1. Introduction
Thymus is an herbaceous perennials or subshrubs of the Lamiaceae family and is widely distributed worldwide. The name of thyme was obtained because it has rich aromatic aroma during flowering [
1]. Thyme species are important aromatic and medicinal plants that have been used as traditional medicine for thousands of years in the Mediterranean basin [
2]. Thyme essential oils (TEOs) have strong anti-inflammatory, antioxidant, antimicrobial, and antifungal functions [
3,
4,
5,
6]. The Chinese native thyme species
Thymus quinquecostatus is also widely used in folk medicine for the treatment of stroke, cold, dyspepsia, toothache, acute gastroenteritis, hypertension, chronic eczema, and other diseases [
2]. In 1986, the World Health Organization identified thyme as an important medicinal plant and included it in the list of medicinal plants. In the
European Pharmacopoeia (published in 2001), it was stipulated that the sum of thymol and carvacrol contents must be greater than 40% of the total essential oil contents from dried plant materials in order to be used as medicinal purposes [
7]. Essential oils are thought to be safe and are commonly employed in medications, agricultural products and food preservation agents because of high volatility, biodegradability, and ephemeral characteristics [
8].
Chemical type is used to describe the differences, diversity, and complexity of secondary metabolites between individuals or even populations, and TEOs have significant differences in chemical composition, exhibiting chemical polymorphism [
9,
10]. The main components of TEOs belong to the chemical classes of terpenoids, terpene alcohols, phenolic derivatives, ketones, aldehydes, ethers, and esters [
11]. Kim et al. [
12] evaluated compositional analysis of essential oils from six commercial species of
T. vulgaris and three
T.quinquecostatus cultivars. The results showed that 'Lemon', 'Silver', and 'Odae' belong to the thymol type, with thymol contents of 43.91%, 66.24%, and 30.54%, respectively. 'Creeping', 'Golden', 'Orange', and 'Wolchul' belong to the geraniol type, with geraniol contents of 29.57%, 65.99%, 44.70%, and 42.94%, respectively. 'Carpet' and 'Jiri' belong to the linalool type, with linalool contents of 48.16% and 47.89% respectively. In addition, Trendafilova et al. [
13] found new chemotypes of
T. atticus (caryophyllene oxide/β-caryophyllene),
T. leucotrichus (β-caryophyllene/elemol/germacrene D) and
T. striatus (β-caryophyllene/germacrene D/caryophyllene oxide).
The classification of chemical types is usually determined by the proportion of the component in the essential oil [
14]. And the proportion of the component in the essential oil determine the function of essential oils. However, the wide range of chemical compounds display various biological activity because of their different mechanism. Thymol can help maintain quality and reduce the decay of fruits and vegetables by inhibiting microbial growth during postharvest storage, the antimicrobial effect has been mainly linked to the reduction of ergosterol, resulting in the disruption of cell membrane integrity of microorganisms [
15,
16,
17]. Carvacrol is a biocidal product leading to bacterial membrane perturbations, in turn, it can result in the leakage of intracellular ATP and potassium ions and ultimately, cell death [
18]. Thymol, γ-terpinene, and p-cymene exhibit potent antioxidant, antibacterial, as well as ability to reduce cellular glucose intake and block lactate synthesis [
3,
4,
5]. The α-terpineol has anticonvulsant properties, can attenuate mechanical hypernociception and inflammatory responses, and has effects on the heart and stomach [
19]. The antiviral, anti-inflammatory, antioxidant, and antibacterial properties of 1,8-cineole are well known [
20]. The blood-brain barrier permeability is increased by borneol, which also possesses anti-inflammatory and antioxidant properties [
6]. A sesquiterpene with insecticidal action is germacrene-D [
21]. Another sesquiterpene with significant pharmacological action is β-Caryophyllene [
22]. By removing duplicate information, integrated multivariate methods can efficiently characterize EO chemical signatures [
23].
In this study, we used various thyme samples collected from Europe and China native species to extract the essential oils, we categorized these samples chemically by measuring the volatile components using GC/MS. Then, we assessed the chemical profiles of eight TEOs from various thyme species using a multidimensional assessment method including correlation, dendrogram, principal component analysis (PCA). The antioxidant activity of the eight TEOs were investigated by the DPPH free radical scavenging test and four standards of main chemical type were used to evaluate antibacterial, cytotoxicity and cancer cell inhibitory activity. This research not only can show breeding target by selecting the dominant chemical type of thyme, but also can provide theoretical basis for further exploring the function of natural products.
2. Results
2.1. Phenotypic evaluation of different thyme species
According to the growth habits of thyme species, the plant can be divided into two types: erect type and creeping type. These two types exhibit striking morphological distinctions.
Figure 1 displays the plant forms of eight distinct thyme species. Among these species,
Thymus vulgaris ‘Fausitinoi’ (TvF),
Thymus pulegioides (Tp),
Thymus rotundifolia (Tr),
Thymus thracicus (Tt),
Thymus longicaulis (Tl) were erect type,
Thymus vulgaris (Tv),
Thymus quinquecostatus (Tq),
Thymus serpyllum (Ts) belonged to creeping type. In addition to the differences in plant type, leaf color, leaf type, flower color and pattern were different. The flower colors of
T. pulegioides,
T. rotundifolia,
T. longicaulis,
T. quinquecostatus were pink, the flower colors of
T. vulgaris ‘Fausitinoi’,
T. thracicus,
T. vulgaris were white to light pink. The leaf colors of
T. vulgaris ‘Fausitinoi’,
T. rotundifolia,
T. thracicus,
T. vulgaris were dark green and leaf blade shape were ovate, the leaves of
T. pulegioides were oil-green and triangular in shape.
T. longicaulis,
T. quinquecostatus were green in color and ovale in shape. The leaf color of
T. serpyllum was yellow green.
2.2. Essential oil components from eight thyme species
The main components in eight essential oils of thyme were determined by GC-MS. Statistical analysis was conducted on 25 components (these compounds showed relative contents were greater than 0.01%) for 91.54–96.18% of total TEO components, and the results were shown in
Table 1. From the data in
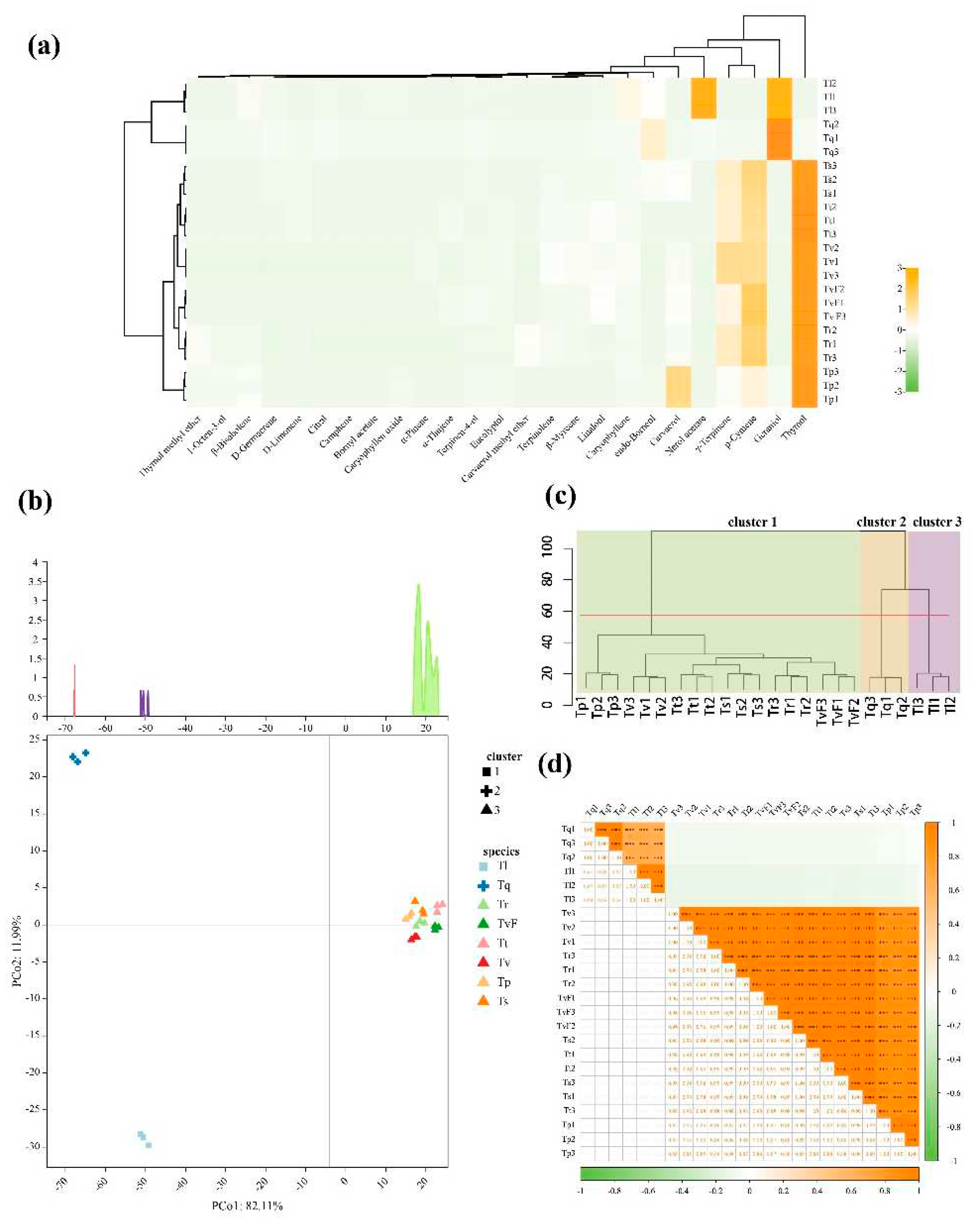
Table 1, we found that the compositions of TEOs varied greatly among eight species. Furthermore, the details of these differences were visualized via a heatmap. As shown in
Figure 2a and
Table 1, the chemical components of TEOs were mainly terpenoids, with a relative percentage of 90.25–95.06%, including monoterpene hydrocarbons (0–41.94%), oxygenated monoterpenes (49.42–92.23%), sesquiterpene hydrocarbons (1.64–10.11%) and oxygenated sesquiterpenes (0–0.61%). Among them, oxygenated monoterpenes were the main components of TEOs, followed by monoterpene hydrocarbons, and the two types of components had relatively large variation. The contents of sesquiterpene hydrocarbons and oxygenated sesquiterpenes were relatively low in most species detected, especially oxygenated sesquiterpenes, which were almost non-existent. In monoterpene hydrocarbons, p-cymene and γ-terpinene were the main component, showed high percentages in TvF (25.60% and 7.74%, respectively), Tr (21.81% and 11.66%, respectively), Tt (19.22% and 10.51%, respectively), Ts (21.15% and 11.16%, respectively), and Tv (17.25% and 17.80%, respectively). In oxygenated monoterpenes, geraniol, thymol, carvacrol and nerol acetate were the main component. Geraniol showed high percentages in Tl (25.60%) and Tq (21.81%). Among the six of the eight TEOs tested, the percentage of thymol exceeded 40%. In addition, it was found that carvacrol contained 20.61% in Tp essential oil. Interestingly, we observed a high percentage of nerol acetate (41.02%) in the EOs of Tl with both thymol and carvacrol as 0. Only three sesquiterpene hydrocarbons were detected, with caryophyllene containing a high percentage. Only caryophylllen oxide was detected in oxygenated sesquiterpenes, and the highest percentage was found in Tp (0.5%).
According to
Table 1, the yields of TEOs ranged from 0.53% to 1.63% dry matter (v/w). Tt had the highest yield (1.63%) of all the samples, with thymol (51.68%) being the most dominant. Other important components included p-cymene (19.22%), γ-terpinene (10.51%), linalool (3.25%), caryophyllene (1.76%), terpinolene (1.62%), β-myrcene (1.50%) and α-thujene (1.21%). The EO yields of Tr and Ts were more than 1.5%, and the main chemical components were thymol (42.75% and 47.31%, respectively), p-cymene (21.81% and 21.15%, respectively) and γ-terpinene (11.66% and 11.16%, respectively). Tp had the lowest EO yield (0.53%) of all the samples, and with the thymol (48.32%), carvacrol (20.61%), and p-cymene (8.70%) being the top three components in this species. All in all, TEO yields decreased in the following order: Tt > Tr > Ts > Tl > TvF > Tv > Tq > Tp.
2.3. Multivariate statistical analysis of essential oil components from eight thyme species
The primary components present in the essential oils of all eight species of thyme were subjected to principal component analysis (PCA) analysis. (
Figure 2b). The results showed that the first PC axis and the secondary PC axis explained 94.10% of the overall variance (
Figure 2b). Tl and Tq were clearly separated from Tt, Tr, Ts, TvF, Tv and Tp according to PC1, and Tl and Tq were clearly separated according to PC2. In conclusion, the essential oil components variation between Tt, Tr, Ts, TvF, Tv and Tp were obviously high. Dendrogram analysis confirmed the results of PCA analysis in which the 25 main volatile components from TEOs were clustered into 3 different clusters (
Figure 2c). The first cluster was mainly composed of thymol, Tt, Tr, Ts, TvF, Tv and Tp belonged to the group, which can be defined as thymol-type; Tq was clustered into second cluster because of their main components was geraniol, defined as geraniol-type; Tl was clustered into third cluster because of their main components was nerol acetate, defined as nerol acetate-type (
Figure 2d). In terms of thyme species correlations, the strongest positive correlation was observed in first cluster, all
P of which were outweigh 0.8. In addition, three chemical type standards were used for antibacterial activity and anti-tumor cell migration, and the results showed that thyme has strong antibacterial activity and antimigratory activity (Figure S1 and S2).
2.4. Characterization of shared and unique TEO components from eight thyme species
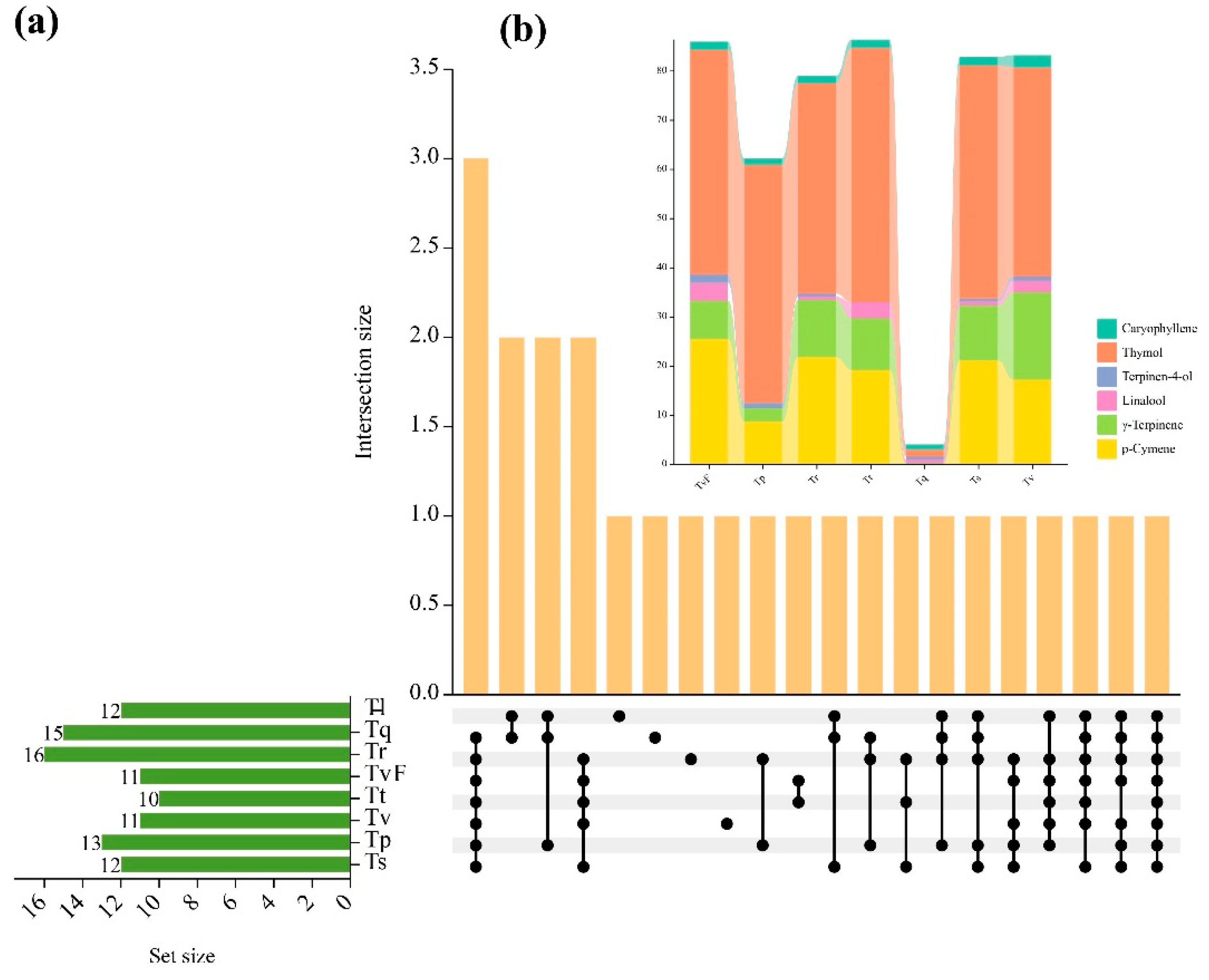
By analyzing the shared and unique components of eight TEOs, the results were shown in
Figure 3a. The findings revealed that the number of components present in each TEO ranged from 10 to 16. Furthermore, we found that a majority of components were shared by more than one cultivar. For example, p-cymene, γ-terpinene, linalool, terpinen-4-ol, thymol and caryophyllene were present in seven samples. Of these, only caryophyllene was shared in all samples. Four components, such as α-pinene, camphene, carvacrol methylether and bornyl acetate, were unique to specific thyme species. The chemical profiles of the p-cymene, γ-terpinene, linalool, terpinen-4-ol, thymol and caryophyllene shared seven TEO components are presented in
Figure 3b. Tt, Tr, Ts, TvF, Tv and Tp showed the highest p-cymene (19.22%, 21.81%, 21.15%, 25.6%, 17.25% and 8.7%, respectively) and thymol (51.68%, 42.75%, 47.31%, 45.74%, 42.44% and 48.32%, respectively). In EO of Tq, although all six compounds were contained, the contents of six compounds were low. These results suggested that the composition of Tq essential oil was quite different from that of the other six TEOs, but the types of compounds were similar.
2.5. Variation analysis of TEO components from eight thyme species
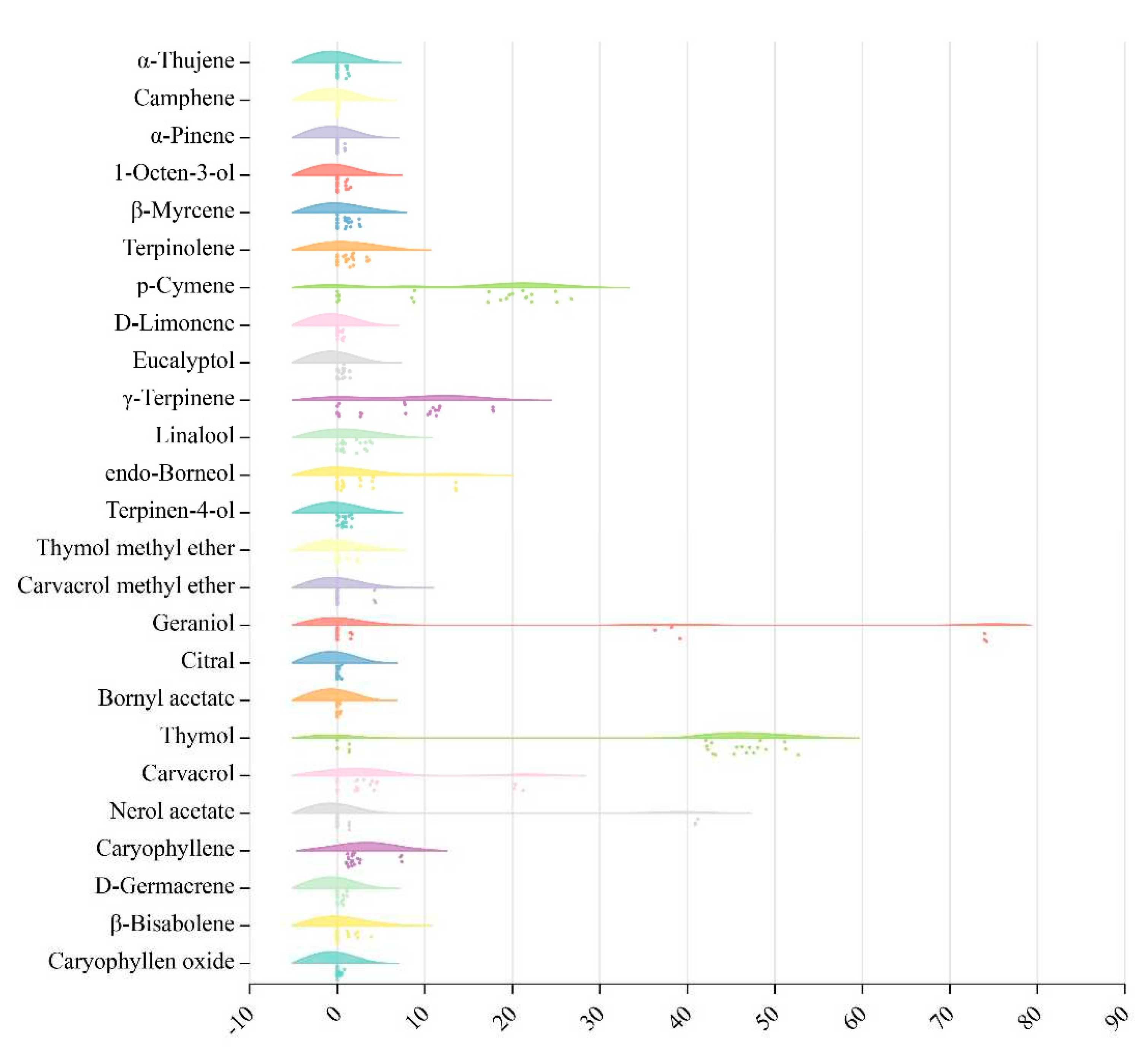
Given the above analysis results, 25 main volatile components were identified and a majority of components had different degrees of variation. The results about variation analysis of TEO components from eight thyme species were showed in
Figure 4. The percentage contents of 22 components in the 25 main volatile components was mainly concentrated in 0-10%. It was noted that geraniol was the most variable of all components (0-74.04%), followed by thymol (0-51.68%) and nerol acetate (0-41.02%), and then p-cymene (0-25.60%). Of these, thymol was mainly concentrated between 40-50%, while p-cymene was mainly concentrated at 20%.
2.6. Evaluation of antioxidant activity of eight thyme species
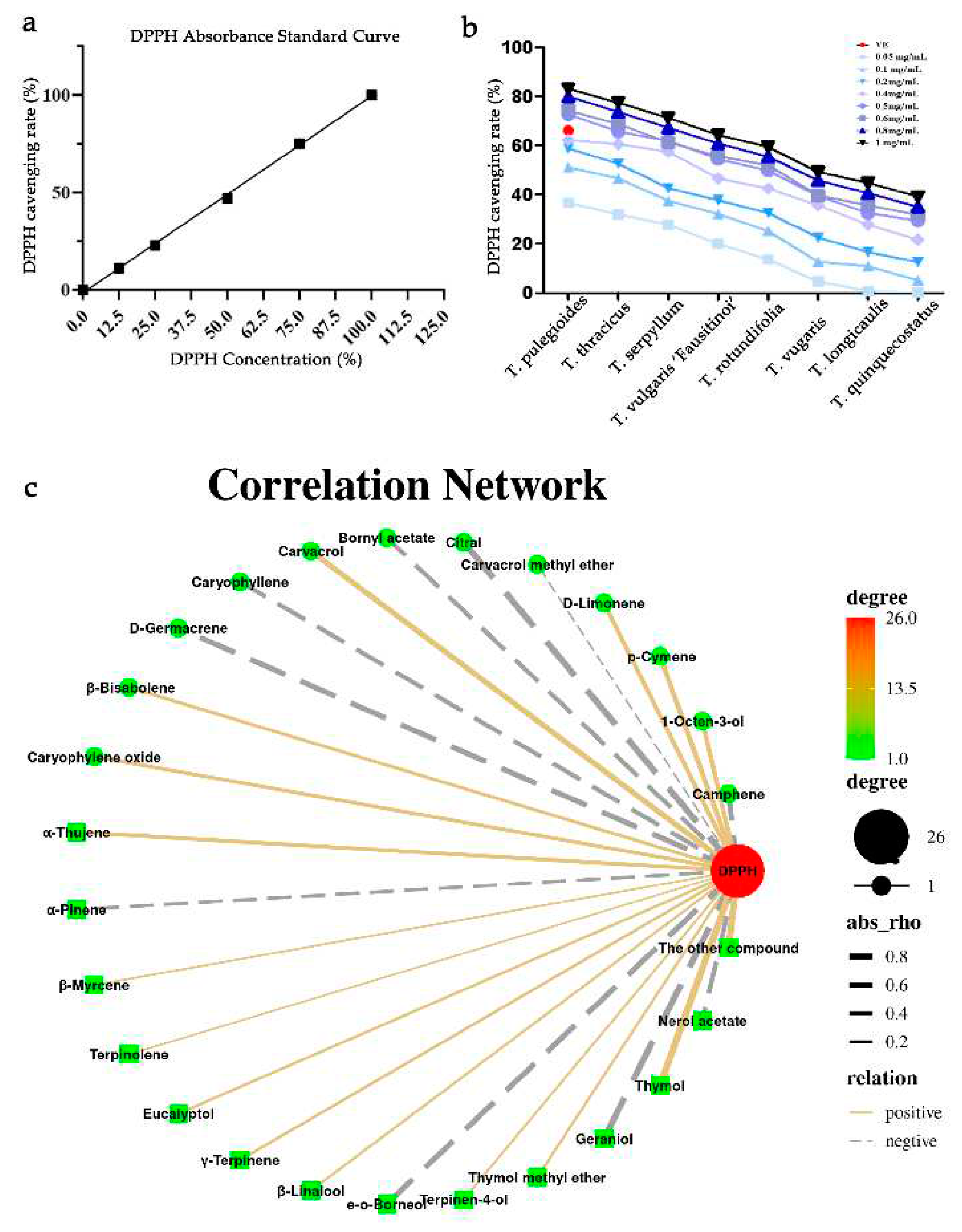
DPPH free radical scavenging results showed that all eight essential oils had significant antioxidant activity in a dose-dependent manner, and antioxidant activity increased with the increase of essential oil concentration, as shown in
Figure 5a, b. When the concentration of essential oils was 0.05 mg/mL, the antioxidant activity of essential oils were the weakest, and when the concentration of essential oils was 1 mg/mL, the antioxidant activity of essential oils was the strongest. In addition, when the concentration of essential oils was 0.5 mg/mL and 0.6 mg/mL, there was no significant difference in the free radical scavenging rates of different kinds of thyme, especially in TvF and Tv, their free radical scavenging rates were nearly the same. And four of eight TEOs had stronger antioxidant activity than vitamin E in the concentration of 1 mg/mL. The eight essential oils were ranked in order of antioxidant activity: Tp > Tt > Ts > TvF > Tr > Tv > Tl > Tq.
Then we analyzed the volatile components and DPPH activity of thyme in conjunction (
Figure 5c). A total of 16 compounds were positively correlated with DPPH scavenging ability, which were ranked in descending order of correlation: thymol, carvacrol, p-cymene, 1-octen-3-ol, D-limonene, α-thujene, caryophyllen oxide, β-bisabolene, γ-terpinene, eucalyptol, thymol methyl ether, linalool, terpinen-4-ol, β-myrcene, terpinolene. There were 9 compounds negatively correlated with DPPH scavenging ability, which were ranked in descending order of correlation, as follows: geraniol, endo-borneol, germacrene D, camphene, caryophyllene, α-pinene. In conclusion, thymol and carvacrol played a key role in antioxidant activity, while geraniol and nerol acetate had weak antioxidant activity, which further indicated that the difference in antioxidant capacity of these eight essential oils may be due to the difference in the proportion of moniterpenoids and the best essential oil with antioxidant activity was thymol/carvavol-type, followed by geraniol/nerol acetate-type, while geraniol type essential oil had relatively weak antioxidant activity.
3. Discussion
The main chemical categories of components in TEOs include monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, ketones, aldehydes, ethers and esters [
24,
25]. Among them, thymol and carvacrol are phenolic derivatives and the most common chemical types in TEOs [
26,
27]. In this study, six out of eight TEOs were thymol type. In addition, some chemotypes are rare, for example, the chemotypes of α-terpenyl acetate (accumulate to 50–70% of α-terpenyl acetate) and cis-sabinene hydrate (accumulate to 63.2% of cis-sabinene hydrate) were detected in Denmark and Lithuania, respectively [
28,
29]. Previous studies have shown that the qualitative composition of TEOs are controlled by genetically [
30]. However, the different environmental conditions (climate, soil chemistry), pest control, fertilization method, extraction method and harvesting time can influence the quantitative composition of its essential oils, as well as the percentage composition [
31,
32,
33]. For instance, the contents of carvacrol or thymol increases as the change of temperature, whereas cis-sabinene hydrate is not influenced by temperature [
33]. In
Zataria multiflora, increasing calcium contents in soil tends to increase essential oil yield and carvacrol contents but decrease linalool contents [
34]. In oregano, it can synthesized more thymol than other chemical components at high altitudes [
35]. In bergamot fruit, the composition of the main chemical components of essential oil changed dynamically during ripening [
36]. In lavender, the contents and chemical composition of essential oil were influenced by flower development, temperature, gene expression [
37]. In these eight thyme species from the same cultivation conditions,
T. thracicus included highest thymol contents and
T. pulegioides included highest carvacrol contents, but there were no thymol and carvacrol in
T. longicaulis. This may also relate to its terpenoid metabolic pathways, therefore different chemical types of thyme can be used as good research materials for different biogynthesis pathways. Over a long period of time, several of the genes responsible for volatile synthesis may be impacted by internal genetic factors connected to anatomical and physiological characteristics of the plants and the biosynthetic pathways of the volatiles. The same plant species can have different ecotypes or chemotypes as a result of those circumstances. [
38].
In our investigation, the abundances of numerous components differed significantly among the eight TEOs we tested, which is similar with findings from the oregano study [
39]. However, the yields of oregano essential oils were positively correlated with the content of carvacrol/thymol. According to Lukas et al. [
40], the active cymyl pathway typically results in the formation of significant amounts of phenolic monoterpenes (mostly carvacrol and/or thymol) while concurrently maintaining high EO yields. But this phenomenon was not found in this study. Characterization of shared and unique TEO components from eight thyme species showed six shared components among the seven thyme species. In addition, β-caryophyllene was shared in all thyme species tested. Previous studies have shown that the formation of shared components may be due to these species encountering the same selection factors, such as antimicrobial, antioxidant, antiparasitic, and pro-pollinator effects [
41,
42]. Our examination of the shared elements revealed vital information about the traits that all thyme species share.
Every active cell is a small factory, constantly undergoing a large number of chemical reactions, and free radicals are inevitably present during the chemical reaction process, which can cause varying degrees of damage to the cells. Antioxidants can act by reducing activity of their structure, allowing the neutralization of free radicals, decomposition of peroxides and chelation of transition-metal ions [
43,
44,
45]. And Brewer [
46] found that the aromatic rings and the arrangement and number of hydroxyl groups affected antioxidant activity. Previous studies have shown that thymol and carvacrol have strong antioxidant effects [
47,
48,
49]. In this study, it was found that eight TEOs had antioxidant capacity, and four of them had stronger antioxidant capacity than vitamin E. Furthermore, it was found that the antioxidant capacity of eight TEOs was related to the sum of thymol and thymol, for example, Tp having the strongest antioxidant capacity and the highest contents of thymol and thymol (68.92%); the antioxidant capacity of Tt was second, and the sum of thymol and carvacrol contents was 51.68%; Tq had the worst antioxidant capacity, and the contents of carvacrol and thymol in Tq was the lowest (1.36%).
4. Materials and Methods
4.1. Plant Materials
The eight thyme materials were obtained from the Institute of Botany, Chinese Academy of Sciences (IB-CAS), Beijing, China (
Figure 5).
T. vulgaris ‘Fausitinoi’,
T. pulegioides,
T. rotundifolia,
T. thracicus,
T. longicaulis,
T. serpyllum,
T. vulgaris were collected from Europe,
T. quinquecostatus was Chinese native thyme. All the above-ground part of the plants in full bloom were collected and rinsed well with water, then stored in the shade for future use.
4.2. Extraction of Essential Oils
Eight thyme samples dried were crushed into a powder. The 100 g of powdered samples were combined with 1000 mL of distilled water to extract the essential oils by steam distillation. After boiling, the extraction procedure was carried out for three hours. The essential oils were extracted, dried with anhydrous sodium sulfate, and kept at 4°C in an amber bottle. As the dry weight of plant materials (in% v/w of 100 g dried raw material), the yields were calculated.
4.3. Gas Chromatography-mass Spectrometry Analysis of Essential Oils
The essential were filtered via filter membrane of 0.22 μm and diluted with n-hexane. The 7890A-7000B GC-MS (Agilent Technologies, Santa Clara, CA, USA) outfitted with an HP-5MS column (30 m × 0.25 mm × 0.25 μm; Agilent Technologies) was then used to conduct gas chromatography-mass spectrometry (GC-MS). The temperature of the injector was 280°C. The oven program has the following settings: The temperature was kept at 40°C for 2 min, then increased linearly to 260°C at a rate of 4°C/min; the temperature was then increased to 260°C at a rate of 4°C/min; and finally, the temperature was increased to 310°C at a rate of 60°C/min. The following are the MS symptoms: quadrupole, electronic impaction source temperature of 230 °C, ionization energy of 70 eV and 50–500 u of mass range.
The RI values were determined using n-alkane (C7-C40) hydrocarbons under the same conditions. The relative percentage of essential oil components was determined based on the peak area. Retention index (RI) values and the spectra from the 17.0 library of the National Institute of Standards and Technology were compared to identify the compounds [
50]. Under the same circumstances, the RI values were calculated using n-alkane (C7-C40) hydrocarbons. Based on the peak area, the relative percentage of the essential oil components was calculated.
4.4. In Vitro Antioxidant Activity
Using a previously described approach, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity of extracts from 8 essential oils was evaluated [
51]. Essential oils and 0.1 mM DPPH ethanol solution totaling 0.5 mL each were added to 96-well plates and incubated for 30 minutes in the dark before the absorbance at 517 nm was measured.
4.5. Statistical Analysis
The statistical analysis was performed with office 2010, R version:4.2.0 and a onlin software (
https://www.chiplot.online). Values are represented as the mean ± SEM. All experiments were independently performed in triplicate. Asterisk (*) indicates significant differences (*P < 0.05, **P < 0.01, ***P < 0.001).
5. Conclusions
This study established a multidimensional analytical method to evaluate differences in the chemical profiles and potential biological functions of EOs from eight thyme species. The results showed that TEOs exhibited chemical diversity, and three main clusters were identified (thymol-, geraniol- and nerol acetate-type) and thymol was main type. The percentage contents of 22 components in the 25 main volatile components was mainly concentrated in 0-10%. The geraniol was the most variable of all components (0-74.04%), followed by thymol (0-51.68%) and nerol acetate (0-41.02%), and then p-cymene (0-25.60%). In addition, eight TEOs had some common compounds, such as thymol and p-cymene, which may encounter the same selection factors. The eight TEOs had antioxidant activity, and the contents of thymol and carvacrol were positively correlated with antioxidant activity of TEOs. Overall, our results contributed to understanding of the TEOs and can provide theoretical basis for further exploring the function of natural products from thyme essential oils.
Author Contributions
Conceptualization, Y.D., L.S. and H.L.; methodology, Y.D., Z.W., Y.Z. and R.Y.; software, Z.W., Y.D., Y.Z. and R.Y.; validation R.Y.; formal analysis, Y.D., Z.W. and Y.Z.; investigation, R.Y. and Y.Z.; resources, H.L., M.S. and H.B.; data curation, H.L. and L.S.; writing—original draft preparation, Y.D., Y.Z., Z.W and R.Y.; writing—review and editing, Y.D., Z.W., C.Y. and M.M; visualization, Z. W. and Y.D.; supervision, H.L. and L.S.; project administration, M.S. and L.S.; funding acquisition, M.M., C.Y. and L.S.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the International Partnership Program of the Chinese Academy of Sciences. (Grant No. 063GJHZ2022038GC), Strategic Priority Research Program of the Chinese Academy of Sciences, grant number XDA23080603 and the R&D Program of Sinno Cosmetics Co., Ltd. [Grant No. 2019C079].
Acknowledgments
We would thank Jin-Dan Zhang and Yan Zhu from the Plant Science Facility of the Institute of Botany, Chinese Academy of Sciences, for their excellent technical assistance with flow cytometry and GC-MS analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lorenzo, J.M.; Mousavi Khaneghah, A.; Gavahian, M.; Marszałek, K.; Eş, I.; Munekata, P.E.S.; Ferreira, I.C.F.R.; Barba, F.J. Understanding the potential benefits of thyme and its derived products for food industry and consumer health: from extraction of value-added compounds to the evaluation of bioaccessibility, bioavailability, anti-inflammatory, and antimicrobial activities. Crit. Rev. Food Sci. Nutr. 2019, 59, 2879–2895. [Google Scholar] [CrossRef]
- Salehi, B.; Abu-Darwish, M.S.; Tarawneh, A.H.; Cabral, C.; Gadetskaya, A.V.; Salgueiro, L.; Hosseinabadi, T.; Rajabi, S.; Chanda, W.; Sharifi-Rad, M.; et al. Thymus Spp. plants - food applications and phytopharmacy properties. Trends in Food Science & Technology 2019, 85, 287–306. [Google Scholar] [CrossRef]
- Hong, J.Y.; Kim, H.; Jeon, W.-J.; Baek, S.; Ha, I.-H. Antioxidative effects of Thymus quinquecostatus Celak. through mitochondrial biogenesis improvement in RAW 264.7 macrophages. Antioxidants 2020, 9, 548. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, S.J.; Hwang, J.W.; Kim, E.K.; Kim, S.E.; Kim, E.H.; Moon, S.H.; Jeon, B.T.; Park, P.J. In vitro protective effects of Thymus quinquecostatus Celak. extracts on T-BHP-induced cell damage through antioxidant activity. Food Chem. Toxicol. 2012, 50, 4191–4198. [Google Scholar] [CrossRef]
- Evans, J.D.; Martin, S.A. Effects of thymol on ruminal microorganisms. Curr. Microbiol. 2000, 41, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Hu, C.L.; Zhang, Z.P.; Chen, R.; Yang, S.B.; Miao, Z.Y.; Sun, L.N.; Wang, Y.Q. Development and validation an LC-MS/MS method to quantify (+)-borneol in rat plasma: application to a pharmacokinetic study. J. Chromatogr. B 2019, 1109, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Stahl-Biskup, E.; Francisco, S. Thyme: The genus thymus. In essential oil chemistry of the genus thymus-A global view. Los Angeles: CRC Press 2002, 75–124.
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential Oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Variations in chemical composition and bioactive compounds of Thymus kotschyanus Boiss. & Hohen populations originated from different collection sites. J. Essent. Oil Bear. Plants 2018, 21, 1272–1283. [Google Scholar] [CrossRef]
- Bigdeloo, M.; Hadian, J.; Nazeri, V. Composition of essential oil compounds from different populations of Thymus caramanicus Jalas. J. Appl. Res. Med. Aromat. Plants 2017, 7, 95–98. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Marchese, A.; Izadi, M.; Curti, V.; Daglia, M.; Nabavi, S.F. Plants belonging to the genus thymus as antibacterial agents: from farm to pharmacy. Food Chem. 2015, 173, 339–347. [Google Scholar] [CrossRef]
- Kim, M.; Moon, J.C.; Kim, S.; Sowndhararajan, K. Morphological, chemical, and genetic characteristics of korean native thyme Bak-Ri-Hyang (Thymus quinquecostatus Celak.). Antibiotics 2020, 9, 289. [Google Scholar] [CrossRef]
- Trendafilova, A.; Todorova, M.; Ivanova, V.; Zhelev, P.; Aneva, I. Essential oil composition of five thymus species from Bulgaria. Chem Biodivers 2021, 18, e21004. [Google Scholar] [CrossRef]
- Thompson, J.D.; Chalchat, J.C.; Michet, A.; Linhart, Y.B.; Ehlers, B. Qualitative and quantitative variation in monoterpene co-occurrence and composition in the essential oil of Thymus vulgaris chemotypes. Journal of Chemical Ecology 2003, 29, 859–880. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur J Clin Microbiol Infect Dis 2011, 30, 41–50. [Google Scholar] [CrossRef]
- De Lira Mota, K.; De Oliveira Pereira, F.; De Oliveira, W.; Lima, I.; De Oliveira Lima, E. Antifungal activity of Thymus vulgaris L. Essential oil and its constituent phytochemicals against Rhizopus oryzae: Interaction with ergosterol. Molecules 2012, 17, 14418–14433. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.S.; Tupe, S.G. Antifungal activity and mechanism of action of carvacrol and thymol against vineyard and wine spoilage yeasts. Food Control 2014, 46, 115–120. [Google Scholar] [CrossRef]
- A, U.; EPW, K.; EJ, S. Mechanisms of action of carvacrol on the food-borne pathogen bacillus cereus. Applied and Environmental Microbiology 65, 4606–4610. [CrossRef]
- Dos Santos Negreiros, P.; Da Costa, D.S.; Da Silva, V.G.; De Carvalho Lima, I.B.; Nunes, D.B.; De Melo Sousa, F.B.; De Souza Lopes Araújo, T.; Medeiros, J.V.R.; Dos Santos, R.F.; De Cássia Meneses Oliveira, R. Antidiarrheal activity of α-terpineol in mice. Biomed. Pharmacother. 2019, 110, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Juergens, L.J.; Worth, H.; Juergens, U.R. New perspectives for mucolytic, anti-inflammatory and adjunctive therapy with 1,8-cineole in COPD and asthma: review on the new therapeutic approach. Adv. Ther. 2020, 37, 1737–1753. [Google Scholar] [CrossRef]
- Stranden, M. Receptor neuron discrimination of the germacrene D enantiomers in the mothhelicoverpa armigera. Chem. Senses 2002, 27, 143–152. [Google Scholar] [CrossRef]
- Machado, K.D.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phytother. Res. 2018, 32, 2376–2388. [Google Scholar] [CrossRef]
- Hao, Y.; Kang, J.; Guo, X.; Yang, R.; Chen, Y.; Li, J.; Shi, L. Comparison of nutritional compositions and essential oil profiles of different parts of a dill and two fennel cultivars. Foods 2021, 10, 1784. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and thyme essential oil—new insights into selected therapeutic applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Marchese, A.; Izadi, M.; Curti, V.; Daglia, M.; Nabavi, S.F. Plants belonging to the genus thymus as antibacterial agents: From farm to pharmacy. Food Chem. 2015, 173, 339–347. [Google Scholar] [CrossRef]
- Ben El Hadj Ali, I.; Guetat, A.; Boussaid, M. Chemical and Genetic Variability of Thymus Algeriensis Boiss. et Reut. (Lamiaceae), a north african endemic species. Ind. Crops Prod. 2012, 40, 277–284. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Mockut, D.; Bernotien, G. The alpha-terpenyl acetate chemotype of essential oil of thymus pulegioides L. Biochem. Syst. Ecol. 2001, 29, 69–76. [Google Scholar] [CrossRef]
- Groendahl, E.; Ehlers, B.K.; Keefover-Ring, K. A new cis-sabinene hydrate chemotype detected in large thyme (Thymus pulegioides L.) growing wild in Denmark. J. Ess. Oil Res. 2008, 20, 40–41.
- Mártonfi, P.; Grejtovský, A.; Repčák, M. Chemotype pattern differentiation of Thymus pulegioides on different substrates. Biochem. Syst. Ecol. 1994, 22, 819–825. [Google Scholar] [CrossRef]
- Senatore, F. Influence of harvesting time on yield and composition of the essential oil of a thyme (Thymus Pulegioides L.) growing wild in campania (southern Italy). J. Agric. Food Chem. 1996, 44, 1327–1332. [Google Scholar] [CrossRef]
- Ložienė, K.; Venskutonis, P.R. Influence of environmental and genetic factors on the stability of essential oil composition of Thymus pulegioides. Biochem. Syst. Ecol. 2005, 33, 517–525. [Google Scholar] [CrossRef]
- Novak, J.; Lukas, B.; Franz, C. Temperature Influences thymol and carvacrol differentially in Origanum Spp. (Lamiaceae). Journal of Essential Oil Research 2010, 22, 412–415. [Google Scholar] [CrossRef]
- Karimi, A.; Krähmer, A.; Herwig, N.; Schulz, H.; Hadian, J.; Meiners, T. Variation of secondary metabolite profile of zataria multiflora boiss. Populations linked to geographic, climatic, and edaphic factors. Front. Plant Sci. 2020, 11, 969. [Google Scholar] [CrossRef] [PubMed]
- Mastro, G.D.; Tarraf, W.; Verdini, L.; Brunetti, G.; Ruta, C. Essential oil diversity of Origanum vulgare L. Populations from southern Italy. Food Chemistry 2017, 235, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gioffrè, G.; Ursino, D.; Labate, M.L.C.; Giuffrè, A.M. The peel essential oil composition of bergamot fruit (Citrus bergamia, Risso) of Reggio Calabria (Italy): A review. Emir J Food Agric 2020, 835. [Google Scholar] [CrossRef]
- Hassiotis, C.N.; Ntana, F.; Lazari, D.M.; Poulios, S.; Vlachonasios, K.E. Environmental and developmental factors affect essential oil production and quality of Lavandula angustifolia during flowering period. Industrial Crops and Products 2014, 62, 359–366. [Google Scholar] [CrossRef]
- Andrea, B. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commu. 2009, 4, 1147–1154. [Google Scholar]
- Hao, Y.; Kang, J.; Yang, R.; Li, H.; Cui, H.; Bai, H.; Tsitsilin, A.; Li, J.; Shi, L. Multidimensional exploration of essential oils generated via eight oregano cultivars: compositions, chemodiversities, and antibacterial capacities. Food Chem. 2022, 374, 131629. [Google Scholar] [CrossRef] [PubMed]
- Lukas, B.; Schmiderer, C.; Novak, J. Essential oil diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Coutinho, T. A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef]
- Moore, B.D.; Andrew, R.L.; Külheim, C.; Foley, W.J. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol. 2014, 201, 733–750. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Su, L.; Yin, J.J.; Charles, D.; Zhou, K.; Moore, J.; Yu, L. (Lucy) Total phenolic contents, chelating capacities, and radical-scavenging properties of black peppercorn, nutmeg, rosehip, cinnamon and oregano leaf. Food Chem. 2007, 100, 990–997. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: natural sources of antioxidants – a mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Aristatile, B.; Al-Numair, K.S.; Veeramani, C.; Pugalendi, K.V. Effect of carvacrol on hepatic marker enzymes and antioxidant status in d-galactosamine-induced hepatotoxicity in rats. Fundam. Clin. Pharmacol. 2009, 23, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Botsoglou, N.A.; Grigoropoulou, S.H.; Botsoglou, E.; Govaris, A.; Papageorgiou, G. The effects of dietary oregano essential oil and α-tocopheryl acetate on lipid oxidation in raw and cooked turkey during refrigerated storage. Meat Sci. 2003, 65, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. Journal of Physical and Chemical Reference Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Park, C.; Yeo, H.; Park, Y.; Kim, Y.; Park, C.; Kim, J.; Park, S. Integrated analysis of transcriptome and metabolome and evaluation of antioxidant activities in Lavandula pubescens. Antioxidants 2021, 10, 1027. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).