1. Introduction
In late December 2019, the world witnessed the emergence of Coronavirus Disease (COVID-19), stemming from the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Wuhan, Hubei province, China. The World Health Organization (WHO) officially declared it a pandemic on March 11, 2020. COVID-19 has left an indelible mark on healthcare systems across the globe, affecting them at every level and increased the existing risk of burnout for doctors [
1,
2]. The disease spectrum associated with this pathogen spans from mild, self-limiting infections to potentially fatal illnesses. Individuals with coexisting morbidities such as diabetes mellitus, obesity, and other lifestyle diseases face significantly elevated morbidity and mortality rates. Furthermore, patients with respiratory comorbidities like chronic obstructive pulmonary disease (COPD) experience poorer outcomes. Finally, anti-cancer treatments, including radiation therapy, chemotherapy, stem cell transplantation, and chimeric antigen receptor T-cell therapy, substantially elevate the risk of contracting COVID-19 due to their immunosuppressive effects. These therapies can occasionally mask prodromal symptoms like fever, potentially resulting in a delay in diagnosis [
3]. Hyperinflammation and cytokine storm syndrome represent significant contributors to the development of acute respiratory distress syndrome, multiorgan failure, and ultimately, mortality in individuals infected with SARS-CoV-2. It is essential for the field of therapeutics to continually advance and develop new and enhanced vaccines to address the constantly evolving nature of the virus [
4].
Numerous opportunistic infections have been documented in COVID-19 patients. These include
Aspergillus spp.,
Candida spp.,
Cryptococcus neoformans,
Pneumocystis jiroveci (
carinii),
Cytomegalovirus (CMV),
Herpes simplex virus (HSV),
Strongyloides stercoralis,
Mycobacterium tuberculosis, and
Toxoplasma gondii. Within COVID-19 pandemic lies another rising worldwide challenge, namely COVID-19 associated mucormycosis (CAM) [
5]. There is evidence of a rising number of cases among patients who have successfully recovered from initial COVID-19 infection, with the vast majority originating in the subcontinent region [
6]. Patients may have an increased susceptibility to CAM due to their compromised immune status, which can result from the viral infection itself, previous corticosteroid therapy, or uncontrolled blood sugar levels [
7]. Studies have indicated that the median duration for the development of mucormycosis is approximately 10 to 15 days following a COVID-19 diagnosis [
8]. Mucormycosis can present clinically as rhino-cerebral-orbital mucormycosis (RCOM), pulmonary, gastrointestinal, cutaneous, or disseminated mucormycosis. However, unlike other forms, cutaneous mucormycosis typically does not require an underlying impaired immune response for its development [
5]. It is often triggered by trauma or burns to the skin, leading to local edema and discoloration, which can progress to gangrene and the formation of nodular skin lesions [
9].
In addition to mucormycosis, COVID-19 patients should maintain a high level of suspicion for cytomegalovirus (CMV) reactivation [
10]. Reactivation can be attributed to drug-related immunosuppression, including prior corticosteroid use, tocilizumab, or hydroxychloroquine, or it may arise directly due to COVID-19 infection and the associated immunosuppression [
11,
12]. CMV reactivation can manifest in various ways, such as myocarditis, pneumonitis, proctitis, or disseminated CMV.
Opportunistic infections triggered by COVID-19 are a critical problem, and a diagnosis of mucormycosis is often delayed. Herein, we present a case of post-COVID pneumonia complicated by cutaneous mucormycosis and CMV infection.
2. Case Report
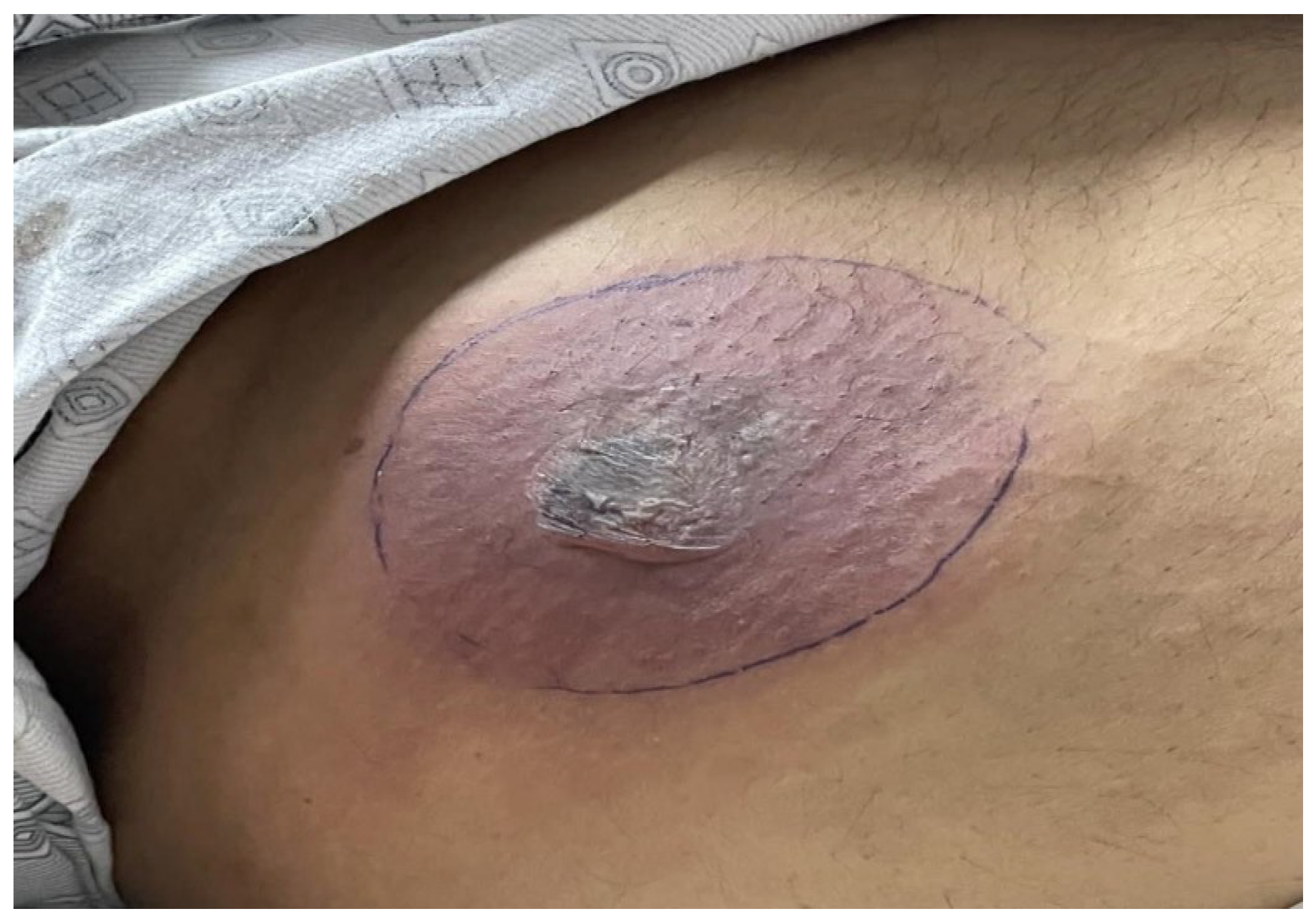
A 56-year-old male with a medical history of diabetes and hypertension, was admitted due to post-COVID pneumonia. Prior to this admission, the patient had received treatment for COVID-19 pneumonia at a nearby isolation center, where he had tested positive on a reverse transcriptase-polymerase chain reaction (RT-PCR) test. A high-resolution computerized tomography (CT) scan of the chest had revealed extensive lung fibrosis. Upon his admission to our medical facility, the patient was promptly initiated on appropriate treatment, including non-invasive ventilation (NIV). Of note that he presented with an anterior abdominal wound, as illustrated in
Figure 1. There was no accompanying fever or localized pain, and the patient did not report any recent trauma in the area, except for occasional discomfort related to insulin injections. The lesion appeared non-tender, with no associated discharge. There were no significant abnormalities in the standard laboratory tests (total leukocyte count 7.2*10
9/L, lactate dehydrogenase 481 U/L), apart from the increased C-reactive protein (227 mg/L) and HBA1c-glycosylated hemoglobin (10,1 %).
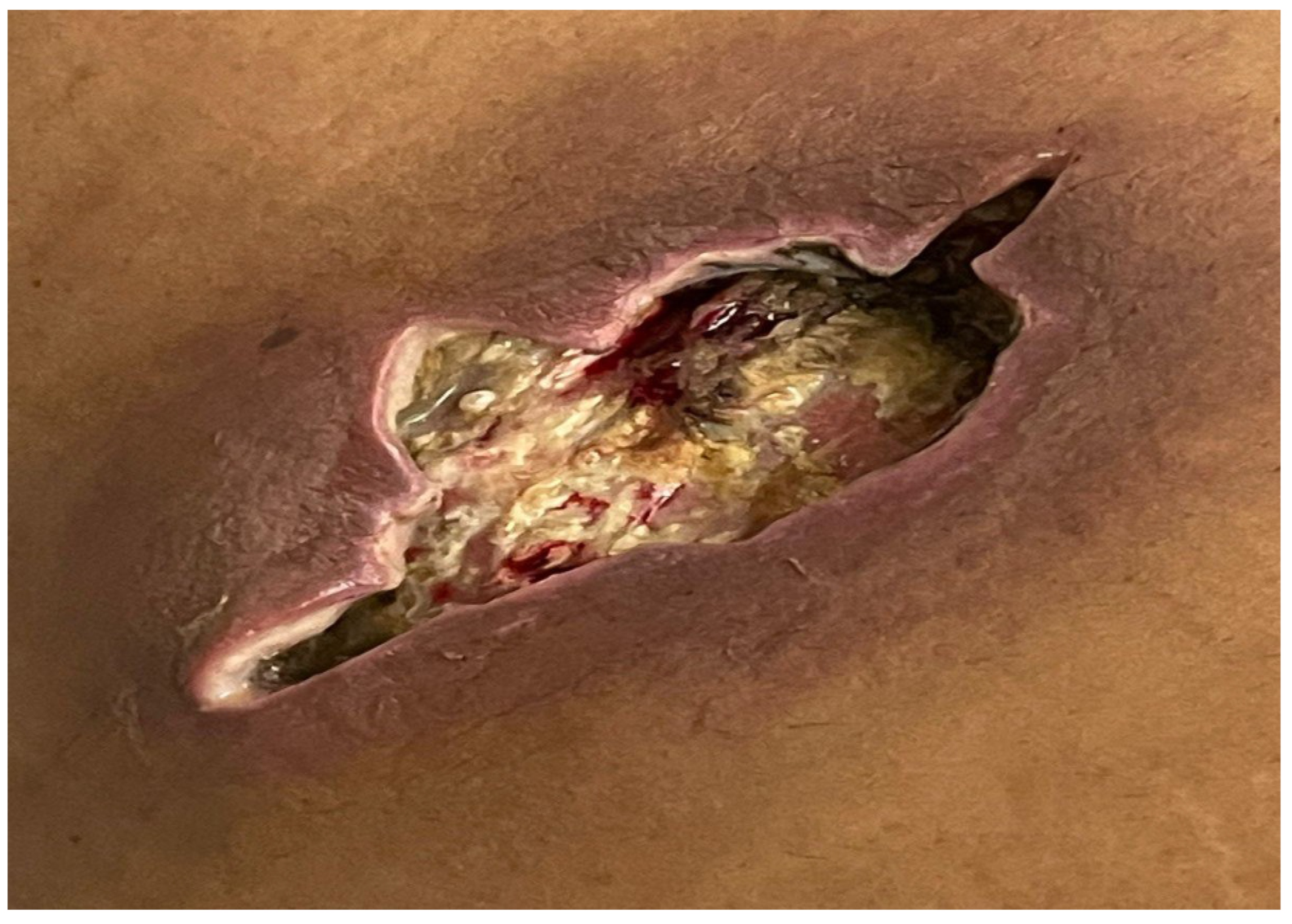
An abdominal ultrasound revealed an ill-defined, hyper-echoic, wide oval-shaped lesion in the subcutaneous soft tissues of the right hypochondrial region of the abdominal wall, measuring approximately 5.4*1.9 cm, likely of infectious origin. Two days later, a follow-up ultrasound showed an increase in the lesion's size to 7.5*2.1 cm. The patient subsequently underwent incision and drainage of the lesion, as depicted in
Figure 2, and a tissue specimen was collected for histopathological analysis. The patient was safely discharged the following day.
Later on that same day, the patient had to be readmitted due to severe hypoxemia. Over the next two days, the patient's respiratory condition significantly improved while continuing the previous treatment, leading to a transfer to a medical floor. Histopathological analysis of the abdominal wound specimen revealed invasive fungal growth, strongly suggestive of mucormycosis. Considering the increasing prevalence and high mortality associated with CAM worldwide, the patient was initiated on a 3-week regimen of intravenous liposomal amphotericin B at a daily dose of 5 mg/kg. Regular monitoring of serum electrolytes and renal function was conducted, with adjustments made to the amphotericin B dosage as needed. An abdominal CT scan, with and without contrast, was performed to assess the extent of the infection. The findings indicated a significant open wound within the dermal and subcutaneous soft tissues, localized to the anterior abdominal wall, with no remaining drainable collection or abscess and no intra-peritoneal extension. Following unanimous recommendations from the multidisciplinary team, the patient underwent surgical debridement of the necrotic tissue in the wound. Subsequently, daily abdominal wound dressings with Eusol and magnesium sulfate were initiated, along with oral posaconazole at a dose of 300 mg twice daily.
In the following weeks, the patient faced recurrent readmissions due to sepsis. A pus culture from the abdominal wound revealed multiple bacterial strains sensitive to several tested antibiotics. The patient was promptly started on appropriate antimicrobial agents. However, as there was limited clinical improvement, the possibility of a CMV infection was considered in the differential diagnosis. Subsequently, a positive PCR test confirmed the presence of CMV infection, likely associated with prior use of tocilizumab and corticosteroid therapy used to manage the initial COVID-19 infection. Consequently, intravenous ganciclovir therapy was initiated. Despite aggressive treatment with both antifungal and antiviral therapies, the patient's clinical condition continued to deteriorate and ultimately resulted in a fatal outcome.
3. Discussion
While systemic corticosteroid treatment has proven effective in reducing mortality among individuals with the most severe forms of COVID-19, it also carries the risk of predisposing patients to secondary fungal infections, along with various immunological and clinical factors. Although the literature on COVID-19 secondary infections has primarily focused on COVID-19-associated pulmonary aspergillosis, it is important to acknowledge that other fungal super-infections, such as candida infections, rare mold infections like fusariosis, and CAM, are likely to be under-reported [
13]. A recent analysis of both published and unpublished data reveals that CAM has made its presence felt in over 18 countries, including Pakistan, with a notable concentration of cases originating in India [
13]. While precise statistics detailing the extent of CAM in Pakistan are yet to be confirmed definitively, there has been a marked and alarming increase in the number of cases, closely paralleling the trend observed in its neighboring nation [
14].
Predisposing factors contributing to CAM include a post-COVID immunocompromised state, uncontrolled blood sugar levels, and prior corticosteroid therapy. Indeed, systemic corticosteroids have been recognized as a leading cause of drug-induced hyperglycemia. Individuals with diabetes and hyperglycemia frequently exhibit an inflammatory condition characterized by continuous recruitment and local activation of immune cells, such as macrophages and neutrophils. These immune cells release proinflammatory cytokines, sustaining persistent inflammation [
15]. In these cases, the activation of antiviral immunity against SARS-CoV-2 could potentially enhance this inflammatory phenotype, thereby creating conditions conducive to secondary infections. Of note that iron serves as an indispensable element for the growth of Mucorales. During episodes of ketoacidosis, free iron becomes abundantly available in the bloodstream. This surplus endogenous iron is readily absorbed by Mucorales, thereby augmenting their virulence. Conditions like hemochromatosis and the use of iron chelators such as deferoxamine elevate the risk of mucormycosis. Our patient had poorly controlled blood sugar levels, and was pretreated with corticosteroids before manifesting symptoms of cutaneous mucormycosis [
7]. As reported by Song G et al., severely ill COVID-19 patients who require prolonged hospitalization, admission to the intensive care unit (ICU), or invasive mechanical ventilation are particularly vulnerable to invasive mycoses [
16]. This aligns with our case, where our patient's deteriorating respiratory condition necessitated frequent readmissions, both in ICU and on the medical floor.
While cutaneous mucormycosis typically does not necessitate an underlying compromised immune response, our patient exhibited significant immunosuppression, which could indicate an impending poor clinical outcome. Trauma or burns to the skin are commonly associated with cutaneous mucormycosis [
5,
9]. Interestingly, similar to the case report by Tambe RR et al. and the five cases described by Rrapi R et al., our patient denied any prior trauma history [
17,
18]. Therefore, it is conceivable that even mild skin trauma resulting from repeated subcutaneous insulin injections may be adequate to predispose patients to mucormycosis, particularly when coupled with concurrent immunosuppression.
No biomarker currently exists for mucormycosis, making negative results for galactomannan and (1,3)-β-D-glucan assays unhelpful in ruling out the diagnosis. In the majority of cases, biopsy remains the primary diagnostic method. According to Rrapi R et al., persistent skin lesions in post-COVID immunosuppressed patients without prior trauma are often initially misdiagnosed as superficial fungal infections, such as Candida intertrigo [
18]. In such cases, it is imperative to promptly consider the possibility of cutaneous mucormycosis, using fungal cultures and direct microscopic analysis of tissue specimens, without delaying treatment while awaiting results [
19]. The identification of thick, non-septated, ribbon-like fungal hyphae invading tissue and inducing necrosis and angioinvasion in tissue sections stained with hematoxylin-eosin, periodic acid-Schiff stain, and Grocott-Gomori's methenamine silver stain proves valuable in distinguishing a genuine infection from tissue colonization. Moreover, the presence of an inflammatory reaction signifies that the organism represents an infection rather than a commensal presence [
20]. An evaluation of the infection's extent using CT and/or magnetic resonance imaging (MRI) scans is crucial, given that disseminated mucormycosis, albeit the rarest form, carries the highest overall mortality [
20,
21]. Nevertheless, patients with localized infections require aggressive treatment as they can experience a substantial rate of morbidity and mortality if not diagnosed and treated promptly [
22].
A multidisciplinary team approach is essential for optimizing the management of mucormycosis cases. Effective control of hyperglycemia and the avoidance of diabetic ketoacidosis are critical steps in preventing the progression of the disease. The use of high-dose corticosteroids, especially in poorly controlled diabetic patients, should be approached with caution. Even when indicated for severe COVID-19 cases, corticosteroid treatment should be administered judiciously, and unnecessary corticosteroid use in non-hypoxemic COVID-19 patients should be avoided. Effective management necessitates the combination of amphotericin B along with surgical debridement, a combination directly linked to significantly improved survival rates, whereas patients who do not receive treatment face nearly certain death [
23]. Among the intravenous amphotericin B options, lipid-based preparations, such as the liposomal formulation administered to our patient, are favored over the less expensive but more toxic amphotericin B deoxycholate [
24]. Despite aggressive surgical debridement and systemic antifungal therapy, mortality rates vary widely, ranging from 33.3% to 80%. In cases of disseminated disease with delayed intervention, mortality can reach up to 100% [
20]. Posaconazole can be used as an alternative therapy or in combination with amphotericin B in refractory cases, although there is no strong evidence of its superiority, and further studies are required. Additionally, following the initial treatment, step-down therapy with posaconazole or isavuconazole may be considered, as was the approach taken in our patient's case [
25].
While undergoing treatment for cutaneous mucormycosis, our patient was incidentally diagnosed with CMV co-infection. The differential diagnosis encompasses CMV co-infection due to the deteriorating clinical condition of the patient. A French retrospective analysis conducted by Simonnet A. et al. revealed that among 34 patients who received intensive care for COVID-19, five subsequently had PCR-confirmed CMV reactivation [
26]. Individuals with underlying COVID-induced immunosuppression, like our patient, and those who had received tocilizumab therapy are particularly susceptible to CMV reactivation [
27]. In a case series by Kim M. et al., eleven patients experienced post-COVID CMV reactivation, with 4 having previously received tocilizumab, and all of them had received prolonged courses of intravenous corticosteroids [
28]. However, among the 9 patients who received intravenous ganciclovir therapy, seven ultimately succumbed, indicating a poor prognosis despite treatment. Conversely, Aldehaim AY et al. described a case of post-COVID CMV pneumonitis that responded successfully to ganciclovir treatment, ultimately leading to complete recovery [
27]. Furthermore, a systematic review conducted by Wei LW et al. reported a mortality rate of 17.1% for cutaneous mucormycosis, which is notably lower than other types of mucormycosis [
29]. Unfortunately, despite aggressive antiviral, antifungal, and surgical therapy, like in the majority of previously documented cases of post-COVID cutaneous mucormycosis and CMV reactivation, our patient passed away within 2 months from the original diagnosis [
18,
28,
30]. Physicians should be aware of the increased risk of mucormycosis among COVID-19 patients. Prompt suspicion, early diagnosis, and timely treatment are vital factors for improving outcomes in affected individuals.