Submitted:
02 November 2023
Posted:
03 November 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Results
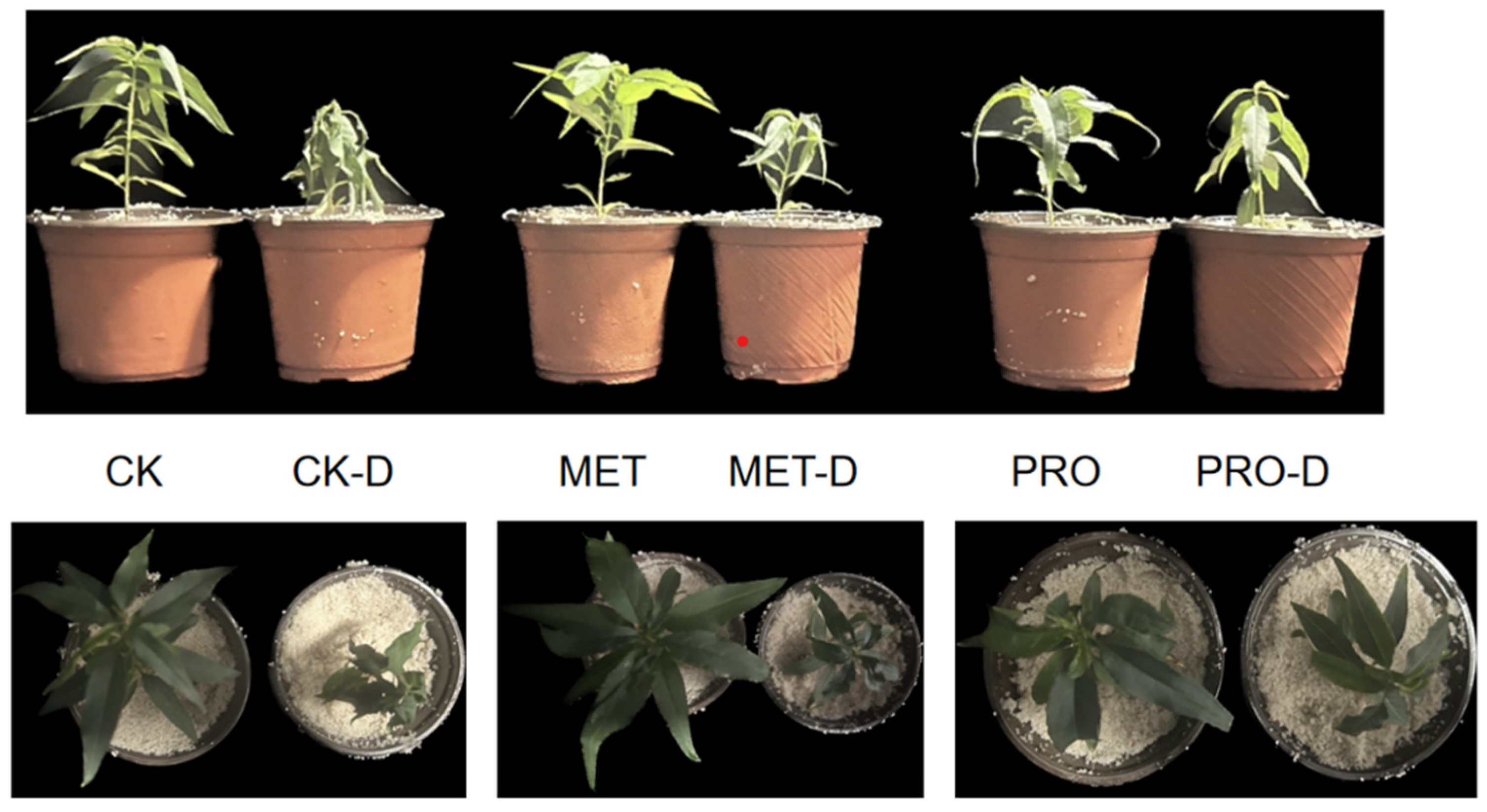
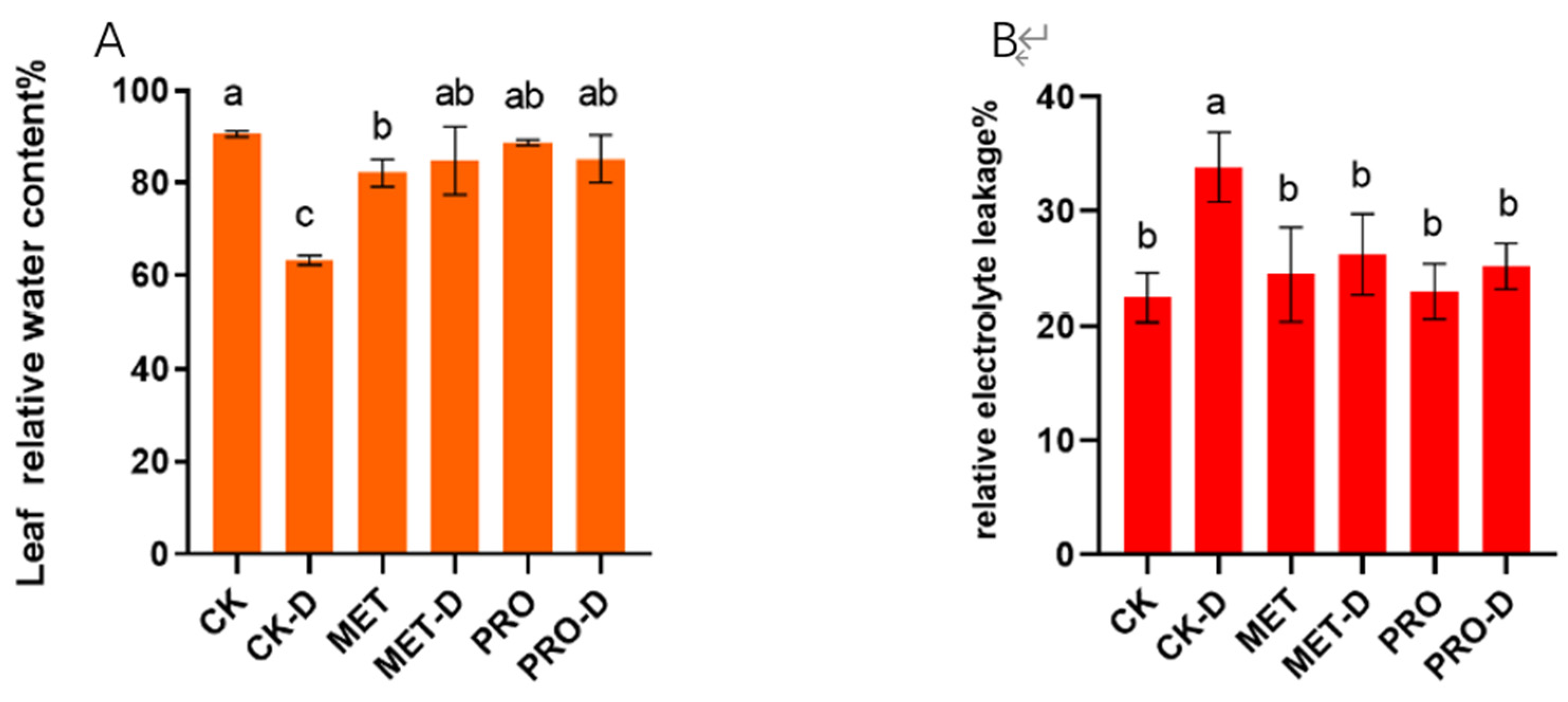
2.1. Methionine and Proline Could Relieve Drought Stress of Peach Seedlings
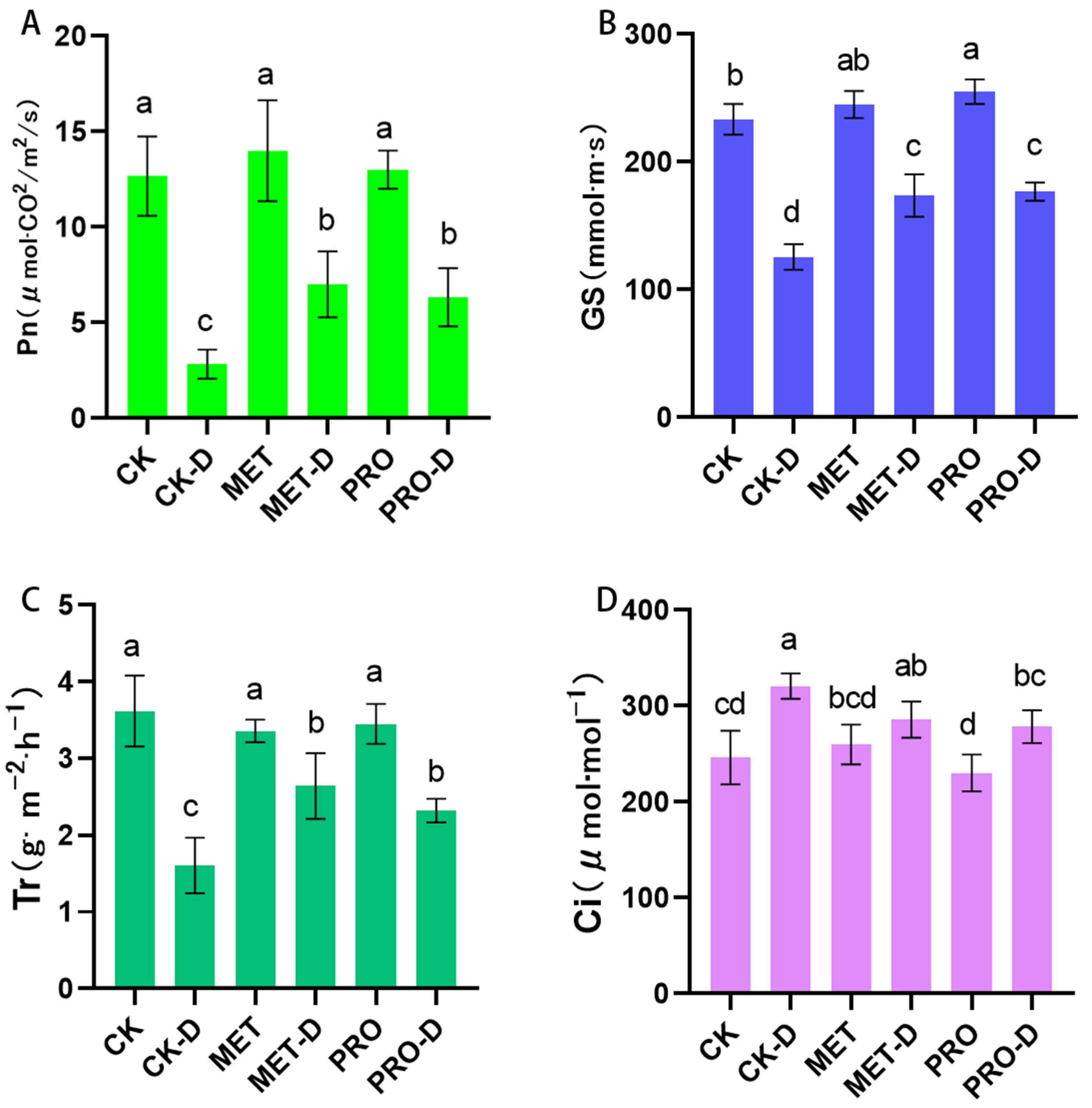
3.2. Effects of Exogenous Amino Acids on Leaf Photosynthetic Parameter
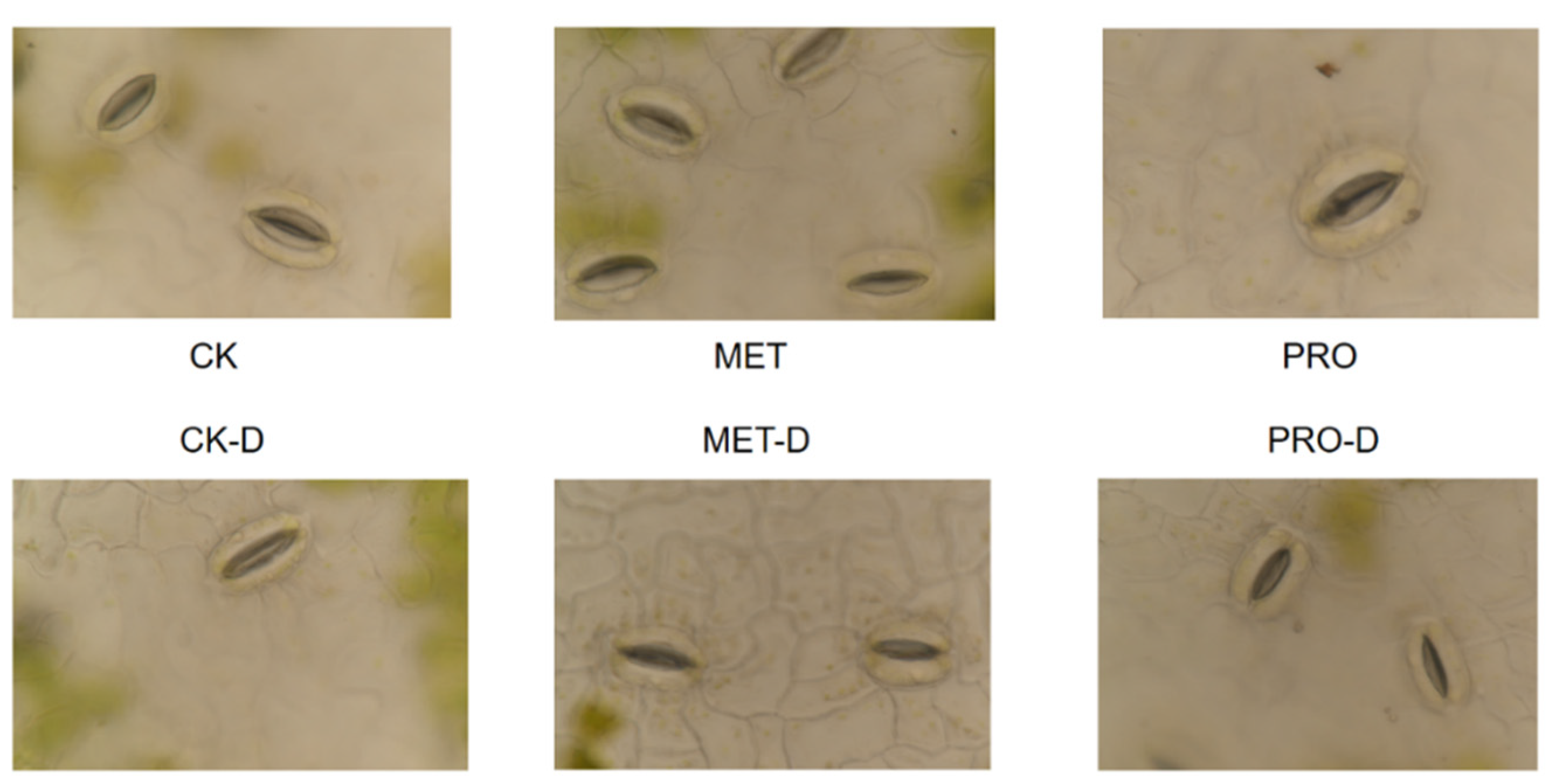
3.3. Effects of Exogenous Amino Acids on Stomata in Leaves
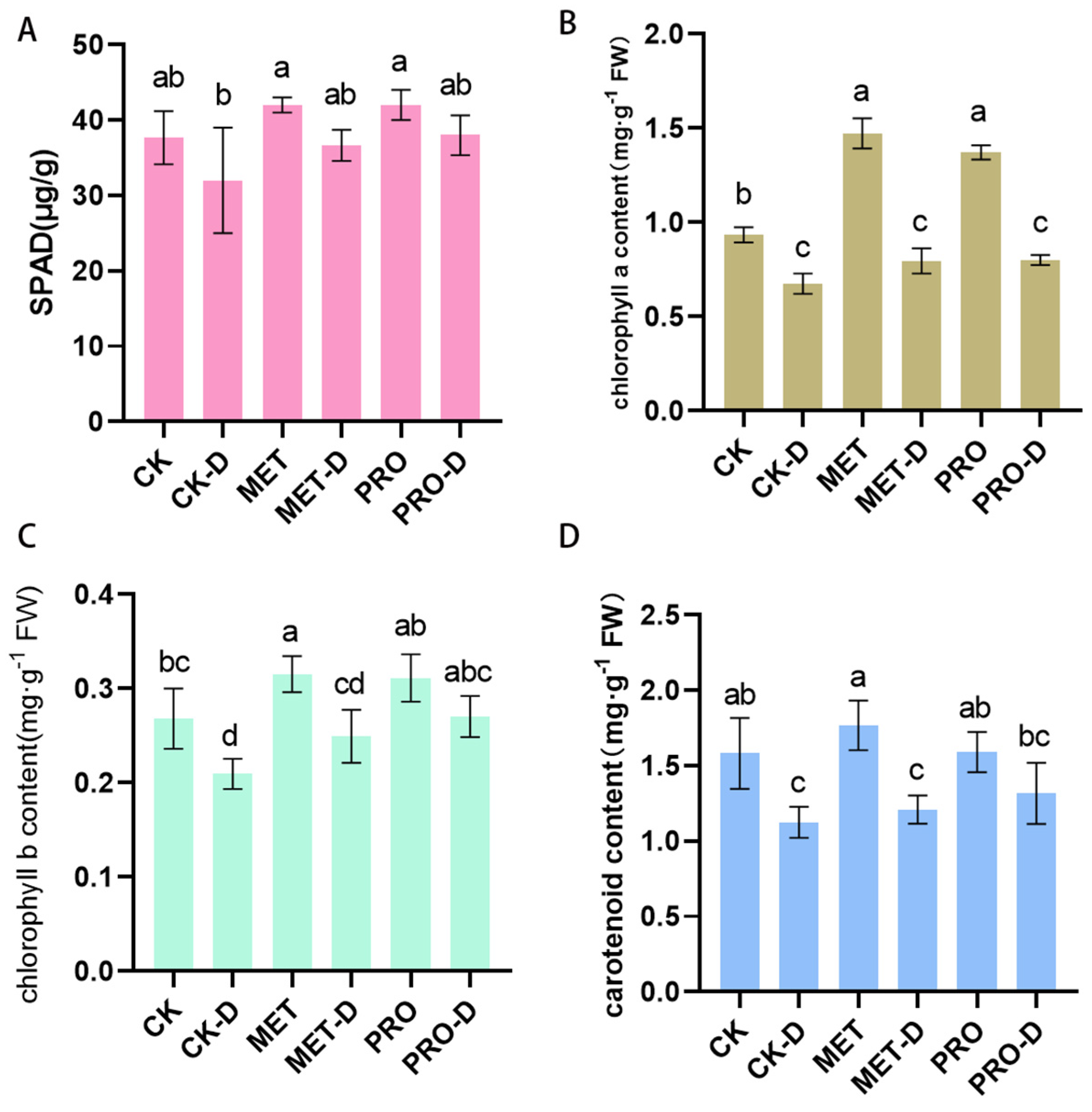
3.4. Effects of Exogenous Amino Acids on Leaf Pigment
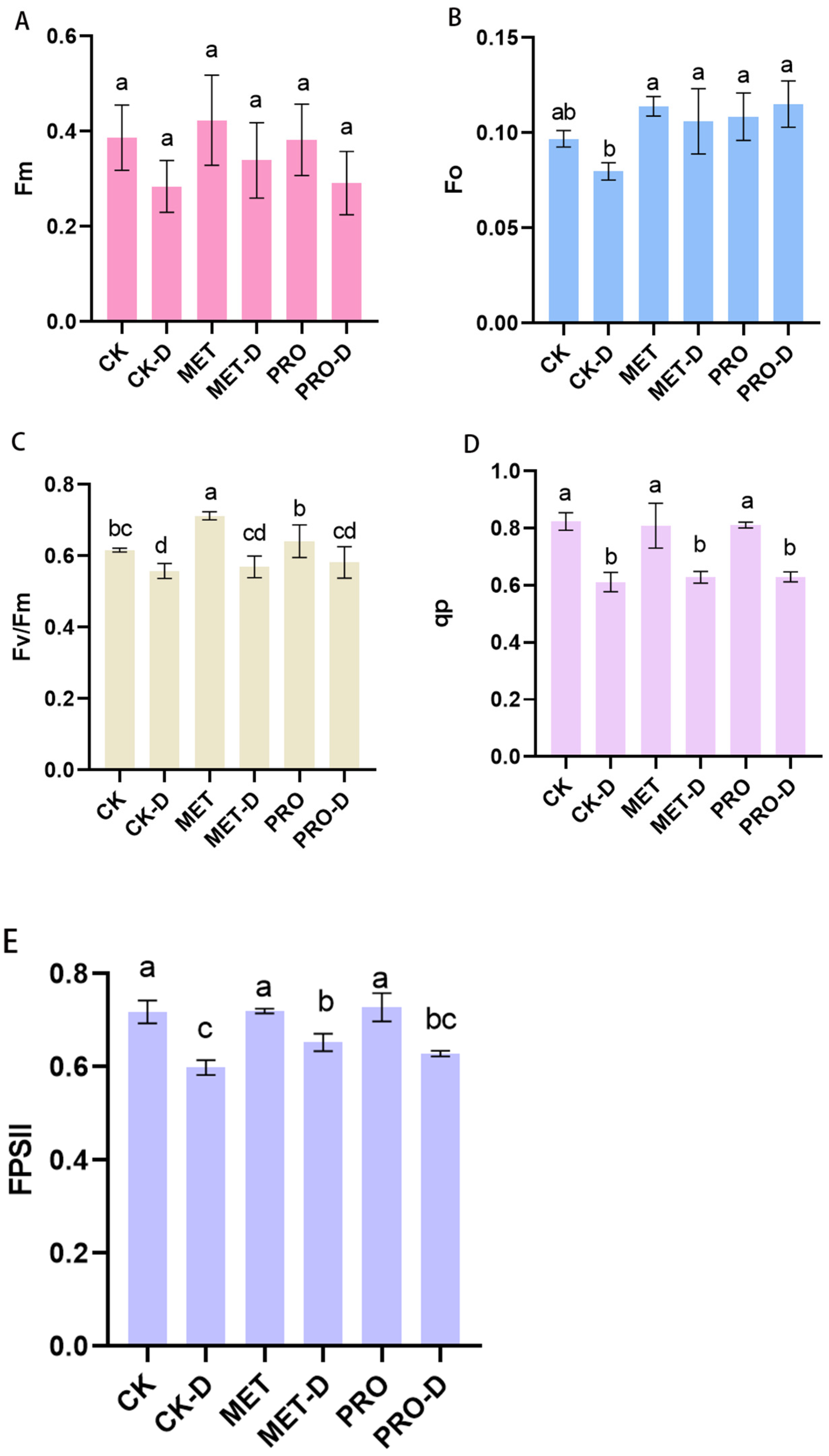
3.5. Effects of Exogenous Amino Acids on Chlorophyll Fluorescence of Peach Seedlings
3.6. Effects of Exogenous Amino Acids on the Function of Leaf Penetration System
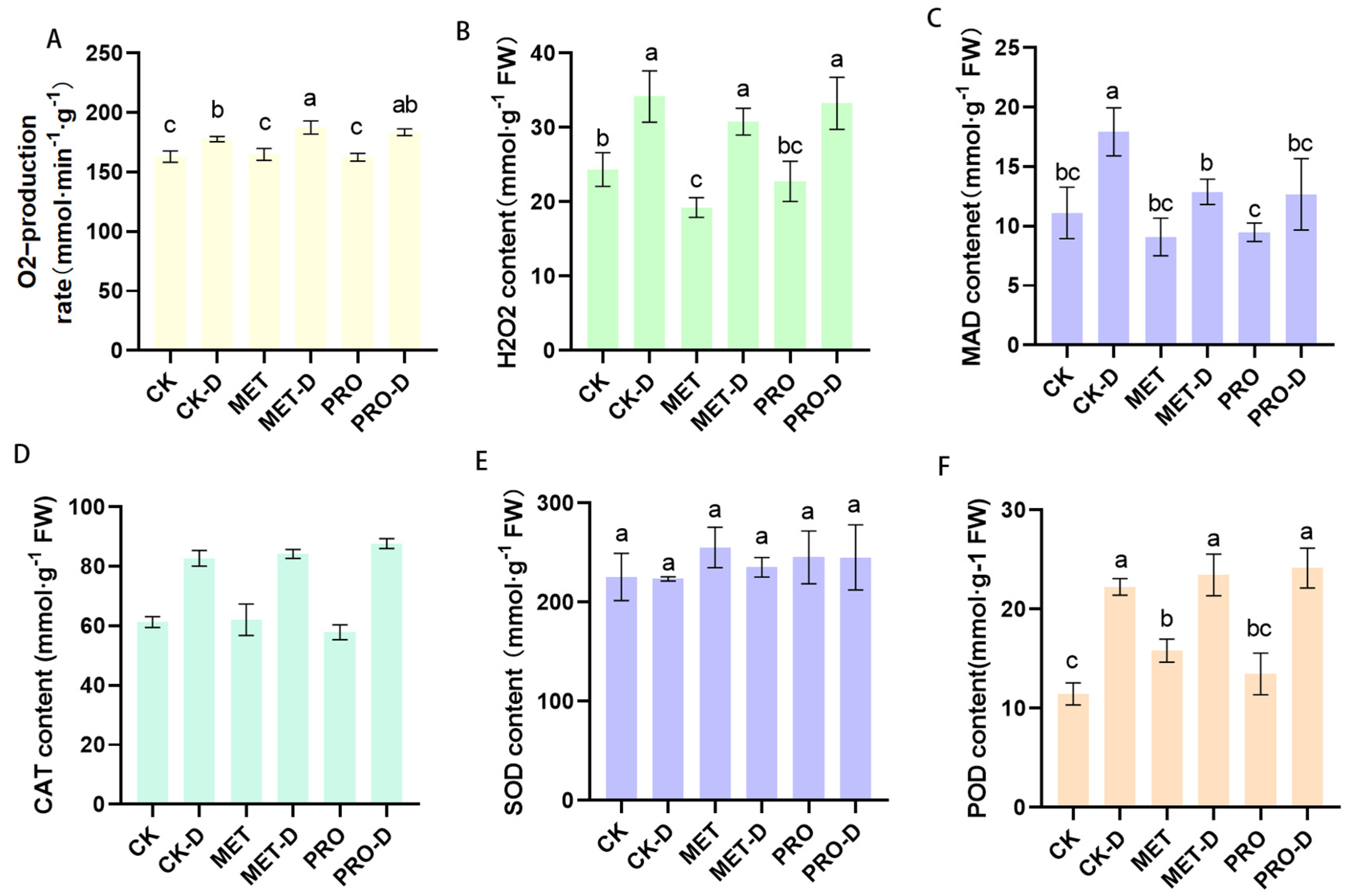
3.7. Effects of Exogenous Amino Acids on Active Oxygen Content and Antioxidant Enzyme Activity in Leaves
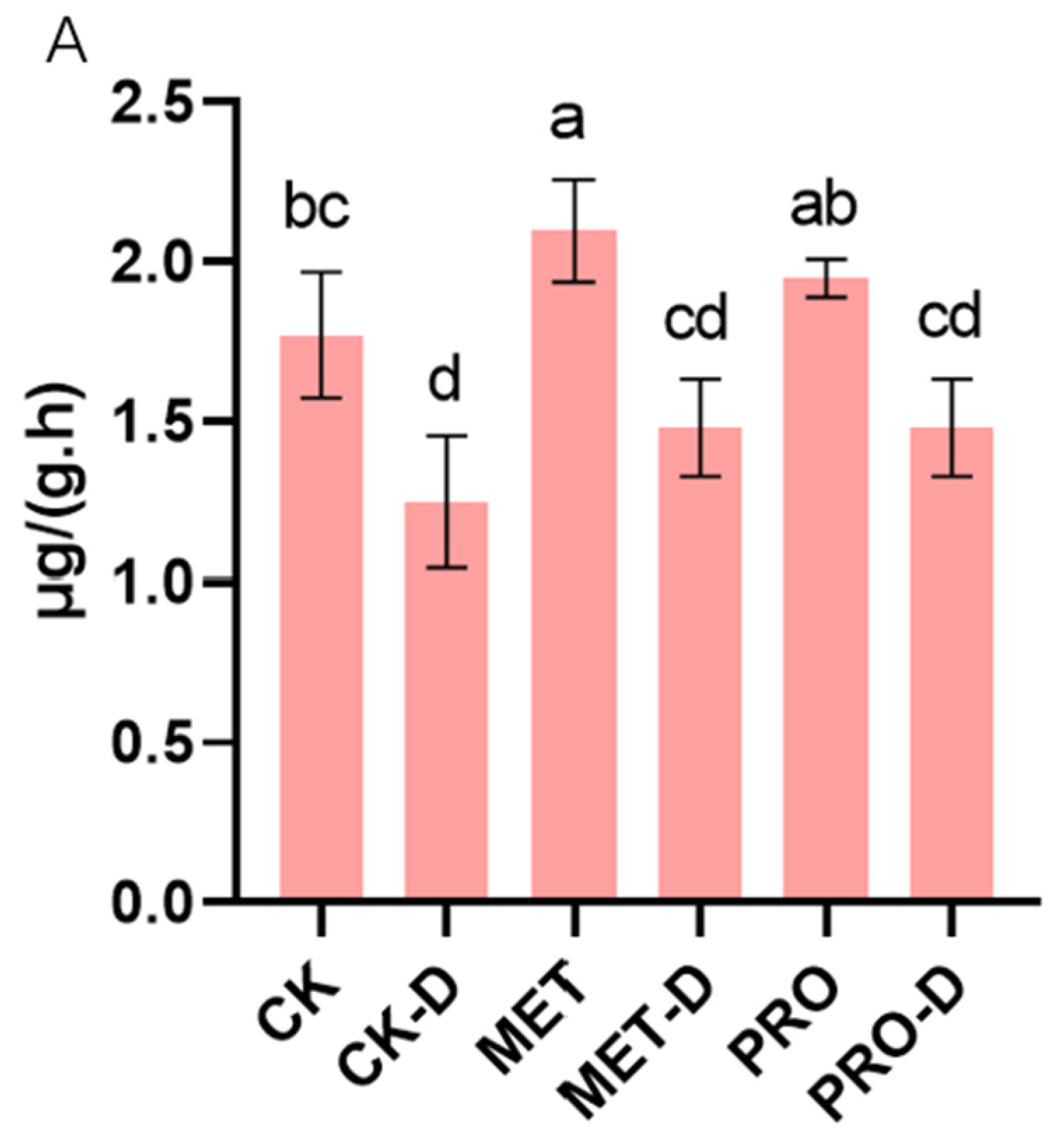
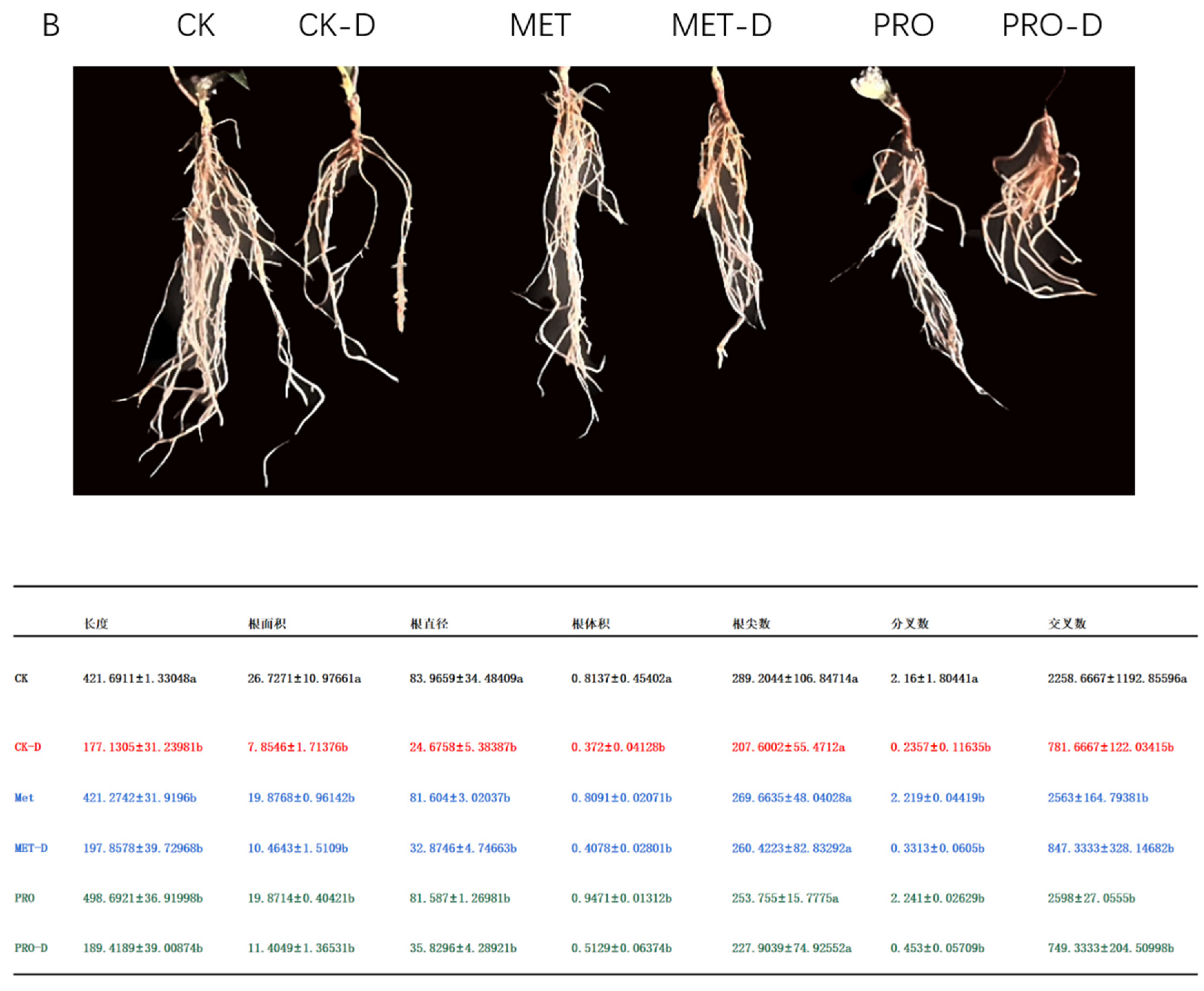
3.8. Effects of Exogenous Amino Acids on Root Growth of Peach Seedlings
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Drought Treatment
4.3. Measurements of Physiological and Biochemical Indicators
4.3.1. Light and Index Measurements
4.3.1.1. Measurements of the Photosynthetic Parameters
4.3.1.2. Chlorophyll Fluorescence Parameter Determination:
4.3.2. Relative water content and relative electrical conductivity of blades
4.3.3. Determination of Leaf Stomata
4.3.4. Determination of Root Vitality and Configureuration Indexes of Peach Seedlings
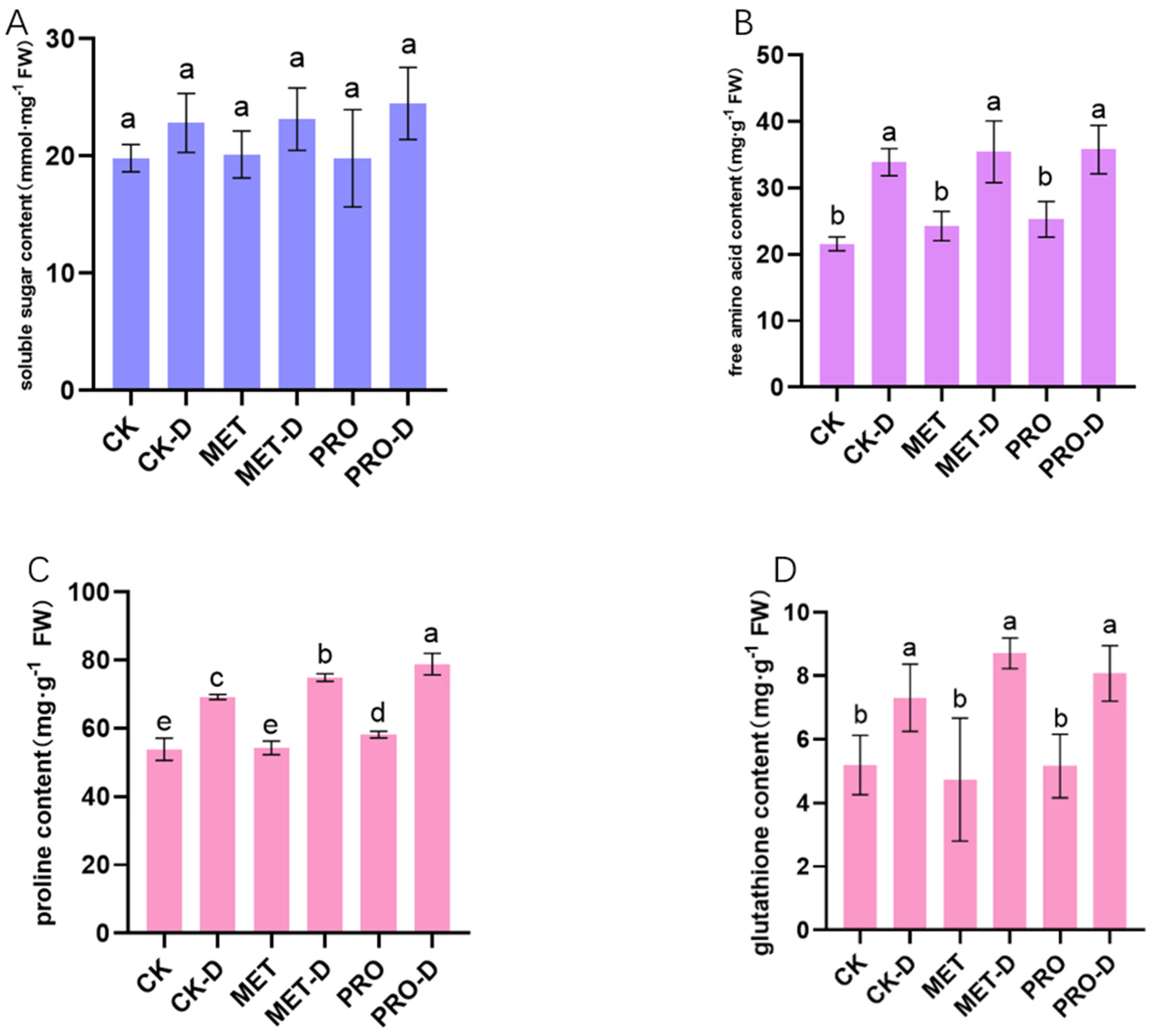
4.3.5. Determination of Osmotic Regulatory Substances
4.3.5.1. Determination of Soluble Sugar Content
4.3.5.2. Determination of Proline Content
4.3.5.3. Determination of Free Amino Acid Content
4.3.5.4. Determination of Glutathione Content
4.3.6. Determination of Active Oxygen Species in Leaves
4.3.7. Determination of MAD Content,CAT,POD and SOD Activity in Leaves
4.3.7.1. Determination of MAD Content
4.3.7.2. Determination of SOD Activity of CAT POD
5. Conclusions
References
- Yu Ming-liang, WANG Li-Rong, WANG Zhi-qiang, Peng Futian, Zhang Fan, YE Zhengwen. 70 years of fruit science research in New China -- Peach [J]. Journal of Fruit Science, 2019,36, 1283-1291.
- Najla Ksouri, Sergio Jiménez, Christina E. Wells,Bruno Contreras-Moreira and Yolanda Gogorcena1.Transcriptional Responses in Root and Leaf of Prunus persica under Drought Stress Using RNA Sequencing. Frontiers in Plant Science. [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.-X.; Naz, S.; Altaf, M.M.; Khan, L.U.; Tiwari, R.K.; Lal, M.K.; Shahid, M.A.; Kumar, R.; et al. Melatonin Improves Drought Stress Tolerance of Tomato by Modulating Plant Growth, Root Architecture, Photosynthesis, and Antioxidant Defense System. Antioxidants 2022, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxifification during salt stress in mung bean. Biologia Plantarum. 2015, 59, 745–756. [Google Scholar] [CrossRef]
- Liu, X.Q., Ko K.Y., Kim S.H., Lee K.S. 2008. Effect of amino acid fertilization on nitrate assimi lation of leafy radish and soil chemical properties in high nitrate soil. Commun Soil Sci Plant Anal 39: 269-281. [CrossRef]
- Sarojnee D.Y., Navindra B., Chandrabose S. 2009. Effect of naturally occurring amino acid stimulants on the growth and yield of hot peppers. J Anim Plant Sci, 5, 414-424.
- Rennenberg H, Herschbach C (2014) A detailed view on sulphur metabolism at the cellular and whole-plant level illustrates challenges in metabolite fux analyses. J Exp Bot 65:5711–5724.
- Hildebrandt TM, Nunes-Nesi A, Araújo WL, Braun HP (2015) Amino acid catabolism in plants. Mol Plant 8:1563–1579.
- Bakhoum GS, Badr EA, Sadak MS, Kabesh MO, Amin GA (2019) Improving growth, some biochemical aspects and yield of three cultivars of soybean plant by methionine treatment under sandy soil condition. Int J Enviro Res 13,35–43.
- Zhengxuan Wang, Liang Cai, Hui Li,Mingcai Liang, Yan Zhang,Qiong Wu,Lin Yang(2020) Rice protein stimulates endogenous antioxidant response attributed to methionine availability in growing rats. Journal Of Food Biochemistry. [CrossRef]
- El-Awadi M, El-Bassiony A, Fawzy Z, El-Nemr M (2011) Response of snap bean (Phaseolus vulgaris L) plants to nitrogen fertilizer and foliar application with methionine and tryptophan. Nat Sci 9,87–94.
- Paungfoo-Lonhienne C, Thierry G, Lonhienne A, Doris R, Nicole R, Michael C, Richard IW, Gamage HK, Bernard JC, Peer MS (2008) Plants can use protein as a N source without assistance from other organisms. Proc Natl Acad Sci 105:4524–4529.
- Yakhin OI, Aleksandr AL, Ildus AY, Patrick HB (2017) Biostimulants in plant science: a global perspective. Front Plant Sci 7:2049.
- Hasegawa, P.M., Bressan, R.A., Zhu, J.K., Bohnert, H.J., 2000. Plant cellular and molecular response to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 463–469.
- Wated, A.F., Reinhard, L., Erner, H.R.L., 1983. Comparison between a stabile NaCl selected Nicotiana cell line and the wide type. Na, K and proline pools as a function of salinity. Plant Physiol. 73, 624–629.
- Hare PD, Cress WA (1997) Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul 21:79–102.
- Hare PD, Cress WA, Van Staden J (1998) Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ 21:535–553.
- Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216.
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ 33:453–467.
- Akbudak M.A.and Filiz E..Genome-wide investigation of proline transporter (ProT) gene family in tomato: Bioinformatics and expression analyses in response to droughtstress. Plant Physiology and Biochemistry, 2020, 157, 13-22.
- Moe, L.A. (2013). Amino acids in the rhizosphere: from plants to microbes. Am. J. Bot. 100:1692–1705.
- Watanabe, M., Balazadeh, S., Tohge, T., Erban, A., Giavalisco, P., Kopka, J., Mueller-Roeber, B., Fernie, A.R., and Hoefgen, R. (2013). Comprehensive dissection of spatiotemporal metabolic shifts in primary, secondary, and lipid metabolism during developmental senescence in Arabidopsis. Plant Physiol. 162:1290–1310.
- Zeier, J. (2013). New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ. 36:2085–2103.
- Fagard, M., Launay, A., Cle´ ment, G., Courtial, J., Dellagi, A., Farjad, M., Krapp, A., Soulie´ , M.C., and Masclaux-Daubresse, C. (2014). Nitrogen metabolism meets phytopathology. J. Exp. Bot. 65:5643– 5656.
- Galili, G., Avin-Wittenberg, T., Angelovici, R., and Fernie, A.R. (2014). The role of photosynthesis and amino acid metabolism in the energy status during seed development. Front. Plant Sci. 5:447.
- Hausler, R.E., Ludewig, F., and Krueger, S. (2014). Amino acids - a life between metabolism and signaling. Plant Sci. 229:225–237.
- Pratelli, R., and Pilot, G. (2014). Regulation of amino acid metabolic enzymes and transporters in plants. J. Exp. Bot. 65:5535–5556.
- Ravanel S, Gakière B, Job D, Douce R. The specifific features of methionine biosynthesis and metabolism in plants. Proc Natl Acad Sci USA 1998;95:7805–12.
- Levine RL, Moskovitz J, Stadtman ER. Oxidation of methionine in proteins: roles in antioxidant defense and cellular regulation. IUBMB Life 2000;50:301–7.
- Luo S, Levine RL. Methionine in proteins defends against oxidative stress. FASEB J 2009;23:464–72.
- Rehman, A.U., Bashir, F., Ayaydin, F., Kota, Z., Pali, T., Vass, I., 2021. Proline is a quencher of singlet oxygen and superoxide both in in vitro systems and isolated thylakoids. Physiol. Plant 172, 7–18.
- Szabados, L., Savoure, A., 2010. Proline: a multifunctional amino acid. Trends Plant Sci. 15, 89–97.
- Han, D., Tu, S., Dai, Z., Huang, W., Jia, W., Xu, Z., et al. (2022). Comparison of selenite and selenate in alleviation of drought stress in nicotiana tabacum l. Chemosphere. 287, 132136. [CrossRef]
- un, X., Wang, P., Jia, X., Huo, L., Che, R., and Ma, F. (2018). Improvement of drought tolerance by overexpressing MdATG18a is mediated by modifified antioxidant system and activated autophagy in transgenic apple. Plant Biotechnol. J. 16, 545–557. [CrossRef]
- Muhammad, I., Shalmani, A., Ali, M., Yang, Q.-H., Ahmad, H., and Li, F. B. (2021). Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front. Plant Sci. 11, 2310. [CrossRef]
- Liu, E., Mei, X., Yan, C., Gong, D., and Zhang, Y. (2016). Effects of water stress on photosynthetic characteristics, dry matter translocation and WUE in two winter wheat genotypes. Agric. Water Management. 167, 75–85. [CrossRef]
- Li, C., Zhao, Q., Gao, T., Wang, H., Zhang, Z., Liang, B., et al. (2018). The mitigation effects of exogenous melatonin on replant disease in apple. J. pineal Res. 65, e12523. [CrossRef]
- Liang, B., Ma, C., Zhang, Z., Wei, Z., Gao, T., Zhao, Q., et al. (2018). Long-term exogenous application of melatonin improves nutrient uptake flfluxes in apple plants under moderate drought stress. Environ. Exp. botany. 155, 650–661. [CrossRef]
- Moaveni, P. (2011). Effect of water defificit stress on some physiological traits of wheat (Triticum aestivum). Agric. Sci. Res. J. 1, 64–68.
- Shahid, M. A., Balal, R. M., Pervez, M. A., Garcia-Sanchez, F., Gimeno, V., Abbas, T., et al (2014). Treatment with 24-epibrassinolide mitigates NaCl-induced toxicity by enhancing carbohydrate metabolism, osmolyte accumulation, and antioxidant activity in Pisum sativum. Turk J Bot. 38, 511–525. [CrossRef]
- Maxwell, K., and Johnson, G. N. (2000). Chlorophyll flfluorescence–a practical guide. J. Exp. botany. 51, 659–668. [CrossRef]
- Rao, L., Li, S., and Cui, X. (2021). Leaf morphology and chlorophyll flfluorescence characteristics of mulberry seedlings under waterlogging stress. Sci. Rep. 11, 1–11. [CrossRef]
- Pettigrew, W. (2004). Physiological consequences of moisture defificit stress in cotton. Crop Sci. 44, 1265–1272. [CrossRef]
- Raja, V., Qadir, S. U., Alyemeni, M. N., and Ahmad, P. (2020). Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in solanum lycopersicum. 3 Biotech. 10, 1–18. [CrossRef]
- Jespersen, D., Yu, J., and Huang, B. (2017). Metabolic effects of acibenzolar-smethyl for improving heat or drought stress in creeping bentgrass. Front. Plant sci. 8, 1224. [CrossRef]
- Gao, D., Shi, C., Li, Q., Wei, Z., Liu, L., and Feng, J. (2021). Drought tolerance monitoring of apple rootstock m. 9-T337 based on infrared and flfluorescence imaging. Photosynthetica. 59, 458–467. [CrossRef]
- Khan, M., Khan, N. A., Masood, A., Per, T. S., and Asgher, M. (2016). Hydrogen peroxide alleviates nickel-inhibited photosynthetic responses through increase in use-effificiency of nitrogen and sulfur, and glutathione production in mustard. Front. Plant sci. 7, 44. [CrossRef]
- Wang, P., Sun, X., Li, C., Wei, Z., Liang, D., and Ma, F. (2013). Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. pineal Res. 54, 292–302. [CrossRef]
- Gao, T., Zhang, Z., Liu, X., Wu, Q., Chen, Q., Liu, Q., et al. (2020). Physiological and transcriptome analyses of the effects of exogenous dopamine on drought tolerance in apple. Plant Physiol. Biochem. 148, 260–272. [CrossRef]
- La V. H., Lee B. R., Islam M., Mamun M., Park S. H., Bae D. W. and Kim, T. H.. Characterization of glutamate-mediated hormonal regulatory pathway of the droughtresponses in relation to proline metabolism in Brassica napus L. Plants, 2020, 9 (4),512.
- Bielsa, B., Sanz, M.A, and Rubio-Cabetas, M. J. (2021). ‘Garnem’and myrobalan‘P. 2175’: Two different drought responses and their implications in drought tolerance. Horticulturae. 7, 299. [CrossRef]
- Zhang Xiong. Determination of wheat root and pollen activity and its application [J]. Plant Physiology Bulletin, 1982, 48-50.
- Li,Y., Lv,Y., Lian,M., Peng, F., andXiao,Y.(2021). Effects of combined glycine and urea fertilizer application on the photosynthesis, sucrose metabolism, and fruit development of peach. Scientia Horticulturae. 289, 110504. [CrossRef]
- Mehdizadeh, L., Farsaraei, S., and Moghaddam, M. (2021). Biochar application modifified growth and physiological parameters of ocimum ciliatum l. and reduced human risk assessment under cadmium stress. J. Hazardous Materials. 409, 124954. [CrossRef]
- Vardharajula, S., Zulfifikar Ali, S., Grover, M., Reddy, G., and Bandi, V. (2011). Drought-tolerant plant growth promoting bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interactions. 6, 1–14. [CrossRef]
- Han, D., Tu, S., Dai, Z., Huang, W., Jia, W., Xu, Z., et al. (2022). Comparison of selenite and selenate in alleviation of drought stress in nicotiana tabacum l. Chemosphere. 287, 132136. [CrossRef]
- Zhang, X., Wang, X., Zhong, J., Zhou, Q., Wang, X., Cai, J., et al (2016). Drought priming induces thermo-tolerance to post-anthesis high-temperature in offspring of winter wheat. Environ. Exp. botany. 127, 26–36. [CrossRef]
- Li, Y., Zhao, H., Duan, B., Korpelainen, H., and Li, C. (2011). Effect of drought and ABA on growth, photosynthesis and antioxidant system of cotinus coggygria seedlings under two different light conditions. Environ. Exp. Botany. 71, 107–113. [CrossRef]
- Liang, B., Ma, C., Zhang, Z., Wei, Z., Gao, T., Zhao, Q., et al. (2018). Long-term exogenous application of melatonin improves nutrient uptake flfluxes in apple plants under moderate drought stress. Environ. Exp. botany. 155, 650–661. [CrossRef]
- Zhang, X., Wang, X., Zhong, J., Zhou, Q., Wang, X., Cai, J., et al (2016). Drought priming induces thermo-tolerance to post-anthesis high-temperature in offspring of winter wheat. Environ. Exp. botany. 127, 26–36. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




