Submitted:
01 November 2023
Posted:
01 November 2023
You are already at the latest version
Abstract
Keywords:
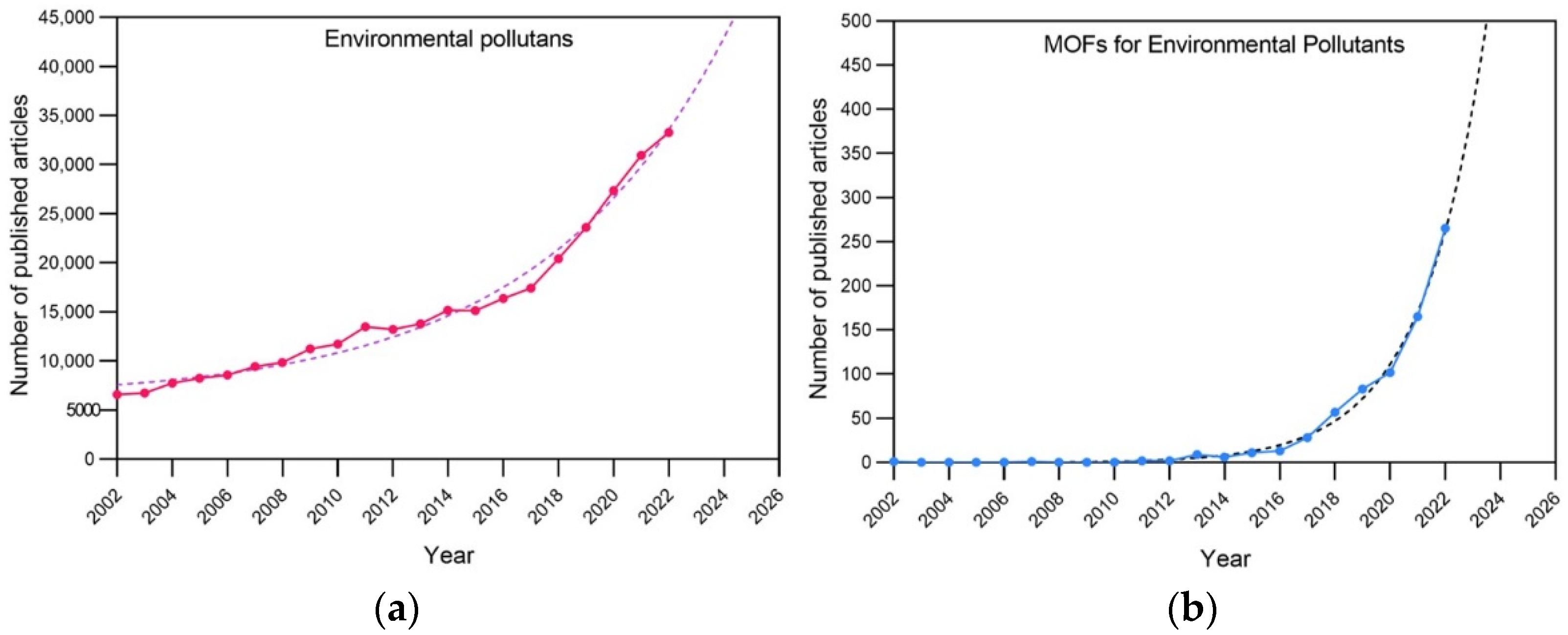
1. Introduction
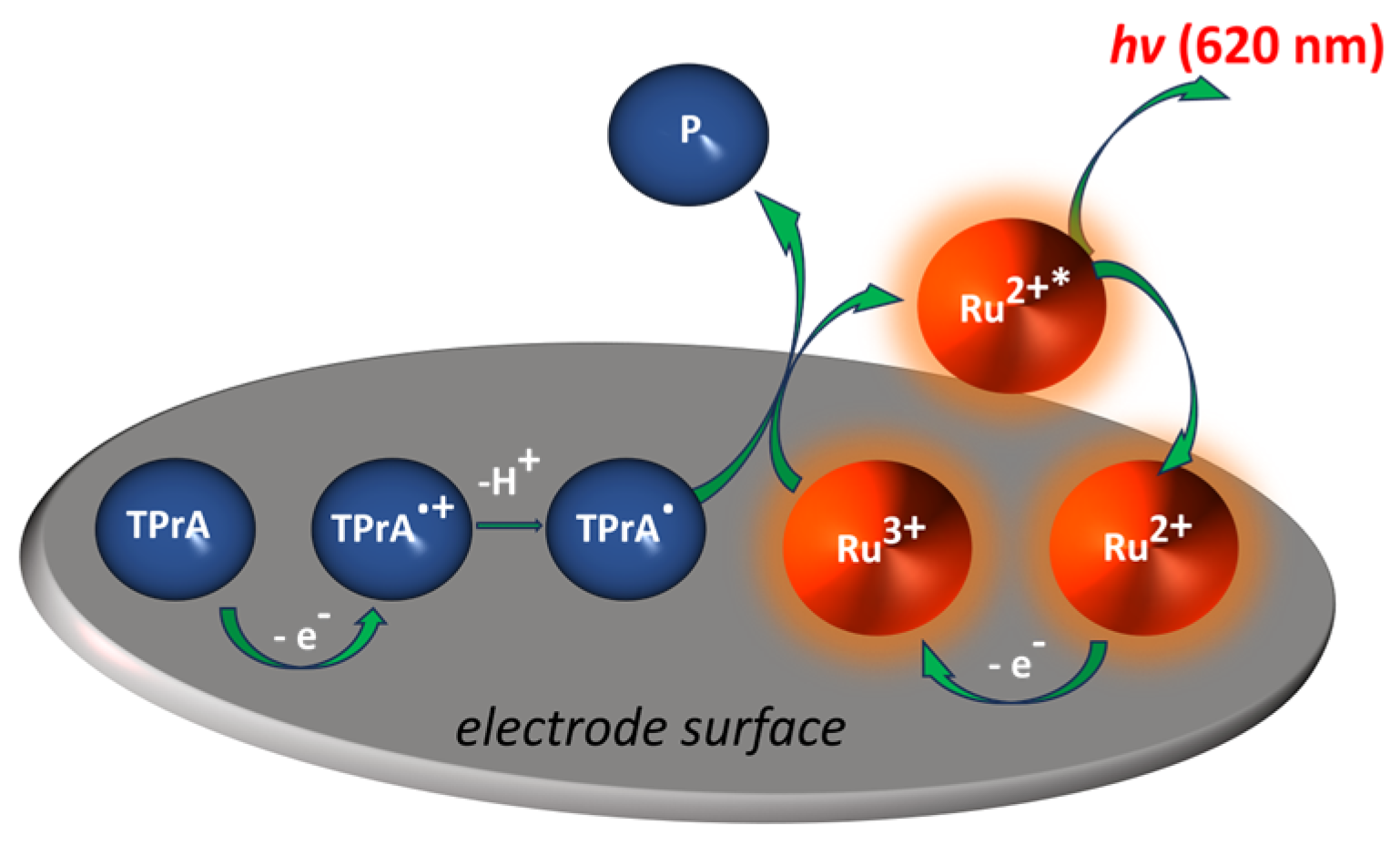
2. ECL sensors
3. MOFs for ECL sensors
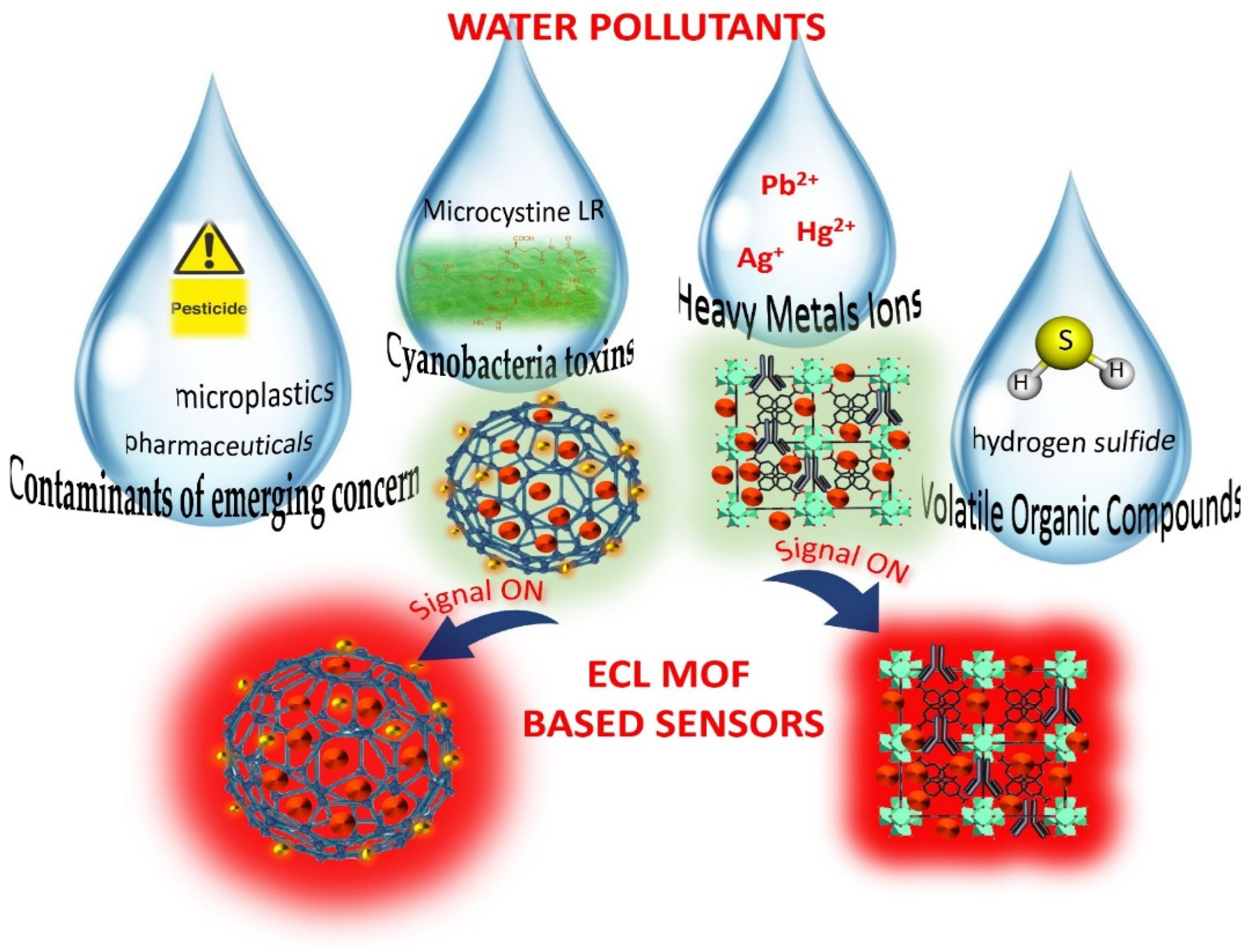
4. Applying ECL-active MOFs in water pollutant sensing
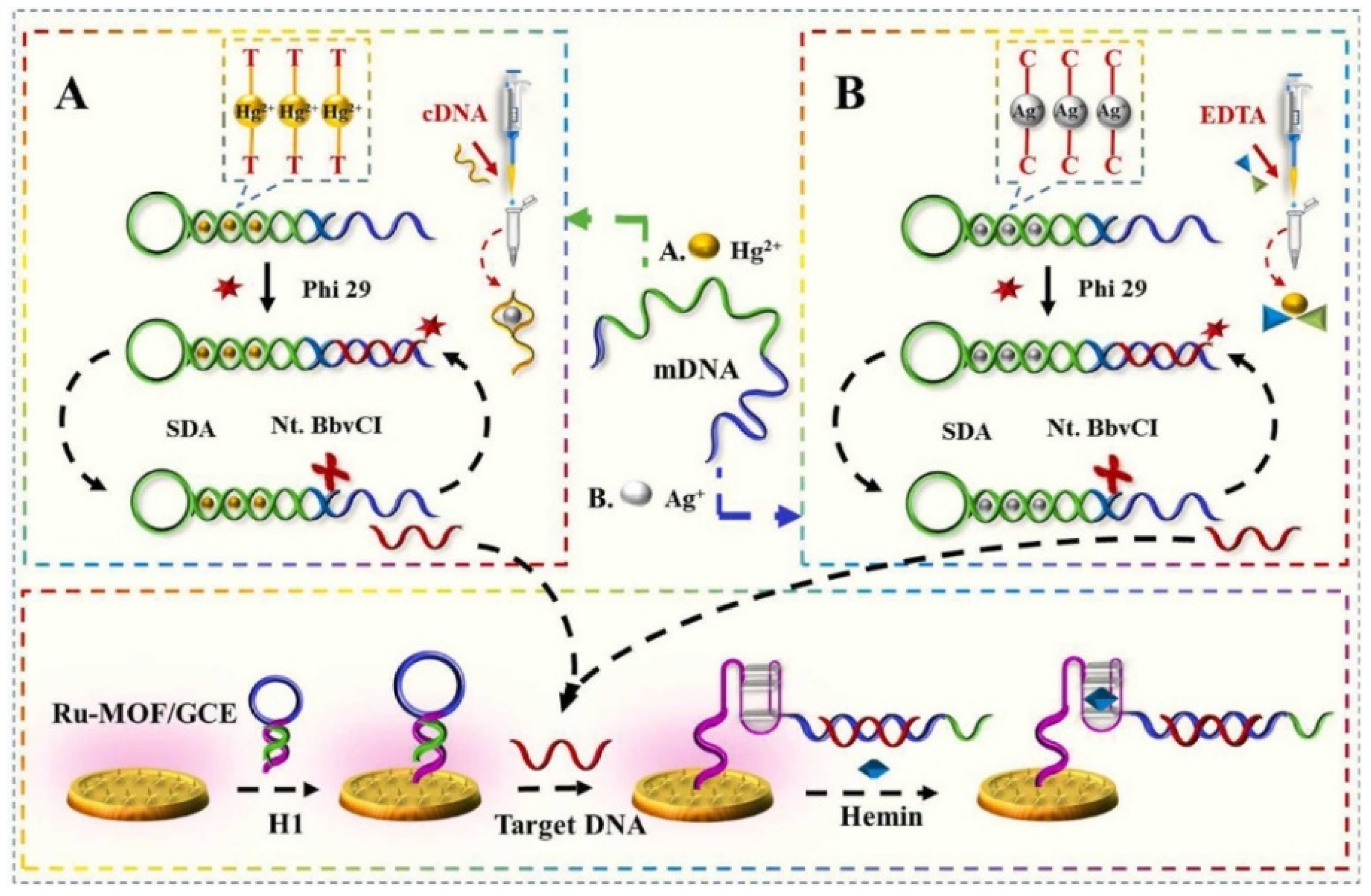
4.1. ECL MOF sensors for heavy metals detection
4.2. ECL MOF sensors for CEC detection
4.3. ECL MOF sensors for VOC detection
4.4. ECL MOF sensors for cyanotoxin detection in water
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Inglezakis, V.; Poulopoulos, S.; Arkhangelsky, E.; Zorpas, A.; Menegaki, A. Aquatic environment. In Environment and development, Elsevier: 2016; pp 137-212.
- Miletić, A.; Lučić, M.; Onjia, A. Exposure Factors in Health Risk Assessment of Heavy Metal (loid) s in Soil and Sediment. Metals 2023, 13, 1266. [Google Scholar] [CrossRef]
- Milošković, A.; Branković, S.; Simić, V.; Kovačević, S.; Ćirković, M.; Manojlović, D. The accumulation and distribution of metals in water, sediment, aquatic macrophytes and fishes of the Gruža Reservoir, Serbia. Bulletin of environmental contamination and toxicology 2013, 90, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Sakan, S.; Dević, G.; Relić, D.; Anđelković, I.; Sakan, N.; Đorđević, D. Evaluation of sediment contamination with heavy metals: The importance of determining appropriate background content and suitable element for normalization. Environmental geochemistry and health 2015, 37, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R. K.; Mentha, S. S.; Misra, Y.; Dwivedi, N. Emerging pollutants of severe environmental concern in water and wastewater: A comprehensive review on current developments and future research. Water-Ene.g., Nexus, 2023. [Google Scholar]
- Sangkham, S.; Islam, M. A.; Sarndhong, K.; Vongruang, P.; Hasan, M. N.; Tiwari, A.; Bhattacharya, P. Case Studies in Chemical and Environmental Engineering. Face mask and medical waste disposal during the novel Covid-19 pandemic in Asia, 2020. [Google Scholar]
- Knežević, S.; Jovanović, N. T.; Vlahović, F.; Ajdačić, V.; Costache, V.; Vidić, J.; Opsenica, I.; Stanković, D. Direct glyphosate soil monitoring at the triazine-based covalent organic framework with the theoretical study of sensing principle. Chemosphere 2023, 341, 139930. [Google Scholar] [CrossRef] [PubMed]
- Pantelić, D.; Svirčev, Z.; Simeunović, J.; Vidović, M.; Trajković, I. Cyanotoxins: Characteristics, production and degradation routes in drinking water treatment with reference to the situation in Serbia. Chemosphere 2013, 91, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Organization, W. H. Guidelines for drinking-water quality. World Health Organization: 2004; Vol. 1.
- Chorus, I.; Fastner, J.; Welker, M. Cyanobacteria and cyanotoxins in a changing environment: Concepts, controversies, challenges. Water 2021, 13, 2463. [Google Scholar] [CrossRef]
- Sharma, V. K.; Triantis, T. M.; Antoniou, M. G.; He, X.; Pelaez, M.; Han, C.; Song, W.; O’Shea, K. E.; de la Cruz, A. A.; Kaloudis, T. Destruction of microcystins by conventional and advanced oxidation processes: A review. Separation and Purification Technology 2012, 91, 3–17. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Bester, K.; Kümmerer, K. Xenobiotics in the urban water cycle: Mass flows, environmental processes, mitigation and treatment strategies. Springer Science & Business Media: 2010; Vol. 16.
- Jeong, C.; Ansari, Z.; Anwer, A. H.; Kim, S.-H.; Nasar, A.; Shoeb, M.; Mashkoor, F. A review on metal-organic frameworks for the removal of hazardous environmental contaminants. Separation and Purification Technology 2022, 122416. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, M. Nanomaterials in pollution trace detection and environmental improvement. Nano Today 2010, 5, 128–142. [Google Scholar] [CrossRef]
- Govindhan, M.; Adhikari, B.-R.; Chen, A. Nanomaterials-based electrochemical detection of chemical contaminants. RSC Advances 2014, 4, 63741–63760. [Google Scholar] [CrossRef]
- Bobbitt, N. S.; Mendonca, M. L.; Howarth, A. J.; Islamoglu, T.; Hupp, J. T.; Farha, O. K.; Snurr, R. Q. Metal–organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents. Chemical Society Reviews 2017, 46, 3357–3385. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. TrAC Trends in Analytical Chemistry 2018, 100, 155–166. [Google Scholar] [CrossRef]
- Grubišić, S.; Dahmani, R.; Djordjević, I.; Sentić, M.; Hochlaf, M. Selective adsorption of sulphur dioxide and hydrogen sulphide by metal–organic frameworks. Physical Chemistry Chemical Physics 2023, 25, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Mohan, B.; Kumar, S.; Kumar, V.; Jiao, T.; Sharma, H. K.; Chen, Q. Electrochemiluminescence metal-organic frameworks biosensing materials for detecting cancer biomarkers. TrAC Trends in Analytical Chemistry 2022, 116735. [Google Scholar] [CrossRef]
- Bobrinetskiy, I.; Radovic, M.; Rizzotto, F.; Vizzini, P.; Jaric, S.; Pavlovic, Z.; Radonic, V.; Nikolic, M. V.; Vidic, J. Advances in nanomaterials-based electrochemical biosensors for foodborne pathogen detection. Nanomaterials 2021, 11, 2700. [Google Scholar] [CrossRef] [PubMed]
- Hesari, M.; Ding, Z. Electrogenerated chemiluminescence: Light years ahead. Journal of The Electrochemical Society 2015, 163, H3116. [Google Scholar] [CrossRef]
- Sojic, N. Analytical electrogenerated chemiluminescence: From fundamentals to bioassays. Royal Society of Chemistry: 2019; Vol. 15.
- Liu, Z.; Qi, W.; Xu, G. Recent advances in electrochemiluminescence. Chemical Society Reviews 2015, 44, 3117–3142. [Google Scholar] [CrossRef] [PubMed]
- Rebeccani, S.; Zanut, A.; Santo, C. I.; Valenti, G.; Paolucci, F. A guide inside electrochemiluminescent microscopy mechanisms for analytical performance improvement. Analytical Chemistry 2021, 94, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, J.; Zhou, P.; Liu, J.; Qiao, Z.; Yu, K.; Jiang, J.; Su, B. Electrochemiluminescence Distance and Reactivity of Coreactants Determine the Sensitivity of Bead-Based Immunoassays. Angewandte Chemie International Edition 2023, 62, e202216525. [Google Scholar] [CrossRef]
- Zanut, A.; Fiorani, A.; Canola, S.; Saito, T.; Ziebart, N.; Rapino, S.; Rebeccani, S.; Barbon, A.; Irie, T.; Josel, H.-P. Insights into the mechanism of coreactant electrochemiluminescence facilitating enhanced bioanalytical performance. Nature communications 2020, 11, 2668. [Google Scholar] [CrossRef]
- Ma, X.; Gao, W.; Du, F.; Yuan, F.; Yu, J.; Guan, Y.; Sojic, N.; Xu, G. Rational design of electrochemiluminescent devices. Accounts of Chemical Research 2021, 54, 2936–2945. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, A.; Han, D.; Jiang, D.; Fang, D.; Paolucci, F.; Sojic, N.; Valenti, G. Spatially resolved electrochemiluminescence through a chemical lens. Chemical science 2020, 11, 10496–10500. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Han, S.; Hu, L.; Parveen, S.; Xu, G. Coreactants of tris (2, 2′-bipyridyl) ruthenium (II) electrogenerated chemiluminescence. Electrochimica acta 2012, 82, 484–492. [Google Scholar] [CrossRef]
- Ma, C.; Cao, Y.; Gou, X.; Zhu, J.-J. Recent progress in electrochemiluminescence sensing and imaging. Analytical chemistry 2019, 92, 431–454. [Google Scholar] [CrossRef]
- Zanut, A.; Palomba, F.; Rossi Scota, M.; Rebeccani, S.; Marcaccio, M.; Genovese, D.; Rampazzo, E.; Valenti, G.; Paolucci, F.; Prodi, L. Dye-doped Silica nanoparticles for enhanced ECL-based immunoassay analytical performance. Angewandte Chemie International Edition 2020, 59, 21858–21863. [Google Scholar] [CrossRef] [PubMed]
- Valenti, G.; Rampazzo, E.; Kesarkar, S.; Genovese, D.; Fiorani, A.; Zanut, A.; Palomba, F.; Marcaccio, M.; Paolucci, F.; Prodi, L. Electrogenerated chemiluminescence from metal complexes-based nanoparticles for highly sensitive sensors applications. Coordination Chemistry Reviews 2018, 367, 65–81. [Google Scholar] [CrossRef]
- Bertoncello, P.; Stewart, A. J.; Dennany, L. Analytical applications of nanomaterials in electrogenerated chemiluminescence. Analytical and bioanalytical chemistry 2014, 406, 5573–5587. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, X.; Wang, S.; Li, B.; Liu, B. Nanoconfinement-Enhanced Electrochemiluminescence for in Situ Imaging of Single Biomolecules. ACS nano 2023, 17, 3809–3817. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Cui, W.-R.; Jiang, Q.-Q.; Wu, Q.; Liang, R.-P.; Luo, Q.-X.; Qiu, J.-D. A general design approach toward covalent organic frameworks for highly efficient electrochemiluminescence. Nature Communications 2021, 12, 4735. [Google Scholar] [CrossRef]
- Zhao, Y.; Bouffier, L.; Xu, G.; Loget, G.; Sojic, N. Electrochemiluminescence with semiconductor (nano) materials. Chemical Science 2022, 13, 2528–2550. [Google Scholar] [CrossRef]
- Luo, R.; Lv, H.; Liao, Q.; Wang, N.; Yang, J.; Li, Y.; Xi, K.; Wu, X.; Ju, H.; Lei, J. Intrareticular charge transfer regulated electrochemiluminescence of donor–acceptor covalent organic frameworks. Nature Communications 2021, 12, 6808. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, Y.; Bao, S.; Wang, N.; Yu, S.; Luo, R.; Ma, J.; Ju, H.; Lei, J. Dual intrareticular oxidation of mixed-ligand metal–organic frameworks for stepwise electrochemiluminescence. Journal of the American Chemical Society 2021, 143, 3049–3053. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Chi, H.; Yang, S.; Niu, Q.; Wu, D.; Cao, W.; Li, T.; Ma, H.; Wei, Q. Self-luminescent lanthanide metal–organic frameworks as signal probes in electrochemiluminescence immunoassay. Journal of the American Chemical Society 2020, 143, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Al-Kutubi, H.; Voci, S.; Rassaei, L.; Sojic, N.; Mathwig, K. Enhanced annihilation electrochemiluminescence by nanofluidic confinement. Chemical Science 2018, 9, 8946–8950. [Google Scholar] [CrossRef]
- Li, H.; Daniel, J.; Verlhac, J. B.; Blanchard-Desce, M.; Sojic, N. Bright Electrogenerated Chemiluminescence of a Bis-Donor Quadrupolar Spirofluorene Dye and Its Nanoparticles. Chemistry–A European Journal 2016, 22, 12702–12714. [Google Scholar] [CrossRef]
- Sentic, M.; Virgilio, F.; Zanut, A.; Manojlovic, D.; Arbault, S.; Tormen, M.; Sojic, N.; Ugo, P. Microscopic imaging and tuning of electrogenerated chemiluminescence with boron-doped diamond nanoelectrode arrays. Analytical and bioanalytical chemistry 2016, 408, 7085–7094. [Google Scholar] [CrossRef]
- Huang, X.; Li, B.; Lu, Y.; Liu, Y.; Wang, S.; Sojic, N.; Jiang, D.; Liu, B. Direct Visualization of Nanoconfinement Effect on Nanoreactor via Electrochemiluminescence Microscopy. Angewandte Chemie International Edition 2023, 62, e202215078. [Google Scholar] [CrossRef]
- Luo, R.; Zhu, D.; Ju, H.; Lei, J. Reticular Electrochemiluminescence Nanoemitters: Structural Design and Enhancement Mechanism. Accounts of Chemical Research 2023, 56, 1920–1930. [Google Scholar] [CrossRef]
- Zhao, L.; Song, X.; Li, Y.; Jia, H.; Zhang, N.; Wei, Q.; Wu, D.; Ju, H. Europium-based metal-organic framework with acid-base buffer structure as electrochemiluminescence luminophore for hyperstatic trenbolone trace monitoring under wide pH range. Biosensors and Bioelectronics 2023, 221, 114925. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Huang, X.; Lu, Y.; Fan, Z.; Li, B.; Jiang, D.; Sojic, N.; Liu, B. High electrochemiluminescence from Ru (bpy) 32+ embedded metal–organic frameworks to visualize single molecule movement at the cellular membrane. Advanced Science 2022, 9, 2204715. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Z.; Ju, H. Copper-doped terbium luminescent metal organic framework as an emitter and a co-reaction promoter for amplified electrochemiluminescence immunoassay. Analytical Chemistry 2021, 93, 14878–14884. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Saada, H.; Abdallah, R.; Loget, G.; Sojic, N. Luminescence amplification at BiVO4 photoanodes by photoinduced electrochemiluminescence. Angewandte Chemie International Edition 2020, 59, 15157–15160. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Descamps, J.; Ababou-Girard, S.; Bergamini, J. F.; Santinacci, L.; Léger, Y.; Sojic, N.; Loget, G. Metal-Insulator-Semiconductor Anodes for Ultrastable and Site-Selective Upconversion Photoinduced Electrochemiluminescence. Angewandte Chemie International Edition 2022, 61, e202201865. [Google Scholar] [CrossRef] [PubMed]
- Descamps, J.; Zhao, Y.; Yu, J.; Xu, G.; Léger, Y.; Loget, G.; Sojic, N. Anti-Stokes photoinduced electrochemiluminescence at a photocathode. Chemical Communications 2022, 58, 6686–6688. [Google Scholar] [CrossRef] [PubMed]
- Zanut, A.; Fiorani, A.; Rebeccani, S.; Kesarkar, S.; Valenti, G. Electrochemiluminescence as emerging microscopy techniques. Analytical and bioanalytical chemistry 2019, 411, 4375–4382. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Goudeau, B.; Manojlovic, D.; Jiang, D.; Fang, D.; Sojic, N. Electrochemiluminescence loss in photobleaching. Angewandte Chemie 2021, 133, 7764–7768. [Google Scholar] [CrossRef]
- Zhang, J.; Arbault, S.; Sojic, N.; Jiang, D. Electrochemiluminescence imaging for bioanalysis. Annual Review of Analytical Chemistry 2019, 12, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gou, X.; Ma, C.; Jiang, D.; Zhu, J.-J. A synergistic coreactant for single-cell electrochemiluminescence imaging: Guanine-rich ssDNA-loaded high-index faceted gold nanoflowers. Analytical Chemistry 2021, 93, 7682–7689. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Xing, Z.; Ma, C.; Zhu, J.-J. A Close Look at Mechanism, Application, and Opportunities of Electrochemiluminescence Microscopy. Chemical & Biomedical Imaging.
- Qi, H.; Zhang, C. Electrogenerated chemiluminescence biosensing. Analytical Chemistry 2019, 92, 524–534. [Google Scholar] [CrossRef]
- Ben Trad, F.; Delacotte, J.; Guille-Collignon, M.; Lemaître, F.; Arbault, S.; Sojic, N.; Burlina, F.; Labbé, E.; Buriez, O. Electrochemiluminescence Imaging of Liposome Permeabilization by an Antimicrobial Peptide: Melittin. Chemical & Biomedical Imaging 2023, 1, 58–65. [Google Scholar]
- Guo, M.; Du, D.; Wang, J.; Ma, Y.; Yang, D.; Haghighatbin, M. A.; Shu, J.; Nie, W.; Zhang, R.; Bian, Z. Three-Biomarker Joint Strategy for Early and Accurate Diagnosis of Acute Myocardial Infarction via a Multiplex Electrochemiluminescence Immunoarray Coupled with Robust Machine Learning. Chemical & Biomedical Imaging 2023, 1, 179–185. [Google Scholar]
- Ding, L.; Zhou, P.; Yan, Y.; Su, B. Electrochemiluminescence Imaging of Cellular Contact Guidance on Microfabricated Substrates. Chemical & Biomedical Imaging 2023, 1, 558–565. [Google Scholar]
- Chemiluminescence, A. E. From Fundamentals to Bioassays. In Royal Society of Chemistry, Cambridge: 2019.
- Liu, X.; Shi, L.; Niu, W.; Li, H.; Xu, G. Environmentally friendly and highly sensitive ruthenium (II) tris (2, 2′-bipyridyl) electrochemiluminescent system using 2-(dibutylamino) ethanol as co-reactant. Angewandte Chemie International Edition 2007, 46, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Kebede, N.; Francis, P. S.; Barbante, G. J.; Hogan, C. F. Electrogenerated chemiluminescence of tris (2, 2′ bipyridine) ruthenium (II) using common biological buffers as co-reactant, pH buffer and supporting electrolyte. Analyst 2015, 140, 7142–7145. [Google Scholar] [CrossRef]
- Kerr, E.; Doeven, E. H.; Wilson, D. J.; Hogan, C. F.; Francis, P. S. Considering the chemical energy requirements of the tri-n-propylamine co-reactant pathways for the judicious design of new electrogenerated chemiluminescence detection systems. Analyst 2016, 141, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Choi, J.-P.; Bard, A. J. Electrogenerated chemiluminescence 69: The Tris (2, 2 ‘-bipyridine) ruthenium (II),(Ru (bpy) 32+)/Tri-n-propylamine (TPrA) system revisited A new route involving TPrA•+ Cation Radicals. Journal of the American Chemical Society 2002, 124, 14478–14485. [Google Scholar] [CrossRef]
- Guo, W.; Ding, H.; Gu, C.; Liu, Y.; Jiang, X.; Su, B.; Shao, Y. Potential-resolved multicolor electrochemiluminescence for multiplex immunoassay in a single sample. Journal of the American Chemical Society 2018, 140, 15904–15915. [Google Scholar] [CrossRef]
- Qiu, R.; Zhang, X.; Luo, H.; Shao, Y. Mass spectrometric snapshots for electrochemical reactions. Chemical Science 2016, 7, 6684–6688. [Google Scholar] [CrossRef]
- Dutta, P.; Han, D.; Goudeau, B.; Jiang, D.; Fang, D.; Sojic, N. Reactivity mapping of luminescence in space: Insights into heterogeneous electrochemiluminescence bioassays. Biosensors and Bioelectronics 2020, 165, 112372. [Google Scholar] [CrossRef]
- Kim, J.; Ha, J.; Lee, J. H.; Moon, H. R. Solid-state phase transformations toward a metal-organic framework of 7-connected Zn 4 O secondary building units. Nano Research 2021, 14, 411–416. [Google Scholar] [CrossRef]
- Zhao, H.; Yi, B.; Si, X.; Cao, L.; Su, L.; Wang, Y.; Chou, L.-Y.; Xie, J. Solid-State Synthesis of Defect-Rich Zr-UiO-66 Metal–Organic Framework Nanoparticles for the Catalytic Ring Opening of Epoxides with Alcohols. ACS Applied Nano Materials 2021, 4, 9752–9759. [Google Scholar] [CrossRef]
- Karve, V. V.; Vieira, A. N.; Stoian, D.; Trukhina, O.; Queen, W. L. Solid-state synthesis of a MOF/polymer composite for hydrodeoxygenation of vanillin. Chemical Communications 2022, 58, 11559–11562. [Google Scholar] [CrossRef] [PubMed]
- Nian, P.; Liu, H.; Zhang, X. Bottom-up synthesis of 2D Co-based metal–organic framework nanosheets by an ammonia-assisted strategy for tuning the crystal morphology. CrystEngComm 2019, 21, 3199–3208. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Wang, W.; Tan, X.; Lu, Z.; Han, H. Metal-organic frameworks-based sensitive electrochemiluminescence biosensing. Biosensors and Bioelectronics 2020, 164, 112332. [Google Scholar] [CrossRef]
- Yan, M.; Ye, J.; Zhu, Q.; Zhu, L.; Huang, J.; Yang, X. Ultrasensitive immunosensor for cardiac troponin I detection based on the electrochemiluminescence of 2D Ru-MOF nanosheets. Analytical chemistry 2019, 91, 10156–10163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Dong, X.; Du, Y.; Zhang, N.; Bai, G.; Wu, D.; Ma, H.; Wang, Y.; Cao, W.; Wei, Q. Enhancing electrochemiluminescence efficiency through introducing atomically dispersed ruthenium in nickel-based metal–organic frameworks. Analytical Chemistry 2022, 94, 10557–10566. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhao, G.; Li, X.; Fang, J.; Miao, J.; Wei, Q.; Cao, W. Electrochemiluminescence immunosensor of “signal-off” for β-amyloid detection based on dual metal-organic frameworks. Talanta 2020, 208, 120376. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhan, Z.; Ding, Z. Progress in electrochemiluminescence biosensors based on organic framework emitters. Current Opinion in Electrochemistry 2023, 101283. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Xu, R.; Wang, H.; Zhang, Y.; Wei, Q. Progress and Prospects of Electrochemiluminescence Biosensors Based on Porous Nanomaterials. Biosensors 2022, 12, 508. [Google Scholar] [CrossRef]
- Jin, Z.; Zhu, X.; Wang, N.; Li, Y.; Ju, H.; Lei, J. Electroactive metal–organic frameworks as emitters for self-enhanced electrochemiluminescence in aqueous medium. Angewandte Chemie 2020, 132, 10532–10536. [Google Scholar] [CrossRef]
- Organization, W. H. Guidelines for drinking-water quality: First addendum to the fourth edition. 2017.
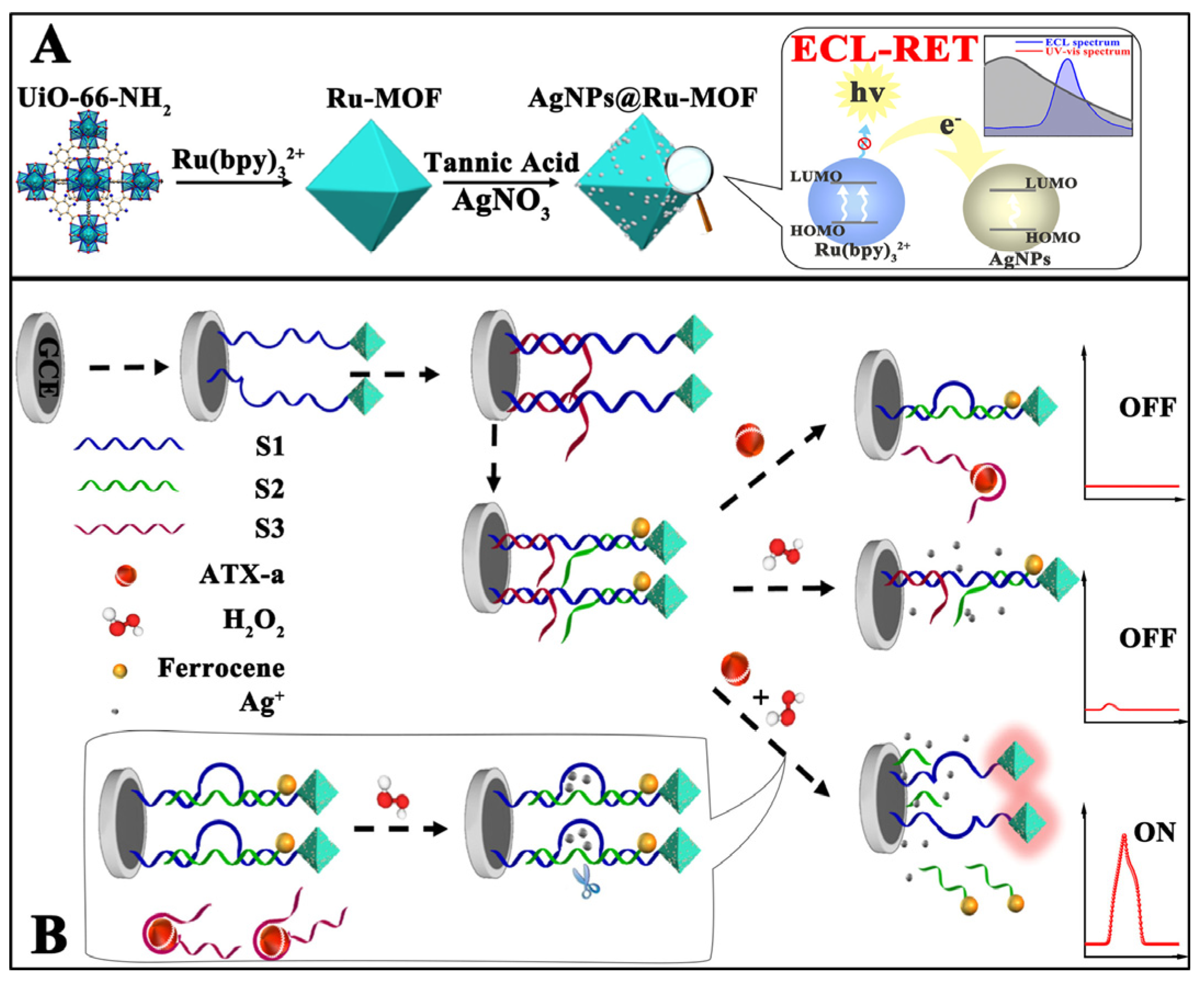
- Cui, J.; Xu, X.; Yang, C.; Wang, J.; Guo, Q.; Nie, G. A difunctional electrochemiluminescence sensor based on Ru-MOFs and strand-displacement-amplification reaction for ultrasensitive detection of Hg2+ and Ag+. Sensors and Actuators B: Chemical 2023, 378, 133141. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Chen, J.-S.; Liu, X.-P.; Mao, C.-J.; Jin, B.-K. An electrochemiluminescence aptasensor based on highly luminescent silver-based MOF and biotin–streptavidin system for mercury ion detection. Analyst 2023, 148, 772–779. [Google Scholar] [CrossRef]
- Shan, X.; Pan, T.; Pan, Y.; Wang, W.; Chen, X.; Shan, X.; Chen, Z. Highly Sensitive and Selective Detection of Pb (II) by NH2− SiO2/Ru (bpy) 32+− UiO66 based Solid-state ECL Sensor. Electroanalysis 2020, 32, 462–469. [Google Scholar] [CrossRef]
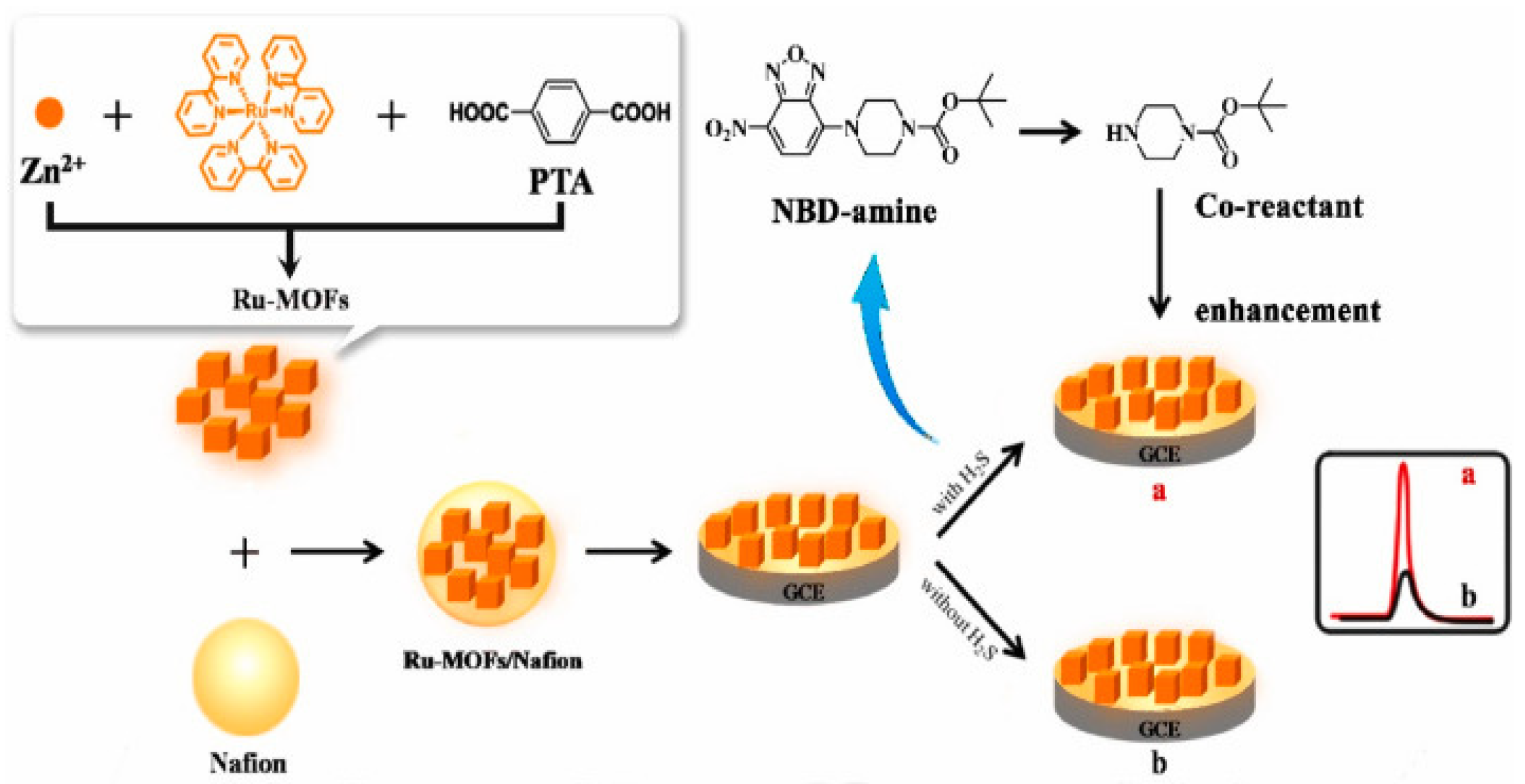
- Xiong, C.; Huang, J.; Liu, H.; Chen, M.-M.; Wen, W.; Zhang, X.; Wang, S. Ruthenium (II) complex encapsulated multifunctional metal organic frameworks based electrochemiluminescence sensor for sensitive detection of hydrogen sulfide. Talanta 2022, 249, 123602. [Google Scholar] [CrossRef]
- Xia, M.; Zhou, F.; Feng, X.; Sun, J.; Wang, L.; Li, N.; Wang, X.; Wang, G. A DNAzyme-based dual-stimuli responsive electrochemiluminescence resonance energy transfer platform for ultrasensitive anatoxin-a detection. Analytical Chemistry 2021, 93, 11284–11290. [Google Scholar] [CrossRef]
- Zhao, G.; Du, Y.; Zhang, N.; Li, Y.; Bai, G.; Ma, H.; Wu, D.; Cao, W.; Wei, Q. Bimetallic Metal–Organic Frameworks as an Efficient Capture Probe in Signal On–Off–On Electrochemiluminescence Aptasensor for Microcystin-LR Detection. Analytical Chemistry 2023. [Google Scholar] [CrossRef]
- Qi, H.; Wang, Z.; Li, H.; Li, F. Directionally In Situ Self-Assembled Iridium (III)-Polyimine Complex-Encapsulated Metal–Organic Framework Two-Dimensional Nanosheet Electrode To Boost Electrochemiluminescence Sensing. Analytical Chemistry 2023, 95, 12024–12031. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, J.; Zhang, X.; Shan, X.; Wu, Q.; Li, C.; Li, H.; Yang, S.; Tian, L. Electrospun nanofibers containing CdTe@ ZnNi-MOF for electrochemiluminescent determination of chlorpyrifos. Microchimica Acta 2022, 189, 473. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, D.; Shan, X.; Wang, W.; Xu, F.; Shiigi, H.; Chen, Z. Ternary electrochemiluminescence biosensor based on black phosphorus quantum dots doped perylene derivative and metal organic frameworks as a coreaction accelerator for the detection of chloramphenicol. Microchemical Journal 2022, 172, 106927. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Yi, J.; Ma, D.; Xia, F.; Tian, D.; Zhou, C. A dual-signal electroluminescence aptasensor based on hollow Cu/Co-MOF-luminol and g-C3N4 for simultaneous detection of acetamiprid and malathion. Sensors and Actuators B: Chemical 2021, 331, 129412. [Google Scholar] [CrossRef]
- Manzoor, R.; Wang, L.; Wang, H.; Lei, Y.; Sehrish, A.; Khan, M. S.; Ali, A.; Wu, D.; Wei, Q. Ultrasensitive competitive electrochemiluminescence immunosensor based on luminol-AuNPs@ Mo2C and upconversion nanoparticles for detection of diethylstilbestrol. Microchemical Journal 2020, 158, 105283. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, G.; Liu, L.; Li, X.; Wei, Q.; Cao, W. Ultrasensitive competitive method-based electrochemiluminescence immunosensor for diethylstilbestrol detection based on Ru (bpy) 32+ as luminophor encapsulated in metal–organic frameworks UiO-67. Biosensors and Bioelectronics 2018, 110, 201–206. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, M.; Song, X.; Liu, X.; Ju, H.; Ai, H.; Wei, Q.; Wu, D. Annihilation luminescent Eu-MOF as a near-infrared electrochemiluminescence probe for trace detection of trenbolone. Chemical Engineering Journal 2022, 434, 134691. [Google Scholar] [CrossRef]
- Zhou, L.; Shan, X.; Jiang, D.; Wang, W.; Chen, Z. Electrochemical luminescence sensor based on CDs@ HKUST-1 composite for detection of catechol. Journal of Electroanalytical Chemistry 2020, 871, 114215. [Google Scholar] [CrossRef]
- Dong, M.; Jiang, D.; Cao, Q.; Wang, W.; Shiigi, H.; Chen, Z. A metal–organic framework regulated graphdiyne-based electrochemiluminescence sensor with a electrocatalytic self-acceleration effect for the detection of di-(2-ethylhexyl) phthalate. Analyst 2023, 148, 4470–4478. [Google Scholar] [CrossRef]
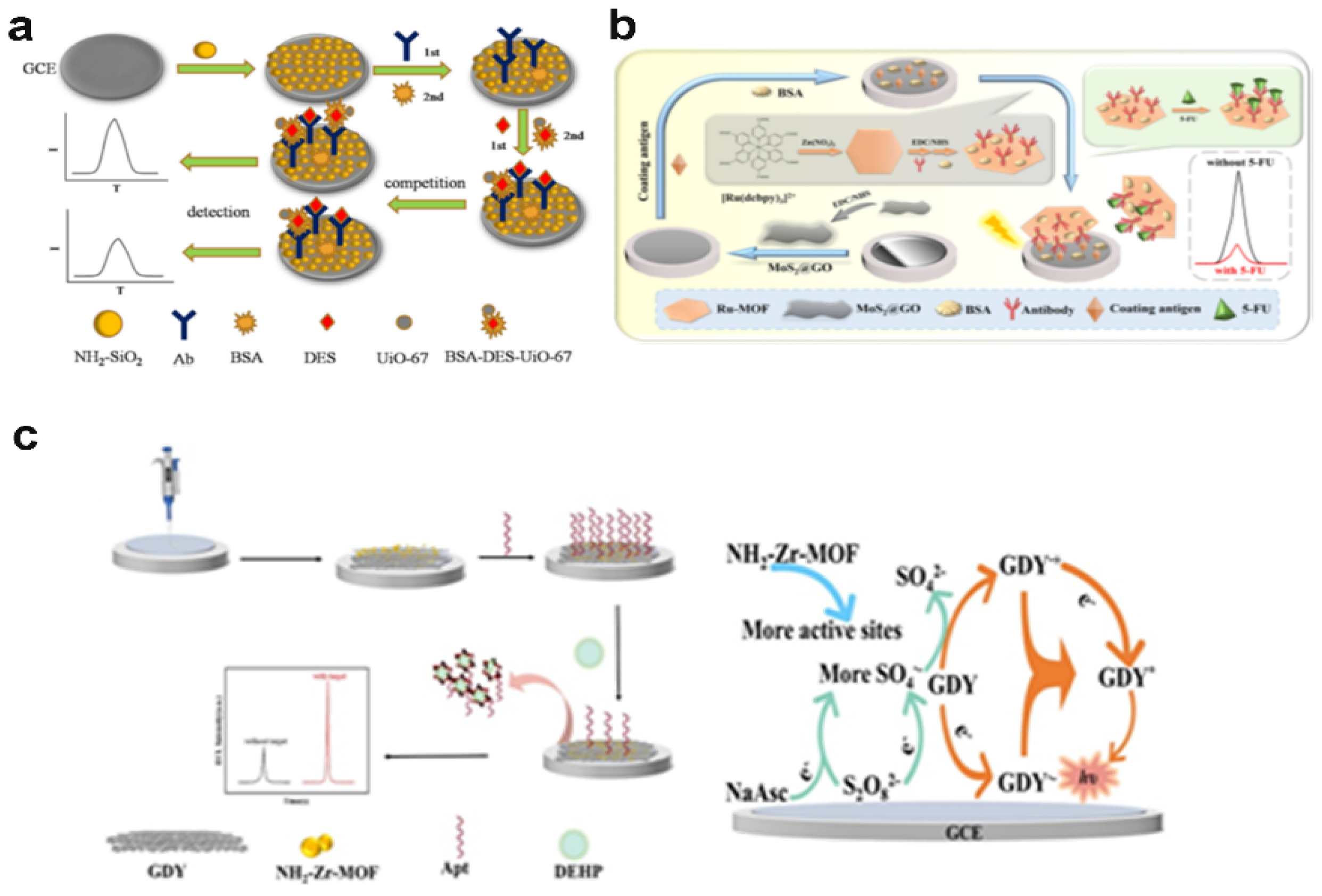
- Ma, G.; Peng, L.; Zhang, S.; Wu, K.; Deng, A.; Li, J. Electrochemiluminescence immunoassay strategies based on a hexagonal Ru-MOF and MoS 2@ GO nanosheets: Detection of 5-fluorouracil in serum samples. Analyst 2023, 148, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
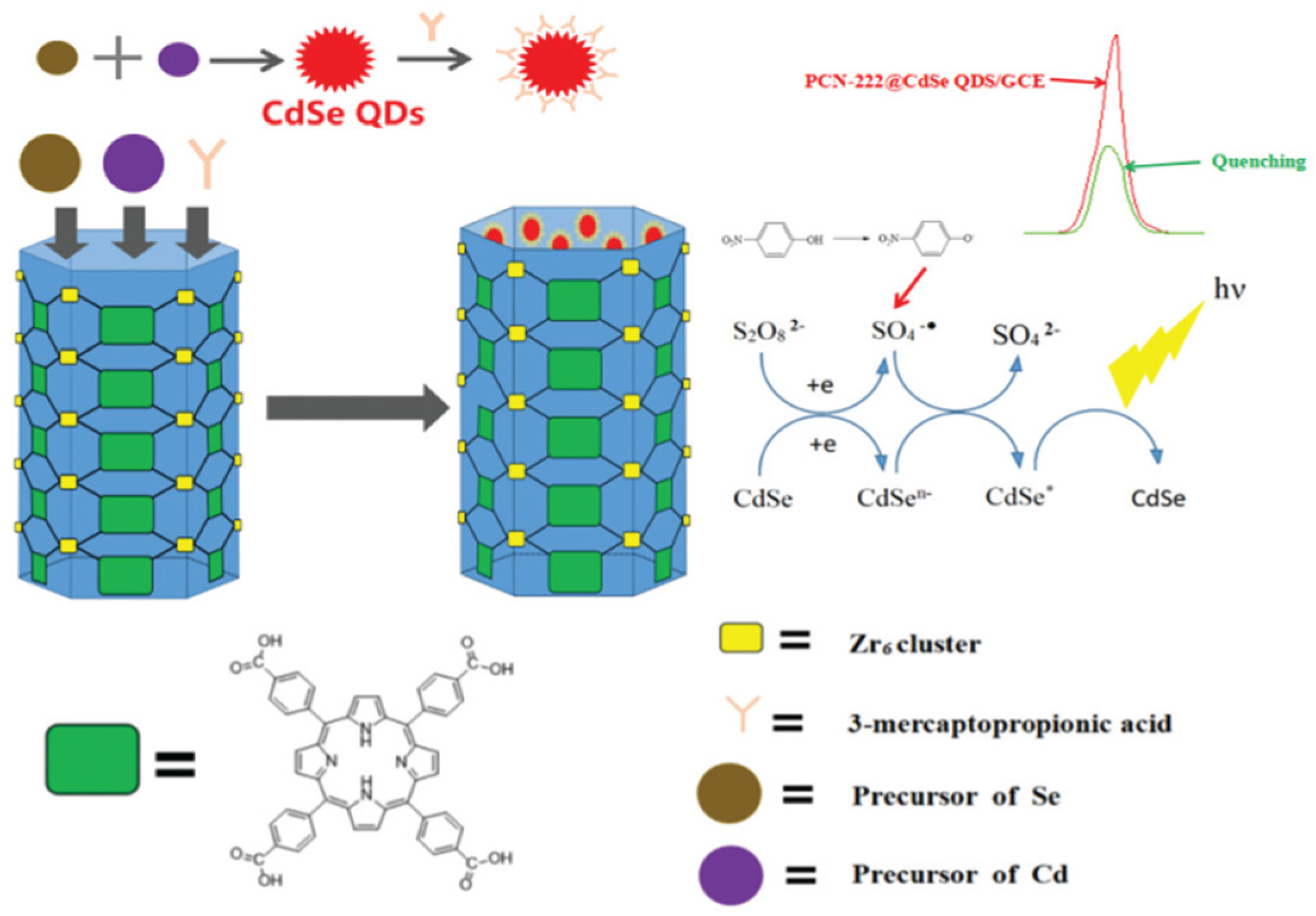
- Li, L.; Chen, J.-S.; Liu, X.-P.; Mao, C.-J.; Jin, B.-K. Functionalized MOF PCN-222-loaded quantum dots as an electrochemiluminescence sensing platform for the sensitive detection of p-nitrophenol. New Journal of Chemistry 2022, 46, 12054–12061. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, L.; Li, Y.; Liu, X.; Fan, D.; Wu, D.; Wei, Q. Porphyrin-based metal-organic frameworks enhanced electrochemiluminescence (ECL) by overcoming aggregation-caused quenching: A new ECL emitter for the detection of trenbolone. Analytica Chimica Acta 2023, 1276, 341616. [Google Scholar] [CrossRef]
- Nguyen, T. N.; Ebrahim, F. M.; Stylianou, K. C. Photoluminescent, upconversion luminescent and nonlinear optical metal-organic frameworks: From fundamental photophysics to potential applications. Coordination Chemistry Reviews 2018, 377, 259–306. [Google Scholar] [CrossRef]
- Wang, X.; Kong, L.; Zhou, S.; Ma, C.; Lin, W.; Sun, X.; Kirsanov, D.; Legin, A.; Wan, H.; Wang, P. Development of QDs-based nanosensors for heavy metal detection: A review on transducer principles and in-situ detection. Talanta 2022, 239, 122903. [Google Scholar] [CrossRef]
- Jin, J.; Xue, J.; Wu, D.; Yang, G.; Wang, Y. Improved performance of the pyrimidine-modified porous In-MOF and an in situ prepared composite Ag@ In-MOF material. Chemical Communications 2022, 58, 7749–7752. [Google Scholar] [CrossRef]
- Zheng, L.; Chi, Y.; Shu, Q.; Dong, Y.; Zhang, L.; Chen, G. Electrochemiluminescent reaction between Ru (bpy) 32+ and oxygen in nafion film. The Journal of Physical Chemistry C 2009, 113, 20316–20321. [Google Scholar] [CrossRef]
- Bian, X.; Guo, B.; Zhao, M.; Han, D.; Cheng, W.; Song, F.; Ding, S. An enzyme-free “ON-OFF” electrochemiluminescence biosensor for ultrasensitive detection of PML/RARα based on target-switched DNA nanotweezer. ACS applied materials & interfaces 2019, 11, 3715–3721. [Google Scholar]
- Deng, S.; Lei, J.; Huang, Y.; Cheng, Y.; Ju, H. Electrochemiluminescent quenching of quantum dots for ultrasensitive immunoassay through oxygen reduction catalyzed by nitrogen-doped graphene-supported hemin. Analytical chemistry 2013, 85, 5390–5396. [Google Scholar] [CrossRef]
- Bai, X.; Lutz, A.; Carroll, R.; Keteles, K.; Dahlin, K.; Murphy, M.; Nguyen, D. Occurrence, distribution, and seasonality of emerging contaminants in urban watersheds. Chemosphere 2018, 200, 133–142. [Google Scholar] [CrossRef]
- Li, J.; Shan, X.; Jiang, D.; Wang, W.; Chen, Z. An electrochemiluminescence aptasensor based on Ru (bpy) 3 2+ encapsulated titanium-MIL-125 metal-organic framework for bisphenol A assay. Microchimica Acta 2020, 187, 1–8. [Google Scholar] [CrossRef]
- Bravo-Linares, C. M.; Mudge, S. M. Analysis of volatile organic compounds (VOCs) in sediments using in situ SPME sampling. Journal of Environmental Monitoring 2007, 9, 411–418. [Google Scholar] [CrossRef]
- Shen, Y.; Tissot, A.; Serre, C. Recent progress on MOF-based optical sensors for VOC sensing. Chemical Science 2022, 13, 13978–14007. [Google Scholar] [CrossRef]
- Huang, I.-S.; Zimba, P. V. Cyanobacterial bioactive metabolites—A review of their chemistry and biology. Harmful algae 2019, 86, 139–209. [Google Scholar] [CrossRef]
- Colas, S.; Marie, B.; Lance, E.; Quiblier, C.; Tricoire-Leignel, H.; Mattei, C. Anatoxin-a: Overview on a harmful cyanobacterial neurotoxin from the environmental scale to the molecular target. Environmental Research 2021, 193, 110590. [Google Scholar] [CrossRef]
- Chia, M. A.; Ameh, I.; George, K. C.; Balogun, E. O.; Akinyemi, S. A.; Lorenzi, A. S. Genetic Diversity of Microcystin Producers (Cyanobacteria) and Microcystin Congeners in Aquatic Resources across Africa: A Review Paper. Toxics 2022, 10, 772. [Google Scholar] [CrossRef]
| MOF type | Analyte | Limit of detection (LOD) | Type of MOF synthesis | Linear detection range (LDR) | Co/reactants | Medium | Reference | |
|---|---|---|---|---|---|---|---|---|
| Ru-Zn: MOF Ru(bpy)32+ 1,3,5-benzentriic acid |
Ag+/Hg2+ | 0.00298 - 0.00032 pM | Electrodeposition, electrochemical synthesis | 0.001–1000 pM/ 0.01–10000 pM |
K2S2O8 | Seawater, water |
[80] | |
| Ag-MOF@CS @(Au-NPs) |
Hg2+ | 66 fM | Ultrasonic, solvothermal |
300 fM – 1 μM | K2S2O8 | Water, lake water | [81] | |
| NH2-SiO2/Ru(bpy)32+- UiO66 | Pb2+ | 1.0 × 10-7 μM | 1.0×10-6 – 1.0×10-2 μM |
TEA | Water, tap water |
[82] | ||
| Ru-MOFs | H2S | 2.5 × 10-12 mol L- 1 | 1.0 × 10-11 mol L-1 - 1.0 × 10- 4 mol L-1 |
NBD-amine 7-nitro-1,2,3-benzoxadiazole amine |
Water, human serum samples |
[83] | ||
| S2-Fc/S3/S1-AgNPs @Ru-MOF |
Anatoxin-a | 0.034 µg/mL |
Solvothermal |
0.001-1 mg/mL |
TPrA |
Lake and river water | [84] | |
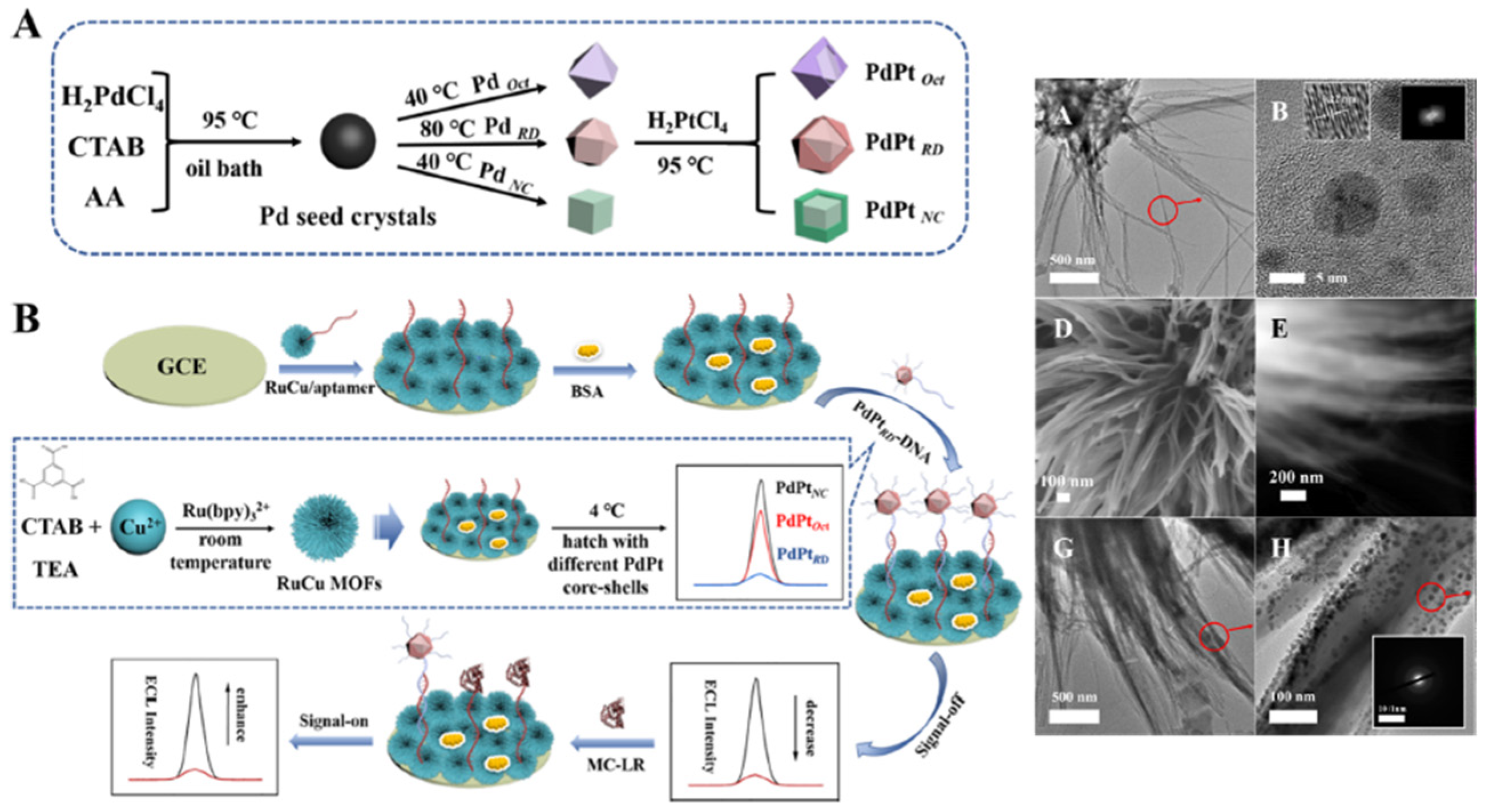
| Ru-Cu MOF | Microcystin-LR | 0.143 pg/mL | Ultasonication | 0.0001 − 50 ng/mL |
TPrA |
Tap water | [85] | |
| Hf-MOF/Ir2PD/APS/ ITO |
Acetamiprid |
0.0025 nM | Directional self-assembling | 0.01 - 10 nM | TPrA |
Pakchoi |
[86] | |
| CdTe@ZnNi-MOF | Chlorpyrifos | 6.23 x 10−17 M | Blending |
1.0 × 10-14 – 1.0 × 10-9 M |
Luminol-O2 | Vegetables |
[87] | |
| Co-Ni/MOF | Chloramphenicol | 2.9 × 10 -14 M | Solovothermal |
1.0 × 10-13 – 1.0 × 10-6 M |
BP/PTC-NH2)/S2O8with K2S2O8 |
Tap water | [88] | |
| Hollow Cu/Co-MOF | Acetamiprid and malathion |
0.015 pM/0.018 pM |
In situ, solvothermal |
0.1 μM - 0.1 pM | Luminol H2O2, K2S2O8 |
Apple and tomato | [89] | |
| UCNPs / Pt@MOF | Diethylstilbestrol | 3.8 fg/mL | Layer-by-layer growth method |
0.1 pg/mL to 30 ng/mL | CBS H2O2 |
Tap and river water | [90] | |
| Ru(bpy)32+/UiO-67 | Diethylstilbestrol | 3.27 fg/mL |
Solvothermal | 0.01 pg/mL to 50 ng/mL | TPrA | Urine | [91] | |
| Eu(II)-MOFs | Trenbolone | 4.42 fg/mL | 10 fg/mL - 100 ng/mL |
TPrA |
River water | [92] | ||
| CDs@HKUST-1 | Catechol | 3.8 × 10−9 mol/L | Hydrothermal synthesis | 5.0×10−9 –2.5×10−5 mol/L | K2S2O8 | Tea sample | [93] | |
| NH2-Zr-MOF | DEHP | 2.43 × 10−13 mg/mL | 1.0 × 10−12 – 1.0 × 10−4 mg/mL |
K2S2O8 | River and urban drinking water | [94] | ||
| Ru-MOF | 5-fluorouracil |
0.031 pg/mL | Ultasonication | 0.0001 –100 ng/mL | K2S2O8 |
Serum |
[95] | |
| PCN-222@CdSe | p-PNP | 0.03 ppb | Solvothermal | 100 ppm to 0.1 ppb | K2S2O8 | Lake and Tap water |
[96] |
|
| PtNPs@Ce-MOFs | Trenbolone | 3.61 fg/mL | One-pot solvothermal | 10 pg/mL - 100 ng/mL | K2S2O8 | River water | [97] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





