1. Introduction
Heart failure (HF) stands as a significant contributor to hospital admissions in individuals aged 65 and above. Its prevalence in developed countries is approximately 1-2%, a figure that escalates to 10% when focusing on individuals over 70 years of age [
1,
2]. Patients admitted with HF have a 20% to 30% risk of death within a year [
3]. While it is common for patients with HF to experience a quick recovery and be discharged within 4 to 5 days of hospitalization, any instances of worsening during their stay can indicate a significant change in the overall trajectory of their condition. The American College of Cardiology advocates for a timely recognition of clinical trajectories in acute heart failure. It is possible to define three main in-hospital trajectories: improving towards target, stalled after initial improvement, or not improved/worsening [
3]. These trajectories translate into different management strategies throughout the hospitalization and post-discharge, by optimizing guideline-based treatment, proceeding with further diagnostic investigation, or increasing diuretic therapy. Ensuring the precision with which the correct trajectory is intercepted is of pivotal importance, especially in the case of elderly patients who carry an elevated risk of experiencing disease recurrence or short-term hospitalization attributed to HF [
4]. However, due to their atypical clinical presentations and their complexity, caused by coexisting comorbidities and frailty, trajectory checks can be challenging in the oldest old affected by acute decompensated heart failure (ADHF).
Point of Care Ultrasound (POCUS) involves non-radiologist healthcare providers performing targeted ultrasound at the patient's bedside to facilitate rapid diagnosis [
5]. POCUS has proved to be a helpful non-invasive tool used for the detection and quantification of pulmonary congestion in both ambulatory and hospitalized patients [
6,
7]. Moreover, several studies suggest that Lung Ultrasound (LUS) outperforms chest radiography in detecting pulmonary edema and it is comparable to chest Computed Tomography in identifying lung pathology in patients with acute respiratory failure [
8,
9,
10]. Nevertheless, only a limited number of studies have endeavored to assess the effectiveness of POCUS in identifying early determinants of in-hospital worsening of ADHF [
11]. Based on these foundations, the primary objective of this study was to examine the potential of POCUS as a predictive tool for the trajectory of ADHF. Specifically, the study aimed to identify the ultrasound early determinants of in-hospital clinical deterioration.
2. Materials and Methods
We consecutively enrolled patients aged 75 years or older hospitalized with ADHF in the Geriatrics Unit of a tertiary care hospital. Exclusion criteria were: (a) patients unable to provide their written or oral consent (b) patients with invasive ventilation at hospital admission. A panel of clinicians adjudicated the diagnosis of congestive heart failure based on clinical symptoms, signs, chest X-ray film results, echocardiographic findings, and therapy at admission in line with recent international guidelines [
1]. All the patients underwent physical examination, complete blood tests, and a comprehensive geriatric assessment (CGA) [
12] including cognitive evaluation using the Short Portable Mental Status Questionnaire (SPMSQ) [
13], level of autonomy in terms of independence in the performance of basic (ADL) [
14], and instrumental (IADL) [
15], activities of daily living. Frailty assessment was evaluated through the Clinical Frailty Scale (CFS) [
16]. The risk of malnutrition was assessed through the Mini Nutritional Assessment-Short Form (MNA-SF) [
17] and Body Mass Index (BMI).
Furthermore, patients underwent a diagnostic examination early during hospitalization (within the first 48 hours) with bedside Point of Care Ultrasound (POCUS) including lung ultrasound, focused cardiac ultrasound, pleural effusion score (PEFs) [
18], and Inferior Vena Cava (IVC) assessment. A convex and a linear covered probe, 3.5 to 7.5 MHz), were used for chest ultrasound examination, a phased array probe with bandwidth of 1.7–3.8 MHz was utilized for focused ultrasound (Esaote Medical System). For patients with severe mobility limitations, two operators were concomitantly involved, according to current guidelines [
19]. 8-zone LUS was performed (anterosuperior, anteroinferior, lateral superior, lateral inferior); the evaluation involved assessing the presence of B-lines, and a score ranging from 0 to 5 was assigned per field based on the quantity observed (max 20 per hemithorax). PEFs was assessed by positioning the probe longitudinally (perpendicular to the ribs) in an intercostal space in the posterior axillary line with the patient in a semi-recumbent position so that the liver or spleen, diaphragm, and lung as well as the spine were visualized in one image. PEFs value ranging from 0 to 4 points for each hemithorax and to estimate the cumulative fluid burden the score of both hemi-thoraces were summed for an overall range of 0-8. Left ventricular function was estimated via Mitral Valve E-Point Septal Separation (EPSS) and by Simpson’s method. We also evaluated the morphometric features of the IVC and its diameter. The diameter was measured during inspiration and expiration to assess collapsibility.
Clinical aggravation was defined as a composite endpoint of:
- I)
at least a 10% reduction in P/F ratio
- II)
an increase on dosage of diuretic therapy,
- III)
onset of pulmonary edema during hospitalization, and
- IV)
in-hospital death. The 30 post-discharge mortality rate was assessed by phone interview.
2.1. Statistical analysis
Statistical analysis was performed with IBM SPSS Statistic (IBM SPSS Statistic version 27.0 lnk IBM Corporation and its licensor 1989-2020) and RStudio (RStudio Team: Integrated Development for R. RStudio, PBC, Boston, MA). Continuous variables are presented as median (interquartile range, IQR) or mean (standard deviation, SD) and categorical variables as counts and percentages. Mann-Whitney and chi-square tests were used for multiple comparisons. A multivariable logistic regression was performed to evaluate the association between statistically significant variables of the univariate model and in-hospital worsening using a priori selected model covariates on the basis of clinical considerations. Covariates included were age, sex, main comorbidities, BMI and LVEF. Estimate odds ratios (ORs) with 95% confidence intervals (CIs) were obtained. As a secondary analysis, we divided patients into three groups on the basis of the sum of baseline B-lines in tertiles in 8 zones: Tertile 1: <7 B-lines, Tertile 2: 7–15 B-lines, Tertile 3: ≥15 B-lines. In a subgroup of 30 patients in whom LUS was performed blindly by the two certified operators (C.O., T.M.), interobserver agreement was calculated.
3. Results
Out of 184 patients hospitalized with ADHF enrolled in the study (mean [SD], 86.8 [5.9] years), 60 (32.6%) experienced HF in-hospital worsening. No differences were found between patients with HF worsening and controls in terms of sex, mean age, body weight, left ventricular ejection fraction, and the number of comorbidities. Regarding comorbidities, patients with in-hospital worsening had a higher prevalence of atrial fibrillation (AF) [78.6% vs 56.9%, p=0.006] and coronary artery disease (CAD) [48.2% vs 29.3%, p=0.001], compared to counterparts.
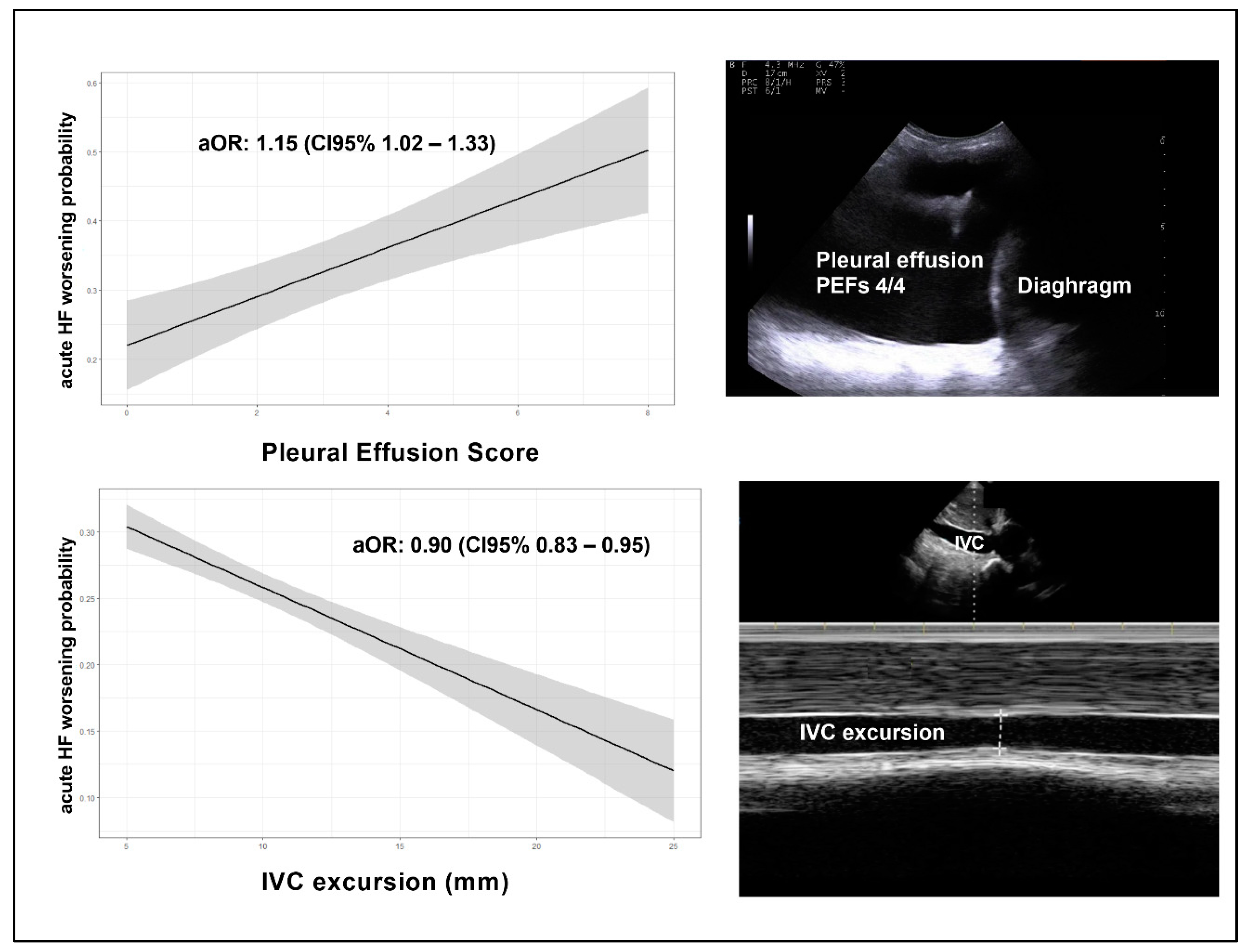
Patients with HF worsening had no differences in terms of frailty detected by CFS [median CFS: 5.5 (IQR=3) vs 6 (IQR=3) respectively, p = 0.55]; moreover, patients with clinical worsening, showed a higher degree of congestion in terms of pleural effusion estimated by PEFs [median cumulative PEFs: 4 (IQR=5) vs 2 (IQR=4) respectively, p=0.005] and reduction of Inferior Vena Cava collapsibility [delta IVC mean: 6 (SD= 4.9) vs 8.9 (IQR= 5.2), respectively, p=0.002] than counterparts; no differences were detected in terms of B-lines number. Moreover, no significant differences in terms of outcomes were found across B-lines tertiles (p=0.59). Interobserver agreement for POCUS, calculated in a subsample of patients was high (k=0. 86).
As shown in
Table 2, by multivariate logistic analysis, total PEFs [adjusted OR: 1.15 (CI95% 1.02– 1.33), p = 0.043], and IVC collapsibility [adjusted OR: 0.90 (CI95% 0.83 – 0.95), p = 0.039] resulted independently associated with HF in-hospital worsening after extensive adjustment for age, sex, main comorbidities, BMI and left ventricular ejection fraction (
Figure 1).
4. Discussion
In the present study, a higher degree of pleural effusion and a reduced IVC collapsibility evaluated by POCUS proved to be strong independent predictors of HF in-hospital worsening in older patients with acutely decompensated heart failure. Moreover, the results suggest that POCUS may provide clinicians with a non-invasive tool for accurately predicting ADHF and deterioration in older patients.
Indeed, previous studies showed how the integration of POCUS with clinical assessment for the diagnosis of ADHF in the emergency department appears to be more accurate than standard diagnostic approaches based on CXR and NT-proBNP [
7,
20]. Furthermore, in patients admitted for ADHF, the ongoing presence of congestion, as identified by LUS before discharge, has been proved to serve as a crucial predictor for both HF-related rehospitalizations and mortality [
11,
21,
22]. In the present investigation, we further demonstrated of the utility of POCUS in the trajectory check of acute HF. This study emphasizes the significance of routinely monitoring and evaluating the pleural effusion and the IVC collapsibility in older patients with acute heart failure. This practice can assist in delivering more efficient care, leading to improved treatment and ultimately better patient outcomes.
Furthermore, POCUS could aid clinicians in tailoring HF diuretic therapy during hospitalization, improving discharge timing. The concept of trajectory check stresses the importance of stepping back to gain perspective of where the patient stands and in which direction the patient is headed. In our study, the odds of adverse outcomes increased by 15% for every 1-unit increase in the total PEFs and reduced by 10% for every 1-unit increase in IVC collapsibility. Even if its role in the diagnosis of acute heart failure is well recognized, the prognostic relevance of pleural effusion has not been investigated systematically. Considering the scarce data available on the mortality risk and associated prognostic factors related to pleural effusion, several studies have successfully linked its presence to an increased risk of mortality [
23,
24,
25]. Moreover, patients with bilateral pleural effusions demonstrated a notably higher risk of mortality when compared to those with unilateral effusions. Numerous studies have demonstrated that thoracic ultrasound outperforms chest radiography in terms of sensitivity and specificity when detecting pleural effusions [
26,
27,
28], and our findings strongly support its potential utility as an indicator of in-hospital deterioration. On the other hand, as it is well-established, the IVC dilates with an increase in right atrial pressure, making it a potential indicator of HF severity that is not dependent on left ventricular ejection fraction (LVEF) [
22]. Previous research has demonstrated that POCUS assessment can identify individuals with chronic heart failure at risk of unfavorable outcomes by detecting IVC diameter enlargement, irrespective of their LVEF status [
21].
The effectiveness of POCUS in addressing acute dyspnea and HF management is widely recognized [
6], yet the predictive significance of early POCUS indicators for in-hospital deterioration in patients with ADHF has not been systematically explored. Previous studies have primarily employed time-consuming 28-zone imaging protocols [
29,
30]. However, Platz et al. recently demonstrated that a 4-zone LUS evaluation can effectively detect pulmonary congestion and that a higher count of B-lines during early admission can identify patients at an elevated risk of in-hospital events [
11], encouraging a simplified approach.
Interestingly, B-lines did not emerge as significant predictors of in-hospital deterioration among the geriatric population with ADHF. This outcome contradicts numerous studies that have shown the quantification of B-lines to be a valuable predictor of HF recurrence and mortality in hospitalized HF patients [
11,
30,
31]. This discrepancy may be attributed to the limited specificity of B-lines themselves. While the detection of a B-pattern on LUS exhibits high sensitivity for diagnosing pulmonary edema, its specificity is notably low. This lack of specificity is particularly evident in the oldest old, where underlying lung diseases could potentially influence LUS findings. Multiple B-lines typically indicate an interstitial syndrome, encompassing conditions such as interstitial lung diseases (e.g., fibrosis), pulmonary manifestations of connective tissue diseases, and pneumonia [
32,
33,
34]. Pneumonia, for instance, is frequently observed in elderly patients admitted for acute HF [
35,
36] and can confound the interpretation of B-lines on LUS due to the combination of cardiogenic and inflammatory edema. Furthermore, occasional B-lines (up to two) can be present in normal lungs, typically at the lung bases. Consequently, patients may exhibit clinical improvement even if they continue to display B-lines during LUS evaluations, irrespective of their ADHF trajectory. No differences were found between patients with in-hospital worsening and controls in terms of left ventricular ejection fraction. In the present study, we observed that patients affected by atrial fibrillation (AF) or coronary artery disease (CAD) presented an increased risk of in-hospital worsening.
However, at least two limitations of the study need to be acknowledged. Firstly, as a single-center investigation, further multicenter studies are warranted to validate the prognostic significance of PEFs and reduced IVC collapsibility evaluated by POCUS as strong independent predictors of acute HF in-hospital worsening; however, the clinical uniformity of the cohort and the clear protocol represent strengths of our investigation. Secondly, POCUS is an operator-dependent methodic, with its limitations.
In conclusion, this study represents the initial documentation of both total pleural effusions and IVC collapsibility as significant indicators for predicting in-hospital deterioration among elderly patients admitted for ADHF. Recognizing predictors of in-hospital deterioration in geriatric patients with ADHF is crucial for clinicians as it can lead to reduced hospitalization durations and better outcomes. Recent research has shown that extended hospital stays following HF hospitalization are linked to higher rates of various types of readmissions [
4]. POCUS can aid medical professionals in enhancing risk assessment and personalizing diuretic treatment for hospitalized heart failure patients, ultimately optimizing the timing of patient discharge.
5. Conclusions
POCUS has the potential to serve a significant role in monitoring pulmonary congestion throughout hospitalizations for ADHF and enhance risk assessment by identifying early indicators of in-hospital deterioration. A poor IVC collapsibility and a higher amount of pleural effusion are markers of worsening acute heart failure during hospitalization.
Author Contributions
Conceptualization, Tessa Mazzarone and Chukwuma Okoye; Data curation, Virginia Morelli, Andrea Giusti, Maria Bianco, Lorenzo Maccioni and Cristina Cargiolli; Formal analysis, Tessa Mazzarone and Chukwuma Okoye; Investigation, Virginia Morelli and Andrea Giusti; Supervision, Agostino Virdis and Chukwuma Okoye; Writing – original draft, Tessa Mazzarone, Virginia Morelli and Chukwuma Okoye; Writing – review & editing, Daniela Guarino and Agostino Virdis.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by local Ethics Committee (Tuscany Regional Ethics Committee for the Clinical Experimentation: FUN-sc 23956).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank all the Cardio-geriatrics Interest Group of the Geriatrics Unit of Pisa University Hospital.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. European heart journal 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Desai, A.S.; Stevenson, L.W. Rehospitalization for Heart Failure: Predict or Prevent? Circulation 2012, 126, 501–506. [Google Scholar] [CrossRef]
- Hollenberg, S.M.; Warner Stevenson, L.; Ahmad, T.; Amin, V.J.; Bozkurt, B.; Butler, J.; Davis, L.L.; Drazner, M.H.; Kirkpatrick, J.N.; Peterson, P.N.; et al. 2019 ACC Expert Consensus Decision Pathway on Risk Assessment, Management, and Clinical Trajectory of Patients Hospitalized With Heart Failure: A Report of the American College of Cardiology Solution Set Oversight Committee. Journal of the American College of Cardiology 2019, 74, 1966–2011. [Google Scholar] [CrossRef]
- Calsolaro, V.; Antognoli, R.; Pasqualetti, G.; Okoye, C.; Aquilini, F.; Cristofano, M.; Briani, S.; Monzani, F. 30-Day Potentially Preventable Hospital Readmissions In Older Patients: Clinical Phenotype And Health Care Related Risk Factors. Clinical interventions in aging 2019, 14, 1851–1858. [Google Scholar] [CrossRef]
- Radonjić, T.; Popović, M.; Zdravković, M.; Jovanović, I.; Popadić, V.; Crnokrak, B.; Klašnja, S.; Mandić, O.; Dukić, M.; Branković, M. Point-of-Care Abdominal Ultrasonography (POCUS) on the Way to the Right and Rapid Diagnosis. Diagnostics (Basel, Switzerland) 2022, 12. [Google Scholar] [CrossRef]
- Qaseem, A.; Etxeandia-Ikobaltzeta, I.; Mustafa, R.A.; Kansagara, D.; Fitterman, N.; Wilt, T.J.; Batur, P.; Cooney, T.G.; Crandall, C.J.; Hicks, L.A.; et al. Appropriate Use of Point-of-Care Ultrasonography in Patients With Acute Dyspnea in Emergency Department or Inpatient Settings: A Clinical Guideline From the American College of Physicians. Annals of internal medicine 2021, 174, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.; Lewis, E.F.; Uno, H.; Peck, J.; Pivetta, E.; Merz, A.A.; Hempel, D.; Wilson, C.; Frasure, S.E.; Jhund, P.S.; et al. Detection and Prognostic Value of Pulmonary Congestion by Lung Ultrasound in Ambulatory Heart Failure Patients. European heart journal 2016, 37, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Scali, M.C.; Ciampi, Q.; Lichtenstein, D. Lung Ultrasound for the Cardiologist. JACC. Cardiovascular imaging 2018, 11, 1692–1705. [Google Scholar] [CrossRef] [PubMed]
- Tierney, D.M.; Huelster, J.S.; Overgaard, J.D.; Plunkett, M.B.; Boland, L.L.; St Hill, C.A.; Agboto, V.K.; Smith, C.S.; Mikel, B.F.; Weise, B.E.; et al. Comparative Performance of Pulmonary Ultrasound, Chest Radiograph, and CT Among Patients With Acute Respiratory Failure. Critical care medicine 2020, 48, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Linsalata, G.; Okoye, C.; Antognoli, R.; Guarino, D.; Ravenna, V.; Orsitto, E.; Calsolaro, V.; Monzani, F. Pneumonia Lung Ultrasound Score (PLUS): A New Tool for Detecting Pneumonia in the Oldest Patients. Journal of the American Geriatrics Society 2020, 68, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.; Campbell, R.T.; Claggett, B.; Lewis, E.F.; Groarke, J.D.; Docherty, K.F.; Lee, M.M.Y.; Merz, A.A.; Silverman, M.; Swamy, V.; et al. Lung Ultrasound in Acute Heart Failure: Prevalence of Pulmonary Congestion and Short- and Long-Term Outcomes. JACC: Heart Failure 2019, 7, 849–858. [Google Scholar] [CrossRef]
- Stuck, A.E.; Siu, A.L.; Wieland, G.D.; Adams, J.; Rubenstein, L.Z. Comprehensive Geriatric Assessment: A Meta-Analysis of Controlled Trials. Lancet (London, England) 1993, 342, 1032–1036. [Google Scholar] [CrossRef]
- Pfeiffer, E. A Short Portable Mental Status Questionnaire for the Assessment of Organic Brain Deficit in Elderly Patients. Journal of the American Geriatrics Society 1975, 23, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies Of Illness In The Aged. The Index Of Adl: A Standardized Measure Of Biological And Psychosocial Function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. The Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Church, S.; Rogers, E.; Rockwood, K.; Theou, O. A Scoping Review of the Clinical Frailty Scale. 2020, 1–18. [CrossRef]
- Guigoz, Y.; Lauque, S.; Vellas, B.J. Identifying the Elderly at Risk for Malnutrition. The Mini Nutritional Assessment. Clinics in geriatric medicine 2002, 18, 737–757. [Google Scholar] [CrossRef]
- Lindner, M.; Thomas, R.; Claggett, B.; Lewis, E.F.; Groarke, J.; Merz, A.A.; Silverman, M.B.; Swamy, V.; Rivero, J.; Hohenstein, C.; et al. Quantification of Pleural Effusions on Thoracic Ultrasound in Acute Heart Failure. European Heart Journal. Acute Cardiovascular Care 2020, 9, 513–521. [Google Scholar] [CrossRef]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International Evidence-Based Recommendations for Point-of-Care Lung Ultrasound. Intensive care medicine 2012, 38, 577–591. [Google Scholar] [CrossRef]
- Pivetta, E.; Goffi, A.; Nazerian, P.; Castagno, D.; Tozzetti, C.; Tizzani, P.; Tizzani, M.; Porrino, G.; Ferreri, E.; Busso, V.; et al. Lung Ultrasound Integrated with Clinical Assessment for the Diagnosis of Acute Decompensated Heart Failure in the Emergency Department: A Randomized Controlled Trial. European journal of heart failure 2019, 21, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.; Cuttitta, F.; Paterna, S.; Garofano, A.; Conti, G.; Pinto, A.; Parrinello, G. Bed-Side Inferior Vena Cava Diameter and Mean Arterial Pressure Predict Long-Term Mortality in Hospitalized Patients with Heart Failure: 36 Months of Follow-Up. European journal of internal medicine 2016, 28, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Pellicori, P.; Carubelli, V.; Zhang, J.; Castiello, T.; Sherwi, N.; Clark, A.L.; Cleland, J.G.F. IVC Diameter in Patients with Chronic Heart Failure: Relationships and Prognostic Significance. JACC. Cardiovascular imaging 2013, 6, 16–28. [Google Scholar] [CrossRef]
- Walker, S.P.; Morley, A.J.; Stadon, L.; De Fonseka, D.; Arnold, D.T.; Medford, A.R.L.; Maskell, N.A. Nonmalignant Pleural Effusions: A Prospective Study of 356 Consecutive Unselected Patients. Chest 2017, 151, 1099–1105. [Google Scholar] [CrossRef]
- DeBiasi, E.M.; Pisani, M.A.; Murphy, T.E.; Araujo, K.; Kookoolis, A.; Argento, A.C.; Puchalski, J. Mortality among Patients with Pleural Effusion Undergoing Thoracentesis. The European respiratory journal 2015, 46, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Claret, P.-G.; Stiell, I.G.; Yan, J.W.; Clement, C.M.; Rowe, B.H.; Calder, L.A.; Perry, J.J. Characteristics and Outcomes for Acute Heart Failure in Elderly Patients Presenting to the ED. The American journal of emergency medicine 2016, 34, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Goldstein, I.; Mourgeon, E.; Cluzel, P.; Grenier, P.; Rouby, J.-J. Comparative Diagnostic Performances of Auscultation, Chest Radiography, and Lung Ultrasonography in Acute Respiratory Distress Syndrome. Anesthesiology 2004, 100, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Takada, S. The Role of Thoracic Ultrasonography for Evaluation of Patients with Decompensated Chronic Heart Failure. Journal of the American College of Cardiology 2000, 35, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A.; Shigueoka, D.C.; Atallah, A.N.; Ajzen, S.; Iared, W. Diagnostic Accuracy of Sonography for Pleural Effusion: Systematic Review. Sao Paulo medical journal = Revista paulista de medicina 2010, 128, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, C.; Casazza, G.; Ceriani, E.; Torzillo, D.; Furlotti, S.; Bossi, I.; Vago, T.; Costantino, G.; Montano, N. Lung Ultrasound and Short-Term Prognosis in Heart Failure Patients. International journal of cardiology 2016, 218, 104–108. [Google Scholar] [CrossRef]
- Gargani, L.; Pang, P.S.; Frassi, F.; Miglioranza, M.H.; Dini, F.L.; Landi, P.; Picano, E. Persistent Pulmonary Congestion before Discharge Predicts Rehospitalization in Heart Failure: A Lung Ultrasound Study. Cardiovascular ultrasound 2015, 13, 40. [Google Scholar] [CrossRef]
- Coiro, S.; Rossignol, P.; Ambrosio, G.; Carluccio, E.; Alunni, G.; Murrone, A.; Tritto, I.; Zannad, F.; Girerd, N. Prognostic Value of Residual Pulmonary Congestion at Discharge Assessed by Lung Ultrasound Imaging in Heart Failure. European journal of heart failure 2015, 17, 1172–1181. [Google Scholar] [CrossRef]
- Wang, Y.; Gargani, L.; Barskova, T.; Furst, D.E.; Cerinic, M.M. Usefulness of Lung Ultrasound B-Lines in Connective Tissue Disease-Associated Interstitial Lung Disease: A Literature Review. Arthritis research & therapy 2017, 19, 206. [Google Scholar] [CrossRef]
- Pitsidianakis, G.; Vassalou, E.E.; Vasarmidi, E.; Bolaki, M.; Klontzas, M.E.; Xirouchaki, N.; Georgopoulos, D.; Karantanas, A.H.; Tzanakis, N.; Antoniou, K.M. Performance of Lung Ultrasound for Monitoring Interstitial Lung Disease. Journal of ultrasound in medicine: official journal of the American Institute of Ultrasound in Medicine 2022, 41, 1077–1084. [Google Scholar] [CrossRef]
- Allinovi, M.; Parise, A.; Giacalone, M.; Amerio, A.; Delsante, M.; Odone, A.; Franci, A.; Gigliotti, F.; Amadasi, S.; Delmonte, D.; et al. Lung Ultrasound May Support Diagnosis and Monitoring of COVID-19 Pneumonia. Ultrasound in medicine & biology 2020, 46, 2908–2917. [Google Scholar] [CrossRef]
- Jobs, A.; Simon, R.; de Waha, S.; Rogacev, K.; Katalinic, A.; Babaev, V.; Thiele, H. Pneumonia and Inflammation in Acute Decompensated Heart Failure: A Registry-Based Analysis of 1939 Patients. European heart journal. Acute cardiovascular care 2018, 7, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, J.R.; Kapoor, R.; Ju, C.; Heidenreich, P.A.; Eapen, Z.J.; Hernandez, A.F.; Butler, J.; Yancy, C.W.; Fonarow, G.C. Precipitating Clinical Factors, Heart Failure Characterization, and Outcomes in Patients Hospitalized With Heart Failure With Reduced, Borderline, and Preserved Ejection Fraction. JACC. Heart failure 2016, 4, 464–472. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).