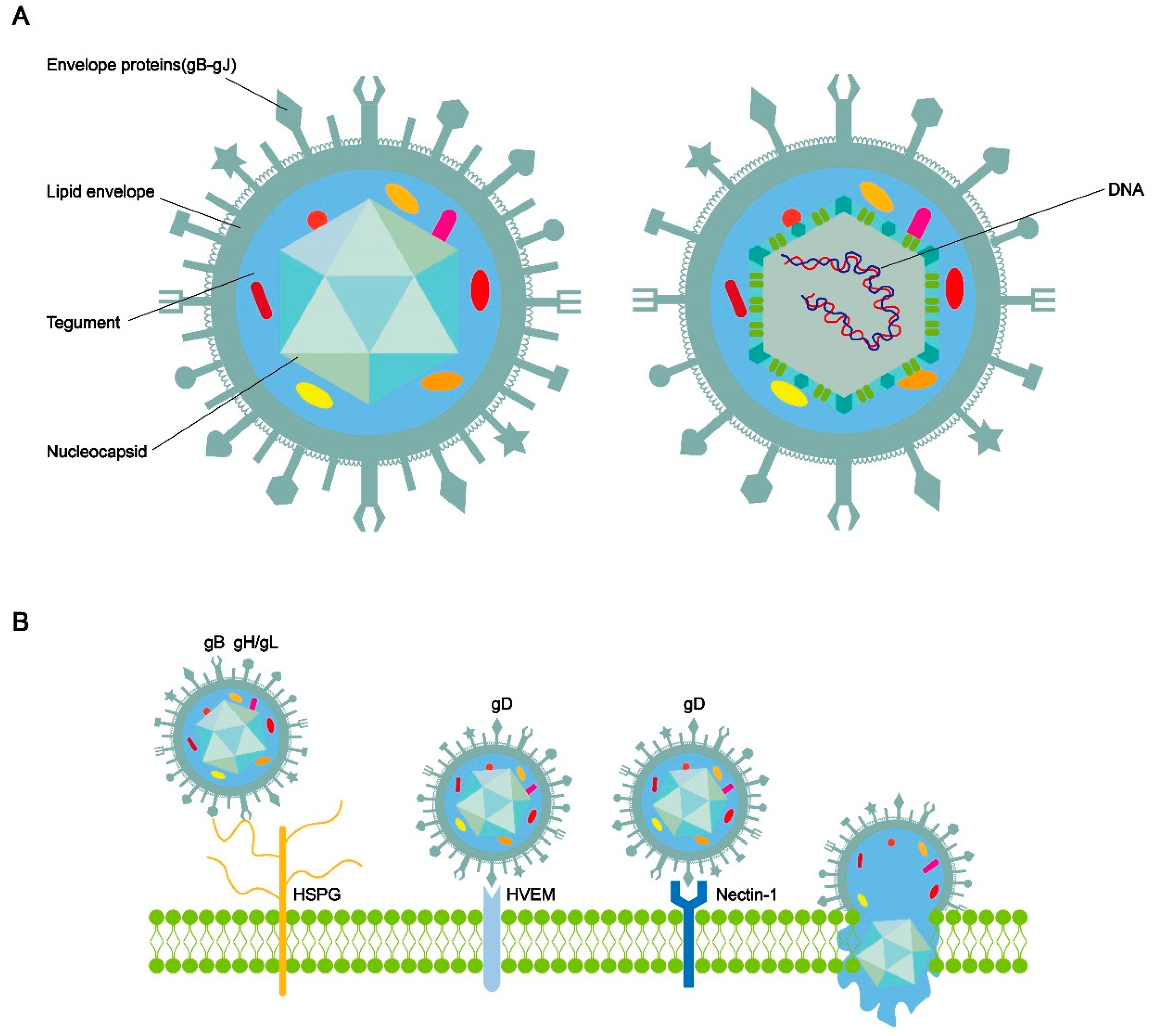
2.1. The process of HSV-1 entering host cells, replication and assembly
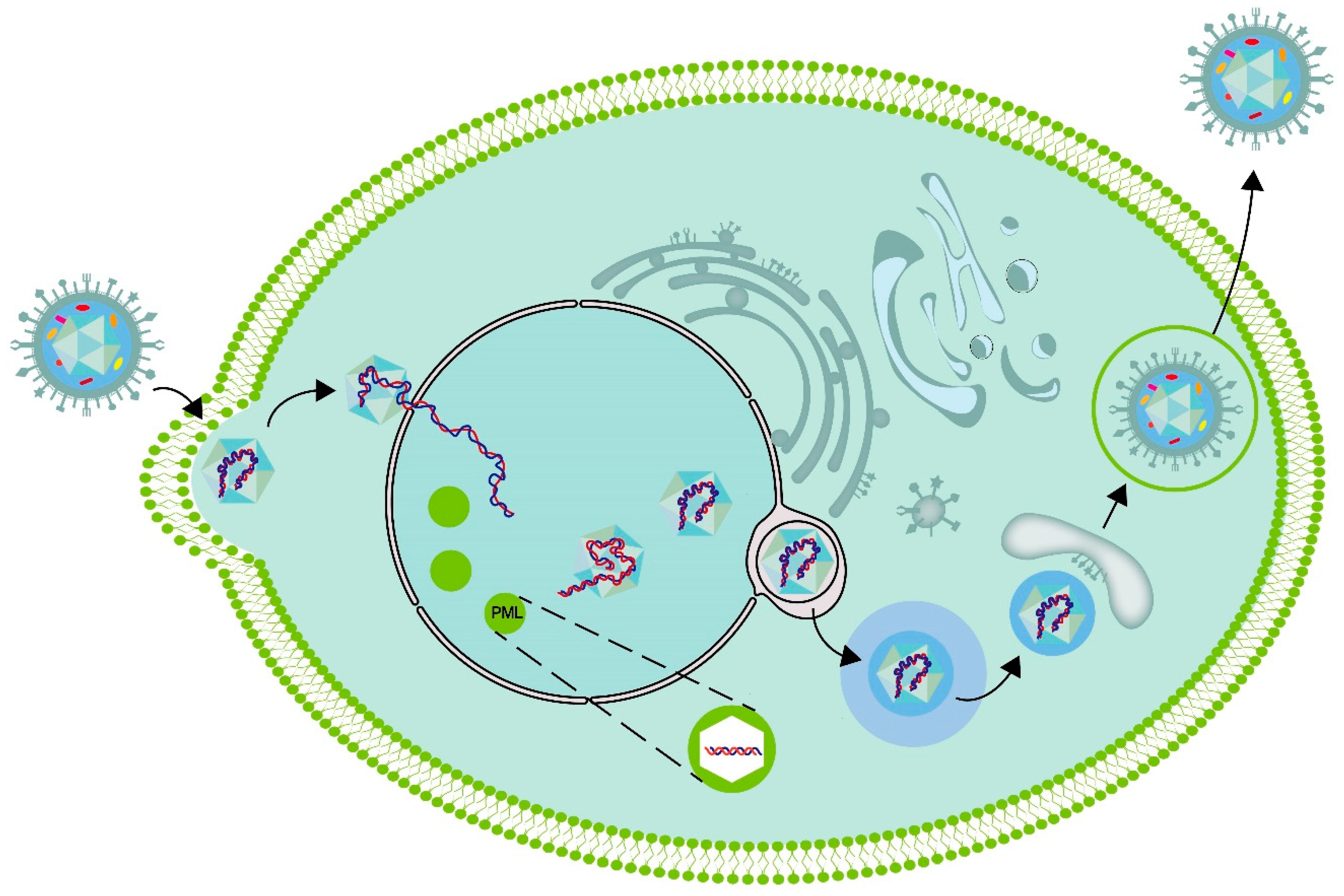
2.2. Host cell processes caused by HSV-1 infection
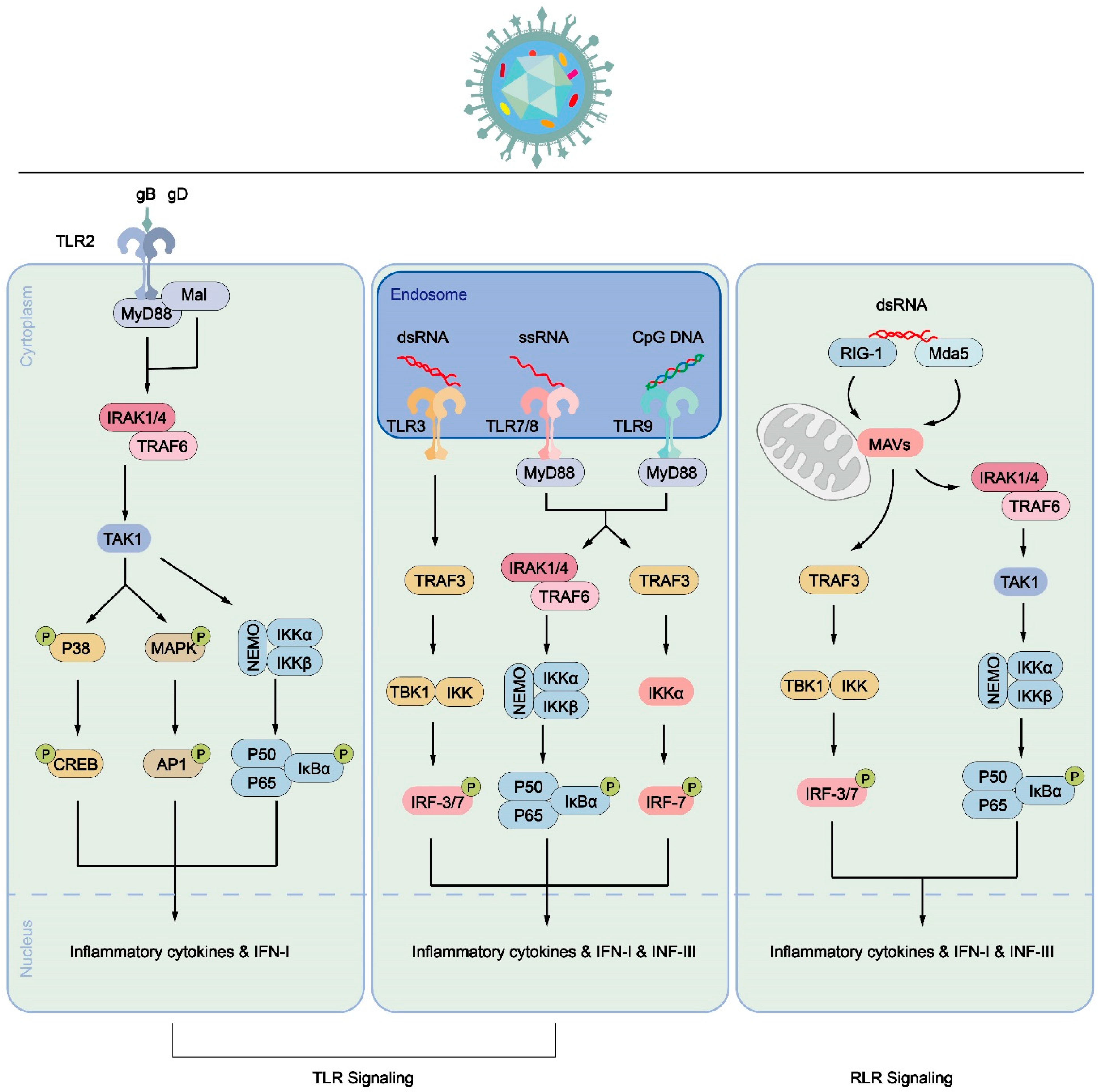
2.3. Immune cell process caused by HSV-1 infection
3. Human Cytomegalovirus
3.1. The process of HCMV entering host cells, replication and assembly
3.2. Host cell processes caused by HCMV infection
3.3. Immune cell process caused by HCMV infection
4. Conclusion
5. Patents
Author Contributions
Funding
References
- Weidner-Glunde, M.; Kruminis-Kaszkiel, E.; Savanagouder, M., Herpesviral Latency-Common Themes. Pathogens 2020, 9, (2). [CrossRef]
- Theil, D.; Arbusow, V.; Derfuss, T.; Strupp, M.; Pfeiffer, M.; Mascolo, A.; Brandt, T., Prevalence of HSV-1 LAT in human trigeminal, geniculate, and vestibular ganglia and its implication for cranial nerve syndromes. Brain Pathol 2001, 11, (4), 408-13. [CrossRef]
- Arbusow, V.; Schulz, P.; Strupp, M.; Dieterich, M.; von Reinhardstoettner, A.; Rauch, E.; Brandt, T., Distribution of herpes simplex virus type 1 in human geniculate and vestibular ganglia: implications for vestibular neuritis. Ann Neurol 1999, 46, (3), 416-9. [CrossRef]
- Furuta, Y.; Takasu, T.; Fukuda, S.; Inuyama, Y.; Sato, K. C.; Nagashima, K., Latent herpes simplex virus type 1 in human vestibular ganglia. Acta Otolaryngol Suppl 1993, 503, 85-9. [CrossRef]
- Schulz, P.; Arbusow, V.; Strupp, M.; Dieterich, M.; Rauch, E.; Brandt, T., Highly variable distribution of HSV-1-specific DNA in human geniculate, vestibular and spiral ganglia. Neurosci Lett 1998, 252, (2), 139-42. [CrossRef]
- Morgenstein, K. M.; Seung, H. I., Vestibular neuronitis. Laryngoscope 1971, 81, (1), 131-9.
- Davis, L. E., Viruses and vestibular neuritis: review of human and animal studies. Acta Otolaryngol Suppl 1993, 503, 70-3. [CrossRef]
- Arbusow, V.; Strupp, M.; Wasicky, R.; Horn, A. K.; Schulz, P.; Brandt, T., Detection of herpes simplex virus type 1 in human vestibular nuclei. Neurology 2000, 55, (6), 880-2. [CrossRef]
- Suzuki, S., [Detection of latent herpes simplex virus in human vestibular ganglia]. Hokkaido Igaku Zasshi 1996, 71, (5), 561-71.
- Esaki, S.; Goshima, F.; Kimura, H.; Ikeda, S.; Katsumi, S.; Kabaya, K.; Watanabe, N.; Hashiba, M.; Nishiyama, Y.; Murakami, S., Auditory and vestibular defects induced by experimental labyrinthitis following herpes simplex virus in mice. Acta Otolaryngol 2011, 131, (7), 684-91. [CrossRef]
- Hirata, Y., [An experimental herpes simplex virus infection in the vestibular nerve]. Nihon Jibiinkoka Gakkai Kaiho 1994, 97, (7), 1191-9. [CrossRef]
- Karasneh, G. A.; Shukla, D., Herpes simplex virus infects most cell types in vitro: clues to its success. Virol J 2011, 8, 481. [CrossRef]
- AlMukdad, S.; Harfouche, M.; Farooqui, U. S.; Aldos, L.; Abu-Raddad, L. J., Epidemiology of herpes simplex virus type 1 and genital herpes in Australia and New Zealand: systematic review, meta-analyses and meta-regressions. Epidemiol Infect 2023, 151, e33. [CrossRef]
- Vallbracht, M.; Backovic, M.; Klupp, B. G.; Rey, F. A.; Mettenleiter, T. C., Common characteristics and unique features: A comparison of the fusion machinery of the alphaherpesviruses Pseudorabies virus and Herpes simplex virus. Adv Virus Res 2019, 104, 225-281.
- Möhl, B. S.; Chen, J.; Longnecker, R., Gammaherpesvirus entry and fusion: A tale how two human pathogenic viruses enter their host cells. Adv Virus Res 2019, 104, 313-343.
- Nishimura, M.; Mori, Y., Entry of betaherpesviruses. Adv Virus Res 2019, 104, 283-312.
- Connolly, S. A.; Jardetzky, T. S.; Longnecker, R., The structural basis of herpesvirus entry. Nat Rev Microbiol 2021, 19, (2), 110-121. [CrossRef]
- Atanasiu, D.; Saw, W. T.; Cohen, G. H.; Eisenberg, R. J., Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J Virol 2010, 84, (23), 12292-9. [CrossRef]
- Cooper, R. S.; Heldwein, E. E., Herpesvirus gB: A Finely Tuned Fusion Machine. Viruses 2015, 7, (12), 6552-69. [CrossRef]
- Fontana, J.; Atanasiu, D.; Saw, W. T.; Gallagher, J. R.; Cox, R. G.; Whitbeck, J. C.; Brown, L. M.; Eisenberg, R. J.; Cohen, G. H., The Fusion Loops of the Initial Prefusion Conformation of Herpes Simplex Virus 1 Fusion Protein Point Toward the Membrane. mBio 2017, 8, (4). [CrossRef]
- Saksena, M. M.; Wakisaka, H.; Tijono, B.; Boadle, R. A.; Rixon, F.; Takahashi, H.; Cunningham, A. L., Herpes simplex virus type 1 accumulation, envelopment, and exit in growth cones and varicosities in mid-distal regions of axons. J Virol 2006, 80, (7), 3592-606. [CrossRef]
- Lee, G. E.; Murray, J. W.; Wolkoff, A. W.; Wilson, D. W., Reconstitution of herpes simplex virus microtubule-dependent trafficking in vitro. J Virol 2006, 80, (9), 4264-75. [CrossRef]
- Kharkwal, H.; Smith, C. G.; Wilson, D. W., Blocking ESCRT-mediated envelopment inhibits microtubule-dependent trafficking of alphaherpesviruses in vitro. J Virol 2014, 88, (24), 14467-78.
- Miranda-Saksena, M.; Armati, P.; Boadle, R. A.; Holland, D. J.; Cunningham, A. L., Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J Virol 2000, 74, (4), 1827-39. [CrossRef]
- Miranda-Saksena, M.; Denes, C. E.; Diefenbach, R. J.; Cunningham, A. L., Infection and Transport of Herpes Simplex Virus Type 1 in Neurons: Role of the Cytoskeleton. Viruses 2018, 10, (2). [CrossRef]
- Maday, S.; Twelvetrees, A. E.; Moughamian, A. J.; Holzbaur, E. L., Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron 2014, 84, (2), 292-309. [CrossRef]
- Liu, J. J., Regulation of dynein-dynactin-driven vesicular transport. Traffic 2017, 18, (6), 336-347.
- Hirokawa, N.; Niwa, S.; Tanaka, Y., Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron 2010, 68, (4), 610-38. [CrossRef]
- Antinone, S. E.; Zaichick, S. V.; Smith, G. A., Resolving the assembly state of herpes simplex virus during axon transport by live-cell imaging. J Virol 2010, 84, (24), 13019-30. [CrossRef]
- Smith, G. A.; Gross, S. P.; Enquist, L. W., Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc Natl Acad Sci U S A 2001, 98, (6), 3466-70. [CrossRef]
- Villanueva-Valencia, J. R.; Tsimtsirakis, E.; Evilevitch, A., Role of HSV-1 Capsid Vertex-Specific Component (CVSC) and Viral Terminal DNA in Capsid Docking at the Nuclear Pore. Viruses 2021, 13, (12). [CrossRef]
- Schmid, M.; Speiseder, T.; Dobner, T.; Gonzalez, R. A., DNA virus replication compartments. J Virol 2014, 88, (3), 1404-20. [CrossRef]
- Charman, M.; Weitzman, M. D., Replication Compartments of DNA Viruses in the Nucleus: Location, Location, Location. Viruses 2020, 12, (2). [CrossRef]
- Hidalgo, P.; Gonzalez, R. A., Formation of adenovirus DNA replication compartments. FEBS Lett 2019, 593, (24), 3518-3530. [CrossRef]
- Feric, M.; Vaidya, N.; Harmon, T. S.; Mitrea, D. M.; Zhu, L.; Richardson, T. M.; Kriwacki, R. W.; Pappu, R. V.; Brangwynne, C. P., Coexisting Liquid Phases Underlie Nucleolar Subcompartments. Cell 2016, 165, (7), 1686-1697. [CrossRef]
- Brangwynne, C. P.; Eckmann, C. R.; Courson, D. S.; Rybarska, A.; Hoege, C.; Gharakhani, J.; Jülicher, F.; Hyman, A. A., Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 2009, 324, (5935), 1729-32. [CrossRef]
- Hyman, A. A.; Weber, C. A.; Jülicher, F., Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol 2014, 30, 39-58. [CrossRef]
- Kobiler, O.; Weitzman, M. D., Herpes simplex virus replication compartments: From naked release to recombining together. PLoS Pathog 2019, 15, (6), e1007714. [CrossRef]
- Seyffert, M.; Georgi, F.; Tobler, K.; Bourqui, L.; Anfossi, M.; Michaelsen, K.; Vogt, B.; Greber, U. F.; Fraefel, C., The HSV-1 Transcription Factor ICP4 Confers Liquid-Like Properties to Viral Replication Compartments. Int J Mol Sci 2021, 22, (9). [CrossRef]
- Steiner, I.; Kennedy, P. G.; Pachner, A. R., The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol 2007, 6, (11), 1015-28. [CrossRef]
- Kramer, T.; Enquist, L. W., Directional spread of alphaherpesviruses in the nervous system. Viruses 2013, 5, (2), 678-707.
- Chayavichitsilp, P.; Buckwalter, J. V.; Krakowski, A. C.; Friedlander, S. F., Herpes simplex. Pediatr Rev 2009, 30, (4), 119-29; quiz 130.
- Roizman, B., Polykaryocytosis induced by viruses. Proc Natl Acad Sci U S A 1962, 48, (2), 228-34. [CrossRef]
- Avitabile, E.; Di Gaeta, S.; Torrisi, M. R.; Ward, P. L.; Roizman, B.; Campadelli-Fiume, G., Redistribution of microtubules and Golgi apparatus in herpes simplex virus-infected cells and their role in viral exocytosis. J Virol 1995, 69, (12), 7472-82. [CrossRef]
- Heeg, U.; Dienes, H. P.; Müller, S.; Falke, D., Involvement of actin-containing microfilaments in HSV-induced cytopathology and the influence of inhibitors of glycosylation. Arch Virol 1986, 91, (3-4), 257-70. [CrossRef]
- Hampar, B.; Ellison, S. A., Chromosomal aberrations induced by an animal virus. Nature 1961, 192, 145-7. [CrossRef]
- Roizman, B., The programming of herpes virus multiplication in doubly-infected and in puromycin-treated cells. Proc Natl Acad Sci U S A 1963, 49, (2), 165-71. [CrossRef]
- Roizman, B.; Roane, P. R., Jr., THE MULTIPLICATION OF HERPES SIMPLEX VIRUS. II. THE RELATION BETWEEN PROTEIN SYNTHESIS AND THE DUPLICATION OF VIRAL DNA IN INFECTED HEP-2 CELLS. Virology 1964, 22, 262-9.
- Aubert, M.; Blaho, J. A., The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J Virol 1999, 73, (4), 2803-13. [CrossRef]
- Finlay, D.; Cantrell, D. A., Metabolism, migration and memory in cytotoxic T cells. Nat Rev Immunol 2011, 11, (2), 109-17. [CrossRef]
- Dufour, F.; Sasseville, A. M.; Chabaud, S.; Massie, B.; Siegel, R. M.; Langelier, Y., The ribonucleotide reductase R1 subunits of herpes simplex virus types 1 and 2 protect cells against TNFα- and FasL-induced apoptosis by interacting with caspase-8. Apoptosis 2011, 16, (3), 256-71.
- Dufour, F.; Bertrand, L.; Pearson, A.; Grandvaux, N.; Langelier, Y., The ribonucleotide reductase R1 subunits of herpes simplex virus 1 and 2 protect cells against poly(I · C)-induced apoptosis. J Virol 2011, 85, (17), 8689-701. [CrossRef]
- Finnen, R. L.; Johnston, S. M.; Neron, C. E.; Banfield, B. W., Nucleocytoplasmic shuttling of the HSV-2 serine/threonine kinase Us3. Virology 2011, 417, (1), 229-37. [CrossRef]
- Mori, I., Herpes simplex virus US3 protein kinase regulates host responses and determines neurovirulence. Microbiol Immunol 2012, 56, (6), 351-5. [CrossRef]
- Benetti, L.; Roizman, B., Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis. Proc Natl Acad Sci U S A 2004, 101, (25), 9411-6.
- Lussignol, M.; Queval, C.; Bernet-Camard, M. F.; Cotte-Laffitte, J.; Beau, I.; Codogno, P.; Esclatine, A., The herpes simplex virus 1 Us11 protein inhibits autophagy through its interaction with the protein kinase PKR. J Virol 2013, 87, (2), 859-71. [CrossRef]
- Li, Y.; Zhang, C.; Chen, X.; Yu, J.; Wang, Y.; Yang, Y.; Du, M.; Jin, H.; Ma, Y.; He, B.; Cao, Y., ICP34.5 protein of herpes simplex virus facilitates the initiation of protein translation by bridging eukaryotic initiation factor 2alpha (eIF2alpha) and protein phosphatase 1. J Biol Chem 2011, 286, (28), 24785-92. [CrossRef]
- Orvedahl, A.; Alexander, D.; Tallóczy, Z.; Sun, Q.; Wei, Y.; Zhang, W.; Burns, D.; Leib, D. A.; Levine, B., HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 2007, 1, (1), 23-35. [CrossRef]
- Zhu, S.; Viejo-Borbolla, A., Pathogenesis and virulence of herpes simplex virus. Virulence 2021, 12, (1), 2670-2702. [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O., Pathogen recognition and innate immunity. Cell 2006, 124, (4), 783-801.
- Paludan, S. R.; Bowie, A. G.; Horan, K. A.; Fitzgerald, K. A., Recognition of herpesviruses by the innate immune system. Nat Rev Immunol 2011, 11, (2), 143-54.
- Takeda, K.; Akira, S., TLR signaling pathways. Semin Immunol 2004, 16, (1), 3-9.
- Vandevenne, P.; Sadzot-Delvaux, C.; Piette, J., Innate immune response and viral interference strategies developed by human herpesviruses. Biochem Pharmacol 2010, 80, (12), 1955-72. [CrossRef]
- Gianni, T.; Leoni, V.; Campadelli-Fiume, G., Type I interferon and NF-κB activation elicited by herpes simplex virus gH/gL via αvβ3 integrin in epithelial and neuronal cell lines. J Virol 2013, 87, (24), 13911-6.
- Leoni, V.; Gianni, T.; Salvioli, S.; Campadelli-Fiume, G., Herpes simplex virus glycoproteins gH/gL and gB bind Toll-like receptor 2, and soluble gH/gL is sufficient to activate NF-κB. J Virol 2012, 86, (12), 6555-62. [CrossRef]
- Kurt-Jones, E. A.; Chan, M.; Zhou, S.; Wang, J.; Reed, G.; Bronson, R.; Arnold, M. M.; Knipe, D. M.; Finberg, R. W., Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A 2004, 101, (5), 1315-20. [CrossRef]
- Marino, A.; Pergolizzi, S.; Cimino, F.; Lauriano, E. R.; Speciale, A.; D'Angelo, V.; Sicurella, M.; Argnani, R.; Manservigi, R.; Marconi, P., Role of Herpes Simplex Envelope Glycoprotein B and Toll-Like Receptor 2 in Ocular Inflammation: An ex vivo Organotypic Rabbit Corneal Model. Viruses 2019, 11, (9). [CrossRef]
- Gantt, S.; Muller, W. J., The immunologic basis for severe neonatal herpes disease and potential strategies for therapeutic intervention. Clin Dev Immunol 2013, 2013, 369172. [CrossRef]
- Casanova, J. L., Severe infectious diseases of childhood as monogenic inborn errors of immunity. Proc Natl Acad Sci U S A 2015, 112, (51), E7128-37. [CrossRef]
- Reinert, L. S.; Harder, L.; Holm, C. K.; Iversen, M. B.; Horan, K. A.; Dagnæs-Hansen, F.; Ulhøi, B. P.; Holm, T. H.; Mogensen, T. H.; Owens, T.; Nyengaard, J. R.; Thomsen, A. R.; Paludan, S. R., TLR3 deficiency renders astrocytes permissive to herpes simplex virus infection and facilitates establishment of CNS infection in mice. J Clin Invest 2012, 122, (4), 1368-76. [CrossRef]
- Leib, D. A., Herpes simplex virus encephalitis: toll-free access to the brain. Cell Host Microbe 2012, 12, (6), 731-2. [CrossRef]
- Lund, J.; Sato, A.; Akira, S.; Medzhitov, R.; Iwasaki, A., Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med 2003, 198, (3), 513-20. [CrossRef]
- Krug, A.; Luker, G. D.; Barchet, W.; Leib, D. A.; Akira, S.; Colonna, M., Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 2004, 103, (4), 1433-7. [CrossRef]
- Verjans, G. M.; Hintzen, R. Q.; van Dun, J. M.; Poot, A.; Milikan, J. C.; Laman, J. D.; Langerak, A. W.; Kinchington, P. R.; Osterhaus, A. D., Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A 2007, 104, (9), 3496-501. [CrossRef]
- van Velzen, M.; Jing, L.; Osterhaus, A. D.; Sette, A.; Koelle, D. M.; Verjans, G. M., Local CD4 and CD8 T-cell reactivity to HSV-1 antigens documents broad viral protein expression and immune competence in latently infected human trigeminal ganglia. PLoS Pathog 2013, 9, (8), e1003547. [CrossRef]
- Zhu, J.; Koelle, D. M.; Cao, J.; Vazquez, J.; Huang, M. L.; Hladik, F.; Wald, A.; Corey, L., Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med 2007, 204, (3), 595-603. [CrossRef]
- Zhu, J.; Peng, T.; Johnston, C.; Phasouk, K.; Kask, A. S.; Klock, A.; Jin, L.; Diem, K.; Koelle, D. M.; Wald, A.; Robins, H.; Corey, L., Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature 2013, 497, (7450), 494-7.
- Posavad, C. M.; Zhao, L.; Dong, L.; Jin, L.; Stevens, C. E.; Magaret, A. S.; Johnston, C.; Wald, A.; Zhu, J.; Corey, L.; Koelle, D. M., Enrichment of herpes simplex virus type 2 (HSV-2) reactive mucosal T cells in the human female genital tract. Mucosal Immunol 2017, 10, (5), 1259-1269. [CrossRef]
- Jing, L.; Haas, J.; Chong, T. M.; Bruckner, J. J.; Dann, G. C.; Dong, L.; Marshak, J. O.; McClurkan, C. L.; Yamamoto, T. N.; Bailer, S. M.; Laing, K. J.; Wald, A.; Verjans, G. M.; Koelle, D. M., Cross-presentation and genome-wide screening reveal candidate T cells antigens for a herpes simplex virus type 1 vaccine. J Clin Invest 2012, 122, (2), 654-73.
- Moss, N. J.; Magaret, A.; Laing, K. J.; Kask, A. S.; Wang, M.; Mark, K. E.; Schiffer, J. T.; Wald, A.; Koelle, D. M., Peripheral blood CD4 T-cell and plasmacytoid dendritic cell (pDC) reactivity to herpes simplex virus 2 and pDC number do not correlate with the clinical or virologic severity of recurrent genital herpes. J Virol 2012, 86, (18), 9952-63. [CrossRef]
- Laing, K. J.; Magaret, A. S.; Mueller, D. E.; Zhao, L.; Johnston, C.; De Rosa, S. C.; Koelle, D. M.; Wald, A.; Corey, L., Diversity in CD8(+) T cell function and epitope breadth among persons with genital herpes. J Clin Immunol 2010, 30, (5), 703-22. [CrossRef]
- Koelle, D. M.; Corey, L.; Burke, R. L.; Eisenberg, R. J.; Cohen, G. H.; Pichyangkura, R.; Triezenberg, S. J., Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J Virol 1994, 68, (5), 2803-10. [CrossRef]
- Valyi-Nagy, T.; Deshmane, S. L.; Raengsakulrach, B.; Nicosia, M.; Gesser, R. M.; Wysocka, M.; Dillner, A.; Fraser, N. W., Herpes simplex virus type 1 mutant strain in1814 establishes a unique, slowly progressing infection in SCID mice. J Virol 1992, 66, (12), 7336-45. [CrossRef]
- Wakim, L. M.; Jones, C. M.; Gebhardt, T.; Preston, C. M.; Carbone, F. R., CD8(+) T-cell attenuation of cutaneous herpes simplex virus infection reduces the average viral copy number of the ensuing latent infection. Immunol Cell Biol 2008, 86, (8), 666-75. [CrossRef]
- Hoshino, Y.; Pesnicak, L.; Cohen, J. I.; Straus, S. E., Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. J Virol 2007, 81, (15), 8157-64. [CrossRef]
- Derfuss, T.; Segerer, S.; Herberger, S.; Sinicina, I.; Hüfner, K.; Ebelt, K.; Knaus, H. G.; Steiner, I.; Meinl, E.; Dornmair, K.; Arbusow, V.; Strupp, M.; Brandt, T.; Theil, D., Presence of HSV-1 immediate early genes and clonally expanded T-cells with a memory effector phenotype in human trigeminal ganglia. Brain Pathol 2007, 17, (4), 389-98. [CrossRef]
- Geiger, K. D.; Nash, T. C.; Sawyer, S.; Krahl, T.; Patstone, G.; Reed, J. C.; Krajewski, S.; Dalton, D.; Buchmeier, M. J.; Sarvetnick, N., Interferon-gamma protects against herpes simplex virus type 1-mediated neuronal death. Virology 1997, 238, (2), 189-97.
- Knickelbein, J. E.; Khanna, K. M.; Yee, M. B.; Baty, C. J.; Kinchington, P. R.; Hendricks, R. L., Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 2008, 322, (5899), 268-71. [CrossRef]
- Frank, G. M.; Lepisto, A. J.; Freeman, M. L.; Sheridan, B. S.; Cherpes, T. L.; Hendricks, R. L., Early CD4(+) T cell help prevents partial CD8(+) T cell exhaustion and promotes maintenance of Herpes Simplex Virus 1 latency. J Immunol 2010, 184, (1), 277-86. [CrossRef]
- Singh, N.; Tscharke, D. C., Herpes Simplex Virus Latency Is Noisier the Closer We Look. J Virol 2020, 94, (4). [CrossRef]
- Liu, T.; Khanna, K. M.; Chen, X.; Fink, D. J.; Hendricks, R. L., CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med 2000, 191, (9), 1459-66. [CrossRef]
- van Velzen, M.; Laman, J. D.; Kleinjan, A.; Poot, A.; Osterhaus, A. D.; Verjans, G. M., Neuron-interacting satellite glial cells in human trigeminal ganglia have an APC phenotype. J Immunol 2009, 183, (4), 2456-61. [CrossRef]
- Gobeil, P. A.; Leib, D. A., Herpes simplex virus γ34.5 interferes with autophagosome maturation and antigen presentation in dendritic cells. mBio 2012, 3, (5), e00267-12. [CrossRef]
- Lubinski, J. M.; Jiang, M.; Hook, L.; Chang, Y.; Sarver, C.; Mastellos, D.; Lambris, J. D.; Cohen, G. H.; Eisenberg, R. J.; Friedman, H. M., Herpes simplex virus type 1 evades the effects of antibody and complement in vivo. J Virol 2002, 76, (18), 9232-41. [CrossRef]
- Weisblum, Y.; Panet, A.; Haimov-Kochman, R.; Wolf, D. G., Models of vertical cytomegalovirus (CMV) transmission and pathogenesis. Semin Immunopathol 2014, 36, (6), 615-25. [CrossRef]
- Navti, O. B.; Al-Belushi, M.; Konje, J. C., Cytomegalovirus infection in pregnancy - An update. Eur J Obstet Gynecol Reprod Biol 2021, 258, 216-222. [CrossRef]
- Chandler, S. H.; Holmes, K. K.; Wentworth, B. B.; Gutman, L. T.; Wiesner, P. J.; Alexander, E. R.; Handsfield, H. H., The epidemiology of cytomegaloviral infection in women attending a sexually transmitted disease clinic. J Infect Dis 1985, 152, (3), 597-605. [CrossRef]
- Gerna, G.; Baldanti, F.; Revello, M. G., Pathogenesis of human cytomegalovirus infection and cellular targets. Hum Immunol 2004, 65, (5), 381-6. [CrossRef]
- Gretch, D. R.; Kari, B.; Rasmussen, L.; Gehrz, R. C.; Stinski, M. F., Identification and characterization of three distinct families of glycoprotein complexes in the envelopes of human cytomegalovirus. J Virol 1988, 62, (3), 875-81. [CrossRef]
- Mach, M.; Kropff, B.; Dal Monte, P.; Britt, W., Complex formation by human cytomegalovirus glycoproteins M (gpUL100) and N (gpUL73). J Virol 2000, 74, (24), 11881-92. [CrossRef]
- Mach, M.; Kropff, B.; Kryzaniak, M.; Britt, W., Complex formation by glycoproteins M and N of human cytomegalovirus: structural and functional aspects. J Virol 2005, 79, (4), 2160-70. [CrossRef]
- Kari, B.; Gehrz, R., A human cytomegalovirus glycoprotein complex designated gC-II is a major heparin-binding component of the envelope. J Virol 1992, 66, (3), 1761-4.
- Ciferri, C.; Chandramouli, S.; Donnarumma, D.; Nikitin, P. A.; Cianfrocco, M. A.; Gerrein, R.; Feire, A. L.; Barnett, S. W.; Lilja, A. E.; Rappuoli, R.; Norais, N.; Settembre, E. C.; Carfi, A., Structural and biochemical studies of HCMV gH/gL/gO and Pentamer reveal mutually exclusive cell entry complexes. Proc Natl Acad Sci U S A 2015, 112, (6), 1767-72. [CrossRef]
- Wang, D.; Shenk, T., Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol 2005, 79, (16), 10330-8. [CrossRef]
- Wang, D.; Shenk, T., Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A 2005, 102, (50), 18153-8. [CrossRef]
- Lin, E.; Spear, P. G., Random linker-insertion mutagenesis to identify functional domains of herpes simplex virus type 1 glycoprotein B. Proc Natl Acad Sci U S A 2007, 104, (32), 13140-5. [CrossRef]
- Reimer, J. J.; Backovic, M.; Deshpande, C. G.; Jardetzky, T.; Longnecker, R., Analysis of Epstein-Barr virus glycoprotein B functional domains via linker insertion mutagenesis. J Virol 2009, 83, (2), 734-47. [CrossRef]
- Maurer, U. E.; Zeev-Ben-Mordehai, T.; Pandurangan, A. P.; Cairns, T. M.; Hannah, B. P.; Whitbeck, J. C.; Eisenberg, R. J.; Cohen, G. H.; Topf, M.; Huiskonen, J. T.; Grünewald, K., The structure of herpesvirus fusion glycoprotein B-bilayer complex reveals the protein-membrane and lateral protein-protein interaction. Structure 2013, 21, (8), 1396-405.
- Soroceanu, L.; Akhavan, A.; Cobbs, C. S., Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature 2008, 455, (7211), 391-5. [CrossRef]
- Wang, X.; Huong, S. M.; Chiu, M. L.; Raab-Traub, N.; Huang, E. S., Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 2003, 424, (6947), 456-61. [CrossRef]
- Feire, A. L.; Roy, R. M.; Manley, K.; Compton, T., The glycoprotein B disintegrin-like domain binds beta 1 integrin to mediate cytomegalovirus entry. J Virol 2010, 84, (19), 10026-37. [CrossRef]
- Feire, A. L.; Koss, H.; Compton, T., Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci U S A 2004, 101, (43), 15470-5. [CrossRef]
- Ryckman, B. J.; Jarvis, M. A.; Drummond, D. D.; Nelson, J. A.; Johnson, D. C., Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J Virol 2006, 80, (2), 710-22.
- Martinez-Martin, N.; Marcandalli, J.; Huang, C. S.; Arthur, C. P.; Perotti, M.; Foglierini, M.; Ho, H.; Dosey, A. M.; Shriver, S.; Payandeh, J.; Leitner, A.; Lanzavecchia, A.; Perez, L.; Ciferri, C., An Unbiased Screen for Human Cytomegalovirus Identifies Neuropilin-2 as a Central Viral Receptor. Cell 2018, 174, (5), 1158-1171.e19. [CrossRef]
- Kabanova, A.; Marcandalli, J.; Zhou, T.; Bianchi, S.; Baxa, U.; Tsybovsky, Y.; Lilleri, D.; Silacci-Fregni, C.; Foglierini, M.; Fernandez-Rodriguez, B. M.; Druz, A.; Zhang, B.; Geiger, R.; Pagani, M.; Sallusto, F.; Kwong, P. D.; Corti, D.; Lanzavecchia, A.; Perez, L., Platelet-derived growth factor-α receptor is the cellular receptor for human cytomegalovirus gHgLgO trimer. Nat Microbiol 2016, 1, (8), 16082.
- Wu, Y.; Prager, A.; Boos, S.; Resch, M.; Brizic, I.; Mach, M.; Wildner, S.; Scrivano, L.; Adler, B., Human cytomegalovirus glycoprotein complex gH/gL/gO uses PDGFR-α as a key for entry. PLoS Pathog 2017, 13, (4), e1006281.
- Compton, T.; Nepomuceno, R. R.; Nowlin, D. M., Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 1992, 191, (1), 387-95. [CrossRef]
- Scherer, M.; Stamminger, T., Emerging Role of PML Nuclear Bodies in Innate Immune Signaling. J Virol 2016, 90, (13), 5850-5854. [CrossRef]
- Caragliano, E.; Bonazza, S.; Frascaroli, G.; Tang, J.; Soh, T. K.; Grünewald, K.; Bosse, J. B.; Brune, W., Human cytomegalovirus forms phase-separated compartments at viral genomes to facilitate viral replication. Cell Rep 2022, 38, (10), 110469. [CrossRef]
- Sanchez, V.; Greis, K. D.; Sztul, E.; Britt, W. J., Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J Virol 2000, 74, (2), 975-86. [CrossRef]
- Stagno, S.; Reynolds, D. W.; Tsiantos, A.; Fuccillo, D. A.; Long, W.; Alford, C. A., Comparative serial virologic and serologic studies of symptomatic and subclinical congenitally and natally acquired cytomegalovirus infections. J Infect Dis 1975, 132, (5), 568-77. [CrossRef]
- Griffiths, P.; Baraniak, I.; Reeves, M., The pathogenesis of human cytomegalovirus. J Pathol 2015, 235, (2), 288-97. [CrossRef]
- Cope, A. V.; Sweny, P.; Sabin, C.; Rees, L.; Griffiths, P. D.; Emery, V. C., Quantity of cytomegalovirus viruria is a major risk factor for cytomegalovirus disease after renal transplantation. J Med Virol 1997, 52, (2), 200-5.
- Cope, A. V.; Sabin, C.; Burroughs, A.; Rolles, K.; Griffiths, P. D.; Emery, V. C., Interrelationships among quantity of human cytomegalovirus (HCMV) DNA in blood, donor-recipient serostatus, and administration of methylprednisolone as risk factors for HCMV disease following liver transplantation. J Infect Dis 1997, 176, (6), 1484-90. [CrossRef]
- Emery, V. C.; Cope, A. V.; Bowen, E. F.; Gor, D.; Griffiths, P. D., The dynamics of human cytomegalovirus replication in vivo. J Exp Med 1999, 190, (2), 177-82. [CrossRef]
- Reeves, M.; Sinclair, J., Aspects of human cytomegalovirus latency and reactivation. Curr Top Microbiol Immunol 2008, 325, 297-313.
- Mocarski, E. S.; Kemble, G. W.; Lyle, J. M.; Greaves, R. F., A deletion mutant in the human cytomegalovirus gene encoding IE1(491aa) is replication defective due to a failure in autoregulation. Proc Natl Acad Sci U S A 1996, 93, (21), 11321-6. [CrossRef]
- Marchini, A.; Liu, H.; Zhu, H., Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J Virol 2001, 75, (4), 1870-8. [CrossRef]
- Martínez, F. P.; Cruz, R.; Lu, F.; Plasschaert, R.; Deng, Z.; Rivera-Molina, Y. A.; Bartolomei, M. S.; Lieberman, P. M.; Tang, Q., CTCF binding to the first intron of the major immediate early (MIE) gene of human cytomegalovirus (HCMV) negatively regulates MIE gene expression and HCMV replication. J Virol 2014, 88, (13), 7389-401. [CrossRef]
- Lau, B.; Poole, E.; Krishna, B.; Sellart, I.; Wills, M. R.; Murphy, E.; Sinclair, J., The Expression of Human Cytomegalovirus MicroRNA MiR-UL148D during Latent Infection in Primary Myeloid Cells Inhibits Activin A-triggered Secretion of IL-6. Sci Rep 2016, 6, 31205.
- Buehler, J.; Zeltzer, S.; Reitsma, J.; Petrucelli, A.; Umashankar, M.; Rak, M.; Zagallo, P.; Schroeder, J.; Terhune, S.; Goodrum, F., Opposing Regulation of the EGF Receptor: A Molecular Switch Controlling Cytomegalovirus Latency and Replication. PLoS Pathog 2016, 12, (5), e1005655.
- Diggins, N. L.; Skalsky, R. L.; Hancock, M. H., Regulation of Latency and Reactivation by Human Cytomegalovirus miRNAs. Pathogens 2021, 10, (2). [CrossRef]
- Pan, C.; Zhu, D.; Wang, Y.; Li, L.; Li, D.; Liu, F.; Zhang, C. Y.; Zen, K., Human Cytomegalovirus miR-UL148D Facilitates Latent Viral Infection by Targeting Host Cell Immediate Early Response Gene 5. PLoS Pathog 2016, 12, (11), e1006007.
- Diggins, N. L.; Crawford, L. B.; Hancock, M. H.; Mitchell, J.; Nelson, J. A., Human Cytomegalovirus miR-US25-1 Targets the GTPase RhoA To Inhibit CD34(+) Hematopoietic Progenitor Cell Proliferation To Maintain the Latent Viral Genome. mBio 2021, 12, (2).
- Hancock, M. H.; Crawford, L. B.; Perez, W.; Struthers, H. M.; Mitchell, J.; Caposio, P., Human Cytomegalovirus UL7, miR-US5-1, and miR-UL112-3p Inactivation of FOXO3a Protects CD34(+) Hematopoietic Progenitor Cells from Apoptosis. mSphere 2021, 6, (1).
- Anderson, D.; DeFor, T.; Burns, L.; McGlave, P.; Miller, J.; Wagner, J.; Weisdorf, D., A comparison of related donor peripheral blood and bone marrow transplants: importance of late-onset chronic graft-versus-host disease and infections. Biol Blood Marrow Transplant 2003, 9, (1), 52-9. [CrossRef]
- Hancock, M. H.; Crawford, L. B.; Pham, A. H.; Mitchell, J.; Struthers, H. M.; Yurochko, A. D.; Caposio, P.; Nelson, J. A., Human Cytomegalovirus miRNAs Regulate TGF-β to Mediate Myelosuppression while Maintaining Viral Latency in CD34(+) Hematopoietic Progenitor Cells. Cell Host Microbe 2020, 27, (1), 104-114.e4. [CrossRef]
- Hancock, M. H.; Mitchell, J.; Goodrum, F. D.; Nelson, J. A., Human Cytomegalovirus miR-US5-2 Downregulation of GAB1 Regulates Cellular Proliferation and UL138 Expression through Modulation of Epidermal Growth Factor Receptor Signaling Pathways. mSphere 2020, 5, (4).
- Crawford, L. B.; Kim, J. H.; Collins-McMillen, D.; Lee, B. J.; Landais, I.; Held, C.; Nelson, J. A.; Yurochko, A. D.; Caposio, P., Human Cytomegalovirus Encodes a Novel FLT3 Receptor Ligand Necessary for Hematopoietic Cell Differentiation and Viral Reactivation. mBio 2018, 9, (2). [CrossRef]
- Boehme, K. W.; Guerrero, M.; Compton, T., Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J Immunol 2006, 177, (10), 7094-102. [CrossRef]
- Compton, T.; Kurt-Jones, E. A.; Boehme, K. W.; Belko, J.; Latz, E.; Golenbock, D. T.; Finberg, R. W., Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol 2003, 77, (8), 4588-96. [CrossRef]
- Landais, I.; Pelton, C.; Streblow, D.; DeFilippis, V.; McWeeney, S.; Nelson, J. A., Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway. PLoS Pathog 2015, 11, (5), e1004881.
- Park, A.; Ra, E. A.; Lee, T. A.; Choi, H. J.; Lee, E.; Kang, S.; Seo, J. Y.; Lee, S.; Park, B., HCMV-encoded US7 and US8 act as antagonists of innate immunity by distinctively targeting TLR-signaling pathways. Nat Commun 2019, 10, (1), 4670. [CrossRef]
- Krug, A.; French, A. R.; Barchet, W.; Fischer, J. A.; Dzionek, A.; Pingel, J. T.; Orihuela, M. M.; Akira, S.; Yokoyama, W. M.; Colonna, M., TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 2004, 21, (1), 107-19. [CrossRef]
- Loh, J.; Chu, D. T.; O'Guin, A. K.; Yokoyama, W. M.; Virgin, H. W. t., Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol 2005, 79, (1), 661-7. [CrossRef]
- Iversen, A. C.; Norris, P. S.; Ware, C. F.; Benedict, C. A., Human NK cells inhibit cytomegalovirus replication through a noncytolytic mechanism involving lymphotoxin-dependent induction of IFN-beta. J Immunol 2005, 175, (11), 7568-74.
- Wu, Z.; Sinzger, C.; Reichel, J. J.; Just, M.; Mertens, T., Natural killer cells can inhibit the transmission of human cytomegalovirus in cell culture by using mechanisms from innate and adaptive immune responses. J Virol 2015, 89, (5), 2906-17. [CrossRef]
- Arnon, T. I.; Achdout, H.; Levi, O.; Markel, G.; Saleh, N.; Katz, G.; Gazit, R.; Gonen-Gross, T.; Hanna, J.; Nahari, E.; Porgador, A.; Honigman, A.; Plachter, B.; Mevorach, D.; Wolf, D. G.; Mandelboim, O., Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat Immunol 2005, 6, (5), 515-23. [CrossRef]
- Rölle, A.; Mousavi-Jazi, M.; Eriksson, M.; Odeberg, J.; Söderberg-Nauclér, C.; Cosman, D.; Kärre, K.; Cerboni, C., Effects of human cytomegalovirus infection on ligands for the activating NKG2D receptor of NK cells: up-regulation of UL16-binding protein (ULBP)1 and ULBP2 is counteracted by the viral UL16 protein. J Immunol 2003, 171, (2), 902-8. [CrossRef]
- Eagle, R. A.; Traherne, J. A.; Hair, J. R.; Jafferji, I.; Trowsdale, J., ULBP6/RAET1L is an additional human NKG2D ligand. Eur J Immunol 2009, 39, (11), 3207-16.
- Wu, J.; Chalupny, N. J.; Manley, T. J.; Riddell, S. R.; Cosman, D.; Spies, T., Intracellular retention of the MHC class I-related chain B ligand of NKG2D by the human cytomegalovirus UL16 glycoprotein. J Immunol 2003, 170, (8), 4196-200. [CrossRef]
- Dassa, L.; Seidel, E.; Oiknine-Djian, E.; Yamin, R.; Wolf, D. G.; Le-Trilling, V. T. K.; Mandelboim, O., The Human Cytomegalovirus Protein UL148A Downregulates the NK Cell-Activating Ligand MICA To Avoid NK Cell Attack. J Virol 2018, 92, (17). [CrossRef]
- Stern-Ginossar, N.; Elefant, N.; Zimmermann, A.; Wolf, D. G.; Saleh, N.; Biton, M.; Horwitz, E.; Prokocimer, Z.; Prichard, M.; Hahn, G.; Goldman-Wohl, D.; Greenfield, C.; Yagel, S.; Hengel, H.; Altuvia, Y.; Margalit, H.; Mandelboim, O., Host immune system gene targeting by a viral miRNA. Science 2007, 317, (5836), 376-81. [CrossRef]
- Mena-Romo, J. D.; Pérez Romero, P.; Martín-Gandul, C.; Gentil, M.; Suárez-Artacho, G.; Lage, E.; Sánchez, M.; Cordero, E., CMV-specific T-cell immunity in solid organ transplant recipients at low risk of CMV infection. Chronology and applicability in preemptive therapy. J Infect 2017, 75, (4), 336-345. [CrossRef]
- Díaz, J.; Henao, J.; Rodelo, J.; García, A.; Arbeláez, M.; Jaimes, F., Incidence and risk factors for cytomegalovirus disease in a Colombian cohort of kidney transplant recipients. Transplant Proc 2014, 46, (1), 160-6. [CrossRef]
- Lu, L. L.; Suscovich, T. J.; Fortune, S. M.; Alter, G., Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol 2018, 18, (1), 46-61. [CrossRef]
- Lilleri, D.; Kabanova, A.; Lanzavecchia, A.; Gerna, G., Antibodies against neutralization epitopes of human cytomegalovirus gH/gL/pUL128-130-131 complex and virus spreading may correlate with virus control in vivo. J Clin Immunol 2012, 32, (6), 1324-31. [CrossRef]
- Gerna, G.; Percivalle, E.; Perez, L.; Lanzavecchia, A.; Lilleri, D., Monoclonal Antibodies to Different Components of the Human Cytomegalovirus (HCMV) Pentamer gH/gL/pUL128L and Trimer gH/gL/gO as well as Antibodies Elicited during Primary HCMV Infection Prevent Epithelial Cell Syncytium Formation. J Virol 2016, 90, (14), 6216-6223. [CrossRef]
- Gerna, G.; Sarasini, A.; Patrone, M.; Percivalle, E.; Fiorina, L.; Campanini, G.; Gallina, A.; Baldanti, F.; Revello, M. G., Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J Gen Virol 2008, 89, (Pt 4), 853-865. [CrossRef]
- Cui, X.; Cao, Z.; Wang, S.; Lee, R. B.; Wang, X.; Murata, H.; Adler, S. P.; McVoy, M. A.; Snapper, C. M., Novel trimeric human cytomegalovirus glycoprotein B elicits a high-titer neutralizing antibody response. Vaccine 2018, 36, (37), 5580-5590. [CrossRef]
- Elkington, R.; Walker, S.; Crough, T.; Menzies, M.; Tellam, J.; Bharadwaj, M.; Khanna, R., Ex vivo profiling of CD8+-T-cell responses to human cytomegalovirus reveals broad and multispecific reactivities in healthy virus carriers. J Virol 2003, 77, (9), 5226-40. [CrossRef]
- Crough, T.; Khanna, R., Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev 2009, 22, (1), 76-98, Table of Contents. [CrossRef]
- van Lier, R. A.; ten Berge, I. J.; Gamadia, L. E., Human CD8(+) T-cell differentiation in response to viruses. Nat Rev Immunol 2003, 3, (12), 931-9. [CrossRef]
- Day, E. K.; Carmichael, A. J.; ten Berge, I. J.; Waller, E. C.; Sissons, J. G.; Wills, M. R., Rapid CD8+ T cell repertoire focusing and selection of high-affinity clones into memory following primary infection with a persistent human virus: human cytomegalovirus. J Immunol 2007, 179, (5), 3203-13. [CrossRef]
- Ouyang, Q.; Wagner, W. M.; Wikby, A.; Walter, S.; Aubert, G.; Dodi, A. I.; Travers, P.; Pawelec, G., Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J Clin Immunol 2003, 23, (4), 247-57. [CrossRef]
- Khan, N.; Shariff, N.; Cobbold, M.; Bruton, R.; Ainsworth, J. A.; Sinclair, A. J.; Nayak, L.; Moss, P. A., Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol 2002, 169, (4), 1984-92. [CrossRef]
- Pourgheysari, B.; Khan, N.; Best, D.; Bruton, R.; Nayak, L.; Moss, P. A., The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol 2007, 81, (14), 7759-65. [CrossRef]
- Pawelec, G.; Akbar, A.; Caruso, C.; Solana, R.; Grubeck-Loebenstein, B.; Wikby, A., Human immunosenescence: is it infectious? Immunol Rev 2005, 205, 257-68.
- Chiu, Y. L.; Lin, C. H.; Sung, B. Y.; Chuang, Y. F.; Schneck, J. P.; Kern, F.; Pawelec, G.; Wang, G. C., Cytotoxic polyfunctionality maturation of cytomegalovirus-pp65-specific CD4 + and CD8 + T-cell responses in older adults positively correlates with response size. Sci Rep 2016, 6, 19227. [CrossRef]
- Costa-García, M.; Ataya, M.; Moraru, M.; Vilches, C.; López-Botet, M.; Muntasell, A., Human Cytomegalovirus Antigen Presentation by HLA-DR+ NKG2C+ Adaptive NK Cells Specifically Activates Polyfunctional Effector Memory CD4+ T Lymphocytes. Front Immunol 2019, 10, 687. [CrossRef]
- Aandahl, E. M.; Michaëlsson, J.; Moretto, W. J.; Hecht, F. M.; Nixon, D. F., Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol 2004, 78, (5), 2454-9. [CrossRef]
- Basta, S.; Bennink, J. R., A survival game of hide and seek: cytomegaloviruses and MHC class I antigen presentation pathways. Viral Immunol 2003, 16, (3), 231-42. [CrossRef]
- Ahn, K.; Angulo, A.; Ghazal, P.; Peterson, P. A.; Yang, Y.; Früh, K., Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc Natl Acad Sci U S A 1996, 93, (20), 10990-5. [CrossRef]
- Ahn, K.; Gruhler, A.; Galocha, B.; Jones, T. R.; Wiertz, E. J.; Ploegh, H. L.; Peterson, P. A.; Yang, Y.; Früh, K., The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity 1997, 6, (5), 613-21. [CrossRef]
- Jones, T. R.; Sun, L., Human cytomegalovirus US2 destabilizes major histocompatibility complex class I heavy chains. J Virol 1997, 71, (4), 2970-9. [CrossRef]
- Wiertz, E. J.; Jones, T. R.; Sun, L.; Bogyo, M.; Geuze, H. J.; Ploegh, H. L., The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell 1996, 84, (5), 769-79. [CrossRef]
- Tomazin, R.; Boname, J.; Hegde, N. R.; Lewinsohn, D. M.; Altschuler, Y.; Jones, T. R.; Cresswell, P.; Nelson, J. A.; Riddell, S. R.; Johnson, D. C., Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat Med 1999, 5, (9), 1039-43. [CrossRef]
- Hegde, N. R.; Tomazin, R. A.; Wisner, T. W.; Dunn, C.; Boname, J. M.; Lewinsohn, D. M.; Johnson, D. C., Inhibition of HLA-DR assembly, transport, and loading by human cytomegalovirus glycoprotein US3: a novel mechanism for evading major histocompatibility complex class II antigen presentation. J Virol 2002, 76, (21), 10929-41. [CrossRef]
- Lim, E. Y.; Jackson, S. E.; Wills, M. R., The CD4+ T Cell Response to Human Cytomegalovirus in Healthy and Immunocompromised People. Front Cell Infect Microbiol 2020, 10, 202.
- Miller, D. M.; Rahill, B. M.; Boss, J. M.; Lairmore, M. D.; Durbin, J. E.; Waldman, J. W.; Sedmak, D. D., Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J Exp Med 1998, 187, (5), 675-83. [CrossRef]
- Lee, A. W.; Wang, N.; Hornell, T. M.; Harding, J. J.; Deshpande, C.; Hertel, L.; Lacaille, V.; Pashine, A.; Macaubas, C.; Mocarski, E. S.; Mellins, E. D., Human cytomegalovirus decreases constitutive transcription of MHC class II genes in mature Langerhans cells by reducing CIITA transcript levels. Mol Immunol 2011, 48, (9-10), 1160-7. [CrossRef]
- Sandhu, P. K.; Buchkovich, N. J., Human Cytomegalovirus Decreases Major Histocompatibility Complex Class II by Regulating Class II Transactivator Transcript Levels in a Myeloid Cell Line. J Virol 2020, 94, (7). [CrossRef]
- Poole, E.; Neves, T. C.; Oliveira, M. T.; Sinclair, J.; da Silva, M. C. C., Human Cytomegalovirus Interleukin 10 Homologs: Facing the Immune System. Front Cell Infect Microbiol 2020, 10, 245. [CrossRef]
- Mittal, S. K.; Roche, P. A., Suppression of antigen presentation by IL-10. Curr Opin Immunol 2015, 34, 22-7. [CrossRef]
| Viruses associated with VN | Family of viruses |
|---|---|
| Herpes simplex virus | Alphaherpesvirinae |
| Varicella-zoster virus | |
| Human Cytomegalovirus | Betaherpesvirinae |
| Epstein-Barr virus | Gammaherpesvirinae |
| Influenza virus A | Non-herpesvirus family |
| Influenza virus B | |
| Adenoviruses | |
| Rubella virus | |
| Parainfluenza virus |
|
R
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
|
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





