1. Introduction
Nanotechnology has been increasingly used in medicine, including scar removal [
1]. Scar removal is a complex process that involves the regeneration of damaged skin tissue, and nanotechnology offers unique solutions to this problem [
2]. One promising application of nanotechnology in scar removal is nanofibers [
3]. Nanofibers are tiny fibres with diameters in the nanometre range, which can be produced from various materials such as polymers, ceramics, and metals [
4]. These fibres can be used to create scaffolds that support the growth of new skin tissue [
5]. Nanofibers can also be loaded with drugs or growth factors to promote tissue regeneration and reduce scarring [
6]. Another application of nanotechnology in scar removal is using nanocarriers for drug delivery [
7]. Nanocarriers are tiny particles that can be loaded with drugs and targeted to specific areas of the body [
8]. In scar removal, nanocarriers can deliver drugs or growth factors directly to the site of the scar, promoting tissue regeneration and reducing scarring [
2]. Nanotechnology has also been used to develop new materials for skin regeneration [
9].
While many of these applications are still experimental, they offer exciting possibilities for the future of scar removal and skin repair. With continued research and development, nanotechnology could provide more effective and efficient solutions for scar removal and skin regeneration.
2. Understanding Scar Formation and Wound Healing: A Molecular Perspective
Scar formation is a complex process during wound healing involving a series of molecular and cellular events. The initial phase of wound healing is the inflammatory phase, where damaged tissues release pro-inflammatory cytokines and growth factors. This results in the recruitment of immune cells, such as neutrophils and macrophages, to the wound site [
10]. During the subsequent proliferative phase, fibroblasts migrate to the wound site and synthesize extracellular matrix (ECM) proteins, such as collagen and fibronectin. This is accompanied by the formation of granulation tissue, which provides a scaffold for new tissue growth [
11]. Finally, during the remodelling phase, the ECM is reorganized and degraded, forming a scar [
12]. The balance between ECM synthesis and degradation during this phase is critical in determining the quality of the scar tissue [
13]. Several molecular pathways that play important roles in scar formation and wound healing have been identified. For example, transforming growth factor-beta (TGF-β) signalling is critical in regulating ECM synthesis during the proliferative phase [
14]. In addition, the Wnt signalling pathway has been implicated in regulating cell proliferation and differentiation during wound healing [
15].
Understanding the molecular mechanisms underlying scar formation and wound healing is essential for developing new therapies for scar removal and tissue regeneration. By targeting specific molecular pathways, it may be possible to promote tissue regeneration while reducing scar formation.
2. Wound Healing Mechanism
The human skin is the body's largest organ, which serves as a protective barrier against the external environment and prevents dehydration and injuries. When the skin is injured, a sequence of events begins immediately. Cutaneous wound repair consists of several phases overlapping, including the inflammatory response, formation of granulation tissue that involves angiogenesis and re-epithelialization, and matrix remodelling [
16,
17].
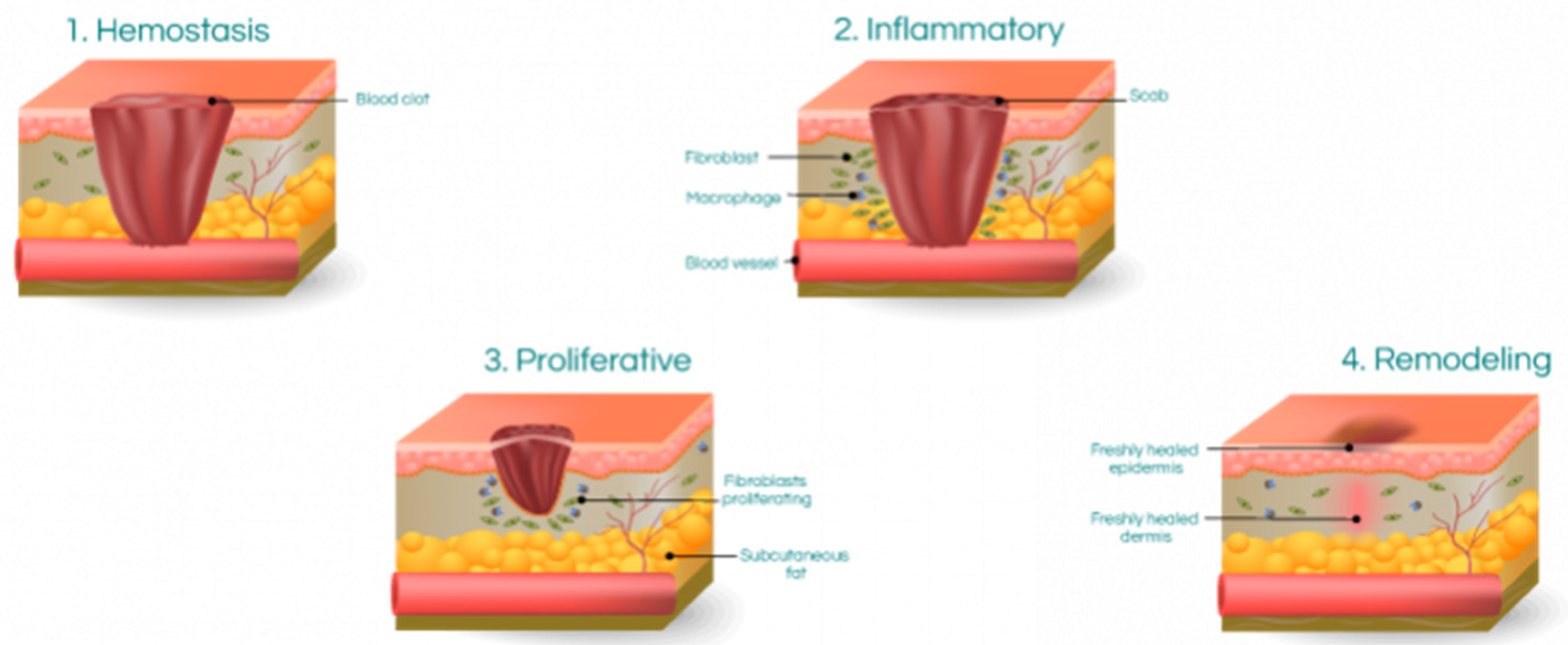
Figure 1 illustrates the three fundamental phases of wound healing.
Integumental injuries refer to outer wounds, while inner or closed wounds involve injuries or ruptures of inner organs and tissues with intact skin. Skin wounds can be closed by either regeneration or repair. Regeneration involves the specific replacement of the tissue, such as superficial epidermis, mucosa, or fetal skin, whereas repair is an unspecific form of healing that involves fibrosis and scar formation. Unfortunately, scar formation is the predominant form of healing in adult skin wounds [
18,
19]. The interplay between cells, growth factors, and cytokines leads to skin closure after an injury. Although disruptions in this delicate balance can occur, recent findings suggest that the absence of a particular cell type or mediator can be compensated for by others involved in wound healing, allowing the repair process to proceed [
20].
2.1. Wound Healing Phases
The process of wound healing involves four interdependent and interrelated phases: hemostasis, inflammation, proliferation, and tissue remodelling or resolution. These phases are highly integrated and overlap with each other [
21]. For successful wound healing to occur, these phases with their physiological functions must take place in a specific order, at precise timing, and for a specific duration with optimal intensity [
22].
Table 1 shows wound healing phases and their timeframe.
The wound-healing process is divided into four phases: hemostasis, inflammation, proliferation, and tissue remodelling or resolution. During the hemostasis phase, the blood vessels constrict to reduce blood loss, and platelets aggregate to form a clot. In the inflammation phase, immune cells, such as neutrophils and macrophages, are recruited to the wound site to clear debris and pathogens. Growth factors and cytokines are also released during this phase, stimulating cell proliferation and angiogenesis.
The proliferation phase is characterized by the migration and proliferation of fibroblasts and keratinocytes, leading to the formation of granulation tissue and re-epithelialization of the wound. During the tissue remodelling or resolution phase, the extracellular matrix is reorganized and degraded, resulting in scar formation or tissue regeneration, depending on the injury's extent and the wound's location. Fibroblasts, originating from mesenchymal cells, are vital in wound healing by producing new extracellular matrix components. Matrix production results in the restoration of tissue homeostasis [
25]. The tissue remodelling phase is the final stage of wound healing and can last up to one year after the injury. During this phase, collagenase enzymes secreted by fibroblasts, macrophages, and neutrophils break down collagen molecules, leading to degradation [
26]. As collagen is broken down during the tissue remodelling phase, it is gradually replaced by Type I collagen. This replacement increases the tensile strength of the new tissue over time [
12]. Collagen fibres in wound tissue are thinner than those of normal dermal collagen. Over time, these thinner fibres will thicken and organize themselves along the stress lines of the injury. However, the resulting scar tissue will never be as muscular as the normal tissue that preceded it [
23,
27,
28]. Several studies have indicated that variations in inflammation during the wound-healing process are strongly linked to the extent of scar tissue formation [
29]. Fetal wound healing is an example of the correlation between inflammation and scar tissue formation. Fetal wounds lack typical inflammatory markers and are known to be
"scarless" up to a certain age [
12]. In adult wound healing, polymorphonuclear leukocytes are the first immune cells recruited to the wound site, followed by macrophages and lymphocytes. In contrast, fetal wounds lack polymorphonuclear leukocytes, and as the healing process progresses, fetal macrophages enter the wound site in smaller numbers than those found in adults [
2]. The lack of an inflammatory response in fetal wounds may be due to a deficiency in appropriate signalling and the immature state of fetal inflammatory cell populations. In non-healing wounds, the failure to transition from the inflammatory phase to the proliferative phase can result in abnormal wound repair.
2.2. Factors Affecting Wound Healing
Several factors can affect wound healing, including age, underlying medical conditions, medications, nutrition, and lifestyle factors. For example, older adults may experience delayed wound healing due to reduced cell proliferation and decreased growth factor production. Diabetes, hypertension, and other chronic diseases can also impair wound healing by affecting blood flow and immune function [
30]. Medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs), can impair wound healing by reducing the inflammatory response and inhibiting the production of growth factors [
31]. Nutritional deficiencies, such as vitamin C deficiency, can impair wound healing by affecting collagen synthesis and angiogenesis [
19]. Lifestyle factors, such as smoking and alcohol consumption, can also impair wound healing by reducing blood flow and oxygen delivery to the wound site [
30]. Impaired wound healing can have significant consequences, such as chronic wounds, infection, and scarring. Chronic wounds, such as pressure ulcers and diabetic foot ulcers, can result from impaired wound healing due to underlying medical conditions or other factors [
32]. Infection can also occur when the inflammatory response is inadequate or when the wound is contaminated with bacteria. Scarring can result from excessive collagen deposition during tissue remodelling, leading to hypertrophic or keloid scars [
24].
The wound-healing process is a complex series of events that involves the coordinated effort of cells, growth factors, cytokines, and extracellular matrix components. Numerous factors can influence wound healing, leading to impaired or improper tissue repair.
3. Scar Formation
Scarring is a significant burden on healthcare systems worldwide and has prompted extensive research to prevent and reduce its occurrence [
33,
34]. A fully mature scar comprises Type I collagen [
35]. In scar tissue, Type I collagen is arranged in bundles parallel to the skin's surface, unlike the non-parallel arrangement in normal skin [
36]. The epidermal basement membrane in scar tissue is flatter than normal skin since it lacks the rete pegs that usually penetrate the dermis [
9]. Scar tissue lacks other typical dermal structures, such as hair follicles or sweat glands [
37,
38]. As scar tissue matures, the concentration of fibroblasts within the tissue decreases. Scar tissue also has less elastin in its extracellular matrix than healthy tissue, which contributes to the loss of tensile strength and increases the likelihood of re-injury [
39]. The degree of fibrosis after an injury varies depending on the organ or tissue affected. When the molecular regulation of the tissue remodelling phase of wound healing is inefficient or disrupted, problematic scars such as hypertrophic scars and keloids can develop. Hypertrophic scars, such as burns, usually arise following surgery or trauma [
40]. Scars are formed due to wound healing, which involves four phases: hemostasis, inflammation, proliferation, and tissue remodelling. The tissue remodelling phase is the final stage of wound healing, during which collagen is broken down and replaced by Type I collagen. The parallel arrangement of collagen fibres, reduced tensile strength, and the absence of typical dermal structures such as hair follicles and sweat glands characterize scar tissue. Scar tissue can vary in its degree of fibrosis depending on the tissue or organ affected, and inefficient regulation of the tissue remodelling phase can lead to problematic scars such as hypertrophic scars and keloids.
3.1. Scar Types
3.1.1. Acne Scars
Acne scarring is a common concern among patients seeking facial rejuvenation. While acne and its resulting scars typically occur in one's teenage or early adulthood, patients may request treatment for acne scarring at any age. The three main types of acne scars are ice pick, rolling, and boxcar, and the ideal treatment for each subtype may vary. However, most patients have a combination of these subtypes, making determining the optimal treatment approach challenging. One of the primary obstacles to developing a standardized approach for treating acne scars is the lack of high-quality studies. Existing studies are often small, biased, lack uniform baseline variables and outcomes, or have a limited follow-up period [
41].
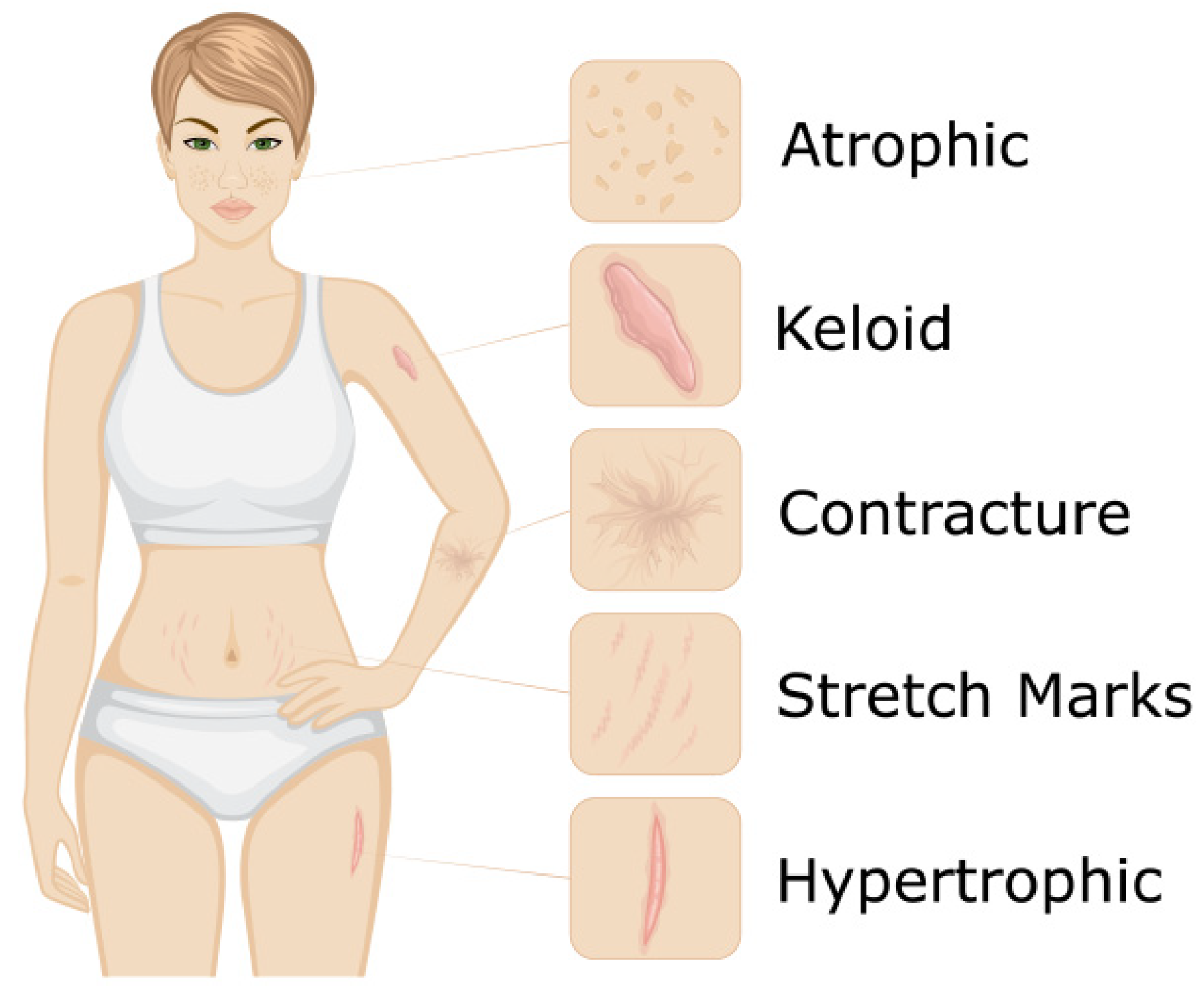
Figure 2 illustrates of type of scars.
Ice Pick Scars
Ice pick scars are narrow, deep scars that resemble the puncture made by an ice pick. Due to their depth, they can be more challenging to treat than rolling or boxcar scars. However, some treatment options can provide excellent results. Punch excision is an effective treatment for Ice Pick scars. Although this procedure involves creating a new scar, the scars from the punch excision can often heal to barely noticeable [
42]. Scars should be at least 4-5 mm apart to be treated simultaneously. If scars are too close to each other, the skin's surface will experience too much tension, which can hinder optimal healing. In cases where scars are located within 4-5 mm of one another, it is best to wait four weeks between treatments to achieve the best long-term results [
43].
Rolling Scars
Rolling scars are broad depressions in the skin with sloping edges, creating a rolling or wave-like appearance. They are typically caused by damage beneath the skin's surface, which can cause the subcutaneous tissue to adhere to deeper structures. As a result, the surface of the skin appears uneven and dimpled. Rolling scars can be treated with several modalities, including micro-needling and laser resurfacing. These treatments can help to break up the underlying fibrous tissue, stimulate collagen production, and promote smoother skin texture. However, the ideal treatment approach may vary based on the individual's skin type and scar severity [
44].
Boxcar scars
Boxcar scars are angular or rectangular-shaped depressions in the skin with well-defined edges. They are typically wider than Ice Pick scars and have a flat base. Boxcar scars are caused by the destruction of collagen, resulting in a depression in the skin. The ideal treatment approach for boxcar scars may vary depending on the severity of the scarring, but commonly used treatments include punch excision, dermal fillers, and laser resurfacing. Punch excision involves surgically removing the scar and then suturing the surrounding skin together, while dermal fillers can help to lift the scar and create a smoother skin surface. Laser resurfacing can help to promote collagen production and improve the overall appearance of the skin [
45,
46].
Erythematous Scars
Erythematous scars are pink or red and are caused by excess blood flow to the scar tissue. They are typically raised, thick, and itchy and can be caused by various factors, including acne, surgery, or injury. Treatment options for erythematous scars include topical creams, silicone sheets or gels, and laser therapy. Topical creams and silicone sheets or gels can help to reduce redness and inflammation and promote healing. Laser therapy can help reduce the scar's size and stimulate collagen production, resulting in a smoother, less noticeable scar. The ideal treatment approach may vary depending on the severity and cause of the scar [
47].
3.1.2. Surgical Scars
Treating surgical scars requires consideration of various factors, such as the timing of interventions and the specific type of scar. Scars can be erythematous, raised, or depressed and may have different treatments. Patients may inquire about the benefits of silicone gel sheeting in the immediate aftermath of surgery, but evidence for its effectiveness is weak and susceptible to bias. Physicians must thoroughly understand scar management techniques, including topical treatments, injections, surgery, and laser therapy, to provide optimal patient care. Early intervention and wound care can also improve scar outcomes [
48].
Hypertrophic Scars
Hypertrophic scars are raised, red, and thick scars that form due to an overproduction of collagen during healing. They are common after surgery, burns, or trauma and can cause physical and emotional discomfort for patients. Treatment options for hypertrophic scars include topical creams, corticosteroid injections, silicone sheets or gels, laser therapy, and surgery. Surgery may be considered if other treatments are ineffective, but it carries a risk of recurrence or further scarring. A combination of these treatments may be used to achieve the best results for each patient [
49].
Atrophic Scars
Atrophic surgical scars are characterized by depression or indentation in the skin and present different challenges than hypertrophic scars. They can be more difficult to treat and require a tailored approach. One treatment option for atrophic surgical scars is fractional laser therapy. This therapy involves using either non-ablative or fully ablative lasers to target the scar tissue, stimulate collagen production, and improve the scar's colour, texture, thickness, and patient satisfaction. Other treatments for atrophic surgical scars may include dermal fillers, micro-needling, or surgical scar revision. The choice of treatment depends on the severity and location of the scar, as well as the patient's skin type and medical history. Physicians must have a thorough understanding of scar management techniques to provide optimal care for their patients with atrophic surgical scars [
50,
51,
52].
4. Current Scar Treatments
There are various options for scar treatment, depending on the type and severity of the scar. Topical treatments such as creams, gels, and silicone sheets can help improve the appearance of scars by reducing redness, flattening the scar, and improving texture [
47]. Injections of corticosteroids can treat hypertrophic and keloid scars by reducing inflammation and flattening the scar [
53]. Surgical excision is a standard treatment for hypertrophic and keloid scars, while laser therapy can improve the scars' appearance and stimulate collagen production [
35]. Cryotherapy involves freezing the scar tissue, which can help to reduce inflammation and flatten the scar [
36]. Pressure therapy involves applying pressure to the scar using a special bandage or dressing to reduce inflammation and flatten the scar. Radiation therapy can also be used to treat keloid scars by reducing the size of the scar and preventing its recurrence [
43].
When treating acne scars, ice-pick scars may be treated with punch excision, while rolling and boxcar scars can be treated with various methods such as dermal fillers, chemical peels, or micro-needling. Atrophic surgical scars can be treated with fractional laser therapy, dermal fillers, micro-needling, or surgical scar revision.
Table 2 shows general treatment methods for the scar.
5. Nanoparticle-Based Topical Treatments for Scar Removal: Mechanisms and Efficacy
Nanoparticle-based topical treatments have emerged as a promising approach for scar removal due to their unique properties, such as high surface area-to-volume ratio and the ability to encapsulate drugs and growth factors [
63]. These nanoparticles can target the specific cells involved in wound healing and modulate their behaviour to promote scar reduction and tissue regeneration [
6]. Some nanoparticles that have shown promise in scar removal include liposomes, solid lipid nanoparticles, and polymeric nanoparticles. These nanoparticles can be loaded with various therapeutic agents, such as anti-inflammatory drugs, growth factors, and antioxidants, further enhancing their efficacy [
39]. One of the mechanisms by which nanoparticle-based topical treatments work is through the modulation of the inflammatory response in the wound healing process [
19]. By reducing inflammation, these nanoparticles can prevent excessive scar formation and promote tissue regeneration [
28]. Additionally, these nanoparticles can enhance collagen synthesis and remodelling, further improving the appearance of scars. Several studies have demonstrated the efficacy of nanoparticle-based topical treatments in scar removal. A study demonstrated that polymeric nanoparticles loaded with an anti-inflammatory drug reduced scar formation and improved tissue regeneration in a mouse model [
64]. Nanoparticle-based topical treatments are an exciting area of research for scar removal and tissue regeneration. These treatments have the potential to improve the efficacy of traditional scar treatments by targeting specific cellular and molecular mechanisms involved in scar formation [
65]. Different types of nanoparticles, including liposomes, gold nanoparticles, and quantum dots, have been shown to effectively deliver therapeutic agents to the scar site and promote wound healing through various mechanisms, such as reducing inflammation, enhancing collagen production, and promoting angiogenesis [
66]. However, more research is needed to fully understand these treatments' safety, efficacy, and long-term effects. Nanoparticle-based topical treatments offer a promising avenue for scar removal and tissue regeneration.
Table 3. General overview of nanoparticles on scar treatment.
Nanomaterials for wound healing have become increasingly popular due to their unique physicochemical and biological properties [
67]. These nanoparticles have shown potential for promoting hemostasis, anti-infection, immunoregulation, and proliferation and can be used in wound dressings for the sustained delivery of therapeutic agents. Additionally, nanoparticles can detect and treat bacterial infections by absorbing light and transforming it into heat, resulting in bacterial death [
68]. However, there are challenges in translating nanoparticle-based wound dressings from laboratory experiments to clinical applications, including reproducibility, toxicity, and histocompatibility [
69]. Animal trials are often used to examine the behaviour of nanoparticle-based wound dressings, but there is a need for alternative preclinical studies due to differences between human and animal models. Intelligent wound dressings that use nanoparticles and chitosan-based formulations to detect and treat bacterial infections show promise for future wound healing [
70].
6. Biocompatibility and Safety Considerations of Nanotechnology-Based Scar Removal
Nanotechnology has significantly shifted wound healing and scar removal [
63]. Nanotechnology-based approaches have revolutionized the treatment of wounds and scars, providing faster healing and better cosmetic outcomes. However, the biocompatibility and safety of these nanotechnology-based approaches remain a concern. One of the primary concerns is the potential toxicity of nanoparticles (NPs) used in wound healing and scar removal. Some studies have shown that NPs can cause damage to cells and tissues, leading to inflammation and oxidative stress. However, the toxicity of NPs depends on their size, shape, and surface properties [
71]. The biodistribution of NPs in the body is another concern. NPs can accumulate in organs and tissues, leading to long-term adverse effects. The accumulation of NPs in the liver, lungs, and spleen has been reported in some studies. However, more research is needed to fully understand the biodistribution of NPs and potential toxicity [
72]. Immune response to NPs is also a concern. The immune system can recognize NPs as foreign particles and mount an immune response against them. This immune response can lead to inflammation and tissue damage. However, the immune response to NPs depends on their size, shape, and surface properties [
73].
The potential for NPs to induce genotoxicity and mutagenesis is also a concern. Some studies have shown that NPs can damage DNA and lead to mutations. However, the genotoxicity and mutagenesis of NPs depend on their size, shape, and surface properties [
74]. The biodegradability of NPs is an important consideration for their safety. NPs that are not biodegradable can accumulate in the body, leading to long-term adverse effects [
75]. Therefore, it is essential to develop biodegradable NPs that can be eliminated from the body. The interaction of NPs with other drugs or chemicals is another concern [
76]. NPs can interact with other drugs or chemicals, leading to unexpected toxicity or adverse effects. Therefore, it is essential to consider the potential interactions of NPs with other drugs or chemicals when developing nanotechnology-based approaches for wound healing and scar removal. The potential for NPs to enter the bloodstream is also a concern. NPs can enter the bloodstream and travel to other body parts, leading to systemic toxicity. Therefore, it is essential to develop NPs that can be localized to the site of the wound or scar. The potential for NPs to cause allergic reactions is another concern [
77]. NPs can induce allergic reactions in some individuals, leading to inflammation and tissue damage. Therefore, it is essential to consider the potential for NPs to cause allergic reactions when developing nanotechnology-based approaches for wound healing and scar removal [
53]. The long-term effects of NPs on the environment are also a concern. NPs can enter the environment through wastewater, leading to potential ecological damage. Therefore, it is essential to develop biodegradable NPs that do not have adverse effects on the environment. The regulatory approval of nanotechnology-based approaches for wound healing and scar removal is a concern. The safety and efficacy of these approaches must be demonstrated through rigorous preclinical and clinical trials before regulatory approval can be obtained.
The biocompatibility and safety of nanotechnology-based approaches for wound healing and scar removal remain a concern. Potential toxicity, biodistribution, immune response, genotoxicity, mutagenesis, biodegradability, interactions with other drugs or chemicals, the potential for systemic toxicity and allergic reactions, long-term environmental effects, regulatory effects, and regulatory approval are all crucial factors to consider when developing and evaluating new medical treatments or substances. Understanding these aspects helps ensure the safety and efficacy of new therapies, minimize adverse side effects, and protect both human health and the environment. Therefore, it is essential to conduct rigorous research and development to ensure the safety and efficacy of these approaches.
7. Conclusion: Future Perspectives and Clinical Implications of Nanotechnology in Scar Removal
Nanotechnology-based scar removal holds great promise for improving the outcomes of scar treatments. While the field is still in its early stages, significant progress has been made in developing nanomaterials that can promote wound healing, reduce inflammation, and enhance the delivery of therapeutic agents. As the understanding of the mechanisms of nanomaterials in scar removal continues to grow, more targeted and effective treatments will likely emerge. One key focus area will be developing nanomaterials that can penetrate deeper into the skin to target the underlying fibrosis that causes scars.
Another promising area of research is using nanomaterials in combination with other therapies, such as laser therapy, to enhance their efficacy. For example, combining nanoparticle-based drug delivery with laser therapy has significantly improved scar treatment outcomes. In addition to their potential for scar removal, nanomaterials offer other applications in wound healing and regenerative medicine. For example, they can be used to develop advanced wound dressings that can deliver therapeutic agents in a sustained and controlled manner.
Despite their potential, significant challenges remain to be overcome before nanomaterial-based scar removal becomes a clinical reality. One of the main challenges is ensuring the safety and biocompatibility of these materials. While many nanomaterials have been shown to be safe and biocompatible in vitro, long-term in vivo studies cannot still evaluate their safety in humans. It is also essential to consider the potential for toxicity to the patient and the environment, as these materials are often difficult to degrade. In addition, there is a need for standardized protocols for the synthesis, characterization, and testing of nanomaterials for scar removal. This will help ensure that these materials are developed and tested consistently and reproducibly and will enable comparison between different studies. Besides these challenges, the potential benefits of nanotechnology-based scar removal are significant, and we will likely see continued growth and development in this field in the coming years. With suitable approaches to safety and regulation, it is possible that these materials could become a routine part of scar removal treatments in the future.
In summary, nanotechnology-based scar removal offers a promising approach to improving the outcomes of scar treatments. As research advances, more targeted and effective treatments will likely emerge. However, it is crucial to ensure the safety and biocompatibility of these materials before they can become a clinical reality. With suitable approaches to regulation and safety, nanomaterials could become an essential tool in treating scars and other wound-healing applications.
References
- Haleem A, Javaid M, Singh RP, Rab S, Suman R. Applications of nanotechnology in medical field: a brief review. Global Health Journal 2023. [CrossRef]
- Mackool RJ, Gittes GK, Longaker MT. Scarless healing. The fetal wound. Clin Plast Surg 1998;25:357–65.
- Su C, Chen J, Xie X, Gao Z, Guan Z, Mo X, et al. Functionalized Electrospun Double-Layer Nanofibrous Scaffold for Wound Healing and Scar Inhibition. ACS Omega 2022;7:30137–48. [CrossRef]
- Barhoum A, Bechelany M, Hamdy Makhlouf AS. Handbook of Nanofibers. 2019. [CrossRef]
- Cavo M, Serio F, Kale NR, D’Amone E, Gigli G, del Mercato LL. Electrospun nanofibers in cancer research: from engineering of in vitro 3D cancer models to therapy. Biomater Sci 2020;8:4887–905. [CrossRef]
- Pandey VK, Ajmal G, Upadhyay SN, Mishra PK. Nano-fibrous scaffold with curcumin for anti-scar wound healing. Int J Pharm 2020;589:119858. [CrossRef]
- Contreras-Cáceres R, Cabeza L, Perazzoli G, Díaz A, López-Romero JM, Melguizo C, et al. Electrospun Nanofibers: Recent Applications in Drug Delivery and Cancer Therapy. Nanomaterials 2019;9:656. [CrossRef]
- Contreras-Cáceres R, Cabeza L, Perazzoli G, Díaz A, López-Romero JM, Melguizo C, et al. Electrospun Nanofibers: Recent Applications in Drug Delivery and Cancer Therapy. Nanomaterials 2019;9:656. [CrossRef]
- Takeo M, Lee W, Ito M. Wound healing and skin regeneration. Cold Spring Harb Perspect Med 2015;5:a023267. [CrossRef]
- Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol 2005;15:599–607. [CrossRef]
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 2014;6:265sr6. [CrossRef]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–21. [CrossRef]
- Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech 2010;43:146–55. [CrossRef]
- Wipff P-J, Rifkin DB, Meister J-J, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol 2007;179:1311–23. [CrossRef]
- Alarcon-Barrera JC, Kostidis S, Ondo-Mendez A, Giera M. Recent advances in metabolomics analysis for early drug development. Drug Discovery Today 2022;27:1763–73. [CrossRef]
- Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol 2007;25:9–18. [CrossRef]
- Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res 2012;49:35–43. [CrossRef]
- Krafts KP. Tissue repair: The hidden drama. Organogenesis 2010;6:225–33. [CrossRef]
- Enoch S, Leaper D. Basic science of wound healing. Surgery (Oxford) 2006;23:31–7. [CrossRef]
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 2007;127:514–25. [CrossRef]
- Gosain A, DiPietro LA. Aging and wound healing. World J Surg 2004;28:321–6. [CrossRef]
- Mathieu D, Linke J-C, Wattel F. Non-Healing Wounds. Handbook on Hyperbaric Medicine, 2006, p. 401–28. [CrossRef]
- Schilling JA. Wound healing. Surg Clin North Am 1976;56:859–74. [CrossRef]
- Broughton G, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg 2006;117:1e-S-32e-S. [CrossRef]
- Darby IA, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol 2014;7:301–11. [CrossRef]
- Caley MP, Martins VLC, O'Toole EA. Metalloproteinases and Wound Healing. Adv Wound Care (New Rochelle) 2015;4:225–34. [CrossRef]
- White WL, Brody GS, Glaser AA, Marangoni RD, Beckwith TG, Must JS, et al. Tensiometric studies of unwounded and wounded skin: results using a standardized testing method. Ann Surg 1971;173:19–25. [CrossRef]
- Corr DT, Gallant-Behm CL, Shrive NG, Hart DA. Biomechanical behavior of scar tissue and uninjured skin in a porcine model. Wound Repair Regen 2009;17:250–9. [CrossRef]
- Lim X, Tateya I, Tateya T, Muñoz-Del-Río A, Bless DM. Immediate inflammatory response and scar formation in wounded vocal folds. Ann Otol Rhinol Laryngol 2006;115:921–9. [CrossRef]
- Guo S, DiPietro LA. Factors Affecting Wound Healing. J Dent Res 2010;89:219–29. [CrossRef]
- Anderson K, Hamm RL. Factors That Impair Wound Healing. J Am Coll Clin Wound Spec 2014;4:84–91. [CrossRef]
- Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol 2020;10:200223. [CrossRef]
- Mirastschijski U, Sander JT, Zier U, Rennekampff HO, Weyand B, Vogt PM. The cost of post-burn scarring. Ann Burns Fire Disasters 2015;28:215–22.
- Barnes LA, Marshall CD, Leavitt T, Hu MS, Moore AL, Gonzalez JG, et al. Mechanical Forces in Cutaneous Wound Healing: Emerging Therapies to Minimize Scar Formation. Adv Wound Care (New Rochelle) 2018;7:47–56. [CrossRef]
- Marshall CD, Hu MS, Leavitt T, Barnes LA, Lorenz HP, Longaker MT. Cutaneous Scarring: Basic Science, Current Treatments, and Future Directions. Adv Wound Care (New Rochelle) 2018;7:29–45. [CrossRef]
- van Zuijlen PPM, Ruurda JJB, van Veen HA, van Marle J, van Trier AJM, Groenevelt F, et al. Collagen morphology in human skin and scar tissue: no adaptations in response to mechanical loading at joints. Burns 2003;29:423–31. [CrossRef]
- Kiani MT, Higgins CA, Almquist BD. The Hair Follicle: An Underutilized Source of Cells and Materials for Regenerative Medicine. ACS Biomater Sci Eng 2018;4:1193–207. [CrossRef]
- Fu X-B, Sun T-Z, Li X-K, Sheng Z-Y. Morphological and distribution characteristics of sweat glands in hypertrophic scar and their possible effects on sweat gland regeneration. Chin Med J (Engl) 2005;118:186–91.
- Mulholland EJ. Electrospun Biomaterials in the Treatment and Prevention of Scars in Skin Wound Healing. Frontiers in Bioengineering and Biotechnology 2020;8.
- Carswell L, Borger J. Hypertrophic Scarring Keloids. StatPearls, Treasure Island (FL): StatPearls Publishing; 2023.
- Abdel Hay R, Shalaby K, Zaher H, Hafez V, Chi C-C, Dimitri S, et al. Interventions for acne scars. Cochrane Database Syst Rev 2016;4:CD011946. [CrossRef]
- Levy LL, Zeichner JA. Management of acne scarring, part II: a comparative review of non-laser-based, minimally invasive approaches. Am J Clin Dermatol 2012;13:331–40. [CrossRef]
- Jacob CI, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol 2001;45:109–17. [CrossRef]
- Barikbin B, Akbari Z, Yousefi M, Dowlati Y. Blunt Blade Subcision: An Evolution in the Treatment of Atrophic Acne Scars. Dermatol Surg 2017;43 Suppl 1:S57–63. [CrossRef]
- Alam M, Han S, Pongprutthipan M, Disphanurat W, Kakar R, Nodzenski M, et al. Efficacy of a needling device for the treatment of acne scars: a randomized clinical trial. JAMA Dermatol 2014;150:844–9. [CrossRef]
- Cachafeiro T, Escobar G, Maldonado G, Cestari T, Corleta O. Comparison of Nonablative Fractional Erbium Laser 1,340 nm and Microneedling for the Treatment of Atrophic Acne Scars: A Randomized Clinical Trial. Dermatol Surg 2016;42:232–41. [CrossRef]
- Callaghan DJ. Review on the treatment of scars. Plastic and Aesthetic Research 2020;7:66. [CrossRef]
- O'Brien L, Jones DJ. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev 2013;2013:CD003826. [CrossRef]
- Morelli Coppola M, Salzillo R, Segreto F, Persichetti P. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol 2018;11:387–96. [CrossRef]
- Tidwell WJ, Owen CE, Kulp-Shorten C, Maity A, McCall M, Brown TS. Fractionated Er:YAG laser versus fully ablative Er:YAG laser for scar revision: Results of a split scar, double blinded, prospective trial. Lasers Surg Med 2016;48:837–43. [CrossRef]
- Cohen JL. Minimizing skin cancer surgical scars using ablative fractional Er:YAG laser treatment. J Drugs Dermatol 2013;12:1171–3.
- Gokalp H. Evaluation of non-ablative fractional laser treatment in scar reduction. Lasers Med Sci 2017;32:1629–35. [CrossRef]
- González N, Goldberg DJ. Update on the Treatment of Scars. J Drugs Dermatol 2019;18:550–5.
- Li K, Nicoli F, Cui C, Xi WJ, Al-Mousawi A, Zhang Z, et al. Treatment of hypertrophic scars and keloids using an intralesional 1470 nm bare-fibre diode laser: a novel efficient minimally-invasive technique. Sci Rep 2020;10:21694. [CrossRef]
- Mustoe TA, Cooter RD, Gold MH, Hobbs FDR, Ramelet A-A, Shakespeare PG, et al. International clinical recommendations on scar management. Plast Reconstr Surg 2002;110:560–71. [CrossRef]
- Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med 2011;17:113–25. [CrossRef]
- Monstrey S, Middelkoop E, Vranckx JJ, Bassetto F, Ziegler UE, Meaume S, et al. Updated scar management practical guidelines: non-invasive and invasive measures. J Plast Reconstr Aesthet Surg 2014;67:1017–25. [CrossRef]
- Zouboulis CC. Cryosurgery in dermatology. Eur J Dermatol 1998;8:466–74.
- Rabello FB, Souza CD, Júnior JAF. Update on hypertrophic scar treatment. Clinics (Sao Paulo) 2014;69:565–73. [CrossRef]
- Shaffer JJ, Taylor SC, Cook-Bolden F. Keloidal scars: a review with a critical look at therapeutic options. J Am Acad Dermatol 2002;46:S63-97. [CrossRef]
- Hession MT, Graber EM. Atrophic Acne Scarring. J Clin Aesthet Dermatol 2015;8:50–8.
- Magnani LR, Schweiger ES. Fractional CO2 lasers for the treatment of atrophic acne scars: a review of the literature. J Cosmet Laser Ther 2014;16:48–56. [CrossRef]
- Paiva-Santos AC, Mascarenhas-Melo F, Coimbra SC, Pawar KD, Peixoto D, Chá-Chá R, et al. Nanotechnology-based formulations toward the improved topical delivery of anti-acne active ingredients. Expert Opinion on Drug Delivery 2021;18:1435–54. [CrossRef]
- Lin T-K, Zhong L, Santiago JL. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int J Mol Sci 2017;19:70. [CrossRef]
- Crucho CIC, Barros MT. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Materials Science and Engineering: C 2017;80:771–84. [CrossRef]
- Mauricio MD, Guerra-Ojeda S, Marchio P, Valles SL, Aldasoro M, Escribano-Lopez I, et al. Nanoparticles in Medicine: A Focus on Vascular Oxidative Stress. Oxidative Medicine and Cellular Longevity 2018;2018:e6231482. [CrossRef]
- Kushwaha A, Goswami L, Kim BS. Nanomaterial-Based Therapy for Wound Healing. Nanomaterials 2022;12:618. [CrossRef]
- Fahmy HM, Salah Eldin RE, Abu Serea ES, Gomaa NM, AboElmagd GM, Salem SA, et al. Advances in nanotechnology and antibacterial properties of biodegradable food packaging materials. RSC Adv 2020;10:20467–84. [CrossRef]
- Kolanthai E, Fu Y, Kumar U, Babu B, Venkatesan AK, Liechty KW, et al. Nanoparticle mediated RNA delivery for wound healing. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2022;14:e1741. [CrossRef]
- Rashki S, Asgarpour K, Tarrahimofrad H, Hashemipour M, Ebrahimi MS, Fathizadeh H, et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr Polym 2021;251:117108. [CrossRef]
- Hornos Carneiro MF, Barbosa F. Gold nanoparticles: A critical review of therapeutic applications and toxicological aspects. J Toxicol Environ Health B Crit Rev 2016;19:129–48. [CrossRef]
- Kumar M, Kulkarni P, Liu S, Chemuturi N, Shah DK. Nanoparticle biodistribution coefficients: A quantitative approach for understanding the tissue distribution of nanoparticles. Adv Drug Deliv Rev 2023;194:114708. [CrossRef]
- Mohammapdour R, Ghandehari H. Mechanisms of immune response to inorganic nanoparticles and their degradation products. Adv Drug Deliv Rev 2022;180:114022. [CrossRef]
- Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small 2008;4:26–49. [CrossRef]
- Balfourier A, Luciani N, Wang G, Lelong G, Ersen O, Khelfa A, et al. Unexpected intracellular biodegradation and recrystallization of gold nanoparticles. Proc Natl Acad Sci U S A 2020;117:103–13. [CrossRef]
- Hasan A, Morshed M, Memic A, Hassan S, Webster TJ, Marei HE-S. Nanoparticles in tissue engineering: applications, challenges and prospects. Int J Nanomedicine 2018;13:5637–55. [CrossRef]
- Maurer-Jones MA, Bantz KC, Love SA, Marquis BJ, Haynes CL. Toxicity of therapeutic nanoparticles. Nanomedicine (Lond) 2009;4:219–41. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).