Submitted:
02 November 2023
Posted:
03 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animal Preparation
2.2. Blood Sample Collection
2.3. Whole Blood Viscosity, Hematological, and Serum Chemical Analysis
2.4. Statistical analysis
3. Results
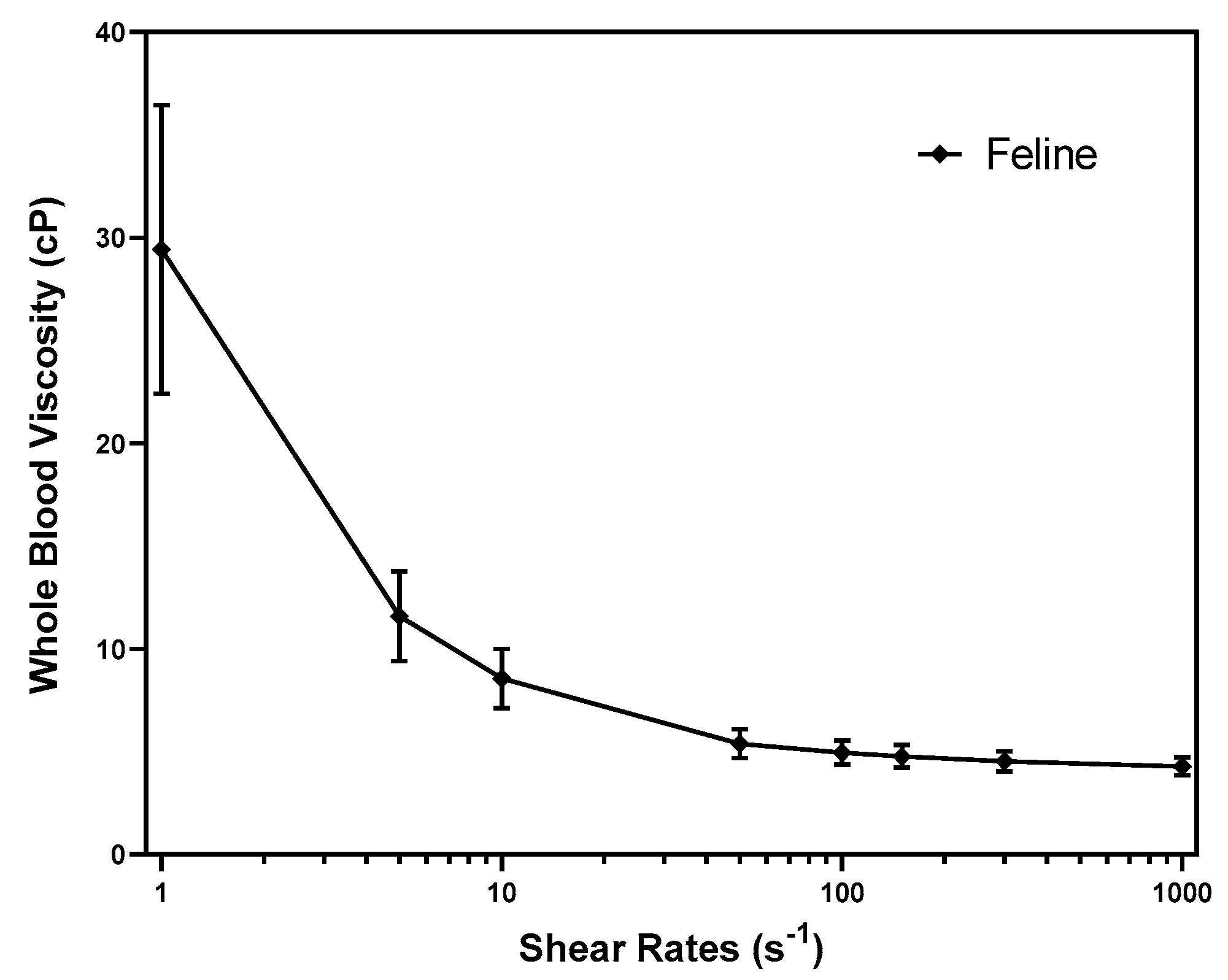
3.1. Reference intervals of WBV
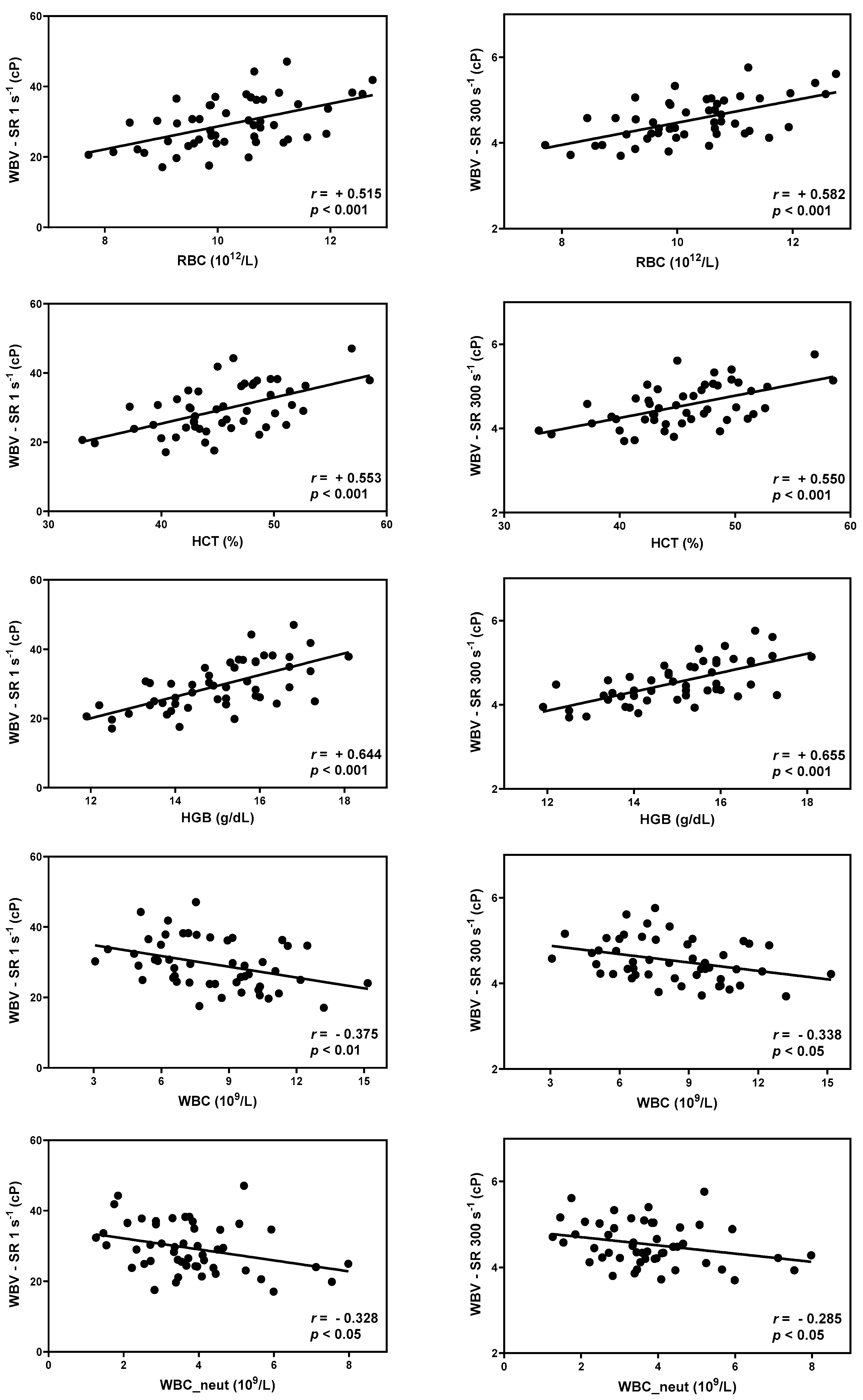
3.2. Correlation between WBV and hematology
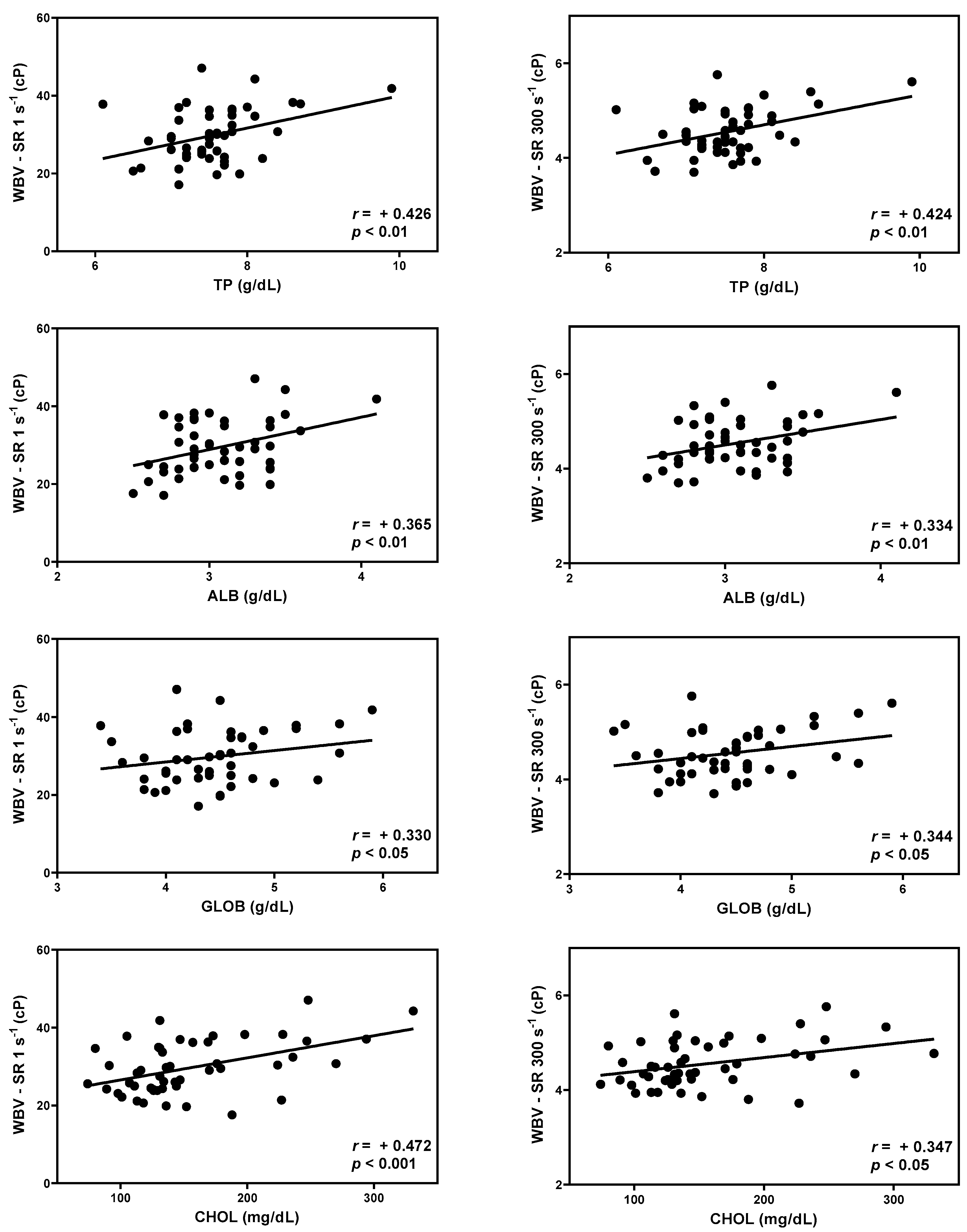
3.3. Correlation between WBV and serum chemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Copley, A.L. Fluid mechanics and biorheology. Clin Hemorheol Micro 1990, 10, 3–19. [Google Scholar] [CrossRef]
- Cho, Y.-I.; Cho, D.J. Hemorheology and microvascular disorders. Korean Circ J 2011, 41, 287–295. [Google Scholar] [CrossRef]
- Reinhart, W.H.; Gaudenz, R.; Walter, R. Acidosis induced by lactate, pyruvate, or HCl increases blood viscosity. J Crit Care 2002, 17, 68–73. [Google Scholar] [CrossRef]
- Kim, S.; Cho, Y.I.; Jeon, A.H.; Hogenauer, B.; Kensey, K.R. A new method for blood viscosity measurement. J Non-newton Fluid 2000, 94, 47–56. [Google Scholar] [CrossRef]
- Kim, H.; Cho, Y.I.; Lee, D.-H.; Park, C.-M.; Moon, H.-W.; Hur, M.; Kim, J.Q.; Yun, Y.-M. Analytical performance evaluation of the scanning capillary tube viscometer for measurement of whole blood viscosity. Clin Biochem 2013, 46, 139–142. [Google Scholar] [CrossRef]
- Lowe, G.; Lee, A.; Rumley, A.; Price, J.; Fowkes, F. Blood viscosity and risk of cardiovascular events: the Edinburgh Artery Study. Brit J Haematol 1997, 96, 168–173. [Google Scholar] [CrossRef]
- Galea, G.; Davidson, R. Haematological and haemorheological changes associated with cigarette smoking. J Clin Pathol 1985, 38, 978–984. [Google Scholar] [CrossRef]
- Gertz, M.A.; Kyle, R.A. Hyperviscosity syndrome. J Intensive Care Med 1995, 10, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Ciuffetti, G.; Schillaci, G.; Lombardini, R.; Pirro, M.; Vaudo, G.; Mannarino, E. Prognostic impact of low-shear whole blood viscosity in hypertensive men. Eur J Clin Invest 2005, 35, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Windberger, U.; Bartholovitsch, A.; Plasenzotti, R.; Korak, K.; Heinze, G. Whole blood viscosity, plasma viscosity and erythrocyte aggregation in nine mammalian species: reference values and comparison of data. Exp Physiol 2003, 88, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Lee, D.; Lee, D.H.; Kim, N.S. Reference Values of Whole Blood Viscosity and Its Correlation with Hematology and Serum Chemistry in Beagle Dogs. J Vet Clin 2018, 35, 77–82. [Google Scholar] [CrossRef]
- Friedrichs, K.R.; Harr, K.E.; Freeman, K.P.; Szladovits, B.; Walton, R.M.; Barnhart, K.F.; Blanco-Chavez, J. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 2012, 41, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y. Biostatistics 104: correlational analysis. Singapore Med J 2003, 44, 614–619. [Google Scholar] [PubMed]
- Akoglu, H. User's guide to correlation coefficients. Turk J Emerg Med 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Lee, D.; Kim, K.; Choi, M.; Cho, Y.; Lee, H.; Choi, S.; Lee, S.; Kim, D. Reference intervals for whole blood viscosity using the analytical performance-evaluated scanning capillary tube viscometer. Clin Biochem 2014, 47, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Mccormick, A.; Uretz, E.F. Distribution of blood viscosity values and biochemical correlates in healthy adults. Clin Chem 1996, 42, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-J.; Lee, J.H.; Moon, J.; Kim, H.-R.; Choi, H.-J.; Kim, S.-H.; Won, Y.; Shin, M.-G. Analytical performance and reference interval of a microfluidic viscometer, Viscore-300 for the measurement of whole blood viscosity. Lab Med 8, 1-6. [CrossRef]
- Kampmann, J.D.J. Whole-blood viscosity, hematocrit and plasma protein in normal subjects at different ages. Acta Physiol Scand 1971, 81, 264–268. [Google Scholar] [CrossRef]
- Kameneva, M.; Watach, M.; Borovetz, H. Gender difference in rheologic properties of blood and risk of cardiovascular diseases. Clin Hemorheol Micro 1999, 21, 357–363. [Google Scholar]
- Tvedten, H.W. Hematology of the normal dog and cat. Vet Clin North Am Small Anim Pract 1981, 11, 209–217. [Google Scholar] [CrossRef]
- Nemeth, N.; Kiss, F.; Furka, I.; Miko, I. Gender differences of blood rheological parameters in laboratory animals. Clin Hemorheol Micro 2010, 45, 263–272. [Google Scholar] [CrossRef]
- Chien, S.; Sung, L.A. Physicochemical basis and clinical implications of red cell aggregation. Clin Hemorheol Micro 1987, 7, 71–91. [Google Scholar] [CrossRef]
- Chien, S. Red cell deformability and its relevance to blood flow. Annu Rev Physiol 1987, 49, 177–192. [Google Scholar] [CrossRef]
- Eppihimer, M.; Lipowsky, H. Effects of leukocyte-capillary plugging on the resistance to flow in the microvasculature of cremaster muscle for normal and activated leukocytes. Microvasc Res 1996, 51, 187–201. [Google Scholar] [CrossRef]
- Ho, C.-H. White blood cell and platelet counts could affect whole blood viscosity. J Chin Med Assoc 2004, 67, 394–397. [Google Scholar]
- Kwaan, H.C. Role of plasma proteins in whole blood viscosity: a brief clinical review. Clin Hemorheol Micro 2010, 44, 167–176. [Google Scholar] [CrossRef]
- Patel, R.T.; Caceres, A.; French, A.F.; McManus, P.M. Multiple myeloma in 16 cats: a retrospective study. Vet Clin Pathol 2005, 34, 341–352. [Google Scholar] [CrossRef]
- Sloop, G.D.; Garber, D.W. The effects of low-density lipoprotein and high-density lipoprotein on blood viscosity correlate with their association with risk of atherosclerosis in humans. Clin Sci 1997, 92, 473–479. [Google Scholar] [CrossRef]
- Watson, T.; Barrie, J. Lipoprotein metabolism and hyperlipidaemia in the dog and cat: A review. J Small Anim Pract 1993, 34, 479–487. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Herbert, T.B.; Patterson, S.M.; Kameneva, M.; Raible, R.; Manuck, S.B. Effects of acute psychological stress on serum lipid levels, hemoconcentration, and blood viscosity. Arch Intern Med 1995, 155, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Rand, J.S.; Kinnaird, E.; Baglioni, A.; Blackshaw, J.; Priest, J. Acute stress hyperglycemia in cats is associated with struggling and increased concentrations of lactate and norepinephrine. J Vet Intern Med 2002, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.I.; Mooney, M.P.; Cho, D.J. Hemorheological disorders in diabetes mellitus. J diabetes Sci Technol 2008, 2, 1130–1138. [Google Scholar] [CrossRef]
| Shear rate | Descriptive Statistics | RI within 90% CI | ||||
|---|---|---|---|---|---|---|
| Mean | SD | RI | Lower Limit | Upper Limit | ||
| Whole Blood Viscosity (cP) | SR 1 s-1 | 29.427 | 7.025 | 15.169 - 43.684 | 12.574 - 17.883 | 40.826 - 46.423 |
| SR 5 s-1 | 11.591 | 2.196 | 7.133 - 16.048 | 6.223 - 8.129 | 15.092 - 17.061 | |
| SR 10 s-1 | 8.565 | 1.446 | 5.630 - 11.501 | 5.004 - 6.258 | 10.910 - 12.117 | |
| SR 50 s-1 | 5.393 | 0.707 | 3.957 - 6.829 | 3.675 - 4.264 | 6.541 - 7.106 | |
| SR 100 s-1 | 4.955 | 0.595 | 3.747 - 6.163 | 3.499 - 4.003 | 5.901 - 6.406 | |
| SR 150 s-1 | 4.770 | 0.551 | 3.653 - 5.888 | 3.434 - 3.877 | 5.658 - 6.108 | |
| SR 300 s-1 | 4.534 | 0.498 | 3.524 - 5.544 | 3.302 - 3.725 | 5.328 - 5.729 | |
| SR 1000 s-1 | 4.285 | 0.448 | 3.375 - 5.195 | 3.198 - 3.574 | 5.013 - 5.380 | |
| Shear rates | RBC | HCT | HGB | WBC | WBC_Neut | |
|---|---|---|---|---|---|---|
| Whole Blood Viscosity (cP) | SR 1 s-1 | 0.515*** | 0.553*** | 0.644*** | -0.375** | -0.328* |
| SR 5 s-1 | 0.521*** | 0.554*** | 0.640*** | -0.353* | -0.316* | |
| SR 10 s-1 | 0.520*** | 0.549*** | 0.632*** | -0.337* | -0.306* | |
| SR 50 s-1 | 0.543*** | 0.538*** | 0.621*** | -0.329* | -0.292* | |
| SR 100 s-1 | 0.565*** | 0.547*** | 0.640*** | -0.337* | -0.296* | |
| SR 150 s-1 | 0.575*** | 0.550*** | 0.649*** | -0.337* | -0.292* | |
| SR 300 s-1 | 0.582*** | 0.550*** | 0.655*** | -0.338* | -0.285* | |
| SR 1000 s-1 | 0.587*** | 0.543*** | 0.658*** | -0.334* | -0.275* |
| Shear rates | TP | ALB | GLOB | CHOL | |
|---|---|---|---|---|---|
| Whole Blood Viscosity (cP) | SR 1 s-1 | 0.426** | 0.365** | 0.330* | 0.472*** |
| SR 5 s-1 | 0.439** | 0.358* | 0.350* | 0.461*** | |
| SR 10 s-1 | 0.444** | 0.352* | 0.359* | 0.453** | |
| SR 50 s-1 | 0.453** | 0.349* | 0.371** | 0.417** | |
| SR 100 s-1 | 0.442** | 0.344* | 0.360* | 0.390** | |
| SR 150 s-1 | 0.437** | 0.342* | 0.356* | 0.374** | |
| SR 300 s-1 | 0.424** | 0.334* | 0.344* | 0.347* | |
| SR 1000 s-1 | 0.403** | 0.319* | 0.326* | 0.311* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





