1. Introduction
Water electrolysis (WE) stands at the forefront of hydrogen (H
2) production technology. By utilizing electrical energy to split water into hydrogen and oxygen, WE taps into the potential of renewable energy sources (RES). In contrast to the dominant method of steam methane reforming [
1], which contributes to global emissions, WE for green hydrogen production harnesses RES-generated electricity. This shift not only decarbonizes hydrogen production but also serves as a vital energy storage solution. Electrolytic hydrogen acts as a linchpin, balancing surplus green energy, bolstering grid stability, and transforming sectors resistant to conventional electrification. Notably, it holds the key to decarbonizing challenging domains like long-haul transport and industries where emissions reduction poses a formidable challenge, like hard-to-abate sectors [
2]. This transformative potential underscores the pivotal role of water electrolysis in shaping a sustainable and low-carbon future. In the realm of energy, hydrogen is a widely debated subject. However, discussions often suffer from oversimplification, lacking a comprehensive perspective that illuminates its broader implications. There’s a need to transcend surface-level analysis and embrace a holistic view, acknowledging the profound implications of hydrogen within the larger energy landscape. In the energy sector, confusion often arises between resources, energy carriers, and end uses. The recent hydrogen debate is no exception. Hydrogen is indeed a carrier and, while it can aid energy transition, it cannot solve all problems. Developing green hydrogen is crucial. It can boost renewable energy generation, even for unconventional uses. Advancements in electrolysis will undoubtedly enhance the production of green hydrogen [
3].
Water electrolysis relevance is evident just today: the electrolytic process involves many variants and variables, such as the type of ion transport medium, electrodes and catalysts, the operating temperature, pressure, voltage and current density, and the electrolyser size, thus, analysis of cell, stack, and system levels are necessary. Many scientific and technological problems are still open and, thus, are to be investigated, such as electrolysers’ size scaling up, performance measurement, and association between electrolytic H2 production and RES. In today’s scientific and technical landscape, numerous projects focus on hydrogen and its related domains. Yet, amidst this vast realm, numerous themes and microtopics remain open and often elusive. Distinguishing between established knowledge and ongoing research objectives poses a challenge. This article endeavours to provide clarity, meticulously assessing the existing body of knowledge while highlighting debated topics. We aim to delineate the current state-of-the-art, discerning what is consolidated and shed light on the persistently open avenues of research.
In particular, the objective of this paper is to provide an elucidating critical analysis of water electrolysis for green hydrogen production. This includes the description of the main electrolysis technologies and energy balance considerations about the various techniques. An incisive analysis of the electrolytic cell physics and main technologies irreversibility is carried out, to identify the aspects that would require more investigation to increase the electrolytic process efficiency.
The existing literature offers extensive material on hydrogen production by various electrolysis technologies [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13]. However, the focus tends to be primarily on the analysis of technologies’ specific aspects. While this is valuable, it is equally important to construct a methodological framework rooted in system energy analysis to evaluate the green hydrogen integration in real energetic systems. This approach can enable a more accurate assessment of water electrolysis environmental impact, and economic feasibility.
The electricity consumption associated with electrolysis currently stands at around 55 kWh per kilogram of hydrogen produced. H2 lower calorific value is approximately 33 kWh per kilogram of hydrogen. This disparity underscores the critical need for optimization strategies targeting various elements within the electrolysis system, including electrodes, electrolytes, catalysts and membranes. Through meticulous research and development, advancements in these components can lead to substantial reductions in energy consumption, paving the way for more energy-efficient and sustainable hydrogen production methods. Our work seeks to bridge the gap between detailed technological analyses and the larger energy system context. By incorporating the broader perspective and considering the whole electrolytic process, we aim to provide an insightful contribution to the existing knowledge on green hydrogen production and its potential applications.
The paper is organized as follows. In
Section 2, a brief overview of electrolysis, and its significance in sustainable energy production, is provided.
In
Section 3 the fundamentals of water electrolysis for hydrogen production are outlined. Electrochemical analysis of the electrolytic cell is carried out for a better understanding of the process. The Section focuses on crucial aspects related to technology, scalability, and efficiency of the main routes of implementing H
2 production by WE.
Section 4 illustrates the main technologies for electrolysis and open fronts of research and development.
In
Section 5 the commercially available technologies, the problem of scaling up the sizes of electrolysers, and the association between electrolytic hydrogen production and energy sources are investigated. Considerations about green energy sources necessary for future scenarios of electrolytic hydrogen production are proposed.
Finally, the Conclusions serve as a synthesis of the paper’s analysis, highlighting the prospects and potential of electrolysis and green hydrogen integration in real energy systems. This section encapsulates key points, and implications, offering a comprehensive understanding of the challenges, and opportunities associated with hydrogen production by water electrolysis for the energy transition. Our article pioneers a transformative approach by adopting a holistic perspective grounded in energy systems and methodology. Through extensive literature synthesis and innovative concept integration, we strive for an authentic evaluation of developments in green hydrogen production.
2. Brief Overview of Electrolysis and its Significance in Sustainable Energy Issues: A Careful Energy Analysis
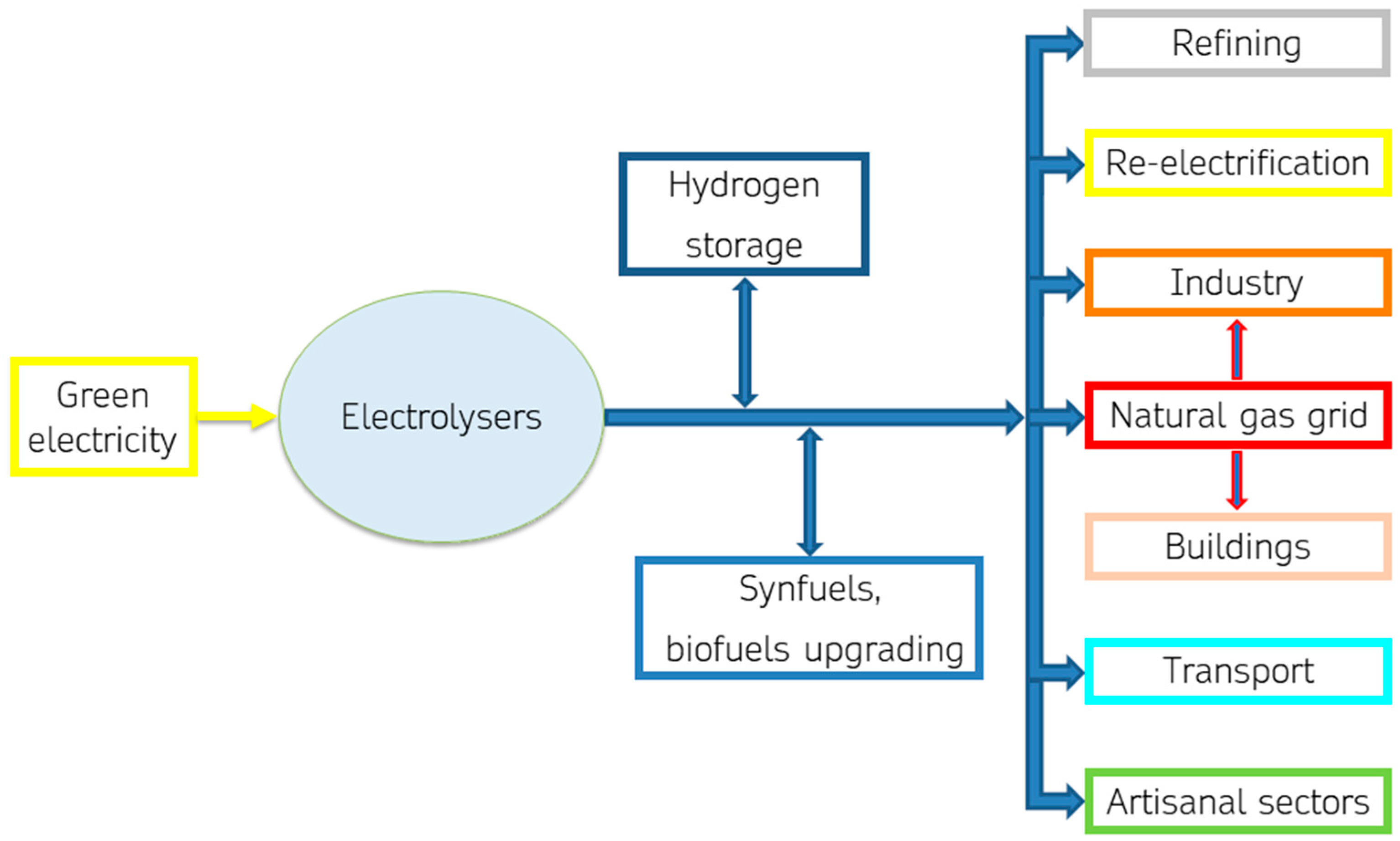
Green hydrogen has an important potential in the process of scaling up renewable energy sources. Electrolysis is a key technology for integrating renewable energy sources into various sectors. It serves as an energy storage solution, enabling the utilization of surplus renewable energy during peak production times. Electrolysis-produced hydrogen finds applications in various sectors, including transportation, industry, and energy storage solutions for grid stabilization. Green hydrogen can play a key role in the energy transition by helping to meet industrial and civil demand for heat and electricity, integrate renewable energy sources into the electricity system, make natural gas infrastructure more sustainable and decarbonize the transport sector, as shown in
Figure 1.
A small scale market for electrolytic H2 production has been established in handicrafts, such as gold working. Larger scale production can have as leading end-uses the re-electrification, transport sector, and industry.
In re-electrification, electrolytic hydrogen can be used to produce electrical power on a large scale through fuel cells, internal combustion engines, or turbines, thus, turning this power to the grid to adjust demand and generation. H2 is an energy carrier which can support the integration of variable RES in the electricity system, being one of the few options for storing energy over days, weeks or months.
Electrolytic hydrogen can be fundamental for the decarbonization of highly energy-intensive sectors, such as heavy transport and hard-to-abate industry [
2]. Electrolysers are tested and in production for refuelling stations for public transport, cars and heavy road transport. H
2 can be used as an alternative fuel in fuel cell vehicles, offering a transport option low in carbon to improve air quality, especially interesting in the heavy-duty vehicles sector (e.g., buses, ships, trains).
Hydrogen is currently used as a raw material in different industrial sectors on a large scale, therefore, the decarbonization of the process in which it is applied is achieved if it is produced from low-carbon sources.
Industrial experimentation of electrolytic hydrogen integration in the productive processes as feedstock, reducing agent, or/and alternative fuel in burners is underway. Hydrogen has the highest mass heating value among fuels, and an invaluable decarbonization potential [
14] since its combustion generates no carbon dioxide emissions. Green hydrogen can also be used as a renewable energy storage system, linked to the electrification of the processes maximizing the use of green energy from RES. Industrial activities already using hydrogen as a feedstock or/and process agent are favourite for electrolytic H
2 integration in their productive processes, because security and safety protocols about hydrogen use are known and managed yet. Furthermore, gradual decarbonization of the natural gas (ng) infrastructure could take place, by injection of increasing percentages of green hydrogen or synthetic methane in the transmission natural gas grid; experimentation up to 10% in volume of hydrogen are ongoing.
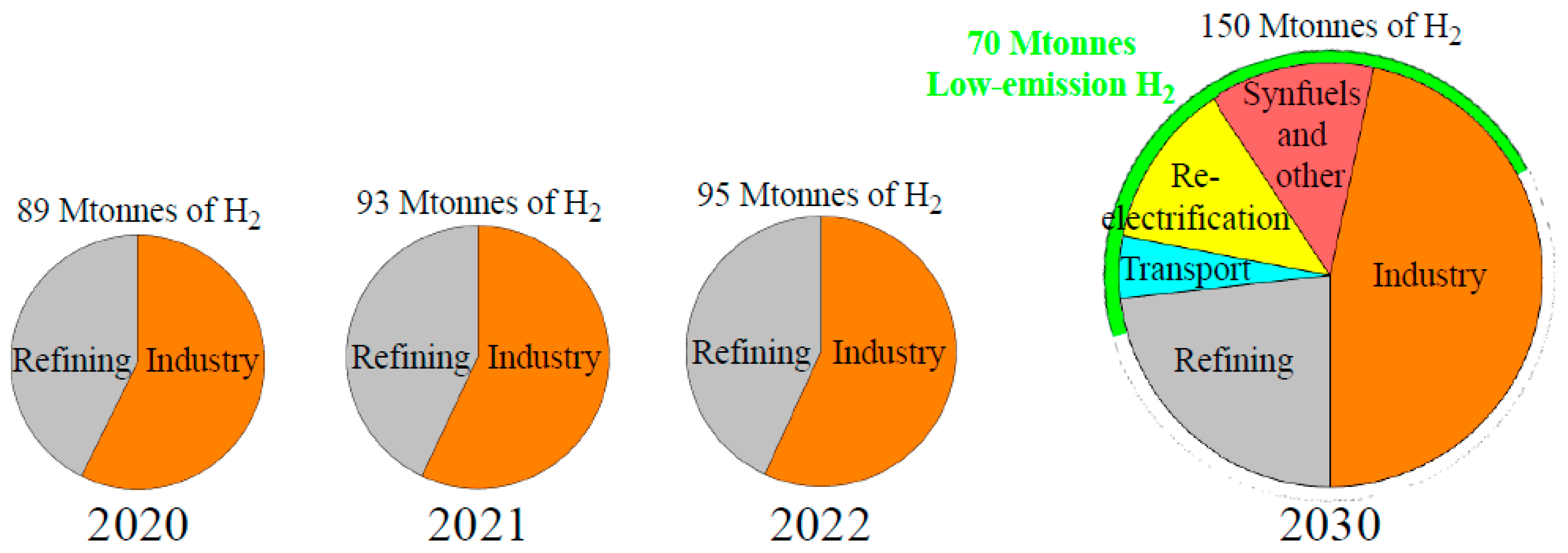
As in everything related to energy fields, growth scenarios are absolutely difficult to predict because they often follow a non-linear trend or are in any case driven by sudden technological innovations or external mechanisms, such as economic incentives. However, the growth in the impact of hydrogen on energy systems is predictable to contribute materially to the energy transition. Within these considerations,
Figure 2 shows global recent hydrogen uses, and low-emission H
2 targets in the Net Zero Emissions by 2050 Scenario (NZE) by 2030, indicating the direction to follow for decarbonisation. The bigger part of low-emission H
2 will be produced by water electrolysis [
1,
3]. In 2022, global hydrogen production grew by 2–3% to reach 95 Mtonnes (
Figure 2), but low-emission H
2 was about 0.7% (less than 1 Mtonne) of the global production, almost all from fossil fuels with carbon capture, utilisation and storage (CCUS), with only 0.1% from water electrolysis. The amount of electrolytic H
2, while still below 100 ktonnes in 2022, increased by 35% compared to 2021 [
1]. H
2 industrial use accounted for 53 Mtonnes [
1], of which about 90% was for chemicals (about 60% for ammonia production and 30% for methanol), and 10% for direct reduced iron process in the industrial subsector of iron and steel.
In the NZE, demand for low-emission hydrogen reaches 70 Mtonnes by 2030 (
Figure 2), compared to the 3–14 Mtonnes that could be achieved through current government and private sector activities [
1]; Net Zero Emissions by 2050 Scenario would require installed electrolysis capacity to reach more than 550 GW by 2030, while the realisation of all the projects could lead to an installed electrolyser capacity of 170–365 GW. Investment in electrolysers is growing but would require 70% annual growth until 2030 to get on track with Net Zero Emissions by 2050 Scenario [
1].
Electrolysis capacity for hydrogen production has been growing in the past few years; in 2022, installed capacity grew by more than 20%, with about 130 MW of new capacity entering operation in 2022 [
3]. Electrolysers’ capacity could reach almost 3 GW by the end of 2023, a more than four-fold increase in total capacity compared to 2022 [
3].
Electrolyser manufacturing capacity could support projects’ developments to achieve targets set out in national strategies. Electrolyser manufacturing capacity increased by more than 25% since last year, reaching nearly 11 GW per year in 2022 [
3].
In the iron and steel industry, hydrogen blending in blast furnaces and hydrogen-based direct reduced iron process are proposed. In the industry of chemicals, electrolysis by variable renewable electricity for ammonia production is between demonstration and market uptake, and in the demonstration phase for methanol. Hydrogen’s use for cement kiln blending is in large prototyping. For re-electrification, H
2 blending in ng turbine and high-temperature fuel cell is market uptaking. Hydrogen refuelling stations and hydrogen fuel cells for light- and heavy-duty road transport are market uptaking: Internal combustion engines for light-duty road transport are also proposed but with less convincing perspectives, while hydrogen fuel cells for shipping and rail applications are between demonstration and market uptake. Numerous demonstrative projects have been developed or are ongoing in the world [
1,
15], e.g., Hydrogen Valleys, in which the electrolytic production of H
2 is integrated with renewable energy sources, especially wind and solar. Green H
2 needs the guarantee of green electricity supply, by a dedicated RES plant or a renewable energy mix from the electrical grid with Guarantees of origin (traceability of renewable energies throughout the energy system).
3. Fundamentals of Electrolysis
In an electrolytic cell, electricity is used to dissociate water (H2O) into hydrogen (H2) and oxygen (O2) molecules. When sufficient electric current is passed between two electrodes separated by an ion transport medium (conductive electrolyte), H2 is produced at the negative electrode (cathode) and O2 at the positive electrode (anode).
The electrolytic cells, and electrical, gas processing, ventilation, cooling, monitoring equipment and controls are contained within an enclosure. Gas compression, feed water conditioning, and auxiliary equipment may also be included.
In the process of hydrogen production through electrolysis, energy is required: the energy consumption varies depending on several factors, including the electrolysis technology used and the operating conditions.
Different electrolysis technologies are available, each with unique advantages and challenges: low-temperature technologies (50–80 °C), such as alkaline (ALK), proton exchange membrane (PEM), and anion exchange membrane (AEM) electrolysers, employ liquid water, while high-temperature technologies (500–1000 °C), like solid oxide (SO) electrolysers, use steam. Each of these technologies offers unique advantages in terms of operational temperature, efficiency, scalability, and suitability for specific applications. Many research activities focus on improving the performance, durability, and cost-effectiveness of these technologies to enable their widespread adoption in H2 production.
The following first Subsection sheds light on electrolytic cell physics (analysis of the electrolysis process), and the second Subsection on electrolyser analysis at stack level.
3.1. Electrolytic Cell Physics
Examining water electrolysis from a chemical-physical perspective may seem straightforward, given the extensive literature available in university textbooks. However, the simplicity diminishes when considering the intricacies. The fundamental challenge lies in determining the energy required to produce a unit of hydrogen mass under specific operational conditions.
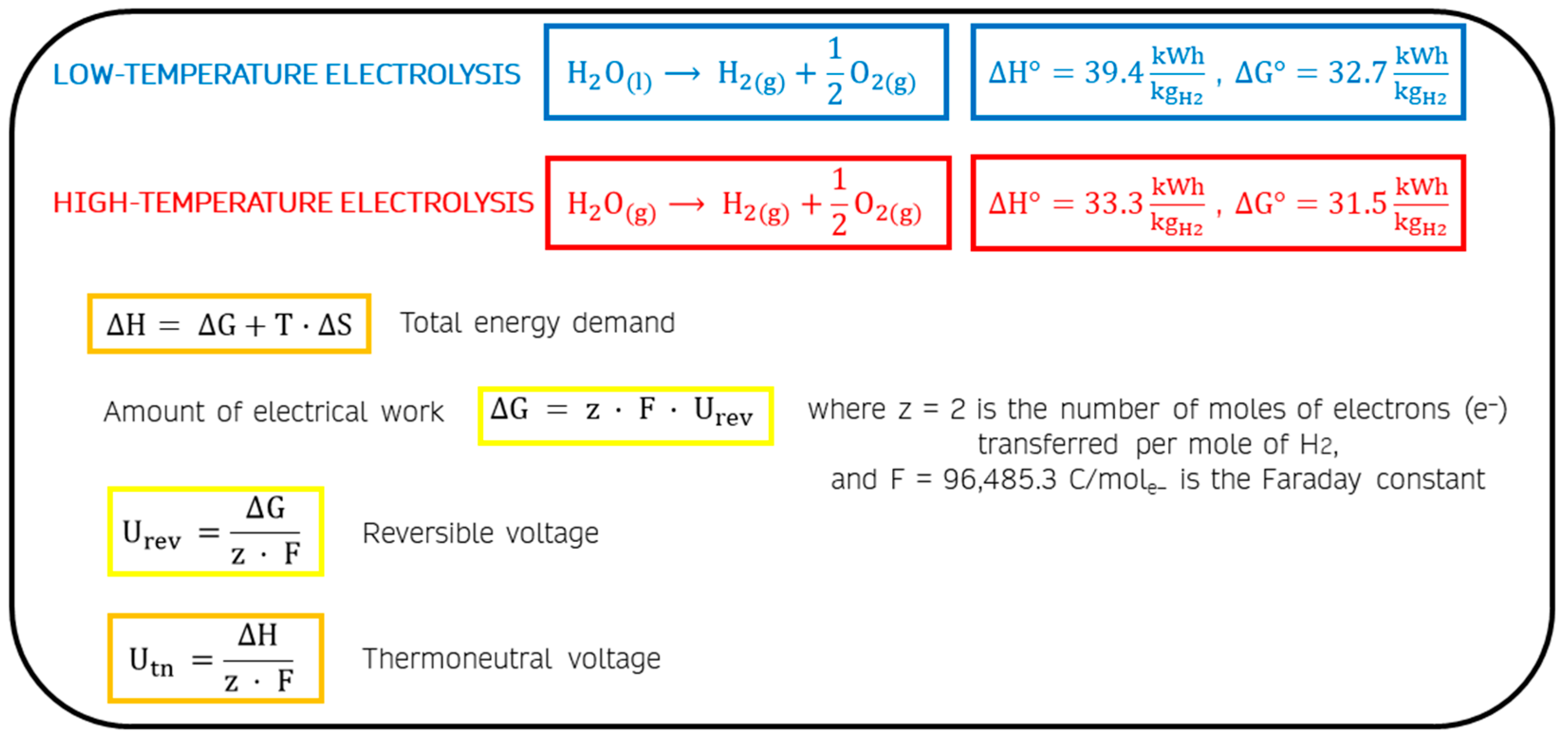
Figure 3 shows the main electrochemical equations. Low-temperature electrolysis is described from the splitting reaction of the liquid water molecule, while high-temperature electrolysis employs steam. The enthalpy change in standard conditions (ΔH°) of the reverse reaction of high-temperature electrolysis, the H
2 combustion, corresponds to the lower calorific value (LHV) of hydrogen, about 33.3 kWh/kg.
Water electrolysis is a nonspontaneous process, and the reaction free energy change (ΔG) is a large positive quantity. The change in free energy of a system for a constant-temperature process is the difference between the enthalpy change and the product of the absolute temperature and the entropy change (T∙ΔS). ΔG corresponds with the electrical energy demand of the electrolytic process, T∙ΔS the thermal energy demand, and ΔH the total energy required. In standard conditions, the electrical energy demand (ΔG°) of liquid water electrolysis equals 32.7 kWh per kg of hydrogen [
16], instead 31.5 kWh/kg
H2 are necessary for steam electrolysis.
Various researchers have analyzed the influence of temperature on WE thermodynamic parameters: the electrical demand decreases with increasing the temperature at which electrolysis is carried out, especially when the process happens with steam instead of liquid water [
4]. For temperatures above 100 °C, the total energy demand increases slightly as the temperature increases, even if the increase in the heat demand is greater than the decrease in the required electricity.
The change in free energy represents the amount of electrical work that must be provided in a reaction (
Figure 3): assuming the reaction is reversible and carrying out the electrolytic process at constant temperature and pressure, the difference between the electrodes’ potential corresponds with the reversible cell voltage (U
rev), which is defined as the minimum voltage to be applied between the electrodes for electrolysis to take place.
By the respective standard free-energy change value (
Figure 3), U°
rev is calculated to be 1.23 V for liquid water electrolysis and 1.18 V for steam electrolysis.
In not ideal conditions, the cell voltage is always higher than U
rev due to the irreversibilities of the real electrolytic process. These efficiency losses lead to an increase of the voltage (overpotentials, η
˅) required for WE compared to the reversible one, as shown in Equation 1 to calculate the operating cell voltage:
where η
˅act is the activation overpotential, η
˅ohm is the ohmic overpotential, and η
˅conc is the concentration or diffusion overpotential. The activation overpotential is related to the reactions’ activation energy; catalysts and operational temperature increase can reduce this efficiency loss. The ohmic overpotential is due to electrical, ionic, and contact resistances in the electrolytic cell; the current density, cell materials and design, and temperature affect this overpotential. η
˅conc is related to mass transport, which is more difficult at high current densities; if H
2 and O
2 are not removed as fast as they are produced, their concentration increases decreasing the reaction kinetics [
5]. It is accurate to say that the combination of the various overpotentials leads to an increase in energy consumption for electrolysis. Reducing these overpotentials through various means is a focus of research to enhance the efficiency of electrolysis for hydrogen production.
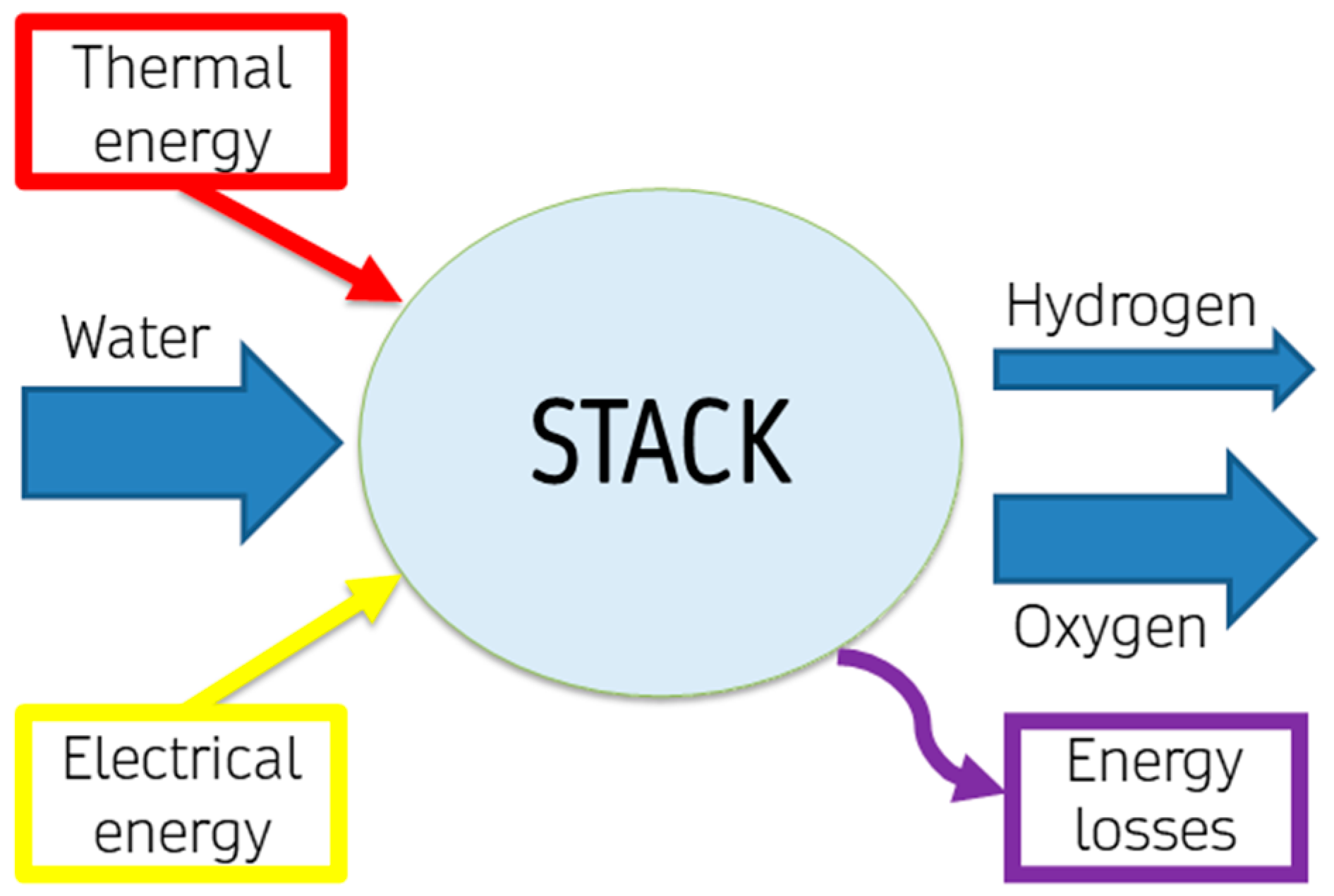
3.2. Electrolyser Analysis at Stack Level
The individual cell, while significant, does not fully represent the complexity of a stack, which comprises multiple cells operating in tandem. Understanding the behaviour and efficiency of this stack is paramount, as it reflects the operational dynamics of the electrolyser system, as depicted in
Figure 4.
Energy consumption before the application of voltage to the apparatus (e.g., energy consumption of water demineralization, and system’s pressurization), electrolyser ageing effects (e.g., corrosion and wear effects, such as membrane thinning and anode oxidation) and transients, thus performance degradation during electrolysers’ lifetime and with changing operating conditions, are neglected in this first energy analysis of water electrolysis. Considering an electrolytic stack, a mass balance can be written as in Equations 2 and 3, in mol, or Equation 4, in mass:
and by respective molar masses and Equation 4, per kg of hydrogen produced, the mass balance results as follows:
The electrical power in input to an electrolytic cell (W
cell) is equal to the product of the operational cell voltage (U
cell) and the electrical current (I), thus, by Equation 1 it can be described as in Equation 6:
where W
irr is the electrical power due to the various irreversibilities. In commercialized stacks, electrolytic cells are assembled in series, thus Equations 7 and 8 characterize the stack voltage, U
stack, and the electrical current in the stack, I
stack:
with N as the number of electrolytic cells in the stack.
Therefore, the electrical power in input to the stack, W
stack, can be identified by:
Thus, the irreversibilities due to the cells’ assembly result as:
As a result of the process irreversibilities, the generated thermal power, Q
gen, can be evaluated by:
where a part of the thermal power generated by the process irreversibilities satisfy the thermal energy demand (T∙ΔS), in the time unit, of the WE reaction (
Figure 3; z = 2 is the number of moles of electrons, e
-, transferred per mole of hydrogen in the overall redox equation, and F = 96,485.3 C/mol
e− [
16] is the Faraday constant). While considering the electrochemical schematization mentioned above and the need to split water molecules into hydrogen and oxygen, there are various technologies available for electrolysis. These technologies result in significant differences and a wide variety of solutions. Choosing the appropriate technology depends on factors such as required hydrogen purity, energy source availability, scalability, and economic considerations, highlighting the need for a comprehensive understanding of each technology’s strengths and limitations.
Beyond the electrochemical description, the essence of electrolysis lies in the need for energy input, primarily electrical and sometimes thermal. The minimum theoretical values, as identified in the previously discussed model, are often far from being achieved.
4. Electrolysis: The Main Technologies and the Relevant Differences
A general classification of electrolysis technologies can be carried out by the operating temperature and the ion transport medium (electrolyte) employed.
Technologies such as alkaline (ALK), proton exchange membrane (PEM) and anion exchange membrane (AEM) electrolysis have operating temperatures in the range of 50–80 °C. High-temperature technologies like solid oxide (SO) cells, molten carbonate (MC) cells and proton conductive cells (PCC) typically work at 500–1000 °C.
Low-temperature WE technologies employ a basic ionic transport medium, such as a concentrated solution of hydroxide in ALK electrolysis, or an acid medium, such as a solid polymer electrolyte in PEM electrolysers. In AEM technology, both a polymeric membrane and an aqueous solution of hydroxide (a few percentage points by weight) are used, trying to combine the advantages of both technologies. In SO electrolysis, the main high-temperature technology, oxygen ions are responsible for the ionic transport and ceramic membranes are utilized.
ALK and PEM electrolysis technologies are indeed commercially available and widely used for hydrogen production. These technologies have been in commercial use for several years and are considered mature and established. SO and AEM electrolysis technologies, on the other hand, are still considered emerging or maturing technologies. While there has been significant research and development in these areas, they are not as widely commercialized as ALK and PEM technologies.
4.1. ALK Electrolysis
Alkaline WE is the more mature technology with a long history of deployment in the chlor-alkali industry. For an ALK electrolysis cell,
Figure 5, the half-cell reactions at the electrodes are the reduction half-reaction at the cathode, Equation 12, and the oxidation half-reaction at the anode, Equation 13:
where the overall reaction is liquid water electrolysis (
Figure 3). The reduction reaction of H
2O to H
2 happens in the cathode, generating hydroxyl ions (OH
-); the OH
- ions move toward the anode where they donate their electrons and oxygen is produced. About the potential difference between the electrodes in standard conditions, U°
cathode is 0.83 V and U°
anode is 0.40 V [
16], thus U°
rev equals 1.23 V, as calculated by the standard free-energy change value of liquid water electrolysis.
The main components of an ALK electrolysis cell (
Figure 5) are two electrodes, an aqueous solution of an inert strong electrolyte (usually potassium hydroxide, KOH, or sodium hydroxide, NaOH), and a porous medium (diaphragm) as the separator of the anode and cathode chambers. Electrocatalysts are usually employed, e.g., nickel-foam sprayed with platinum. The diaphragm is not perfectly impermeable: at low current densities, the cross-contamination phenomenon (crossover) could occur, i.e., some of the gases at the ends of the diaphragm could pass through it, implying explosion risk. The crossover in alkaline cells implies the need for hydrogen purification (substantially, deoxygenation by pressure swing adsorption). If coupling with non-stationary power sources, a lower limit to current density is needed (around 20% of rated power).
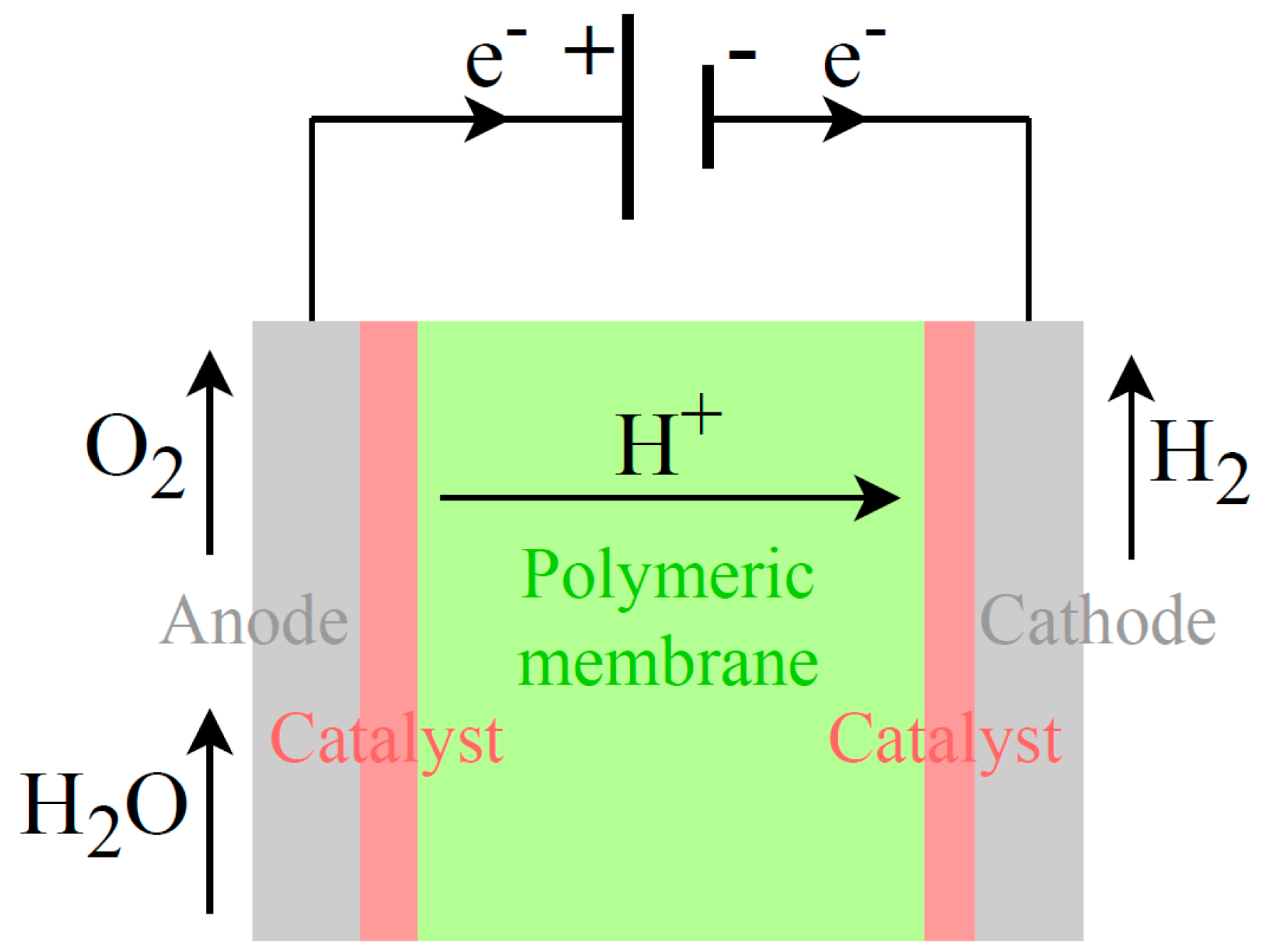
4.2. PEM electrolysis
In PEM electrolysis,
Figure 6, the two half-reactions, respectively at the cathode and anode, are described by Equations 14 and 15:
In standard conditions, U°
cathode is 0 V and U°
anode is 1.23 V [
16], thus U°
rev equals 1.23 V (as calculated by ∆G° of liquid water electrolysis in
Section 3.1).
In a PEM electrolysis cell (
Figure 6), a polymeric membrane acts as the electrolyte and separator, allowing only proton ions (H
+) transport from the anode to the cathode. Platinum Group Metals (usually iridium and platinum) are used as catalysts to accelerate the splitting process. Scarce materials can represent a barrier to electrolysers’ scaling up.
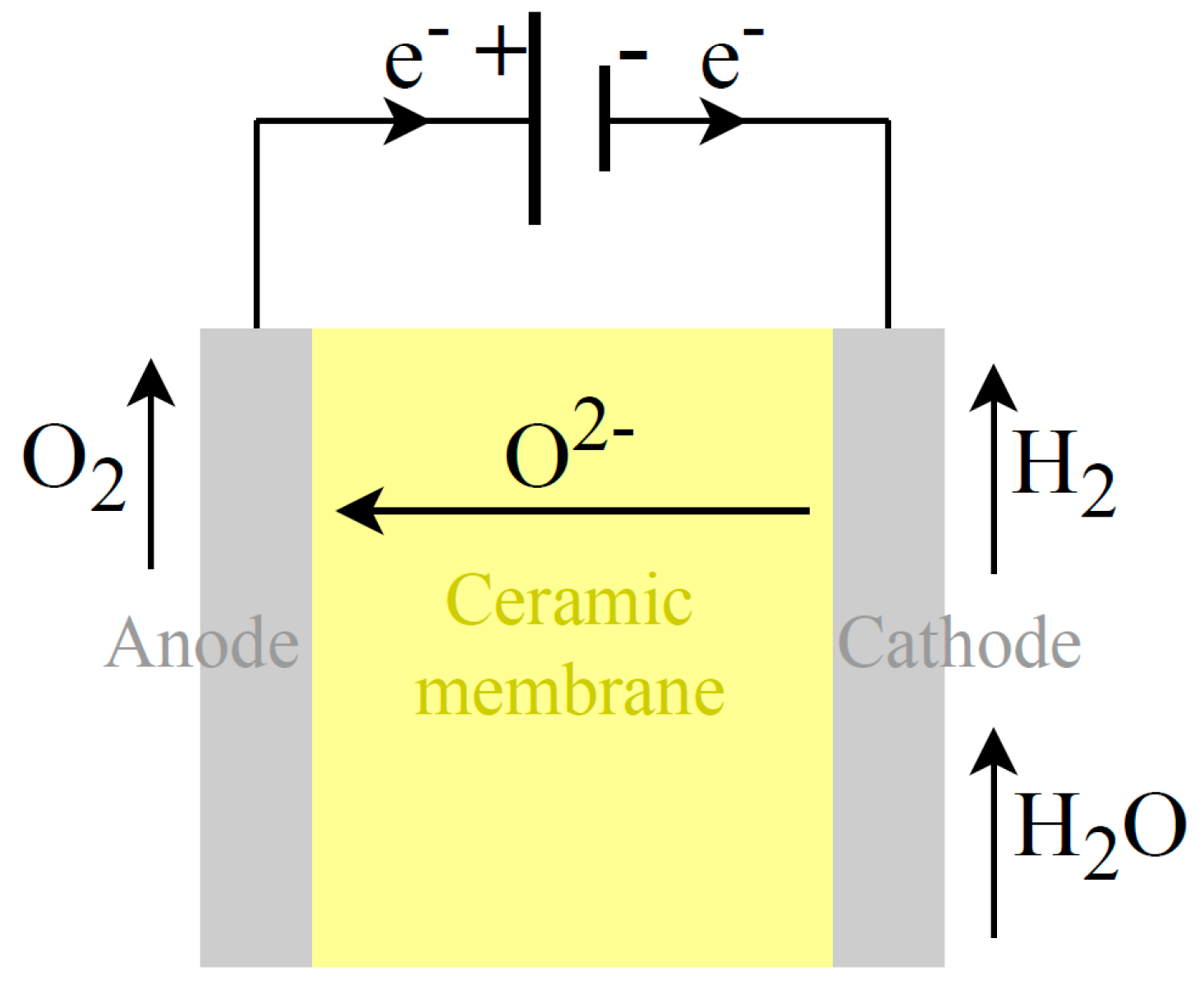
4.3. SO Electrolysis
Increasing temperature in electrolysis processes can lead to significant reductions in electrical consumption but introduce the use of thermal energy. High-temperature electrolysis, such as by SO cells,
Figure 7, is a promising approach that utilizes elevated temperatures, typically above 600 °C often in the range of 800 to 1000 °C. The reduction half-reaction at the cathode, Equation 16, and the oxidation half-reaction at the anode, Equation 17, are written as follows:
where the overall reaction is steam electrolysis (
Figure 3). At 1000 K, U
rev is equal to 0.94 V [
12], a much smaller value than U°
rev (1.18 V) calculated in
Section 3.1, thanks to the strong reduction in electricity demand (∆G) as the temperature increases.
In SO electrolysis (
Figure 6), a ceramic membrane is used (usually YSZ, i.e., yttria-stabilized zirconia), which acts as the electrolyte and separator, allowing only oxygen ions (O
2-) transport from the cathode to the anode. A preheated air flow is normally introduced through the anode. This air stream is used to control the temperature and guarantee the correct temperature distribution inside the stack, thus, avoiding a degradation increase. By leveraging the benefits of elevated temperatures, SO electrolysers look promising in reducing electricity consumption, making it an area of active research. However, solid oxide electrolysis needs process heat and steam in input, which should be considered in an evaluation of the First Law efficiency and system performance. If industrial waste heat or heat from RES is used as process heat, overall efficiency improvement is possible.
Thermal control is indeed a significant concern in solid oxide electrolyzers (SO electrolyzers). Maintaining precise operational temperatures is crucial for the efficiency and stability of SO electrolyzers. These devices rely on high operating temperatures, typically around 700-900 °C, for the solid oxide electrolyte to conduct ions effectively.
Accurate thermal management is essential to prevent overheating, ensure uniform temperature distribution across the electrolyzer cells, and avoid thermal stress on the materials, which can impact the electrolyzer’s performance and lifespan. Additionally, efficient thermal control is vital for safety reasons, as high temperatures can pose risks if not properly managed. Developing effective thermal management systems is a key area of research and innovation in the field of SO electrolysis.
4.4. The Operational Performance of Electrolysers
The operative conditions and the electrodes, ion transport medium, and catalysts employed characterize the performance of the various electrolysers. A lot of research on electrolytic cells’ performance is carried out to increase the efficiency of electrolysers [
4,
5,
6,
7,
8,
9,
10,
11,
12] and WE plants [
13]. Electrical efficiency losses can be identified as a function of many variables, as shown in
Table 1. The thermal energy required is fundamental too, from warming up to heat requirements during electrolysers’ operation, through components’ heat transfer properties and heat dissipation or usage.
In literature, the ohmic losses have been identified as predominant both for low- and high-temperature technologies in the range of operational current densities. Much research still has to be conducted to identify the configurations, materials and operating conditions that maximize hydrogen production while minimizing the associated energy consumption of each technology. The main characteristics, traced in literature and by market analysis, of the leading electrolysis technologies are provided in
Table 2.
The main limits and positive characteristics of each technology are reported in
Table 3. ALK technology advantage is the condensation recovery, which implies a lower water requirement; on the other hand, corrosive electrolytes are employed, low current densities entail bigger volumes, and a hydrogen purification stage is necessary due to the cross-contamination phenomenon. PEM electrolysers seem to be interesting for specific utilization; for example, they can harness cooling system heat. Unfortunately, they incur higher costs due to components (catalysts, membranes) and operation (e.g., water use).
SO electrolysers boast superior electrical efficiency and reversibility. However, they require high-temperature steam and thermal sources (even if, it would be possible to use industrial waste heat or heat from RES as process heat), warm-up times are long because high operational temperatures must be reached, and the useful life is limited due to thermal cycles. Selecting the ideal WE technology depends on specific operating conditions set by electricity and heat sources, and hydrogen end-uses. SO electrolysis, when fully developed, appears optimal for industrial use, proven ALK technology is suitable for re-electrification or for developing the idea of promoting hydrogen injection into natural gas grids, while smaller PEM electrolysers could find applicability in the transport and civil sectors. Hydrogen still faces numerous limitations, and upon closer examination, data in certain application areas show practically negligible levels. Certainly, it is accurate to assert that electrolysis technology still faces limitations, as outlined in the following
Table 3. This highlights the existing challenges and areas that require further development and innovation. The estimated cost of green hydrogen ranges from 3 to 6
$ per kilogram of produced H
2, while ongoing advancements aim to reduce it to 2
$/kg
H2 or lower [
17,
18]. In the literature it is possible to find estimates on the useful life of ALK and PEM electrolysers longer than those reported in
Table 3 (e.g., 60,000–95,000 h for ALK, 50,000–80,000 h for PEM [
5,
17]); however, tests performed to predict stacks’ long-term performance (e.g., according to Accelerated Stress Test methodology) are around a few thousand hours, extrapolating performance data up to 40,000 hours. Components lifetime is usually tested in the order of some to several thousands of hours [
19,
20]. SO electrolysers’ lifespan is still in research, nowadays estimated to be at most 10,000–20,000 h [
5,
17].
4.5. The Actual Problems Connected to Electrolysis and Electrolyser Development
The recent advancements in electrolysis demand continuous research to enhance efficiency, scalability, and cost-effectiveness in hydrogen production. Existing challenges include integrating electrolysis with renewable energy sources and energy grids while ensuring stability. Current data, often from commercial sources, lack standardized methodologies, highlighting the need for universal testing protocols.
The ISO 22734:2019(E) is a document intended to be used for certification purposes of ALK, PEM and AEM electrolysers for industrial, commercial and residential applications [
21]. The ISO Technical Committee ISO/TC 197 is developing standards for hydrogen technologies; in particular, the Working Group ISO/TC 197/WG 34 deals with electrolysers’ test protocols and safety requirements. System equipment requirements, test methods, and the ongoing work of ISO/TC 197/WG 34 emphasize the necessity for standardized methods to ensure accurate performance evaluation in electrolysis. The development of hydrogen production through water electrolysis necessitates a well-structured system of regulations and certification, addressing challenges in standardization, data reliability, and performance measurement methodologies.
Table 4 provides a summary of the main research activities and objective in water electrolysis.
Beyond the established successes, it meticulously scrutinizes the intricate challenges tied to seamlessly integrating electrolysis systems with existing energy grids and renewable sources. As we explore the intersection of energy analysis and electrolysis, we confront the pressing issue of scaling up.
This multifaceted investigation navigates the complexities of amplifying electrolysis capacities, ensuring not only increased production efficiency but also harmonious integration within larger energy infrastructures. By delving into these critical areas, this research aims to illuminate pathways for a sustainable and efficient hydrogen production future, mainly based on general integration with renewable energy. In the next Section we will try to investigate this further problem.
5. Energy Analysis of Electrolysis: A Commercial State-of-the-Art and the Additional Problems Connected with Energy System Integration and scaling Up
Electrolysis is often measured in terms of energy intensity, representing the energy required to produce a unit of hydrogen (typically in kilowatt-hours per kilogram, kWh/kg). Typically, real-world electrical energy demand for hydrogen production via water electrolysis falls in the range of 50 to 60 kWh per kg of hydrogen produced. However, ongoing research and advancements in electrolysis technologies aim to reduce these losses, bringing the actual energy demand closer to the theoretical minimum.
According to the data available from the literature and market, summarized in
Table 5, it is evident that the energy consumption related to hydrogen production is notably higher than the theoretical values discussed above. For electrolysers with nominal powers from 100 kW to 1 MW and above, the average specific electricity consumption (ASEC) falls within the range of 55 to 60 kWh per kilogram of hydrogen produced for low-temperature electrolysers and 40–42 kWh/kg for high-temperature electrolysers.
These figures highlight that there are efficiency losses and energy requirements beyond the idealized values in practical hydrogen production systems.
The producible hydrogen per MW of installed electrolysis capacity is about 18 kg/h for low-temperature multi-MW scale electrolysers; the solid oxide technology aims for values greater than 20 kg/h per installed MW. The energy consumption for hydrogen production is partially dependent on the size of the electrolyser. When considering the implementation of H2 production systems, it is essential to take into account the specific characteristics and scale of the electrolyser to accurately assess its energy requirements.
Technological advancements in low-temperature and high-temperature electrolysis and optimizations can lead to improvements in energy efficiency, potentially reducing the energy consumption per unitary mass of hydrogen produced. The performances of an electrolyser can be evaluated by means of the First Law efficiency as:
where the useful effect of the process is evaluated through hydrogen (mass) lower calorific value, LHV
H2, and (mass) flow rate, ṁ
H2, and W is the electrical power and Q is the thermal power in input to the electrolyser. Considering only electricity consumption data available in the literature and commercial catalogues, efficiency results in around 60% for low-temperature electrolysers and about 80% for high-temperature electrolysers, with the current ASECs of large scale electrolysers identified in
Table 5. By the declared electricity consumption of electrolysers, it is possible to define an electrical efficiency, η
el, as the ratio of the electricity demand of the electrolytic process and the average specific electricity consumption of the electrolyser [
2]:
Evaluating the electricity demand in standard conditions, ΔG°, to have a fixed term of comparison depending only on operating electrolysis at low or high temperatures, and through ASECs identified in
Table 5, the electrical efficiency is around 57% for low-temperature electrolysers and 77% for high-temperature electrolysers. The electrical energy demand decreases steadily as the temperature increases, and it decreases significantly above 100 °C; if the ΔG were evaluated at the operating temperature instead of in standard conditions, the electrical efficiency would surely assume lower values, especially for high-temperature electrolysers.
Table 6 summarizes the main data and results.
By the current average specific electricity consumptions (
Table 5 and
Table 6), an upper bound for the operating cell voltage in large scale electrolysers can be estimated as in
Table 2, by the ratio between U°
rev and U
cell and the average electrical efficiency, in analogy to the amount of electrical work shown in
Figure 3 (thus, ASEC
cell as a function of U
cell) and Eq. 19. Considering low-temperature electrolysis applications, such as ALK, PEM, and AEM electrolysers, energy consumption is of the order of 55–60 kWh per kg of hydrogen produced, and this means that in the first step about 40% of the energy is lost. At elevated temperatures, the electrolysis reactions become more favourable. The higher operating temperature allows for faster reaction kinetics, enabling higher production rates of hydrogen. All this leads to lower overpotentials required; thus, less electrical energy is needed to drive the reactions, resulting in reduced electricity consumption. However, it’s important to note that high-temperature electrolysis comes with its challenges, such as material compatibility, thermal management, and system integration. High temperatures require appropriate materials for cell components and sealing, and efficient heat transfer mechanisms are necessary for obtaining the desired operating conditions. These technical considerations need to be addressed to realize the full potential of high-temperature electrolysis.
5.1. System Level and Integration Problem
An additional problem connected with hydrogen production by means of water electrolysis is the fact that electrolysers are integrated into complex energy systems where additional energy consumption and inefficiencies are inherent. This intricate interplay demands meticulous consideration to optimize efficiency and minimize overall energy losses.
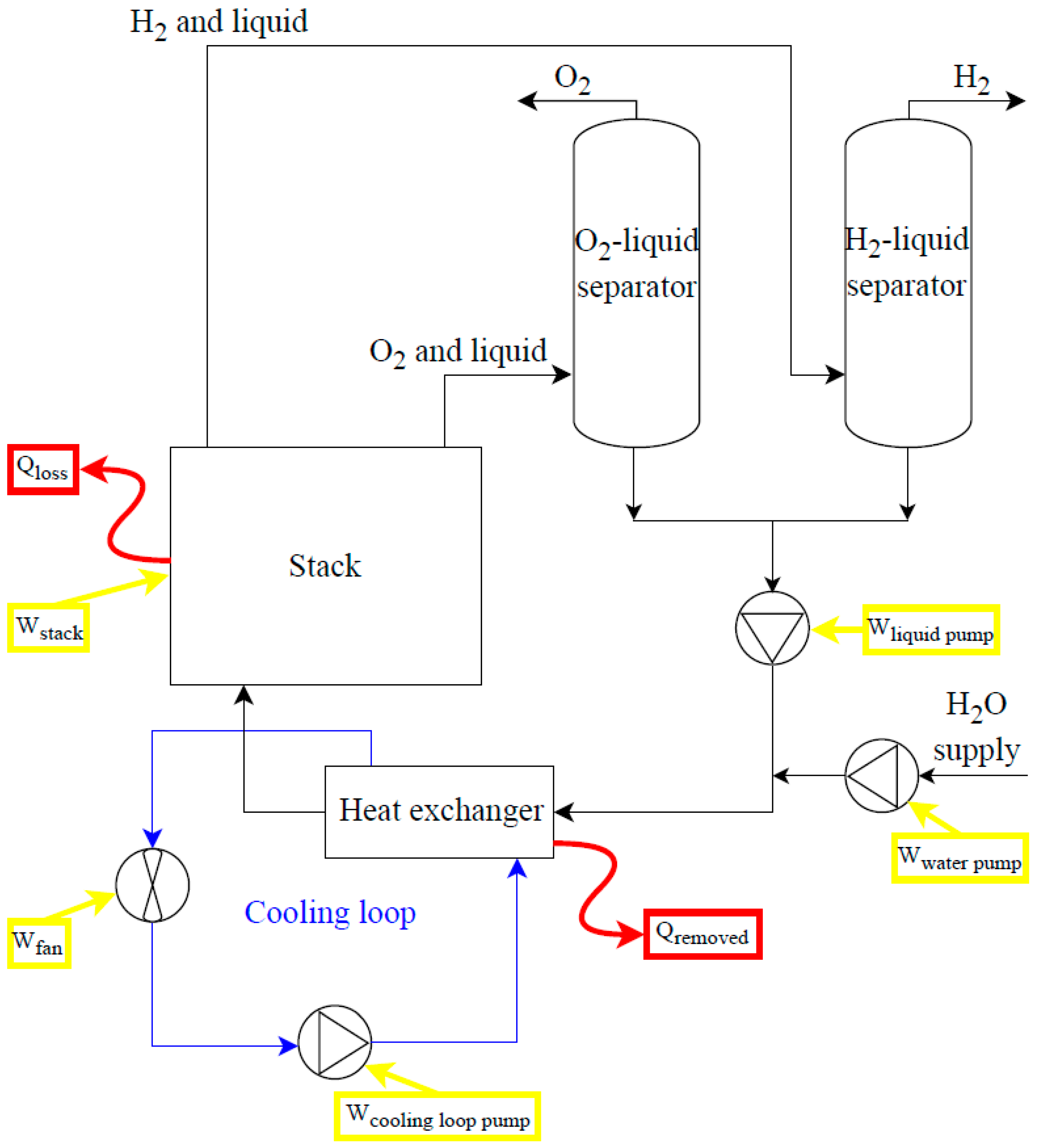
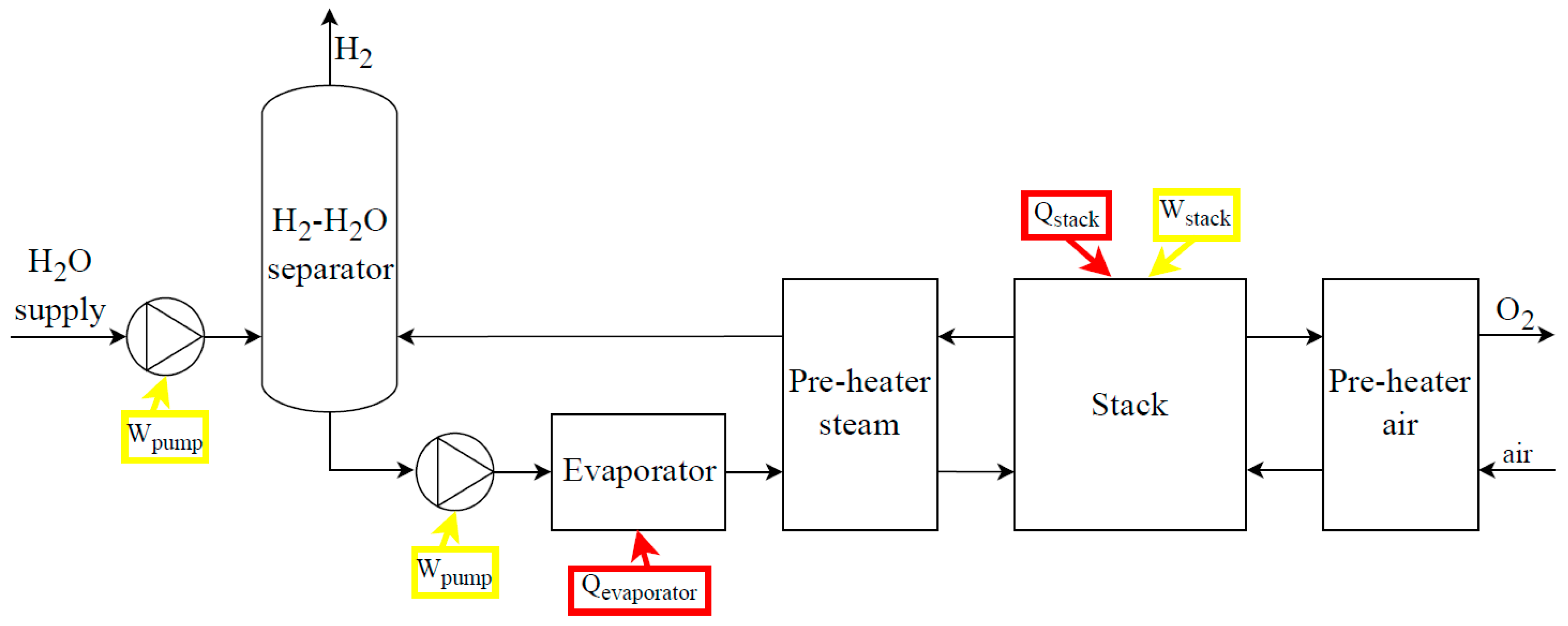
Figure 8 and
Figure 9 are simplified schemes of, respectively, low-temperature and solid oxide electrolysis systems. The energy consumption of auxiliaries (e.g., fans and pumps) must be considered: their percentage of total consumption decreases as the rated power of the electrolyser increases. The set of auxiliary systems constitutes the balance of plant. The main components of ALK and PEM electrolysis systems (
Figure 8), in addition to the stack, are the deionized water subsystem, the power supply subsystem, the gas-liquid separation subsystem, the pressure control subsystem, and the thermal management subsystem.
Considering the case of an ALK electrolysis system, energy balances can be written as in Equations 20 and 21:
with W
pumps as the electrical consumption of water, electrolyte solution and cooling loop pumps, Q
rem as the thermal power removed by the cooling system to maintain the stack temperature at operating values, and Q
loss as the thermal losses of the stack,
Figure 8. In the case of cogeneration purposes, the electrical consumption of the fan and cooling loop pump could be avoided, and the thermal power generated in the stack could be recovered, how it is evaluated for PEM electrolysis systems.
Main SO electrolysis systems’ components (
Figure 9) are the power supply subsystem, the deionized water subsystem, the steam production subsystem, the subsystem of air supply, the stack, the subsystem of stack heating, the H
2/steam supply subsystem, the gas-liquid separation subsystem, and the pressure control and thermal management subsystems. All electrolysis systems (
Figure 8 and
Figure 9) may require drying subsystems (condensate traps, cooling systems, and coalescence/desiccant filters) and purification subsystems (de-oxo reactors) depending on the quality of the hydrogen required. A monitoring and control subsystem is always necessary, to collect all the information coming from sensors and take the necessary actions to enable the correct and safe operation of the system.
5.2. Electrolysers’ Scaling Up
ALK and PEM electrolysers of the size of 100 kW are commercially available and already marketed, but larger sizes are needed for massive hydrogen production to meet future demand scenarios for decarbonization, as discussed in
Section 2. MW-size projects are currently in the demonstration phase, and GW scale projects are announced for next years [
1,
15].
Water electrolysis is a modular technology, and electrolyser manufacturers are evaluating different choices in terms of stack dimensions and number of stacks to connect to reach the MW size. Scaling up of stack components is a difficult challenge for the various electrolysis technologies.
Investigating electrolysers literature and the market, it is evident that smaller sizes have higher nominal energy consumption. For large electrolysers, more performing materials and configurations can be used, since a higher initial capital cost can be compensated by lower operating energy costs in massive hydrogen production. However, cells’ assembly losses are important and the balance of plant needs to be carefully optimized.
Moreover, advancements in electrolysis technology will lead to the development of more efficient and sophisticated electrolysers, bringing the technology from the megawatt (MW) scale to the gigawatt (GW) scale. The need to increase electrolysers’ manufacturing capacity is a priority to materially contribute to energy transition by water electrolysis [
17]. For green hydrogen production, electrolysers’ scaling up travels with RES exploitation’s growth and with H
2 uses’ development.
Adequate strategies, policies and financial support from the key stakeholders (i.e., Governments, Industry and International Organisations) are fundamental for the implementation of the entire green hydrogen value chain. Manufacturing capacity’ scaling up and costs reduction are necessary for, and possible only if, electrolysers’ scaling up takes place. Technological improvement in terms of electrolysers’ efficiency and durability are fundamental to reducing the specific cost of the hydrogen produced, decreasing the amount of energy required to produce one unit of hydrogen, and extending the equipment lifetime thus spreading the cost of the electrolyser facility over a larger hydrogen production volume. Cost reduction in electrolysers’ manufacturing and balance of plant could be possible by economies of scale. Design and manufacturing should be simplified and standardised to allow for industrialization.
Uncertainties about the demand for green hydrogen versus fossil-fuels based hydrogen with CCUS are a big obstacle to electrolysers’ scaling up [
17]: policymakers should carefully assess this balance in investments.
Water and land use should not represent barriers to electrolysers’ scaling up. Sea water desalination could be used with limited penalties on cost or efficiency [
17]. Scarce materials can be a barrier: e.g., Platinum Group Metals used as catalysts are an important part of the capital cost, thus, they might represent a bottleneck for scaling up. Research on recycling of these materials could have a positive impact on large scale applications. The choice of concentrated or distributed electrolytic hydrogen production influences the electrolysers’ size to use. For example, an industrial district could be an agglomerate of hydrogen production and demand to favour the economies of scale, spreading capital and operating costs, thus lowering the levelised cost of H
2.
5.3. Integration with Renewable Energy Sources in the Electrical Energy System
As mentioned earlier, hydrogen will play a pivotal role in the energy transition only if it successfully facilitates the integration of renewable energy sources. Renewable energy storage is fundamental. Electrochemical storage systems are not well suited if energy needs to be stored for long periods, such as weeks and months. One of the best solutions for long-term storage of renewable energy is green hydrogen, because of its limited self-discharge rate (leakage and/or permeation) and decoupling of energy rating from the power rating. Green power can be used to produce electrolytic hydrogen when electricity production from RES is higher than electricity consumption. The stored H
2 can then be used to produce electricity when production from RES is lower than demand (re-electrification) or can be employed in all the applications discussed in
Section 2.
However, RES variability and intermittency, therefore uncertainty, can be a problem for electrolysers, which adapt poorly to sudden load variations (potential problems of corrosion, changes in the internal temperatures, pressure drops, explosive mixture generation) and have better performance at full or high loads. Together with suitable power electronics, batteries’ utilization is probably the best strategy capable of guaranteeing green electricity supply to electrolysers in the most stable and controlled way possible.
RES low density is a challenge for green hydrogen production too.
It is estimated that a large scale electrolyser will be able to work around 5000 h/y [
1]. Assuming a WE specific electricity consumption of 55 kWh/kg, a balance of system typical of big photovoltaic (PV) generation, and an annual specific solar energy equal to 1500 kWh, 250 kW of installed PV peak power could be necessary to produce 1 kg/h of electrolytic hydrogen.
The efficiency of green hydrogen production systems by wind energy or photovoltaic generation and low-temperature electrolysis is estimated to be around 10–12% [
15].
Considering current average efficiencies of photovoltaic modules, charge regulators, batteries, inverters, low-temperature electrolysers and balance of plant, an overall efficiency (η
PV+WE) can be evaluated between 8% and 10%, as the product of the average efficiency of the photovoltaic generation and the average efficiency of the electrolytic hydrogen production system. Assuming an overall efficiency of around 10%, about 330 kWh of solar energy per kg of H
2 are needed, according to the general indication given by a kind of extended Balance of System efficiency, as in Equation 22:
The topic of integrating hydrogen into energy systems based on intermittent renewable sources, such as photovoltaics, must be approached with great care and a keen eye on overall energy balances, considering the significant losses during the conversion from electrical power to hydrogen. It certainly requires improvements, especially concerning electrolysis, but it needs to be evaluated with utmost caution in every aspect.
5.4. Integration of WE with Renewable Energy Sources in the Hard-to-Abate Industrial Context
A perhaps more feasible possibility, although challenging to implement, could be the direct connection between some highly energy-intensive industrial processes and renewable-based generation facilities (such as photovoltaics) using hydrogen. However, even in this scenario, certain dimensional aspects need to be clear, namely the high power demands in these sectors. Let’s consider, for illustrative purposes, a specific case - the steel industry - and conduct evaluations referencing specific sites with significant steel plants.
The authors of this article have already explored in a recent study the potential use of hydrogen in the steel industry, which can be employed both as a direct fuel or to innovate the production process through new technologies, such as the Direct Reduced Iron process [
2]. For example, 60 kg of H
2 generated by low-temperature WE are necessary per tonne of steel produced by hydrogen-based direct reduced iron process (H
2DRI) and electric arc furnace (EAF) process [
2].
The producible hydrogen per MW of installed electrolysis capacity is about 18 kg/h for multi-MW scale low-temperature electrolysers; about 3.3 MW of electrolysis capacity would be necessary per tonne/h of steel produced by H2DRI-EAF.
Table 7 provides some qualitative estimations for photovoltaic generation per tonne of steel, as produced by the H
2DRI-EAF process utilizing hydrogen generated through low-temperature water electrolysis [
2], in several major steel production sites worldwide. Evaluating the minimum PV surface that would be necessary in some big steel production sites by local hydrogen production, thus the respective Global tilted irradiation at the
optimum angle [
22], and assuming annual production, 0.2–0.3 m
2 are needed per kg
H2, thus 12–18 m
2/tonne
steel. In favourite sites, around 0.2 m
2/kg
H2 thus more than 10 km
2/Mtonne
steel, with current process efficiencies, are required: these very big numbers are due to low PV+WE efficiency and relatively low energy density of solar source. Indeed, concentrated green hydrogen production is evaluated in greater irradiation sites. While working on improving all hydrogen processes’ efficiency, green H
2 blending in the direct reduced iron process would need to be evaluated to reduce the iron and steel sector’s emissions.
6. Conclusions
Electrolysis accounts for approximately 0.1% of the world’s hydrogen production while the majority of hydrogen is still produced through natural gas reforming. Although electrolysis’s contribution remains marginal, its development becomes crucial for green hydrogen production, marking a small step toward industrial decarbonization.
Electrolysis systems are typically 60–80% efficient, meaning they convert 60–80% of the input electrical energy into chemical energy stored in hydrogen gas.
On average, low-temperature MW scale electrolysers need 55–60 kWh of electricity to produce 1 kilogram of hydrogen. High-temperature electrolysis can be more efficient, and the target is to reduce energy consumption at 40 kWh of electricity to produce 1 kilogram of hydrogen, but it requires steam and process heat in input. The producible hydrogen per MW of installed electrolysis capacity is about 18 kg/h for low-temperature multi-MW scale electrolysers and about 25 kgH2/h/MWWE for high-temperature ones.
Realistic estimates of ALK and PEM electrolysers’ lifespan are 10,000–40,000 operational hours. Nowadays, AEM and SO electrolysis devices have a useful lifetime shorter. Advanced materials and engineering could extend the lifespan of electrolysers of each WE technology. Increasing electrolysers’ useful life can reduce the hydrogen production cost reduction and promote electrolysers’ scaling up.
The cost of green hydrogen production through electrolysis varies widely based on factors such as electricity prices, electrolyser efficiency, and scale. The estimated cost ranges from 3–6 $/kgH2. Ongoing advancements aim to reduce the cost to 2 $/kgH2 or lower, making green hydrogen more economically competitive.
Research in various areas aimed at increasing the efficiency of electrolysis processes, bringing them as close as possible to theoretical values (around 33 kWh of energy and about 9 kg of water per 1 kg of hydrogen produced), cost reduction of electrolysers, and prolonging their lifespan (a useful life of 10,000 hours seems rather low to justify economic investments) is crucial to positioning hydrogen as a significant player in the future energy transition. However, it is essential to recognize that hydrogen is an energy carrier, and the primary challenge remains the energy resource itself. The examples discussed, particularly the integrations between hydrogen and photovoltaics in various contexts, including specific facilities like steel plants, highlight the difficulties and complexity of the challenge ahead. Nevertheless, this study aims to showcase the ongoing efforts in electrolysis process improvement and the goals set for future research.
Author Contributions
Conceptualization, A.F. and C.G.; formal analysis, A.F. and C.G.; methodology, A.F. and C.G.; data curation, C.G.; supervision, A.F.; writing, review, and editing, A.F. and C.G. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3 - Call for tender No. 1561 of 11.10.2022 of Ministero dell’Università e della Ricerca (MUR); Project funded by the European Union – NextGenerationEU. Award Number: Project code PE0000021, Concession Decree No. 1561 of 11.10.2022 adopted by Ministero dell’Università e della Ricerca (MUR), CUP I53C22001450006, according to attachment E of Decree No. 1561/2022, Project title “Network 4 Energy Sustainable Transition – NEST”.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| ASEC |
average specific electricity consumption, kWh/kgH2
|
| ∆G |
Gibbs free-energy change, J/molH2 or kWh/kgH2
|
| ∆H |
enthalpy change, J/molH2 or kWh/kgH2
|
| ∆S |
entropy change, J/molH2/K |
| Ecell
|
cell voltage, V |
| η |
efficiency, % or dimensionless |
| η˅
|
overpotential, V |
| F |
Faraday constant, C/mole−
|
| I |
electrical current, A |
| LHV |
mass lower heating value, kWh/kg |
| m |
mass, kg |
| ṁ |
mass flow rate, kg/h |
| N |
number of cells, dimensionless |
| n |
number of moles, mol |
| P |
power, kW |
| Q |
thermal power, kW or W |
| T |
temperature, °C or K |
| U |
voltage, V |
| W |
electrical power, kW or W |
| z |
number of moles of electrons per mole of H2, mole−/molH2
|
| Subscripts, superscripts, acronyms and abbreviations |
| AEM |
anion exchange membrane |
| ALK |
alkaline |
| (aq) |
aqueous solution |
| CCUS |
carbon capture, utilisation and storage |
| e−
|
electron |
| EAF |
electric arc furnace |
| el |
electrical |
| I |
defined through the First Law of Thermodynamics |
| (g) |
gaseous state |
| gen |
generated |
| H2DRI |
hydrogen-based direct reduced iron process |
| irr |
irreversibilities |
| (l) |
liquid state |
| MC |
molten carbonate |
| Me |
metal |
| ng |
natural gas |
| NZE |
net zero emissions by 2050 |
| PCC |
proton conductive cell |
| PEM |
proton exchange membrane |
| PV |
photovoltaic |
| rem |
removed |
| RES |
renewable energy sources |
| rev |
reversible |
| SO |
solid oxide |
| ° |
standard conditions (1 atm and 25 °C) |
| TRL |
technology readiness level |
| WE |
water electrolysis |
| YSZ |
yttria-stabilized zirconia |
References
- International Energy Agency, IEA Report, Global Hydrogen Review 2023. Available online: https://www.iea.org/reports/global-hydrogen-review-2023 (accessed on 29 September 2023).
- Franco, A.; Giovannini, C. Routes for Hydrogen Introduction in the Industrial Hard-to-Abate Sectors for Promoting Energy Transition. Energies 2023, 16, 6098. [Google Scholar] [CrossRef]
- International Energy Agency, Electrolysers. Available online: https://www.iea.org/energy-system/low-emission-fuels/electrolysers (accessed on 29 September 2023).
- Millet, P.; Grigoriev, S. Chapter 2 - Water Electrolysis Technologies. In Renewable Hydrogen Technologies; Gandía, L.M., Arzamendi, G., Diéguez, P.M., Eds.; Elsevier: Amsterdam, NL, USA, 2013; pp. 19–41. [Google Scholar] [CrossRef]
- Amores, E.; Sánchez, M.; Rojas, N.; Sánchez-Molina, M. Renewable hydrogen production by water electrolysis. In Sustainable Fuel Technologies Handbook; Academic Press: Cambridge, MA, USA, 2021; pp. 271–313. [Google Scholar] [CrossRef]
- Phillips, R.; Edwards, A.; Rome, B.; Jones, D.R.; Dunnill, C.W. Minimising the ohmic resistance of an alkaline electrolysis cell through effective cell design. Int. J. Hydrog. Energy 2017, 42, 23986–23994. [Google Scholar] [CrossRef]
- Sakas, G.; Ibáñez-Rioja, A.; Ruuskanen, V.; Kosonen, A.; Ahola, J.; Bergmann, O. Dynamic energy and mass balance model for an industrial alkaline water electrolyzer plant process. Int. J. Hydrog. Energy 2022, 47, 4328–4345. [Google Scholar] [CrossRef]
- Roggi, A.; Guazzelli, E.; Resta, C.; Agonigi, G.; Filpi, A.; Martinelli, E. Vinylbenzyl Chloride/Styrene-Grafted SBS Copolymers via TEMPO-Mediated Polymerization for the Fabrication of Anion Exchange Membranes for Water Electrolysis. Polymers 2023, 15, 1826. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.; Smith, K.; Dodwell, J.; Hinds, G.; Shearing, P.R.; Brett, D.J.L. Mass transport in PEM water electrolysers: A review. Int. J. Hydrog. Energy 2022, 47, 30–56. [Google Scholar] [CrossRef]
- Noor Azam, A.M.I.; Li, N.K.; Zulkefli, N.N.; Masdar, M.S.; Majlan, E.H.; Baharuddin, N.A.; Mohd Zainoodin, A.; Mohamad Yunus, R.; Shamsul, N.S.; Husaini, T.; et al. Parametric Study and Electrocatalyst of Polymer Electrolyte Membrane (PEM) Electrolysis Performance. Polymers 2023, 15, 560. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.S.; Peppley, B.A.; Burheim, O.S. Tuning transport mechanisms in fuel-assisted solid oxide electrolysis cells for enhanced performance and product selectivity: Thermodynamic and kinetic modeling. Chem. Eng. J. 2023, 452, 139079. [Google Scholar] [CrossRef]
- Beale, S.B.; Andersson, M.; Boigues-Muñoz, C.; Frandsen, H.L.; Lin, Z.; McPhail, S.J.; Ni, M.; Sundén, B.; Weber, A.; Weber, A.Z. Continuum scale modelling and complementary experimentation of solid oxide cells. Progr. Energy Combust. Sci. 2021, 85, 100902. [Google Scholar] [CrossRef]
- Rizwan, M.; Alstad, V.; Jäschke, J. Design considerations for industrial water electrolyzer plants. Int. J. Hydrog. Energy 2021, 46, 37120–37136. [Google Scholar] [CrossRef]
- Giacomazzi, E.; Troiani, G.; Di Nardo, A.; Calchetti, G.; Cecere, D.; Messina, G.; Carpenella, S. Hydrogen Combustion: Features and Barriers to its Exploitation in the Energy Transition. Energies 2023, 16, 7174. [Google Scholar] [CrossRef]
- Fuel Cells and Hydrogen Joint Undertaking, FCH Report, Annual Activity Report 2020. Available online: https://www.europarl.europa.eu/cmsdata/237843/FCH_CAAR_2020.pdf (accessed on 4 October 2023).
- Chang, R.; Overby, J. Chemistry, 13th ed.; McGraw-Hill Education: New York, NY, USA, 2019; pp. 806–841. [Google Scholar]
- International Renewable Energy Agency, IRENA Report, Green Hydrogen Cost Reduction. Available online: https://www.irena.org/publications/2020/Dec/Green-hydrogen-cost-reduction (accessed on 4 October 2023).
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The Role of Green and Blue Hydrogen in the Energy Transition—A Technological and Geopolitical Perspective. Sustainability 2021, 13, 298. [Google Scholar] [CrossRef]
- Lohmann-Richters, F.P.; Renz, S.; Lehnert, W.; Müller, M.; Carmo, M. Challenges and Opportunities for Increased Current Density in Alkaline Electrolysis by Increasing the Operating Temperature. J. Electrochem. Soc. 2021, 168, 114501. [Google Scholar] [CrossRef]
- Ahmed, K.W.; Jang, M.J.; Park, M.G.; Chen, Z.; Fowler, M. Effect of Components and Operating Conditions on the Performance of PEM Electrolyzers: A Review. Electrochem 2022, 3, 581–612. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 22734:2019 - Hydrogen generators using water electrolysis — Industrial, commercial, and residential applications. Available online: https://www.iso.org/standard/69212.html (accessed on 4 October 2023).
- Global solar atlas. Available online: https://globalsolaratlas.info/map?s=21.943046,43.769531&m=site (accessed on 30 August 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).