Submitted:
02 November 2023
Posted:
02 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
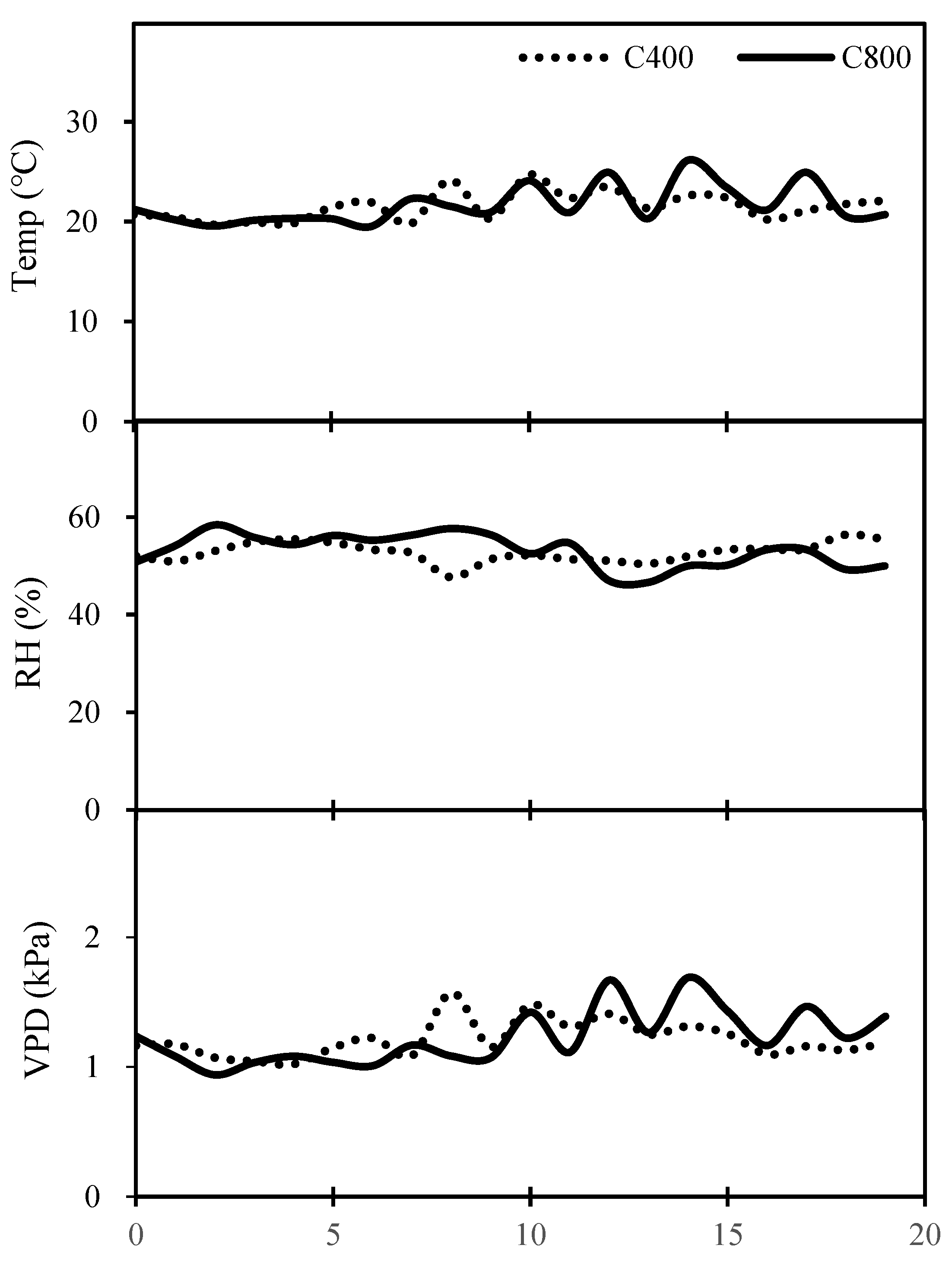
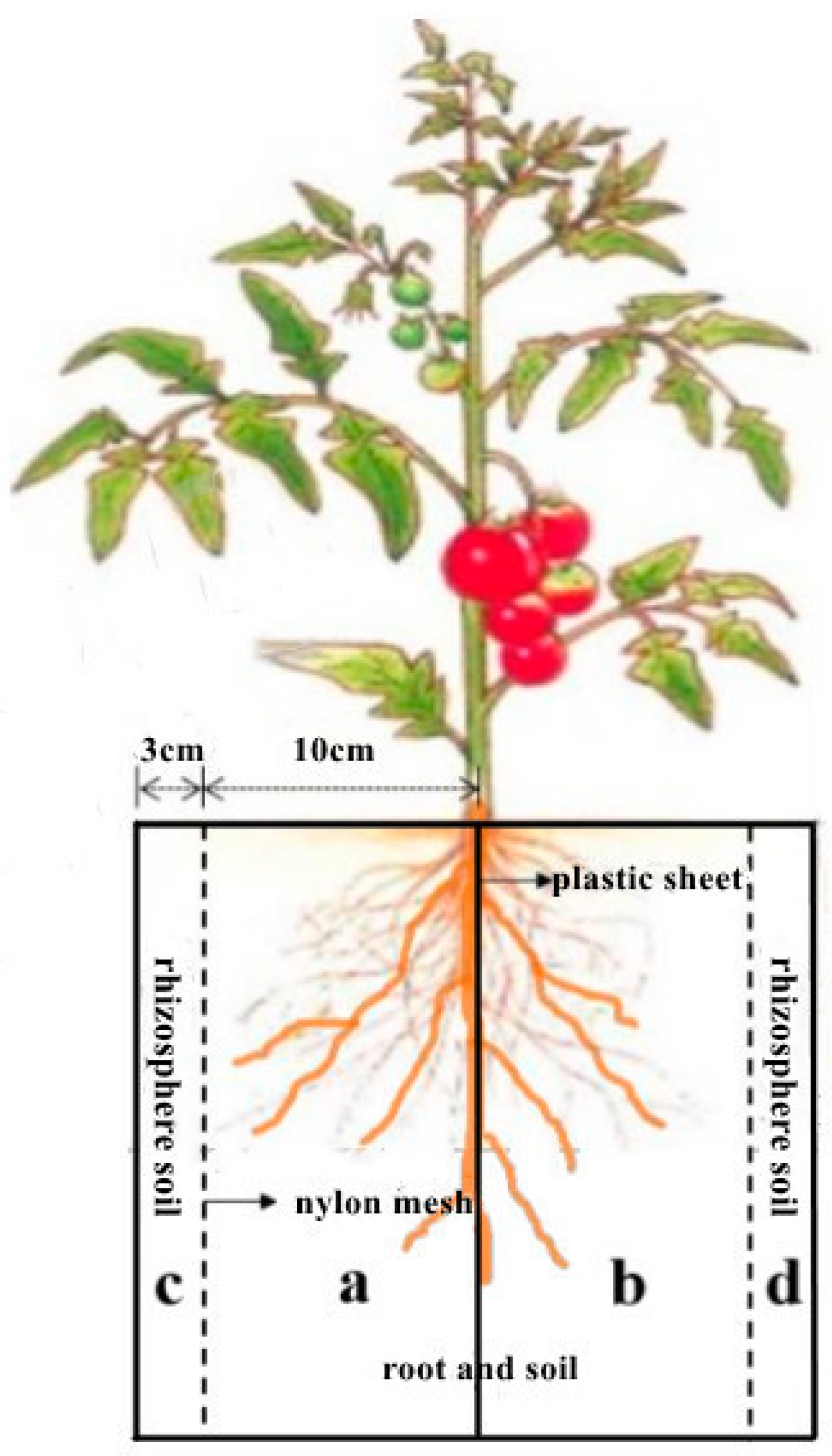
2.2. Treatments
2.3. Measurements
2.3.1. Harvest
2.3.2. Root morphological analyses
2.3.3. Plant biomass, water use, water use efficiency
2.3.4. Elemental analyses
2.4. Statistical analysis
3. Results
3.1. Plant dry matter accumulation (ΔDM), Dry biomass allocation, shoot dry mass, Plant water use (WU)and plant water use efficiency (WUE
| [CO2] | Irrigation regimes | ∆DM (g plant-1) | biomass allocation (%) | SDW (g plant-1) | LDW (g plant-1) | WU (L plant-1) | WUE (g L-1) | |||
| leaf | stem | root | ||||||||
| C400 | FI | 25.9 ± 1.6 | 73.0 ± 1.5 | 21.6 ± 1.4 | 5.4 ± 0.2 | 26.6 ± 1.5 | 20.5 ± 1.0 | 7.0 ± 0.3 | 3.7 ± 0.3 | |
| DI | 25.7 ± 0.5 | 68.5 ± 1.2 | 24.9 ± 1.2 | 6.7 ± 0.2 | 26.2 ± 1.8 | 19.2 ± 1.2 | 4.9 ± 0 | 5.2 ± 0.1 | ||
| PRI | 25.8 ± 2.0 | 67.6 ± 1.2 | 25.6 ± 1.2 | 6.8 ± 0.1 | 26.1 ± 0.5 | 18.9 ± 0.3 | 4.9 ± 0 | 5.3 ± 0.4 | ||
| C800 | FI | 53.8 ± 2.1 | 74.3 ± 1.3 | 19.2 ± 1.4 | 6.5 ± 0.2 | 52.6 ± 2.1 | 41.8 ± 1.9 | 9.7 ± 0.7 | 5.6 ± 0.3 | |
| DI | 50.3 ± 1.6 | 70.7 ± 1.1 | 21.5 ± 1.0 | 7.9 ± 0.4 | 50.2 ± 2.4 | 38.4 ± 1.3 | 6.8 ± 0 | 7.4 ± 0.2 | ||
| PRI | 52.1 ± 2.7 | 72.0 ± 0.7 | 21.8 ± 0.3 | 7.3 ± 0.4 | 48.8 ± 1.3 | 37.8 ± 0.8 | 6.8 ± 0 | 7.6 ± 0.4 | ||
| Output of two-way ANOVA | ||||||||||
| [CO2] (C) | *** | * | ** | ** | *** | *** | *** | *** | ||
| Irrigation regimes (IR) | ns | ** | * | *** | ns | ** | *** | *** | ||
| C×IR | ns | ns | ns | ns | ns | ns | ns | ns | ||
3.2. Root growth and morphological traits
| [CO2] | Irrigation regimes | RL (m) | RS (cm2) | RD (mm) | RV (cm3) | SRL (m g-1) | RTD (g cm-3) | R/S | RDW (g plant-1) | |
| C400 | FI | 12.9 ± 1.1 | 280.5 ± 28.6 | 0.54 ± 0.02 | 4.5 ± 0.6 | 9.9 ± 0.8c | 0.3 ± 0.02 | 0.06±0.003 | 1.5 ± 0.1 | |
| DI | 60.8 ± 4.6 | 718.0 ± 55.8 | 0.38 ± 0.01 | 7.1 ± 0.5 | 35.9 ± 2.7a | 0.2 ± 0.02 | 0.07±0.003 | 1.9 ± 0.2 | ||
| PRI | 43.1 ± 6.4 | 609.8 ± 62.7 | 0.45 ± 0.03 | 7.4 ± 0.6 | 25.8 ± 2.9ab | 0.2 ± 0.01 | 0.07±0.003 | 1.9 ± 0.0 | ||
| C800 | FI | 75.0 ± 19.7 | 1045.5 ± 132.7 | 0.47 ± 0.04 | 12.4 ± 0.6 | 21.9 ± 5.9b | 0.3 ± 0.01 | 0.07±0.003 | 3.6 ± 0.1 | |
| DI | 99.5 ± 24.5 | 1324.4 ± 240.7 | 0.47 ± 0.05 | 15.2 ± 1.6 | 26.3 ± 4.5ab | 0.2 ± 0.01 | 0.08±0.005 | 4.3 ± 0.4 | ||
| PRI | 123.0 ± 14.3 | 1569.4 ± 123.7 | 0.43 ± 0.03 | 17.0 ± 1.1 | 30.8 ± 4.6ab | 0.2 ± 0.01 | 0.09±0.005 | 3.8 ± 0.3 | ||
| Output of two-way ANOVA | ||||||||||
| [CO2] (C) | *** | *** | ns | *** | ns | ns | ** | *** | ||
| Irrigation regimes (IR) | * | ** | ns | ** | ** | ** | ** | ns | ||
| C×IR | ns | ns | ns | ns | * | ns | ns | ns | ||
3.3. Leaf element concentrations.
| [CO2] | Irrigation regimes | [C] (mg g-1) | [N] (mg g-1) | [P] (mg g-1) | [K] (mg g-1) | [Ca] (mg g-1) | [Mg] (mg g-1) | [S] (mg g-1) | [15N] (mg g-1) | |
| C400 | FI | 412.2 ± 4.1 | 50.9 ± 0.9 | 4.2 ± 0.09 | 25.0 ± 1.9 | 51.2 ± 1.1 | 4.1 ± 0.2 | 7.2 ± 0.5 | 1.5 ± 0.1 | |
| DI | 421.4 ± 2.9 | 54.8 ± 1.1 | 3.8 ± 0.1 | 24.6 ± 2.0 | 49.5 ± 1.5 | 3.9 ± 0.2 | 5.7 ± 0.3 | 2.2 ± 0.3 | ||
| PRI | 420.4 ± 1.1 | 54.0 ± 1.8 | 3.8 ± 0.05 | 22.5 ± 1.4 | 47.1 ± 1.7 | 3.6 ± 0.1 | 5.9 ± 0.4 | 1.4 ± 0.2 | ||
| C800 | FI | 417.6 ± 1.4 | 45.4 ± 2.2 | 4.2 ± 0.16 | 25.0 ± 0.7 | 55.2 ± 1.7 | 3.7 ± 0.2 | 7.8 ± 0.1 | 1.1 ± 0.1 | |
| DI | 418.4 ± 3.1 | 43.7 ± 1.6 | 3.7 ± 0.27 | 24.5 ± 1.8 | 54.2 ± 1.9 | 3.7 ± 0.1 | 7.6 ± 0.4 | 1.6 ± 0.1 | ||
| PRI | 423.9 ± 1.7 | 49.6 ± 3.3 | 3.0 ± 0.39 | 22.7 ± 1.2 | 50.5 ± 1.6 | 3.4 ± 0.1 | 7.2 ± 0.7 | 1.5 ± 0.1 | ||
| Output of two-way ANOVA | ||||||||||
| [CO2] (C) | ns | *** | ns | ns | ** | * | ** | 0.052 | ||
| Irrigation regimes (IR) | * | ns | ** | ns | * | * | ns | ** | ||
| C×IR | ns | ns | ns | ns | ns | ns | ns | ns | ||
3.4. Leaf element uptakes
| [CO2] | Irrigation regimes | C (g plant-1) | N (g plant-1) | P (mg plant-1) | K (mg plant-1) | Ca (g plant-1) | Mg (mg plant-1) | S (mg plant-1) | 15N (mg plant-1) |
| C400 | FI | 8.5 ± 0.5 | 1.0 ± 0.05 | 86.4 ± 5.1d | 506.8 ± 17.2 | 1.1 ± 0.1 | 82.5 ± 3.1 | 147.7 ± 13.2 | 31.0 ± 2.2 |
| DI | 8 ± 0.1 | 1.0 ± 0.02 | 71.2 ± 1.5d | 463.6 ± 31.8 | 0.9 ± 0.0 | 74.3 ± 2.4 | 107.5 ± 6.6 | 41.0 ± 5.2 | |
| PRI | 8.1 ± 0.5 | 1.0 ± 0.06 | 73.2 ± 3.9d | 431.9 ± 41.5 | 0.9 ± 0.1 | 68.7 ± 5.5 | 112.4 ± 8.2 | 26.9 ± 4.7 | |
| C800 | FI | 17.5 ± 0.8 | 1.9 ± 0.05 | 175.8 ± 11.2a | 1041.3 ± 43.5 | 2.3 ± 0.1 | 155.2 ± 7.9 | 328.0 ±17.6 | 45.7 ± 5.8 |
| DI | 15.8 ± 0.4 | 1.7 ± 0.04 | 141.4 ± 13.3b | 929.5 ± 89.3 | 2.0 ± 0.0 | 138.7 ± 6.3 | 287.9 ± 21.9 | 61.7 ± 3.5 | |
| PRI | 16.3 ± 0.6 | 1.9 ± 0.11 | 113.3 ± 11.1c | 867.4 ± 18.5 | 1.9 ± 0.1 | 129.5 ± 5.8 | 273.9 ± 21.1 | 58.2 ± 4.2 | |
| Output of two-way ANOVA | |||||||||
| [CO2] (C) | *** | *** | *** | *** | *** | *** | *** | *** | |
| Irrigation regimes (IR) | ns | ns | ** | * | ** | ** | * | * | |
| C×IR | ns | ns | * | ns | ns | ns | ns | ns | |
3.5. C/N, C/P, C/K, C/Ca, C/Mg, C/S. N/P and N/K in tomato leaves
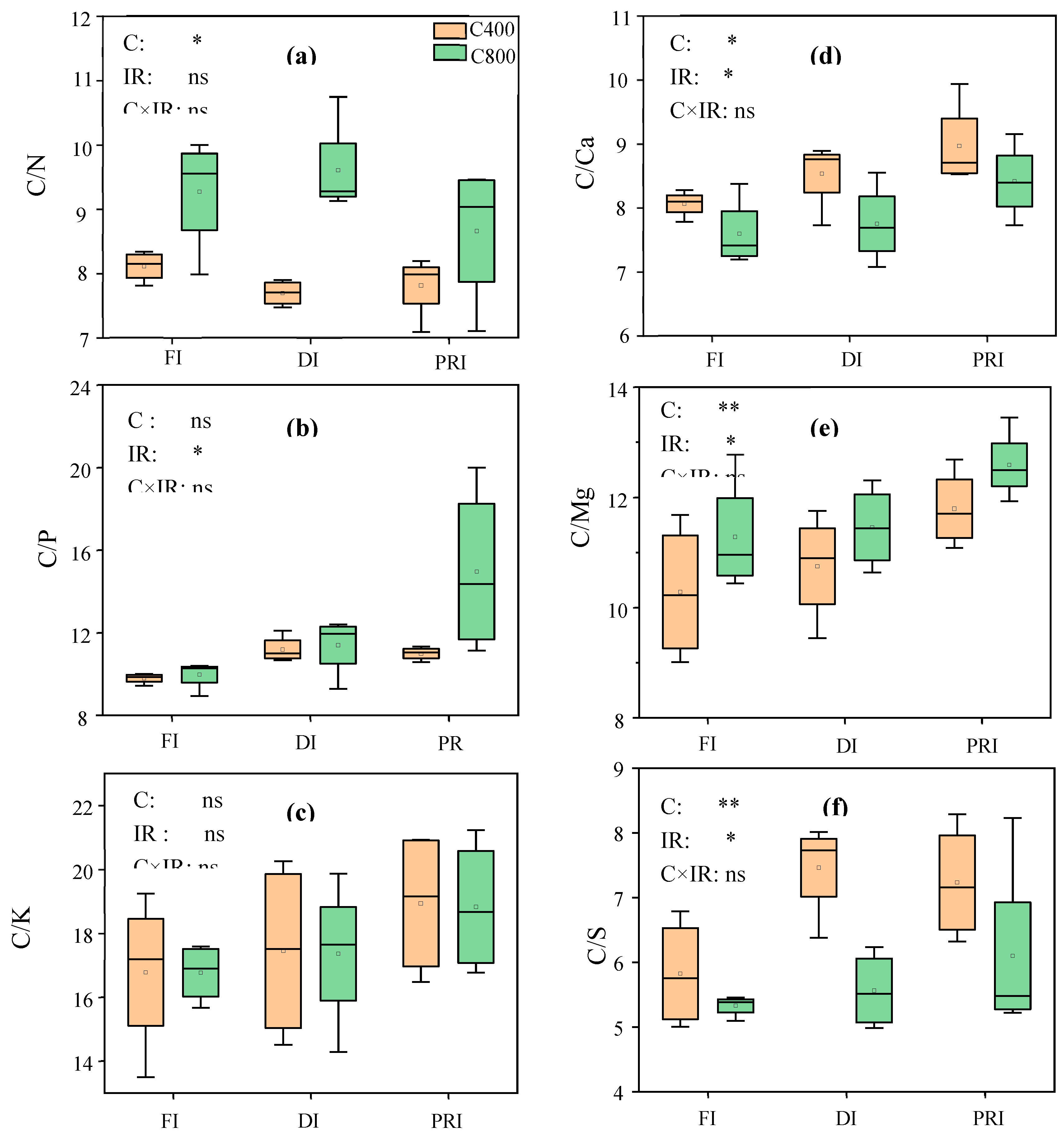
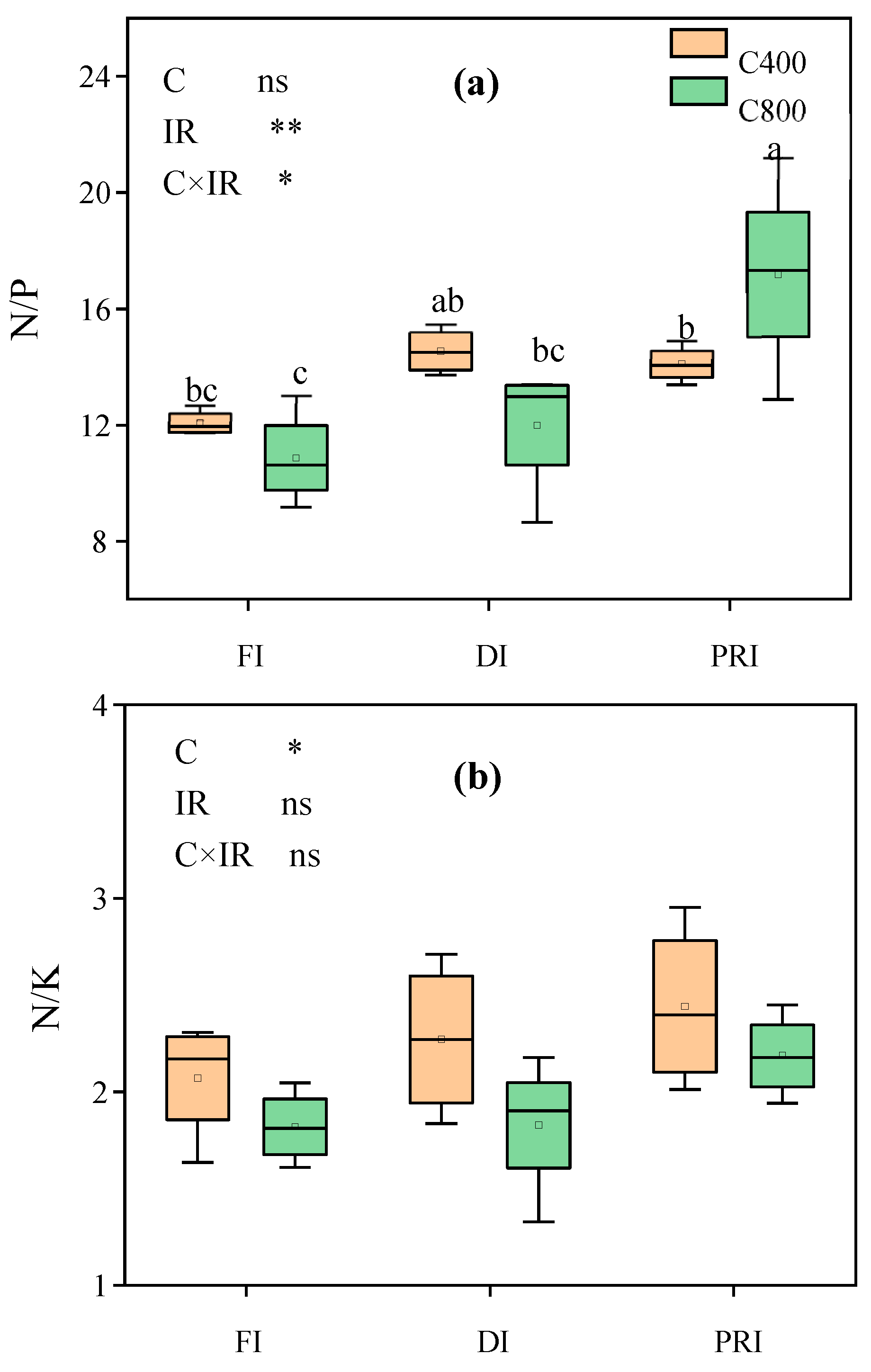
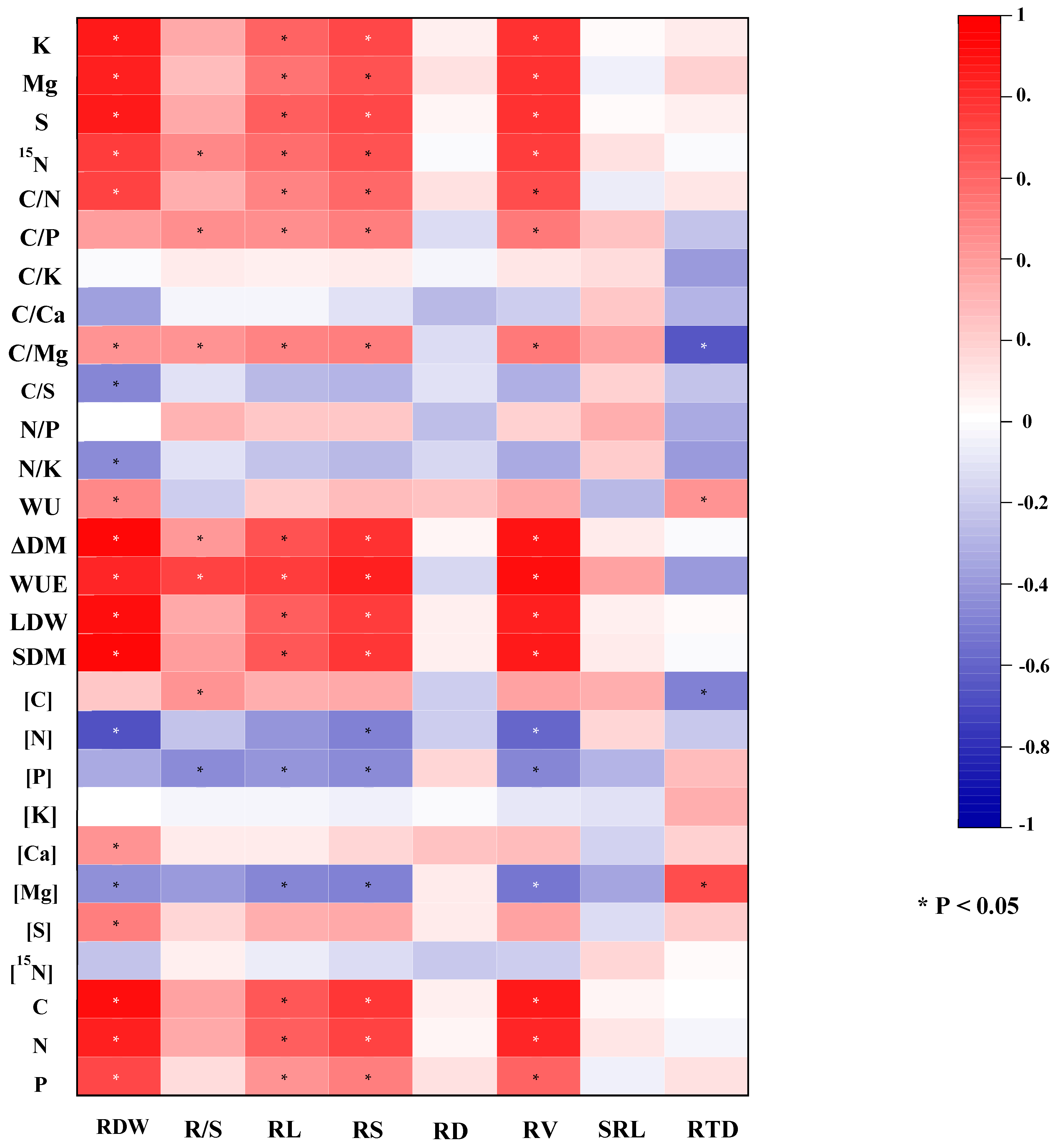
3.6. The Pearson correlation analysis between root morphology traits and tomato growth, element concentrations, element uptakes and stoichiometric ratios
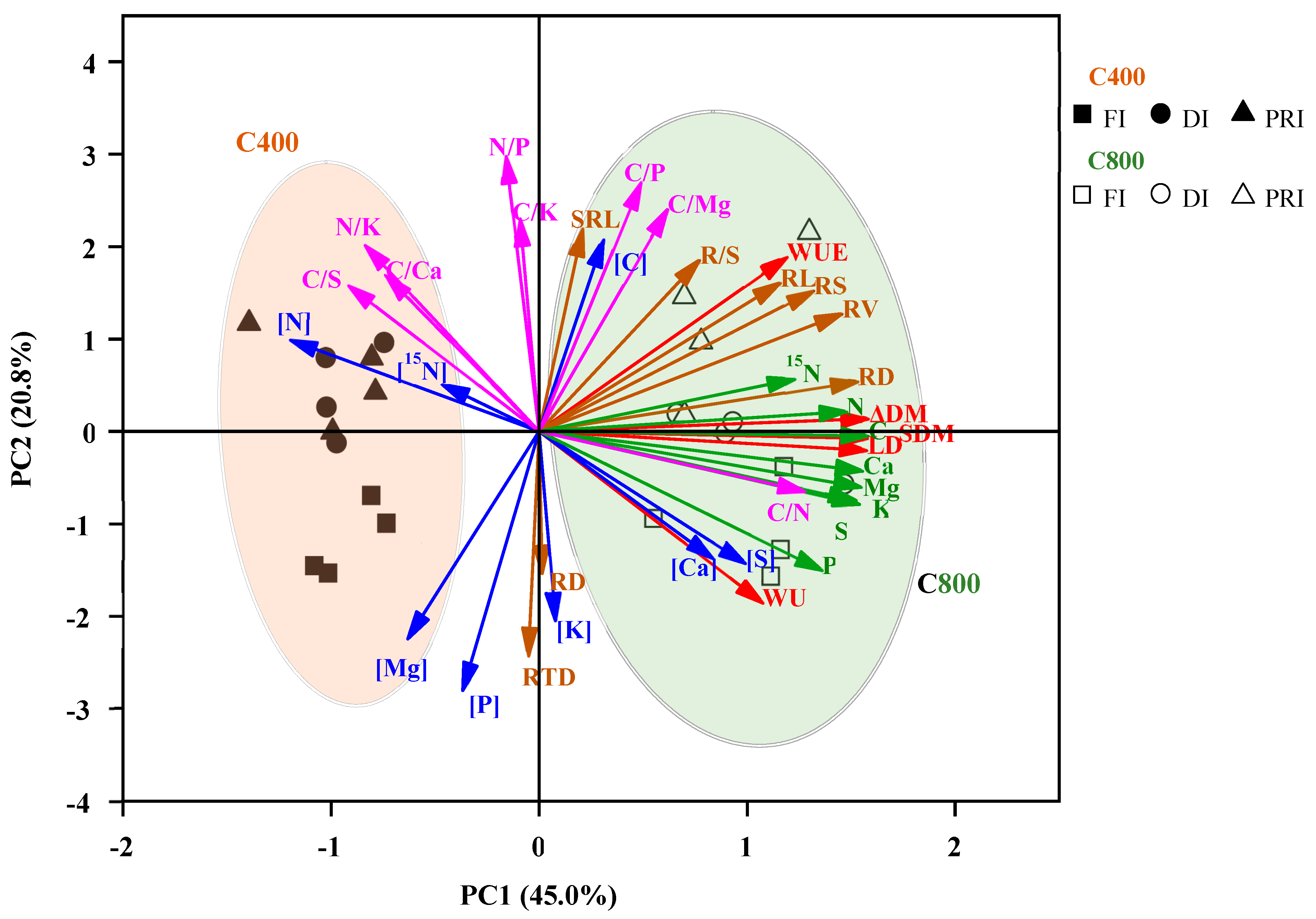
3.7. PCA analysis of tomato parameters
4. Discussion
4.1. The effects of PRI on Plant biomass, biomass allocation and water use efficiency under e[CO2]
4.2. The effects of PRI on root morphological traits of tomato leaves under e[CO2]
4.3. The effects of PRI on leaf nutrient concentration, nutrient uptake, nutrient stoiochiometry of tomato leaves under e[CO2]
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate change 2014: synthesis report. . In CoreWriting Team, Pachauri, R. K., Meyer, L.A. (Eds.), Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Geneva., 2014.
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 2005, 165, 351–371. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Glob Chang Biol 2021, 27, 27–49. [Google Scholar] [CrossRef]
- Leakey, A.D.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot 2009, 60, 2859–2876. [Google Scholar] [CrossRef]
- Wei, Z.; Du, T.; Li, X.; Fang, L.; Liu, F. Interactive effects of CO2 concentration elevation and nitrogen fertilization on water and nitrogen use efficiency of tomato grown under reduced irrigation regimes. Agricultural Water Management 2018, 202, 174–182. [Google Scholar] [CrossRef]
- Yang, X.; Bornø, M.L.; Wei, Z.; Liu, F. Combined effect of partial root drying and elevated atmospheric CO2 on the physiology and fruit quality of two genotypes of tomato plants with contrasting endogenous ABA levels. Agricultural Water Management 2021, 254. [Google Scholar] [CrossRef]
- McDonald, E.P.; Erickson, J.E.; Kruger, E.L. Can decreased transpiration limit plant nitrogen acquisition in elevated CO2? Functional Plant Biology 2002, 22, 1115–1120. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; Holbrook, N.M.; Nelson, R.L.; Ottman, M.J.; Raboy, V.; Sakai, H.; Sartor, K.A.; Schwartz, J.; Seneweera, S.; Tausz, M.; Usui, Y. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef]
- Loladze, I. Hidden shift of the ionome of plants exposed to elevated CO(2)depletes minerals at the base of human nutrition. Elife 2014, 3, e02245. [Google Scholar] [CrossRef]
- Müller, C.; Elliott, J.; Levermann, A. Fertilizing hidden hunger. Nature Climate Change 2014, 4, 540–541. [Google Scholar] [CrossRef]
- Loladze, I. Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry? Trends in Ecology & Evolution 2002, 17, 457–461. [Google Scholar] [CrossRef]
- Du, C.; Wang, X.; Zhang, M.; Jing, J.; Gao, Y. Effects of elevated CO2 on plant C-N-P stoichiometry in terrestrial ecosystems: A meta-analysis. Sci Total Environ 2019, 650, 697–708. [Google Scholar] [CrossRef]
- Kundu, P.; Goel, K.; Zinta, G. Nutritional imbalance in plants under rising atmospheric CO2. In Plant Nutrition and Food Security in the Era of Climate Change, 2022; pp 513-536.
- Xing, K.; Zhao, M.; Niinemets, U.; Niu, S.; Tian, J.; Jiang, Y.; Chen, H.Y.H.; White, P.J.; Guo, D.; Ma, Z. Relationships Between Leaf Carbon and Macronutrients Across Woody Species and Forest Ecosystems Highlight How Carbon Is Allocated to Leaf Structural Function. Front Plant Sci 2021, 12, 674932. [Google Scholar] [CrossRef]
- Benlloch-Gonzalez, M.; Bochicchio, R.; Berger, J.; Bramley, H.; Palta, J.A. High temperature reduces the positive effect of elevated CO2 on wheat root system growth. Field Crops Research 2014, 165, 71–79. [Google Scholar] [CrossRef]
- Chaudhuri, U.N.; Kirkham, M.B.; Kanemasu, E.T. Root Growth of Winter Wheat under Elevated Carbon Dioxide and Drought. Crop Sci 1990, 30, 853–857. [Google Scholar] [CrossRef]
- Uddin, S.; Low, M.; Parvin, S.; Fitzgerald, G.J.; Tausz-Posch, S.; Armstrong, R.; O'Leary, G.; Tausz, M. Elevated [CO2] mitigates the effect of surface drought by stimulating root growth to access sub-soil water. PLoS One 2018, 13, e0198928. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, J. Controlled alternate partial root-zone irrigation: its physiological consequences and impact on water use efficiency. J Exp Bot 2004, 55, 2437–2446. [Google Scholar] [CrossRef]
- Wang, Y.S.; Liu, F.L.; Andersen, M.N.; Jensen, C.R. Improved plant nitrogen nutrition contributes to higher water use efficiency in tomatoes under alternate partial root-zone irrigation. Functional Plant Biology 2010, 37, 175–182. [Google Scholar] [CrossRef]
- Dodd, I.C. Soil moisture heterogeneity during deficit irrigation alters root-to-shoot signalling of abscisic acid. Funct Plant Biol 2007, 34, 439–448. [Google Scholar] [CrossRef]
- Wang, Y.S.; Liu, F.L.; Jensen, C.R. Comparative effects of partial root-zone irrigation and deficit irrigation on phosphorus uptake in tomato plants. Journal of Horticultural Science & Biotechnology 2012, 87, 600–604. [Google Scholar] [CrossRef]
- Kang, S.Z.; Liang, Z.S.; Hu, W.; Zhang, J.H. Water use efficiency of controlled alternate irrigation on root-divided maize plants. Agricultural Water Management 1998, 38, 69–76. [Google Scholar] [CrossRef]
- Kang, S.Z.; Shi, W.J.; Cao, H.X.; Zhang, J.H. Alternate watering in soil vertical profile improved water use effciency of maize (Zea mays). Field Crops Research 2002, 77, 31–41. [Google Scholar] [CrossRef]
- Liu, F.; Shahnazari, A.; Andersen, M.N.; Jacobsen, S.-E.; Jensen, C.R. Effects of deficit irrigation (DI) and partial root drying (PRD) on gas exchange, biomass partitioning, and water use efficiency in potato. Scientia Horticulturae 2006, 109, 113–117. [Google Scholar] [CrossRef]
- Wang, L.; de Kroon, H.; Bogemann, G.M.; Smits, A.J.M. Partial root drying effects on biomass production in Brassica napus and the significance of root responses. Plant and Soil 2005, 276, 313–326. [Google Scholar] [CrossRef]
- Dodd, I.C. Rhizosphere manipulations to maximize 'crop per drop' during deficit irrigation. J Exp Bot 2009, 60, 2454–2459. [Google Scholar] [CrossRef]
- Birch, H.F. The Effec tOf Soi lDrying On Humus Decomposition and Nitrogen availability. Plant and Soil 1958, 1, 9–31. [Google Scholar] [CrossRef]
- Wang, Y.S.; Jensen, C.R.; Liu, F.L. Nutritional responses to soil drying and rewetting cycles under partial root-zone drying irrigation. Agricultural Water Management 2017, 179, 254–259. [Google Scholar] [CrossRef]
- Wang, Y.S.; Liu, F.L.; Jensen, C.R. Comparative effects of deficit irrigation and alternate partial root-zone irrigation on xylem pH, ABA and ionic concentrations in tomatoes. J Exp Bot 2012, 63, 1907–1917. [Google Scholar] [CrossRef]
- Wang, Y.S.; Liu, F.L.; de Neergaard, A.; Jensen, L.S.; Luxhøi, J.; Jensen, C.R. Alternate partial root-zone irrigation induced dry/wet cycles of soils stimulate N mineralization and improve N nutrition in tomatoes. Plant and Soil 2010, 337, 167–177. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Cui, X.Y.; Liu, F.L. Effect of irrigation regimes and phosphorus rates on water and phosphorus use efficiencies in potato. Scientia Horticulturae 2015, 190, 64–69. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Keller, B.; Hoop, D.; Jud, K.; Boivin, P.; Frossard, E. Increased availability of phosphorus after drying and rewetting of a grassland soil: processes and plant use. Plant and Soil 2013, 370, 511–526. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, M.; Cui, B.; Wei, Z.; Liu, F. Ammonium nitrogen combined with partial root-zone drying enhanced fruit quality of tomato under elevated atmospheric CO2. Scientia Horticulturae 2024, 323. [Google Scholar] [CrossRef]
- Wei, Z.; Du, T.; Li, X.; Fang, L.; Liu, F. Interactive Effects of Elevated CO2 and N Fertilization on Yield and Quality of Tomato Grown Under Reduced Irrigation Regimes. Front Plant Sci 2018, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Bornø, M.L.; Eduah, J.O.; Müller-Stöver, D.S.; Liu, F. Effect of different biochars on phosphorus (P) dynamics in the rhizosphere of Zea mays L. (maize). Plant and Soil 2018, 431, 257–272. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Manevski, K.; Andersen, M.N.; Li, Y.; Wei, Z.; Liu, F. Biochar and alternate wetting-drying cycles improving rhizosphere soil nutrients availability and tobacco growth by altering root growth strategy in Ferralsol and Anthrosol. Sci Total Environ 2021, 806, 150513. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, P.; Wei, Z.; Liu, J.; Hu, X.; Liu, F. Effects of elevated CO2 and nitrogen supply on leaf gas exchange, plant water relations and nutrient uptake of tomato plants exposed to progressive soil drying. Scientia Horticulturae 2022, 292. [Google Scholar] [CrossRef]
- Pazzagli, P.T.; Weiner, J.; Liu, F. Effects of CO2 elevation and irrigation regimes on leaf gas exchange, plant water relations, and water use efficiency of two tomato cultivars. Agricultural Water Management 2016, 169, 26–33. [Google Scholar] [CrossRef]
- Elhani, S.; Haddadi, M.; Csákvári, E.; Zantar, S.; Hamim, A.; Villányi, V.; Douaik, A.; Bánfalvi, Z. Effects of partial root-zone drying and deficit irrigation on yield, irrigation water-use efficiency and some potato (Solanum tuberosum L.) quality traits under glasshouse conditions. Agricultural Water Management 2019, 224. [Google Scholar] [CrossRef]
- Reddy, A.R.; Rasineni, G.K.; Raghavendra, A.S. The impact of global elevated CO2 concentration on photosynthesis and plant productivity. Current Science 2010, 99, 46–57. [Google Scholar]
- Fleisher, D.H.; Timlin, D.J.; Reddy, V.R. Interactive Effects of Carbon Dioxide and Water Stress on Potato Canopy Growth and Development. Agronomy Journal 2008, 100, 711–719. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Aust. J. Plant Physiol 2000, 27, 595–607. [Google Scholar] [CrossRef]
- Kizildeniz, T.; Pascual, I.; Irigoyen, J.J.; Morales, F. Future CO2, warming and water deficit impact white and red Tempranillo grapevine: Photosynthetic acclimation to elevated CO2 and biomass allocation. Physiol Plant 2021, 172, 1779–1794. [Google Scholar] [CrossRef]
- Brouwer, R. Distribution of dry matter in the plant. Netherlands Journal of Agricultural Science 1962, 10, 361–376. [Google Scholar] [CrossRef]
- Benlloch-Gonzalez, M.; Berger, J.; Bramley, H.; Rebetzke, G.; Palta, J.A. The plasticity of the growth and proliferation of wheat root system under elevated CO2. Plant and Soil 2013, 374, 963–976. [Google Scholar] [CrossRef]
- Madhu, M.; Hatfield, J.L. Dynamics of Plant Root Growth under Increased Atmospheric Carbon Dioxide. Agronomy Journal 2013, 105, 657–669. [Google Scholar] [CrossRef]
- Rogers, H.H.; Peterson, C.M.; Mccrimmon, J.N.; CURE, J.D. Response of plant roots to elevated atmospheric carbondioxide. Plant, Celt and Environment 1992, 15, 749–752. [Google Scholar] [CrossRef]
- Mingo, D.M.; Theobald, J.C.; Bacon, M.A.; Davies, W.J.; Dodd, I.C. Biomass allocation in tomato (Lycopersicon esculentum) plants grown under partial rootzone drying: enhancement of root growth. Funct Plant Biol 2004, 31, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xu, F.; Zhu, J.R.; Wang, R.F.; Xu, Z.H.; Shu, L.Z.; Xu, W.W. Nitrogen forms affect root growth, photosynthesis, and yield of tomato under alternate partial root-zone irrigation. Journal of Plant Nutrition and Soil Science 2015, 179, 104–112. [Google Scholar] [CrossRef]
- Wang, H.; Inukai, Y.; Yamauchi, A. Root Development and Nutrient Uptake. Critical Reviews in Plant Sciences 2007, 25, 279–301. [Google Scholar] [CrossRef]
- Ogawa, A.; Kawashima, C.; Yamauchi, A. Sugar Accumulation along the Seminal Root Axis, as Affected by Osmotic Stress in Maize: A Possible Physiological Basis for Plastic Lateral Root Development. Plant Production Science 2015, 8, 173–180. [Google Scholar] [CrossRef]
- Ristova, D.; Busch, W. Natural variation of root traits: from development to nutrient uptake. Plant Physiol 2014, 166, 518–527. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Singla-Pareek, S.L.; Pareek, A.; Singh, A.K. Shaping the root system architecture in plants for adaptation to drought stress. Physiol Plant 2022, 174, e13651. [Google Scholar] [CrossRef] [PubMed]
- Birouste, M.; Zamora-Ledezma, E.; Bossard, C.; Pérez-Ramos, I.M.; Roumet, C. Measurement of fine root tissue density: a comparison of three methods reveals the potential of root dry matter content. Plant and Soil 2013, 374, 299–313. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Wu, H.; Valverde-Barrantes, O.J.; Wang, R.; Zeng, H.; Kardol, P.; Zhang, H.; Feng, Y. Nonlinearity of root trait relationships and the root economics spectrum. Nat Commun 2019, 10, 2203. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, C.M.; Lindahl, B.; Wardle, D.A.; Sundqvist, M.K.; Gundale, M.J.; Fanin, N.; Kardol, P. Root trait-microbial relationships across tundra plant species. New Phytol 2021, 229, 1508–1520. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, C.; Zhang, Y.; Wang, B.; Ran, Q.; Zhang, J. The bHLH family member ZmPTF1 regulates drought tolerance in maize by promoting root development and abscisic acid synthesis. J Exp Bot 2019, 70, 5471–5486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yuan, W.; Wang, Q.; Cao, Y.; Xu, F.; Dodd, I.C.; Xu, W. ABA regulation of root growth during soil drying and recovery can involve auxin response. Plant Cell Environ 2022, 45, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.H.; Topping, J.F.; Liu, J.; Lindsey, K. Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin. New Phytol 2016, 211, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Wasaya, A.; Zhang, X.; Fang, Q.; Yan, Z. Root Phenotyping for Drought Tolerance: A Review. Agronomy 2018, 8. [Google Scholar] [CrossRef]
- Ho, M.D.; Rosas, J.C.; Brown, K.M.; Lynch, J.P. Root architectural tradeoffs for water and phosphorus acquisition. Funct Plant Biol 2005, 32, 737–748. [Google Scholar] [CrossRef]
- Nie, M.; Lu, M.; Bell, J.; Raut, S.; Pendall, E. Altered root traits due to elevated CO2: a meta-analysis. Global Ecology and Biogeography 2013, 22, 1095–1105. [Google Scholar] [CrossRef]
- Pokorný, R.; Tomášková, I.; Marek, M.V. Response of Norway spruce root system to elevated atmospheric CO2 concentration. Acta Physiologiae Plantarum 2013, 35, 1807–1816. [Google Scholar] [CrossRef]
- Wang, N.; Gao, G.; Wang, Y.; Wang, D.; Wang, Z.; Gu, J. Coordinated responses of leaf and absorptive root traits under elevated CO2 concentration in temperate woody and herbaceous species. Environmental and Experimental Botany 2020, 179. [Google Scholar] [CrossRef]
- Li, X.; Jiang, D.; Liu, F. Soil warming enhances the hidden shift of elemental stoichiometry by elevated CO2 in wheat. Scientific Reports 2016, 6. [Google Scholar] [CrossRef]
- Taub, D.R.; Wang, X. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J Integr Plant Biol 2008, 50, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.E.; LeCain, D.R.; McCormack, M.L.; Pendall, E.; Carlson, M.; Blumenthal, D.M.; Lamb, E. Root responses to elevated CO 2, warming and irrigation in a semi-arid grassland: Integrating biomass, length and life span in a 5-year field experiment. Journal of Ecology 2018, 106, 2176–2189. [Google Scholar] [CrossRef]
- Jungk, A. Root hairs and the acquisition of plant nutrients from soil. Journal of Plant Nutrition and Soil Science 2001, 164, 121–129. [Google Scholar] [CrossRef]
- McGrath, J.M.; Lobell, D.B. Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO(2) concentrations. Plant Cell Environ 2013, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Ito, O.; Engelaar, W.M.H.G. Uptake, metabolism and distribution of nitrogen in crop plants traced by enriched and natural 15N: Progress over the last 30 years. Phytochemistry Reviews 2003, 2. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Rubio Asensio, J.S.; Cousins, A.B. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 2010, 328, 899–903. [Google Scholar] [CrossRef]
- Ullah, S.; Zhao, Q.; Wu, K.; Ali, I.; Liang, H.; Iqbal, A.; Wei, S.; Cheng, F.; Ahmad, S.; Jiang, L.; Gillani, S.W.; Amanullah, *!!! REPLACE !!!*; Anwar, S. ; Khan, Z. Biochar application to rice with (15)N-labelled fertilizers, enhanced leaf nitrogen concentration and assimilation by improving morpho-physiological traits and soil quality. Saudi J Biol Sci 2021, 28, 3399–3413. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, C.-S.; Li, J.; Christie, P.; He, X.-H.; Ju, X.-T. Natural 15N Abundance in Winter Wheat Amended with Urea and Compost: A Long-Term Experiment. Pedosphere 2013, 23, 835–843. [Google Scholar] [CrossRef]
- Fratte, M.D.; Pierce, S.; Zanzottera, M.; Cerabolini, B.E.L. The association of leaf sulfur content with the leaf economics spectrum and plant adaptive strategies. Funct Plant Biol 2021, 48, 924–935. [Google Scholar] [CrossRef]
- Gusewell, S.; Koerselman, W. Variation in nitrogen and phosphorus concentrations of wetland plants. Perspectives in Plant Ecology Evolution and Systematics 2002, 5, 37–61. [Google Scholar] [CrossRef]
- Craine, J.M.; Morrow, C.; Stock, W.D. Nutrient concentration ratios and co-limitation in South African grasslands. New Phytol 2008, 179, 829–836. [Google Scholar] [CrossRef]
- Olde Venterink, H.; Wassen, M.J.; Verkroost, A.W.M.; De Ruiter, P.C. Species Richness–Productivity Patterns Differ between N-, P-, and K-Limited Wetlands. Ecology 2003, 84, 2191–2199. [Google Scholar] [CrossRef]
- Sun, Y.; Holm, P.E.; Liu, F. Alternate partial root-zone drying irrigation improves fruit quality in tomatoes. Horticultural Science 2014, 41, 185–191. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, F.; Cui, X.; Liu, F. Plasticity in stomatal size and density of potato leaves under different irrigation and phosphorus regimes. J Plant Physiol 2014, 171, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Rubæk, G.H.; Liu, F.; Andersen, M.N. Effect of partial root zone drying and deficit irrigation on nitrogen and phosphorus uptake in potato. Agricultural Water Management 2015, 159, 66–76. [Google Scholar] [CrossRef]
- Oliveira, E.M.M.; Ruiz, H.A.; Alvarez V, V.H.; Ferreira, P.A.; Costa, F.O.; Almeida, I.C.C. Nutrient supply by mass flow and diffusion to maize plants in response to soil aggregate size and water potential. Revista Brasileira de Ciência do Solo 2010, 34, 317–328. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





