1. Introduction:
Tuberculosis (TB) infection (TBI) is an important reservoir of the TB disease, and it is important to treat individuals with TBI to break the chain of transmission and prevent the further spread of the disease in the community [
1]. It is estimated that over one-fourth of the world's population is infected with
Mycobacterium Tuberculosis, the bacteria responsible for TB disease [
2]. If TB infection remains untreated, 5–10% of individuals with TBI develop active TB in their lifetime, with 50% developing the active disease within two years after infection [
3,
4]. A study by Dye et al. has shown that to meet WHO End TB targets, TBI treatment will need to be incorporated in TB programs, as merely treating the active disease will not result in a significant reduction in the burden given the large number of individuals infected with TB. These individuals with TBI will continue to give rise to TB cases due to reactivation [
5]. The WHO recommends that TPT should be prioritized for contacts of TB patients [
6]. Thus, prevention of new TB infections and their progression to active TB disease is critical to reduce the burden of the disease and resulting deaths, as well as to achieve the United Nations high-level meeting (UNHLM) on TB and End TB Strategy targets for 2022 and 2030/2035.
TB remains a major public health concern in Bangladesh, with an estimated 360,000 people who developed TB and 44,000 died from TB in 2020 in the country [
7]. The country annual report on TB shows that though TB case detection and notification in Bangladesh is increasing steadily, the estimated TB incidence has remained static - between 225/100,000 and 221/100,000 since 2001 [
8]. The current National Tuberculosis Control Program (NTP) in Bangladesh focuses heavily on detecting new TB cases and treating the new patients with a limited focus on TPT. Children younger than five years and people living with HIV are prioritized for isoniazid preventive treatment (IPT) on a daily dose for six months. Although the NTP and partners have been implementing IPT among children <5 years for years, the coverage is only 51% among all eligible children [
7]. Global evidence also suggests that the acceptance and completion rates of IPT are often low (30-64%) because of the long duration of treatment [
9], and implementation of TPT is challenging. The current initiatives are not enough to achieve a significant reduction in TB infection in line with the UNHLM and End TB targets for TPT.
Recent WHO guideline recommends several shorter regimens, which can minimize the burden on both patients and health systems. One of the recommended TPT regimens is Rifapentine and Isoniazid once-weekly dose for three months (3HP). This regimen has comparable adverse events and better treatment completion rates [
10,
11,
12,
13,
14,
15,
16,
17]. However, the experience with this regimen in low-resource programmatic settings is lacking but given once-weekly dosing and higher completion rates as observed in other trials, and it is expected to improve adherence and address the operational challenges associated with IPT. At present, there is no data available in Bangladesh on the eligibility, initiation, and completion of TPT for child contacts and the feasibility of TPT for other HH contacts. Understanding this implementation feasibility can potentially inform the effective development of future TPT programs and thus warrants comprehensive programmatic research. The current study was conducted to assess the uptake and completion of the 3HP regimen and better understand the programmatic challenges with intervention delivery, uptake, and completion of the 3HP regimen for TPT.
2. Materials and Methods:
Ethical approval
The ethical approval for the study was obtained from the Bangladesh Medical Research Council (Registration Number 127 14 06 2018). Written informed consent for participation was obtained by project staff prior to enrollment into the study from adults and from guardians/parents of children. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee. All participants enrolled in this study received free services, including costs for investigation and TPT with 3HP.
Study design and location
A community-based implementation study was conducted under routine programmatic settings in Dhaka, Bangladesh, between February 2018 and March 2019 to assess the uptake and completion of TPT with 3HP. The design of this study and the intervention packages were supported by a prior qualitative study on barriers to and facilitators of existing IPT program in Bangladesh [
18].
Study population
The study population comprised the household (HH) contacts of bacteriologically confirmed (B+) pulmonary drug-susceptible TB (DS-TB) patients (index patients) enrolled for TB treatment at selected 12 NTP-linked treatment facilities during the study period.
Inclusion and exclusion criteria
All HH contacts of index DS-TB patients were considered eligible if i) both the index DS-TB patients and their families living in our selected study sites, and ii) the individual is aged over two years. The HH contacts were excluded from the study only if: i) they were already receiving TB disease treatment or IPT at the time of the HH contact investigation, and ii) women who were pregnant or planning pregnancy during the study period. iii) HH contact under two years of age as rifapentine is not recommended for this age group.
Initiation of TPT with 3HP
The HH contacts who were willing to participate and met the inclusion criteria were considered eligible. The eligible HH contacts took the recommended first dose of the TPT in front of the physician within seven days of the initial evaluation. The 3HP regimen was used among eligible contacts > 2 years old and as a self-administered treatment procedure by the participants with support from the project team and the CHWs [
11,
12].
Treatment support and monitoring of adverse events
A trained CHW (acted as treatment supporter) from a local non-governmental organization (NGO) visited the participant at home bi-weekly to follow up on treatment progress and to assess for any adverse drug reactions. The treatment adherence was assessed through self-reported pill intake by the HH contacts and reconfirmed by pill count by CHWs during follow-up household visits. The treatment completion was defined as completing at least 11 doses of 3HP within 3 months period. The TCs called the HH contacts on TPT every two weeks during the treatment period. In addition, the FSs visited all HHs monthly, quantified adherence, asked about adverse events, and recorded the results. If either CHWs or FSs identified a possible adverse event, they immediately communicated with the physician and referred the participant to the hospital for clinical evaluation if needed. Contacts on 3HP visited the health facility every month for follow-up evaluation by the physician. Enablers (USD 36 per month) to promote treatment adherence were provided to CHWs, and travel and investigation costs were reimbursed to the participating families. The HH contacts also could self-report any adverse events to the CHWs or to the project physician which was recorded on a standardized open-ended adverse event reporting tool. The reported adverse events were immediately assessed, graded, and managed by the physicians. In cases of drop-out from the TPT, the CHWs explored the reason for discontinuation and recorded the reasons.
Data Analysis
All data were analyzed using the Statistical Package for Social Sciences, version 24 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to report the data. The data involving continuous variables (age, schooling, income, etc.) were analyzed using the rank-sum test, and results are presented as the median value plus the minimum and maximum values. Sex, residence, occupation, comorbidity, etc., variables are presented as frequency and percentage. The cumulative probability of a HH contact completing all stages of the TB preventive care cascade was assessed in all eligible participants, and the proportion of HH contacts completing 3HP was assessed among all those who initiated the regimen. Univariate logistic regression analysis was used to analyze the relationships between various factors and subjects' completion of TPT, and results were calculated as an odds ratio (OR) and its 95% confidence interval (95% CI). A multivariable non-conditional logistic regression analysis was then performed on univariate variables that were statistically significant, with the criteria for inclusion being a P-value ≤ 0.050.
3. Results:
Demographic and clinical characteristics
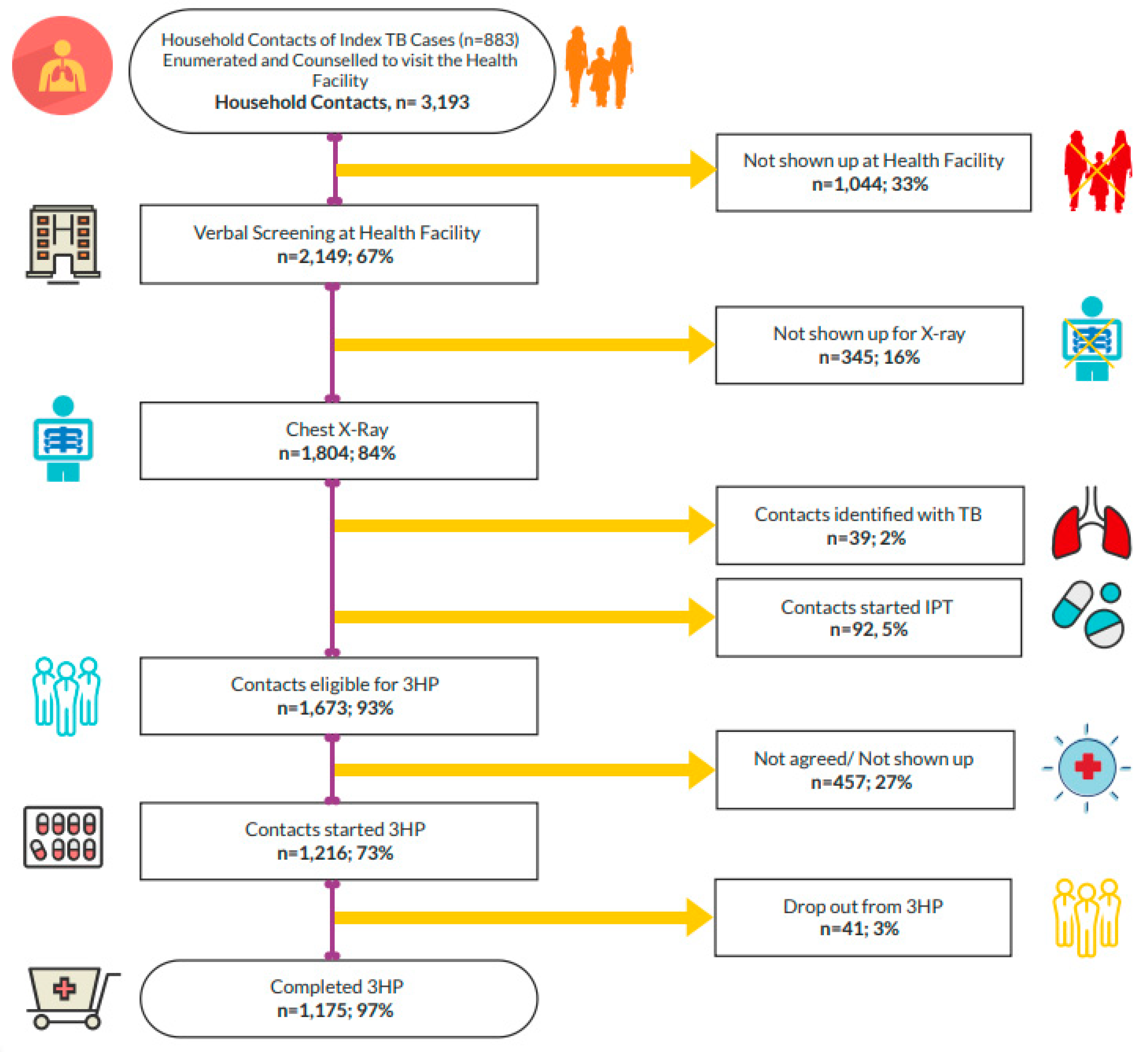
During the study period, 3,193 HH contacts of 883 index TB patients were enumerated and counseled to visit the health facilities for evaluation. Of 3,193 HH contacts, 67% (n = 2,149) showed up at the health facilities and were verbally screened, of whom 54% (n = 1,167) were female, and the mean age of the contacts was 21.2 years (SD ±17.5). Of the 1,216 contacts initiated on TPT, the mean age was 27.4 (SD ±23.8), and 56% (n = 675) were female. Diabetes was the predominant comorbidity among contacts. It was observed that most of the contacts needed two phone calls from the project team to get them to the facilities for evaluation (
Table 1).
Reported adverse events
During the TPT, 5.3% (n = 65) of HH contacts on TPT experienced adverse events. However, no major adverse events were observed, nor were any hospitalizations required. Most of the adverse events were grade 1, and only symptomatic management resolved the presenting issues (
Table 2). Majority of the adverse events were reported in subjects aged 15 years and older (n = 51; 4.2%) and among females (n = 35; 2.9%). Of the three contacts who stopped TPT as per suggestions of the physician, one was hypertensive and had poor adherence to antihypertensive medications, and two female patients had gynecologic symptoms (spotting).
Factors associated with TPT completion with the 3HP regimen
Table 3 presents bivariate and multivariable logistic regression models, examining the association between TPT completion with 3HP and demographic characteristics and clinical factors. The multivariable model reveals that the TPT completion was higher in contacts aged 15 years or more (OR 1.5; 95% CI 1.1-2.0; p 0.043); female contacts (OR 1.7; 95% CI 1.3-2.1; p 0.009); contacts with higher education (OR 1.4; 95% CI 1.1-1.9; p 0.044), contacts with low HH income (OR 1.5; 95% CI 1.0-2.1; p 0.047), contacts those with no comorbidities (OR 1.7; 95% CI 1.1-2.2; p 0.046), and who have not had experienced any adverse events while on 3HP regimen (OR 1.6; 95% CI 1.2-2.1; p 0.009).
The multivariable logistic regression model identified the significant independent predictors of TPT completion with the 3HP regimen. Female sex, higher schooling, higher income, older age, contacts with no comorbidities, and contacts who have not experienced any adverse events while on a 3HP regimen - were all found to be independent predictors for TPT completion with a 3HP regimen.
Intervention approaches
We used multiple intervention approaches for contact investigation, TPT enrolment, and during treatment. They include - phone calls only, phone calls plus counseling, and phone calls plus counseling plus home visit. Among those who attended the health facility (n = 2,149); 88%
(n=1,890) came based on the phone calls made to the index patients by the project staff; 9.1% (n= 196) based on the phone calls plus counseling; and the remaining 2.9% (n= 63) as a result of the combined efforts of the phone calls, counseling, and HH visits. Further, during the treatment, reminder phone calls to inform the next dose schedule, phone-based and/or face-to-face treatment counseling, and follow-up home visit
s by the project staff and NGO CHWs were all found associated with the completion of the 3HP regimen (
Table 3).
4. Discussion:
This is the first population-based study in Bangladesh that has assessed the implementation feasibility of TPT under routine programmatic settings with a 12-dose, weekly 3HP regimen among the HH contacts of DS-TB patients. The use of 3HP for the treatment of LTBI was found feasible and well accepted. Consistent with the findings of other studies, we found that the weekly 3HP regimen has higher treatment completion rates (97.3%) compared to the under-five children in the routine IPT program (74.6%) in Bangladesh with fewer adverse events, and results were similar across subgroups of people without HIV [
19,
20,
21].
Several other studies that included a shorter TPT regimen showed a better completion rate compared to longer regimens [
11,
12,
22]. The treatment completion rates in this study were also higher than in other large randomized controlled studies, phase 4 studies, and other cohort studies done among adults and children in the developed and developing country settings [
11,
12,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32]. A recent prospective cohort study conducted on children and adolescents in Pakistan using a 1HP regimen reported a 94% completion rate [
33].
In Bangladesh, the national TB program provides IPT to children aged under five years who are contacts of B+ TB patients, and there is no provision of TPT for adult contacts. The majority (about 62%) of the HH contacts who were enrolled for the TPT in our study were adults, and treatment completion rates among the adults were also as high as children. Our study generated solid evidence that it is possible to implement TPT among adult populations through the existing routine TB program in Bangladesh. We observed that a higher percentage of our study population (56%) were female, and TPT completion was significantly higher among the female participants. This learning will help in shaping the healthcare-seeking behavior of the female HH contacts.
We noted a low frequency (5.3%) of adverse events with 3HP among the study participants, mostly being of mild severity and were comparable with the previous studies [
22,
24,
34,
35,
36]. A recent systematic review including data from 23 randomized and 55 non-randomized studies also reported a similar low frequency of adverse events with 3HP compared to INH monotherapy [
36]. The low adverse events as observed in our study might also be attributed to the low drop-out and high treatment completion rates among the contacts who initiated the TPT with 3HP.
The project field activities were carried out by the project field staff and NGO CHWs alongside their regular community health interventions. Data indicated that intervention approaches resulted in a high level of TPT enrollment, adherence to treatment, and TPT completion. The possible explanations for this high participation and completion rate may be related to the use of multiple approaches like counseling, phone calls only, phone calls plus counseling, and phone calls plus counseling plus home visits. During the treatment period, we also used reminder phone calls to inform about the next dose schedule, phone-based and face-to-face treatment support and counseling, and follow-up home visit
s – all of these may have cumulative effects on outcomes and helped the participants to make informed decision to adhere to and completion of the TPT. Moreover, CHWs used in this study were involved in the community mobilization, and they were well accepted and trusted by the community, which created an enabling environment for the target community [
37]. The study demonstrated that it is feasible to implement a TPT intervention utilizing ongoing TB program infrastructure and facilities of the Government and NGOs in a resource-limited setting.
Prevention of the active TB disease by TPT is a critical component of the WHO End TB Strategy. This study proved that a convenient and easy to administer TPT regimen could be considered to achieve END TB and UNHLM targets. Considering the high completion rate of TPT, the cascade of care for managing the HH contacts (evaluation and enrollment to TPT) and the interventions to support the TPT can be adopted by the national program. However, the NTP should also consider critical issues with programmatic scale-up of TPT, including policy considerations, ruling out active TB, diagnostic tests and evaluation, time to start treatment, safety, uninterrupted drug supplies, treatment adherence monitoring, and recording and reporting, etc. before going for the countrywide TPT imitative.
One of the limitations of the study was that it only implemented TPT in the urban settings of Dhaka. This may not represent the entire country and limits the generalizability of our results. However, considering urban TB as the most challenging piece of TB control efforts and the presence of extensive community programs (Sasthya Sebika model) in the rural area of Bangladesh [
38], we believe this study would help in formulating appropriately targeted measures and future TPT programs in Bangladesh. The study also did not have a true comparative group for the HH contacts for whom TPT was initiated using 3HP. Further, the study did not use any test to confirm the presence of LTBI. Interferon-gamma Release Assay is not practiced in the country, and the tuberculin skin test (TST) was the test of choice that could be used. However, mass population-wide LTBI testing was not feasible considering the high TB burden setting, and the TST is inconclusive.
5. Conclusions:
In this community-based implementation study, TPT using 3HP was found to have a high completion rate. The convenient weekly regimen of 3HP, shorter treatment duration, and minimal adverse events have resulted in higher treatment adherence among those who were enrolled in this study. The study demonstrated that identification of potential HH contacts for TPT in urban areas and high treatment completion could be achieved through a well-designed, community-based program using the existing program structure and involving appropriately trained CHWs. The awareness creation, counseling, rigorous follow-up, and self-administered TPT option also contributed to achieving a higher TPT adherence.
Author Contributions
Conceptualization, Hamidah Hussain, Md. Toufiq Rahman and Tapash Roy; Methodology, Md. Toufiq Rahman, Rupali Sisir Banu, Md. Shamiul Islam, Abu Jamil Faisel and Hamid Salim ; Data curation, Farzana Hossain and Shamsher Alam; Formal analysis, Tapash Roy; Investigation, Farzana Hossain and Shamsher Alam; Project administration, Md. Toufiq Rahman, Farzana Hossain and Shamsher Alam; Visualization, Md. Toufiq Rahman; Writing – original draft, Md. Toufiq Rahman, Hamidah Hussain and Tapash Roy; Writing – review & editing, Md. Shamiul Islam, Rupali Sisir Banu, Abu Jamil Faisel, Hamid Salim, Oscar Cordon, Pedro Suarez, Farzana Hossain and Shamsher Alam
Funding
The Global Health Bureau, Office of Infectious Disease, United States Agency for International Development (USAID), financially supported this work through Challenge TB (CTB) Project under the terms of Agreement No. AID-OAA-A-14-00029. This work is made possible by the generous support of the American People through USAID. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The contents are the responsibility of the authors, CTB Bangladesh (Management Sciences for Health, Arlington, USA, and IRD Global, Singapore), and do not necessarily reflect the views of USAID or the United States Government.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and the ethical approval was obtained from the Bangladesh Medical Research Council (Registration Number 127 14 06 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are fully available without restriction. However, data cannot be shared publicly because the detailed data is generated under programmatic condition. Data are available from the National TB Control Program (directormbdc@gmail.com) for researchers who meet the criteria for access to confidential data.
Acknowledgments
We are very grateful to the National Tuberculosis Control Program (NTP) Bangladesh for strategic guidance and support in various aspects of this implantation study.
Conflicts of Interest
The authors have declared that no competing interests exist.
References
- Uplekar M, Weil D, Lonnroth K, Jaramillo E, Lienhardt C, Dias HM, Falzon D, Floyd K, Gargioni G, Getahun H: WHO's new end TB strategy. The Lancet 2015, 385(9979):1799-1801.
- Houben RM, Dodd PJ: The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS medicine 2016, 13(10):e1002152.
- World Health Organization: Latent TB infection: updated and consolidated guidelines for programmatic management. Geneva: World Health Organization 2018.
- Styblo K: The relationship between the risk of tuberculous infection and the risk of developing infectious tuberculosis. Bull IUAT 1985, 60(3):117-119.
- Dye C, Glaziou P, Floyd K, Raviglione M: Prospects for tuberculosis elimination. Annual review of public health 2013, 34.
- Getahun H, Matteelli A, Abubakar I, Aziz MA, Baddeley A, Barreira D, Den Boon S, Gutierrez SMB, Bruchfeld J, Burhan E: Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. European Respiratory Journal 2015, 46(6):1563-1576.
- World Health Organization: Global tuberculosis report 2020. Glob Tuberc Rep 2020, 2020.
- National TB Control Program: Tuberculosis Control in Bangladesh : Annual report 2019. Dhaka: Directorate General of Health Services; 2019.
- Stuurman AL, Noordegraaf-Schouten MV, van Kessel F, Oordt-Speets AM, Sandgren A, van der Werf MJ: Interventions for improving adherence to treatment for latent tuberculosis infection: a systematic review. BMC infectious diseases 2016, 16(1):1-17.
- Martinson NA, Barnes GL, Moulton LH, Msandiwa R, Hausler H, Ram M, McIntyre JA, Gray GE, Chaisson RE: New regimens to prevent tuberculosis in adults with HIV infection. New England Journal of Medicine 2011, 365(1):11-20.
- Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, Hackman J, Hamilton CD, Menzies D, Kerrigan A: Three months of rifapentine and isoniazid for latent tuberculosis infection. New England Journal of Medicine 2011, 365(23):2155-2166.
- Villarino ME, Scott NA, Weis SE, Weiner M, Conde MB, Jones B, Nachman S, Oliveira R, Moro RN, Shang N: Treatment for preventing tuberculosis in children and adolescents: a randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and isoniazid. JAMA pediatrics 2015, 169(3):247-255.
- Sharma SK, Sharma A, Kadhiravan T, Tharyan P: Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Evidence-Based Child Health: A Cochrane Review Journal 2014, 9(1):169-294.
- Njie GJ, Morris SB, Woodruff RY, Moro RN, Vernon AA, Borisov AS: Isoniazid-rifapentine for latent tuberculosis infection: a systematic review and meta-analysis. American journal of preventive medicine 2018, 55(2):244-252.
- Zenner D, Beer N, Harris RJ, Lipman MC, Stagg HR, Van Der Werf MJ: Treatment of latent tuberculosis infection: an updated network meta-analysis. Annals of internal medicine 2017, 167(4):248-255.
- Semitala FC, Kadota JL, Musinguzi A, Nabunje J, Welishe F, Nakitende A, Akello L, Bishop O, Patel D, Sammann A: Completion of isoniazid–rifapentine (3HP) for tuberculosis prevention among people living with HIV: Interim analysis of a hybrid type 3 effectiveness–implementation randomized trial. PLoS medicine 2021, 18(12):e1003875.
- Yuen CM, Majidulla A, Jaswal M, Safdar N, Malik AA, Khan AJ, Becerra MC, Keshavjee S, Lu C, Hussain H: Cost of delivering 12-dose isoniazid and rifapentine versus 6 months of isoniazid for tuberculosis infection in a high-burden setting. Clinical Infectious Diseases 2021, 73(5):e1135-e1141.
- Project CT: Contextual Factors Affecting Implementation and Uptake of Preventive Treatment in Urban Settings: A Qualitative Study. In. Dhaka, Bangladesh: IRD, MSH, KNCV; June 2018.
- Hamada Y, Ford N, Schenkel K, Getahun H: Three-month weekly rifapentine plus isoniazid for tuberculosis preventive treatment: a systematic review. The International Journal of Tuberculosis and Lung Disease 2018, 22(12):1422-1428.
- Harries AD, Kumar AM, Satyanarayana S, Takarinda KC, Timire C, Dlodlo RA: Treatment for latent tuberculosis infection in low-and middle-income countries: progress and challenges with implementation and scale-up. Expert review of respiratory medicine 2020, 14(2):195-208.
- Pease C, Hutton B, Yazdi F, Wolfe D, Hamel C, Quach P, Skidmore B, Moher D, Alvarez GG: Efficacy and completion rates of rifapentine and isoniazid (3HP) compared to other treatment regimens for latent tuberculosis infection: a systematic review with network meta-analyses. BMC infectious diseases 2017, 17(1):1-11.
- Sandul AL, Nwana N, Holcombe JM, Lobato MN, Marks S, Webb R, Wang S-H, Stewart B, Griffin P, Hunt G: High rate of treatment completion in program settings with 12-dose weekly isoniazid and rifapentine for latent Mycobacterium tuberculosis infection. Clinical Infectious Diseases 2017, 65(7):1085-1093.
- Belknap R, Holland D, Feng P, Millet J, Caylà J, Martinson N, Wright A, Chen M, Moro R, Scott N: TB Trials Consortium iAdhere Study Team Self-administered versus directly observed once-weekly isoniazid and rifapentine treatment of latent tuberculosis infection: a randomized trial. Ann Intern Med 2017, 167(10):689-697.
- Haas MK, Aiona K, Erlandson KM, Belknap RW: Higher Completion Rates with Self-administered Once-weekly Isoniazid-Rifapentine versus Daily Rifampin in Adults with Latent Tuberculosis. Clinical Infectious Diseases 2021, 73(9):e3459-e3467.
- Cruz AT, Starke JR: Completion rate and safety of tuberculosis infection treatment with shorter regimens. Pediatrics 2018, 141(2).
- Yang H, Yang Y, Hu Z-d, Xia L, Liu X-h, Yu X, Ma J-y, Li T, Lu S-h: High rate of completion for weekly rifapentine plus isoniazid treatment in Chinese children with latent tuberculosis infection—A single center study. Plos one 2021, 16(6):e0253159.
- Surey J, Stagg HR, Yates TA, Lipman M, White PJ, Charlett A, Muñoz L, Gosce L, Rangaka MX, Francis M: An open label, randomised controlled trial of rifapentine versus rifampicin based short course regimens for the treatment of latent tuberculosis in England: the HALT LTBI pilot study. BMC infectious diseases 2021, 21(1):1-8.
- Stennis NL, Burzynski JN, Herbert C, Nilsen D, Macaraig M: Treatment for tuberculosis infection with 3 months of isoniazid and rifapentine in New York City health department clinics. Clinical Infectious Diseases 2016, 62(1):53-59.
- Huang H-L, Lee M-R, Cheng M-H, Lu P-L, Huang C-K, Sheu C-C, Lai P-C, Chen T-C, Wang J-Y, Chong I-W: Impact of age on outcome of rifapentine-based weekly therapy for latent tuberculosis infection. Clinical Infectious Diseases 2021, 73(5):e1064-e1071.
- Walker RE, Bass S, Srinivas P, Miranda C, Johnson L, Pallotta AM: Evaluation of 3 months of once-weekly rifapentine and isoniazid for latent tuberculosis infection. Annals of Pharmacotherapy 2020, 54(5):457-463.
- Murphy AM, Thomas A, Crinion SJ, Kent BD, Tambuwala MM, Fabre A, Pepin J-L, Roche HM, Arnaud C, Ryan S: Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. European Respiratory Journal 2017, 49(4).
- Sun H-Y, Huang Y-W, Huang W-C, Chang L-Y, Chan P-C, Chuang Y-C, Ruan S-Y, Wang J-Y, Wang J-T: Twelve-dose weekly rifapentine plus isoniazid for latent tuberculosis infection: a multicentre randomised controlled trial in Taiwan. Tuberculosis 2018, 111:121-126.
- Malik AA, Farooq S, Jaswal M, Khan H, Nasir K, Fareed U, Shahbaz S, Amanullah F, Safdar N, Khan AJ: Safety and feasibility of 1 month of daily rifapentine plus isoniazid to prevent tuberculosis in children and adolescents: a prospective cohort study. The Lancet Child & Adolescent Health 2021, 5(5):350-356.
- Schmit KM, Wortham JM, Ho CS, Powell KM: Analysis of severe adverse events reported among patients receiving isoniazid-rifapentine treatment for latent Mycobacterium tuberculosis infection—United States, 2012–2016. Clinical Infectious Diseases 2020, 71(9):2502-2505.
- Yu Y-Y, Tsao S-M, Yang W-T, Huang W-C, Lin C-H, Chen W-W, Yang S-F, Chiou H-L, Huang Y-W: Association of drug metabolic enzyme genetic polymorphisms and adverse drug reactions in patients receiving rifapentine and isoniazid therapy for latent tuberculosis. International journal of environmental research and public health 2020, 17(1):210.
- Pease C, Hutton B, Yazdi F, Wolfe D, Hamel C, Barbeau P, Skidmore B, Alvarez GG: A systematic review of adverse events of rifapentine and isoniazid compared to other treatments for latent tuberculosis infection. Pharmacoepidemiology and drug safety 2018, 27(6):557-566.
- Terpstra J, Coleman KJ, Simon G, Nebeker C: The role of community health workers (CHWs) in health promotion research: ethical challenges and practical solutions. Health promotion practice 2011, 12(1):86-93.
- Mistry SK, Harris-Roxas B, Yadav UN, Shabnam S, Rawal LB, Harris MF: Community health workers can provide psychosocial support to the people during COVID-19 and beyond in low-and middle-income countries. Frontiers in Public Health 2021:800.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).