Submitted:
02 November 2023
Posted:
02 November 2023
You are already at the latest version
Abstract
Keywords:
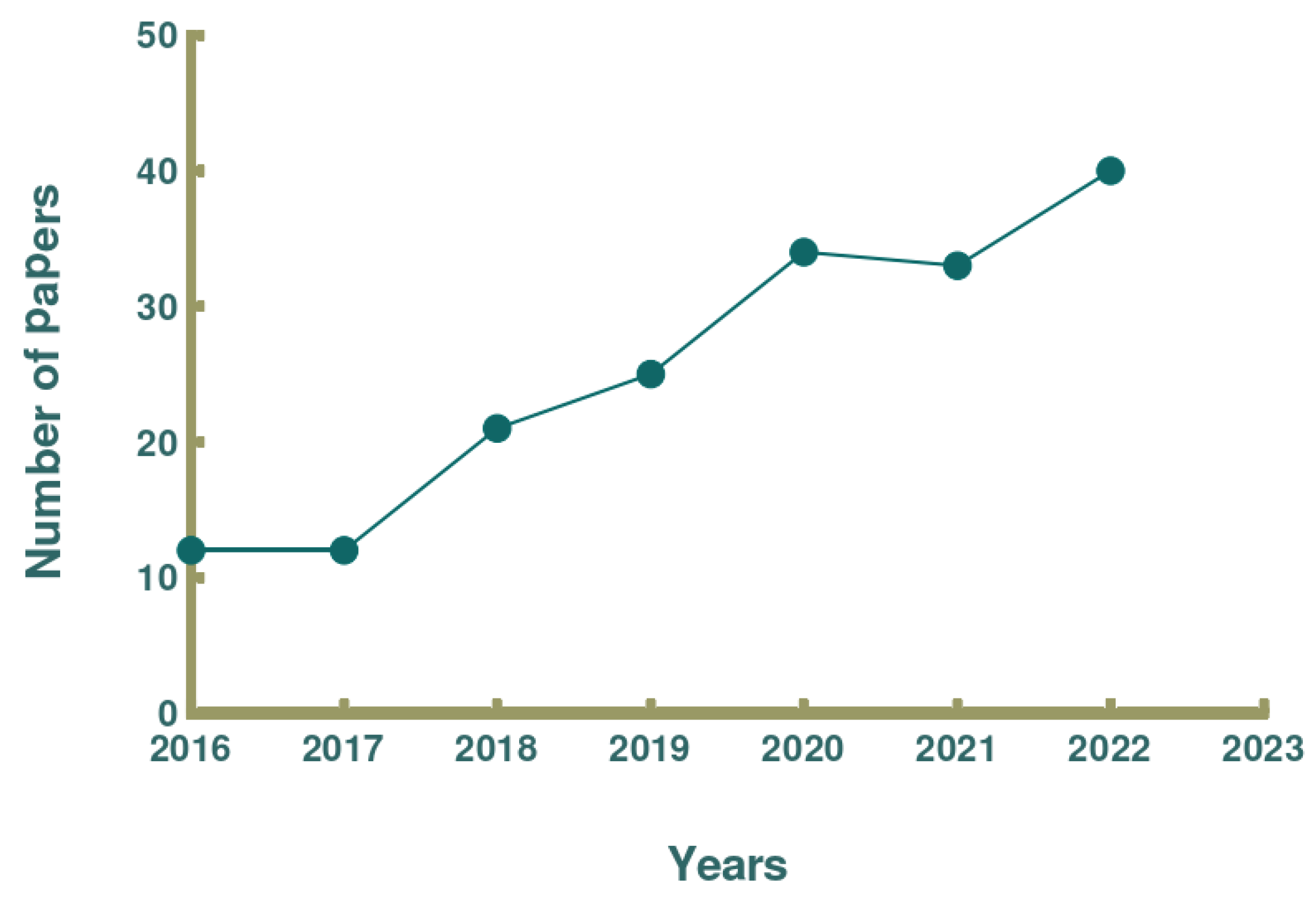
1. Introduction
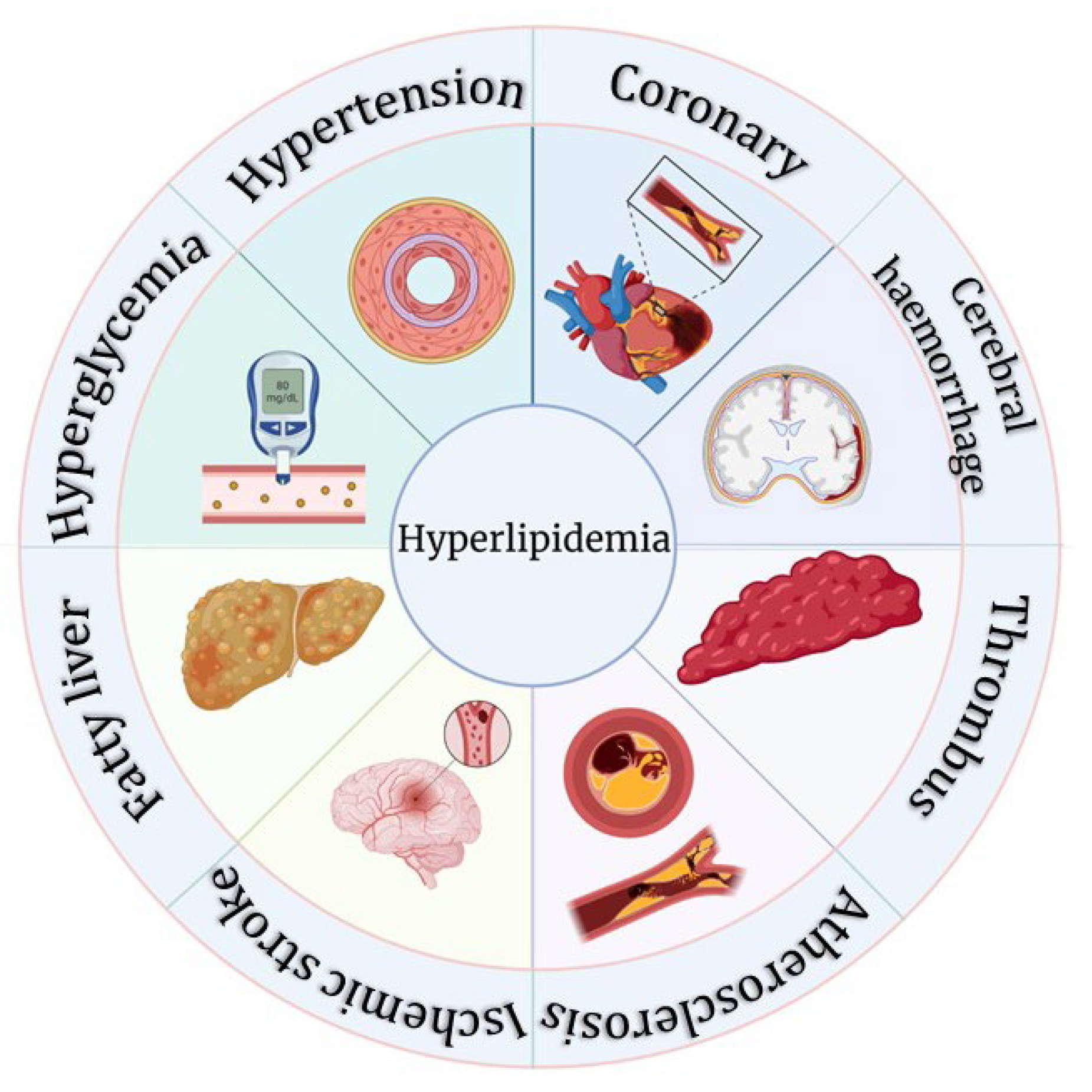
2. Current Status of Research on Hyperlipidemia
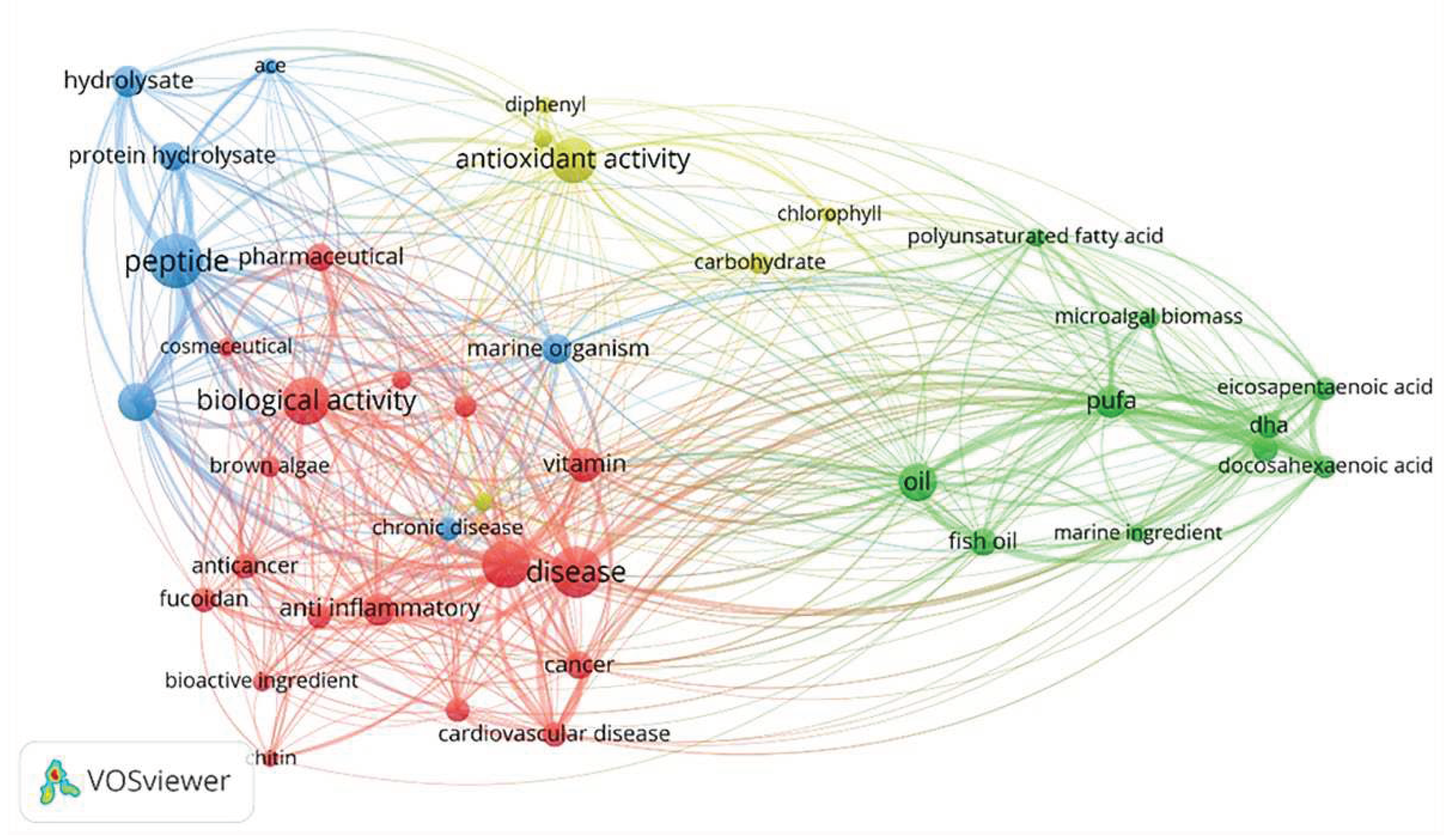
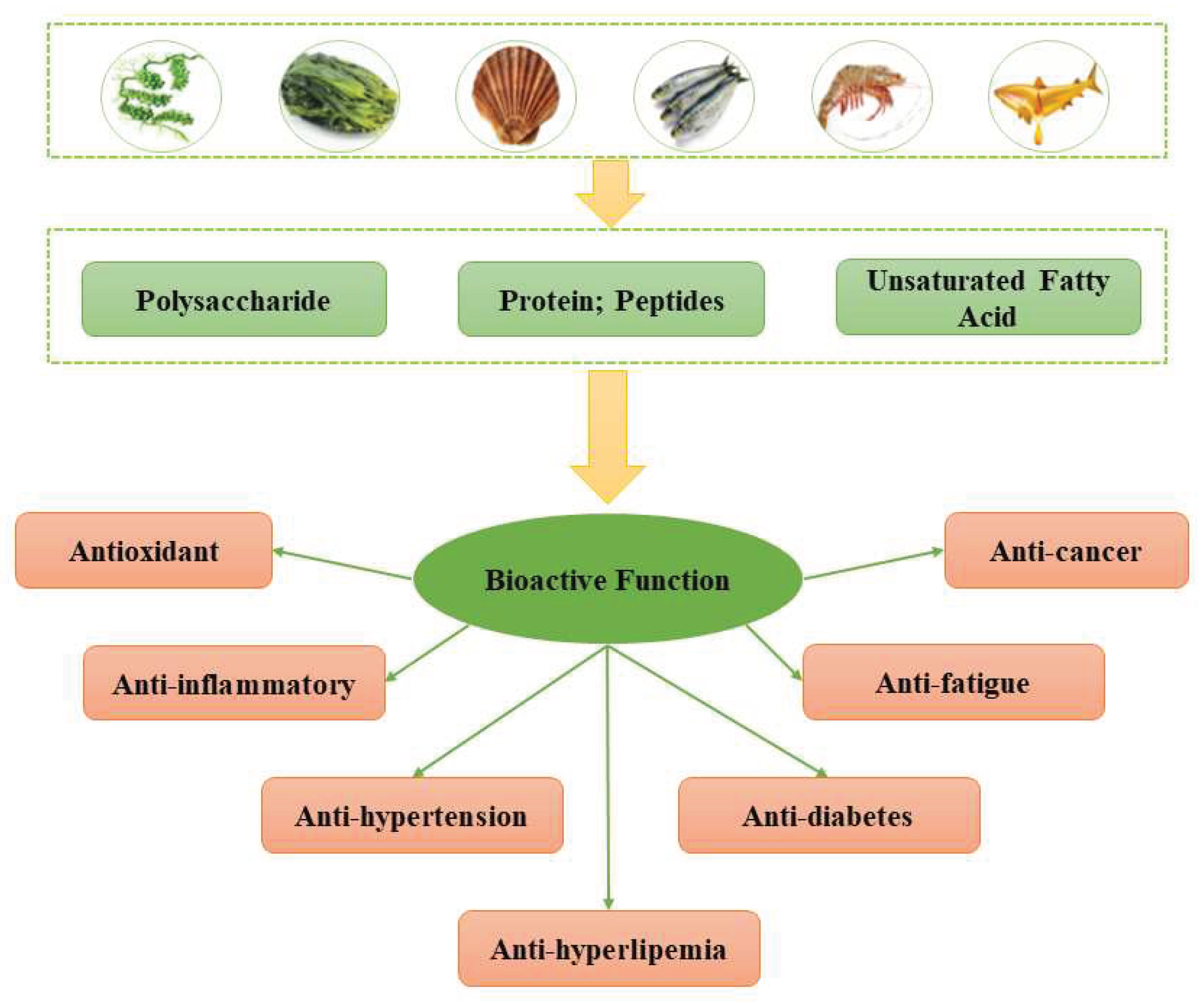
3. Source Classification of Marine-Derived Hypolipidemic Actives
3.1. Marine Polysaccharides
3.2. Marine-Derived Unsaturated Fatty Acids
3.3. Marine Bioactive Peptides
3.4. Others
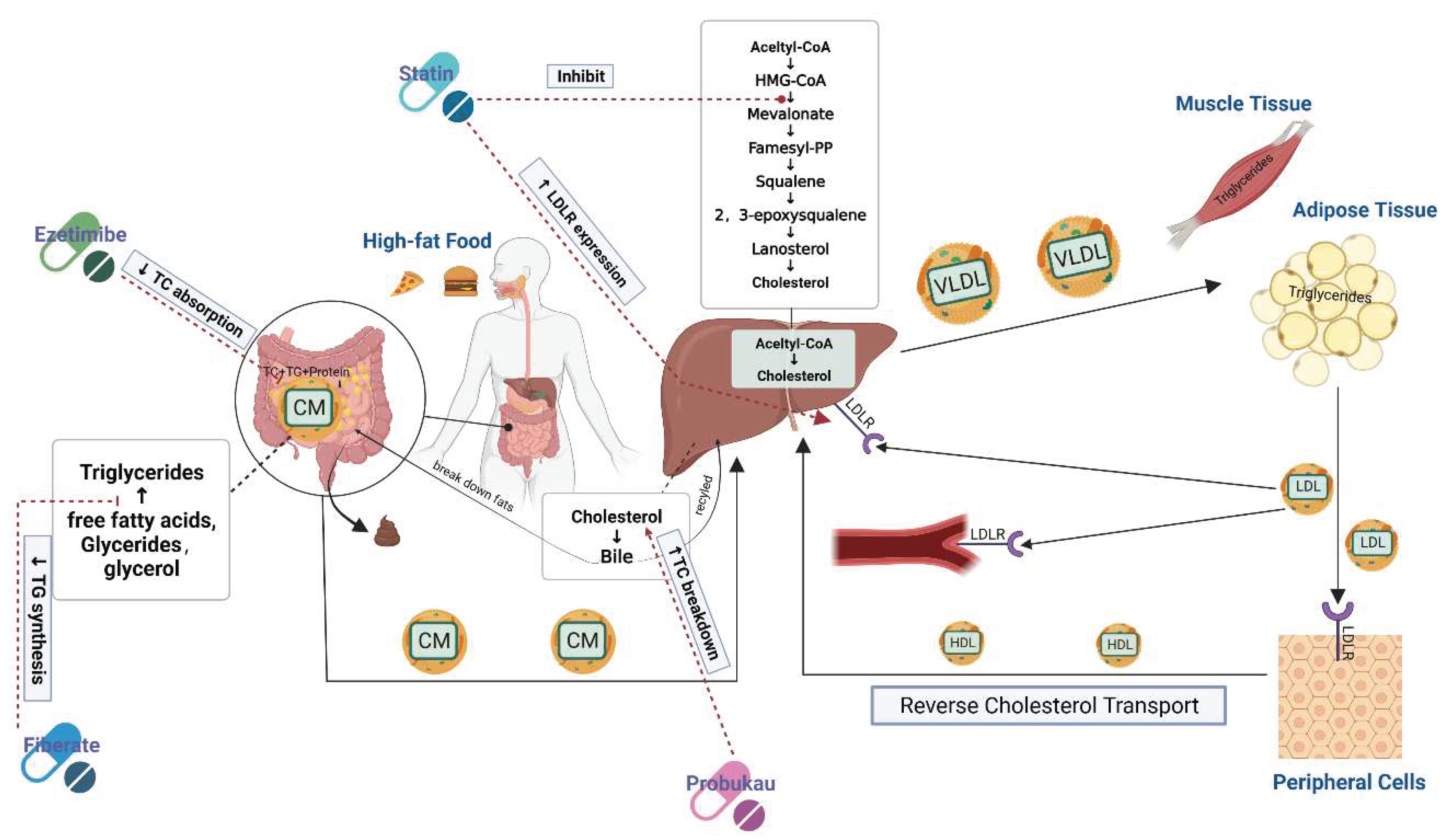
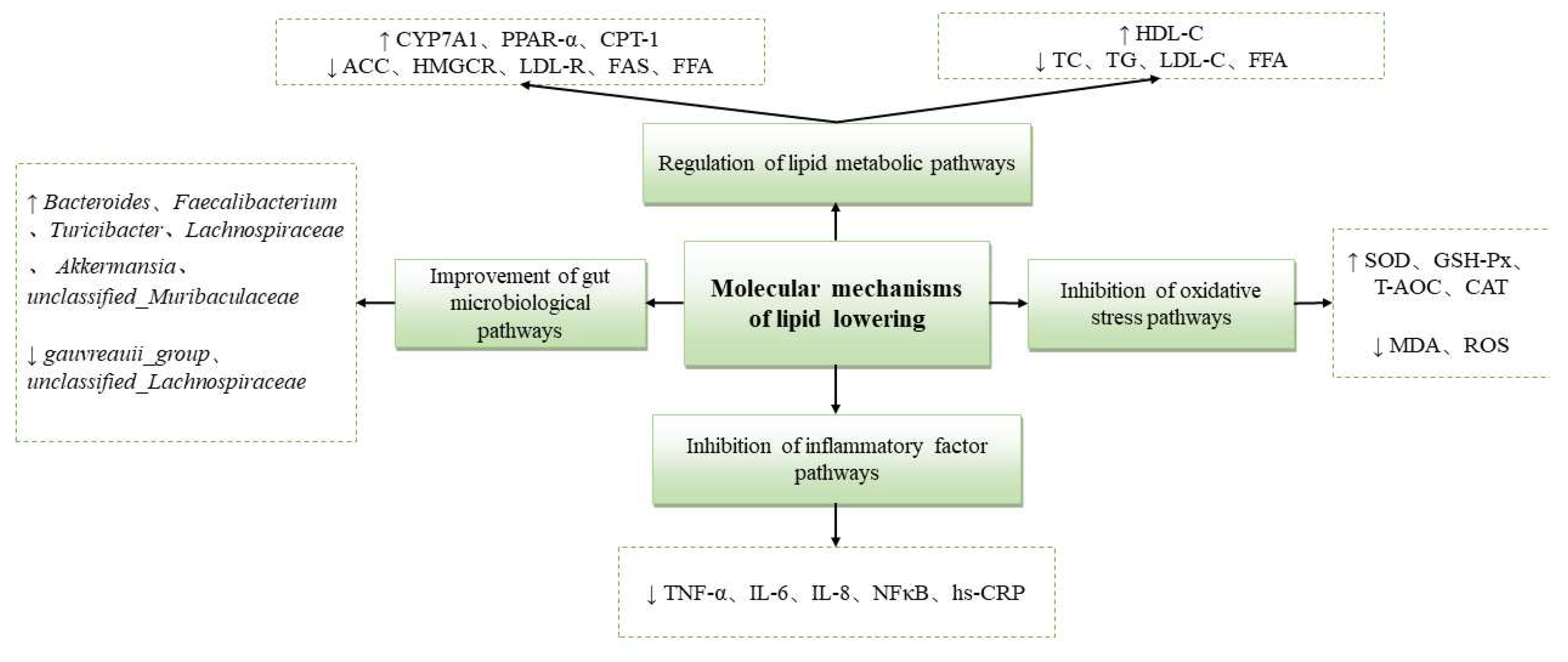
4. Mechanisms of Marine-Derived Hypolipidemic Active Substances
4.1. Inhibition of Cholesterol, TG, and Fatty Acid Pathways
4.2. Inhibition of Oxidative Damage Pathways
4.3. Inhibition of Inflammatory Factor Pathways
4.4. Improvement of Gut Microbial Pathways
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Muhammad, J.; Garrison, . S.; Rachel, N.; Deliana, K.; Patricia, R. The cost-effectiveness of hyperlipidemia medication in low- and middle-income countries: A review. Global Heart. 2022, 17, 18. [Google Scholar] [CrossRef]
- Sun, L.; Robert, C.; Bennett, d.; Guo, Y.; Walters, R.; Hill, M.; Parish, S.; Millwood, I.; Bian, Z.; Chen, Y.; Yu, C.; et al. Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat. Med. 2019, 25, 569–574. [Google Scholar] [CrossRef]
- Manh H P, Huy T V, Van S H, et al. Adherence to hypertension and dyslipidemia treatment and its implication on control of cardiovascular disease in Vietnam: A semi-systematic review Medicine. 2022, 101, e32137. [CrossRef]
- Silvia, A.; Olimpia, M.; Daniele, V.; et al. Statins neuromuscular adverse effects. Int. J. Mol. Sci. 2022, 23, 8364. [Google Scholar] [CrossRef] [PubMed]
- Gabriela, P.; Andreea, F.; Anca, Butucă.; Claudiu, M.; Anca, M.; Manuela, P.; Minodora, T.; Felicia, G. Post-Marketing surveillance of statins—A descriptive analysis of psychiatric adverse reactions in EudraVigilance. Pharmaceuticals. 2022, 15, 1536. [Google Scholar] [CrossRef]
- Manue, U.; Tatiana, P.; Carol, P.; Miguel, U. Statin associated adverse reactions in Latin America: a scoping review. BMJ. open. 2021, 11, e050675. [Google Scholar] [CrossRef]
- Thompson, C.; Kruger, H.; Thompson, F. Unlocking marine biotechnology in the developing world. Trends. Biotechnol. 2017, 35, 1119–1121. [Google Scholar] [CrossRef]
- Florean, C.; Dicato, M.; Diederich, M. Immune-modulating and anti-inflammatory marine compounds against cancer. Semin. Cancer. Biol. 2020, 80, 58–72. [Google Scholar] [CrossRef]
- Lobine, D.; Rengasamy, K.; Mahomoodally, F. Functional foods and bioactive ingredients harnessed from the ocean: current status and future perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 5794–5823. [Google Scholar] [CrossRef]
- Correia-da-Silva, M.; Sousa, E.; Pinto, M. Anticancer and cancer preventive compounds from edible marine organisms. Semin. Cancer Biol. 2017, 46, 55–64. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, T.; Yu, Z.; Wang, C.; Zhao, Y.; Wang, Y.; Xue, C. Taurine alleviates trimethylamine N-Oxide-Induced atherosclerosis by regulating bile acid metabolism in ApoE(-/-) mice. J. Agric. Food Chem. 2022, 70, 5738–5747. [Google Scholar] [CrossRef]
- Yang, Y.; Woo, J.; Seo, Y.; Lee, K.; Lim, Y.; Choi, J. Protective effect of brown alga phlorotannins against hyper-inflammatory responses in lipopolysaccharide-induced sepsis models. J. Agric. Food Chem. 2016, 64, 570–578. [Google Scholar] [CrossRef]
- Kalita, P.; Ahmed, A.; Sen, S.; Chakraborty, R. A comprehensive review on polysaccharides with hypolipidemic activity: Occurrence, chemistry and molecular mechanism. Int. J. Biol. Macromol. 2022, 206, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, M.; Alcaide, P. Vascular Inflammation and hyperlipidemia: The neutrophil within. JACC. Basic to translational science, 2021, 6, 524–526. [Google Scholar] [CrossRef]
- Song, D.; Jiang, J. Hypolipidemic components from medicine food homology species used in china: pharmacological and health effects. Arch. Med. Res. 2017, 48, 569–581. [Google Scholar] [CrossRef]
- Salyamova, A.; Khromova, A.; Kvasova, O.; Burko, N.; Oleinikov, V. The incidence of muscle tissue damage during therapy with atorvastatin in patients after STEMI. Eur. Heart J. 2021, 4. [Google Scholar] [CrossRef]
- Carlson, L. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J. Intern. Med. 2010, 258, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Durham, S.; Covington, E.; Clemmons, K. Hepatotoxicity upon using niacin to pass a drug test: A case report. J. Am. Pharm. Assoc. 2018, 58, 564–567. [Google Scholar] [CrossRef]
- Soo, H.; Brian, B.; Anand, B.; Sankar, N. 196 Fenofibrate-induced acute kidney injury: An under-recognized adverse effect. Am. J. Kidney Dis. 2022, 79, 2. [Google Scholar]
- Huang, X.; Sun, J.; Bian, C.; Ji, S.; Ji, H. Docosahexaenoic acid lessens hepatic lipid accumulation and inflammation via the AMP-activated protein kinase and endoplasmic reticulum stress signaling pathways in grass carp (Ctenopharyngodon idella). Food Funct. 2022, 13, 1846–1859. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Xue, C.; Wang, J.; Wang, Y.; Xu, J.; Li, Z. Exogenous natural EPA-enriched phosphatidylcholine and phosphatidylethanolamine ameliorate lipid accumulation and insulin resistance via activation of PPARα/γ in mice. Food Funct. 2020, 1, 8248–8258. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Song, Y.; Kang, K.; Noh, J. Anti-Obesity effects of collagen peptide derived from skate (Raja kenojei) skin through regulation of lipid metabolism. Mar. Drugs. 2018, 16, 306. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cardoso, K.; Josep, M.; Caimari, A.; Lama, C.; Torres, S.; Mantecón, L.; Infante, C. TetraSOD®, a unique marine microalgae ingredient, promotes an antioxidant and anti-Inflammatory status in a metabolic syndrome-Induced model in rats. Nutrients. 2022, 14, 4028. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Raman, S.; Dara, P.; Jacob, R.; Mathew, S.; Rangasamy, A.; Nagarajarao, R. In vivo anti-lipidemic and antioxidant potential of collagen peptides obtained from great hammerhead shark skin waste. J Food. Sci. Technol. 2022, 59, 1140–1151. [Google Scholar]
- Wang, Z.; Xu, Z.; Yang, X.; Li, M.; Yip, R.; Li, Y.; Chen, H. Current application and modification strategy of marine polysaccharides in tissue regeneration: A review. Biomater. Adv. 2023, 154, 213580. [Google Scholar] [CrossRef] [PubMed]
- Arnosti, C.; Wietz, M.; Brinkhoff, T.; Hehemann, J.; Probandt, D.; Zeugner, L.; Amann, R. The biogeochemistry of marine polysaccharides: sources, inventories, and bacterial drivers of the carbohydrate cycle. Annu. Rev. Mar. Sci. 2021, 13, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Perumal, P.; Dong, C.; Chauhan, A.; Anisha, G.; Kadri, M.; Chen, C.; Singhania, R.; Patel, A. Advances in oligosaccharides production from algal sources and potential applications. Biotechnol. Adv. 2023, 67, 108195. [Google Scholar] [CrossRef]
- Liu, Z.; Ai, C.; Lin, X.; Guo, X.; Song, S.; Zhu, B. Sea cucumber sulfated polysaccharides and Lactobacillus gasseri synergistically ameliorate the overweight induced by altered gut microbiota in mice. Food Funct. 2023, 2023. 14, 4106–4116. [Google Scholar] [CrossRef]
- Qu, H.; Wu, Y.; Luo, Z.; Dai, C. An efficient approach for extraction of polysaccharide from abalone (Haliotis Discus Hannai Ino) viscera by natural deep eutectic solvent. Int. J. Biol. Macromol. 2023, 244, 125336. [Google Scholar] [CrossRef]
- Sun, J.; Song, S.; Ai, C.; Zhu, B. A sulfated abalone polysaccharide inhibited SARS-CoV-2 infection of Vero E6 Cells in vitro. Foods. 2022, 11, 2865. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Z.; Li, C.; Chen, J.; Fang, T. Hypolipidemic and anti-atherogenic activities of crude polysaccharides from abalone viscera. Food Sci. Nutr. 2020, 8, 2524–2534. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, J.; Ahmad, A.; Daneshwar, P.; Nadeem, N.; Abdulwahed, F.; Zhang, Y. Marine Microbial Polysaccharides: An untapped resource for biotechnological applications. Mar. Drugs. 2023, 21, 420. [Google Scholar] [CrossRef]
- Yan, M.; Mao, M.; Liu, X.; Wang, S.; Xia, Z.; Cao, S.; Li, J.; Qin, L.; Xian, H. Extracellular polysaccharide with novel structure and antioxidant property produced by the deep-sea fungus Aspergillus versicolor N(2)bC. Carbohydr. Polym. 2016, 147, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Shi, W.; Miao, J.; Hu, H.; Gao, Y. Extraction, isolation, screening, and preliminary characterization of polysaccharides with anti-oxidant activities from Oudemansiella raphanipies. Polymers. 2023, 15, 2917. [Google Scholar] [CrossRef] [PubMed]
- Liu X, Sun Z, Zhang M, et al. Antioxidant and antihyperlipidemic activities of polysaccharides from sea cucumber Apostichopus japonicus. Carbohydr. Polym. 2012, 90, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Z.; Zhang, M.; Meng, X.; Xia, X.; Yuan, W.; Xue, F.; Liu, C. Macromolecular properties and hypolipidemic effects of four sulfated polysaccharides from sea cucumbers. Carbohydr. Polym. 2017, 173, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Abdo, A.; Zhang, C.; Al-Dalali, S.; Hou, Y.; Gao, J.; Yahya, M.; Saleh, A.; Aleryani, H.; Al-Zamani, Z.; Sang, Y. Marine chitosan-oligosaccharide ameliorated plasma cholesterol in hypercholesterolemic hamsters by modifying the gut microflora, bile acids, and short-chain fatty acids. Nutrients. 2023, 15, 2923. [Google Scholar] [CrossRef]
- Wan, X.; Ai, C.; Chen, Y.; Gao, X.; Zhong, R.; Liu, B.; Chen, X.; Zhao, C. Physicochemical characterization of a polysaccharide from green microalga chlorella pyrenoidosa and its hypolipidemic activity via gut microbiota regulation in rats. J. Agric. Food Chem. 2020, 2020. 68, 1186–1197. [Google Scholar] [CrossRef]
- Yuan, D.; Huang, Q.; Li, C.; Fu, X. A polysaccharide from Sargassum pallidum reduces obesity in high-fat diet-induced obese mice by modulating glycolipid metabolism. Food Funct. 2022, 2022.13, 7181–7191. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Z.; Li, C.; Chen, J.; Fang, T. Hypolipidemic and anti-atherogenic activities of crude polysaccharides from abalone viscera. Food Sci. Nutr. 2020, 8, 2524–2534. [Google Scholar] [CrossRef]
- Kim, M.; Yang, I.; Lee, H.; Lee, J.; Kim, K. Lipid-modifying effects of krill oil vs fish oil: a network meta-analysis. Nutr. Rev. 2020, 78, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, C.; Yan, C.; Chen, H.; You, S.; Sheng, S.; Wu, F.; Wang, J. Silkworm pupa oil exerts hypolipidemic and antioxidative effects on rat model of high-fat diet induced hyperlipidemia. FASEB J. 2015, 29, 589–4. [Google Scholar] [CrossRef]
- Oršolić, N.; Jurčević, N.; Đikić, D.; Rogić, D.; Odeh, D.; Balta, V.; Junaković, E.; Terzić, S.; Jutrić, D. Effect of propolis on diet-induced hyperlipidemia and atherogenic indices in mice. Antioxidants. 2019, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.; Yun, J.; Kim, D.; Park, S.; Lee, C.; Go, E.; Kim, J.; Park, S.; Lee, J. Krill oil inhibits cholesterol synthesis and stimulated cholesterol excretion in hypercholesterolemic rats. Mar. Drugs. 2022, 20, 609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, J.; Wang, Y.; Xue, C. Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog. Lipid Res. 2019, 75, 100997. [Google Scholar] [CrossRef] [PubMed]
- Yang, S. A New Perspective on Fish Oil: The prevention of alcoholic liver disease: Review. J. Oleo Sci. 2021, 70, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Q.; Wang, H.; Zhao, X.; Li, N.; Zhang, H.; Chen, G.; Liu, Z. Fish oil alleviates circadian bile composition dysregulation in male mice with NAFLD. J. Nutr. Biochem. 2019, 69, 53–62. [Google Scholar] [CrossRef]
- Calder, P. Beneficial Outcomes of omega-6 and omega-3 polyunsaturated fatty aacids on human health: An update for 2021. Nutrients, 2021, 13, 2421. [Google Scholar] [CrossRef]
- Kimura, R.; Takahashi, N.; Lin, S.; Goto, T.; Murota, K.; Nakata, R.; Inoue, H.; Kawada, T. DHA attenuates postprandial hyperlipidemia via activating PPARα in intestinal epithelial cells. J. Lipid Res. 2013, 54, 3258–3268. [Google Scholar] [CrossRef]
- Chong, S.; Wang, X.; Bloois, L.; et al. Injectable liposomal docosahexaenoic acid alleviates atherosclerosis progression and enhances plaque stability. J. Controlled Release. 2023, 360, 344–364. [Google Scholar] [CrossRef]
- Skulas-Ray, A.; et al. Omega-3 fatty acids for the management of hypertriglyceridemia: A science advisory from the American Heart Association. Circulation. 2019, 14, e673–e691. [Google Scholar] [CrossRef] [PubMed]
- Grundy. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association task force on clinical practice guideline. Circulation. 2019, 773, e285–e350. [Google Scholar] [CrossRef]
- François, M.; Colin, B.; Catapano, A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Sabarinathan, S.; Rani, R. Protective effect of fish oil targeting hadhaa (3-ketoacyl-CoA thiolase, alpha subunit a and hadhb (3-ketoacyl-CoA thiolase, beta subunit) against high cholesterol diet (HCD) induced atherosclerosis on Zebrafish. Obesity Medicine. 2022, 34, 100447. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, Y.; Kim, J.; Chun, Y.; Kwon, Y.; Ku, S.; Song, C. Preventive and therapeutic effects of krill oil on obesity and obesity-induced metabolic syndromes in high-fat diet-fed mice. Mar. Drugs. 2022, 20, 483. [Google Scholar] [CrossRef]
- Stine, M.; Kirsten, B. Comparison of bioavailability of krill oil versus fish oil and health effect. Vasc. Health. Risk. Manag. 2015, 11, 511–524. [Google Scholar]
- Guo, P.; Xue, M.; Teng, X.; Wang, Y.; Ren, R.; Han, J.; Zhang, H.; Tian, Y.; Liang, H. Antarctic krill oil ameliorates liver injury in rats exposed to alcohol by regulating bile acids metabolism and gut microbiota. J. Nutr. Biochem. 2022, 107, 109061. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, Z.; Lv, Y.; Lu, H.; Liu, T.; Yi, H.; Zhao, M.; Zhang, L.; Gong, P. Krill oil combined with Bifidobacterium animalis subsp. lactis F1-7 alleviates the atherosclerosis of ApoE−/− mice. Foods. 2021, 10, 2374. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, S.; Yang, X.; Zhao, X.; Wang, C.; Xu, H. Immunomodulatory effects of different molecular weight sporisorium reilianum polypeptides on LPS-induced RAW264.7 macrophages. Food Biosci. 2021, 43, 101322. [Google Scholar] [CrossRef]
- Ma, J.; Zeng, X.; Zhou, M.; Cheng, L.; Ren, D. Inhibitory effect of low-molecular-weight peptides (0–3kDa) from Spirulina platensis on H2O2-induced oxidative damage in L02 human liver cells. Bioresour. Bioprocess. 2021, 8, 1–9. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Zhang, C.; Liu, C. Angiotensin converting enzyme (ACE) inhibitory, antihypertensive and antihyperlipidaemic activities of protein hydrolysates from Rhopilema esculentum. Food Chem. 2012, 134, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Tsai, J.; Hung, L.; Pan, B. Plasma lipid regulatory effect of compounded freshwater clam hydrolysate and Gracilaria insoluble dietary fibre. Food Chem. 2011, 125, 397–401. [Google Scholar] [CrossRef]
- Khaled, H.; Ghlissi, Z.; Chtourou, Y.; Hakim, A.; Ktari, N.; Fatma, M.; Barkia, A.; Sahnoun, Z.; Nasri, M. Effect of protein hydrolysates from sardinelle (Sardinella aurita) on the oxidative status and blood lipid profile of cholesterol-fed rats. Food Res. Int. 2012, 45, 60–68. [Google Scholar] [CrossRef]
- Wergedahl, H.; Liaset, B.; Gudbrandsen, O.; Lied, E.; Espe, M.; Muna, Z.; Mørk, S.; Berge, R. Fish protein hydrolysate reduces plasma total cholesterol, increases the proportion of HDL cholesterol, and lowers acyl-CoA:cholesterol acyltransferase activity in liver of Zucker rats. J. Nutr. 2004, 134, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Hur, J.; Ham, S.; Jo, Y.; Lee, S.; Choi, M.; Seo, H. Fish collagen peptide inhibits the adipogenic differentiation of preadipocytes and ameliorates obesity in high fat diet-fed mice. Int. J. Biol. Macromol. 2017, 104, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, X.; He, Y.; Chi, C.; Wang, B. Hypolipidemic activities of two pentapeptides (VIAPW and IRWWW) from Miiuy Croaker (Miichthys miiuy) muscle on lipid accumulation in HepG2 Cells through regulation of AMPK pathway. Appl. Sci. 2020, 10, 817. [Google Scholar] [CrossRef]
- Zhao, Q.; Jiang, X.; Mao, Z.; Zhang, J.; Sun, J.; Mao, X. Exploration, sequence optimization and mechanism analysis of novel xanthine oxidase inhibitory peptide from Ostrea rivularis Gould. Food Chem. 2023, 404, 134537. [Google Scholar] [CrossRef]
- Margaret. M.; Aimee, L.; Maxine, P.; Lisa, R. Do marine algal polyphenols have antidiabetic, antihyperlipidemic or anti-inflammatory effects in humans? A systematic review. Crit. Rev. Food. Sci. 2018, 58, 2039–2054. [CrossRef]
- Manuel, G.; Alba, R.; Francesca, A.; Julio, G. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs. 2018, 16, 250. [Google Scholar] [CrossRef]
- Choi, H.; Jeon, H.; Le, O.; Lee, B. Dieckol, a major phlorotannin in Ecklonia cava, suppresses lipid accumulation in the adipocytes of high-fat diet-fed zebrafish and mice: Inhibition of early adipogenesis via cell-cycle arrest and AMPKα activation. Mol. Nutr. Food Res. 2015, 59, 1458–1471. [Google Scholar] [CrossRef]
- Wells, M.; Potin, P.; Craigie, J.; et al. Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, A.; Wu, C.; Guo, G.; Proksch, P.; Lin, W. Lipid-lowering effects of farnesylquinone and related analogues from the marine-derived Streptomyces nitrosporeus. Bioorg. Med. Chem. Lett. 2014, 24, 5288–5293. [Google Scholar] [CrossRef] [PubMed]
- Abebe, W.; Mozaffari, M. Role of taurine in the vasculature: An overview of experimental and human studies. Am. J. Cardiovasc. Dis. 2011, 1, 293–311. [Google Scholar] [PubMed]
- Dong, Y.; Li, X.; Liu, Y.; Gao, J.; Tao, J. The molecular targets of taurine confer anti-hyperlipidemic effects. Life Sci. 2021, 278, 119579. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Oh, D.; Kim, J.; et al. Taurine ameliorates hyperglycemia and dyslipidemia by reducing insulin resistance and leptin level in Otsuka Long-Evans Tokushima fatty (OLETF) rats with long-term diabetes. Exp Mol Med. 2012, 44, 665–73. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yanai, H.; Ito, K.; et al. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis. 2010, 209, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yue, Q.; Fang, F.; et al. Effect of dual residual risk of cholesterol and inflammation on all-cause mortality in patients with cardiovascular disease. Cardiovasc. Diabetol. 2023, 22, 96. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.; Koudstaal, S.; Mosterd, A.; et al. C-Reactive protein and risk of incident heart failure in patients with cardiovascular disease. J. Am. Coll. Cardiol. 2023, 82, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Gori, T.; Bruno, R. Is oxidative stress a therapeutic target in cardiovascular disease? Eur. Heart J. 2010, 31, 2741–2748. [Google Scholar] [CrossRef]
- Wan, X.; Ai, C. Chen, Y.; Gao, X.; Zhong, R.; Liu, B.; Chen, X.; Zhao, C. Physicochemical characterization of a polysaccharide from green microalga chlorella pyrenoidosa and its hypolipidemic activity via gut microbiota regulation in rats. J. Agric. Food. Chem. 2020, 68, 1186–1197. [Google Scholar] [CrossRef]
- Uppin, V.; Acharya, P.; Kempaiah, B.; Talahalli, R. Zerumbone augments cognitive enhancement potentials of EPA+DHA: insight from a hyperlipidaemic rat model. Br. J. Nutr. 2020, 124, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, N.; Sun, X.; et al. Effect of intake pattern of sulfated polysaccharides on its biological activity in high fat diet-fed mice. Int. J. Biol. Macromol. 2019, 132, 9–16. [Google Scholar] [CrossRef]
- Jia, R.; Li, Z.; Wu, J.; Ou, Z.; Zhu, Q.; Sun, B.; Lin, L.; Zhao, M. Physicochemical properties of polysaccharide fractions from Sargassum fusiforme and their hypoglycemic and hypolipidemic activities in type 2 diabetic rats - ScienceDirect. Int. J. Biol. Macromol. 2020, 147, 428–438. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Ma, G. Hypolipidemic effect of the polysaccharide from Pholiota nameko. Nutrition. 2010, 26, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Urbatzka, R. Potential anti-obesity, anti-steatosis, and anti-inflammatory properties of extracts from the microalgae chlorella vulgaris and chlorococcum amblystomatis under different growth conditions. Mar. Drugs., 2021, 20, 9. [Google Scholar] [CrossRef]
- Tietge, U. Hyperlipidemia and cardiovascular disease: Inflammation, dyslipidemia, and atherosclerosis. Curr. Opin. Lipidol. 2014, 25, 94–95. [Google Scholar] [CrossRef]
- Hofmaenner, D.; Kleyman, A.; Press, A.; Bauer, M.; Singe, M. The many roles of cholesterol in sepsis: A review. Am. J. Respir. Crit. Care. Med. 2022, 205, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.; Badimon, L.; Hansson, G.; Deanfield, J.; Bittencourt, M.; Tokgözoğlu, L.; Lewis, E. Atherosclerosis. Nat. Rev. Dis. Primers. 2019, 5, 56. [Google Scholar] [CrossRef]
- Zou, B.; Ge, Z.; Zhang, Y.; Du, J.; Xua, Z.; Li, C. Persimmon Tannin accounts for hypolipidemic effects of persimmon through activating of AMPK and suppressing NF-kB activation and inflammatory responses in High-Fat Diet Rats. Food. Funct. 2014, 5, 1536–1546. [Google Scholar] [CrossRef]
- Siasos, G.; Tousoulis, D.; Oikonomou, E.; Zaromitidou, M.; Stefanadis, C.; Papavassiliou, A. inflammatory markers in hyperlipidemia: from experimental models to clinical practice. Curr. Pharm. Des. 2011, 17, 4132–46. [Google Scholar] [CrossRef]
- Wang, S.; Wu, D.; Matthan, N.; Lamon-Fava, S.; Lecker, J.; Aortic, A. Macrophage lipid accumulation and inflammatory response in LDL receptor Null mice fed an atherogenic diet. Lipids. 2010, 45, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Zwartjes, M.; Gerdes, V.; Nieuwdorp, M. The role of gut microbiota and its produced metabolites in obesity, dyslipidemia, adipocyte dysfunction, and its interventions. Metabolites, 2021, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature, 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Gu, W.; Lee, I.; Joh, E.; Kim, D. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. Plos One. 2012, 7, e47713. [Google Scholar] [CrossRef] [PubMed]
- Aroa, L. .; Jose, M.; Alicia, D.; Alexandre, L.; Alejandra, C..; Carlos, M.; Alberto, C. Potential Use of Marine seaweeds as prebiotics: A review. Molecules, 2020, 25, 1004. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Mao, G.; et al. Effect of the sulfation pattern of sea cucumber-derived fucoidan oligosaccharides on modulating metabolic syndromes and gut microbiota dysbiosis caused by HFD in mice. J. Funct. Foods. 2019, 55, 193–210. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




