Submitted:
12 June 2024
Posted:
13 June 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
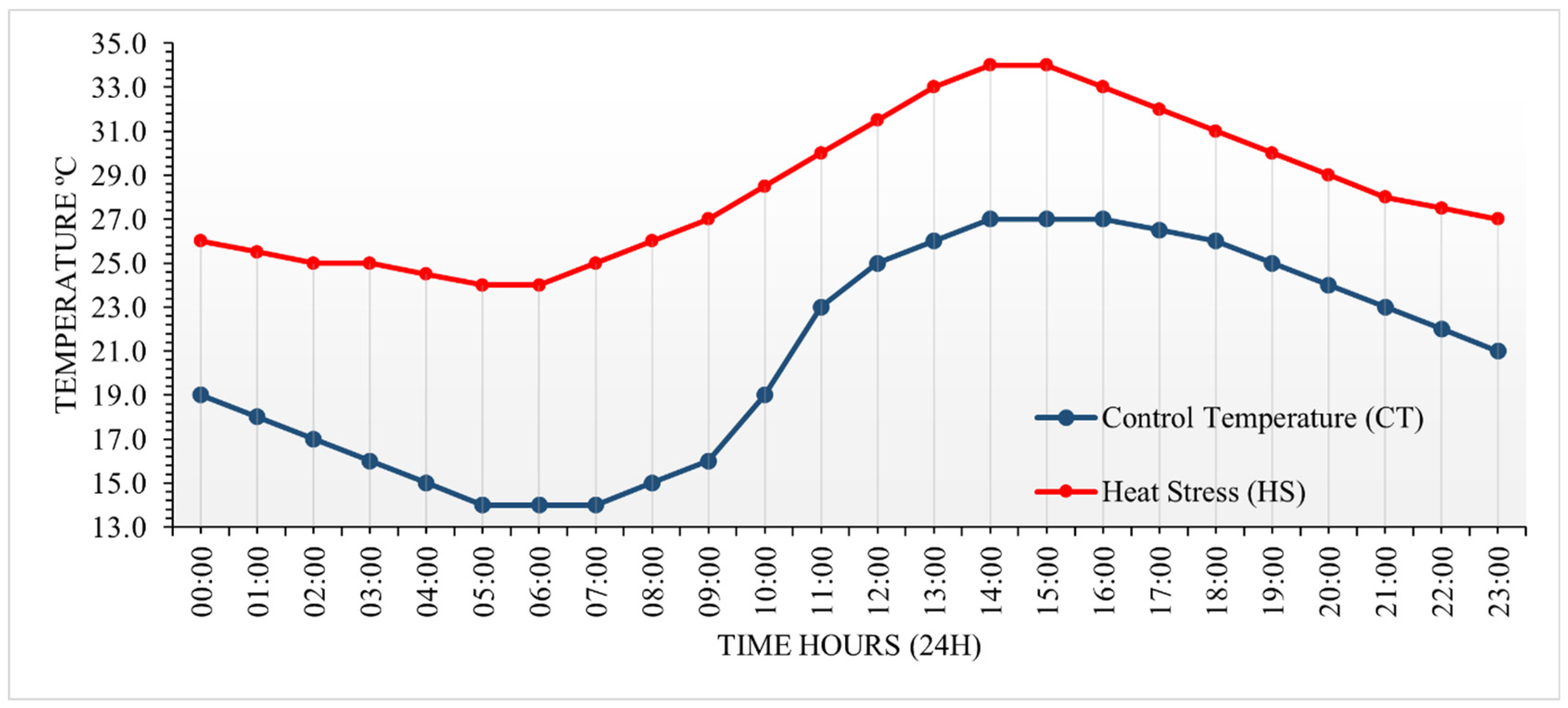
Plant Material
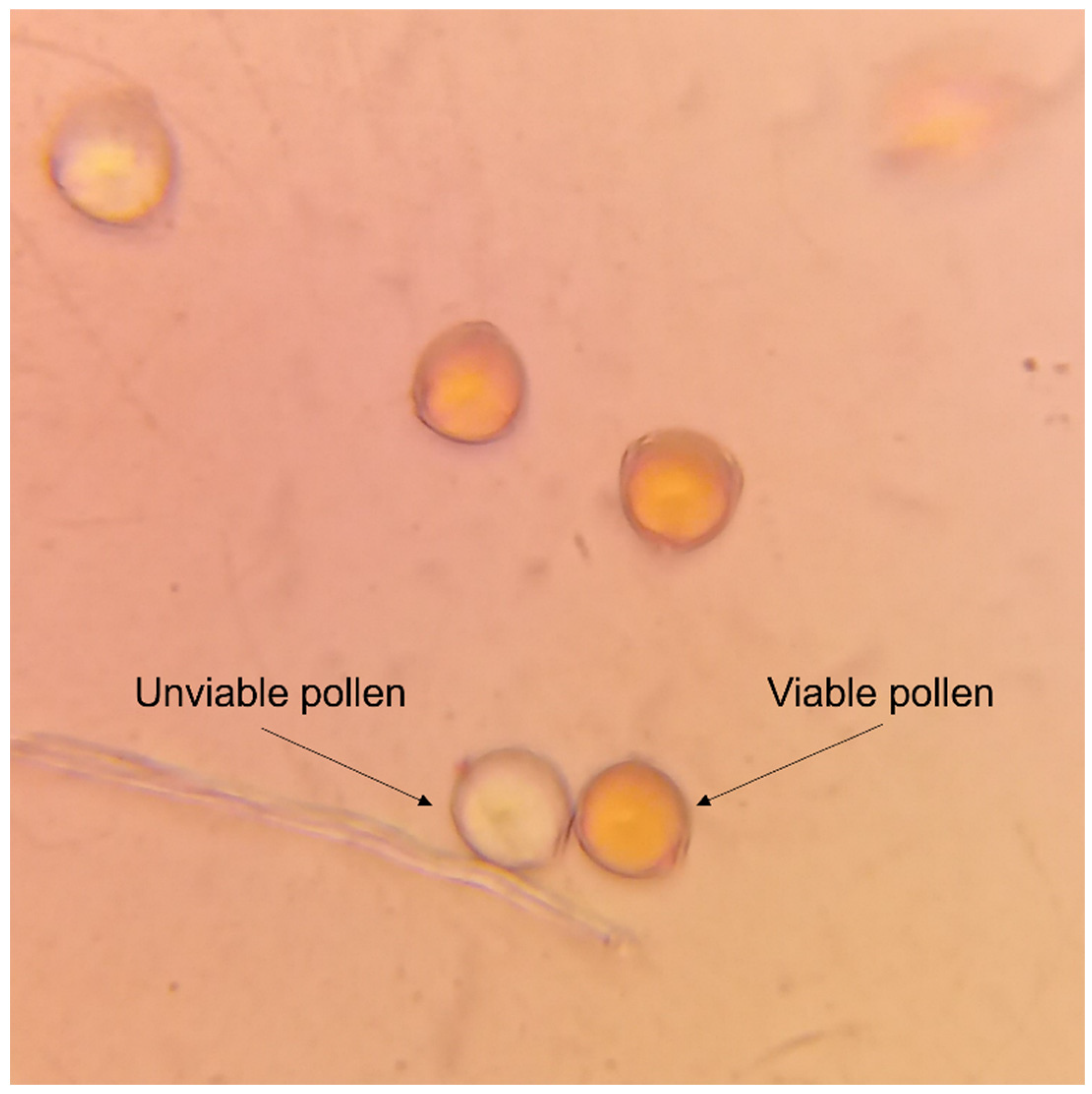
Pollen Viability Assessment
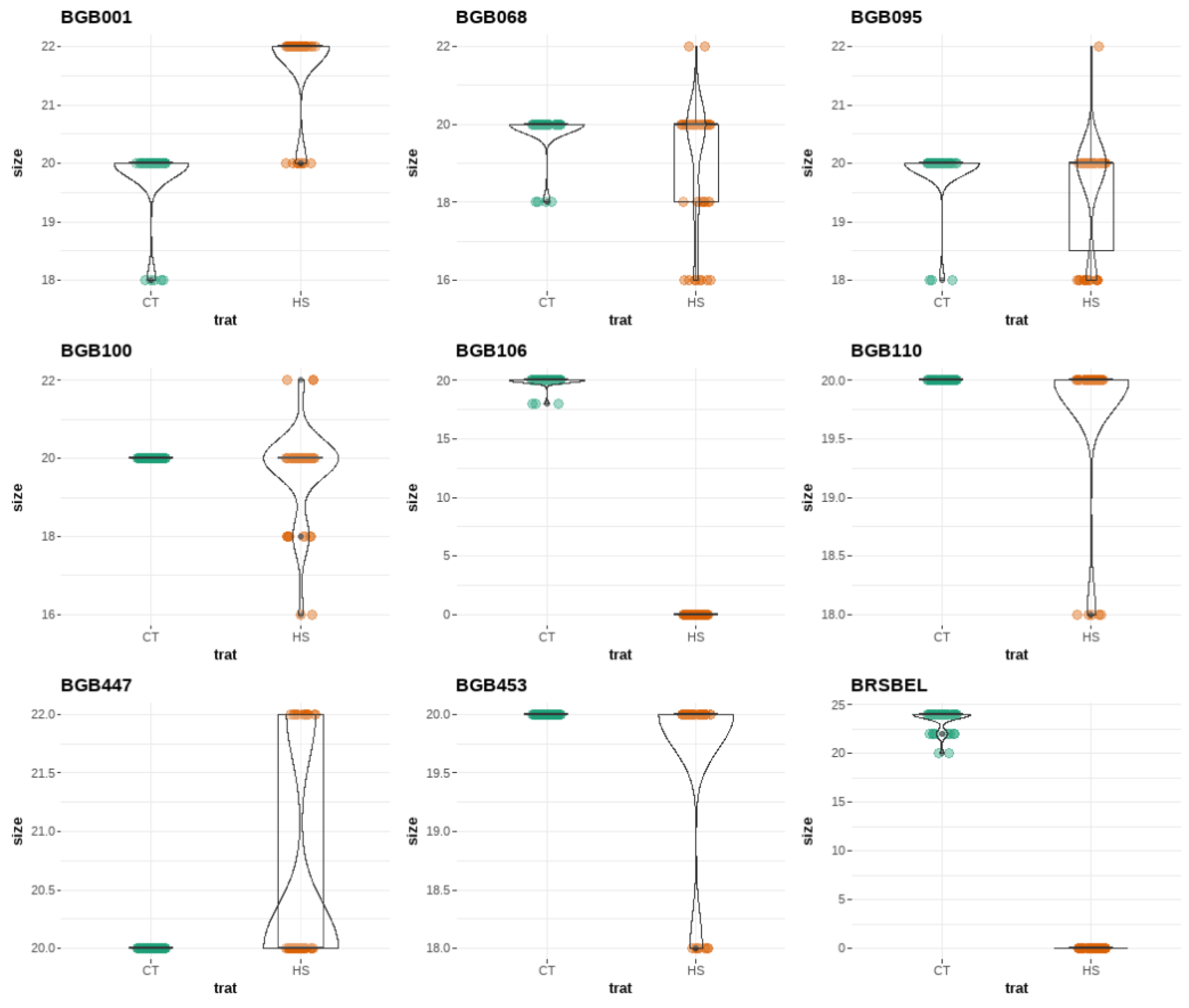
Pollen Size
Pollen Viability-Based Heat Susceptibility Index (HSIpv)
Genetic Parameters
Phenotypic Variance
Heritability
Statistical Analysis
Results and Discussion
Analysis of Variance
Pollen Viability-Based Heat Susceptibility Index (HSIpv)
Heritability for Pollen Viability
Pollen Size
Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li D, Lu X, Zhu Y, Pan J, Zhou S, Zhang X, Zhu G, Shang Y, Huang S, Zhang C (2022) The multi-omics basis of potato heterosis. Journal of Integrative Plant Biology 64:3, 671-687. [CrossRef]
- Malagamba, P. (1988) Potato production from true seed in tropical climates. HortScience 23:3, 495-500.
- Buckseth T, Tiwari JK, Singh RK, Kumar V, Sharma AK, Dalamu D, Bhardwaj V, Sood S, Kumar M, Sadawarti M, Challam CL, Naik S, Pandey NK (2022) Advances in innovative seed potato production systems in India. Frontiers in Agronomy 4:956667. [CrossRef]
- Bloomfield JA, Rose TJ, King GJ (2014) Sustainable harvest: managing plasticity for resilient crops. Plant Biotechnology Journal 12:517–533.
- IPCC: Masson-Delmotte, V, Zhai, P, Pörtner, H. O, Roberts, D, Skea, J, Shukla, P.R, Pirani, A, Moufouma-Okia, W, Péan, C, Pidcock, R, Connors, S, Matthews, J. B. R, Chen, Y, Zhou, X, Gomis, M. I, Lonnoy, E, Maycock, T, Tignor, M, Waterfield, T. (eds.)]. Global warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty 2018 In Press.
- Pörtner HO, Roberts DC, Adams H, Adler C, Aldunce P, Ibrahim ZZ (2022) Climate Change 2022: Impacts, Adaptation and Vulnerability. IPCC: Geneva, Switzerland. Available online: https://www.ipcc.ch/report/ar6/wg2/downloads/report/IPCC_AR6_WGII_FrontMatter.pdf (accessed on 1 January 2023).
- Jarvis A, Lane A, Hijmans RJ (2008) The effect of climate change on crop wild relatives. Agriculture, Ecosystem and Environment 126:13–23. [CrossRef]
- Vincent H, Amri A, Castañeda-Álvarez NP, Dempewolf H, Dulloo E, Guarino L, Hole D, Mba C, Toledo A, Maxted N (2019) Modeling of crop wild relative species identifies areas globally for in situ conservation. Communications Biology 2:1, 1-8. [CrossRef]
- Silva GO, Lopes CA (2015) Sistema de produção da Batata. Embrapa, Brasília 2. Available online: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1028425.
- Stokstad E (2019) The new potato. Science 363:6427, 574-577. [CrossRef]
- Hardigan MA, Laimbeer FPE, Newron L, Crisovan E, Hamilton JP, Vaillancourt B, Wiegert-Rininger K, Wood JC, Douches DS, Farré EM, Veilleux RE, Nuell CR (2017) Genome diversity of tuber-bearing Solanum uncovers complex evolutionary history and targets of domestication in the cultivated potato. Proceedings of the National Academy of Sciences of the United States of America 114:46, 9999–10008. [CrossRef]
- Spooner DM, Núñez J, Trujillo G, Herrera MDR, Guzmán F, Ghislain M, Nunez J, del Rosario Herrera M, Guzma F (2007) Extensive simple sequence repeat genotyping of potato landraces supports a major reevaluation of their gene pool structure and classification. Proceedings of the National Academy of Sciences 104:19398–19403. [CrossRef]
- Spooner DM, Ghislain M, Simon R, Jansky SH, Gavrilenko T (2014) Systematics, diversity, genetics, and evolution of wild and cultivated potatoes. Botanical Review 80:283–383. [CrossRef]
- Ovchinnikova A, Krylova E, Gavrilenko T, Smekalova T, Zhuk M, Knapp S, Spooner DM (2011) Taxonomy of cultivated potatoes (Solanum section Petota: Solanaceae). Botanical Journal of the Linnean Society 165:107–155. [CrossRef]
- Hawkes JG, Hjerting JP (1969) The potatoes of Argentina, Brazil, Paraguay and Uruguay. A biosystematics study. Oxford University Press, London.
- Hijmans RJ, Spooner DM (2001) Geographic distribution of wild potato species. American Journal of Botany 88, 2101–2112. [CrossRef]
- Magar BT, Acharya S, Gyawali B, Timilsena K, Upadhayaya J, Shrestha J (2021) Genetic variability and trait association in maize (Zea mays L.) varieties for growth and yield traits. Heliyon 7(9). [CrossRef]
- Bashir I, Nicolao R, Heiden G (2021) Wild potatoes: A genetic reservoir for potato breeding. In Wild germplasm for genetic improvement in crop plants, Academic Press, pp. 215-240.
- Singh B, Sharma J, Sood S, Bhardwaj V, Siddappa S, Dalamu, Kardile HS, Sharma V, Dipta B, Kumar V, Dua VK, Goutam U, Pandey NK (2023) Genetic Variations in Tuber Dry Matter (%), Yield and Mineral Concentrations in a Diversity Panel of Tetraploid Potatoes. Potato Research 66(1):179-193. [CrossRef]
- Singer SD, Laurie JD, Bilichak A, Kumar S, Singh J (2021) Genetic variation and unintended risk in the context of old and new breeding techniques. Critical Reviews in Plant Sciences 40(1):68-108. [CrossRef]
- Dempewolf H, Baute G, Anderson J, Kilian B, Smith C, Guarino L (2017) Past and future use of wild relatives in crop breeding Crop Science 57:3, 1070-1082. [CrossRef]
- Warschefsky E, Penmetsa RV, Cook DR, Von Wettberg EJ (2014) Back to the wilds: tapping evolutionary adaptations for resilient crops through systematic hybridization with crop wild relatives. American journal of Botany 101:10, 1791-1800. [CrossRef]
- Jansky S (2006) Overcoming hybridization barriers in potato. Plant Breeding 125:1, 1–12. [CrossRef]
- Bethke PC, Halterman DA, Jansky S (2017) Are we getting better at using wild potato species in light of new tools?. Crop Science 57:3, 1241-1258. [CrossRef]
- Hawkes JG (1958) Significance of wild species and primitive forms for potato breeding. Euphytica 7:3, 257–270. [CrossRef]
- Correll D (1962) The potato and its wild relatives. Texas Research Foundation, Renner, USA .
- Lindhout P, Meijer D, Schotte T, Hutten RC, Visser RG, van Eck HJ (2011) Towards F1 hybrid seed potato breeding. Potato Research 54:4, 301-312. [CrossRef]
- Jansky SH, Charkowski AO, Douches DS, Gusmini G, Richael C, Bethke PC, Spooner DM, Novy RG, de Jong H, de Jong WS, Bamberg JB, Thompson AL, Bizimungu B, Holm DG, Brown CR, Haynes KG, Sathuvalli VR, Veilleux RE, Miller JC, Bradeen JM, Jiang J (2016) Reinventing potato as a diploid inbred line–based crop. Crop Science 56:1412–1422. [CrossRef]
- Bradshaw JE (2022a) Breeding diploid F1 hybrid potatoes for propagation from botanical seed (TPS): comparisons with theory and other crops. Plants 11:9, 1121. [CrossRef]
- Bradshaw JE (2022b) A brief history of the impact of potato genetics on the breeding of tetraploid potato cultivars for tuber propagation. Potato Research 65(3): 461-501. [CrossRef]
- De Vries M, ter Maat M, Lindhout P (2016) The potential of hybrid potato for East-Africa. Open Agriculture 1:1:151-156. [CrossRef]
- Bethke PC, Halterman DA, Francis DM, Jiang J, Douches DS, Charkowski AO, Parsons J (2022) Diploid potatoes as a catalyst for change in the potato industry. American Journal of Potato Research 99:5-6, 337-357. [CrossRef]
- Endelman JB, Jansky SH (2016) Genetic mapping with an inbred line-derived F2 population in potato. Theoretical and Applied Genetics 129, 935-943. [CrossRef]
- Meijer D, Viquez-Zamora M, Van Eck HJ, Hutten RCB, Su Y, Rothengatter R, Visser RGF, Lindhout WH, Van Heusden AW (2018) QTL mapping in diploid potato by using selfed progenies of the cross S. tuberosum× S. chacoense. Euphytica 214:1-18. [CrossRef]
- Song L, Endelman JB (2023) Using haplotype and QTL analysis to fix favorable alleles in diploid potato breeding. The Plant Genome e20339. [CrossRef]
- Bethke PC, Halterman DA, Jansky SH (2019) Potato germplasm enhancement enters the genomics era. Agronomy 9:10, 575. [CrossRef]
- Watson A, Ghosh S, Williams MJ, Cuddy WS, Simmonds J, Rey MD, Hatta MAM, Hinchliffe A, Steed A, Reynolds D, Adamsky NM, Breakspear A, Korolev A, Rayuner T, Dixon LE, Riaz A, Martin W, Ryan M, Edward D, Batley J, Raman H, Carter J, Rogers C, Domoney C, Moore G, Harwood W, Nicholson P, Dieters MJ, DeLacy IH, Zhou J, Uauy C, Boden SA, Park RF, Wulff BBH, Hickey LT (2018) Speed breeding is a powerful tool to accelerate crop research and breeding. Nature Plants 4:1, 23-29. [CrossRef]
- Pécrix Y, Rallo G, Folzer H, Cigna M, Gudin S, Le Bris, M (2011) Polyploidization mechanisms: temperature environment can induce diploid gamete formation in Rosa sp. Journal of Experimental Botany 62:3587–3597. [CrossRef]
- Schindfessel C, De Storme N, Trinh HK, Geelen D (2023) Asynapsis and meiotic restitution in tomato male meiosis induced by heat stress. Frontiers in Plant Science 14. [CrossRef]
- Omidi M, Siahpoosh MR, Mamghani R, Modarresi M (2014) The influence of terminal heat stress on meiosis abnormalities in pollen mother cells of wheat. Cytologia 79:1, 49-58. [CrossRef]
- Alam MA, Seetharam K, Zaidi PH, Dinesh A, Vinayan MT, Nath UK (2017) Dissecting heat stress tolerance in tropical maize (Zea mays L.). Field Crops Research 204:110-119. [CrossRef]
- Jiang Y, Lahlali R, Karunakaran C, Kumar S, Davis AR, Bueckert RA (2015) Seed set, pollen morphology and pollen surface composition response to heat stress in field pea. Plant, Cell & Environment (11):2387-2397. [CrossRef]
- Jiang Y, Lahlali R, Karunakaran C, Warkentin TD, Davis AR, Bueckert RA (2019) Pollen, ovules, and pollination in pea: Success, failure, and resilience in heat. Plant, Cell & Environment 42(1):354-372. [CrossRef]
- Zhou Q, Cheng X, Kong B, Zhao Y, Li Z, Sang Y, Wu J, Zhang P (2022) Heat shock-induced failure of meiosis I to meiosis II transition leads to 2n pollen formation in a woody plant. Plant Physiology 189:4:2110-2127. [CrossRef]
- Hasanuzzaman M, Nahar K, Alam M, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. International Journal of Molecular Sciences 14:5, 9643-9684. [CrossRef]
- Paupière MJ, Van Heusden AW, Bovy AG (2014) The metabolic basis of pollen thermos-tolerance: perspectives for breeding. Metabolites 4:4, 889-920. [CrossRef]
- Paupière MJ, van Haperen P, Rieu I, Visser RG, Tikunov YM, Bovy AG (2017) Screening for pollen tolerance to high temperatures in tomato. Euphytica 213:1-8. [CrossRef]
- Mesihovic A, Iannacone R, Firon N, Fragkostefanakis S (2016) Heat stress regimes for the investigation of pollen thermotolerance in crop plants. Plant Reproduction 29:93-105. [CrossRef]
- Ahmed Z, Khalid M, Ghafoor A, Shah MKN, Raja GK, Rana RM, Mahmood T, Thompson AM (2022) SNP-Based Ge-nome-wide association mapping of pollen viability under heat stress in tropical Zea mays L. inbred lines. Frontiers in Genetics 13:819849. [CrossRef]
- Alexander MP (1969) Differential staining of aborted and non-aborted pollen. Stain Technology 44:117–122. [CrossRef]
- Marks GE (1954) An aceto-carmine glycerol jelly for use in pollen-fertility counts. Stain Technology 29:5, 277-277. [CrossRef]
- Ordoñez B, Orrillo M, Bonierbale MW (2017) Technical manual potato reproductive and cytological biology. International Pota-to Center (CIP) pp.69. Available online: https://cgspace.cgiar.org/server/api/core/bitstreams/5d4bb61f-c49d-40e8-92ee-e41a512f2da1/content.
- Quinn AA, Mok DWS, Peloquin SJ (1974) Distribution and significance of diplandroids among the diploid Solanums. American Potato Journal 51:16-21. [CrossRef]
- Fischer RA, Maurer R (1978) Drought resistance in spring wheat cultivars. I. Grain yield responses. Australian Journal of Agricultural Research 29:5, 897-912. [CrossRef]
- Khan I, Wu J, Sajjad M (2022) Pollen viability-based heat susceptibility index (HSIpv): A useful selection criterion for heat-tolerant genotypes in wheat. Frontiers in Plant Science 13:1064569. [CrossRef]
- Burton GW (1952) Quantitative inheritance in grasses. In Proceedings of the 6th International Grassland Congress, State College, PA, USA 17–23, pp 277–283.
- Allard RW (1960) Principles of plant breeding. John Willey and Sons. Inc. New York.
- Robinson HF, Cornstock RE, Harvey PH (1949) Estimates of heritability and degree of dominance in corn. Agronomy Journal 41:353–359.
- Ferreira EB, Cavalcanti PP, Nogueira DA (2021) ExpDes.pt: Experimental Designs package (Portuguese). R package version 1.1.2. Available online: https://cran.utstat.utoronto.ca/web/packages/ExpDes.pt/ExpDes.pt.pdf (accessed on 1 August 2023).
- RStudio Team (2020) Rstudio: Integrated Development for R. Rstudio, PBC, Boston MA. Available online: http://www.rstudio.com/ (accessed on 1 August 2023).
- Johnson HW, Robinson HF, Comstock RE (1955) Estimation of genetic and environmental variability in soybean. Agronomy Journal 47:314-318.
- Bernardo R (2010) Breeding for quantitative traits in plants. 2nd ed. Woodbury: Stemma Press.
- Begcy K, Nosenko T, Zhou LZ, Fragner L, Weckwerth W, Dresselhaus T (2019) Male sterility in maize after transient heat stress during the tetrad stage of pollen development. Plant Physiology 181:2, 683-700. [CrossRef]
- Ullah A, Nadeem F, Nawaz A, Siddique KH, Farooq M (2022) Heat stress effects on the reproductive physiology and yield of wheat. Journal of Agronomy and Crop Science 208:1, 1-17. [CrossRef]
- Kumar N, Kumar N, Shukla A, Shankhdhar SC, Shankhdhar D (2015) Impact of terminal heat stress on pollen viability and yield attributes of rice (Oryza sativa L.). Cereal Research Communications 43:4, 616-626. [CrossRef]
- Paupière MJ, Müller F, Li H, Rieu I, Tikunov YM, Visser RG, Bovy AG (2017) Untargeted metabolomic analysis of tomato pollen development and heat stress response. Plant Reproduction 30:2, 81-94. [CrossRef]
- Bamberg JB (1995) Screening potato (Solanum) species for male fertility under heat stress. American Potato Journal 72:1, 23-33. [CrossRef]
- Allard RW, Hansche PE (1964) Some parameters of population variability and their implications in plant breeding. Advances in Agronomy 16:281-325. [CrossRef]
- Nagalakshmi RM, Ravikesavan R, Paranidharan V, Manivannan N, Firoz H, Vignesh M, Senthil N (2018) Genetic variability, heritability and genetic advance studies in backcross populations of maize (Zea mays L.). Electronic Journal of Biotechnology 9:1137–1145.
- Ganesan KN, Nallathambi G, Senthil N, Tamilarasi PM (2010) Genetic divergence (Zea mays L.) Analysis in indigenous maize. Electronic Journal of Biotechnology 1:1241–1243.
- Schoper JB, Lambert RJ, Vasilas BL (1986) Maize pollen viability and ear receptivity under water and high temperature stress 1. Crop Science 26:5, 1029-1033. [CrossRef]
- Panthee DR, Kressin JP, Piotrowski A (2018) Heritability of flower number and fruit set under heat stress in tomato. HortScience 53:9, 1294-1299. [CrossRef]
- Hazra P, Ansary SH (2008) Genetics of heat tolerance for floral and fruit set to high temperature stress in tomato (Lycopersicon esculentum Mill.). SABRAO Journal of Breeding & Genetics 40(2), p 117.
- Visscher P, Hill W, Wray N (2008) Heritability in the genomics era — concepts and misconceptions. Nature Review Genetics 9:255–266. [CrossRef]
- Petr FC, Frey KJ (1966) Genotypic Correlations, Dominance, and Heritability of Quantitative Characters in Oats 1. Crop Science 6:3, 259-262. [CrossRef]
- Olakojo SA, Olaoye G (2011) Correlation and heritability estimates of maize agronomic traits for yield improvement and Striga asiatica (L.) Kuntze tolerance. African Journal of Plant Science 5:365–369. Available online: https://academicjournals.org/article/article1380018174_Olakojo%20and%20Olaoye.pdf.
- Aminu D, Izge AU (2012) Heritability and correlation estimates in maize (Zea mays L.) Under drought conditions in northern guinea and sudan savannas of Nigeria. World Journal of Agricultural Sciences 8:598–602. [CrossRef]
- Azam MG, Sarker U, Maniruzzaman M, Banik BR (2015) Genetic variability of yield and its contributing characters on CIMMYT maize inbreds under drought stress. Bangladesh Journal of Agricultural Research 39:419–426.
- Hill WG, Goddard ME, Visscher PM (2008) Data and theory point to mainly additive genetic variance for complex traits. PloS Genetics 4:2, e1000008. [CrossRef]
- Nyquist WE, Baker RJ (1991) Estimation of heritability and prediction of selection response in plant populations. Critical Reviews in Plant Sciences 10(3):235-322. [CrossRef]
- Divakara BN, Upadhyaya HD, Wani SP, Gowda CL (2010) Biology and genetic improvement of Jatropha curcas L.: a review. Applied Energy 87:3, 732-742. [CrossRef]
- Kalloo G (2012) Genetic improvement of tomato. Springer Science & Business Media.
- Liu Y, Li J, Zhu Y, Jones A, Rose RJ, Song Y (2019) Heat stress in legume seed setting: effects, causes, and future prospects. Frontiers in Plant Science 10:938. [CrossRef]
- Narayanan S, Prasad PVV, Welti R (2018) Alterations in wheat pollen lipidome during high day and night temperature stress. Plant, Cell & Environment 41:1749–1761. [CrossRef]
- Djanaguiraman M, Perumal R, Ciampitti IA, Gupta SK, Prasad PVV (2018) Quantifying pearl millet response to high temperature stress: thresholds, sensitive stages, genetic variability and relative sensitivity of pollen and pistil. Plant, Cell & Environment 41:993–1007. [CrossRef]
- Cecchetti V, Celebrin D, Napoli N, Ghelli R, Brunetti P, Costantino P, Cardarelli M (2013) An auxin maximum in the middle layer controls stamen development and pollen maturation in Arabidopsis. New Phytologist 1194–1207. [CrossRef]
- Aiqing S, Somayanda I, Sebastian SV, Singhm K, Gill K, Prasad PV, Jagadish SV (2018) Heat stress during flowering affects time of day of flowering, seed set, and grain quality in spring wheat. Crop Science 58:1, 380–392. [CrossRef]
- Chaturvedi P, Wiese AJ, Ghatak A, Zaveska DL, Weckwerth W, Honys D (2021) Heat stress response mechanisms in pollen development. New Phytologist 231:2, 571-585. [CrossRef]
- Delph LF, Johannsson MH, Stephenson AG (1997) How environmental factors affect pollen performance ecological and evolutionary perspectives. Ecology 78:1632- 1639. [CrossRef]
- Djanaguiraman M, Prasad PV, Boyle D, Schapaugh W (2013) Soybean pollen anatomy, viability and pod set under high temperature stress. Journal of Agronomy and Crop Science 199:171–177. [CrossRef]
- Djanaguiraman M, Prasad PV, Murugan M, Perumal R, Reddy UK (2014) Physiological differences among sorghum (Sorghum bicolor L. Moench) genotypes under high temperature stress. Environmental and Experimental Botany 100:43–54. [CrossRef]
- Endo M, Tsuchiya T, Hamada K, Kawamura S, Yano K, Ohshima M, Higashitani A, Watanabe M, Kawagishi-Kobayashi M (2009) High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant & Cell Physiology 50:1911–1922. [CrossRef]
- Jain M, Chourey PS, Boote KJ, Allen LHJr (2010) Short-term high temperature growth conditions during vegetative-to-reproductive phase transition irreversibly compromise cell wall invertase-mediated sucrose catalysis and microspore meiosis in grain sorghum (Sorghum bicolor). Journal of Plant Physiology 167:578–582. [CrossRef]
- Pécrix Y, Rallo G, Folzer H, Cigna M, Gudin S, Le Bris M (2011) Polyploidization mechanisms: temperature environment can induce diploid gamete formation in Rosa sp. Journal of Experimental Botany 62:3587–3597. [CrossRef]
| Local code* | Genesys code** | Species | Origin | Lat | Lon |
| BGB447 | BRA 00183755-8 | S. malmeanum | Porto Lucena, RS, Brazil | -27.856100 | -55.016400 |
| BGB100 | BRA 00167017-3 | S. chacoense | Catamarca, Argentina | -28.471588 | -65.787721 |
| BGB110 | BRA 00167028-0 | S. chacoense | Unknown | - | - |
| BGB106 | BRA 00167023-1 | S. chacoense | Unknown | - | - |
| BGB095 | BRA 00167447-2 | S. chacoense | Cordoba, Argentina | -31.13333 | -64.48333 |
| BRSIPR-BEL | BRA 00167251-8 | S. tuberosum | Brazil | - | - |
| BGB001 | BRA 00167007-4 | S. commersonii | Ijuí, RS, Brazil | -28.388000 | -53.915000 |
| BGB453 | BRA 00183760-8 | S. commersonii | Herval, RS, Brazil | -32.023600 | -53.395600 |
| BGB068 | BRA 00167420-9 | S. commersonii | São Gabriel, RS, Brazil | -30.336000 | -54.32000 |
| Df | Sum Sq | Mean Sq | F-value | Pr (>F) | |
|---|---|---|---|---|---|
| Block | 1 | 82.3 | 5 | 2.450 | 0.141 |
| Genotype | 6 | 4278.7 | 4 | 21.228 | 0.000* |
| Temperature | 1 | 869.1 | 6 | 25.872 | 0.000* |
| Genotype×Temperature | 6 | 340.9 | 3 | 1.532 | 0.200 |
| Residue | 13 | 436.7 | 2 | ||
| Total | 27 | 6007.7 | 1 | ||
| CV (%) | 7.13 |
| Components | Pollen Viability |
|---|---|
| phenotypic variance | 210.29 |
| genotypic variance | 164.10 |
| residual variance | 35.66 |
| genotype × treatment interaction variance | 10.54 |
| broad sense heritability (%) | 78% |
| accuracy in genotypic selection | 0.96 |
| phenotypic coefficient of variation | 17.84 |
| genotypic coefficient of variation | 15.76 |
| genetic gain % | 28.68 |
| Genetic advance | 23.30 |
| General average | 81.29 |
| Genotype | Genesys code | Species | Temperature treatment | HSIpv | Score | Heritability (h2) % | |
|---|---|---|---|---|---|---|---|
| CT | HS | ||||||
| BGB447 | BRA 00183755-8 | S. malmeanum | 100 aA* | 91.5 abcA | 0.66 | Moderately tolerant | 91.32 |
| BGB100 | BRA 00167017-3 | S. chacoense | 98.5 aA | 94.0 abA | 0.36 | Tolerant | 88.35 |
| BGB110 | BRA 00167028-0 | S. chacoense | 96.0 aA | 83.5 abcdA | 1.01 | Susceptible | 88.56 |
| BGB095 | BRA 00167447-2 | S. chacoense | 89.5 abcdA | 71.5 bcdeA | 1.57 | Susceptible | 83.16 |
| BGB001 | BRA 00167007-4 | S. commersonii | 86.5 abcdA | 65.5 deA | 1.89 | Susceptible | 58.82 |
| BGB453 | BRA 00183760-8 | S. commersonii | 69.0 bA | 69.5 bcA | 0.06 | Tolerant | 83.82 |
| BGB068 | BRA 00167420-9 | S. commersonii | 68.5 cdeA | 54.5 eA | 1.59 | Susceptible | 87.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





