1. Introduction
Fever in humans is characterized by an oral temperature of 37.7℃ or higher, as measured by a thermometer (1). Various factors can contribute to fever, including infection (2), (3), trauma (4), metabolic disease (5), and autoimmune disease (6), (7). Both infectious and non-infectious causes can result in systemic inflammatory response syndrome (SIRS). However, SIRS associated with infection tends to have higher mortality rates compared to SIRS caused by non-infectious conditions (8). Consequently, infection-related fever has been recognized as a predictor for sepsis development. Molecular and cellular biomarkers can aid in differentiating sepsis from SIRS, even in the presence of fever (9). Cellular biomarkers, in particular, are preferred due to their ease of use, immediate application, high reproducibility, and cost-effectiveness (10).
In sepsis, an important characteristic leukocyte change is the appearance of immature neutrophils (11). Building upon this observation, the delta neutrophil index (DNI) has shown promise as a cellular biomarker for early sepsis differentiation, as demonstrated in our preclinical study (12) and other clinical reports (13), (14) for the early differentiation of sepsis. In acute severe sepsis or septic shock, DNI peaks at the time of onset and gradually decreases 24 hours after onset (15), and in non-infectious inflammation such as severe acute pancreatitis, DNI values are also observed to increase and then decrease in milder cases (16). Similarly, platelet activation in sepsis plays a crucial role in host defence against pathogens and holds predictive value for sepsis outcomes. Mean platelet component (MPC) is a recognized cellular biomarker for sepsis (17). Fortunately, automated blood analyzers can readily measure both leukocyte indices like DNI and platelet indices such as MPC (18). Although both DNI and MPC have demonstrated prognostic capabilities for sepsis, there has been limited evaluation of their simultaneous predictive potential. We hypothesize that assessing DNI and MPC together in patients with suspected sepsis can provide a more robust and accurate diagnosis of sepsis and prognostic prediction compared to using DNI or MPC alone, as well as other biomarkers like CRP and procalcitonin. To investigate this, we compared cellular markers such as DNI and MPC with molecular biomarkers like procalcitonin and C-reactive protein (CRP) in patients presenting with suspected sepsis.
2. Material and Methods
2.1. Sample Size Calculation:
To determine the sample size of patients with suspected sepsis, we utilized a significance level (Type I error) of 0.1% and a type II error of 0.2%. Previous studies (19), (20) reported the incidence of confirmed sepsis among suspected sepsis patients ranging from 0.39 to 0.43. Additionally, we assumed that the control group would consist of 1.5 times more individuals than the confirmed sepsis group among suspected sepsis patients. Using the appropriate formula, the calculated total sample size was determined to be 180. Considering a dropout rate of 10%, the final total sample size was determined to be 198.
q1 = Proportion of febrile septic patients in febrile suspected septic patients
q2 = Proportion of non-febrile septic patients in non-febrile suspected septic patients
P1 = Proportion of patients expected to have febrile sepsis in febrile suspected septic patients
P2 = Proportion of patients expected to have non-febrile sepsis in non-febrile suspected septic patients
Zα = The standard normal deviate for α
Zβ = The standard normal deviate for β
N = Total number of febrile suspected septic patients
P = q1 P1 + q2 P2
Then,
N = [Zα √P(1−P)(1/q1 + 1/q2) + Zβ √P1(1−P1)(1/q1) + P2(1−P2)(1/ q2)]2/(P1−P2)2
2.2. Study population
This study was carried out at Chuncheon Sacred Heart Hospital, a 400-bed hospital located in Chuncheon, South Korea. The study was approved by the Institutional Review Board of Chuncheon Sacred Heart Hospital (IRB No. 2021-04-001). As this study was retrospectively designed, informed consent was not obtained from the institutional review board. We subsequently enrolled all febrile patients who visited the emergency department between January 2021 and March 2021.
Inclusion Criteria for patients with suspected sepsis
- body temperature>38℃
- patient’s age ≥19 years
Exclusion criteria for patients with suspected sepsis
- age <19 years old
2.3. Data definitions, data collection, and definitions of infection, sepsis, suspected sepsis and organ failure.
Basic demographic data of patients, along with laboratory results including procalcitonin level (PCT), C-reactive protein (CRP), mean platelet component (MPC), hemoglobin level, blood urea nitrogen, serum creatinine, leukocyte counts, and platelet counts, were collected from the electronic medical record (EMR). The highest recorded temperature on the day of the emergency department visit was noted. Clinical variables such as mortality, organ failure, underlying disease, admission to the Intensive Care Unit (ICU), hospital days, surgeries, readmissions within 30 days of discharge, as well as culture results from blood, urine, sputum, and wounds, were also extracted from the EMR.
Sepsis was defined as the presence of both infection and a systemic inflammatory response syndrome (21). Suspected sepsis was defined as a patient admitted to the emergency department with a fever of 38℃ or higher. Infection was defined as the identification of causative organisms through culture of fluids collected from sites of infection, including the lung, kidney, skin, bone, soft tissue, and liver. The criteria for systemic inflammatory response syndrome were defined based on a previous study (20). Organ failure was defined in accordance with the sequential organ failure assessment (22).
2.4. DNI calculation and other blood test measurements
Blood samples for DNI measurement were collected in ethylenediamine tetraacetic acid (EDTA) tubes. DNI measurements were performed within 1 minute of blood collection using an automatic cell analyzer (ADVIA 2120 Hematology System, Siemens Healthcare Diagnostics, Forchheim, Germany). The calculation of DNI involves two independent white blood cell (WBC) analysis methods: the MPO channel and the lobularity/nuclear density channel. The DNI calculation formula incorporates the neutrophil and eosinophil subfractions from the MPO channel, as well as the polymorphonuclear neutrophil (PMN) subfraction from the nuclear lobularity channel.
2.5. Statistical analysis
We used the Kolmogorov Smirnov test to determine the distribution of continuous variables, which are presented as mean ± standard deviation (SD), Student’s t-test for normally distributed variables, and Mann-Whitney U test for non-normally distributed variables. Categorical variables are presented as percentages and compared using the chi-square test or Fisher’s exact test.
Pearson’s rank correlation coefficient was used to evaluate the association between DNI, MPC, PCT and CRP. The area under the curves (AUCs) are shown to distinguish DNI from other markers. Receiver-operating characteristics (ROC) curve is constructed and the Youden’s index is applied to measure sensitivity and specificity at optimal cutoff values for DNI. A P-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA) and R studio ,Version 4.3 (Rstudio, Inc, Boston, MA, USA).
3. Results
A total of 215 patients were enrolled in the study. We categorized the causes of septic and non-septic cases (
Table 1 and
Table 2).
Among the nonseptic group, the gastrointestinal cause was enteritis in more than 90% of cases, and enteritis was diagnosed by the presence of symptoms such as diarrhea and abdominal pain, but no fever of other causes on computed tomography or evidence of severe infection on hematology tests, and upper respiratory tract cause was diagnosed by the absence of lower respiratory tract infection, such as no lung infiltrates on chest X-ray and no RALE on breath sounds. Unexplained fever was diagnosed in the absence of severe infection on blood tests and no evidence of bacteria on X-ray or culture. Musculoskeletal and soft tissue cases were diagnosed when there was tissue inflammation or redness but no infection. The basic clinical characteristics of the patients included in the study was as follows (
Table 3 and
Table 4).
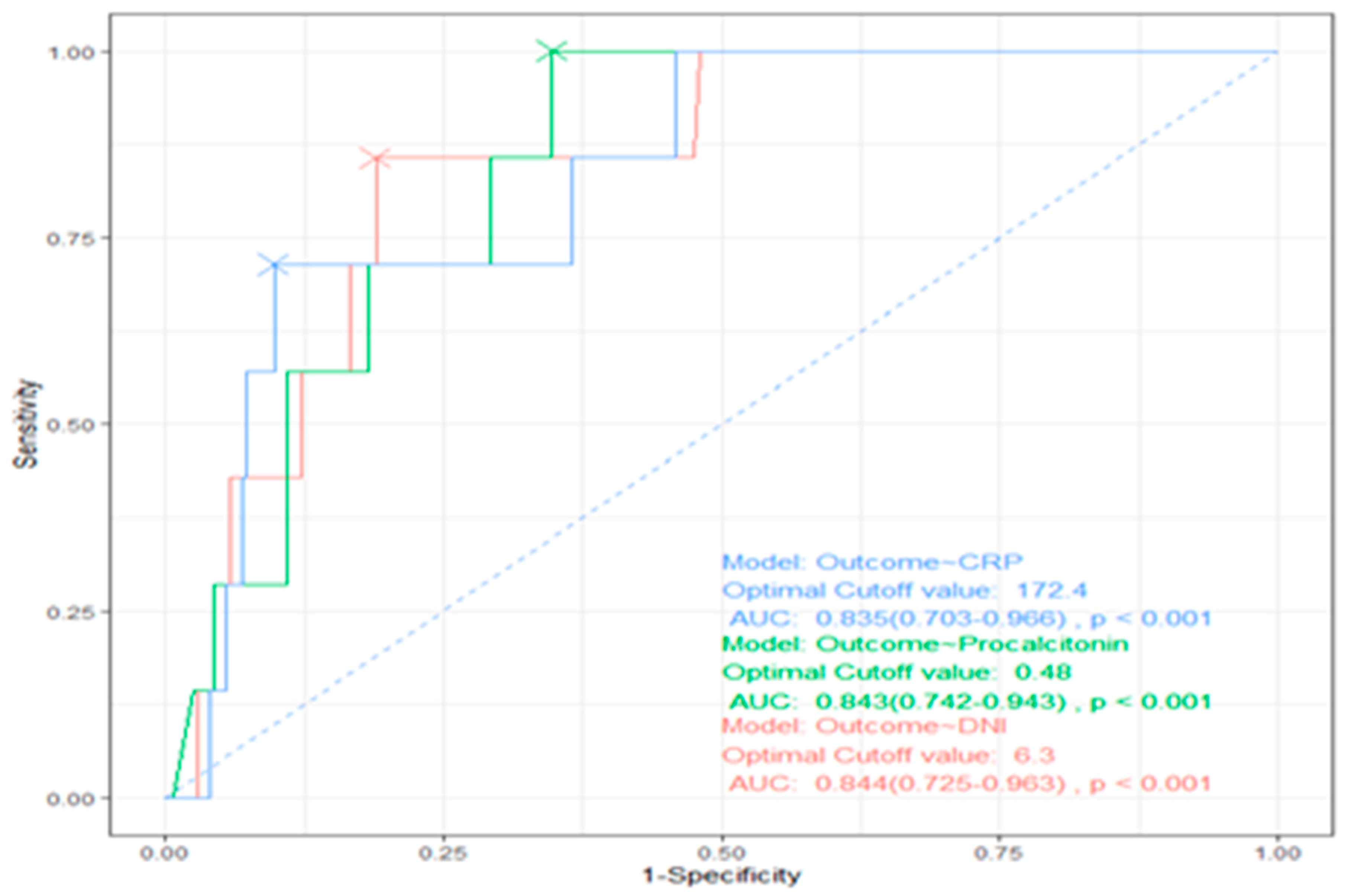
CRP, procalcitonin, and DNI levels in the sepsis group were higher than in nonsepsis group (103.0±91.6 vs 34.9±45.7, P=0.000, 8.2±21.1 vs 1.8±6.8, P=0.004, and 6.7±7.8 vs 2.1±2.2, P=0.000). When the cutoff value for DNI was 2.65%, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of DNI in sepsis were 69%, 73.9%, 77.9%, and 64.1% respectively. A comparison of the accuracy of diagnosing sepsis using DNI, crp, and procalcitonin is shown in
Figure 1.
Using a cutoff value of 6.3% for DNI, compared to a cutoff value of 0.48 ng/mL for procalcitonin and 172.4 mg/dl for crp, the AUC values were 0.844, 0.843, and 0.835 (P <0.001), respectively, with significant differences, and DNI had the highest AUC (
Figure 1). The sepsis group had a lower MPC than the non-sepsis group (26.0±1.9 vs 26.8±1.4, P=0.002). The sepsis group had a longer total length of stay than the non-sepsis group (8.6±7.4 vs 4.0±6.6, P=0.000). ICU group also had a higher DNI than the non-ICU group (8.7±10.8 vs 3.9±4.9, P=0.015). In addition, MPC level in the ICU group was significantly lower than in non-ICU group (25.1±2.3 vs 26.6±1.5, P=0.001). Looking at the effect of the source of sepsis, MPC level was lowest in sepsis that originated in the lung (
Table 5).
(
Table 6) represents the Pearson correlation rankings of Delta Neutrophil Index (DNI) with other inflammatory markers, including Mean Platelet Component (MPC), C-reactive protein (CRP), and procalcitonin (PCT), categorized by the cause of sepsis. In all three categories (all causes, gastrointestinal, and pulmonary), DNI exhibits a negative correlation with MPC.
The ROC curves illustrated in
Figure 1 demonstrate that DNI is the most effective factor in predicting mortality during sepsis, with an area under the curve (AUC) of 0.88 (95% confidence interval [CI], 0.81-0.95).
The baseline clinical characteristics of patients comparing the survival and non-survival group were shown in
Table 7.
DNI level in the non-survival group was higher than in the survival group (p=0.013). Consistently, ICU length of stay, and number of ICU admissions (%) were shorter (p=0.008), and lower (p=0.003) in the survival group compared to the non-survival group. Accordingly, the mortality rate in the ICU admission group was higher than in the non-ICU admission group (11.4% vs 0.6%, P=0.003).
3.1. Differentiating DNI and Other Markers Associated with Mortality in Sepsis Patients:
Using a cutoff level of 6.2% for DNI, it was found to be superior in predicting sepsis-related mortality compared to other markers such as CRP and procalcitonin (as shown in
Table 8).
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of DNI for mortality prediction were 100%, 80.3%, 10.9%, and 100%, respectively (as shown in
Table 9).
The incidence of multiorgan failure was significantly higher in the group with a DNI of 6.2 or higher (19.6%, 9/46) compared to the group with a DNI less than 6.2 (0%, 0/167) (p=0.000). Additionally, patients in the DNI ≥ 6.2 group had a longer length of stay in the ICU compared to those in the DNI < 6.2 group (2.0 ± 3.8 days versus 0.8 ± 2.8 days, p=0.056) (as shown in
Table 7).
4. Discussion
Previous studies(23) have shown that a complete blood count can identify early risk of developing sepsis and distinguish a poor prognosis. Among them, total white blood cells are composed of lymphocytes, monocytes, neutrophils, eosinophils, and basophils, and their increase is known to be caused by inflammation or infection, but it can also be caused by non-infectious diseases such as rheumatoid arthritis or cancer, so it is known to have low specificity in sepsis. Neutrophils are the most abundant in peripheral blood and quickly migrate to the site of infection to remove infectious agents in the event of infection, and are increased in sepsis, but can be caused by smoking or stress, and can also be increased in inflammatory bowel disease or rheumatic diseases, so they are not good for diagnosis and prognosis of sepsis, and eosinophils also migrate to the site of infection in infection and secrete cytokines, Eosinophil reduction has shown good sensitivity and specificity in the diagnosis of sepsis in some studies(24), but in a recent analysis (25) it was not found to be superior to CRP or procalcitonin. Furthermore, lymphocytes are an important component of the adaptive immune response, and although lymphopenia is associated with poor prognosis in sepsis, it can also be seen in nutritional deficiencies and autoimmune diseases (26). On the other hand, the ratio of neutrophils to lymphocytes reflects the diagnosis and prognosis of sepsis and is known to be superior to CRP and inferior to procalcitonin (27), but it is also increased in coronary artery disease and cancer, limiting its use (28).
Previous studies(29) have shown that platelet dysfunction in sepsis is present and associated with poor prognosis, and that dysfunction includes thrombocytopenia, mean platelet volume, and immature platelet fraction. Of these, thrombocytopenia was associated with a higher risk of death in the intensive care unit (30). However, thrombocytopenia is also associated with non-infectious diseases such as idiopathic thrombocytopenic purpura and systemic lupus erythematosus. Mean platelet volume is higher in sepsis than in localized infections, and increased mean platelet volume has been reported to be associated with more severe infections or antibiotic treatment failure, but it is also increased in heart disease and cerebral infarction, limiting its applicability to sepsis.
The present study aimed to assess the effectiveness of Delta Neutrophil Index (DNI) compared to other molecular biomarkers, such as procalcitonin (PCT) and C-reactive protein (CRP), in differentiating febrile septic patients from febrile non-septic patients in the emergency department and predicting prognosis. It is worth noting that previous studies (31) have established the utility of procalcitonin as a biomarker for diagnosing sepsis and predicting prognosis. However, our study demonstrates that DNI is a superior cytological marker for diagnosing and predicting sepsis when compared to procalcitonin.
Furthermore, our findings reveal a negative correlation between Mean Platelet Component (MPC) levels and DNI levels, indicating the potential of MPC in differentiating the causes of sepsis. These conclusions are drawn from the statistical analysis and comparative measurements of two molecular markers (PCT and CRP) and two cellular markers (DNI and MPC).
In our study, DNI levels increased above the baseline (1%) in both the febrile sepsis and non-sepsis groups. However, the increase was more pronounced in the febrile sepsis group compared to the febrile non-sepsis group (110 out of 123 (89.4%) vs. 61 out of 92 (66.3%), p=0.000). There was a significant difference in DNI levels between the febrile sepsis and non-sepsis groups (6.7±7.8% vs. 2.1±2.2%, p=0.000).
The elevated DNI levels observed in the febrile non-sepsis group may be attributed to non-infectious inflammation, such as trauma-related tissue damage, metabolic disorders, or autoimmune diseases. However, DNI levels were significantly higher in the febrile sepsis group compared to the febrile non-sepsis group, which supports previous reports highlighting the effectiveness of DNI in assessing sepsis (32). Accumulative studies have also suggested that DNI can be a diagnostic marker for sepsis ((14), (33), (15), (34)).
Consistent with prior research, our results demonstrate that DNI is effective in differentiating sepsis-related fever from non-sepsis-related fever. Furthermore, the cutoff value of 6.2% for DNI is more effective than CRP and PCT in predicting the prognosis of febrile sepsis, including outcomes such as mortality, organ failure, length of stay, and in-hospital surgery. Patients with a DNI value greater than 6.2% have a worse prognosis.
It is well-known that platelets are activated in various infectious and non-infectious pathological conditions, such as acute myocardial infarction (35), cerebrovascular disease (36), and diabetes mellitus (37). Similar to the aberrant neutrophil behaviour observed in sepsis, platelet activation plays a significant role in these conditions.
Patients with febrile sepsis and lower MPC levels are at an increased risk of death. This association can be attributed to the detrimental effects of platelet activation, including platelet depletion, thrombosis, and the development of disseminated intravascular coagulopathy (38). These complications can ultimately lead to multi-organ failure and even death (39).
These findings have significant clinical implications, suggesting that patients admitted to the emergency department with fever and suspected sepsis, particularly those with low MPC levels, should undergo prompt evaluation to identify the source of sepsis and initiate appropriate management strategies as early as possible.
Finally, inferential statistics revealed a significant negative correlation between DNI and MPC, and the strength of this inverse correlation varied depending on the origin of sepsis. Notably, this is the first report to demonstrate this relationship in the context of human sepsis. The strongest negative correlation between DNI and MPC was observed in cases of sepsis originating from the gastrointestinal tract and lungs (Pearson correlation coefficients: -0.611 for gastrointestinal tract origin, -0.492 for lung origin, and -0.312 for all sources of sepsis). This finding may provide valuable insights into the clinical application of identifying the focus of infection in sepsis. However, the consideration of using a panel of the best candidate markers may be superior to the use of each of crp, procalcitonin, and DNI alone in assessing the diagnosis and prognosis of sepsis, but there is not much room for consideration at this time because each biomarker alone is excellent for the diagnosis and prognosis of sepsis.
One possible explanation for the strong negative correlation between DNI and MPC in gastrointestinal and lung sepsis could be the dysbiosis of gut microbiota associated with the gut-lung axis (40). The human gut harbors a vast number of microbiomes, accounting for over 80% of the body’s lymphocytes, and plays a crucial role in the development and function of immune cells (41). In septic conditions, the gut microbiota undergoes dysbiosis (42) and increased permeability, leading to platelet activation, severe tissue damage, and ultimately multi-organ failure and death (43). Similarly, the gut-lung axis (GLA) can significantly impact the lungs during sepsis (44). Gut microbiota can modulate pulmonary immune responses and diseases through the mesenteric lymphatic system (41), (45). Therefore, we speculate that these intricate networks contribute to the more pronounced negative correlations observed in gastrointestinal and lung-origin sepsis compared to other causes of sepsis.
Our study demonstrated the predictive value of DNI and MPC in febrile sepsis and highlighted the potential benefit of utilizing both markers together to enhance the probability of predicting sepsis. Nevertheless, it is important to acknowledge the limitations of this study. Firstly, there were cases where initial cultures were not obtained in less than 5% of patients. Additionally, follow-up data was unavailable for less than 10% of eligible patients. Furthermore, patients with sepsis without fever were not evaluated. The one-year follow-up period in this study may not provide a comprehensive understanding of the long-term prognosis and outcome assessment of febrile patients with suspected sepsis. Therefore, future research with longer follow-up durations, ideally one year or more, is warranted. Lastly, it is important to note that this study utilized a retrospective design.
In summary: this study highlights the superiority of DNI over other molecular biomarkers, such as procalcitonin and CRP, in differentiating febrile septic patients from nonseptic febrile patients in the emergency department and predicting prognosis. MPC also contributes to the differentiation of causes in febrile sepsis. The clinical significance of DNI/MPC lies in their cost-effectiveness and applicability in various impending sepsis scenarios. Importantly, this study is the first to evaluate the diagnostic validity of simultaneously employing DNI and MPC in human cases of febrile sepsis.
Author Contributions
HDL designed this study. HDL, THL collected data of the patients and reviewed electronic medical record. JWL, SKK, HDL and DHS analysed the data and conducted literature review. HDL, THL wrote the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
Hyungdon Lee, Jongwook Lee, Soo-Ki Kim, Dong Hoon Shin and Taehun Lee declare that they have no conflict of interest.
Human and animal rights
This study was approved by the Institutional Review of Board of Chuncheon Sacred Heart Hospital (IRB approval No: 2021-04-001 ). Informed consent was not obtained, because this study was retrospective study.
References
- Mackowiak PA, Chervenak FA, Grunebaum A. Defining Fever. Open Forum Infect Dis. 2021;8(6):ofab161. [CrossRef]
- Bossink AW, Groeneveld AB, Hack CE, Thijs LG. The clinical host response to microbial infection in medical patients with fever. Chest. 1999;116(2):380-90. [CrossRef]
- Wrotek S, Sobocinska J, Kozlowski HM, Pawlikowska M, Jedrzejewski T, Dzialuk A. New Insights into the Role of Glutathione in the Mechanism of Fever. Int J Mol Sci. 2020;21(4). [CrossRef]
- Scott HF, Deakyne SJ, Woods JM, Bajaj L. The prevalence and diagnostic utility of systemic inflammatory response syndrome vital signs in a pediatric emergency department. Acad Emerg Med. 2015;22(4):381-9. [CrossRef]
- Berardinelli F, De Francesco P, Marchioni M, Cera N, Proietti S, Hennessey D, et al. Infective complications after retrograde intrarenal surgery: a new standardized classification system. Int Urol Nephrol. 2016;48(11):1757-62. [CrossRef]
- Verrecchia E, Zampetti A, Antuzzi D, Ricci R, Ferri L, Morrone A, et al. The impact of fever/hyperthermia in the diagnosis of Fabry: A retrospective analysis. Eur J Intern Med. 2016;32:26-30. [CrossRef]
- Burdette BE, Esparza AN, Zhu H, Wang S. Gasdermin D in pyroptosis. Acta Pharm Sin B. 2021;11(9):2768-82. [CrossRef]
- Shiraishi A, Gando S, Abe T, Kushimoto S, Mayumi T, Fujishima S, et al. Quick sequential organ failure assessment versus systemic inflammatory response syndrome criteria for emergency department patients with suspected infection. Sci Rep. 2021;11(1):5347. [CrossRef]
- Agnello L, Iacona A, Maestri S, Lo Sasso B, Giglio RV, Mancuso S, et al. Independent Validation of Sepsis Index for Sepsis Screening in the Emergency Department. Diagnostics (Basel). 2021;11(7). [CrossRef]
- Park SJ, Park J, Lee MJ, Seo JS, Ahn JY, Cho JW. Time series analysis of delta neutrophil index as the predictor of sepsis in patients with acute poisoning. Hum Exp Toxicol. 2020;39(1):86-94. [CrossRef]
- Kim H, Kim Y, Lee HK, Kim KH, Yeo CD. Comparison of the delta neutrophil index with procalcitonin and C-reactive protein in sepsis. Clin Lab. 2014;60(12):2015-21. [CrossRef]
- Lee H, Lim JM, Lee J, Kim SK, Lee T. Positive Role of Delta Neutrophil Index (DNI) as a Prodiagnostic Marker in Cecal Ligation and Puncture (CLP)-Induced Sepsis Murine Model. Medicina (Kaunas). 2022;58(3). [CrossRef]
- Peneva P, Nikolova S, Bocheva Y. Delta neutrophil index: in search of an early indicator of sepsis. Folia Med (Plovdiv). 2021;63(4):496-501. [CrossRef]
- Kim JH, Park YS, Yoon CY, Lee HS, Kim S, Lee JW, et al. Delta Neutrophil Index for the Prediction of the Development of Sepsis-Induced Acute Kidney Injury in the Emergency Department. Shock. 2019;52(4):414-22. [CrossRef]
- Park BH, Kang YA, Park MS, Jung WJ, Lee SH, Lee SK, et al. Delta neutrophil index as an early marker of disease severity in critically ill patients with sepsis. BMC Infect Dis. 2011;11:299. [CrossRef]
- Kim TY, Kim SJ, Kim YS, Lee JW, Park EJ, Lee SJ, et al. Delta neutrophil index as an early predictive marker of severe acute pancreatitis in the emergency department. United European Gastroenterol J. 2019;7(4):488-95. [CrossRef]
- Platanaki C, Zareifopoulos N, Lagadinou M, Tsiotsios K, Velissaris D. Correlation of Positive Blood Cultures with Peripherally Inserted Central Catheter Line Infection in Oncology Patients. Cureus. 2021;13(1):e12858. [CrossRef]
- Nam M, Son BH, Seo JE, Kim IR, Park CK, Kim HK. Improved Diagnostic and Prognostic Power of Combined Delta Neutrophil Index and Mean Platelet Volume in Pediatric Sepsis. Ann Clin Lab Sci. 2018;48(2):223-30.
- Schrijver IT, Kemperman H, Roest M, Kesecioglu J, de Lange DW. Myeloperoxidase can differentiate between sepsis and non-infectious SIRS and predicts mortality in intensive care patients with SIRS. Intensive Care Med Exp. 2017;5(1):43. [CrossRef]
- Comstedt P, Storgaard M, Lassen AT. The Systemic Inflammatory Response Syndrome (SIRS) in acutely hospitalised medical patients: a cohort study. Scand J Trauma Resusc Emerg Med. 2009;17:67. [CrossRef]
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-6. [CrossRef]
- Jones AE, Trzeciak S, Kline JA. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. 2009;37(5):1649-54. [CrossRef]
- Agnello L, Giglio RV, Bivona G, Scazzone C, Gambino CM, Iacona A, et al. The Value of a Complete Blood Count (CBC) for Sepsis Diagnosis and Prognosis. Diagnostics (Basel). 2021;11(10). [CrossRef]
- Abidi K, Khoudri I, Belayachi J, Madani N, Zekraoui A, Zeggwagh AA, et al. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit Care. 2008;12(2):R59. [CrossRef]
- Lin Y, Rong J, Zhang Z. Silent existence of eosinopenia in sepsis: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):471. [CrossRef]
- Boomer JS, Shuherk-Shaffer J, Hotchkiss RS, Green JM. A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit Care. 2012;16(3):R112. [CrossRef]
- Dragoescu AN, Padureanu V, Stanculescu AD, Chiutu LC, Tomescu P, Geormaneanu C, et al. Neutrophil to Lymphocyte Ratio (NLR)-A Useful Tool for the Prognosis of Sepsis in the ICU. Biomedicines. 2021;10(1). [CrossRef]
- Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int J Mol Sci. 2022;23(7). [CrossRef]
- Pigozzi L, Aron JP, Ball J, Cecconi M. Understanding platelet dysfunction in sepsis. Intensive Care Med. 2016;42(4):583-6. [CrossRef]
- Akca S, Haji-Michael P, de Mendonca A, Suter P, Levi M, Vincent JL. Time course of platelet counts in critically ill patients. Crit Care Med. 2002;30(4):753-6. [CrossRef]
- Gregoriano C, Heilmann E, Molitor A, Schuetz P. Role of procalcitonin use in the management of sepsis. J Thorac Dis. 2020;12(Suppl 1):S5-S15. [CrossRef]
- Ahn C, Kim W, Lim TH, Cho Y, Choi KS, Jang BH. The delta neutrophil index (DNI) as a prognostic marker for mortality in adults with sepsis: a systematic review and meta-analysis. Sci Rep. 2018;8(1):6621. [CrossRef]
- Park HJ, Ha YJ, Pyo JY, Park YB, Lee SK, Lee SW. Delta neutrophil index as an early marker for differential diagnosis of adult-onset Still’s disease and sepsis. Yonsei Med J. 2014;55(3):753-9. [CrossRef]
- Kim JW, Park JH, Kim DJ, Choi WH, Cheong JC, Kim JY. The delta neutrophil index is a prognostic factor for postoperative mortality in patients with sepsis caused by peritonitis. PLoS One. 2017;12(8):e0182325. [CrossRef]
- Chung I, Choudhury A, Patel J, Lip GY. Soluble CD40L, platelet surface CD40L and total platelet CD40L in congestive heart failure: relationship to platelet volume, mass and granularity. J Intern Med. 2008;263(3):313-21. [CrossRef]
- Ghodsi H, Abouei Mehrizi MA, Khoshdel AR, Shekarchi B. Evaluation of combining Alberta Stroke Program Early CT Score (ASPECTS) with mean platelet volume, plateletcrit, and platelet count in predicting short- and long-term prognosis of patients with acute ischemic stroke. Clin Neurol Neurosurg. 2021;208:106830. [CrossRef]
- Ji S, Zhang J, Fan X, Wang X, Ning X, Zhang B, et al. The relationship between mean platelet volume and diabetic retinopathy: a systematic review and meta-analysis. Diabetol Metab Syndr. 2019;11:25. [CrossRef]
- Cay N, Ipek A, Gumus M, Birkan Z, Ozmen E. Platelet activity indices in patients with deep vein thrombosis. Clin Appl Thromb Hemost. 2012;18(2):206-10. [CrossRef]
- Boos CJ, Beevers GD, Lip GY. Assessment of platelet activation indices using the ADVIATM 120 amongst ’high-risk’ patients with hypertension. Ann Med. 2007;39(1):72-8. [CrossRef]
- Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieers G, Guery B, et al. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front Cell Infect Microbiol. 2020;10:9. [CrossRef]
- Wang C, Li Q, Tang C, Zhao X, He Q, Tang X, et al. Characterization of the blood and neutrophil-specific microbiomes and exploration of potential bacterial biomarkers for sepsis in surgical patients. Immun Inflamm Dis. 2021;9(4):1343-57. [CrossRef]
- Hiengrach P, Panpetch W, Chindamporn A, Leelahavanichkul A. Macrophage depletion alters bacterial gut microbiota partly through fungal overgrowth in feces that worsens cecal ligation and puncture sepsis mice. Sci Rep. 2022;12(1):9345. [CrossRef]
- Fay KT, Ford ML, Coopersmith CM. The intestinal microenvironment in sepsis. Biochim Biophys Acta Mol Basis Dis. 2017;1863(10 Pt B):2574-83. [CrossRef]
- de Oliveira Formiga R, Amaral FC, Souza CF, Mendes D, Wanderley CWS, Lorenzini CB, et al. Neuraminidase is a host-directed approach to regulate neutrophil responses in sepsis and COVID-19. Br J Pharmacol. 2022. [CrossRef]
- Meyer NJ, Reilly JP, Feng R, Christie JD, Hazen SL, Albert CJ, et al. Myeloperoxidase-derived 2-chlorofatty acids contribute to human sepsis mortality via acute respiratory distress syndrome. JCI Insight. 2017;2(23).This is the first study to evaluate the prodiagnostic validity of DNI and MPC together in human febrile sepsis cases. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).