1. Introduction
The exponential surge in global plastic production has engendered a multifaceted crisis. Plastic waste is released into the environment and exposed to the action of microorganisms, thermal stress, oxidative processes, photodegradation, and hydrolytic cleavage. This intricate cascade of events culminates in the formation of minuscule fragments, called microplastics (MPs) (<5 mm) and even smaller fragments, nanoplastics (NPs) (<1000 nm) [
1]. Although the empirical footprint of MPs has been documented across various ecosystems, NPs are recently gaining high attention because of their small size; they are easier to be taken up by organisms and likely to pose higher ecological and health risks than microplastics. The characterization and quantification of NPs faces analytical challenges as there are substantial difficulties in separation, visualization and chemical identification of nanoplastics due to their small sizes, and interferences from coexisting substances [
1]. The ability of NPs to bioaccumulate and cotransport pollutants in the whole organism is one of the most dangerous aspects of this form of plastic debris, defining a new class of emerging pollutants that is still largely unknown. In this context, it is essential to have accurate and representative models of NPs to better understand their toxic effects. Model NPs are a valuable tool needed for hazard and risk assessment estimation, widely used in various in vitro and in vivo biological experiments looking at different adverse outcome pathways of modeled NPs.
Several methods have been described for model NPs manufacturing of different chemical types for the purposes of method development and validation, as well as in vitro toxicity and risk assessment testing [
2]. Particularly challenging is to achieve high quality, low batch-to-batch variation and uniform size, (ideally monodisperse) of the NPs produced. High surface area and intrinsic hydrophobicity of the NPs make them an ideal vector for hydrophobic compounds, such as pesticides, but also proteins, heavy metals [
3]. Manufactured NPs for applications in in vitro testing should be devoid of contaminants, particularly those relevant for cell inflammation or survival.
Many studies investigated toxicity of NPs mostly with commercially available spherical polystyrene NPs (PS-NPs) of known size. However, these NPs were not produced for the purpose of reference materials for NPs found in the environment, and contain additives and surfactants which may account for toxicity observed in cellular assays, making results interpretation challenging [
4,
5]. The publication of Pikuda et al. was the first study to demonstrate that additives in commercial NPs have toxic effects and may introduce artifacts in toxicity assessments [
6]. In addition, manufacturers mainly use surfactants and are often unwilling to provide information on presence and quantities of remaining surfactant, or about details of the surface charge density [
7]. Heinlaan et al. clearly shown that the observed toxicity of commercial PS-NPs was induced by toxic water-soluble additives (e.g., antimicrobial NaN
3 and surfactants) in the PS-NPs preparations as revealed by the disappearance of toxicity after particle dialysis [
8]. Therefore, for studying biological effects of NPs more and more researchers produce and characterize their own NPs material, and in the last several years there is an increasing number of publications reporting preparation of NPs [
2,
7].
NPs are produced by either the “bottom-up” or “top-down” approaches. “Top-down” approach includes laser ablation, photodegradation, ultra-sonication, or mechanical degradation of primary or secondary plastics. Disadvantages of this approach are irregular shape, surface defects and oxidized surface, as well as difficult and time-consuming preparation of large quantities of NPs [
7]. “Bottom-up” approach stems from traditional colloidal chemistry methods such as conventional dispersed media (emulsions and nanoprecipitation), where ionic or non-ionic surfactants enable NPs dispersibility [
7]. However, even after extensive NPs rinsing, the residual traces of surfactants are difficult to remove [
2]. The recent critical review highlighted the importance of purification of PS-NPs suspensions to remove additives, such as surfactants, before performing toxicity evaluations [
9]. Because of the contribution of the surfactants to the observed toxicity, it remains critical to establish auxiliary chemical methods for purity assessment of manufactured NPs correlating toxicity studies with additional relevant parameters such: NPs physicochemical characterization (size, shape, zeta potential) and other parameters related to the properties of the chemical type of plastic polymer and NPs morphological characteristics.
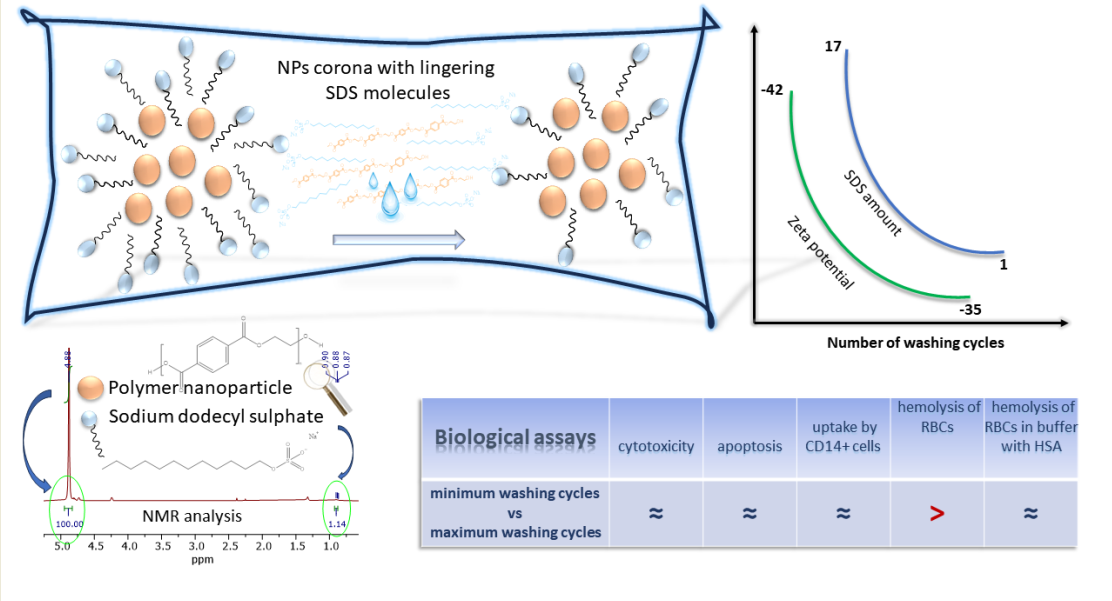
In this work, we have synthesized NPs based on polyethylene terephthalate (PET) by nanoprecipitation method using ionic surfactant sodium dodecyl sulfate (SDS). We have established 1H NMR spectroscopy as a quality control for ionic surfactant presence throughout purification procedure to monitor semi-quantitatively the ratio of surfactant to NPs. The modeled particles of different stages of purification were subject to biological assays, such as red blood cells hemolysis, cytotoxicity, ROS production, apoptosis of primary mononuclear cells and uptake by CD14+ primary mononuclear cells. We demonstrate that for a wide range of surfactant/NP ratio, no difference in cytotoxicity was observed between particles of different purification stages, but the trace amounts of surfactants present accounted for differences observed in protein-free media.
2. Experimental
2.1. Materials
2.1.1. Materials for Preparation and Characterization of PET NPs
Polyethylene terephthalate (PET) (CAS: 25038-59-9, Cat. no. 429252) granules, as well as deuterated chloroform (>99% grade) were obtained from Sigma Aldrich-Merck (St. Louis, MO, USA). Bovine serum albumin (BSA) fraction V was purchased from Pan-Biotech (Germany), while sodium dodecyl sulfate (SDS, analytical grade) was from Serva (Germany). Trifluoroacetic acid (TFA, ≥99.5%, Optima™ LC/MS grade) was obtained from Fisher Chemical (UK). LACE6C low speed centrifuge 6000 rpm (COLO LAbExperts, Slovenia) and ultrasonic bath (Sonorex Super RK52, 230V, 50/60 Hz) from Bandelin electronic GmbH & Co. KG (Berlin, Germany) were used. 1H NMR spectra were recorded with Varian/Agilent NMR 400 MHz. Deuterated chloroform (CDCl3) used for 1H NMR (>99.9%) was from Sigma Aldrich- Merck (St. Louis, MO, USA). Chemical shifts are expressed in ppm, while TMS (tetramethylsilane) was used as an internal standard. Milli-Q water (Smart2Pure 3 UV/UF, Thermo Scientific, USA) was used for all experiments.
2.1.2. Materials for Cellular Assays
Human AB serum, penicillin, streptomycin, L-glutamate, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), trypan blue dye, and dimethyl-sulfoxide (DMSO) were purchased from Sigma Aldrich-Merck (St. Louis, MO, USA). RPMI phenol red free medium was obtained from Life Technologies, Inc. (Rockville, MD; USA). Ficoll-Paque Plus gradient medium was from Cytiva (Uppsala, Sweden). Trypan blue dyer was from Gibco (Thermo Fisher Sci, USA). Annexin V and propidium iodide (PI) were purchased from Becton Dickinson Bioscience (BD, Bioscience, San Jose, CA, USA). 2',7' – dichlorofluorescin diacetate (DCFDA) cellular reactive oxygen species (ROS) assay kit was obtained from Abcam (Abcam, UK). The 20% solution of human serum albumin (HSA, 96% purity, intended for clinical use) was purchased from Baxter (Baxter, Austria). All standard salts and glucose were purchased from Sigma Aldrich-Merck (St. Louis, MO, USA) in p.a. quality. Milli-Q water (Smart2Pure 3 UV/UF, Thermo Scientific, USA) was used for all experiments.
2.2. Methods
2.2.1. Preparation of PET NPs
The model NPs used in this work was produced by nanoprecipitation approach starting from commercial PET pellets. The pellets were used for production of NPs without any shredding.
PET NPs were prepared according to the previously published precipitation protocol [
10], with several modifications from [
11,
12]. Overall scheme of nPET production procedure is presented in
Figure S1 (Supplementary Material). Briefly, 2.00 g of PET pellets was dissolved in 20.0 mL of 90% TFA in Milli-Q water (
v/v), and stirred at 300 rpm for five hours at 50 °C s and next 18h at room temperature. In order to precipitate the NPs 20.0 mL of 20% TFA in Milli-Q water (
v/v) was added dropwise during 110 min to the original high concentration TFA solution (1 drop of 10 μL per 3 s) under vigorous stirring at 1200 rpm using a dropping funnel. After completion of the adding TFA, stirring of suspension was continued for an additional 2 hours, before being sonicated in an ultrasonic bath for 15 min. Suspension was separated into 4 glass centrifuge tubes (each tube with about 10 mL of suspension) and centrifuged at 3000 rpm for 40 min. After the supernatant was carefully removed using a glass pipette, Milli-Q water (10 mL) was added to each tube, sonicated for 10 s and centrifuged at 3000 rpm for 40 min. After removing the supernatant Milli-Q water (10 mL) was added to each tube, sonicated for 10 s, and centrifuged at 4000 rpm for 20 min. The washing of NPs with Milli-Q water was repeated until the pH of the suspension reached 6.
The NPs precipitates from 4 tubes were then combined and resuspended in 200 mL of 0.5 % aqueous solution of sodium dodecyl sulfate (SDS) and ultrasonicated for 40 min. The resulting suspension was quickly transferred into a 250 mL cylinder and allowed to settle for 24 h. Top 100 mL containing a suspension of nanosized PET particles was separated and transferred into another cylinder.
2.2.2. NPs Size Separation and Removal of Impurities
PET NPs suspension in 100 mL of 0.5% SDS was carefully separated into nine fractions (each 10 mL), according their sizes after particles settlement in the presence of surfactant, starting from top 10 mL of suspension in cylinder (
Figure S1), denoted NP-10, NP-20,…NP-90, up to 90 mL, and the last 10 mL of suspension was not used. Each suspension was transferred to glass centrifuge tubes for further purification from the largest particles and impurities like SDS (
Figure S2). Fractions NP-10, NP-20 and NP-30 were used for preparation of PET NPs for further analysis and the assays on cells.
In order to remove the largest particles, samples NP-10, NP-20 and NP-30 were firstly centrifuged at 1000 rpm for 20 min (
Figure S2) and the pellet was removed. After that, in order to remove the smallest particles, obtained supernatants were transferred to new tubes, and centrifuged at 4000 rpm for 20 min and supernatants were removed. The obtained pellets were resuspended in 10 mL of Milli-Q water using ultrasonic bath or 10 s. One aliquot of this suspension (labeled as NPs unwashed in the further text) was used for determination of zeta potential and cellular assays. The rest of the suspension was used for preparation of final NPs (labeled as NPs washed). In the first washing step these suspensions were centrifuged at 4000 rpm for 40 min, the supernatants were removed. Milli-Q water (10 mL) was added to pellets, sonicated for 10 s, and centrifugation was repeated under the same conditions. In order to remove soluble substances, such as surfactant SDS, monomer terephthalic acid (TPA), etc., the step of NPs washing with Milli-Q water was repeated 5 times. Particles at different stages of purification were analyzed by
1H NMR and saved for cellular assays.
After 5 washes, NP-10, NP-20 and NP-30 pellets were combined and resuspended in 4.5 mL of Milli-Q water using an ultrasonic bath for 5 min. After that, the NPs suspension was divided in three equal parts (3 x 1.5 mL). The first 1.5 mL of PET NPs suspension in Milli-Q water (labeled as NPs washed) was transferred to a glass vial, and used for determination of size distribution, zeta potential and assays on cells. In order to exchange the medium in which NPs were suspended, the remaining two parts of NPs suspension (1.5 mL each) were transferred into centrifuge tubes. After addition of Milli-Q water (8.5 mL) suspensions were centrifuged at 4000 rpm for 40 min. The supernatants were discharged and one pellet was suspended in 1.5 mL of 0.05% aqueous solution of BSA (NPs washed in BSA), while the other was suspended in 1.5 mL of 0.1% aqueous solution of SDS (NPs washed in SDS), using ultrasonic bath for 5 min. Samples were stored at 4 °C until further use.
2.2.3. Preparation of NPs for NMR Analysis
1H NMR spectroscopy was applied to check the presence of SDS in the prepared NPs, as well as for the monitoring of the washing process of NPs.
The aliquot (1 mL) of NPs of different purification stages was first centrifuged at 4000 rpm for 40 min, and after removing the supernatant, the remaining water in the pellet was evaporated. The solid residue was dissolved in 0.5 mL of the mixture TFA and deuterated chloroform (CDCl3) mixture (v/v, 4:1). 1H NMR spectrum was recorded on 400 MHz NMR.
2.2.4. Determination of Concentration of NPs in Water Suspensions
The concentration of NPs in water suspensions was determined gravimetrically [
13]. Before the measurement of 0.3 mL suspension of NPs in Milli-Q water into previously weighed glass vials, the suspension was sonified for 10 min. After measuring the mass of suspension on the analytical balance with an error of 0.1 mg, water was completely evaporated, and the remaining NPs were dried using high vacuum (2.25 mmHg) for 1 h. The concentration of NPs was calculated according to the next equation: concentration of NPs (mg/mL) = (mass of vial with dried NPs- mass of empty vial)/(mass of vial with suspension-mass of vial with dried NPs).
2.2.5. Determination of NPs Size
The hydrodynamic diameter of the NPs was measured by dynamic light scattering (DLS) using the Malvern zetasizer Nano-ZS ZEN 3600 (Malvern Panalytical, Great Britain). One hour before the measurement, NP dispersions were held in an ultrasonic bath for 10 min. Measurement was performed with a 60 s equilibration period at 25 °C in triplicate without moving the cuvette (runs of 1 measurement:10; run duration: 10 s; delay between runs: 0 s). Parameters for size calculation: 173º backscatter detection; material RI: 1.636; dispersant: water; dispersant RI: 1.330, viscosity (cP): 0.887.
2.2.6. Determination of Zeta Potential of NPs
Zeta potential of NPs at different stages of purification from the surfactant were measured in MilliQ water or 0.5% SDS using the Malvern zetasizer Nano-ZS ZEN 3600 according to [
14], in a DTS1070 cuvette (MalvernPanalytical, Great Britain). For measurement the same parameters were used as for the determination of NPs size.
2.3. Effects of NPs on Cells
2.3.1. Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
Buffy coats were obtained at the Department of Transfusion Medicine at Karolinska University Hospital and diluted 1:1 with phosphate buffered saline (PBS). The cell solution was carefully layered on top of Ficoll-Paque Plus in 50ml PP tubes and PBMCs were separated by 400 g, 30 min centrifugation without brake. The interphase was carefully collected and washed twice with PBS at 300 g, 10 min. PBMCs were counted using trypan blue in a Bürker counting chamber.
For all experiments PBMCs were incubated with NPs in cRPMI medium (complemented with 2% heat inactivated human AB serum, L-Glutamine, penicillin and streptomycin) was used.
2.3.2. MTT Assay
PBMCs were seeded (150.000 cells per well) in 96-well plates stimulated with different NPs (washed or unwashed) in concentration range (0.001 to 100 µg/mL). Untreated control represented cells without stimulant, while positive control were cells pretreated with 33% of DMSO. After 24h incubation at 37 ̊C in a humidified atmosphere with 5% CO2 a solution of MTT was added to each well and mixed to allow the metabolization of MTT. After the incubation of 4h, the medium was removed by centrifugation for 5min at 300g and aspiration by pipette tips, and formazan crystals were resuspended in 100 μL DMSO. The absorbance was read at 570 nm. All experiments were performed in duplicates.
2.3.3. Annexin V Assay
The induction of apoptosis by NPs on PBMCs was determined by a flow cytometry with Annexin V-FITC and propidium iodide (PI), according to manufacturer's instructions (BD Bioscience, San Jose, CA, USA). Briefly, 2×105 cells, treated with various concentrations of NPs washed and unwashed (0.1-100 µg/mL) for 24 h, were harvested and washed out with PBS. Thereafter, cells were resuspended in a binding buffer containing Annexin V and PI, incubated for 20 minutes, and analyzed by flow cytometry using FACSCanto II cytometer (BD Bioscience) with at least 20.000 events recorded. The FlowJo v10 software (TreeStar Inc., Ashland, USA) was used to analyze the data. Controls were unstained cells, cells stained only with Annexin V and cells stained only with PI.
2.3.4. DCFDA Cellular ROS Assay
For induction of ROS by NPs washed and unwashed in different concentrations (0,1-100 μg/mL), 2x105 cells were stimulated in tube for 4 and 24 h at 37 ̊C in a humidified atmosphere with 5% CO2. Cells were washed with PBS and stained with 5 μM H2DCFDA in PBS for 1h at 37 ̊C. Samples were analyzed by flow cytometry as stated above, where at least 50.000 events per sample were recorded. Positive control for ROS induction was 10 mM H2O2, added to the cells 1h before staining. For proper gating, non stained cells were used. Induction of ROS was analysed based on fold change from unstimulated non stained cells. The experiment was performed in duplicate.
2.3.5. Uptake of NPs by Monocytes
Isolated PBMCs (250.000 cells) were stimulated with different NPs washed and unwashed in concentration range 0.1-100 μg/mL for 4 and 24h at 37 ̊C in a humidified atmosphere with 5% CO2. Cells were washed with PBS and stained with anti-CD14 antibodies in APC (BD Bioscience, USA) for 30 min at 4 ̊C. After the washing step, cells were analysed by flow cytometry, where at least 50.000 events per sample were collected. CD14 gating was based on unstained cells. The uptake of NPs were analysed in FlowJo v 10 looking into the MFI of size scatter channel.
2.3.6. Hemolysis of Red Blood Cells
Preparation of red blood cells (RBCs) suspension
Human blood was obtained from healthy donors using an EDTA vacutainer and 21-gauge needle (BD Vacutainer®) on the day of experiment. The plasma and buffy coat were removed after centrifugation 5 min at 4000 rpm (Eppendorf® Minispin®, Hamburg, Germany). RBCs were washed once with (PBS, twice with 0.99% NaCl and once with Ringer's solution (NaCl 8.6 g/L, KCl 0.3 g/L, CaCl2 0.33g/L, NaHCO3 0.2g/L, pH 7.4). Isolated RBCs (0.150 mL) were resuspended in 50 mL of 0.99% NaCl or Ringer's solution with or without 5mM glucose and 45 g/L HSA.
Preparation of incubation mixture of RBC and NPs and measurement of RBCs hemolysis
The hemolytic effect of NPs on RBCs was measured in supernatant at 540 nm using Shimadzu UV/ViS 1800 (Kyoto, Japan) after incubation of prepared mixtures of RBCs and NPs 3 h at room temperature and centrifugation 2 min at 7000 rpm (Eppendorf® Minispin®, Hamburg, Germany). Incubation mixtures consist of 0.9 mL RBCs suspension in 0.99% NaCl or Ringer's solution with or without 5mM glucose and 45 g/L HSA and 0.1 mL suspension of PET NPs in MilliQ water. PET NPs washed and PET NPs unwashed were used for the preparation of suspensions in MilliQ water in the concentration ranges 1, 10 and 100 μg/mL. The control mixtures were prepared of 0.9 mL RBCs suspension in 0.99% NaCl or Ringer's solution (with or without 5mM glucose, 45 g/L HSA) and 0.1 mL MilliQ water or 0.9% NaCl (negative controls) or 0.1 mL 0.1, 0.03 and 0.003% SDS (surfactant controls). RBCs (0.9 mL) lysed with 1% Triton-X 100 (0.1 mL) were used as a positive control (100% hemolysis). All incubation mixtures were set up in triplicate. The percentage of hemolysis was calculated according to equation: percentage of hemolysis (%) = 100*A540 of sample/A540 of 1% Triton X-100.
2.4. Statistics and Graph Generation
Each assay was, if not stated otherwise, performed in duplicate. The results are expressed as mean ± standard deviation (S.D.). Statistical significance of obtained differences between samples were tested using a two-t-test and ANOVA One-way test with Tukey’s multiple comparison test. All statistical analysis and graphical representations of data were performed using the GraphPad Prism statistical program. P values less than 0.05 were considered as significant.
2.5. Ethics
In this study buffy coats were bought from Karolinska University Hospital where ethical permits are not required. Experiments with human blood were approved by The Swedish Ethics Review authority (Etikprövningsmyndigheten) with permit number No. 20112085-31/4. The study was performed following the declaration of Helsinki.
3. Results and Discussion
3.1. PET NPs Characterization
3.1.1. Yield and Concentration Determination of Synthetized NPs
Using a gravimetric method, yield and concentration of synthetized NPs were estimated. For the combined NPs suspensions, average concentration of 1.13 mg/mL was determined by the gravimetric method, giving an overall yield of 0.76%. Three NPs batches are fractionated according to size (from fractions NP-10 to NP-30, NP-40 to NP-60, NP-70 to NP-90) having the yield of 0.97%, 0.73%, 0.58%, respectively.
3.1.2. PET NPs Size Distribution
According to DLS measurements, synthetized PET NPs washed, dispersed in MilliQ water, BSA (0.05%) and SDS (0.1%), as well as NPs unwashed, showed average size distribution in the 200-300 nm range, with a relatively wide polydispersity index (around 0.4) (
Table 1,
Figures S3 and S4). Moreover, a similar average size distribution was measured also for PET NPs prepared from fractions NP-40 to NP-90 (
Table S1 and
Figures S5–S10). The hydrodynamic average distribution of NPs washed and dispersed in water is just slightly higher than in the presence of SDS and BSA, suggesting that even when NPs are dispersed in the absence of surfactants there is no NPs agglomeration.
3.1.3. Determination of SDS Level in Corona of PET NPs by 1H NMR
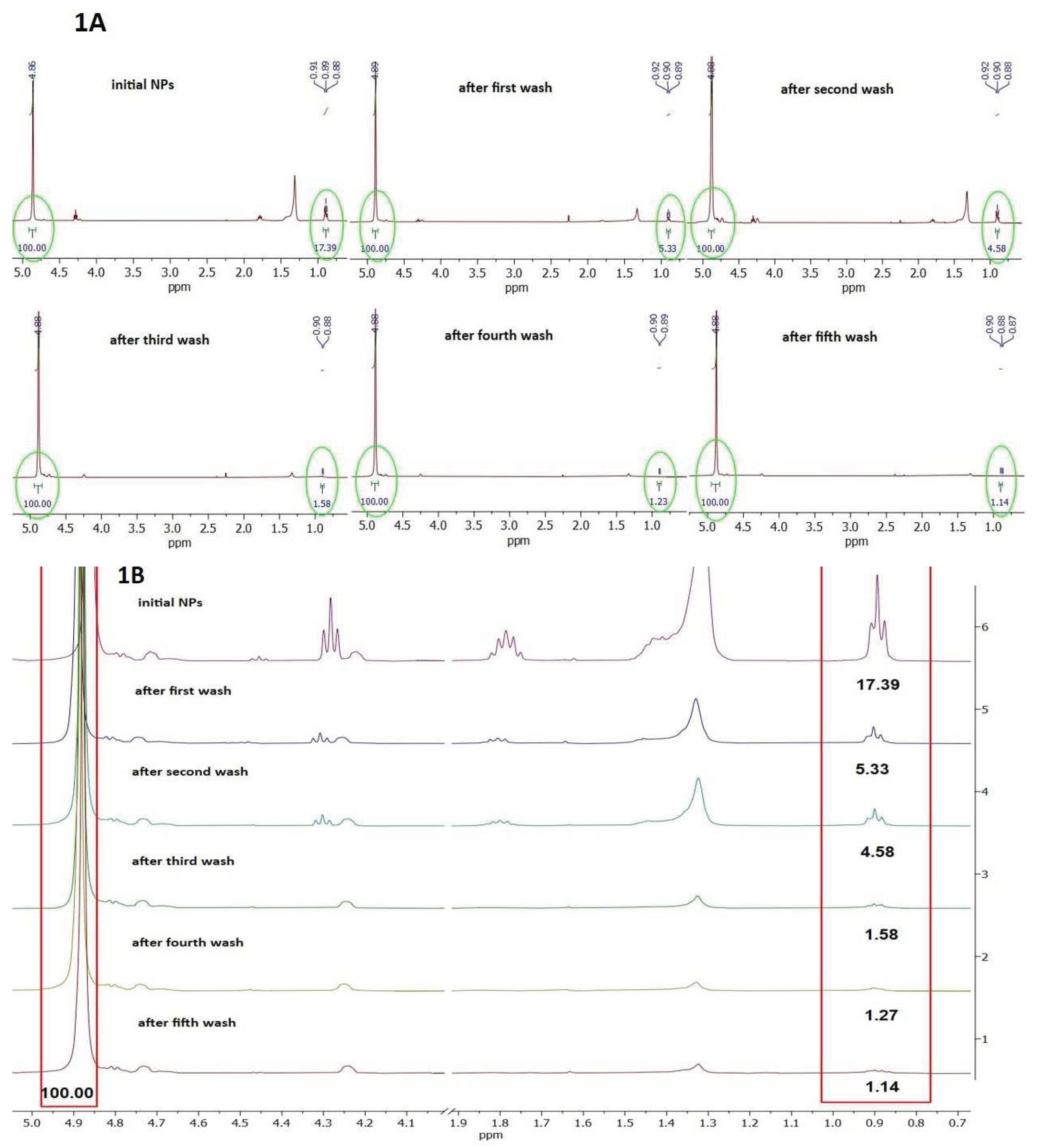
As no particle aggregation was observed when NPs were dispersed in Milli-Q water, we supposed that SDS remaining in NPs corona after extensive washing of NPs could be responsible for their dispersibility. Therefore, we intended to compare the level of SDS in the corona of NPs preparation before and after every washing step by
1H NMR (
Figure 1A,B).
Figure 1A presents
1H NMR spectra of NPs preparation before and during all washing steps in region 5.0 to 0.7 ppm.
Figure 1B presents zoom out spectra in region 0.7 to 1.9 ppm and 4.0 to 5.0 ppm. The peaks at 0.9, 1.35, 1.75 and 4.3. ppm originate from SDS, while peaks at 4.90, 4.73 and 4.26 ppm are from PET (
Figure 1B).
The level of SDS in all samples was estimated based on ratio of characteristic peak of terminal methyl group of SDS (signal multiplicity is triplet) centered at 0.9 ppm (0.88-0.92 ppm) and the peak of internal methylene groups of PET (signal multiplicity is singlet) centered at 4.88 ppm. It can be observed that the ratio of SDS/PET gradually decreases from 17.39 to 1.14 during extensive washing with water. However, this ratio reaches a plateau already after the third wash (ratio 1.58), with only a negligable decrease after further two washings (
Figure 1B and
Figure S11) finally decreasing the level of SDS in NPs corona for 15.25 times. As during every washing step about 200 μL of NPs were washed with 10 mL of water, it is obvious that a certain amount of SDS was adsorbed at NPs surface having low constant of desorption, thus resisting complete removal. These results suggest that adsorbed SDS forms a negatively charged surface on NPs resulting in NPs repulsion, explaining absence of NPs aggregation in water.
1H NMR spectra of solvent mixture, starting material (PET pellet) and SDS are presented in
Figures S12–S14.
3.1.4. Zeta Potential Determination of PET NPs
As SDS remains adsorbed in NPs corona even after their extensive washing, zeta potential of several NPs preparations were compared (
Table 2). NPs washed and dispersed in MilliQ water have a zeta potential of -34.93 mV, demonstrating profound negative charge on NPs surface, pointing to the presence of adsorbed SDS. When NPs washed preparation is dispersed in 0.5% SDS zeta potential is profoundly lower (-63.53 mV), suggesting that additional SDS molecules from dispersant adsorbed on NPs surface making its charge more negative. NPs unwashed preparation had zeta potential -42.10 mV.
Increase in zeta potential after repeated NPs rinsing was also reported for poly(methyl methacrylate) and poly(vinyl chloride) NPs produced by precipitation in the presence of SDS [
15]. Zeta potential of PET NPs obtained by milling and dispersing in 0.1% SDS was −66.7 mV, and in 1000 times diluted SDS (0.0001% SDS) zeta potential increased to -23.2 mV [
11]. Aguilar-Guzmán et al. [
16] produced PET NPs by nanoprecipitation and observed zeta potential of -47.5 mV for PET NPs of a hydrodynamic radius of 221 nm in 0.5% SDS, while after washing twice with ultrapure water and absolute ethanol and resuspension in water zeta potential was +3.6 mV with hydrodynamic radius of 2660 nm. This suggests that if SDS is completely removed insufficient charge on NPs surface results in NPs agglomeration. Interestingly, although Bashirova et al. [
17] did not use surfactants during PET NPs preparation by precipitation method, their zeta potential was −20±5 mV. Similarly, PET NPs prepared by laser ablation also without involvement of surfactants had a zeta potential of -43 mV [
18]. Therefore, it could not be excluded that in our preparation of washed NPs not only adsorbed SDS is contributing to low value of the zeta potential measured in the final preparation.
3.2. Effects of Residual Ionic Surfactant in NPs Preparations in Cellular Assays
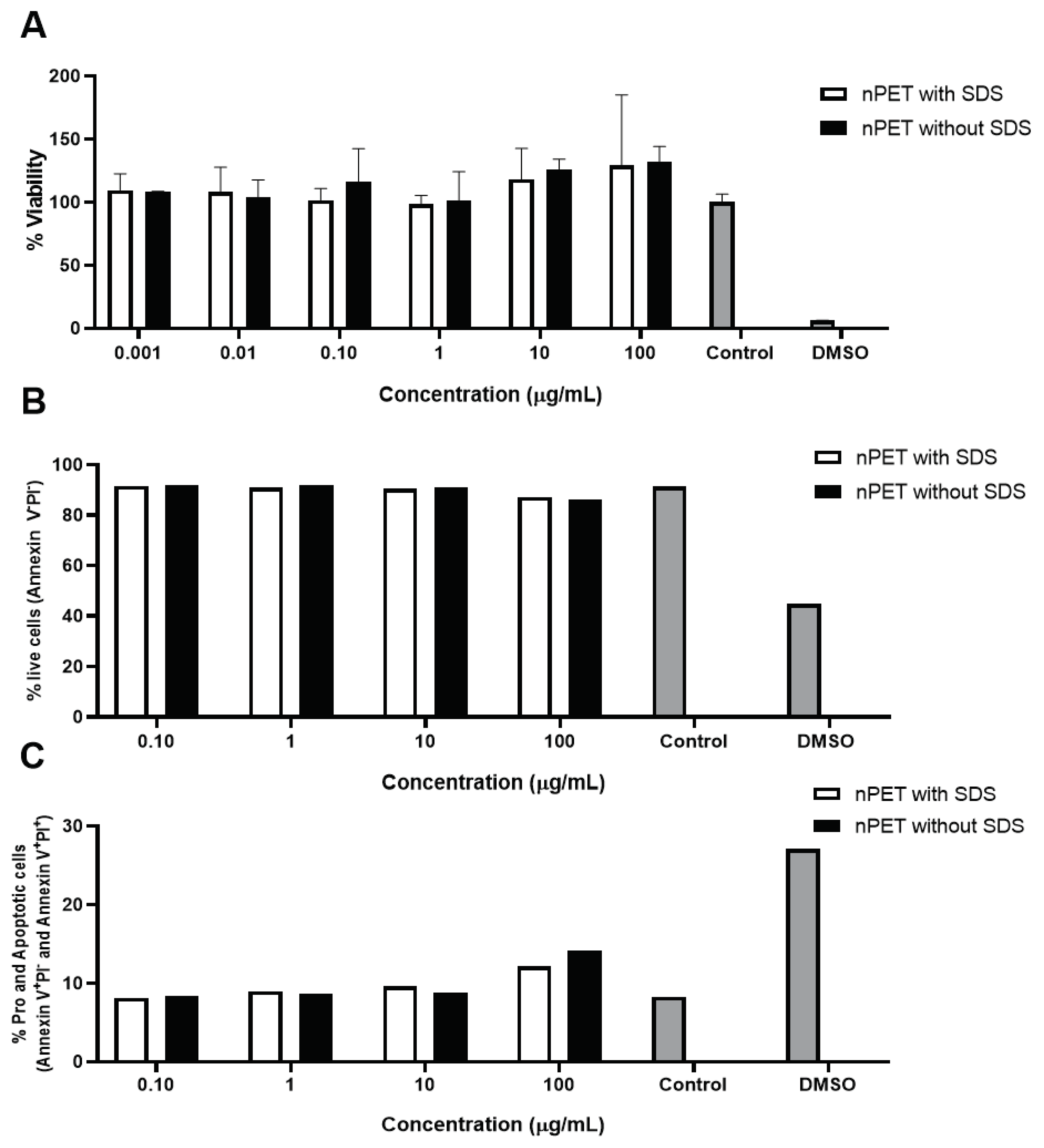
3.2.1. Cytotoxic Effects of PET NPs on Human Peripheral Blood Mononuclear Cells (PBMCs)
The presence of impurities, such as the presence of surfactants (e.g., cetyltrimethyl ammonium bromide or hexadecyl trimethylammonium chloride used to make mesoporous silica) may influence the results of toxicity evaluation [
19]. NPs preparations of high purity from surfactant (PET NPs washed) and intermediate stage of purification (PET NP unwashed) were subject to cytotoxicity testing by MTT assay on peripheral blood mononuclear cells (PBMCs). No acute (24 h) toxicity was observed in both preparations of NPs for all tested concentrations of NPs (
Figure 2A), suggesting that NPs do not exert acute toxicity, nor the trace amounts of SDS present in unwashed NPs contribute to toxicity increase. In the study of Magri et al. no alterations in cell viability was observed after Caco-2 cells treatment nor with PET NPs (136 nm) at 30 μg/mL even for 96 h, neither with PET NP-contaminant complexes (glyphosate, levofloxacin and Hg
2+ ions, suggesting a lack of short-term negative effects to the cells [
18]. By contrary, an apparent increase in % viability was noted in higher concentrations of PET NPs applied in the test, similarly to previously observed effect in macrophages cell line (50-250 nm) [
16], and human lung carcinoma cell A549 cells (164 nm) [
12]. Increase in cell viability was also observed in the presence of PS NPs (100 nm) on gastric adenocarcinoma cells at 10 μg/mL [
20].
Several studies observed no inhibitory effects of secondary PET NPs in similar concentration range (1-100 μg/mL) on different cell lines, such as primary human nasal epithelial cells (NPs 100 nm) [
21] or human lymphoblastic cell lines THP1 and TK6 (NPs 100 nm) [
14]. Inhibitory effect of primary PET NPs (164 nm) on A549 cells viability was observed at concentrations of 100 and 200 μg/mL [
12].
In addition to PET NPs, also NPs of other types (such as PS), and lower sizes (50 nm) have no acute toxic effect at concentrations < 100 μg/mL. For PS NPs (50 nm) the effects on cell viability on peripheral blood lymphocytes were obtained only at very high concentrations, 73.1 % and 39% viability at 500 μg/mL and 2000 μg/mL, respectively [
22]. No effect on Caco-2 cell viability was obtained by PS NPs (50 nm) at 39 μg/cm
2 [
23] and up to 240 μg/mL [
24]. For a 24-h exposure, no pronounced loss of human umbilical vein endothelial cell viability was observed in the presence of 100 nm or 500 nm PS-NPs, even when the PS-NPs concentration was up to 100 μg/mL [
25]. Therefore, regardless of NPs type and size, as well as types of cells (primary or secondary cell line of different types), in general, NPs have cytotoxic effects only at high concentrations (> 100 μg/mL). The main reason for this could be the protective effect of media, e.g., binding of NPs to media components, mainly proteins. Mahadevan et al. demonstrated that even at concentration of 0.4%, SDS was nontoxic to the BHK-21 cells, while 4% SDS decreased cell viability only for 30% [
15], and this seems to be also due to the protective effect of protein-rich cell culture medium.
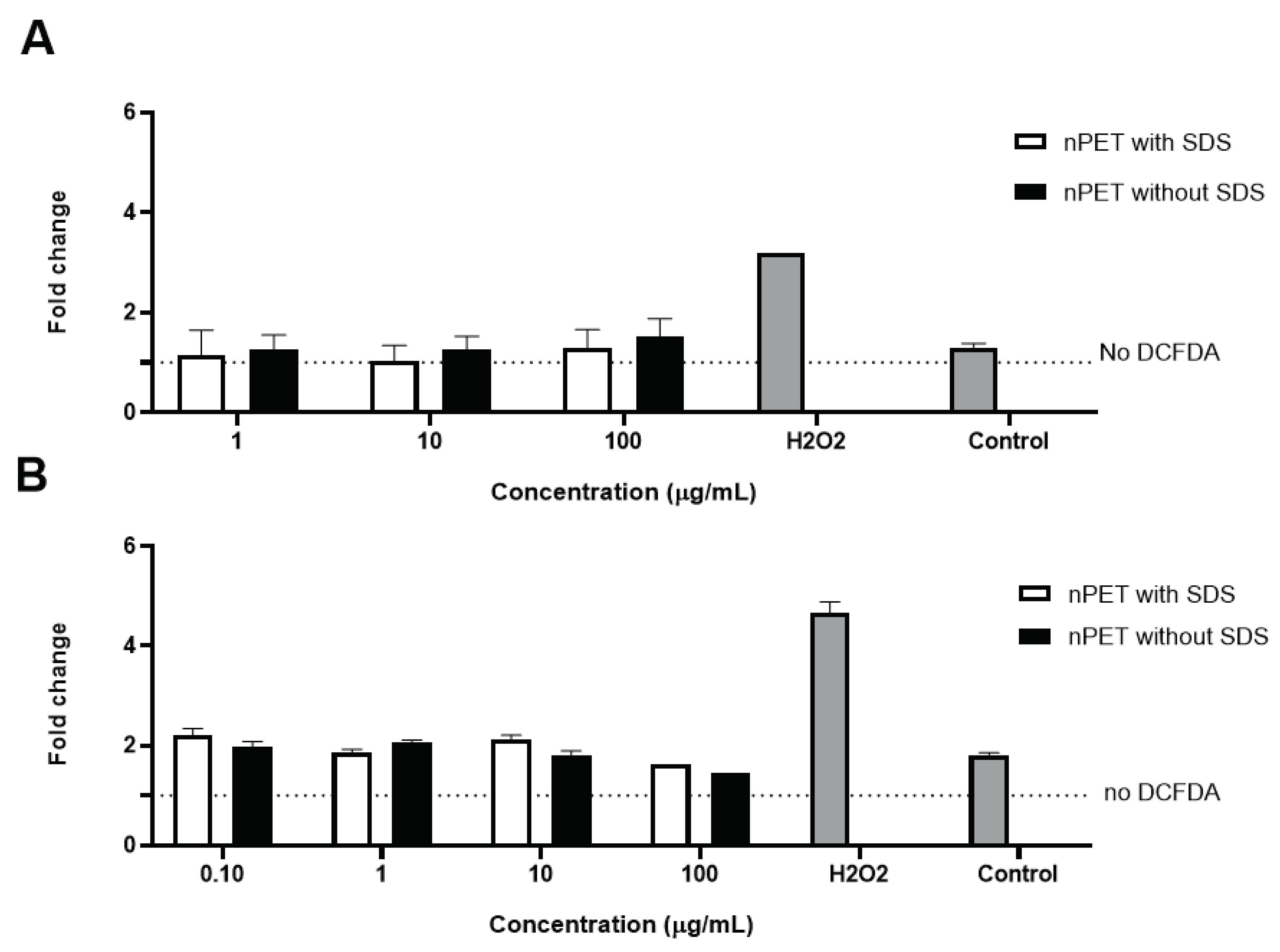
Results of apoptotic measurements (
Figure 2B,C) and ROS determination (
Figure 3) in human PBMCs exposed to NPs were in line with low cytotoxicity of NPs observed in MTT assay (
Figure 2A). Percentage of live cells, pro- and apoptotic cells was analyzed by determination of PI incorporation by flow cytometry. Only the highest concentration (100 μg/mL) of NPs applied showed limited effects on cell apoptosis induction and ROS generation, but it was not statistically significant (
Figure 2B,C and
Figure 3A,B). Similarly, Annangi et al. [
21] have found a significant increase in ROS when the primary human nasal epithelial cells were exposed to 100 µg/mL PET NPs (100 nm). Aguilar-Guzman et al. [
16] reported that, in spite of PET NPs inducing increase in % of viability, PET NPs induced a slight but significantly increased intracellular ROS production at all concentrations assayed, although this ROS increase did not correlate to the PET-NPs concentration increment. Zhang et al. [
12] also have found that PET NPs induced ROS increase at concentration of 50 μg/mL, but without induction of cell apoptosis. However, as PBMCs are resistant to oxidative stress, similarly to macrophages [
26], slightly increased ROS production at the highest NPs concentration tested in our study is probably not deleterious to them, resulting in observed lack of cytotoxic effects. Domenech et al. [
23] found that also PS NPs (up to 6.5 μg/cm2) did not increase ROS in Caco-2 cells after 24 h and even after 8 weeks of exposure.
3.2.2. Hemolytic Effects of PET NPs on Human Red Blood Cells
It has previously been observed that NPs can interact with cell membranes. Severe effects during the process of biological interactions of NPs and cell membranes include damage of membrane structure and cell death. Various factors, such as type and surface charge, play an important role in this process. For example, polyethylene NPs fuse with the hydrophobic core of lipid bilayers and further form a network of disentangled, single polymeric chains. These complexes promote damage on the membrane structure and fluidity, and ultimately cell death [
27]. Red blood cells (RBCs) are particularly sensitive to membrane damage and burst if affected, releasing hemoglobin into solution that can easily be monitored by measuring absorbance at 540 nm to estimate magnitude of hemolysis. Therefore, the hemolytic effect of PET NPs washed and unwashed was determined on RBCs obtained from three healthy donors.
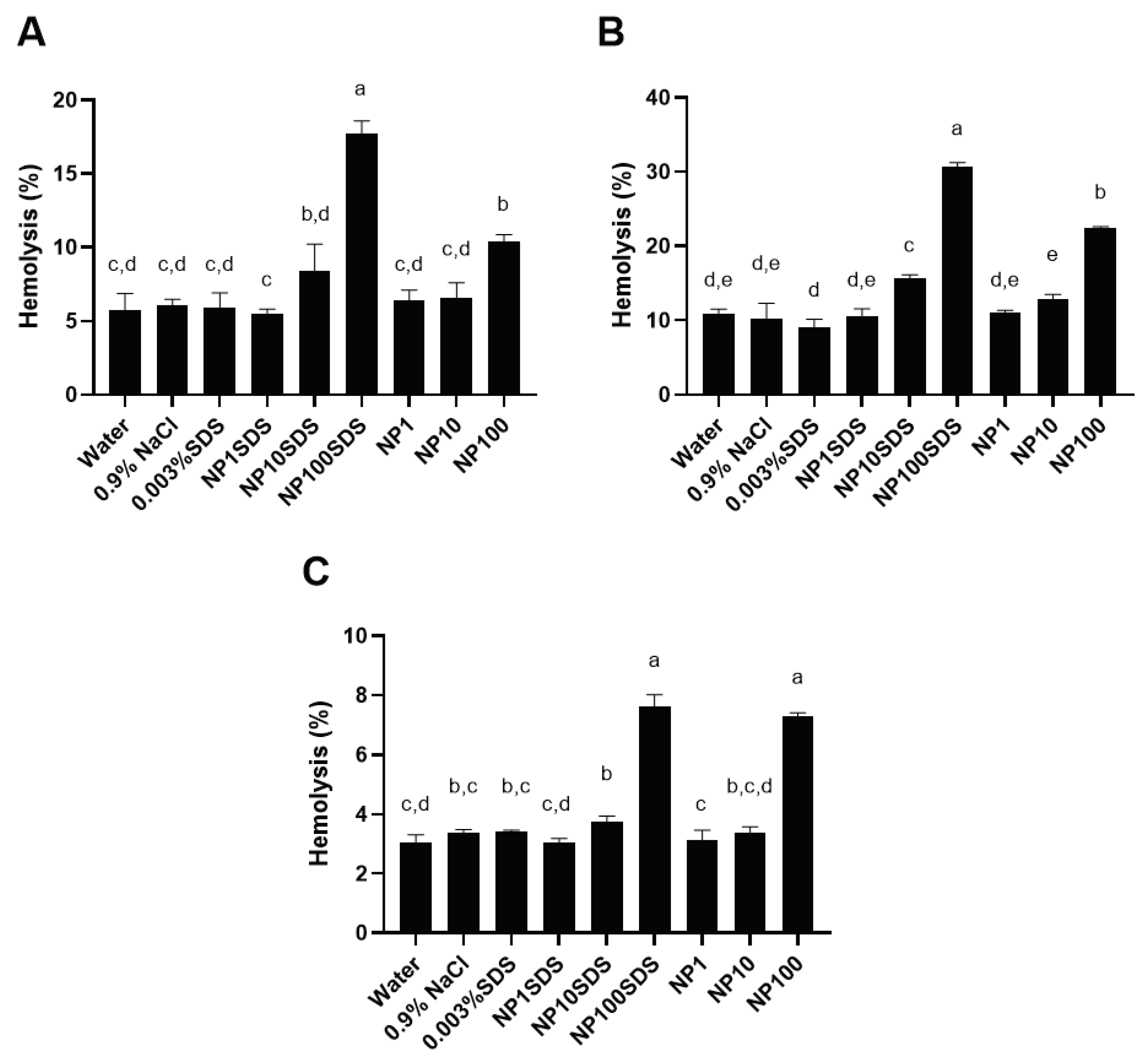
Depending on RBCs donor, presence of SDS in NPs preparation (washed and unwashed) and tested concentration of NPs, the obtained percentages of hemolysis were between 3 and 33% in comparison to 1% Triton X-100 (100% hemolysis (
Figure 4)). Similarly, for amine-modified PS NPs (100 nm) at 50 μg/mL and 100 μg/mL, human RBCs hemolysis of 10% and 26 % was observed when incubated in Tris-buffered saline [
28]. Interestingly, RBCs exposed to 100 µg/mL of 100 nm-sized plain, or carboxyl-modified PS NPs did not cause hemolysis of human RBC [
28].
For all three donors, a statistically significant (p<0.05) increase in hemolysis of RBCs was obtained with 10 µg/mL of unwashed sample of NPs (designated NP10SDS), but not for washed sample of NPs (designated NP10) for the same concentration. Both washed and unwashed NPs at the concentration of 100 µg/mL caused statistically significant increase in hemolysis of RBC. A statistically significant (p<0.05) difference in hemolysis of RBCs between PET NPs without and with SDS was obtained for concentration of 100 µg/mL for two out of three donors (donors 1 and 2 in
Figure 5).
According to these results it could be concluded that increase in RBC hemolysis was caused by increasing the concentration of NPs from 10 to 100 µg/mL and the presence of bound SDS in the soft corona. The more evident effect of bound SDS in the soft corona of NPs on hemolysis of RBC was noticed in NPs unwashed sample for donors 1 and 2 compared to the NPs washed sample. The different ranges of hemolysis percentages obtained for the highest concentration of NPs were expected because of individual differences between blood donors like count of RBCs, content of hemoglobin, and vulnerability of RBCs to hemolysis caused by lifestyle (diet, smoking, alcohol consumption). Besides, when effect of SDS in the concentrations 0.003, 0.3 and 0.1 % on percentages of RBCs hemolysis was tested, it was found that the lowest concentration of SDS had the effect like MilliQ water and 0.9% NaCl while 0.03% and 0.1 % SDS caused 100 % hemolysis (results are not shown), suggesting that there is quite a narrow range of SDS concentrations to which cells respond with 100% hemolysis (10x). Therefore, 15 times difference in amount of SDS adsorbed to NPs clearly caused a statistically significant difference observed in the response of RBC between different NPs preparations (washed vr. unwashed) and for the unwashed NPs decreased the concentration threshold to which cells responded with lysis.
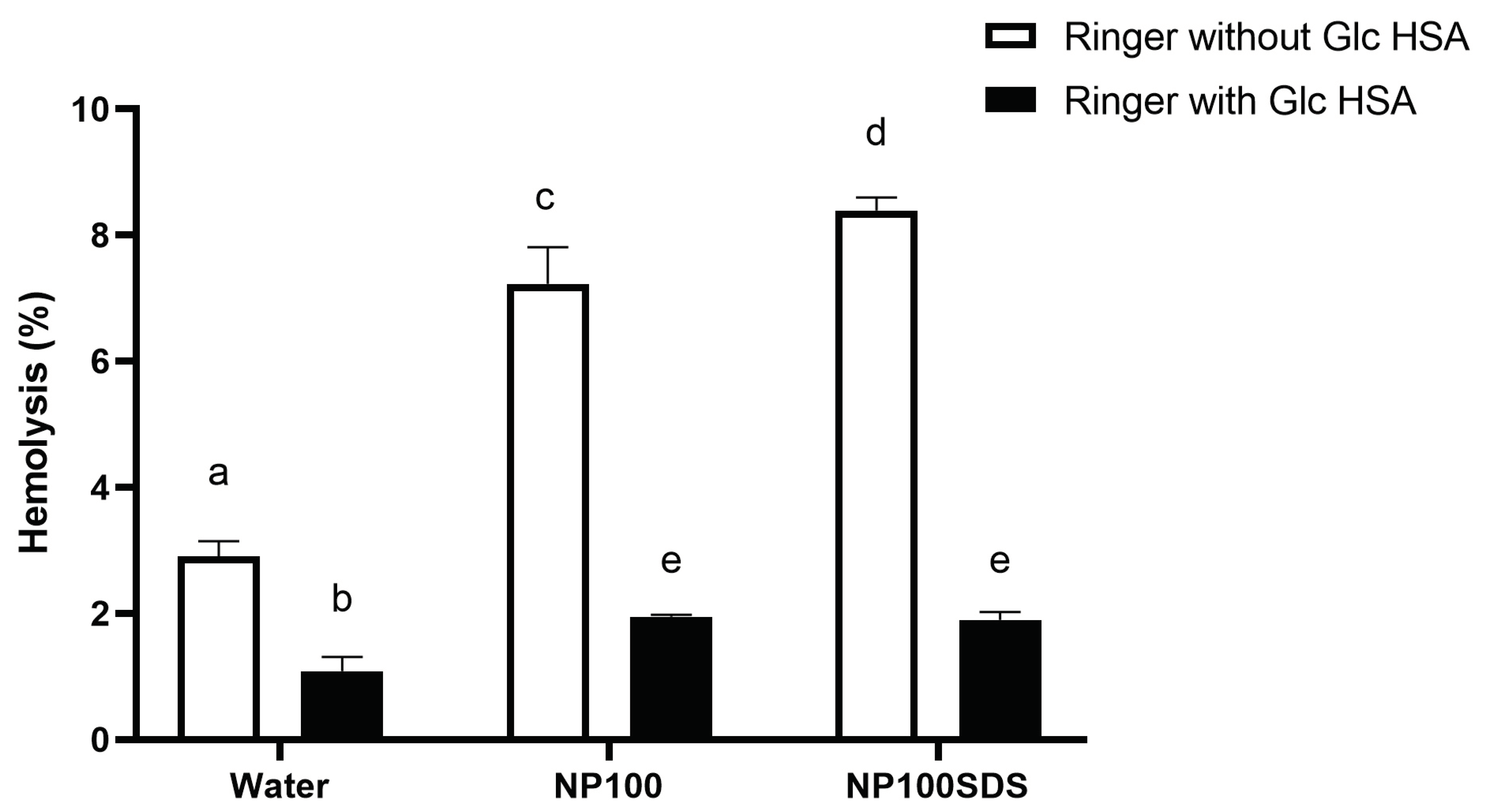
To check possible protective effects of substances, present in plasma on RBCs hemolysis by NPs, in the second experiment RBCs were incubated in Ringer's solutions which contains 5 mM glucose and 45 g/L HSA (
Figure 5). It was found that Ringer's solution with glucose and HSA in the physiological concentrations significantly (p<0.05) reduced hemolysis of RBCs, compared to hemolysis obtained in Ringer's solution for both samples of NPs washed and unwashed, as well as in the negative control (MilliQ water). There was no significant (p<0.05) difference obtained for NPs unwashed and washed samples in Ringer's solution with glucose and HSA. Moreover, the obtained hemolysis for both NPs in the presence of glucose and HSA was significantly (p<0.05) lower compared to the hemolysis obtained in negative control prepared from Ringer's solution. The obtained results confirm the observation of Barshtein et al. [
29] that human RBCs hemolysis by PS NPs (500 μg/mL) is inhibited by supplementation of the suspension medium (PBS) with albumin even at very low concentrations (0.05% wt). This suggests that proteins present in the incubation mixture in physiological concentrations can prevent toxic effects of NPs on RBC, e.g., that presence of protein molecules on the NP surface strongly modulates their interaction with RBCs and/or stabilize the membrane. Therefore, in assays performed in cell culture of primary mononuclear cells, where NPs are added to cell culture medium, formation of protein corona on NPs modulates their effects on cells in similar, most likely in protective, manner (
Figure 2 and
Figure 3). These results can explain why no acute (24 h) toxic effects of different types and size of plastics on different cell lines generally were not observed at the concentration of NPs up to 100 μg/mL, for PET NPs [
12,
14,
16,
18,
21], and for PS NPs [
22,
23,
24,
25].
According to the results of this study, as well as results of other researchers, it seems that besides standardization of type, size and concentration of NPs used for biological assays, also standardization of protocols in the biological assays are needed if we want to understand realistic toxic effects of NPs in biological systems.
On the other hand, Kim et al. [
28] demonstrated the uptake of amine-PS NPs into human RBCs and the morphological and functional changes in RBCs even at non-hemolytic concentrations, with externalization of phosphatidylserine, generation of microvesicles in RBCs, and perturbations in the intracellular microenvironment. This suggests that internalization and morphological/functional changes precede hemolysis, and that presence of SDS in our NPs may favors these changes, finally resulting in hemolysis.
3.2.3. Uptake of NPs by CD14+ Monocytes
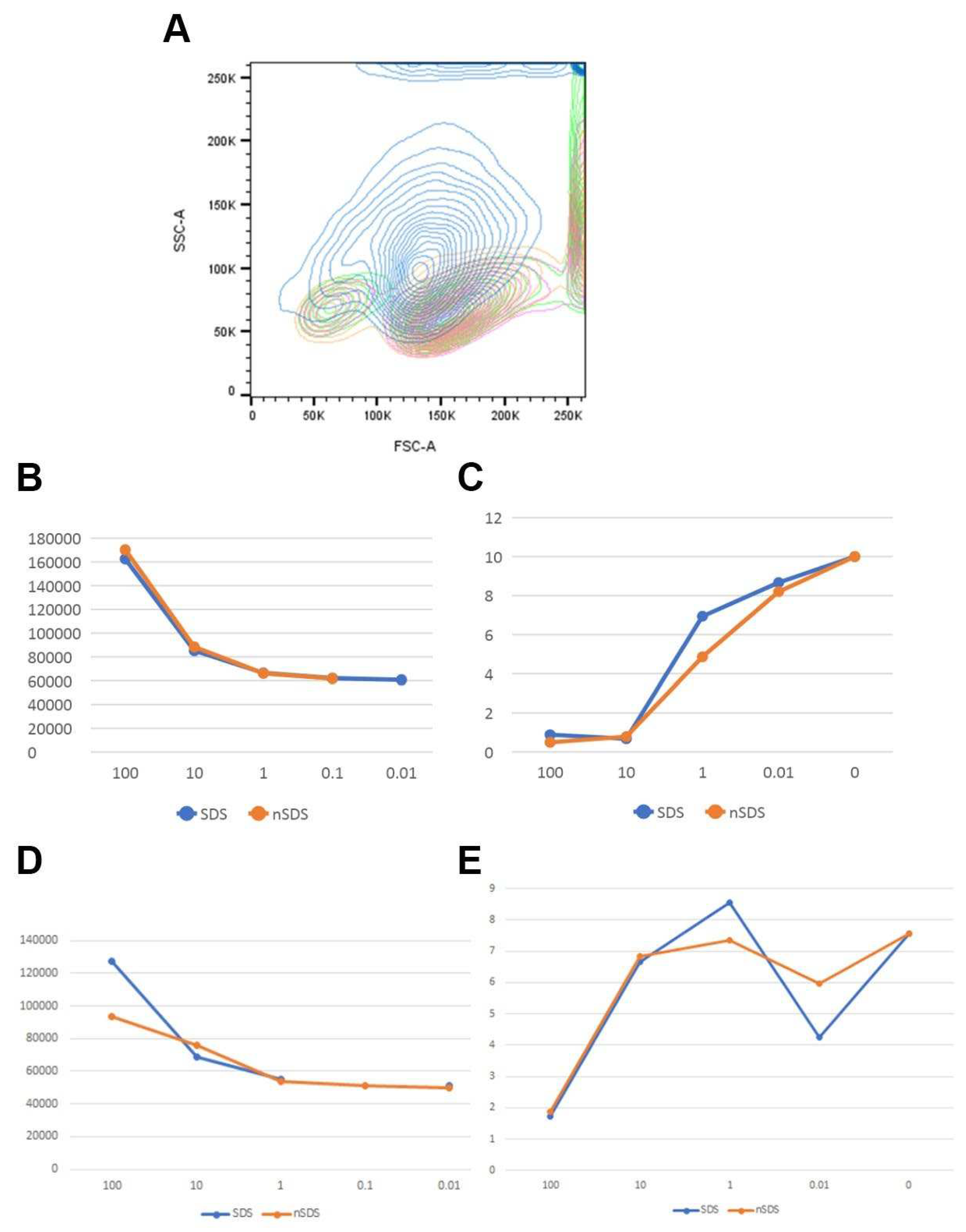
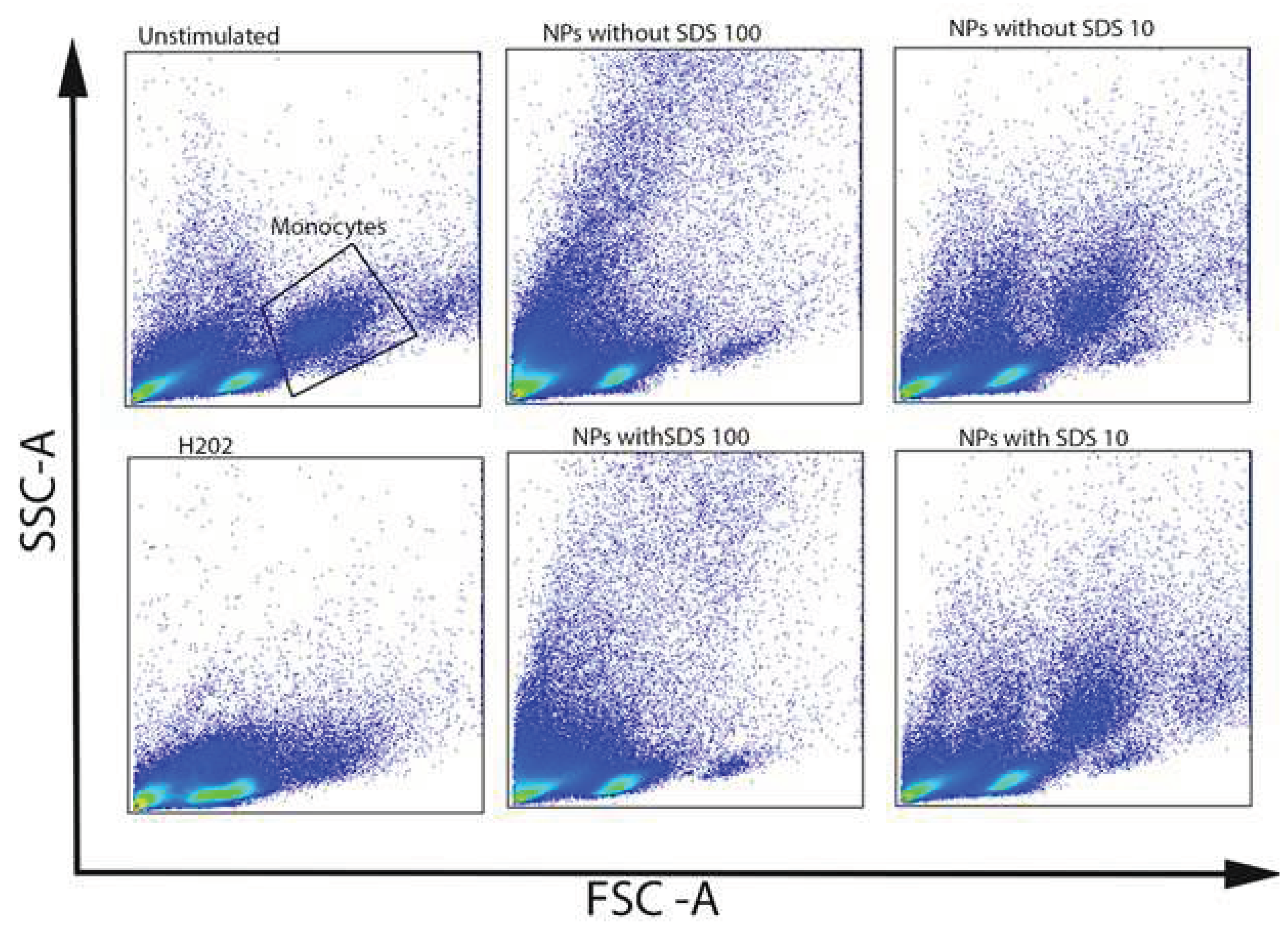
Both in vivo and in vitro experiments indicate that NPs can penetrate cell membranes and can be internalized into cells, inducing intracellular biological effects. It has been shown that the cellular granularity (measured by flow cytometry side scatter) can be predictive of NPs accumulation by cells [
30,
31]. PET NPs of different stages of purity were tested for uptake by CD14+ monocytes of human PBMCs. Side scatter shift, as a measure of uptake, was monitored for different concentrations of NPs, where two donors of PBMC were analyzed (
Figure 6 and
Figure 7). NPs were uptaken in a concentration-dependent manner. High side scatter shift was observed for the highest concentration tested, but it can also be noticed for the low NP concentration (
Figure 6B,D). Concomitantly, % of CD14+ cells drops down with the increase in uptake (
Figure 6C,E), suggesting that apoptotic processes observed in PBMC may preferentially affect the population of antigen-presenting cells, CD14+ monocytes (
Figure 6C,E and
Figure 7). Differences between NPs of different stages of purity with regards to the surfactant contents could not be observed. However, CD14+ monocytes appear to be more sensitive than other PBMCs (
Figure 7) and could not be protected by protein-rich cell medium. There were also donor-specific differences in CD14+ cells sensitivity to NPs, but not with regards to the presence of surfactant (
Figure 6C,E).
Magri et al. [
18] have shown that PET NPs (100 μg/mL) were mostly internalized in the endo-lysosomes of human Caco-2 intestinal epithelial cells. Similarly, Annangi et al. [
21] observed PET NPs (100 μg/mL) internalization in primary human nasal epithelial cells, supposing their localization in endosomes. In the study of Johnson et al. [
13], mouse macrophages cells (RAW 264.7) were exposed to PET NPs (50 μg/mL). NPs were observed inside phagocytic bodies where several cells had formed a tight phagosome around the NPs. Aguilar-Guzmán et al. [
16] observed internalization of PET NPs (15 μg/mL) by RAW 264.7 and revealed mechanism of internalization: macrophage recognize PET NPs as foreign particle, internalize it by macropinocytosis, and once inside, the small NPs cluster is separated into individual vesicles (phagosome). The similar mechanism could be expected for CD14+ monocytes of PBMC, thus explaining NPs concentration-dependent increase in the number of individual phagosomes within cells, as reflected in observed increased cell granularity.
In vivo, antigen presenting cells most likely uptake very low quantities of NPs, but if NPs are not degraded, chronic uptake can result in heavily loaded cells showing a number of disturbances that may impact their function, as demonstrated for PS NPs in mouse macrophage [
26]. Absence of measurable cytotoxicity does not mean that NPs have no effect on cells. The recent study [
32] observed extensive proteome changes in the absence of toxic effects of PS NPs on J774A.1 cell line (mouse macrophages), which are mostly adaptive changes, and proposed that NPs may affect the fine functioning of the innate immune system and have indirect adverse effects. Therefore, although no significant difference in PBMC response to NPs unwashed and NPs washed was observed in our study, it cannot be excluded that presence of SDS induced a different set of adaptive changes in cell proteome, particularly in chronic exposure settings. Ionic surfactant, as well as other cell-metabolism active impurities introduced during NPs production should be carefully monitored and reported to allow meaningful comparison of biological effects of NPs observed in different studies.
4. Conclusions
In this study we have shown that nanoprecipitated PET produced from PET granules of high purity, binds ionic surfactant, needed for NPs dispersion, in its corona throughout the purification procedure. In cellular assays sensitive to ionic surfactant (such as hemolysis of red blood cells) and particularly in the absence of proteins that may mitigate the negative effects, presence of SDS lowers the threshold of the cellular response to NPs and accounts for statistically significant differences observed between NPs of different purity level. We have shown that the relative ratio of surfactant (SDS) to PET NPs could be monitored by 1H NMR on the basis of chemical shift characteristics for PET and SDS. As size, shape and zeta potential of NPs may all be relevant parameters for biological effects of NPs, standardization in NP production methods and quality control of manufactured particles with auxiliary chemical methods that track production impurities is of utmost importance for the ongoing risk assessment of NPs to enable comparison of results obtained in different in vitro and in vivo studies.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 965173, IMPTOX. This research was also supported by the Serbian Academy of Sciences and Arts (grant number F-26) and the Ministry of Science, Innovation and technological development of the Republic of Serbia (Contract number: 451-03-68/2022-14/200168).
References
- Cai, H.; Xu, E.G.; Du, F.; Li, R.; Liu, J.; Shi, H. Analysis of environmental nanoplastics: Progress and challenges. Chem. Eng. J. 2021, 410, 128208. [Google Scholar] [CrossRef]
- Merdy, P.; Delpy, F.; Bonneau, A.; Villain, S.; Iordachescu, L.; Vollertsen, J.; Lucas, Y. Nanoplastic Production Procedure for Scientific Purposes: PP, PVC, PE-LD, PE-HD, and PS. Heliyon 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Cizdziel, J.V.; Wontor, K.; Olubusoye, B.S. Adsorption/Desorption of Mercury (II) by Artificially Weathered Microplastics: Kinetics, Isotherms, and Influencing Factors. Environ. Pollut. 2023, 337, 122621. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, J.; Thünemann, A.F. Aqueous Dispersions of Polypropylene: Toward Reference Materials for Characterizing Nanoplastics. Macromol. Rapid Commun. 2023, 44, 2200874. [Google Scholar] [CrossRef] [PubMed]
- Petersen, Elijah.J.; Barrios, A.C.; Henry, T.B.; Johnson, M.E.; Koelmans, A.A.; Montoro Bustos, A.R.; Matheson, J.; Roesslein, M.; Zhao, J.; Xing, B. Potential Artifacts and Control Experiments in Toxicity Tests of Nanoplastic and Microplastic Particles. Environ. Sci. Technol. 2022, 56, 15192–15206. [Google Scholar] [CrossRef] [PubMed]
- Pikuda, O.; Xu, E.G.; Berk, D.; Tufenkji, N. Toxicity Assessments of Micro- and Nanoplastics Can Be Confounded by Preservatives in Commercial Formulations. Environ. Sci. Technol. Lett. 2019, 6, 21–25. [Google Scholar] [CrossRef]
- Reynaud, S.; Aynard, A.; Grassl, B.; Gigault, J. Nanoplastics: From Model Materials to Colloidal Fate. Curr. Opin. Colloid Interface Sci. 2022, 57, 101528. [Google Scholar] [CrossRef]
- Heinlaan, M.; Kasemets, K.; Aruoja, V.; Blinova, I.; Bondarenko, O.; Lukjanova, A.; Khosrovyan, A.; Kurvet, I.; Pullerits, M.; Sihtmäe, M.; et al. Hazard Evaluation of Polystyrene Nanoplastic with Nine Bioassays Did Not Show Particle-Specific Acute Toxicity. Sci. Total Environ. 2020, 707, 136073. [Google Scholar] [CrossRef]
- Kelpsiene, E.; Ekvall, M.T.; Lundqvist, M.; Torstensson, O.; Hua, J.; Cedervall, T. Review of Ecotoxicological Studies of Widely Used Polystyrene Nanoparticles. Environ. Sci. Process. Impacts 2022, 24, 8–16. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, A.G.; Muñoz-Tabares, J.A.; Aguilar-Guzmán, J.C.; Vazquez-Duhalt, R. A Novel and Simple Method for Polyethylene Terephthalate (PET) Nanoparticle Production. Environ. Sci. Nano 2019, 6, 2031–2036. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, C.; Wang, Y.; Fu, L.; Man, M.; Chen, L. Realistic Polyethylene Terephthalate Nanoplastics and the Size- and Surface Coating-Dependent Toxicological Impacts on Zebrafish Embryos. Environ. Sci. Nano 2020, 7, 2313–2324. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Duan, Z.; Wang, L. Pulmonary Toxicology Assessment of Polyethylene Terephthalate Nanoplastic Particles in Vitro. Environ. Int. 2022, 162, 107177. [Google Scholar] [CrossRef]
- Johnson, L.M.; Mecham, J.B.; Krovi, S.A.; Caffaro, M.M.M.; Aravamudhan, S.; Kovach, A.L.; Fennell, T.R.; Mortensen, N.P. Fabrication of Polyethylene Terephthalate (PET) Nanoparticles with Fluorescent Tracers for Studies in Mammalian Cells. Nanoscale Adv. 2021, 3, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Villacorta, A.; Rubio, L.; Alaraby, M.; López-Mesas, M.; Fuentes-Cebrian, V.; Moriones, O.H.; Marcos, R.; Hernández, A. A New Source of Representative Secondary PET Nanoplastics. Obtention, Characterization, and Hazard Evaluation. J. Hazard. Mater. 2022, 439, 129593. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, G.; Valiyaveettil, S. Understanding the Interactions of Poly(Methyl Methacrylate) and Poly(Vinyl Chloride) Nanoparticles with BHK-21 Cell Line. Sci. Rep. 2021, 11, 2089. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Guzmán, J.C.; Bejtka, K.; Fontana, M.; Valsami-Jones, E.; Villezcas, A.M.; Vazquez-Duhalt, R.; Rodríguez-Hernández, A.G. Polyethylene Terephthalate Nanoparticles Effect on RAW 264. 7 Macrophage Cells. Microplastics Nanoplastics 2022, 2, 9. [Google Scholar] [CrossRef]
- Bashirova, N.; Poppitz, D.; Klüver, N.; Scholz, S.; Matysik, J.; Alia, A. A Mechanistic Understanding of the Effects of Polyethylene Terephthalate Nanoplastics in the Zebrafish (Danio Rerio) Embryo. Sci. Rep. 2023, 13, 1891. [Google Scholar] [CrossRef]
- Magrì, D.; Veronesi, M.; Sánchez-Moreno, P.; Tolardo, V.; Bandiera, T.; Pompa, P.P.; Athanassiou, A.; Fragouli, D. PET Nanoplastics Interactions with Water Contaminants and Their Impact on Human Cells. Environ. Pollut. 2021, 271, 116262. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Zhang, Y.; Zhang, G.; Kang, Y.; Chen, A.; Feng, X.; Shao, L. The Toxicity of Silica Nanoparticles to the Immune System. Nanomed. 2018, 13, 1939–1962. [Google Scholar] [CrossRef]
- Forte, M.; Iachetta, G.; Tussellino, M.; Carotenuto, R.; Prisco, M.; De Falco, M.; Laforgia, V.; Valiante, S. Polystyrene Nanoparticles Internalization in Human Gastric Adenocarcinoma Cells. Toxicol. In Vitro 2016, 31, 126–136. [Google Scholar] [CrossRef]
- Annangi, B.; Villacorta, A.; Vela, L.; Tavakolpournegari, A.; Marcos, R.; Hernández, A. Effects of True-to-Life PET Nanoplastics Using Primary Human Nasal Epithelial Cells. Environ. Toxicol. Pharmacol. 2023, 100, 104140. [Google Scholar] [CrossRef]
- Sarma, D.K.; Dubey, R.; Samarth, R.M.; Shubham, S.; Chowdhury, P.; Kumawat, M.; Verma, V.; Tiwari, R.R.; Kumar, M. The Biological Effects of Polystyrene Nanoplastics on Human Peripheral Blood Lymphocytes. Nanomaterials 2022, 12, 1632. [Google Scholar] [CrossRef] [PubMed]
- Domenech, J.; de Britto, M.; Velázquez, A.; Pastor, S.; Hernández, A.; Marcos, R.; Cortés, C. Long-Term Effects of Polystyrene Nanoplastics in Human Intestinal Caco-2 Cells. Biomolecules 2021, 11, 1442. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Ma, Y.; Han, X.; Chen, Y. Systematic Toxicity Evaluation of Polystyrene Nanoplastics on Mice and Molecular Mechanism Investigation about Their Internalization into Caco-2 Cells. J. Hazard. Mater. 2021, 417, 126092. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-Y.; Li, H.; Ren, H.; Zhang, X.; Huang, F.; Zhang, D.; Huang, Q.; Zhang, X. Size-Dependent Effects of Polystyrene Nanoplastics on Autophagy Response in Human Umbilical Vein Endothelial Cells. J. Hazard. Mater. 2022, 421, 126770. [Google Scholar] [CrossRef] [PubMed]
- Collin-Faure, V.; Vitipon, M.; Torres, A.; Tanyeres, O.; Dalzon, B.; Rabilloud, T. The Internal Dose Makes the Poison: Higher Internalization of Polystyrene Particles Induce Increased Perturbation of Macrophages. Front. Immunol. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Liu, X.; Qu, M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials 2022, 12, 1298. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-H.; Choi, S.; Kim, D.; Park, H.J.; Bian, Y.; Choi, S.H.; Chung, H.Y.; Bae, O.-N. Amine-Modified Nanoplastics Promote the Procoagulant Activation of Isolated Human Red Blood Cells and Thrombus Formation in Rats. Part. Fibre Toxicol. 2022, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Arbell, D.; Yedgar, S. Hemolytic Effect of Polymeric Nanoparticles: Role of Albumin. IEEE Trans. Nanobioscience 2011, 10, 259–261. [Google Scholar] [CrossRef]
- Babonaitė, M.; Čepulis, M.; Kazlauskaitė, J.; Lazutka, J.R. Evaluation of In Vitro Genotoxicity of Polystyrene Nanoparticles in Human Peripheral Blood Mononuclear Cells. Toxics 2023, 11, 627. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Huang, R.; Tang, C.; Hu, C.; Ning, P.; Wang, F. Cytotoxicity and Genotoxicity of Polystyrene Micro- and Nanoplastics with Different Size and Surface Modification in A549 Cells. Int. J. Nanomedicine 2022, 17, 4509. [Google Scholar] [CrossRef] [PubMed]
- Collin-Faure, V.; Dalzon, B.; Devcic, J.; Diemer, H.; Cianférani, S.; Rabilloud, T. Does Size Matter? A Proteomics-Informed Comparison of the Effects of Polystyrene Beads of Different Sizes on Macrophages. Environ. Sci. Nano 2022, 9, 2827–2840. [Google Scholar] [CrossRef]
Figure 1.
1H NMR spectra of PET NPs preparation before and after washing steps. (A). 1H NMR spectra in region 5.0 to 0.6 ppm; (B). Zoom on characteristic chemical shifts of PET and SDS protons taken for relative quantification of SDS present in PET NPs. Relative ratio of SDS: PET was red boxed in the figure.
Figure 1.
1H NMR spectra of PET NPs preparation before and after washing steps. (A). 1H NMR spectra in region 5.0 to 0.6 ppm; (B). Zoom on characteristic chemical shifts of PET and SDS protons taken for relative quantification of SDS present in PET NPs. Relative ratio of SDS: PET was red boxed in the figure.
Figure 2.
Cytotoxicity of PET NP on human PBMCs tested by MTT (A) and apoptosis (B,C) assays obtained after PBMC exposure were exposed to PET NPs of different purity for 24 h, nPET with SDS (unwashed) and nPET without SDS (washed).
Figure 2.
Cytotoxicity of PET NP on human PBMCs tested by MTT (A) and apoptosis (B,C) assays obtained after PBMC exposure were exposed to PET NPs of different purity for 24 h, nPET with SDS (unwashed) and nPET without SDS (washed).
Figure 3.
ROS production in human PBMCs was monitored after 4h (A) and 24h (B) of exposure to PET NPs of different purity, nPET with SDS (unwashed) and nPET without SDS (was.hed)
Figure 3.
ROS production in human PBMCs was monitored after 4h (A) and 24h (B) of exposure to PET NPs of different purity, nPET with SDS (unwashed) and nPET without SDS (was.hed)
Figure 4.
Percentage of hemolysis in the presence of PET NPs washed and NPs unwashed (with SDS) determined by incubation of human red blood cells (RBCs) from three healthy donors (A - Donor No.1, B - Donor No. 2 and C - Donor No. 3) in 0.9% NaCl. The final incubation mixture consists of 0.9 mL RBCs in 0.99 % NaCl and 0.1 mL of tested solutions: water, 0.9% NaCl, 0.003% SDS, PET NPs unwashed 1, 10 and 100 μg/mL and PET NPs washed 1, 10 and 100 μg/mL. The percentage of RBCs hemolysis was calculated in relation to the hemolysis obtained for 1% Triton X-100 (100% hemolysis). Data were analyzed using one-way ANOVA with Tukey's multiple comparison test at a significance level of 0.05.
Figure 4.
Percentage of hemolysis in the presence of PET NPs washed and NPs unwashed (with SDS) determined by incubation of human red blood cells (RBCs) from three healthy donors (A - Donor No.1, B - Donor No. 2 and C - Donor No. 3) in 0.9% NaCl. The final incubation mixture consists of 0.9 mL RBCs in 0.99 % NaCl and 0.1 mL of tested solutions: water, 0.9% NaCl, 0.003% SDS, PET NPs unwashed 1, 10 and 100 μg/mL and PET NPs washed 1, 10 and 100 μg/mL. The percentage of RBCs hemolysis was calculated in relation to the hemolysis obtained for 1% Triton X-100 (100% hemolysis). Data were analyzed using one-way ANOVA with Tukey's multiple comparison test at a significance level of 0.05.
Figure 5.
Percentage of hemolysis in the presence of PET NPs washed and NPs unwashed (with SDS). determined by incubation of human red blood cells (RBCs) from one healthy donor (Donor 1) in Ringer solution with glucose and HSA. The final incubation mixture consists of 0.9 mL RBCs in Ringer's solution with or without 5 mM glucose and 45 g/L HSA and 0.1 mL of tested solutions: water, PET NPs washed 100 μg/mL and PET NPs unwashed 100 μg/mL. The percentage of RBC hemolysis was calculated in relation to the hemolysis obtained for 1% Triton X-100 (100% hemolysis). Data were analyzed using one-way ANOVA with Tukey's multiple comparison test at a significance level of 0..05.
Figure 5.
Percentage of hemolysis in the presence of PET NPs washed and NPs unwashed (with SDS). determined by incubation of human red blood cells (RBCs) from one healthy donor (Donor 1) in Ringer solution with glucose and HSA. The final incubation mixture consists of 0.9 mL RBCs in Ringer's solution with or without 5 mM glucose and 45 g/L HSA and 0.1 mL of tested solutions: water, PET NPs washed 100 μg/mL and PET NPs unwashed 100 μg/mL. The percentage of RBC hemolysis was calculated in relation to the hemolysis obtained for 1% Triton X-100 (100% hemolysis). Data were analyzed using one-way ANOVA with Tukey's multiple comparison test at a significance level of 0..05.
Figure 6.
A. Example of side-scatter shift observed for CD14+ cells of human PBMCs in the presence of different concentrations of NPs (blue - 100 μg/mL, orange - 10 μg/mL, pink - 1 μg/mL, green - control). B. PET NPs concentration-dependent side scatter shift of CD14+ cells of PBMC (SDS-NPs unwashed; nSDS - NPs washed) for donor 1. C. Percentage (%) of CD14+ cells in PBMC in the presence of different concentrations of PET NPs of different purity (SDS -NPs unwashed; nSDS - NPs washed ) for donor 1. B. PET NPs concentration-dependent side scatter shift of CD14+ cells of PBMC (SDS-NPs unwashed; nSDS - NPs washed) for donor 2. C. Percentage (%) of CD14+ cells in PBMC in the presence of different concentrations of PET NPs of different purity (SDS -NPs unwashed; nSDS - NPs washed) for donor 2.
Figure 6.
A. Example of side-scatter shift observed for CD14+ cells of human PBMCs in the presence of different concentrations of NPs (blue - 100 μg/mL, orange - 10 μg/mL, pink - 1 μg/mL, green - control). B. PET NPs concentration-dependent side scatter shift of CD14+ cells of PBMC (SDS-NPs unwashed; nSDS - NPs washed) for donor 1. C. Percentage (%) of CD14+ cells in PBMC in the presence of different concentrations of PET NPs of different purity (SDS -NPs unwashed; nSDS - NPs washed ) for donor 1. B. PET NPs concentration-dependent side scatter shift of CD14+ cells of PBMC (SDS-NPs unwashed; nSDS - NPs washed) for donor 2. C. Percentage (%) of CD14+ cells in PBMC in the presence of different concentrations of PET NPs of different purity (SDS -NPs unwashed; nSDS - NPs washed) for donor 2.
Figure 7.
Forward and side-scatter plots of PBMCs incubated with NPs with a and without SDS at the concentrations of 100 and 10 μg/mL.
Figure 7.
Forward and side-scatter plots of PBMCs incubated with NPs with a and without SDS at the concentrations of 100 and 10 μg/mL.
Table 1.
Size distribution (d. nm) and polydispersity index (PdI) of PET NPs washed dispersed in MilliQ water, BSA (0.05%) and SDS (0.1%), and NPs unwashed dispersed in MilliQ water determined by DLS.
Table 1.
Size distribution (d. nm) and polydispersity index (PdI) of PET NPs washed dispersed in MilliQ water, BSA (0.05%) and SDS (0.1%), and NPs unwashed dispersed in MilliQ water determined by DLS.
| Samples |
Dispersant |
Z- Average
(d.nm) |
Polydispersity Index (PdI) |
| NPs washed |
MilliQ water |
311.7 ± 4.4 |
0.375 ± 0.026 |
| BSA (0.05%) |
243.5 ± 1.3 |
0.352 ± 0.037 |
| SDS (0.1%) |
265.7 ± 5.9 |
0.351 ± 0.041 |
| NPs unwashed |
MilliQ water |
236.4 ± 1.2 |
0.325 ± 0.006 |
Table 2.
Zeta potential, mobility, and conductivity determination in PET NPs at different stages of purification.
Table 2.
Zeta potential, mobility, and conductivity determination in PET NPs at different stages of purification.
| |
NPs Washed
(Dispersed in MilliQ Water) |
NPs Unwashed
(Dispersed in MilliQ Water) |
NPs Washed
(Dispersed
in 0.5% SDS) |
| Zeta potential (mV) |
-34.93 ± 1.18 |
-42.10 ± 1.30 |
-63.53 ± 1.47 |
| Mobility (µmcm/V) |
-2.739 ± 0.094 |
-3.301 ± 0.100 |
-4.980 ± 0.114 |
| Conductivity (mS/cm) |
0.0205 ± 0.0001 |
0.0287 ± 0.0105 |
0.9710 ± 0.0301 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).