1. Introduction
Nitrous oxide (N
2O) is an important greenhouse gas with a warming potential 273 times that of carbon dioxide (CO
2) [
1]. Montane peatlands are huge nitrogen (N) pools, but not a significant source of N
2O emissions due to phosphorus (P) limitation and low temperature [
2,
3,
4,
5,
6,
7]. However, the increase in P deposition and continuous warming caused by global changes will change the current status of peatlands, which may increase the possibility for peatlands to be a hot spot of N
2O emissions [
8,
9,
10]. Nevertheless, the underlying mechanism about the response of N
2O emissions and biotic/abiotic factors in peatlands to the interaction of P addition and warming is rarely studied yet.
Phosphorus is limited in 43% of the global land soil [
11,
12], and it is also a key factor for controlling N
2O emissions in peatlands [
13]. However, the continuous intensification of human activities and the availability of P increase, resulting in P may no longer being the limiting factor for ecological processes in peatlands [
14,
15,
16,
17]. Increase of P input will stimulate the activity of nitrifying and denitrifying bacteria, accelerate N turnover in soil, and promote soil N mineralization, which may produce more N
2O [
3,
18,
19,
20,
21]. Mori’s meta-analysis found that the addition of P in a P limited ecosystem will meet the microbial demand for P, leading to an boom of microorganisms [
22]. Li’s study showed that P addition can stimulate soil enzyme activities, which will promote peat decomposition, accelerate cycling of C and N, thereby providing energy and substrates for nitrifying and denitrifying microorganisms, and promoting N
2O emissions in boreal peatlands [
7,
23].
In addition to directly affecting microorganisms and enzyme activities, P addition can also desorb available C from soil surface, such as dissolved organic carbon (DOC), providing substrates available for microorganisms, stimulating nitrification and denitrification and promoting N
2O emissions [
24,
25]. Phosphorus addition even increases microbial activity in P saturated soil, which may increase N
2O emissions [
26]. This indicates that P addition does not only alleviate microbial P limitations, but also increases N
2O emissions through abiotic C transfer [
27].
Global peatlands are gradually shrinking in area due to climate change and anthropic disturbance [
28]. Under the current global warming, the sink function for the greenhouse gases CO
2 and N
2O in peatlands will be severely weakened [
29]. Warming will stimulate soil enzyme and microbial activity, accelerate peat decomposition, change plant composition, and directly or indirectly promote N
2O emissions from peatlands [
10,
30,
31]. Phosphate monoesters and orthophosphates were the dominant forms of P in wetlands [
32]. Warming will promote the transformation of stable P into unstable P [
33], accelerate soil P cycling, and break the P limitation in the surface soil, leading to a clear increase of P availability in wetlands [
34,
35].
Currently, we still know rather little on how N2O emissions respond to the joint environmental changes, P addition and warming, in wetlands, especially peatland ecosystems. To address this, we conducted a field monitoring experiment in Hani peatland, and combined with laboratory experiments, to investigate the effect of P addition, as well as the interaction with warming on peatland net N2O fluxes and the response of N2O fluxes to changes in biological and abiotic factors. We hypothesized that: 1) P addition would alleviate P limitation, stimulate soil enzyme activities, accelerate peat decomposition, provide reaction substrates and energy sources for the biochemical process of producing N2O, and lead to an increase in the net N2O fluxes in the peatland, and the more P added the more N2O emitted; and 2) warming would amplify the facilitative effect of P addition on N2O emissions to turn the peatland into a significant source of N2O.
2. Materials and Methods
2.1. Study site
Hani peatland (126°31′E, 42°13′N), located in the Changbai Mountains in Northeast China, with an altitude of 900 m and an average peat depth of 4 m. The mean annual temperature is 3℃ and the mean annual precipitation is 800 mm. The vegetation in the peatland is dominated by peat mosses (such as Sphagnum magellanicum Brig., S. imbricatum Hornsch. ex Russow etc.) and vascular plants (such as the shrub Betula ovalifolia Rupr. and the graminoid Carex lasiocarpa Ehrh.).
2.2. Experimental design
The field measurements were conducted at a 12-yr global change simulation experiment platform of Hani peatland during the 2019 growing season (May to September). We selected 20 plots (0.8 m×0.8 m), including 5 treatments {CK (control), P1 (5 kg P ha
-1 yr
-1), P2 (10 kg P ha
-1 yr
-1), P1W (P1+Warming), and P2W (P2+Warming)}, each with 4 replicates. We used NaH
2PO
4⋅2H
2O as the P fertilizer, dissolved it in 300 mL water, and evenly sprayed in plots once a month during the growing season. The same amount of distilled water was sprayed in CK plots. The warming was passively achieved by the open top chamber (OTC), which increased the air temperature about 0.6℃ during the growing season. For more information, see Bu, et al. [
36], Yi, Lu and Bu [
30] and Lu, Wu, Yi, Xu, Wang, Sundberg and Bu [
6].
2.3. Collection and analysis of N2O
The N
2O fluxes were measured by the static chamber-gas chromatography method. Gas samples (60 mL) were collected by a syringe from the static chamber at 10-minute intervals over a 30-minute period (0, 10, 20 and 30 minutes) and then measured by utilizing electron capture detector (ECD) of gas chromatography (GC system, Agilent 7980B, Santa Clara, USA) to calculate the concentration of N
2O, and determined the N
2O flux by establishing a regression equation for gas concentration and sampling time (more details in Yi, Lu and Bu [
30]).
2.4. Environmental factors
Environmental factors of each plot were measured while monitoring N
2O fluxes, including soil temperature at 5 cm and 20 cm below the moss surface, air temperature at 20 cm above the moss surface, soil humidity (SM), water table depth (WTD), and pH of peat water
in situ. The plant covers, including
Sphagnum, vascular plants, and then total plant cover, were estimated by visual inspection at the end of July 2019 [
37]. More details can be seen in Yi, Lu and Bu [
30].
2.5. Water and soil sampling
In August 2019, peat samples were collected from plots and analyzed for their physicochemical indicators, including total nitrogen (TN), total carbon (TC), total phosphorus (TP), and DOC [
30].
2.6. Determination of soil enzyme activities
β-D-glucosidase (BDG), N-acetyl-β-glucosaminidase (NAG) and phosphatase (PHO) are three types of hydrolytic enzymes highly related to the decomposition of organic carbon in peatlands [
38,
39,
40]. The former three enzymes can hydrolyze cellulose into glucose, decompose chitin and catalyze phosphate monoesters to increase C, N and P availability for microorganism and plants, respectively. Phenol oxidase (POX) can partially oxidize phenolic substances into simple organic compounds, and the activity of POX is crucial for the accumulation of organic matter in peatlands [
41,
42]. Microporous plate fluorescence method was used to determine the activities of the above three hydrolases and one oxidase in peat soil [
6,
43].
2.7. Statistical analysis
The data were checked by normality test before analysis, and logarithmic transformation was done when needed. The effects of P addition, and interaction of P addition and warming on mean N2O fluxes during the growing season were analyzed by repeated measures analysis of variance (ANOVA). We used two-way ANOVAs to analyze the effects of P addition and the interaction of P addition and warming on N2O fluxes and biotic/abiotic factors. One-way ANOVA was used to analyze the influences of different treatments on biotic/abiotic factors. Tukey’s HSD test was used to assess the differences among the treatments when ANOVAs have significance (P<0.05). Pearson correlation was used to assess the relationships between N2O fluxes and biotic/abiotic factors, as well as for the correlation between biotic and abiotic factors. We used principal component analysis (PCA) to assess the effects of different treatments on fluxes and biotic/abiotic factors. Structural equation modeling (SEM) was used to establish the relationships between all biotic/abiotic factors. All statistical analyses were performed in R 4.1.4 (Development Core Team, 2019).
3. Results
3.1. N2O fluxes
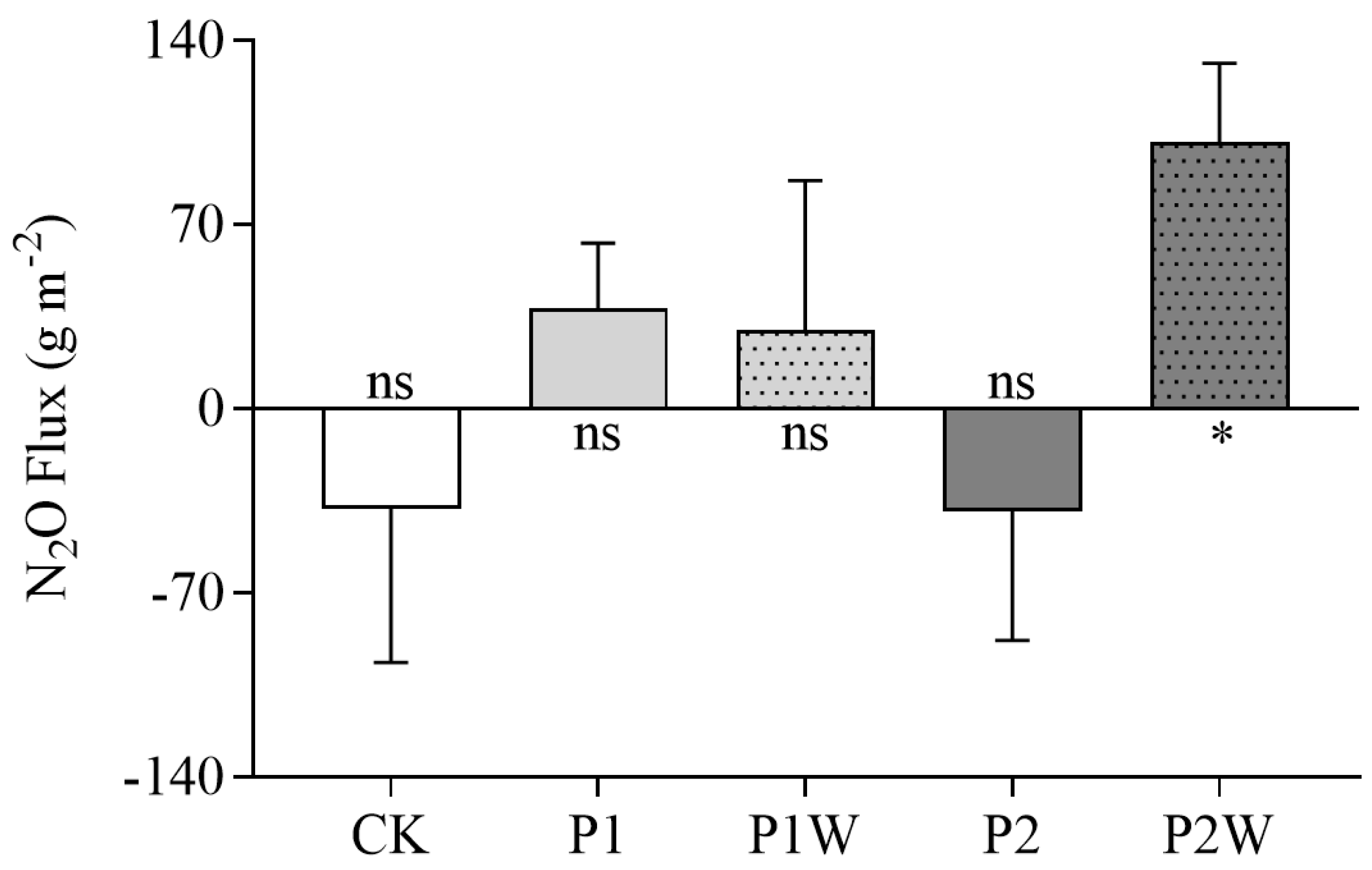
Both low and high levels of P addition alone did not have a significant impact on the N
2O source and sink function of Hani peatland (
Figure 1,
Table 2). The plots with low level of P addition tends to emit N
2O (38 ± 24 g m
-2,
t=1.548,
P=0.219), while those with high level of P addition tends to uptake N
2O (-39 ± 49 g m
-2,
t=0.763,
P=0.484). P addition with warming had a marginal effect on the cumulative N
2O flux during the growing season (
P=0.121,
Table 2), but had a significant impact on the mean N
2O flux (
P=0.010,
Table 2). Low level of P addition with warming did not have a significant impact on the source/sink function of N
2O in the peatland, while high levels of P addition under warming significantly promoted N
2O emissions (
t=3.361,
P=0.041) (
Figure 1), with a flux of 101 ± 30 g m
-2.
Table 1.
Direct and warming interaction-mediating effect of P addition on environmental factors in Hani peatland (Two-way ANOVA).
Table 1.
Direct and warming interaction-mediating effect of P addition on environmental factors in Hani peatland (Two-way ANOVA).
| Parameter |
Warming |
P addition |
Warming × P addition |
| F |
P |
F |
P |
F |
P |
| Tsoil, 5 cm |
1.748 |
0.206 |
0.325 |
0.727 |
0.178 |
0.679 |
| Tsoil, 20 cm |
5.396 |
0.034* |
0.3558 |
0.704 |
2.645 |
0.124 |
| WTD |
4.515 |
0.050* |
8.386 |
0.003*** |
5.671 |
0.031** |
| DOC |
0.052 |
0.822 |
26.020 |
0.000** |
1.834 |
0.196 |
| pH |
0.140 |
0.713 |
2.219 |
0.143 |
1.266 |
0.278 |
| TN |
6.763 |
0.020** |
1.621 |
0.230 |
0.394 |
0.539 |
| TC |
1.899 |
0.188 |
1.707 |
0.367 |
3.248 |
0.091* |
| TP |
19.812 |
0.000*** |
17.754 |
0.000*** |
1.759 |
0.205 |
| N:P |
11.052 |
0.005*** |
18.979 |
0.000*** |
0.356 |
0.560 |
| C:N |
5.396 |
0.034** |
2.256 |
0.139 |
0.056 |
0.816 |
Table 2.
Effect of warming and P addition on dynamic (repeated measurement two-way ANOVA) and cumulative N2O fluxes (two-way ANOVA) in the growth season of 2019 in Hani peatland.
Table 2.
Effect of warming and P addition on dynamic (repeated measurement two-way ANOVA) and cumulative N2O fluxes (two-way ANOVA) in the growth season of 2019 in Hani peatland.
| Treatment |
Dynamic fluxes |
Cumulative fluxes |
| df |
F |
P |
df |
F |
P |
| Warming |
1 |
0.181 |
0.674 |
1 |
3.456 |
0.082* |
| P addition |
2 |
0.003 |
0.997 |
2 |
0.453 |
0.718 |
| Warming × P addition |
1 |
7.658 |
0.010** |
1 |
2.603 |
0.121 |
3.2. Abiotic factors
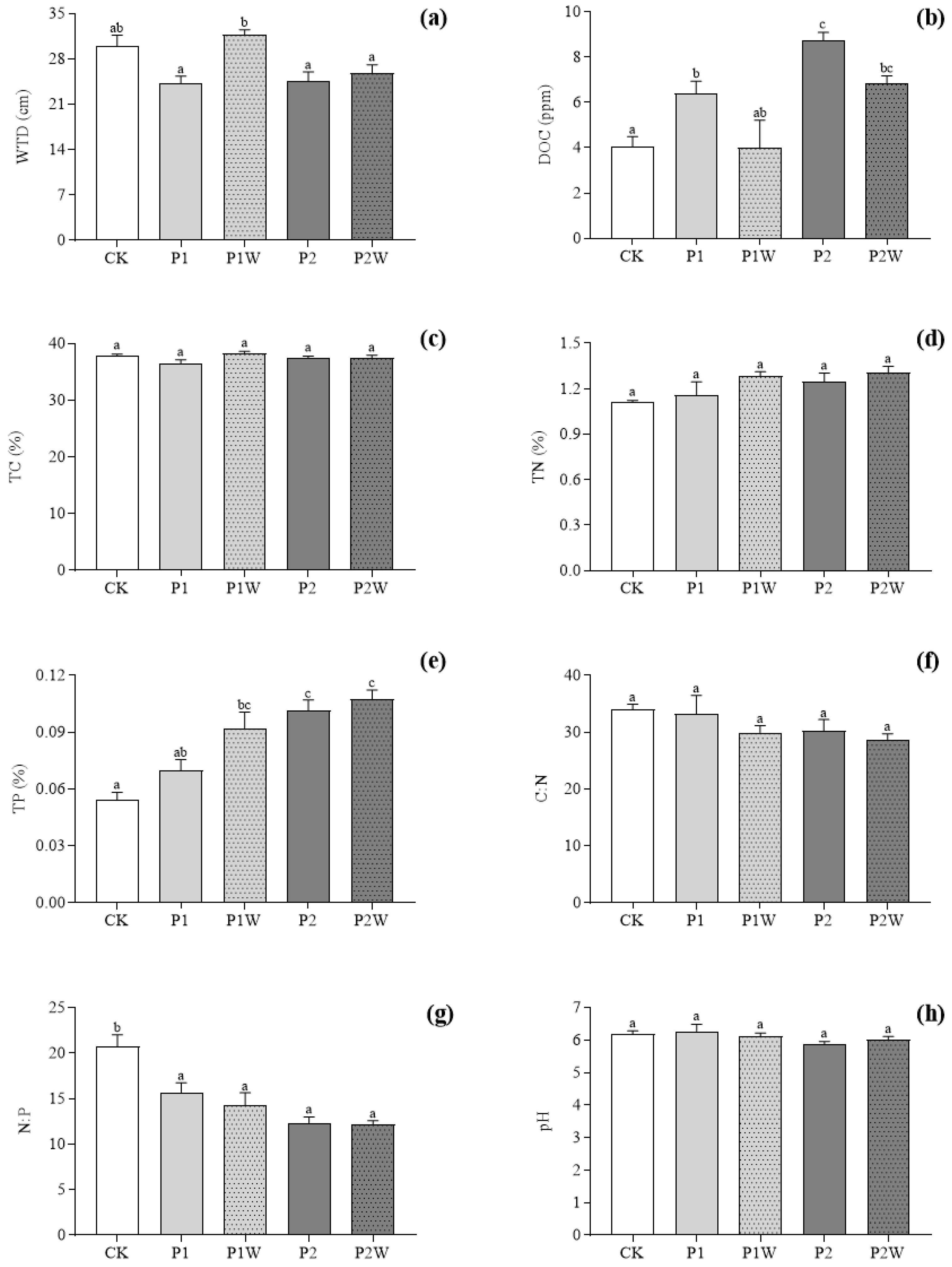
WTD was significantly reduced after P addition (
Table 1,
Figure 2a), but in P1W plots it was significantly higher than that in other treatment plots (
P<0.007) (
Figure 2a). Phosphorus addition alone significantly increased DOC concentration (P1:
P<0.08, P2:
P<0.001) (
Figure 2b), and DOC concentration in P2 plots was the highest among the five treatments (
P<0.09), approximately as twice as that of CK. DOC in P1W treatment was not significantly different from that in CK, while it in P2W plots was significantly higher than that in CK (
P < 0.05,
Figure 2b). There was no significant difference of TN concentration among different treatments, but TN in CK plots was the lowest and in P2W plots was the highest (
Figure 2d). Phosphorus addition singly or jointly with warming significantly increased TP concentration in peat water (
Table 1,
Figure 2e). In addition, there was no significant difference in TC concentration and pH among different treatments (
Figure 2c, h). There was no significant difference in N:P ratio and C:N ratio between different treatments but these two indices showed a decreasing trend with combined P addition and warming (
Figure 2f and g).
3.3. Vegetation
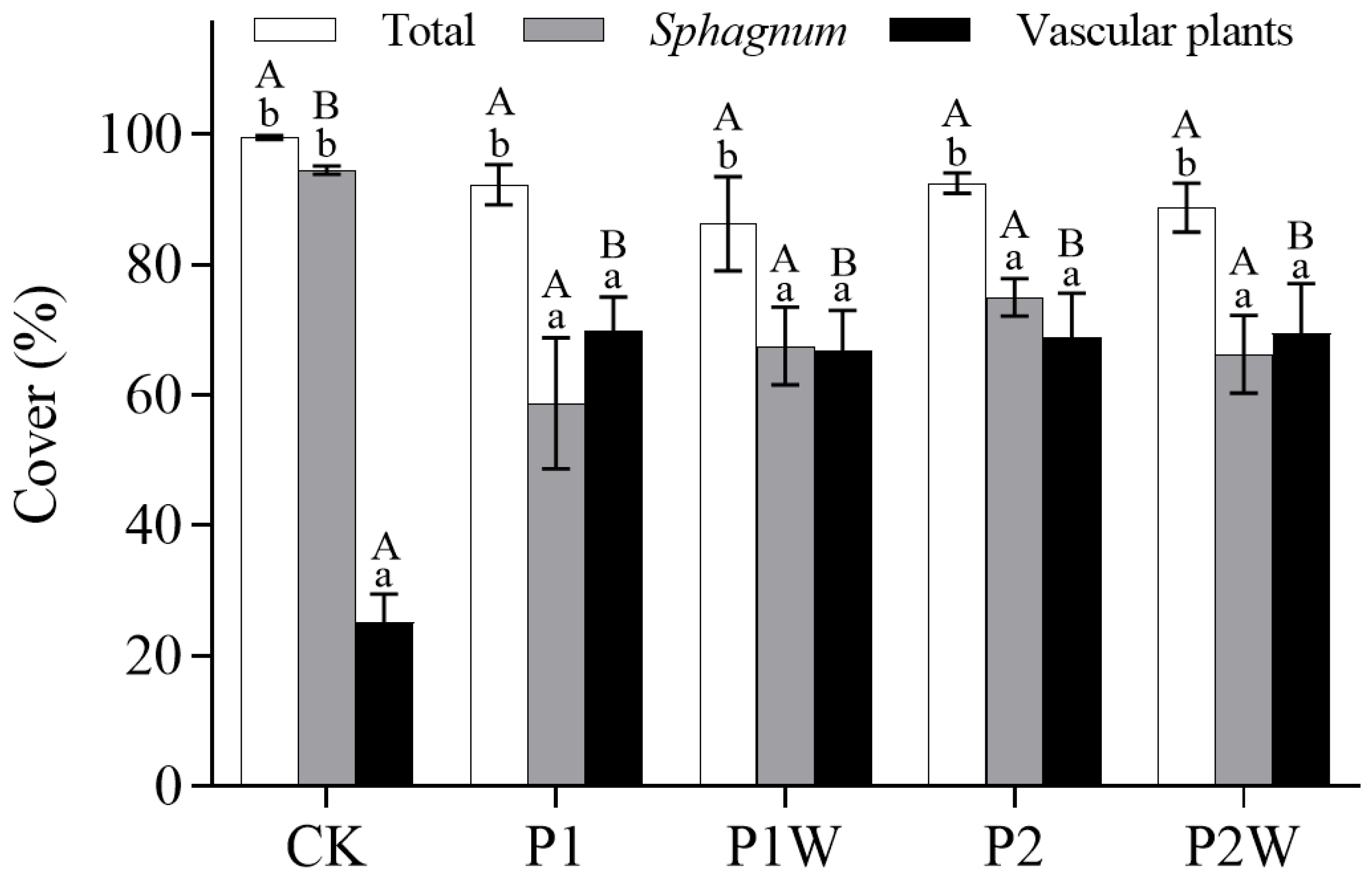
Phosphorus addition significantly reduced
Sphagnum cover but increased vascular plant cover (
P<0.009,
Figure 3). However, the interaction between warming and P addition did not significantly affect total plant cover (
P>0.05). No significant difference in total plant cover among treatments with P addition was found (
Figure 3).
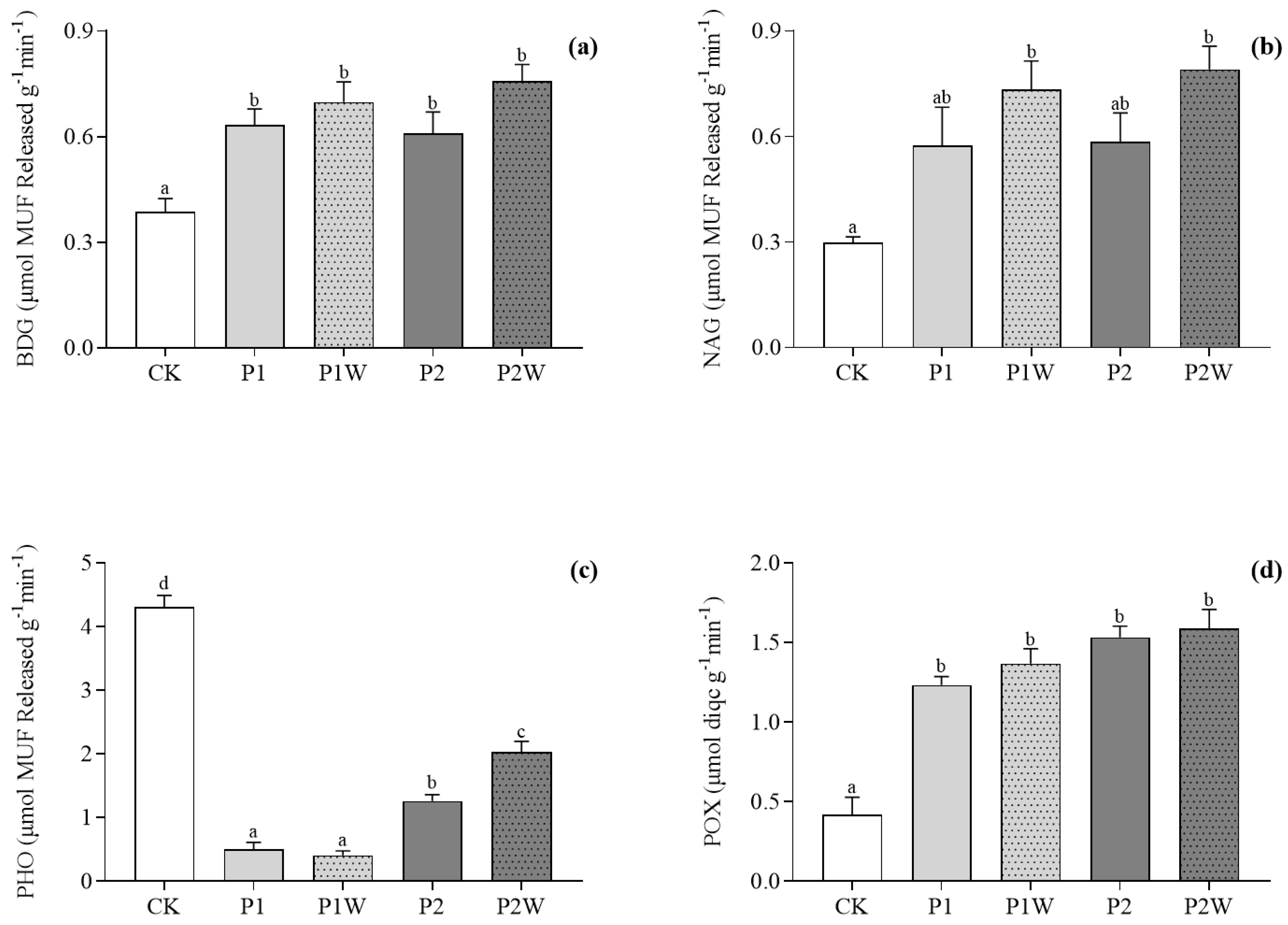
3.4. Enzyme activities
Almost all enzyme activities were promoted under warming condition. There was no significant difference in BDG, NAG and POX activities among the treatments with P addition and they were all significantly higher than CK (
P < 0.05 for all,
Figure 4a, b and d). The PHO activity in the treatment with P addition was significantly lower than that in CK (
P<0.001), but increased with P addition (
Figure 4c).
3.5. PCA and correlation
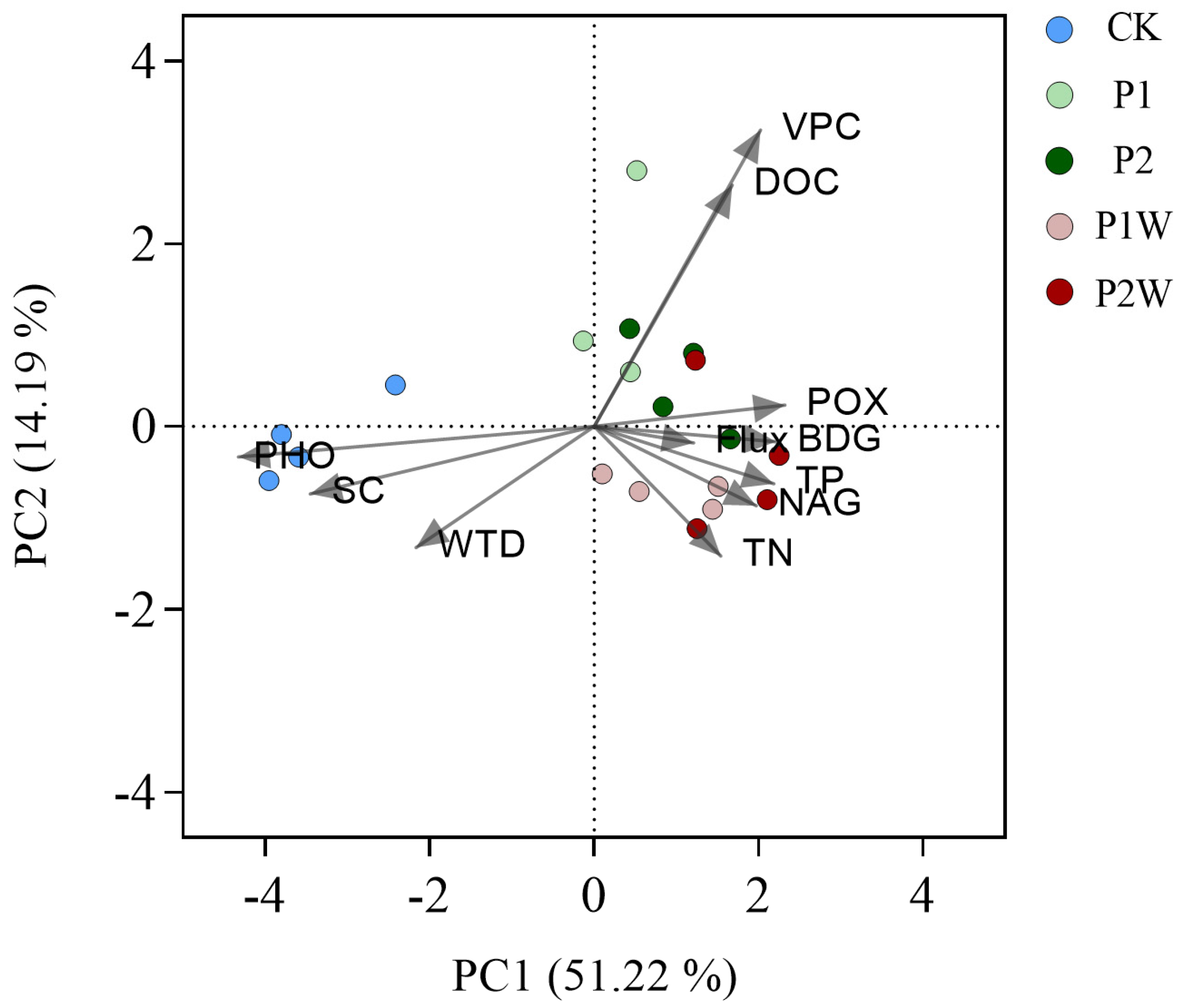
PCA (
Figure 5) showed that the cumulative percentage of variance of PC1 and PC2 reached 65.41%. The control, P addition alone (P1 and P2), and P addition under warming treatments (P1W and P2W) showed a clear cluster, indicating that P addition and warming significantly affected N
2O fluxes and biotic/abiotic factors in Hani peatland. DOC was correlated with vascular plant cover, and BDG, NAG, POX, TN and TP had high correlation (
Figure 5,
Table S1).
4. Discussion
4.1. Phosphorus addition and source/sink function of N2O
Hani peatland is a P limited ecosystem [
36]. Although P addition alone (P1 and P2) did not significantly affect the N
2O flux (
Table 2), it did not drive the peatland to be a significant source or sink of N
2O during the growing season (
Figure 1), the response of both N
2O flux characteristics and biotic and abiotic factors to the two levels of P addition were different. In CK plots, PHO activity was high (
Figure 4c), and it showed a negative correlation with TP, indicating that microorganisms need to decompose monophosphate esters to obtain available P in the P limited peatland [
7]. Since P is an essential nutrient for microbial growth and metabolism, the lack of P will restrict microbial and extracellular enzyme activities, leading to weak denitrification [
44,
45,
46]. This should be one of mechanisms to explain why N
2O fluxes were low in CK plots. Although P1 treatment was not a significant source of N
2O during the growing season, a significant N
2O emission in August was observed (
Figure S1,
P=0.05), indicating that long-term P addition alleviated the P restriction of denitrification, resulting in increased N
2O fluxes [
20,
21,
47], which proved our first hypothesis, to a certain extent.
Phosphorus addition significantly affected the activities of BDG and NAG (
Table 1), and the activity of these two enzymes was positively correlated with TP and DOC concentration (
Table S1), indicating that P addition promoted the decomposition of peat, which was consistent with our first hypothesis. The DOC concentration, BDG and NAG activities in P1 and P2 treatment plots was higher than those in CK, implying that more hydrolases are needed for microorganisms to decompose organic matter to meet their requirement for C and N [
48]. This process finally will provide substrates and energy sources for the biochemical processes of N
2O production and emissions [
49]. Previous studies have shown that the P addition increases the microbial communities in soil [
50,
51], thereby accelerating C mineralization, which provide energy sources for nitrification and denitrification [
52] to enhance N
2O emissions.
Phosphorus addition with high dose (P2 treatment) tended to result in a N
2O uptake, which may be due to the reduction of N
2O to N
2. Dissolved organic C (DOC) concentration in P2 plots was the highest among all treatments, which may indicate that the available C and N of P2 treatment were higher than that of P1. As mentioned above, higher nutrient availability and lower C:N [
53,
54] were more conducive to the reduction of N
2O to N
2 [
55,
56,
57,
58,
59]. As an electron donor in denitrification, the concentration of DOC is critical for the occurrence of denitrification, and higher DOC concentration can stimulate denitrification to consume N
2O, leading to N
2O uptake [
60]. Anderson, et al. [
61] found that the high level addition of P (250 kg P ha
-1) will greatly promote the mineralization of N in grassland ecosystems, thereby stimulating the occurrence of denitrification. O’Neill, et al. [
62] observed that the cumulative N
2O emissions in “low P soil” (with a P addition level of 30 kg P ha
-1 yr
-1) were significantly higher than in “high P soil” (45 kg P ha
-1 yr
-1), because the response of microorganisms to P addition was dose dependent. In addition, as we mentioned above, high level of P addition could also lead to the desorption and transfer of available C from the surface soil, which also promoted the denitrification.
4.2. Source/sink function of N2O under P addition with warming
In our previous study, we found that warming significantly promoted N
2O emissions in Hani peatland [
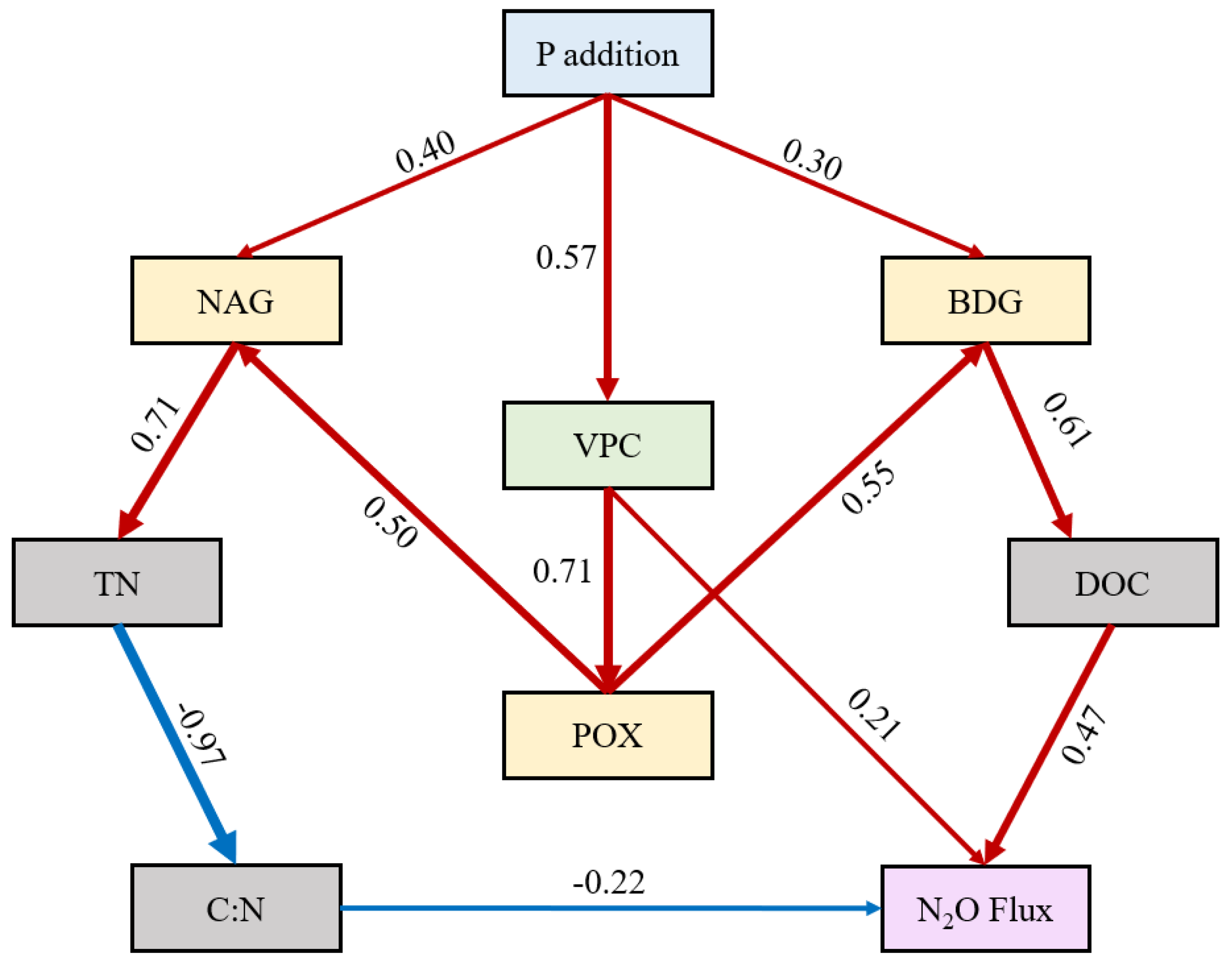
30]. In the current study, the interaction between warming and P addition greatly enhanced both the mean and cumulative N
2O fluxes, which was consistent with our second hypothesis. TN concentration of P1W and P2W was slightly higher than that under P addition alone. Although it is not statistically significant, the slightly more TN can alleviate the N limitation due to the strengthening of decomposition by warming. SEM shows that the addition of P causes an improvement in the activities of BDG and NAG, as well as an increase in DOC and TN concentration, ultimately resulting in an increase in N
2O fluxes. The activities of BDG and NAG are simultaneously affected by warming (
Figure S2), and the dual effect of warming and P addition on enzyme activities leads to further enhancement of hydrolase activities and more intense decomposition of organic matter. A close relation between TN concentration and N
2O fluxes showed in PCA further supports our above view. A study in a boreal peatland found a similar mechanism for warming effect on N
2O fluxes [
9], and a laboratory experiment demonstrated that warming could transform organic P into inorganic P, which may increase the availability of P in peat soil [
63], and further impact N
2O fluxes.
Compared to CK, POX activity clearly increased after P addition and this was related to the increase of vascular plant cover (
Figure 5,
Figure 6). The litter of vascular plants was rich in lignin, which need high POX activity to break down [
64]. According to the “enzyme latch” hypothesis, high POX activity may reduce phenolics to promote the activities of hydrolases, leading to increased peat decomposition and hence DOC concentration to provide an energy source for nitrification and denitrification [
6,
7,
42]. The SEM demonstrates the process how P addition affects N
2O fluxed by affecting vegetation succession, enzyme activity response and then organic matter decomposition. Zeng and Wang [
65] found that the effect of P addition on forest plant litter was greater than that on aboveground biomass. We believe that there may be a similar mechanism in peatlands, where the input of plant litter was important factor affecting N
2O production.
5. Conclusions
In summary, our study clarified the effect of long-term P addition, as well as its interaction with warming on peatland net N2O fluxes. We observed that although long-term P addition did not significantly alter the source/sink function of N2O in the peatland, it altered the plant composition of the peatland, stimulated enzyme activities, and promoted peat decomposition, resulting in a potential source of N2O emissions in the peatland. Warming amplified the effect of P addition to increase N2O emissions by stimulating enzyme activities and changing soil stoichiometry, and even turned the peatland into a significant source of N2O. Our results suggest that current and future warming and P deposition caused by global change will greatly promote peatland N2O emissions and seriously threaten the N pool function in peatlands.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Conceptualization, A.C. and Z.-J.B.; Methodology, Z.-J.B. and B.Y.; Software, B.Y. and F.L.; Validation, A.C.; Investigation, B.Y., F.L. and X.C; Resources, Z.-J.B.; Data curation, B.Y., J.Z. and J.-X.M.; Writing—original draft, B.Y.; Writing—review & editing, A.C. and Z.-J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was made possible by the support of the following funding to Bu Z.-J.: the National Nature Science Foundation of China, China, Grants No. 41871046, No. 41471043 and No. 42371050, and the Jilin Provincial Science and Technology Development, China, Projects No. 20210402032GH and No. 20180101002JC.
Data Availability Statement
Please contact the author of this article for data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC. Summary for Policymakers. In: Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [P.R. Shukla, J. Skea, R. Slade, A. Al Khourdajie, R. van Diemen, D. McCollum, M. Pathak, S. Some, P. Vyas, R. Fradera, M. Belkacemi, A. Hasija, G. Lisboa, S. Luz, J. Malley, (eds.)]; Cambridge University Press, Cambridge, UK and New York, NY, USA, 2022. [CrossRef]
- Limpens, J.; Heijmans, M.; Berendse, F. The nitrogen cycle in boreal peatlands. In: R.K. Wieder and D.H.Vitt (eds.): Boreal Peatland Ecosystem. Springer, Berlin 2006, 195-230. [CrossRef]
- Lund, M.; Christensen, T.; Mastepanov, M.; Lindroth, A.; Ström, L. Effects of N and P fertilization on the greenhouse gas exchange in two northern peatlands with contrasting N deposition rates. Biogeosciences 2009, 6, 2135–2144. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, J. Vegetation composition modulates the interaction of climate warming and elevated nitrogen deposition on nitrous oxide flux in a boreal peatland. Global Change Biology 2021, 27, 5588–5598. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, J.; Vogt, J.; Le, T.B. Warming reduces the increase in N2O emission under nitrogen fertilization in a boreal peatland. Science of the Total Environment 2019, 664, 72–78. [Google Scholar] [CrossRef]
- Lu, F.; Wu, J.; Yi, B.; Xu, Z.; Wang, M.; Sundberg, S.; Bu, Z.-J. Long-term phosphorus addition strongly weakens the carbon sink function of a temperate peatland. Ecosystems 2022, 1–16. [Google Scholar] [CrossRef]
- Li, T.; Bu, Z.; Liu, W.; Zhang, M.; Peng, C.; Zhu, Q.; Shi, S.; Wang, M. Weakening of the ‘enzymatic latch’ mechanism following long-term fertilization in a minerotrophic peatland. Soil Biology and Biochemistry 2019, 136, 1–41. [Google Scholar] [CrossRef]
- Wu, J.; Lu, Y.; Wang, H.; Li, G. Effects of nitrogen and phosphorus additions on CH4 flux in wet meadow of Qinghai-Tibet Plateau. Science of the Total Environment 2023, 887. [Google Scholar] [CrossRef]
- Cui, Q.; Song, C.; Wang, X.; Shi, F.; Yu, X.; Tan, W. Effects of warming on N2O fluxes in a boreal peatland of permafrost region, Northeast China. Science of the Total Environment 2018, 616, 427–434. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, J.; Vogt, J.; Thuong Ba, L.; Yuan, T. Combination of warming and vegetation composition change strengthens the environmental controls on N2O fluxes in a boreal peatland. Atmosphere 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Du, E.; Terrer, C.; Pellegrini, A.F.A.; Ahlstrom, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nature Geoscience 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Liimatainen, M.; Voigt, C.; Martikainen, P.J.; Hytonen, J.; Regina, K.; Oskarsson, H.; Maljanen, M. Factors controlling nitrous oxide emissions from managed northern peat soils with low carbon to nitrogen ratio. Soil Biology & Biochemistry 2018, 122, 186–195. [Google Scholar] [CrossRef]
- Säurich, A.; Tiemeyer, B.; Dettmann, U.; Don, A. How do sand addition, soil moisture and nutrient status influence greenhouse gas fluxes from drained organic soils? Soil Biology and Biochemistry 2019, 135, 71–84. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D. Human alteration of the global nitrogen cycle: Sources and consequences. Ecological Applications 1997, 7, 737–750. [Google Scholar] [CrossRef]
- Frolking, S.; Talbot, J.; Jones, M.C.; Treat, C.C.; Kauffman, J.B.; Tuittila, E.-S.; Roulet, N. Peatlands in the Earth’s 21st century climate system. Environmental Reviews 2011, 19, 371–396. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Tian, D.; Liu, Y.; Dong, J. Nitrogen significantly affected N cycling functional gene abundances compared with phosphorus and drought in an alpine meadow. Agronomy-Basel 2023, 13. [Google Scholar] [CrossRef]
- Wang, G.; Liang, Y.; Ren, F.; Yang, X.; Mi, Z.; Gao, Y.; George, T.S.; Zhang, Z. Greenhouse gas emissions from the Tibetan alpine grassland: effects of nitrogen and phosphorus addition. Sustainability 2018, 10, 4454. [Google Scholar] [CrossRef]
- Sundareshwar, P.; Morris, J.; Koepfler, E.; Fornwalt, B. Phosphorus limitation of coastal ecosystem processes. Science 2003, 299, 563–565. [Google Scholar] [CrossRef]
- Mori, T.; Ohta, S.; Ishizuka, S.; Konda, R.; Wicaksono, A.; Heriyanto, J.; Hardjono, A. Effects of phosphorus addition with and without ammonium, nitrate, or glucose on N2O and NO emissions from soil sampled under Acacia mangium plantation and incubated at 100% of the water-filled pore space. Biology and Fertility of Soils 2013, 49, 13–21. [Google Scholar] [CrossRef]
- Mori, T.; Ohta, S.; Ishizuka, S.; Konda, R.; Wicaksono, A.; Heriyanto, J.; Hardjono, A. Effects of phosphorus addition on N2O and NO emissions from soils of an Acacia mangium plantation. Soil Science and Plant Nutrition 2010, 56, 782–788. [Google Scholar] [CrossRef]
- Mori, T. The ratio of β-1,4-glucosidase activity to phosphomonoesterase activity remains low in phosphorus-fertilized tropical soils: A meta-analysis. Applied Soil Ecology 2022, 180, 1–3. [Google Scholar] [CrossRef]
- Li, T.; Ge, L.; Huang, J.; Yuan, X.; Peng, C.; Wang, S.; Bu, Z.; Zhu, Q.; Wang, Z.; Liu, W. Contrasting responses of soil exoenzymatic interactions and the dissociated carbon transformation to short-and long-term drainage in a minerotrophic peatland. Geoderma 2020, 377, 1–8. [Google Scholar] [CrossRef]
- Mori, T. P fertilization experiments require reinterpretation: Abiotic elevation of available C and N could influence microbial processes in soil. Applied Soil Ecology 2023, 189, 1–3. [Google Scholar] [CrossRef]
- Spohn, M.; Diakova, K.; Aburto, F.; Doetterl, S.; Borovec, J. Sorption and desorption of organic matter in soils as affected by phosphate. Geoderma 2022, 405. [Google Scholar] [CrossRef]
- Spohn, M.; Schleuss, P.-M. Addition of inorganic phosphorus to soil leads to desorption of organic compounds and thus to increased soil respiration. Soil Biology & Biochemistry 2019, 130, 220–226. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.; Lu, X.; Xu, H.; Chen, D.; Li, Y.; Zhou, Z.; Li, Y.; Ma, S.; Yakov, K. Long-term phosphorus addition alleviates CO2 and N2O emissions via altering soil microbial functions in secondary rather primary tropical forests*. Environmental Pollution 2023, 323. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Mao, D.; Wang, Z.; Yu, Z.; Xu, X.; Huang, Y.; Xi, Y.; Luo, L.; Jia, M.; Song, K.; et al. China’s wetland soil organic carbon pool: New estimation on pool size, change, and trajectory. Global Change Biology 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Jia, G.; Xu, X. Weakening greenhouse gas sink of pristine wetlands under warming. Nature Climate Change 2023, 13, 462–469. [Google Scholar] [CrossRef]
- Yi, B.; Lu, F.; Bu, Z.-J. Nitrogen addition turns a temperate peatland from a near-zero source into a strong sink of nitrous oxide. Plant, Soil and Environment 2022, 68, 49–58. [Google Scholar] [CrossRef]
- Yan, W.; Zhong, Y.; Shangguan, Z.; Torn, M.S. Response of soil greenhouse gas fluxes to warming: A global meta-analysis of field studies. Geoderma 2022, 419. [Google Scholar] [CrossRef]
- Teng, C.-Y.; Shen, J.-G.; Wang, Z.; Wang, H.; Li, H.-Y.; Zhang, Z.-J. Effect of simulated climate warming on microbial community and phosphorus forms in wetland soils. Huanjing kexue 2017, 38, 3000–3009. [Google Scholar] [CrossRef]
- Cao, Z.; Xu, L.; Zong, N.; Zhang, J.; He, N. Impacts of climate warming on soil phosphorus forms and transformation in a Tibetan alpine meadow. Journal of Soil Science and Plant Nutrition 2022, 22, 2545–2556. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Li, J.; Xu, X. Characteristics of phosphorus cycling between sediment of the wetlands and water under warming in simulated wetland habitat. Wetland Science 2011, 9, 345–354. [Google Scholar]
- Lie, Z.; Zhou, G.; Huang, W.; Kadowaki, K.; Tissue, D.T.; Ya, J.; Penuelas, J.; Sardans, J.; Li, Y.; Liu, S.; et al. Warming drives sustained plant phosphorus demand in a humid tropical forest. Global Change Biology 2022, 28, 4085–4096. [Google Scholar] [CrossRef] [PubMed]
- Bu, Z.-J.; Rydin, H.; Chen, X. Direct and interaction-mediated effects of environmental changes on peatland bryophytes. Oecologia 2011, 166, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bu, Z.; Ma, J.; Yuan, M.; Feng, L.; Liu, S. Comparative study on the response of deciduous and evergreen shrubs to nitrogen and phosphorus input in Hani Peatland of Changbai Mountains. Chinese Journal of Ecology 2015, 34, 2711–2719. [Google Scholar] [CrossRef]
- Kuijper, B.; Pen, I.; Weissing, F.J. A guide to sexual selection theory. Annual Review of Ecology, Evolution, Systematics 2012, 43, 287–311. [Google Scholar] [CrossRef]
- Freeman, C.; Liska, G.; Ostle, N.J.; Jones, S.; Lock, M. The use of fluorogenic substrates for measuring enzyme activity in peatlands. Plant and Soil 1995, 175, 147–152. [Google Scholar] [CrossRef]
- Shackle, V.; Freeman, C.; Reynolds, B. Carbon supply and the regulation of enzyme activity in constructed wetlands. Soil Biology and Biochemistry 2000, 32, 1935–1940. [Google Scholar] [CrossRef]
- Freeman, C.; Ostle, N.J.; Fenner, N.; Kang, H. A regulatory role for phenol oxidase during decomposition in peatlands. Soil Biology & Biochemistry 2004, 36, 1663–1667. [Google Scholar] [CrossRef]
- Freeman, C.; Ostle, N.; Kang, H. An enzymic’latch’on a global carbon store. Nature 2001, 409, 149–149. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biology & Biochemistry 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Camenzind, T.; Homeier, J.; Dietrich, K.; Hempel, S.; Hertel, D.; Krohn, A.; Leuschner, C.; Oelmann, Y.; Olsson, P.A.; Suarez, J.P.; et al. Opposing effects of nitrogen versus phosphorus additions on mycorrhizal fungal abundance along an elevational gradient in tropical montane forests. Soil Biology & Biochemistry 2016, 94, 37–47. [Google Scholar] [CrossRef]
- Camenzind, T.; Haettenschwiler, S.; Treseder, K.K.; Lehmann, A.; Rillig, M.C. Nutrient limitation of soil microbial processes in tropical forests. Ecological Monographs 2018, 88, 4–21. [Google Scholar] [CrossRef]
- Mehnaz, K.R.; Corneo, P.E.; Keitel, C.; Dijkstra, F.A. Carbon and phosphorus addition effects on microbial carbon use efficiency, soil organic matter priming, gross nitrogen mineralization and nitrous oxide emission from soil. Soil Biology & Biochemistry 2019, 134, 175–186. [Google Scholar] [CrossRef]
- Baral, B.R.; Kuyper, T.W.; Van Groenigen, J.W. Liebig’s law of the minimum applied to a greenhouse gas: alleviation of P-limitation reduces soil N2O emission. Plant and Soil 2014, 374, 539–548. [Google Scholar] [CrossRef]
- Allison, S.D.; Weintraub, M.N.; Gartner, T.B.; Waldrop, M.P. Evolutionary-Economic Principles as Regulators of Soil Enzyme Production and Ecosystem Function. In Soil Enzymology, Shukla, G., Varma, A., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2011; pp. 229–243. [Google Scholar] [CrossRef]
- Kang, H.J.; Freeman, C.; Park, S.S.; Chun, J. N-Acetylglucosaminidase activities in wetlands: a global survey. Hydrobiologia 2005, 532, 103–110. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Wang, F.; Zou, B.; Chen, Y.; Zhao, J.; Mo, Q.; Li, Y.; Li, X.; Xia, H. Effects of nitrogen and phosphorus addition on soil microbial community in a secondary tropical forest of China. Biology and Fertility of Soils 2015, 51, 207–215. [Google Scholar] [CrossRef]
- Liu, L.; Gundersen, P.; Zhang, T.; Mo, J. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biology & Biochemistry 2012, 44, 31–38. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecology Letters 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Prescott, C.E.; Chappell, H.N.; Vesterdal, L. Nitrogen turnover in forest floors of coastal Douglas-fir at sites differing in soil nitrogen capital. Ecology 2000, 81, 1878–1886. [Google Scholar] [CrossRef]
- Yao, Z.; Yan, G.; Ma, L.; Wang, Y.; Zhang, H.; Zheng, X.; Wang, R.; Liu, C.; Wang, Y.; Zhu, B.; et al. Soil C/N ratio is the dominant control of annual N2O fluxes from organic soils of natural and semi-natural ecosystems. Agricultural and Forest Meteorology 2022, 327, 109198. [Google Scholar] [CrossRef]
- Wassen, M.J.; Veterink, H.; Deswart, E. Nutrient concentrations in mire vegetation as a measure of nutrient limitation in mire ecosystems. Journal of Vegetation Science 1995, 6, 5–16. [Google Scholar] [CrossRef]
- Chapuis-Lardy, L.; Wrage, N.; Metay, A.; CHOTTE, J.L.; Bernoux, M. Soils, a sink for N2O? A review. Global Change Biology 2007, 13, 1–17. [Google Scholar] [CrossRef]
- Ward, S.E.; Ostle, N.J.; Oakley, S.; Quirk, H.; Henrys, P.A.; Bardgett, R.D. Warming effects on greenhouse gas fluxes in peatlands are modulated by vegetation composition. Ecology Letters 2013, 16, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Buchen, C.; Roobroeck, D.; Augustin, J.; Behrendt, U.; Boeckx, P.; Ulrich, A. High N2O consumption potential of weakly disturbed fen mires with dissimilar denitrifier community structure. Soil Biology and Biochemistry 2019, 130, 63–72. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, X.; Li, Y.; Yu, J.; Ding, H.; Sveen, T.R.; Zhang, Y. Soil moisture determines nitrous oxide emission and uptake. Science of The Total Environment 2022, 822, 153566. [Google Scholar] [CrossRef]
- Hu, J.; Inglett, K.S.; Wright, A.L.; Reddy, K.R. Nitrous oxide production and reduction in seasonally-flooded cultivated peatland soils. Soil Science Society of America Journal 2016, 80, 783–793. [Google Scholar] [CrossRef]
- Anderson, F.C.; Clough, T.J.; Condron, L.M.; Richards, K.G.; Rousset, C. Nitrous oxide responses to long-term phosphorus application on pasture soil. New Zealand Journal of Agricultural Research 2022. [Google Scholar] [CrossRef]
- O’Neill, R.M.; Girkin, N.T.; Krol, D.J.; Wall, D.P.; Brennan, F.P.; Lanigan, G.J.; Renou-Wilson, F.; Muller, C.; Richards, K.G. The effect of carbon availability on N2O emissions is moderated by soil phosphorus. Soil Biology & Biochemistry 2020, 142. [Google Scholar] [CrossRef]
- Wang, G.; Yu, X.; Bao, K.; Xing, W.; Gao, C.; Lin, Q.; Lu, X. Effect of fire on phosphorus forms in Sphagnum moss and peat soils of ombrotrophic bogs. Chemosphere 2015, 119, 1329–1334. [Google Scholar] [CrossRef]
- Boot, C.M.; Hall, E.K.; Denef, K.; Baron, J.S. Long-term reactive nitrogen loading alters soil carbon and microbial community properties in a subalpine forest ecosystem. Soil Biology and Biochemistry 2016, 92, 211–220. [Google Scholar] [CrossRef]
- Zeng, W.J.; Wang, W. Combination of nitrogen and phosphorus fertilization enhance ecosystem carbon sequestration in a nitrogen-limited temperate plantation of Northern China. Forest Ecology and Management 2015, 341, 59–66. [Google Scholar] [CrossRef]
Figure 1.
Cumulative N2O fluxes (mean ± SEM, n = 4) in Hani peatland in the growing season of 2019. CK, control; P1, P addition with 5 kg P ha-1 yr-1; P2, P addition with 10 kg P ha-1 yr-1; P1W, P1 + warming; P2W, P2 + warming. Asterisks denote N2O flux significantly different from zero. *P < 0.05; ns, no significant difference.
Figure 1.
Cumulative N2O fluxes (mean ± SEM, n = 4) in Hani peatland in the growing season of 2019. CK, control; P1, P addition with 5 kg P ha-1 yr-1; P2, P addition with 10 kg P ha-1 yr-1; P1W, P1 + warming; P2W, P2 + warming. Asterisks denote N2O flux significantly different from zero. *P < 0.05; ns, no significant difference.
Figure 2.
Abiotic factors among the different treatments in Hani peatland in 2019 (mean ± SEM, n = 4). (a) WTD; (b) DOC; (c) TC; (d) TN; (e) TP; (f) C:N; (g) N:P; (h) pH. CK, control; P1, P addition with 5 kg P ha-1 yr-1; P2, P addition with 10 kg P ha-1 yr-1; P1W, P1 + warming; P2W, P2 + warming. WTD, water table depth; DOC, dissolved organic carbon; TC, total carbon; TN, total nitrogen; TP, total phosphorus. Different lowercase letters represent significant differences (P < 0.05) between the treatments.
Figure 2.
Abiotic factors among the different treatments in Hani peatland in 2019 (mean ± SEM, n = 4). (a) WTD; (b) DOC; (c) TC; (d) TN; (e) TP; (f) C:N; (g) N:P; (h) pH. CK, control; P1, P addition with 5 kg P ha-1 yr-1; P2, P addition with 10 kg P ha-1 yr-1; P1W, P1 + warming; P2W, P2 + warming. WTD, water table depth; DOC, dissolved organic carbon; TC, total carbon; TN, total nitrogen; TP, total phosphorus. Different lowercase letters represent significant differences (P < 0.05) between the treatments.
Figure 3.
Plants cover (mean ± SEM, n = 4). CK, control; P1, P addition with 5 kg P ha-1 yr-1; P2, P addition with 10 kg P ha-1 yr-1; P1W, P1 + warming; P2W, P2 + warming. Different capital letters represent significant differences between different treatments of the same plant cover, and different lower-case letters represent significant differences between different plants under the same treatment. P<0.05.
Figure 3.
Plants cover (mean ± SEM, n = 4). CK, control; P1, P addition with 5 kg P ha-1 yr-1; P2, P addition with 10 kg P ha-1 yr-1; P1W, P1 + warming; P2W, P2 + warming. Different capital letters represent significant differences between different treatments of the same plant cover, and different lower-case letters represent significant differences between different plants under the same treatment. P<0.05.
Figure 4.
Soil enzyme activities (mean ± SEM, n = 4). (a) BDG; (b) NAG; (c) PHO; (d) POX. CK, control; P1, P addition with 5 kg P ha-1 yr-1; P2, P addition with 10 kg P ha-1 yr-1; P1W, P1 + warming; P2W, P2 + warming. BDG, β-D-glucosidase activity; NAG, N-acetyl-β-glucosaminidase activity; PHO, phosphatase activity; POX, phenol oxidase activity. Different lowercase letters represent significant differences between treatments. P<0.05.
Figure 4.
Soil enzyme activities (mean ± SEM, n = 4). (a) BDG; (b) NAG; (c) PHO; (d) POX. CK, control; P1, P addition with 5 kg P ha-1 yr-1; P2, P addition with 10 kg P ha-1 yr-1; P1W, P1 + warming; P2W, P2 + warming. BDG, β-D-glucosidase activity; NAG, N-acetyl-β-glucosaminidase activity; PHO, phosphatase activity; POX, phenol oxidase activity. Different lowercase letters represent significant differences between treatments. P<0.05.
Figure 5.
Principal component analysis. DOC, dissolved organic carbon; TP, total phosphorus; TN, total nitrogen; WTD, water table depth; VPC, vascular plant cover; SC, Sphagnum cover; BDG, β-D-glucosidase; NAG, N-acetyl-β-glucosaminidase, PHO, phosphatase, POX, phenol oxidase.
Figure 5.
Principal component analysis. DOC, dissolved organic carbon; TP, total phosphorus; TN, total nitrogen; WTD, water table depth; VPC, vascular plant cover; SC, Sphagnum cover; BDG, β-D-glucosidase; NAG, N-acetyl-β-glucosaminidase, PHO, phosphatase, POX, phenol oxidase.
Figure 6.
Structural equation model between P addition and parameters (Chi-square = 0.494, CFI = 0.990, TLI = 0.984, AIC = 999.11, BIC = 965.01, RMSEA = 0.049, SRMR = 0.007). The red arrow represents positive correlation and the blue arrow represents negative correlation, the numbers on the arrows represent the standardized parameter estimates. DOC, dissolved organic carbon; VPC, vascular plant cover; BDG, β-D-glucosidase activity; NAG, N-acetyl-β-glucosaminidase activity; POX, phenol oxidase activity; TN, total nitrogen.
Figure 6.
Structural equation model between P addition and parameters (Chi-square = 0.494, CFI = 0.990, TLI = 0.984, AIC = 999.11, BIC = 965.01, RMSEA = 0.049, SRMR = 0.007). The red arrow represents positive correlation and the blue arrow represents negative correlation, the numbers on the arrows represent the standardized parameter estimates. DOC, dissolved organic carbon; VPC, vascular plant cover; BDG, β-D-glucosidase activity; NAG, N-acetyl-β-glucosaminidase activity; POX, phenol oxidase activity; TN, total nitrogen.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).