1. Introduction
Resection of skull base meningioma is considered challenging due to the deep location and presence of important surrounding structures such as cranial nerves and blood vessels. Although various approaches to the area are advocated1-6, sufficient internal decompression and mobilization of the tumor are always essential to secure a sufficient workspace and safe tumor resection. Internal decompression often causes bleeding from the tumor, especially in vascular-rich tumors like meningioma, and presents an obstruction to a clear operative field. Particularly in skull base meningioma, the feeding artery often comes from a deeper area of the skull base. This means that an intentional approach to the feeding artery and control of bleeding is needed at an early stage of the surgery. In addition, resection of feeding arteries also provides tumor mobility by releasing the anchor to the skull base2. Thus, in skull base meningioma, treating the feeding artery at an early stage of surgery is a very important step to performing safe surgery, and knowledge of the feeding arteries is essential. Although some studies have described feeding arteries of the dura in healthy subjects7, 8, few reports have provided detailed information on feeding arteries in meningioma patients2. In addition, even studies of preoperative embolization for meningioma have contained little information about the anatomical characteristics and distributions of feeding arteries for meningioma9-12. In particular, skull base meningioma is rare among meningiomas, so relatively few studies have reported details for feeding arteries of skull base meningiomas. This study retrospectively analyzed images from MRI and angiography of skull base meningiomas with attachments around the cavernous sinus, tentorium around the petrosal bone, clivus, and foramen magnum. In this article, we report the distributions and frequency of feeding arteries for skull base meningioma and the relationships between feeding arteries and tumor attachment.
2. Materials and Methods
We searched our institutional database for patients who underwent cerebral angiography for meningioma between September 2015 and October 2022. Contrast-enhanced T1-weighted MRI of each patient was checked and those patients showing tumor attachment at the cavernous sinus, clivus, foramen magnum, petrous edge, or tentorium around the petrous bone were included in this study. Patients who did not undergo contrast-enhanced MRI were excluded from this study. Tumor attachments and feeding arteries in each meningioma were examined retrospectively. The institutional ethics committee approved this study and written informed consent for publication of images was obtained from all patients.
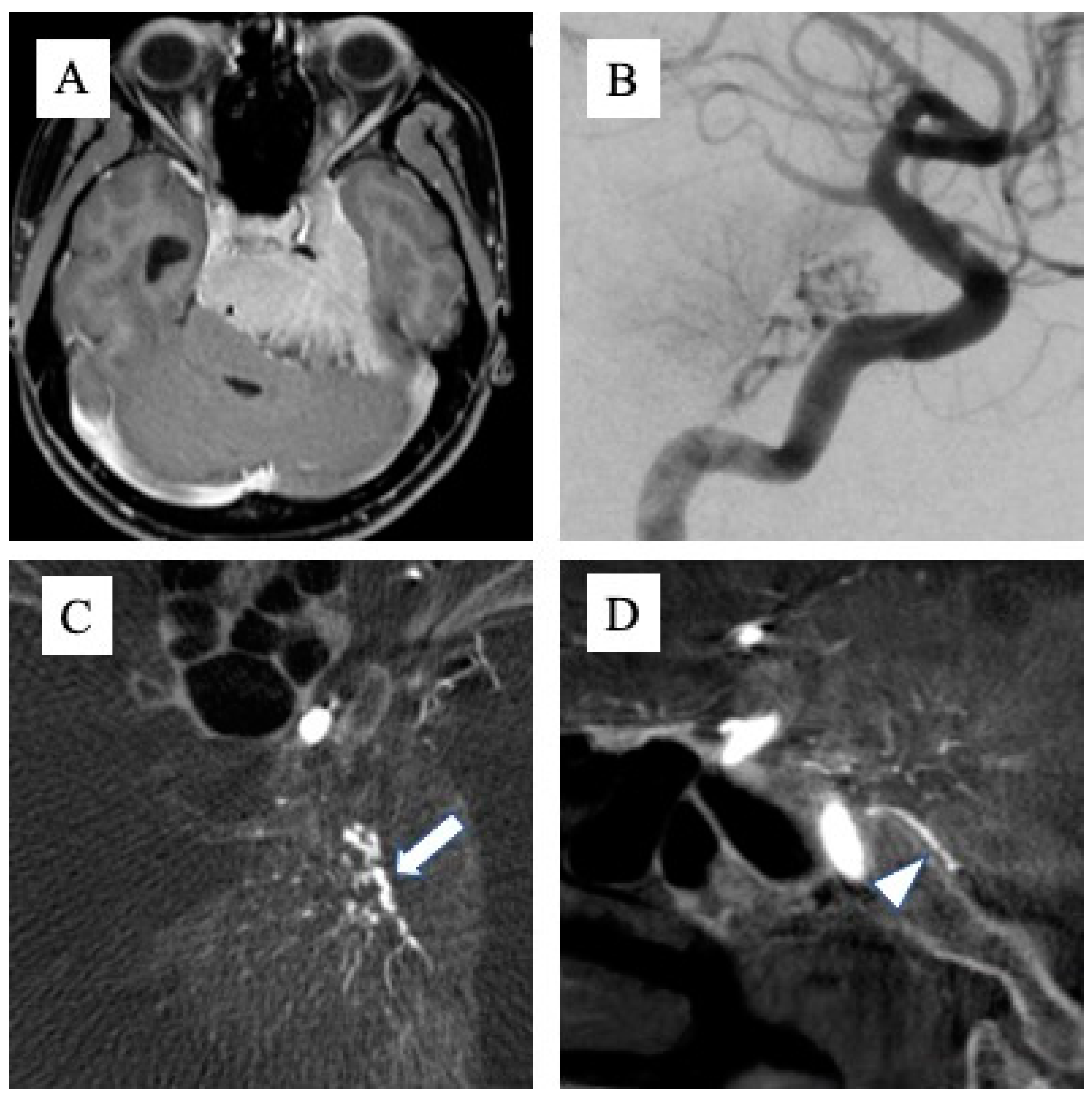
Tumor attachments were defined with reference to MRI T1-enhanced images (MAGNETOM Avanto 1.5T or MAGNETOM Vida 3.0T; Siemens Healthcare GmbH, Forchheim, Germany), and the part with a clear mass more than 2 mm thick and with obvious MRI enhancement was defined as the tumor attachment. The presence or absence of attachment to Areas 1, 2 and 3 was determined for each tumor, and if a wide range of attachments was seen across multiple areas, these were counted as duplicates. The feeding artery was determined from cerebral angiography (angiography system: Artis zee biplane; Siemens Healthcare GmbH). Digital subtraction angiography was performed using a 5-Fr catheter, with 8 ml of contrast medium at a rate of 6 ml/s for the common carotid artery (CCA), 6 ml of contrast medium at a rate of 4 ml/s for the internal carotid artery (ICA), and 5 ml of contrast medium at a rate of 3 ml/s for the external carotid artery (ECA) and vertebral artery (VA). Angiography from the CCA, ICA, or ECA was performed ipsilateral to the tumor in all cases. When tumor attachment was around the foramen magnum, angiograms were obtained from bilateral VAs. Contralaterally, an angiogram from the CCA was obtained first. If tumor staining was evident, angiography was added from the ICA or ECA to clearly visualize the feeding artery. In addition, cone-beam computed tomography (CBCT) from the ipsilateral CCA was taken in all cases, with 56 ml of double-diluted contrast medium injected at 2 ml/sec. When determining the feeding artery by DSA alone proved difficult, we referred to anatomical information that could be confirmed by CBCT, such as the running course of the artery (
Figure 1A–D). Two endovascular specialists checked DSA and CBCT and defined the feeding artery of the tumor.
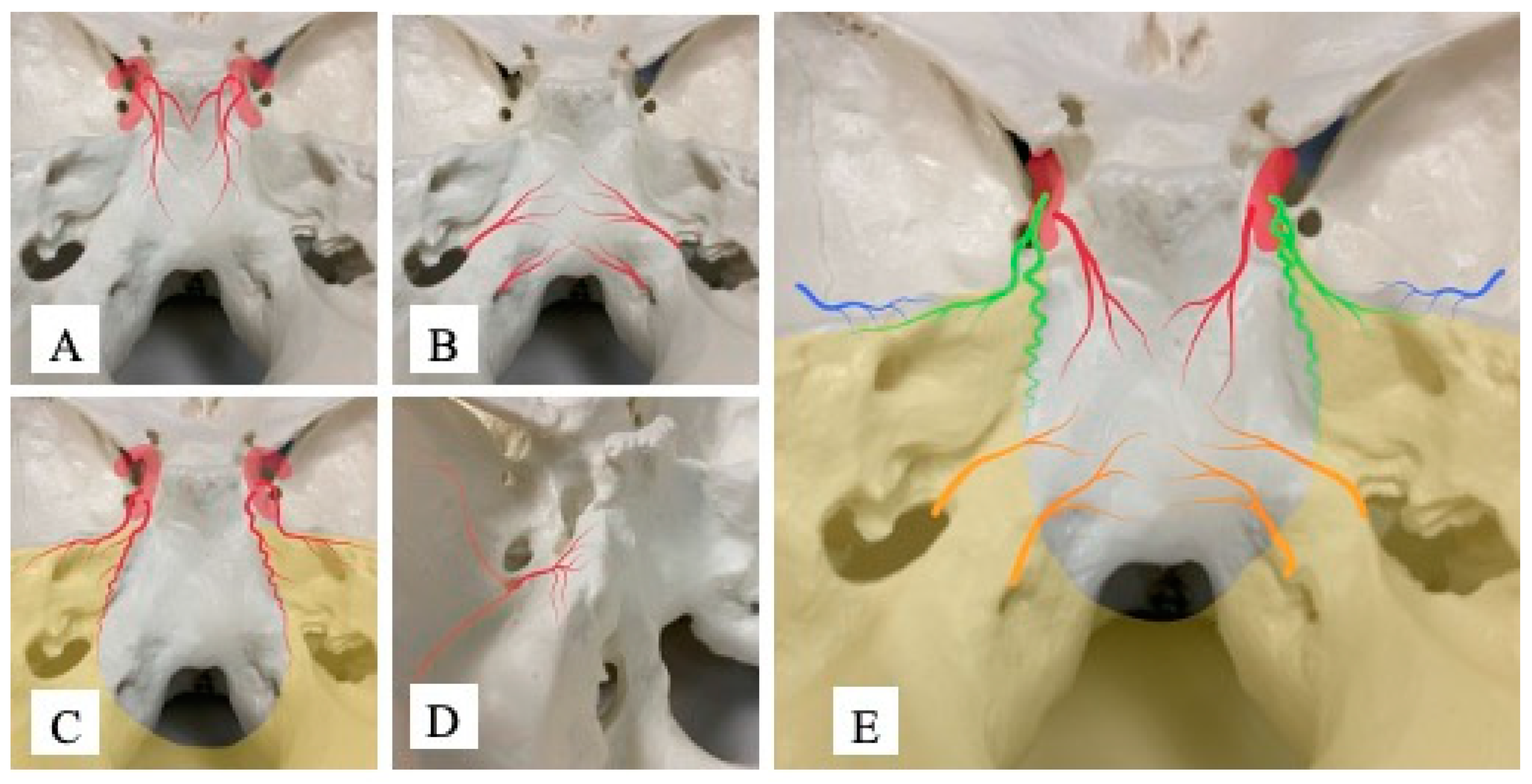
Attachments for meningioma are always continuous, so we intentionally divide the tumor attachment into three areas. The cavernous sinus and upper part of the clivus are defined as Area 1, the lower part of the clivus and the area around the foramen magnum as Area 2, and the tentorium around the petrous bone as Area 3 (
Figure 2). Referring to a report by Khaled et al. [
13], the upper part of the clivus in this study means the rostral area of the clivus from the height of the internal auditory meatus, while the lower part of the clivus represents the caudal area from that height.
The number of feeding arteries detected by angiography was counted, and particularly frequent arteries (DMA, APA, TA, and PB) were analyzed for relationships with tumor attachment in Areas 1–3. Univariate analyses using Pearson’s chi-square test and multivariate analysis using logistic regression analysis were used to test the relationships. All statistical analyses were performed using JMP12 software (SAS Institute, Cary, NC, USA). Values of p<0.05 were accepted as significant.T
3. Results
A total of 168 meningioma patients underwent angiography in our institute between September 2015 and October 2022. Of these, 53 patients were excluded from the study because of a lack of attachments around Areas 1–3. In total, 117 tumors in 115 patients showed attachment in Areas 1, 2, and/or 3, with multiple meningiomas in 2 patients. The 115 patients comprised 32 men and 83 women, with a mean age of 52.7 years (range, 22–84 years). Meningiomas with attachments in Areas 1, 2, and 3 were seen in 92, 50, and 67 of the 117 patients, respectively, and meningiomas with extensive attachments were counted as duplicates.
3.1. Blood Vessels visualized on DSA for Skull Base Meningioma
Key feeding arteries from the ICA were the TA, DMA, and posterior ethmoidal artery. Key feeding vessels from the ECA were the MMA, accessory meningeal artery, artery of the foramen rotundum, sphenopalatine artery, mastoid branch of the occipital artery, and APA. Particularly with the MMA, angiograms showed the PB, petrosquamous branch, anterior branch and recurrent meningeal branch. The posterior meningeal artery and tentorial artery arising from the posterior cerebral artery or superior cerebellar artery were found as feeding arteries from the posterior circulation. As anatomical variations, two cases of TA originating from the recurrent meningeal branch of the MMA and one case of TA originating from the ophthalmic artery were observed, and both were classified as TA. The frequencies of feeding arteries are shown in
Table 1.
3.2. Correspondence between Feeding Artery and Tumor Attachment
Arteries frequently visualized on DSA, such as the DMA, APA, TA, and PB, were analyzed for relationships to Areas 1, 2, and 3.
DMA was strongly associated with Area 1 (univariate analysis, p < 0.001; multivariate analysis, p < 0.001) and was considered an important feeding artery in Area 1 (
Figure 3A). No significant associations were apparent with Area 2 (p = 0.15) or Area 3 (p = 0.76) (
Table 2). In addition, when DMA was present, 97.8% (44/45) of tumors displayed attachments in Area 1. However, even in the absence of DMA, 66.7% (48/72) of cases showed attachment in Area 1 (
Table 3). The presence of DMA is thus specific, but not particularly sensitive for tumor attachment in Area 1.
AphA was strongly associated with Area 2 (univariate analysis, p < 0.001; multivariate analysis, p < 0.001) and represented an important feeding artery in Area 2 (
Figure 3B). In addition, univariate analysis showed a significant association between AphA and Area 1 (p < 0.05), but no significant association with Area 3. When AphA was present, 90.2% of cases (37/41) displayed attachment to Area 2. When AphA was absent, only 17.1% (13/76) of cases showed attachment to Area 2 (
Table 4). The presence of AphA is thus sensitive and specific for Area 2 tumor attachment. When considering the relationship between AphA and Area 1, part of the clivus around the border of Areas 1 and 2 may be supplied by the AphA.
The TA was strongly associated with Area 3 (p < 0.001) and formed an important feeding artery in Area 3 (
Figure 3C). In addition, the TA showed a significant association with Area1 in univariate analysis (p < 0.05), and part of the cavernous sinus in Area 1 may be nourished by the TA. No significant association was found with Area 2 (p = 0.81). When a TA was present, 85.4% (53/62) of cases showed attachment to Area 3. When no TA was present, only 25.5% (14/55) of cases displayed attachment to Area 3 (
Table 5). TA was therefore suggested as a sensitive and specific blood vessel in Area 3 and part of Area 1 may be supplied by the TA.
The PB was associated with Area 3 in multivariate analysis (p<0.05), suggesting that PB is a feeding artery associated with the tentorium. Considering its distribution, the PB nourishes the attachment of the tentorium along the petrosal bone (
Figure 3D), whereas no significant associations with Areas 1 or 2 were seen. When a PB was present, 86.3% of cases (38/44) showed attachment to Area 3, but 74.0% (54/73) displayed attachment to Area 3 even when the PB was not present (
Table 6). The PB was this thought to be a specific feeding artery for Area 3, but does not appear to represent a sensitive blood vessel in this area.
A schematic image of feeding arteries for skull base meningioma based on angiographic information is shown in
Figure 3E. This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
4. Discussion
Skull base meningioma is the most challenging surgery among meningiomas [
1,
14], so tumor devascularization and mobilization are more important for safer tumor resection. Since the feeding arteries are thought to play a role not only in supplying blood flow, but also in anchoring the tumor to the dura mater [
2], effective transection of these arteries makes the tumor bloodless and mobile. A correct understanding of the running course and origin of the feeding artery is thus essential information to achieve safer, more efficient surgery. In an outstanding analysis of the distribution of dural blood vessels in a study of previously healthy cadavers [
7], Martins et al. provided a large amount of information for understanding the normal distribution of blood flows to the dura. Although making such a detailed evaluation by angiogram is difficult, our study provides useful information for understanding the distribution of feeding arteries specifically in patients with skull base meningioma. Our analysis revealed the AphA as a highly sensitive and specific blood vessel in the lower part of the clivus and around the foramen magnum, and the TA as a highly sensitive and specific blood vessel in the tentorium. We also found that the presence of DMA was strongly associated with tumor attachment to the upper part of the clivus. The AphA was also suggested to be associated with the boundaries of the upper and lower clivus, and the PB was associated with the part of the tentorium connected to the petrous edge (
Figure 3,
Table 6). These blood vessels are particularly major feeding arteries in skull base meningiomas and show clear associations with attachment area. Understanding these tendencies and attachments may offer substantial advantages when considering and handling feeding arteries of skull base meningioma before and/or during the operation.
Distributions of the TA, DMA, APA, and PB as described in the report by Martins et al. are very similar to the distributions found in our analysis. Of course there is a possibility of underestimating blood vessels that are too tiny to visualize on angiograms, but even if these un-visualized arteries provide some blood flow to Areas 1–3, the clinical meaning of such feeding arteries would be much less than those of the DMA, APA, TA or PB. Our analyses revealed that feeding arteries for skull base meningioma are very similar to the physiological distributions, so strong visualization of a feeding artery that is usually less involved in the area might lead to consideration of another disease with a high blood supply, such as solitary fibrous tumor.
As described above, physiologically dominant blood vessels may tend to develop prior to or early in meningioma, resulting in a more important role in supplying the tumor compared to small minor vessels. Our analysis suggested that the main blood vessels playing an important role in meningioma with attachments around Areas 1–3 are the TA, DMA, AphA, and PB. In our series, at least one of these vessels was visualized in all cases, without exception. Judging from these results, such knowledge is very important and useful information not only prior to surgery, but also for angiography or preoperative embolization. Advantages of preoperative tumor embolization for meningiomas have been discussed in many reports [
9,
10,
11,
12,
15,
16] and we also support the efficacy of this option. Among all the arteries illustrated in this article, the TA, DMA, AphA, and PB potentially provide blood flow to the cranial nerves or form anastomosis with cerebral vessels [
17,
18]. Maximum care must therefore be taken when performing embolization from these arteries. A report and summary of safety and technical notes on embolization for skull base meningioma in our institute is now in progress.
Blood vessels such as the MMA, recurrent meningeal artery, accessory meningeal artery, and AFR (as shown in
Table 1) are occasionally depicted as feeding arteries, but these vessels were not originally thought to supply blood flow to Areas 1–3. This is suspected to represent an overestimation of feeding arteries particularly for huge meningiomas with attachments not only in Areas 1–3, but to the anterior skull base, middle skull base, or sphenoid ridge. We must therefore pay attention to feeding arteries other than the DMA, AphA, TA and PB, especially when the attachment is extensive and broad.
Among the skull base meningiomas, cavernous sinus meningioma is extremely challenging in terms of tumor resection and the treatment strategy for the area also remains controversial [
14,
19,
20]. In addition to the clinical and surgical difficulties, the anatomical features also make it difficult to clearly define the tumor origin between the cavernous sinus and sphenoid ridge, petrous apex, upper clivus, and tentorium. This is because the dura is continuous around these areas. This article classified the cavernous sinus into Area 1, because the upper clivus and dorsum sellae are continuous, so discriminating a clear tumor border between the cavernous sinus and upper clival tumor is very difficult on MRI. If we try to classify these areas in different parts, the results would be very subjective and concerns about misclassification would increase. On the other hand, the tentorium and cavernous sinus are slightly easier to differentiate because the clivus and petrous bone offer good landmarks for estimating origins. Although our classification gives physicians a relatively easy method for judging borders, there might be some argument regarding this classification from a physiological perspective, because that feeding artery to the cavernous sinus is from the tentorial artery [
7,
21]. In this study, DMA showed a strong correlation with Area 1, but actually 66.7% of patients (48/72) did not show a DMA even if the attachment was in Area 1. This suggests that some part of the CS might be supplied from arteries other than the DMA; this is likely to be the TA. Judging from our analysis and anatomical distributions from a previous report [
22,
23], we speculated that if the main origin of the tumor is the superolateral part of the cavernous sinus, there is a possibility that the tumor is fed by the TA, and if the inferomedial part of the cavernous sinus is involved, the DMA is suspected to supply the tumor. In other words, when the TA is obvious as a feeding artery, the main attachment of the tumor might be at the superolateral part of the CS (
Figure 4), defined as petrotentorial meningioma, and cranial nerves might form the medial border to the tumor. If the feeding artery is the DMA, the tumor origin can be inferred to be at the inferomedial wall of the cavernous sinus, defined as petroclival meningioma. Cranial nerves might then form the lateral part of the tumor. However, there is ample room for further detailed classification and examination of feeding arteries for the cavernous sinus.
Limitations
Although we analyzed a relatively large number of cases with the rare disease of skull base meningioma, we cannot rule out the possibility of selection or information biases, since this was a single-center analysis. Furthermore, only patients with angiography results were included in this study, so possible selection bias may remain, in that small meningiomas that did not need treatment or detailed examination might have been excluded.
5. Conclusions
In skull base meningiomas, cerebral angiography provides very useful information for assessing the tumor origin and feeding artery. The DMA is considered an important blood vessel that nourishes part of the cavernous sinus and upper clivus. The AphA is an important artery for the lower part of the clivus to the area around the foramen magnum. The TA is an important feeding artery for tumor arising from the tentorium around the petrous edge. In addition, the AphA may nourish the border of the upper and lower clivus and the PB may nourish part of the tentorium around the petrous apex. Evaluating and understanding these feeding arteries preoperatively can provide important information for determining adequate surgical strategies.
Author Contributions
Conceptualization, H.A.; methodology, H.A.; validation, Y.W., Y.T., H.M., T.K., T.I. and T.G.; formal analysis, H.A.; investigation, H.A.; resources, H.A. and Y.W.; data curation, A.H.; writing—original draft preparation, A.H.; writing—review and editing, A.H. and T.G.; supervision, T.G.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This retrospective study involving human subjects is in accordance with the principles of the Declaration of Helsinki. This study was approved by Institutional Review Board of Osaka Metropolitan University (2020-105).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to data privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Almefty R, Dunn IF, Pravdenkova S, Abolfotoh M, Al-Mefty O. True petroclival meningiomas: results of surgical management. J Neurosurg 2014, 120, 40–51. [CrossRef] [PubMed]
- Adachi K, Hasegawa M, Tateyama S, Kawazoe Y, Hirose Y. Surgical Strategy for and Anatomic Locations of Petroapex and Petroclival Meningiomas Based on Evaluation of the Feeding Artery. World Neurosurg 2018, 116, e611–e623. [CrossRef] [PubMed]
- Morisako H, Ohata H, Shinde B, Nagahama A, Watanabe Y, Goto T. Minimal anterior and posterior combined transpetrosal approach for large petroclival meningiomas. J Neurosurg 2021, 135(4), 1180–1189. [CrossRef] [PubMed]
- Ohata H, Goto T, Nagm A, Kannepalli NR, Nakajo K, Morisako H, et al. Surgical implementation and efficacy of endoscopic endonasal extradural posterior clinoidectomy. J Neurosurg 2019, 113(1), 135–143. [CrossRef] [PubMed]
- Uda H, Uda T, Kinoshita M, Kishima H, Tanoue Y, Nagahama A, et al. Visualization of Resected Area in Endonasal Endoscopic Approach versus Transcranial Approach for Skull Base Meningiomas by Voxel-Based-Lesion Mapping. Brain Sci 2022, 12(7), 875. [CrossRef] [PubMed]
- Adachi K, Hasegawa M, Hirose Y Prediction of trigeminal nerve position based on the main feeding artery in petroclival meningioma. Neurosurg Rev 2021, 44, 1173–1181. [CrossRef]
- Martins C, Yasuda A, Campero A, Ulm AJ, Tanriover N, Rhoton A, Jr. Microsurgical anatomy of the dural arteries. Neurosurgery 2005, 56, 211–251. [CrossRef]
- Celine Salaud CD, Stephane Ploteau, Antonie Hamel. Dural arteries of the dorsoclival area. Surg Radiol Anat 2019, 42, 179–187. [CrossRef]
- Manaka H, Sakata K, Tatezuki J, Shinohara T, Shimohigoshi W, Yamamoto T. Safety and Efficacy of Preoperative Embolization in Patients with Meningioma. J Neurol Surg B Skull Base 2018, 79, S328–S333. [CrossRef]
- Asai K, Nakamura H, Watanabe Y, Nishida T, Sakai M, Arisawa A, et al. Efficacy of endovascular intratumoral embolization for meningioma: assessment using dynamic susceptibility contrast-enhanced perfusion-weighted imaging. J Neurointerv Surg 2021, 13, 1167–1171. [CrossRef] [PubMed]
- Przybylowski CJ, Zhao X, Baranoski JF, Borba Moreira L, Gandhi S, Chapple KM, et al. Preoperative embolization versus no embolization for WHO grade I intracranial meningioma: a retrospective matched cohort study. J Neurosurg 2020, 134, 693–700. [CrossRef] [PubMed]
- Suzuki K, Nagaishi M, Matsumoto Y, Fujii Y, Inoue Y, Sugiura Y, et al. Preoperative Embolization for Skull Base Meningiomas. J Neurol Surg B Skull Base 2017, 78, 308–314. [CrossRef]
- Aziz KMA, Sanan A, van Loveren HR, Tew JM, Keller JT, Pensak ML. Petroclival Meningiomas: Predictive Parameters for Transpetrosal Approaches. Neurosurgery 2000, 47, 139–152.
- Morisako H, Goto T, Ohata H, Goudihalli SR, Shirosaka K, Ohata K. Safe maximal resection of primary cavernous sinus meningiomas via a minimal anterior and posterior combined transpetrosal approach. Neurosurg Focus 2021, 44, E11. [CrossRef]
- Jumah F, AbuRmilah A, Raju B, Jaber S, Adeeb N, Narayan V, et al. Does preoperative embolization improve outcomes of meningioma resection? A systematic review and meta-analysis. Neurosurg Rev 2021, 44, 3151–3163. [CrossRef]
- Yoon N, Shah A, Couldwell WT, Kalani MYS, Park MS. Preoperative embolization of skull base meningiomas: current indications, techniques, and pearls for complication avoidance. Neurosurg Focus 2018, 44, E5. [CrossRef]
- Hendrix P, Griessenauer CJ, Foreman P, Shoja MM, Loukas M, Tubbs RS. Arterial supply of the upper cranial nerves: a comprehensive review. Clin Anat 2014, 27, 1159–1166. [CrossRef]
- Jittapiromsak P, Sabuncuoglu H, Deshmukh P, McDougall CG, Spetzler RF, Preul MC. Anatomical relationships of intracavernous internal carotid artery to intracavernous neural structures. Skull Base 2010, 20, 327–336. [CrossRef]
- Hafez RF, Morgan MS, Fahmy OM. Stereotactic Gamma Knife surgery safety and efficacy in the management of symptomatic benign confined cavernous sinus meningioma. Acta Neurochir (Wien) 2015, 157, 1559–1564. [CrossRef] [PubMed]
- William TC, Kan P, James KL, Ronald IA. Decompression of cavernous sinus meningioma for preservation and improvement of cranial nerve function. J Neurosurg 2006, 105, 148–152. [CrossRef]
- Masaki, K. Functional Anatomy of the Brain and Spinal Vessels, 2nd ed.; MEDICUS SHUPPAN: Osaka, Japan, 2013; pp. 183–185. [Google Scholar]
- Peltier J, Fichten A, Havet E, Foulon P, Page C, Le Gars D. Microsurgical anatomy of the medial tentorial artery of Bernasconi-Cassinari. Surg Radiol Anat 2010, 32, 919–925. [CrossRef] [PubMed]
- Tubbs RS, Nguyen HS, Shoja MM, Benninger B, Loukas M, Cohen-Gadol AA. The medial tentorial artery of Bernasconi-Cassinari: a comprehensive review of its anatomy and neurosurgical importance. Acta Neurochir (Wien) 2011, 153, 2485–2490. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).