Submitted:
12 March 2024
Posted:
13 March 2024
You are already at the latest version
Abstract
Keywords:
Main Text
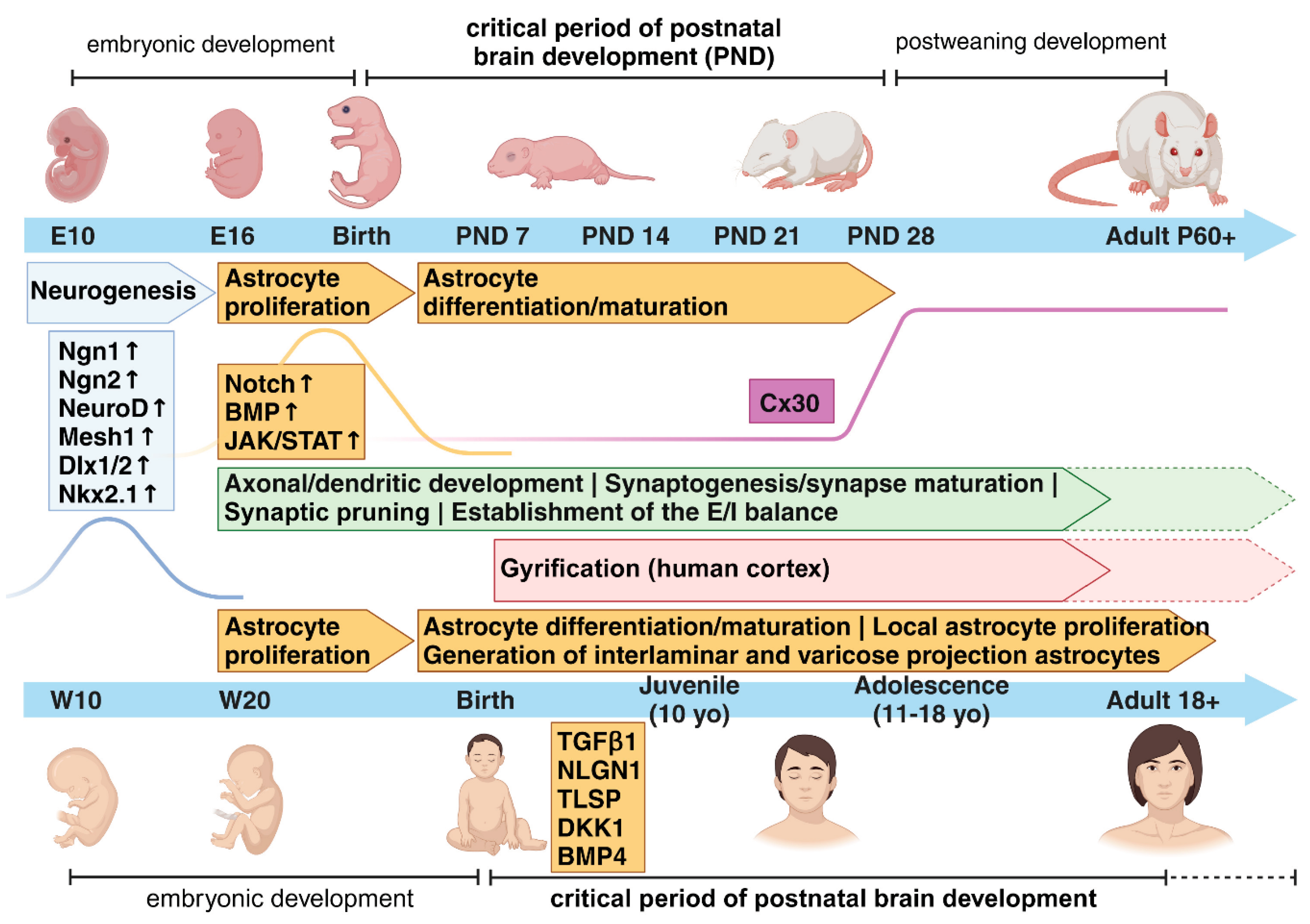
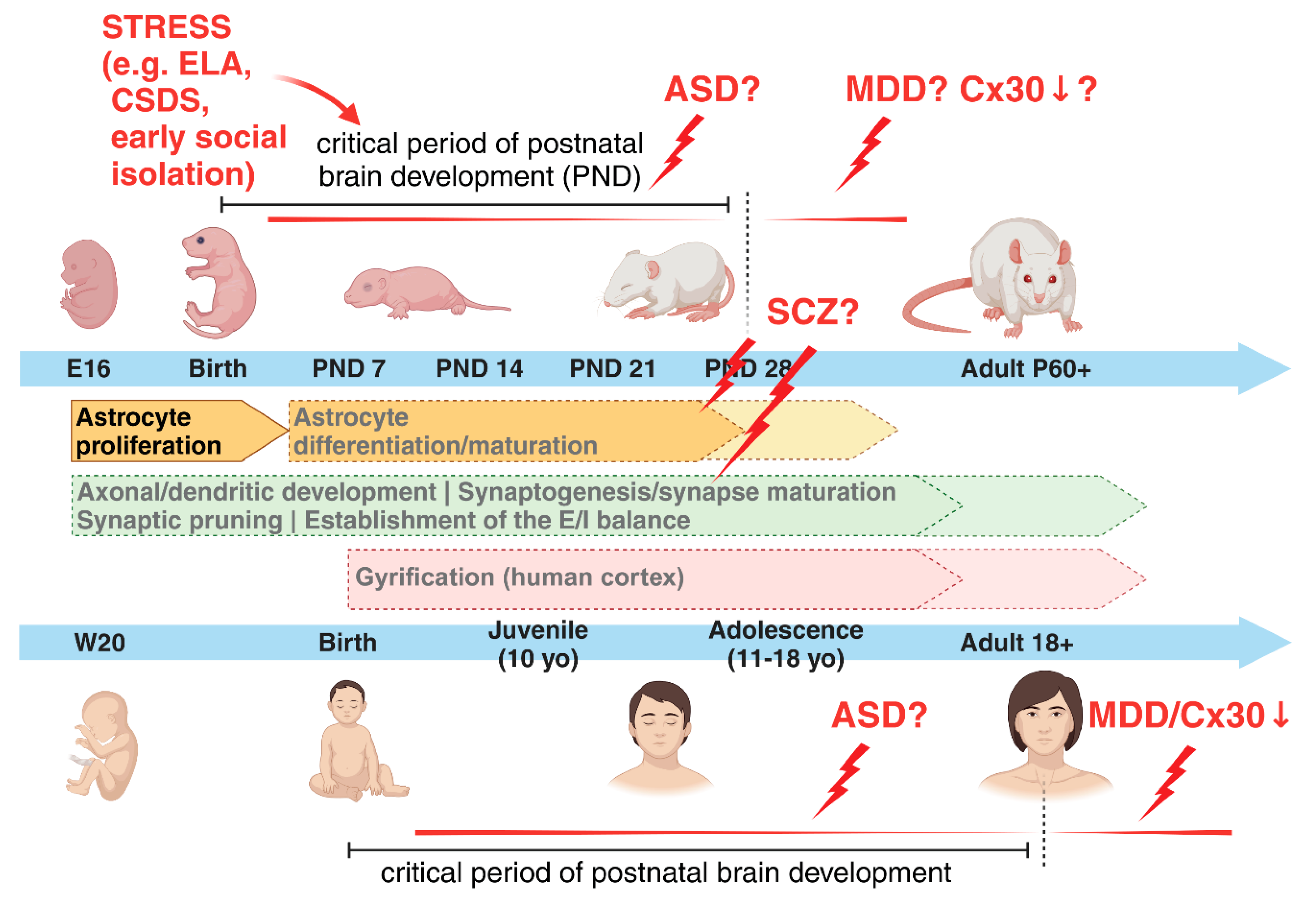
Astrocytes and the Critical Period of Early Postnatal Brain Development in Health and Mental Disorders
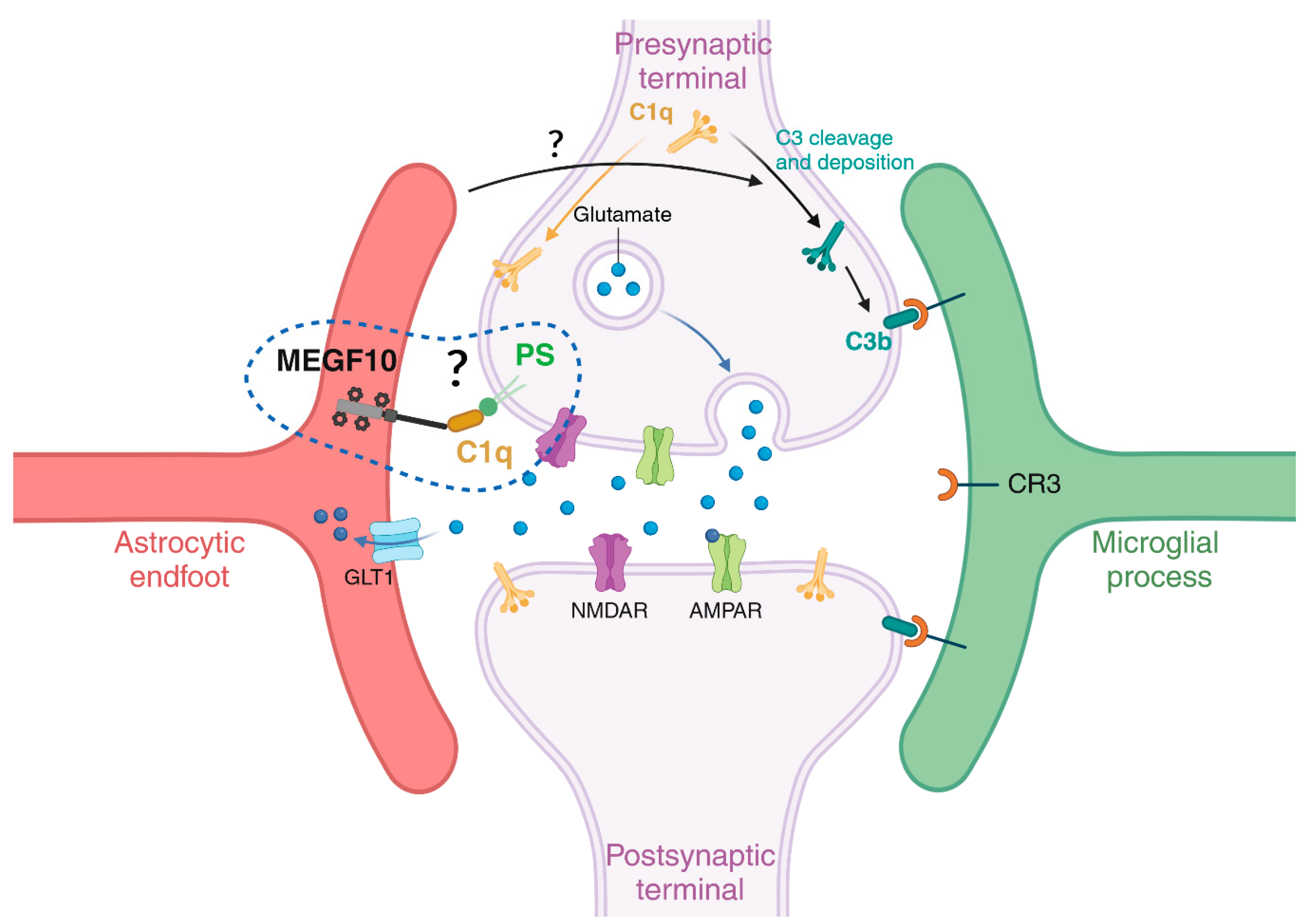
Astrocytes and the Synaptic Compartments: The Relevance of an Astrocyte-Mediated Phagocytosis for Neuronal Circuit Refinement in Health and Disease
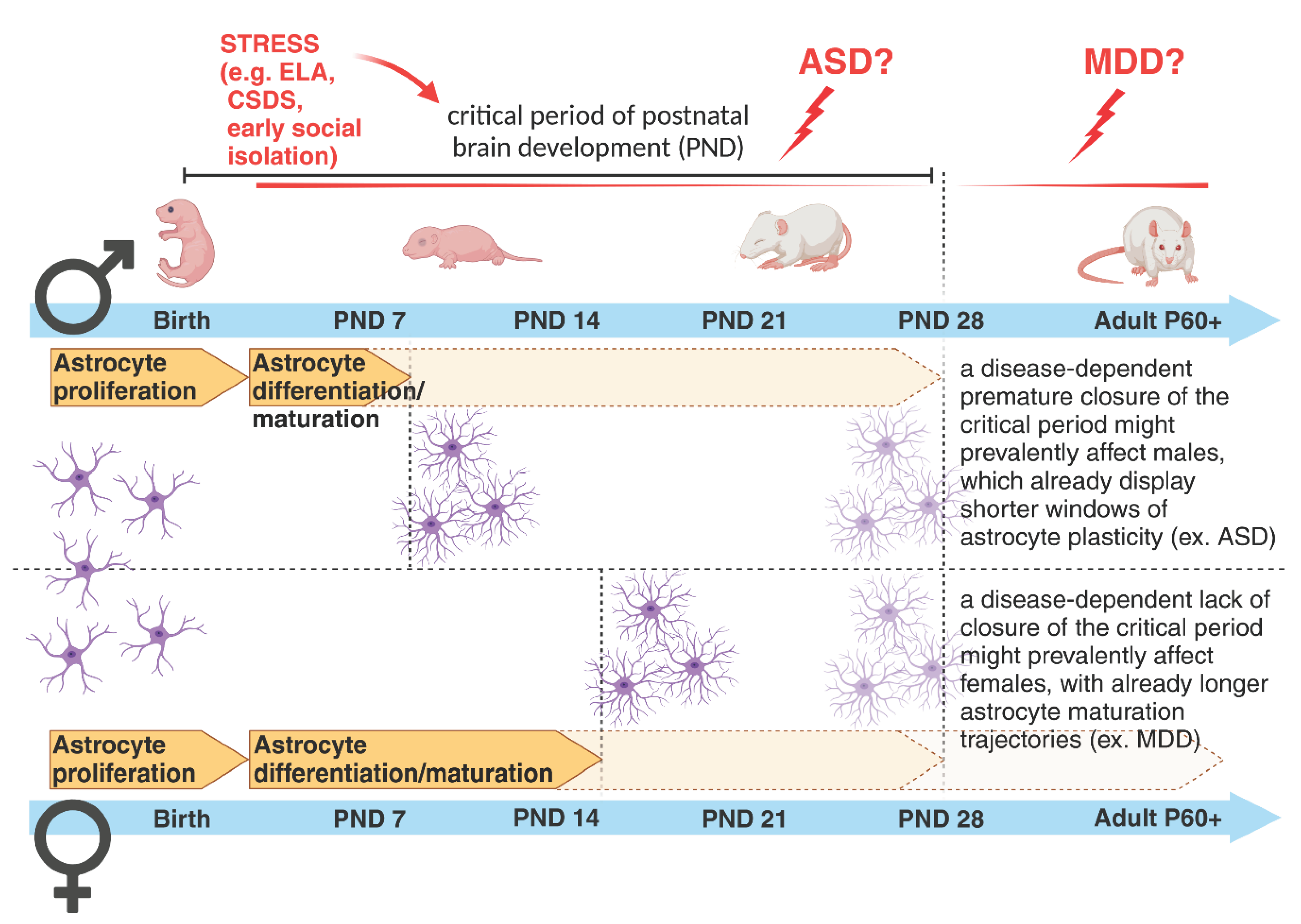
Sex-Dependent Differences in Astrogenesis and in Time Frames of Vulnerability to Perturbations and Disease Onset
Conclusions
Author Contributions
Conflicts of Interest
References
- Bandeira, F.; Lent, R.; Herculano-Houzel, S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc Natl Acad Sci. 2009, 106, 14108–14113. [Google Scholar] [CrossRef]
- Khakh, B.S.; Sofroniew, M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015, 18, 942–952. [Google Scholar] [CrossRef]
- Semyanov, A.; Verkhratsky, A. Astrocytic processes: from tripartite synapses to the active milieu. Trends Neurosci. 2021, 44, 781–792. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Eroglu, C. Cell Biology of Astrocyte-Synapse Interactions. Neuron. 2017, 96, 697–708. [Google Scholar] [CrossRef]
- Bosworth, A.P.; Allen, N.J. The diverse actions of astrocytes during synaptic development. Curr Opin Neurobiol. 2017, 47, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive Astrocytes Protect Tissue and Preserve Function after Spinal Cord Injury. J Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef]
- Iram, T.; Frenkel, D. Targeting the Role of Astrocytes in the Progression of Alzheimers Disease. Curr Signal Transduct Ther. 2012, 7, 20–27. [Google Scholar] [CrossRef]
- Molofsky, A.V.; Kelley, K.W.; Tsai, H.H.; Redmond, S.A.; Chang, S.M.; Madireddy, L.; et al. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature. 2014, 509, 189–194. [Google Scholar] [CrossRef]
- Cabezas, R.; Ávila, M.; Gonzalez, J.; El-Bachá, R.S.; Báez, E.; García-Segura, L.M.; et al. Astrocytic modulation of blood brain barrier: perspectives on Parkinson´s disease. Front Cell Neurosci [Internet]. 2014 Aug 4 [cited 2022 Jan 12];8. Available from: http://journal.frontiersin.org/article/10.3389/fncel.2014.00211/abstract. [CrossRef]
- Boulay, A.C.; Saubaméa, B.; Adam, N.; Chasseigneaux, S.; Mazaré, N.; Gilbert, A.; et al. Translation in astrocyte distal processes sets molecular heterogeneity at the gliovascular interface. Cell Discov. 2017, 3, 17005. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.I.; Katayama, T.; Prat, A. Glial influence on the blood brain barrier. Glia. 2013, 61, 1939–1958. [Google Scholar] [CrossRef]
- Eroglu, C.; Barres, B.A. Regulation of synaptic connectivity by glia. Nature. 2010, 468, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Vasile, F.; Dossi, E.; Rouach, N. Human astrocytes: structure and functions in the healthy brain. Brain Struct Funct. 2017, 222, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.; Wang, F.; et al. Uniquely Hominid Features of Adult Human Astrocytes. J Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef]
- Mohn, T.C.; Koob, A.O. Adult Astrogenesis and the Etiology of Cortical Neurodegeneration. J Exp Neurosci. 2015 Jan;9s2:JEN.S25520. [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Bush, N.; Nedergaard, M.; Butt, A. The Special Case of Human Astrocytes. Neuroglia. 2018, 1, 21–29. [Google Scholar] [CrossRef]
- Götz, M.; Huttner, W.B. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005, 6, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Takouda, J.; Katada, S.; Nakashima, K. Emerging mechanisms underlying astrogenesis in the developing mammalian brain. Proc Jpn Acad Ser, B. 2017, 93, 386–398. [Google Scholar] [CrossRef]
- Ever, L.; Gaiano, N. Radial ‘glial’ progenitors: neurogenesis and signaling. Curr Opin Neurobiol. 2005, 15, 29–33. [Google Scholar] [CrossRef]
- Zhou, C.J.; Zhao, C.; Pleasure, S.J. Wnt Signaling Mutants Have Decreased Dentate Granule Cell Production and Radial Glial Scaffolding Abnormalities. J Neurosci. 2004, 24, 121–126. [Google Scholar] [CrossRef]
- Rowitch, D.H.; Kriegstein, A.R. Developmental genetics of vertebrate glial–cell specification. Nature. 2010, 468, 214–222. [Google Scholar] [CrossRef]
- Eze, U.C.; Bhaduri, A.; Haeussler, M.; Nowakowski, T.J.; Kriegstein, A.R. Single-cell atlas of early human brain development highlights heterogeneity of human neuroepithelial cells and early radial glia. Nat Neurosci. 2021, 24, 584–594. [Google Scholar] [CrossRef]
- Pollen, A.A.; Nowakowski, T.J.; Chen, J.; Retallack, H.; Sandoval-Espinosa, C.; Nicholas, C.R.; et al. Molecular Identity of Human Outer Radial Glia during Cortical Development. Cell. 2015, 163, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.D.; Gauthier, A.S. Timing is everything: making neurons versus glia in the developing cortex. Neuron. 2007, 54, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Morrow, T.; Song, M.R.; Ghosh, A. Sequential specification of neurons and glia by developmentally regulated extracellular factors. Dev Camb Engl. 2001, 128, 3585–3594. [Google Scholar] [CrossRef]
- Ge, W.P.; Miyawaki, A.; Gage, F.H.; Jan, Y.N.; Jan, L.Y. Local generation of glia is a major astrocyte source in postnatal cortex. Nature. 2012, 484, 376–380. [Google Scholar] [CrossRef]
- De Zeeuw, C.I.; Hoogland, T.M. Reappraisal of Bergmann glial cells as modulators of cerebellar circuit function. Front Cell Neurosci [Internet]. 2015 Jul 2 [cited 2023 Jun 6];9. Available from: http://journal.frontiersin.org/Article/10.3389/fncel.2015.00246/abstract. [CrossRef]
- Güngör Kobat, S. Importance of Müller Cells. Beyoglu Eye J [Internet]. 2020 [cited 2023 Jun 6]; Available from: http://beyoglueye.com/jvi.aspx?un=BEJ-28290&volume=.
- Choi, B.H.; Lapham, L.W. Evolution of Bergman glia in developing human fetal cerebellum: A Golgi, electron microscopic and immunofluorescent study. Brain Res. 1980, 190, 369–383. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. Glia of the human retina. Glia. 2020, 68, 768–796. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, F. Contributo allo studio della corteccia cerebrale, ed all ́origine centrale dei nervi. Fratelli Bocca; 1889.
- Andriezen, W.L. The Neuroglia Elements in the Human Brain. BMJ. 1893, 2, 227–230. [Google Scholar] [CrossRef]
- Retzius, G. Die neuroglia des Gehirns beim Menschen und bei Saeugethieren. Jena: Chemie; 1894.
- Colombo, J.A.; Yáñez, A.; Puissant, V.; Lipina, S. Long, interlaminar astroglial cell processes in the cortex of adult monkeys. J Neurosci Res. 1995, 40, 551–556. [Google Scholar] [CrossRef]
- Colombo, J.A. Interlaminar Astroglial Processes in the Cerebral Cortex of Adult Monkeys But Not of Adult Rats. Cells Tissues Organs. 1996, 155, 57–62. [Google Scholar] [CrossRef]
- Colombo, J.A.; Yáñez, A.; Lipina, S.J. Interlaminar astroglial processes in the cerebral cortex of non human primates: response to injury. J Hirnforsch. 1997, 38, 503–512. [Google Scholar]
- Colombo, J.A.; Reisin, H.D. Interlaminar astroglia of the cerebral cortex: a marker of the primate brain. Brain Res. 2004, 1006, 126–131. [Google Scholar] [CrossRef]
- Falcone, C.; Penna, E.; Hong, T.; Tarantal, A.F.; Hof, P.R.; Hopkins, W.D.; et al. Cortical Interlaminar Astrocytes Are Generated Prenatally, Mature Postnatally, and Express Unique Markers in Human and Nonhuman Primates. Cereb Cortex. 2021, 31, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Falcone, C.; Martínez-Cerdeño, V. Astrocyte evolution and human specificity. Neural Regen Res. 2023, 18, 131. [Google Scholar] [PubMed]
- Falcone, C.; McBride, E.L.; Hopkins, W.D.; Hof, P.R.; Manger, P.R.; Sherwood, C.C.; et al. Redefining varicose projection astrocytes in primates. Glia. 2022, 70, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, M.; Wang, F.; Windrem, M.; Wang, S.; Shanz, S.; et al. Forebrain Engraftment by Human Glial Progenitor Cells Enhances Synaptic Plasticity and Learning in Adult Mice. Cell Stem Cell. 2013, 12, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, F.A.C.; Carvalho, L.R.B.; Grinberg, L.T.; Farfel, J.M.; Ferretti, R.E.L.; Leite, R.E.P.; et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009, 513, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Falcone, C. Evolution of astrocytes: From invertebrates to vertebrates. Front Cell Dev Biol. 2022, 10, 931311. [Google Scholar] [CrossRef] [PubMed]
- Degl’Innocenti, E.; Dell’Anno, M.T. Human and mouse cortical astrocytes: a comparative view from development to morphological and functional characterization. Front Neuroanat. 2023, 17, 1130729. [Google Scholar] [CrossRef]
- Namba, T.; Huttner, W.B. Neural progenitor cells and their role in the development and evolutionary expansion of the neocortex. WIREs Dev Biol. 2017, 6, e256. [Google Scholar] [CrossRef] [PubMed]
- Shinmyo, Y.; Saito, K.; Hamabe-Horiike, T.; Kameya, N.; Ando, A.; Kawasaki, K.; et al. Localized astrogenesis regulates gyrification of the cerebral cortex. Sci Adv. 2022, 8, eabi5209. [Google Scholar] [CrossRef] [PubMed]
- Zilles, K.; Palomero-Gallagher, N.; Amunts, K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. 2013, 36, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Farmer, W.T.; Murai, K. Resolving Astrocyte Heterogeneity in the CNS. Front Cell Neurosci. 2017 Sep 27;11:300. [CrossRef]
- Holt, M.G. Astrocyte heterogeneity and interactions with local neural circuits. Bolaños, J.P.; editor. Essays Biochem. 2023, 67, 93–106. [Google Scholar] [PubMed]
- Miller, S.J. Astrocyte Heterogeneity in the Adult Central Nervous System. Front Cell Neurosci. 2018 Nov 15;12:401. [CrossRef]
- Oliveria, J.P.; Li, Z.J. critical role of astrogenesis and neurodevelopment in Fragile X Syndrome and Rett Syndrome. McMaster Univ Med J [Internet]. 2020 Dec 26 [cited 2023 May 31];17(1). Available from: https://journals.mcmaster.ca/mumj/article/view/2338. [CrossRef]
- Kanski, R.; Van Strien, M.E.; Van Tijn, P.; Hol, E.M. A star is born: new insights into the mechanism of astrogenesis. Cell Mol Life Sci. 2014, 71, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Nery, S.; Rutlin, M.L.; Radtke, F.; Fishell, G.; Gaiano, N. Fibroblast Growth Factor Receptor Signaling Promotes Radial Glial Identity and Interacts with Notch1 Signaling in Telencephalic Progenitors. J Neurosci. 2004, 24, 9497–9506. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Martinowich, K.; Chin, M.H.; He, F.; Fouse, S.D.; Hutnick, L.; et al. DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development. 2005, 132, 3345–3356. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Nadal-Vicens, M.; Misono, S.; Lin, M.Z.; Zubiaga, A.; Hua, X.; et al. Neurogenin Promotes Neurogenesis and Inhibits Glial Differentiation by Independent Mechanisms. Cell. 2001, 104, 365–376. [Google Scholar] [CrossRef]
- Bonni, A.; Sun, Y.; Nadal-Vicens, M.; Bhatt, A.; Frank, D.A.; Rozovsky, I.; et al. Regulation of Gliogenesis in the Central Nervous System by the JAK-STAT Signaling Pathway. Science. 1997, 278, 477–483. [Google Scholar] [CrossRef]
- Nieto, M.; Schuurmans, C.; Britz, O.; Guillemot, F. Neural bHLH Genes Control the Neuronal versus Glial Fate Decision in Cortical Progenitors. Neuron. 2001, 29, 401–413. [Google Scholar] [CrossRef]
- Zhang, Y.; Pak, C.; Han, Y.; Ahlenius, H.; Zhang, Z.; Chanda, S.; et al. Rapid Single-Step Induction of Functional Neurons from Human Pluripotent Stem Cells. Neuron. 2013, 78, 785–798. [Google Scholar] [CrossRef]
- Yang, N.; Chanda, S.; Marro, S.; Ng, Y.H.; Janas, J.A.; Haag, D.; et al. Generation of pure GABAergic neurons by transcription factor programming. Nat Methods. 2017, 14, 621–628. [Google Scholar] [CrossRef]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Südhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010, 463, 1035–1041. [Google Scholar] [CrossRef]
- Hatakeyama, J.; Bessho, Y.; Katoh, K.; Ookawara, S.; Fujioka, M.; Guillemot, F.; et al. Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development. 2004, 131, 5539–5550. [Google Scholar] [CrossRef] [PubMed]
- Mizutani K ichi, Saito, T. Progenitors resume generating neurons after temporary inhibition of neurogenesis by Notch activation in the mammalian cerebral cortex. Development. 2005, 132, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Hoch, R.V.; Rubenstein, J.L.R.; Pleasure, S. Genes and signaling events that establish regional patterning of the mammalian forebrain. Semin Cell Dev Biol. 2009, 20, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Leone, D.P.; Srinivasan, K.; Chen, B.; Alcamo, E.; McConnell, S.K. The determination of projection neuron identity in the developing cerebral cortex. Curr Opin Neurobiol. 2008, 18, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, F. Cellular and molecular control of neurogenesis in the mammalian telencephalon. Curr Opin Cell Biol. 2005, 17, 639–647. [Google Scholar] [CrossRef]
- He, F.; Ge, W.; Martinowich, K.; Becker-Catania, S.; Coskun, V.; Zhu, W.; et al. A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat Neurosci. 2005, 8, 616–625. [Google Scholar] [CrossRef]
- Tomita, K. Mammalian achaete-scute and atonal homologs regulate neuronal versus glial fate determination in the central nervous system. EMBO J. 2000, 19, 5460–5472. [Google Scholar] [CrossRef]
- Llorca, A.; Deogracias, R. Origin, Development, and Synaptogenesis of Cortical Interneurons. Front Neurosci. 2022, 16, 929469. [Google Scholar] [CrossRef]
- Styr, B.; Slutsky, I. Imbalance between firing homeostasis and synaptic plasticity drives early-phase Alzheimer’s disease. Nat Neurosci. 2018, 21, 463–473. [Google Scholar] [CrossRef]
- Rubenstein, J.L.R.; Merzenich, M.M. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef]
- Foss-Feig, J.H.; Adkinson, B.D.; Ji, J.L.; Yang, G.; Srihari, V.H.; McPartland, J.C.; et al. Searching for Cross-Diagnostic Convergence: Neural Mechanisms Governing Excitation and Inhibition Balance in Schizophrenia and Autism Spectrum Disorders. Biol Psychiatry. 2017, 81, 848–861. [Google Scholar] [CrossRef] [PubMed]
- Barnabé-Heider, F.; Wasylnka, J.A.; Fernandes, K.J.L.; Porsche, C.; Sendtner, M.; Kaplan, D.R.; et al. Evidence that Embryonic Neurons Regulate the Onset of Cortical Gliogenesis via Cardiotrophin-1. Neuron. 2005, 48, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Farmer, W.T.; Abrahamsson, T.; Chierzi, S.; Lui, C.; Zaelzer, C.; Jones, E.V.; et al. Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science. 2016, 351, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.J.; Lanjewar, S.N.; Sampson, M.M.; King, A.; Hill, E.J.; Sing, A.; et al. Identification of ligand-receptor pairs that drive human astrocyte development. Nat Neurosci. 2023, 26, 1339–1351. [Google Scholar] [CrossRef]
- Bayraktar, O.A.; Bartels, T.; Holmqvist, S.; Kleshchevnikov, V.; Martirosyan, A.; Polioudakis, D.; et al. Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat Neurosci. 2020, 23, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Berardi, N.; Pizzorusso, T.; Maffei, L. Critical periods during sensory development. Curr Opin Neurobiol. 2000, 10, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Chugani, H.T. A Critical Period of Brain Development: Studies of Cerebral Glucose Utilization with PET. Prev Med. 1998, 27, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Sengpiel, F. The critical period. Curr Biol. 2007, 17, R742–3. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.I. Sensitive Periods in the Development of the Brain and Behavior. J Cogn Neurosci. 2004, 16, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.A.; Gabard-Durnam, L.J. Early Adversity and Critical Periods: Neurodevelopmental Consequences of Violating the Expectable Environment. Trends Neurosci. 2020, 43, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Virolainen, S.J.; VonHandorf, A.; Viel KCMF, Weirauch, M. T.; Kottyan, L.C. Gene–environment interactions and their impact on human health. Genes Immun. 2022, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Milbocker, K.A.; Campbell, T.S.; Collins, N.; Kim, S.; Smith, I.F.; Roth, T.L.; et al. Glia-Driven Brain Circuit Refinement Is Altered by Early-Life Adversity: Behavioral Outcomes. Front Behav Neurosci. 2021 Dec 2;15:786234. [CrossRef]
- Christopherson, K.S.; Ullian, E.M.; Stokes, C.C.A.; Mullowney, C.E.; Hell, J.W.; Agah, A.; et al. Thrombospondins Are Astrocyte-Secreted Proteins that Promote CNS Synaptogenesis. Cell. 2005, 120, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Pfrieger, F.W.; Barres, B.A. Synaptic Efficacy Enhanced by Glial Cells in Vitro. Science. 1997, 277, 1684–1687. [Google Scholar] [CrossRef] [PubMed]
- Mauch, D.H.; Nägler, K.; Schumacher, S.; Göritz, C.; Müller, E.C.; Otto, A.; et al. CNS Synaptogenesis Promoted by Glia-Derived Cholesterol. Science. 2001, 294, 1354–1357. [Google Scholar] [CrossRef]
- Eroglu, Ç.; Allen, N.J.; Susman, M.W.; O’Rourke, N.A.; Park, C.Y.; Özkan, E.; et al. Gabapentin Receptor α2δ-1 Is a Neuronal Thrombospondin Receptor Responsible for Excitatory CNS Synaptogenesis. Cell. 2009, 139, 380–392. [Google Scholar] [CrossRef]
- Fossati, G.; Pozzi, D.; Canzi, A.; Mirabella, F.; Valentino, S.; Morini, R.; et al. Pentraxin 3 regulates synaptic function by inducing AMPA receptor clustering via ECM remodeling and β1-integrin. EMBO J. 2019, 38, e99529. [Google Scholar] [CrossRef]
- Allen, N.J.; Bennett, M.L.; Foo, L.C.; Wang, G.X.; Chakraborty, C.; Smith, S.J.; et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012, 486, 410–414. [Google Scholar] [CrossRef]
- Diniz, L.P.; Almeida, J.C.; Tortelli, V.; Vargas Lopes, C.; Setti-Perdigão, P.; Stipursky, J.; et al. Astrocyte-induced Synaptogenesis Is Mediated by Transforming Growth Factor β Signaling through Modulation of d-Serine Levels in Cerebral Cortex Neurons. J Biol Chem. 2012, 287, 41432–41445. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.P.; Tortelli, V.; Garcia, M.N.; Araújo, A.P.B.; Melo, H.M.; Seixas Da Silva, G.S.; et al. Astrocyte transforming growth factor beta 1 promotes inhibitory synapse formation via CaM kinase II signaling. Glia. 2014, 62, 1917–1931. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Casati, M.E.; Murtie, J.C.; Rio, C.; Stankovic, K.; Liberman, M.C.; Corfas, G. Nonneuronal cells regulate synapse formation in the vestibular sensory epithelium via erbB-dependent BDNF expression. Proc Natl Acad Sci. 2010, 107, 17005–17010. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Zhang, T.; Fan, K.; Cai, W.; Liu, H. Astrocyte-Neuron Signaling in Synaptogenesis. Front Cell Dev Biol. 2021, 9, 680301. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.X.; Burrus Lane, C.J.; Eroglu, C. Role of astrocytes in synapse formation and maturation. In: Current Topics in Developmental Biology [Internet]. Elsevier; 2021 [cited 2024 Jan 20]. p. 371–407. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0070215320301435.
- Konishi, H.; Koizumi, S.; Kiyama, H. Phagocytic astrocytes: Emerging from the shadows of microglia. Glia. 2022, 70, 1009–1026. [Google Scholar] [CrossRef] [PubMed]
- Juraska, J.M.; Willing, J. Pubertal onset as a critical transition for neural development and cognition. Brain Res. 2017, 1654, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, P.R.; Dabholkar, A.S. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997, 387, 167–178. [Google Scholar] [CrossRef]
- Peter, R. H. Synaptic density in human frontal cortex — Developmental changes and effects of aging. Brain Res. 1979, 163, 195–205. [Google Scholar] [CrossRef]
- Dosenbach, N.U.F.; Nardos, B.; Cohen, A.L.; Fair, D.A.; Power, J.D.; Church, J.A.; et al. Prediction of Individual Brain Maturity Using fMRI. Science. 2010, 329, 1358–1361. [Google Scholar] [CrossRef]
- Petanjek, Z.; Judaš, M.; Šimić, G.; Rašin, M.R.; Uylings, H.B.M.; Rakic, P.; et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci. 2011, 108, 13281–13286. [Google Scholar] [CrossRef]
- Antoine, M.W.; Langberg, T.; Schnepel, P.; Feldman, D.E. Increased Excitation-Inhibition Ratio Stabilizes Synapse and Circuit Excitability in Four Autism Mouse Models. Neuron. 2019, 101, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Aida, T.; Yoshida, J.; Nomura, M.; Tanimura, A.; Iino, Y.; Soma, M.; et al. Astroglial Glutamate Transporter Deficiency Increases Synaptic Excitability and Leads to Pathological Repetitive Behaviors in Mice. Neuropsychopharmacology. 2015, 40, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Ortinski, P.I.; Dong, J.; Mungenast, A.; Yue, C.; Takano, H.; Watson, D.J.; et al. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010, 13, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Eltokhi, A.; Janmaat, I.E.; Genedi, M.; Haarman, B.C.M.; Sommer, I.E.C. Dysregulation of synaptic pruning as a possible link between intestinal microbiota dysbiosis and neuropsychiatric disorders. J Neurosci Res. 2020, 98, 1335–1369. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, P.L.; De Lima, I.B.Q.; Maciel, E.M.A.; Silva, N.C.; Dobransky, T.; Ribeiro, F.M. Synaptic Elimination in Neurological Disorders. Curr Neuropharmacol. 2019, 17, 1071–1095. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Xu, X.; Li, H.; Niu, F.; Yang, M.; Ge, Q.; et al. Megf10-related engulfment of excitatory postsynapses by astrocytes following severe brain injury. CNS Neurosci Ther. 2023 Apr 20;cns.14223. [CrossRef]
- Iram, T.; Ramirez-Ortiz, Z.; Byrne, M.H.; Coleman, U.A.; Kingery, N.D.; Means, T.K.; et al. Megf10 Is a Receptor for C1Q That Mediates Clearance of Apoptotic Cells by Astrocytes. J Neurosci. 2016, 36, 5185–5192. [Google Scholar] [CrossRef]
- Pattwell, S.S.; Liston, C.; Jing, D.; Ninan, I.; Yang, R.R.; Witztum, J.; et al. Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat Commun. 2016, 7, 11475. [Google Scholar] [CrossRef]
- Honeycutt, J.A.; Demaestri, C.; Peterzell, S.; Silveri, M.M.; Cai, X.; Kulkarni, P.; et al. Altered corticolimbic connectivity reveals sex-specific adolescent outcomes in a rat model of early life adversity. eLife. 2020, 9, e52651. [Google Scholar] [CrossRef]
- Karpova, N.N.; Pickenhagen, A.; Lindholm, J.; Tiraboschi, E.; Kulesskaya, N.; Ágústsdóttir, A.; et al. Fear Erasure in Mice Requires Synergy Between Antidepressant Drugs and Extinction Training. Science. 2011, 334, 1731–1734. [Google Scholar] [CrossRef]
- Vetencourt, J.F.M.; Sale, A.; Viegi, A.; Baroncelli, L.; De Pasquale, R.; F. O’Leary, O.; et al. The Antidepressant Fluoxetine Restores Plasticity in the Adult Visual Cortex. Science. 2008, 320, 385–388. [Google Scholar] [CrossRef]
- Ribot, J.; Breton, R.; Calvo, C.F.; Moulard, J.; Ezan, P.; Zapata, J.; et al. Astrocytes close the mouse critical period for visual plasticity. Science. 2021, 373, 77–81. [Google Scholar] [CrossRef]
- Müller, C.M.; Best, J. Ocular dominance plasticity in adult cat visual cortex after transplantation of cultured astrocytes. Nature. 1989, 342, 427–430. [Google Scholar] [CrossRef]
- Ghézali, G.; Calvo, C.F.; Pillet, L.E.; Llense, F.; Ezan, P.; Pannasch, U.; et al. Connexin 30 controls astroglial polarization during postnatal brain development. Development. 2018, 145, dev155275. [Google Scholar] [CrossRef]
- Abbink, M.R.; Deijk, A.F.; Heine, V.M.; Verheijen, M.H.; Korosi, A. The involvement of astrocytes in early-life adversity induced programming of the brain. Glia. 2019 Apr 30;glia.23625. [CrossRef]
- Codeluppi, S.A.; Chatterjee, D.; Prevot, T.D.; Bansal, Y.; Misquitta, K.A.; Sibille, E.; et al. Chronic Stress Alters Astrocyte Morphology in Mouse Prefrontal Cortex. Int J Neuropsychopharmacol. 2021, 24, 842–853. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. Synaptic and behavioral effects of chronic stress are linked to dynamic and sex-specific changes in microglia function and astrocyte dystrophy. Neurobiol Stress. 2021, 14, 100312. [Google Scholar] [CrossRef] [PubMed]
- Dolotov, O.V.; Inozemtseva, L.S.; Myasoedov, N.F.; Grivennikov, I.A. Stress-Induced Depression and Alzheimer’s Disease: Focus on Astrocytes. Int J Mol Sci. 2022, 23, 4999. [Google Scholar] [CrossRef] [PubMed]
- Naskar, S.; Chattarji, S. Stress Elicits Contrasting Effects on the Structure and Number of Astrocytes in the Amygdala versus Hippocampus. eNeuro. 2019, 6, ENEURO.0338–182019. [Google Scholar] [CrossRef] [PubMed]
- Murphy-Royal, C.; Gordon, G.R.; Bains, J.S. Stress-induced structural and functional modifications of astrocytes—Further implicating glia in the central response to stress. Glia. 2019, 67, 1806–1820. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, K.; Oda, Y.; Kimura, M.; Kimura, H.; Nangaku, M.; Shirayama, Y.; et al. The alterations of glutamate transporter 1 and glutamine synthetase in the rat brain of a learned helplessness model of depression. Psychopharmacology (Berl). 2020, 237, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Tynan, R.J.; Beynon, S.B.; Hinwood, M.; Johnson, S.J.; Nilsson, M.; Woods, J.J.; et al. Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol (Berl). 2013, 126, 75–91. [Google Scholar] [CrossRef]
- Virmani, G.; D’almeida, P.; Nandi, A.; Marathe, S. Subfield-specific effects of chronic mild unpredictable stress on hippocampal astrocytes. Hughes, E.; editor. Eur J Neurosci. 2021, 54, 5730–5746. [Google Scholar] [CrossRef]
- Lu, C.L.; Ren, J.; Mo, J.W.; Fan, J.; Guo, F.; Chen, L.Y.; et al. Glucocorticoid Receptor–Dependent Astrocytes Mediate Stress Vulnerability. Biol Psychiatry. 2022, 92, 204–215. [Google Scholar] [CrossRef]
- Huang, D.; Li, C.; Zhang, W.; Qin, J.; Jiang, W.; Hu, C. Dysfunction of astrocytic connexins 30 and 43 in the medial prefrontal cortex and hippocampus mediates depressive-like behaviours. Behav Brain Res. 2019, 372, 111950. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.G.; Kim, N.S.; Kim, G.; Jeon, Y.S.; Choi, J.B.; Park, C.W.; et al. Stress induces behavioral abnormalities by increasing expression of phagocytic receptor MERTK in astrocytes to promote synapse phagocytosis. Immunity. 2023, 56, 2105–2120. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J.; Moulana, M.; Deloach, P.H.; Rajkowska, G. Chronic Unpredictable Stress Reduces Immunostaining for Connexins 43 and 30 and Myelin Basic Protein in the Rat Prelimbic and Orbitofrontal Cortices. Chronic Stress. 2018, 2, 247054701881418. [Google Scholar] [CrossRef]
- Kang, Y.; Kang, W.; Kim, A.; Tae, W.S.; Ham, B.J.; Han, K.M. Decreased cortical gyrification in major depressive disorder. Psychol Med. 2023, 53, 7512–7524. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.; Li, C.; Gao, L.; Fan, J. Core-Symptom-Defined Cortical Gyrification Differences in Autism Spectrum Disorder. Front Psychiatry. 2021, 12, 619367. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Sasabayashi, D.; Takahashi, T.; Komori, Y.; Furuichi, A.; Kido, M.; et al. Altered brain gyrification in deficit and non-deficit schizophrenia. Psychol Med. 2019, 49, 573–580. [Google Scholar] [CrossRef]
- Cao, B.; Mwangi, B.; Passos, I.C.; Wu, M.J.; Keser, Z.; Zunta-Soares, G.B.; et al. Lifespan Gyrification Trajectories of Human Brain in Healthy Individuals and Patients with Major Psychiatric Disorders. Sci Rep. 2017, 7, 511. [Google Scholar] [CrossRef] [PubMed]
- Sasabayashi, D.; Takahashi, T.; Takayanagi, Y.; Suzuki, M. Anomalous brain gyrification patterns in major psychiatric disorders: a systematic review and transdiagnostic integration. Transl Psychiatry. 2021, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G.; Miguel-Hidalgo, J.J. Glial Pathology in Major Depressive Disorder: An Approach to Investigate the Coverage of Blood Vessels by Astrocyte Endfeet in Human Postmortem Brain. In: Di Benedetto, B.; editor. Astrocytes [Internet]. New York, NY: Springer New York; 2019 [cited 2023 ]. p. 247–54. (Methods in Molecular Biology; vol. 1938). Available from: http://link.springer.com/10. 9 May 1007. [Google Scholar]
- Di Benedetto, B.; Rupprecht, R. Targeting Glia Cells: Novel Perspectives for the Treatment of Neuropsychiatric Diseases. Curr Neuropharmacol. 2013, 11, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Roman, C.; Egert, L.; Di Benedetto, B. Astrocytic-neuronal crosstalk gets jammed: Alternative perspectives on the onset of neuropsychiatric disorders. Eur J Neurosci. 2021, 54, 5717–5729. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Suderman, M.; Yang, J.; Szyf, M.; Mechawar, N.; Ernst, C.; et al. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry. 2015, 20, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Martins-Macedo, J.; Salgado, A.J.; Gomes, E.D.; Pinto, L. Adult brain cytogenesis in the context of mood disorders: From neurogenesis to the emergent role of gliogenesis. Neurosci Biobehav Rev. 2021, 131, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Feresten, A.H.; Barakauskas, V.; Ypsilanti, A.; Barr, A.M.; Beasley, C.L. Increased expression of glial fibrillary acidic protein in prefrontal cortex in psychotic illness. Schizophr Res. 2013, 150, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Sologova, S.S.; Mukhortova, P.; Levushkin, D.; et al. Alterations of Astrocytes in the Context of Schizophrenic Dementia. Front Pharmacol. 2020, 10, 1612. [Google Scholar] [CrossRef] [PubMed]
- Notter, T. Astrocytes in schizophrenia. Brain Neurosci Adv. 2021, 5, 239821282110091. [Google Scholar] [CrossRef]
- Vakilzadeh, G.; Martinez-Cerdeño, V. Pathology and Astrocytes in Autism. Neuropsychiatr Dis Treat. 2023, 19, 841–850. [Google Scholar] [CrossRef]
- Allen, M.; Huang, B.S.; Notaras, M.J.; Lodhi, A.; Barrio-Alonso, E.; Lituma, P.J.; et al. Astrocytes derived from ASD individuals alter behavior and destabilize neuronal activity through aberrant Ca2+ signaling. Mol Psychiatry. 2022, 27, 2470–2484. [Google Scholar] [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J. Gliogenesis and Glial Pathology in Depression. CNS Neurol Disord - Drug Targets. 2007, 6, 219–233. [Google Scholar] [CrossRef]
- Belleau, E.L.; Treadway, M.T.; Pizzagalli, D.A. The Impact of Stress and Major Depressive Disorder on Hippocampal and Medial Prefrontal Cortex Morphology. Biol Psychiatry. 2019, 85, 443–453. [Google Scholar] [CrossRef]
- Malykhin, N.V.; Carter, R.; Seres, P.; Coupland, N.J. Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. J Psychiatry Neurosci. 2010, 35, 337–343. [Google Scholar] [CrossRef]
- Geng, R.; Huang, X. Identification of major depressive disorder disease-related genes and functional pathways based on system dynamic changes of network connectivity. BMC Med Genomics. 2021, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Czéh, B.; Simon, M.; Schmelting, B.; Hiemke, C.; Fuchs, E. Astroglial Plasticity in the Hippocampus is Affected by Chronic Psychosocial Stress and Concomitant Fluoxetine Treatment. Neuropsychopharmacology. 2006, 31, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Czéh, B.; Di Benedetto, B. Antidepressants act directly on astrocytes: Evidences and functional consequences. Eur Neuropsychopharmacol. 2013, 23, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Czéh, B.; Nagy, S.A. Clinical Findings Documenting Cellular and Molecular Abnormalities of Glia in Depressive Disorders. Front Mol Neurosci. 2018, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Papouin, T.; Ladépêche, L.; Ruel, J.; Sacchi, S.; Labasque, M.; Hanini, M.; et al. Synaptic and Extrasynaptic NMDA Receptors Are Gated by Different Endogenous Coagonists. Cell. 2012, 150, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Henneberger, C.; Papouin, T.; Oliet, S.H.R.; Rusakov, D.A. Long-term potentiation depends on release of d-serine from astrocytes. Nature. 2010, 463, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Suarez, E.; Liu, T.F.; Kopelevich, A.; Allen, N.J. Astrocyte-Secreted Chordin-like 1 Drives Synapse Maturation and Limits Plasticity by Increasing Synaptic GluA2 AMPA Receptors. Neuron. 2018, 100, 1116–1132. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, A.L.M.; Sancho, L.; Deng, J.; Bosworth, A.; Miglietta, A.; Diedrich, J.K.; et al. Aberrant astrocyte protein secretion contributes to altered neuronal development in multiple models of neurodevelopmental disorders. Nat Neurosci. 2022, 25, 1163–1178. [Google Scholar] [CrossRef]
- Heresco-Levy, U.; Javitt, D.C.; Ebstein, R.; Vass, A.; Lichtenberg, P.; Bar, G.; et al. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry. 2005, 57, 577–585. [Google Scholar] [CrossRef]
- Kantrowitz, J.T.; Malhotra, A.K.; Cornblatt, B.; Silipo, G.; Balla, A.; Suckow, R.F.; et al. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010, 121, 125–130. [Google Scholar] [CrossRef]
- Ma, T.M.; Abazyan, S.; Abazyan, B.; Nomura, J.; Yang, C.; Seshadri, S.; et al. Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via D-serine depletion. Mol Psychiatry. 2013, 18, 557–567. [Google Scholar] [CrossRef]
- Cardno, A.G.; Gottesman, I.I. Twin studies of schizophrenia: From bow-and-arrow concordances to Star Wars Mx and functional genomics. Am J Med Genet. 2000, 97, 12–17. [Google Scholar] [CrossRef]
- Walker, E.F.; Trotman, H.D.; Pearce, B.D.; Addington, J.; Cadenhead, K.S.; Cornblatt, B.A.; et al. Cortisol Levels and Risk for Psychosis: Initial Findings from the North American Prodrome Longitudinal Study. Biol Psychiatry. 2013, 74, 410–417. [Google Scholar] [CrossRef]
- Selemon, L.D.; Zecevic, N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry. 2015, 5, e623–e623. [Google Scholar] [CrossRef]
- Sheu, J.R.; Hsieh, C.Y.; Jayakumar, T.; Tseng, M.F.; Lee, H.N.; Huang, S.W.; et al. A Critical Period for the Development of Schizophrenia-Like Pathology by Aberrant Postnatal Neurogenesis. Front Neurosci. 2019, 13, 635. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Figueiredo, E.C.; Calì, C.; Petrelli, F.; Bezzi, P. Emerging evidence for astrocyte dysfunction in schizophrenia. Glia. 2022, 70, 1585–1604. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.B.; Freitas, B.C.; Pignatari, G.C.; Fernandes, I.R.; Sebat, J.; Muotri, A.R.; et al. Modeling the Interplay Between Neurons and Astrocytes in Autism Using Human Induced Pluripotent Stem Cells. Biol Psychiatry. 2018, 83, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.M.; Rohn, T.T.; Oxford, J.T. Autism as the Early Closure of a Neuroplastic Critical Period Normally Seen in Adolescence. Biol Syst Open Access [Internet]. 2012 [cited 2023 Jul 23];02(03). Available from: https://www.omicsgroup.org/journals/autism-as-the-early-closure-of-a-neuroplastic-critical-period-normally-seen-in-adolescence-2329-6577-1000118.php?aid=43859.
- Hashimoto, Y.; Greene, C.; Munnich, A.; Campbell, M. The CLDN5 gene at the blood-brain barrier in health and disease. Fluids Barriers CNS. 2023, 20, 22. [Google Scholar] [CrossRef]
- Igarashi, Y.; Utsumi, H.; Chiba, H.; Yamada-Sasamori, Y.; Tobioka, H.; Kamimura, Y.; et al. Glial Cell Line-Derived Neurotrophic Factor Induces Barrier Function of Endothelial Cells Forming the Blood–Brain Barrier. Biochem Biophys Res Commun. 1999, 261, 108–112. [Google Scholar] [CrossRef]
- Rajkowska, G.; Hughes, J.; Stockmeier, C.A.; Javier Miguel-Hidalgo, J.; Maciag, D. Coverage of Blood Vessels by Astrocytic Endfeet Is Reduced in Major Depressive Disorder. Biol Psychiatry. 2013, 73, 613–621. [Google Scholar] [CrossRef]
- Lee, E.; Chung, W.S. Glial Control of Synapse Number in Healthy and Diseased Brain. Front Cell Neurosci. 2019, 13, 42. [Google Scholar] [CrossRef]
- Di Benedetto, B.; Malik, V.A.; Begum, S.; Jablonowski, L.; Gómez-González, G.B.; Neumann, I.D.; et al. Fluoxetine Requires the Endfeet Protein Aquaporin-4 to Enhance Plasticity of Astrocyte Processes. Front Cell Neurosci [Internet]. 2016 Feb 2 [cited 2022 Jan 13];10. Available from: http://journal.frontiersin.org/Article/10.3389/fncel.2016.00008/abstract.
- Malik, V.A.; Zajicek, F.; Mittmann, L.A.; Klaus, J.; Unterseer, S.; Rajkumar, S.; et al. GDF15 promotes simultaneous astrocyte remodeling and tight junction strengthening at the blood–brain barrier. J Neurosci Res. 2020, 98, 1433–1456. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.P.; Price, J. Evidence for multiple precursor cell types in the embryonic rat cerebral cortex. Neuron. 1995, 14, 1181–1188. [Google Scholar] [CrossRef]
- Chung, W.S.; Allen, N.J.; Eroglu, C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb Perspect Biol. 2015, 7, a020370. [Google Scholar] [CrossRef]
- Chung, W.S.; Clarke, L.E.; Wang, G.X.; Stafford, B.K.; Sher, A.; Chakraborty, C.; et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013, 504, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Logan, M.A.; Freeman, M.R. The scoop on the fly brain: glial engulfment functions in Drosophila. Neuron Glia Biol. 2007, 3, 63–74. [Google Scholar] [CrossRef]
- Freeman, M.R.; Delrow, J.; Kim, J.; Johnson, E.; Doe, C.Q. Unwrapping Glial Biology. Neuron. 2003, 38, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Reddien, P.W.; Horvitz, H.R. THE ENGULFMENT PROCESS OF PROGRAMMED CELL DEATH IN CAENORHABDITIS ELEGANS. Annu Rev Cell Dev Biol. 2004, 20, 193–221. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell. 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; et al. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron. 2012, 74, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science. 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Dejanovic, B.; Wu, T.; Tsai, M.C.; Graykowski, D.; Gandham, V.D.; Rose, C.M.; et al. Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer’s disease mouse models. Nat Aging. 2022, 2, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Favuzzi, E.; Huang, S.; Saldi, G.A.; Binan, L.; Ibrahim, L.A.; Fernández-Otero, M.; et al. GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell. 2021, 184, 4048–4063. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Jung, E.; Lee, S.; Sohn, J.; Chung, W. Microglial MERTK eliminates phosphatidylserine-displaying inhibitory post-synapses. EMBO J. 2021, 40, e107121. [Google Scholar] [CrossRef]
- Scott-Hewitt, N.; Perrucci, F.; Morini, R.; Erreni, M.; Mahoney, M.; Witkowska, A.; et al. Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J. 2020, 39, e105380. [Google Scholar] [CrossRef]
- Schmidtner, A.K.; Slattery, D.A.; Gläsner, J.; Hiergeist, A.; Gryksa, K.; Malik, V.A.; et al. Minocycline alters behavior, microglia and the gut microbiome in a trait-anxiety-dependent manner. Transl Psychiatry. 2019, 9, 223. [Google Scholar] [CrossRef]
- Cullheim, S.; Thams, S. The microglial networks of the brain and their role in neuronal network plasticity after lesion. Brain Res Rev. 2007, 55, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Leslie, S.N.; Morozov, Y.M.; Duque, A.; Rakic, P.; Van Dyck, C.H.; et al. Classical complement cascade initiating C1q protein within neurons in the aged rhesus macaque dorsolateral prefrontal cortex. J Neuroinflammation. 2020, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Geloso, M.C.; D’Ambrosi, N. Microglial Pruning: Relevance for Synaptic Dysfunction in Multiple Sclerosis and Related Experimental Models. Cells. 2021, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.R.; Robinton, D.; Stevens, B. Microglia and the Brain: Complementary Partners in Development and Disease. Annu Rev Cell Dev Biol. 2018, 34, 523–544. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim J young, Noh, S. ; Lee, H.; Lee, S.Y.; Mun, J.Y.; et al. Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature. 2021, 590, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Damisah, E.C.; Hill, R.A.; Rai, A.; Chen, F.; Rothlin, C.V.; Ghosh, S.; et al. Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci Adv. 2020, 6, eaba3239. [Google Scholar] [CrossRef] [PubMed]
- Eladl, E.; Tremblay-LeMay, R.; Rastgoo, N.; Musani, R.; Chen, W.; Liu, A.; et al. Role of CD47 in Hematological Malignancies. J Hematol OncolJ Hematol Oncol. 2020, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Lehrman, E.K.; Wilton, D.K.; Litvina, E.Y.; Welsh, C.A.; Chang, S.T.; Frouin, A.; et al. CD47 Protects Synapses from Excess Microglia-Mediated Pruning during Development. Neuron. 2018, 100, 120–134. [Google Scholar] [CrossRef]
- Li, J.; Brickler, T.; Banuelos, A.; Marjon, K.; Shcherbina, A.; Banerjee, S.; et al. Overexpression of CD47 is associated with brain overgrowth and 16p11.2 deletion syndrome. Proc Natl Acad Sci. 2021, 118, e2005483118. [Google Scholar] [CrossRef]
- Chu, Y.; Jin, X.; Parada, I.; Pesic, A.; Stevens, B.; Barres, B.; et al. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci. 2010, 107, 7975–7980. [Google Scholar] [CrossRef]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016, 352, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Dion-Albert, L.; Bandeira Binder, L.; Daigle, B.; Hong-Minh, A.; Lebel, M.; Menard, C. Sex differences in the blood-brain barrier: implications for mental health. Front Neuroendocrinol. 2022 Mar;100989. [CrossRef]
- Blokland, G.A.M.; Grove, J.; Chen, C.Y.; Cotsapas, C.; Tobet, S.; Handa, R.; et al. Sex-Dependent Shared and Nonshared Genetic Architecture Across Mood and Psychotic Disorders. Biol Psychiatry. 2022, 91, 102–117. [Google Scholar] [CrossRef]
- Riecher-Rössler, A. Sex and gender differences in mental disorders. Lancet Psychiatry. 2017, 4, 8–9. [Google Scholar] [CrossRef]
- Ramiro, L.; Faura, J.; Simats, A.; García-Rodríguez, P.; Ma, F.; Martín, L.; et al. Influence of sex, age and diabetes on brain transcriptome and proteome modifications following cerebral ischemia. BMC Neurosci. 2023, 24, 7. [Google Scholar] [CrossRef]
- Iturria-Medina, Y.; Adewale, Q.; Khan, A.F.; Ducharme, S.; Rosa-Neto, P.; O’Donnell, K.; et al. Unified epigenomic, transcriptomic, proteomic, and metabolomic taxonomy of Alzheimer’s disease progression and heterogeneity. Sci Adv. 2022, 8, eabo6764. [Google Scholar] [CrossRef]
- López-Cerdán, A.; Andreu, Z.; Hidalgo, M.R.; Grillo-Risco, R.; Català-Senent, J.F.; Soler-Sáez, I.; et al. Unveiling sex-based differences in Parkinson’s disease: a comprehensive meta-analysis of transcriptomic studies. Biol Sex Differ. 2022, 13, 68. [Google Scholar] [CrossRef]
- Maitra, M.; Mitsuhashi, H.; Rahimian, R.; Chawla, A.; Yang, J.; Fiori, L.M.; et al. Cell type specific transcriptomic differences in depression show similar patterns between males and females but implicate distinct cell types and genes. Nat Commun. 2023, 14, 2912. [Google Scholar] [CrossRef]
- Hyer, M.M.; Phillips, L.L.; Neigh, G.N. Sex Differences in Synaptic Plasticity: Hormones and Beyond. Front Mol Neurosci. 2018, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.; Caspi, A.; Moffitt, T.E. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies: Using sex differences in psychopathology to study causal mechanisms. J Child Psychol Psychiatry. 2003, 44, 1092–1115. [Google Scholar] [CrossRef] [PubMed]
- Ziemka-Nalecz, M.; Pawelec, P.; Ziabska, K.; Zalewska, T. Sex Differences in Brain Disorders. Int J Mol Sci. 2023, 24, 14571. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, A.N.V.; Salimi-Khorshidi, G.; Lai, M.C.; Baron-Cohen, S.; Lombardo, M.V.; Tait, R.J.; et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014, 39, 34–50. [Google Scholar] [CrossRef]
- Knickmeyer, R.C.; Styner, M.; Short, S.J.; Lubach, G.R.; Kang, C.; Hamer, R.; et al. Maturational Trajectories of Cortical Brain Development through the Pubertal Transition: Unique Species and Sex Differences in the Monkey Revealed through Structural Magnetic Resonance Imaging. Cereb Cortex. 2010, 20, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Dehorter, N.; Del Pino, I. Shifting Developmental Trajectories During Critical Periods of Brain Formation. Front Cell Neurosci. 2020, 14, 283. [Google Scholar] [CrossRef] [PubMed]
- Rurak, G.M.; Simard, S.; Freitas-Andrade, M.; Lacoste, B.; Charih, F.; Van Geel, A.; et al. Sex differences in developmental patterns of neocortical astroglia: A mouse translatome database. Cell Rep. 2022, 38, 110310. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, J.; Herbison, A.E. Hypothalamic control of the male neonatal testosterone surge. Philos Trans R Soc Lond B Biol Sci. 2016, 371, 20150115. [Google Scholar] [CrossRef]
- Acaz-Fonseca, E.; Avila-Rodriguez, M.; Garcia-Segura, L.M.; Barreto, G.E. Regulation of astroglia by gonadal steroid hormones under physiological and pathological conditions. Prog Neurobiol. 2016, 144, 5–26. [Google Scholar] [CrossRef]
- Rurak, G.M.; Woodside, B.; Aguilar-Valles, A.; Salmaso, N. Astroglial cells as neuroendocrine targets in forebrain development: Implications for sex differences in psychiatric disease. Front Neuroendocrinol. 2021, 60, 100897. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




