Submitted:
02 November 2023
Posted:
03 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Patients and Methods
2.1. Vaginal Samples
2.2. Culture and Fungal Identification
2.2.1. Isolates Identification by PCR or ID32C
2.3. Molecular Identification of Fungal Species by Multiplex qPCR
2.3.1. Automated DNA Extraction
2.3.2. Primer and Taqman Probe Design for qPCR Assays
2.3.3. Multiplex qPCR
2.4. Antifungal Susceptibility Testing
2.5. Gene Expression Analysis by RT-qPCR
2.6. Sequencing Analysis of ERG11, TAC1, UPC2, MRR1 and MRR2 Genes
2.7. Statistical Analysis
3. Results
3.1. Yeast Identification by Multiplex qPCR
3.1.1. Collection of Microorganisms
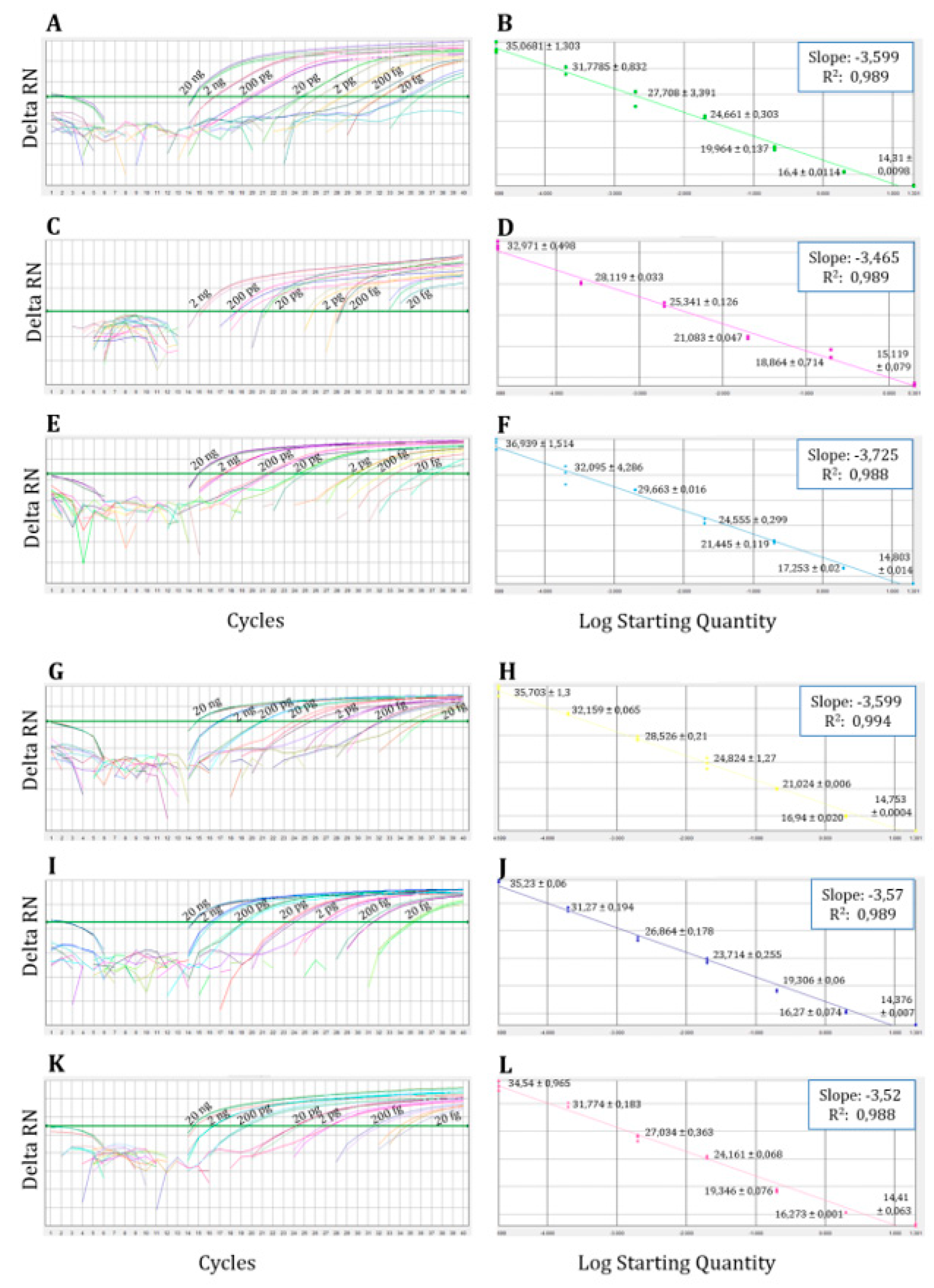
3.1.2. Analytical Sensitivity or Limit of Detection (LOD) of the Multiplex qPCR
3.1.3. Validation of the Multiplex qPCR with Vaginal Swab Samples/Analytical Sensitivity and Specificity of Multiplex qPCR Compared with Conventional Culture
3.2. Antifungal Susceptibility
3.3. Expression of CDR1, CDR2 and MDR1 Genes of Azole Resistant Isolates
3.4. Amino Acid Substitutions in Erg11, Tac1, Upc2, Mrr1 and Mrr2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tardif, K.D.; Schlaberg, R. Development of a real-time PCR assay for the direct detection of Candida species causing Vulvovaginal candidiasis. Diagn. Microbiol. Infect. Dis. 2017, 88, 39-40. Available online: https://www.sciencedirect.com/science/article/pii/S0732889317300317.
- Sobel, J.D. Vulvovaginal candidosis. The Lancet 2007, 369, 1961-1971. Available online: https://www.sciencedirect.com/science/article/pii/S0140673607609179.
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect. Dis. 2018, 18, e339-e347. Available online: https://www.sciencedirect.com/science/article/pii/S1473309918301038.
- Marchaim, D.; Lemanek, L.; Bheemreddy, S.; Kaye, K.S.; Sobel, J.D. Fluconazole-Resistant Candida albicans Vulvovaginitis. Obstet. Gynecol. 2012, 120, 1407-1414. Available online: https://journals.lww.com/greenjournal/fulltext/2012/12000/fluconazole_resistant_candida_albicans.22.aspx.
- Sobel, J.D. Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2016, 214, 15-21. Available online: https://www.sciencedirect.com/science/article/pii/S0002937815007164.
- Sobel, J.D.; Akins, R.A. The Role of PCR in the Diagnosis of Candida Vulvovaginitis—a New Gold Standard? Curr. Infect. Dis. Rep. 2015, 17, 33. [CrossRef]
- Arechavala, A.; Negroni, R.; Santiso, G.; Depardo, R.; Bonvehí, P. Chronic recurrent vulvovaginitis is not only due to Candida. Rev. Iberoam. Micol. 2021, 38, 132-137. Available online: https://www.sciencedirect.com/science/article/pii/S1130140621000243.
- Sobel, J.D.; Akins, R. Determining Susceptibility in Candida Vaginal Isolates. Antimicrob. Agents Chemother. 2022, 66, e0236621. [CrossRef]
- Berkow, E.L.; Lockhart, S.R. Fluconazole resistance in Candida species: a current perspective. Infect. Drug Resist. 2017, 237-245. [CrossRef]
- Coste, A.T.; Karababa, M.; Ischer, F.; Bille, J.; Sanglard, D. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot. Cell 2004, 3, 1639-1652. [CrossRef]
- Dunkel, N.; Blaß, J.; Rogers, P.D.; Morschhäuser, J. Mutations in the multi-drug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol. Microbiol. 2008, 69, 827-840. [CrossRef]
- Flowers, S.A.; Barker, K.S.; Berkow, E.L.; Toner, G.; Chadwick, S.G.; Gygax, S.E.; Morschhäuser, J.; Rogers, P.D. Gain-of-function mutations in UPC2 are a frequent cause of ERG11 upregulation in azole-resistant clinical isolates of Candida albicans. Eukaryot. Cell 2012, 11, 1289-1299. [CrossRef]
- Nishimoto, A.T.; Zhang, Q.; Hazlett, B.; Morschhäuser, J.; Rogers, P.D. Contribution of clinically derived mutations in the gene encoding the zinc cluster transcription factor Mrr2 to fluconazole antifungal resistance and CDR1 expression in Candida albicans. Antimicrob. Agents Chemother. 2019, 63, e00078-19. [CrossRef]
- Nishimoto, A.T.; Sharma, C.; Rogers, P.D. Molecular and genetic basis of azole antifungal resistance in the opportunistic pathogenic fungus Candida albicans. J. Antimicrob. Chemother. 2019, 75, 257-270. [CrossRef]
- Morio, F.; Pagniez, F.; Besse, M.; Gay-andrieu, F.; Miegeville, M.; Le Pape, P. Deciphering azole resistance mechanisms with a focus on transcription factor-encoding genes TAC1, MRR1 and UPC2 in a set of fluconazole-resistant clinical isolates of Candida albicans. Int. J. Antimicrob. Agents 2013, 42, 410-415. Available online: https://www.sciencedirect.com/science/article/pii/S0924857913002720.
- Romeo, O.; Criseo, G. First molecular method for discriminating between Candida africana, Candida albicans, and Candida dubliniensis by using hwp1 gene. Diagn. Microbiol. Infect. Dis. 2008, 62, 230-233. Available online: https://www.sciencedirect.com/science/article/pii/S0732889308002757.
- 17. White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; pp 315-322.
- Edgar, R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792-1797. [CrossRef]
- Khot, P.D.; Fredricks, D.N. PCR-based diagnosis of human fungal infections. Expert Rev. Anti-Infe. 2009, 7, 1201-1221. [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Fourth Edition M27; CLSI: Wayne, PA, USA, 2017.
- Clinical and Laboratory Standards Institute. Performance Standards for Antifungal Susceptibility Testing of Yeasts: Third Edition; CLSI Supplement M27M44S: CLSI, 2022.
- Pelletier René; Joanne, P.; Cynthia, A.; Corina, G.; Lauren, W.; Walsh Thomas, J. Emergence of Resistance of Candida albicans to Clotrimazole in Human Immunodeficiency Virus-Infected Children: In Vitro and Clinical Correlations. J. Clin. Microbiol. 2000, 38, 1563-1568. [CrossRef]
- Morio, F.; Pagniez, F.; Lacroix, C.; Miegeville, M.; Le Pape, P. Amino acid substitutions in the Candida albicans sterol Δ5,6-desaturase (Erg3p) confer azole resistance: characterization of two novel mutants with impaired virulence. J. Antimicrob. Chemother. 2012, 67, 2131-2138. [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402-408. Available online: https://www.sciencedirect.com/science/article/pii/S1046202301912629.
- Coste, A.; T.; Crittin, J.; Bauser, C.; Rohde, B.; Sanglard, D. Functional Analysis of cis- and trans-Acting Elements of the Candida albicans CDR2 Promoter with a Novel Promoter Reporter System. Eukaryot. Cell. 2009, 8, 1250-1267. [CrossRef]
- Wang, Y.; Liu, J.; Shi, C.; Li, W.; Zhao, Y.; Yan, L.; Xiang, M. Mutations in transcription factor Mrr2p contribute to fluconazole resistance in clinical isolates of Candida albicans. Int. J. Antimicrob. Agents 2015, 46, 552-559. Available online: https://www.sciencedirect.com/science/article/pii/S0924857915002897.
- Xiang, M.; Liu, J.; Ni, P.; Wang, S.; Shi, C.; Wei, B.; Ni, Y.; Ge, H. Erg11 mutations associated with azole resistance in clinical isolates of Candida albicans. FEMS Yeast Res. 2013, 13, 386-393. [CrossRef]
- Hiroshi, K.; Yoshitsugu, M.; Haruko, M.; Katherine, N.; Brian, G.; Bennett John, E. Genetic Analysis of Azole Resistance in the Darlington Strain of Candida albicans. Antimicrob. Agents Chemother. 2000, 44, 2985-2990. [CrossRef]
- Kelly, S.L.; Lamb, D.C.; Kelly, D.E. Y132H substitution in Candida albicans sterol 14α-demethylase confers fluconazole resistance by preventing binding to haem. FEMS Microbiol. Lett. 1999, 180, 171-175. [CrossRef]
- Sanglard, D.; Ischer, F.; Koymans, L.; Bille, J. Amino Acid Substitutions in the Cytochrome P-450 Lanosterol 14α-Demethylase (CYP51A1) from Azole-Resistant Candida albicans Clinical Isolates Contribute to Resistance to Azole Antifungal Agents. Antimicrob. Agents Chemother. 1998, 42, 241-253. [CrossRef]
- Giraldo, P.; von Nowaskonski, A.; Gomes, F.A.M.; Linhares, I.; Neves, N.A.; Witkin, S.S. Vaginal colonization by Candida in asymptomatic women with and without a history of recurrent vulvovaginal candidiasis. Obstet. Gynecol. 2000, 95, 413-416. Available online: https://www.sciencedirect.com/science/article/pii/S0029784499005773.
- Weissenbacher, T.; Witkin, S.S.; Ledger, W.J.; Tolbert, V.; Gingelmaier, A.; Scholz, C.; Weissenbacher, E.R.; Friese, K.; Mylonas, I. Relationship between clinical diagnosis of recurrent vulvovaginal candidiasis and detection of Candida species by culture and polymerase chain reaction. Arch. Gynecol. Obstet. 2009, 279, 125-129. [CrossRef]
- Mårdh, P.A.; Novikova, N.; Witkin, S.S.; Korneeva, I.; Rodriques, A.R. Detection of Candida by polymerase chain reaction vs microscopy and culture in women diagnosed as recurrent vulvovaginal cases. Int. J. STD AIDS 2003, 14, 753-756. [CrossRef]
- Cartwright Charles, P.; Lembke Bryndon, D.; Kalpana, R.; Body Barbara, A.; Nye Melinda, B.; Rivers Charles, A.; Schwebke Jane, R. Comparison of Nucleic Acid Amplification Assays with BD Affirm VPIII for Diagnosis of Vaginitis in Symptomatic Women. J. Clin. Microbiol. 2020, 51, 3694-3699. [CrossRef]
- Gaydos, C.A.; Beqaj, S.; Schwebke, J.R.; Lebed, J.; Smith, B.; Davis, T.E.; Fife, K.H.; Nyirjesy, P.; Spurrell, T.; Furgerson, D.; Coleman, J.; Paradis, S.; Cooper, C.K. Clinical Validation of a Test for the Diagnosis of Vaginitis. Obstet. Gynecol. 2017, 130, 181-189. Available online: https://journals.lww.com/greenjournal/fulltext/2017/07000/clinical_validation_of_a_test_for_the_diagnosis_of.25.aspx.
- Arastehfar, A.; Fang, W.; Pan, W.; Liao, W.; Yan, L.; Boekhout, T. Identification of nine cryptic species of Candida albicans, C. glabrata, and C. parapsilosis complexes using one-step multiplex PCR. BMC Infect. Dis. 2018, 18, 480, 10.1186/s12879-018-3381-5. [CrossRef]
- Theill, L.; Dudiuk, C.; Morano, S.; Gamarra, S.; Nardin, M.E.; Méndez, E.; Garcia-Effron, G. Prevalence and antifungal susceptibility of Candida albicans and its related species Candida dubliniensis and Candida africana isolated from vulvovaginal samples in a hospital of Argentina. Rev. Argent. Microbiol. 2016, 48, 43-49. Available online: https://www.sciencedirect.com/science/article/pii/S0325754115001492.
- Kurtzman, C.P.; Robnett, C.J. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5' end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 1997, 35, 1216-1223. [CrossRef]
- Nyirjesy, P.; Brookhart, C.; Lazenby, G.; Schwebke, J.; Sobel, J.D. Vulvovaginal Candidiasis: A Review of the Evidence for the 2021 Centers for Disease Control and Prevention of Sexually Transmitted Infections Treatment Guidelines. Clin. Infect. Dis. 2022, 74, S162-S168. [CrossRef]
- Nyirjesy, P. Vulvovaginal Candidiasis and Bacterial Vaginosis. Infect. Dis. Clin. North Am. 2008, 22, 637-652. Available online: https://www.sciencedirect.com/science/article/pii/S0891552008000524.
- Sobel, J.D.; Sobel, R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin. Pharmacother. 2018, 19, 971-977. [CrossRef]
- Ying, C.; Zhang, H.; Tang, Z.; Chen, H.; Gao, J.; Yue, C. Antifungal susceptibility and molecular typing of 115 Candida albicans isolates obtained from vulvovaginal candidiasis patients in 3 Shanghai maternity hospitals. Med. Mycol. 2015, 54, 394-399. [CrossRef]
- Bulik, C.C.; Sobel, J.D.; Nailor, M.D. Susceptibility profile of vaginal isolates of Candida albicans prior to and following fluconazole introduction – impact of two decades. Mycoses 2011, 54, 34-38. [CrossRef]
- Morio, F.; Jensen, R.H.; Le Pape, P.; Arendrup, M.C. Molecular basis of antifungal drug resistance in yeasts. Int. J. Antimicrob. Agents 2017, 50, 599-606. Available online: https://www.sciencedirect.com/science/article/pii/S0924857917302054.
- Sobel, J.D. Resistance to Fluconazole of Candida albicans in Vaginal Isolates: a 10-Year Study in a Clinical Referral Center. Antimicrob. Agents Chemother. 2023, 67, 181, e00181-23. [CrossRef]
| Primers and probes | Species | Gene | Sequences (5’→ 3’) |
|---|---|---|---|
| Primers | |||
| Diamol-F | - | 18S | TAGGTGAACCTGCGGAAGGA |
| Diamol-R | - | 5.8S | TCGCTGCGTTCTTCATCGAT |
| Probes | |||
| Calb | C. albicans | ITS1 | FAM-CGGTGGGCCCAGCCTGCC-BHQ1 |
| Calb2 | C. albicans | FAM-ATCAA[C]TTGTCACA[C][C]AGA-ZNA4-BHQ1 | |
| Cpar2 | C. parapsilosis | JOE-AGGCC[C]CATA[T]AGAAGG[C]CTA-BHQ1 | |
| Cpar3 | C. parapsilosis | HEX-TGGCAGGCCCCATATAGAAGGCCTAC-BHQ1 | |
| Cgla | N. glabrata | FAM-ATTTCTCCTGCCTGCGCTTAAGTGCG-BHQ1 | |
| Cgla2 | N. glabrata | FAM-TTAAGTGCGCGG[T][T]GGTGG-ZNA4-BHQ1 | |
| Cgui3 | M. guilliermondii | JOE-AA[C]CTA[T]CT[C]TA[G]GC[C]AAA-BHQ1 | |
| Cgui4 | M. guilliermondii | HEX-CAGCGTTTAACTGCGCGGCGA-BHQ1 | |
| Ctro | C. tropicalis | HEX-CGGTAGGATTGCTCCCGCCA-BHQ1 | |
| Ctro2 | C. tropicalis | HEX-CGGTAGGATTGCTCCCGCCACC-BHQ1 | |
| Ckru | P. kudriavzevii | FAM-TTTAGGTGTTGTTGTTTTCGTTCCGCTC-BHQ1 | |
| Ckru2 | P. kudriavzevii | FAM-CTACACTGCGTGAGCGGAACGAAAAC-BHQ1 | |
| Control | |||
| Probe-ICP | - | - | ROX-AACGTGCGACGTTCCGAGCA-BHQ2 |
| IC | - | - | TAGGTGAACCTGCGGAAGGATCGAAACGTGCGACGCTTCCGAGCATGATCACTATGTCCTAATCCCATATATTATTCACTGTGTACTAGCCCTTCTTGGTTCTCGCATCGATGAAGAACGCAGCGA |
| Probes (Ct ± SD)b | ||||||||
|---|---|---|---|---|---|---|---|---|
| Calb | Cgla | Cpar3 | Cgui4 | Ctro2 | Ckru2 | |||
| Species | Straina | |||||||
| C. albicans | NCPF 3153 | 14.26 ± 0.056 | - | - | - | - | - | |
| N. glabrata | NCPF 3203 | - | 13.34 ± 0.056 | - | - | - | - | |
| C. parapsilosis | NCPF 3104 | - | - | 14.4 ± 0.106 | - | - | - | |
| M. guilliermondii | NCPF 3099 | - | - | - | 14.33 ± 0.891 | - | - | |
| C. tropicalis | NCPF 3111 | - | - | - | - | 14.31 ± 0.993 | - | |
| P. kudriavzevii | ATCC 6258 | - | - | - | - | - | 13.64 ± 0.007 | |
| Other yeasts | ||||||||
| Saccharomyces cerevisiae | CECT 1678 | - | 18.37 | - | - | - | - | |
| Saprochaete capitata | IHEM 5666 | - | - | - | - | - | - | |
| Yarrowia lipolytica | UPV 12-097 | - | - | - | - | - | - | |
| Rhodotorula mucilaginosa | CECT 11016 | - | - | - | - | - | - | |
| Filamentous fungi | ||||||||
| Aspergillus fumigatus | Af-293 | - | - | - | - | - | - | |
| Lomentospora prolificans | ATCC 64913 | - | - | - | - | - | - | |
| Cryptococcus neoformans | ATCC 90113 | - | - | - | - | - | - | |
| Bacteria | ||||||||
| Staphylococcus aureus | CECT 435 | - | - | - | - | - | - | |
| Streptococcus pyogenes | CECT 985 | - | - | - | - | - | - | |
| Streptococcus viridans | CECT 804 | - | - | - | - | - | - | |
| Streptococcus pneumoniae | CECT 993 | - | - | - | - | - | - | |
| Escherichia coli | CECT 434 | - | - | - | - | - | - | |
| Klebsiella pneumoniae | CECT 144 | - | - | - | - | - | - | |
| Pseudomonas aeruginosa | CECT 108 | - | - | - | - | - | - | |
| Proteus mirabilis | CECT 4168 | - | - | - | - | - | - | |
| Gardnerella vaginalis | ATCC 14018 | - | - | - | - | - | - | |
| Human DNA | ||||||||
| Human Genomic DNA (Promega, Spain) | G304A* | - | - | - | - | - | - | |
| Isolate | Erg11pa | Tac1pa | Upc2pa | Mrr1a | Mrr2a |
| Be-113 | A114S; Y257H | N396Sh; S758F | - | V341Eh; L592Fh; E1020Qh | - |
| Be-114 | - | A337Vh; N396S; N772K; D776N; E829Qh; S941Ph | - | V341E; E1020Q | A311V; A451A; V582L |
| Be-129 | Y132H; G450E | N396Sh; N772Kh; D776Nh; E829Qh;S935Lh; S941Ph | G648Sh | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





