1. Introduction
Alopecia is the general medical term for hair loss which causes a significant impact in the quality of life of patients [
1]. Traditionally, alopecia is classified into two categories of hair loss disorders: scarring alopecia and non-scarring alopecia [
2]. Scarring alopecia is a rare hair condition that causes destruction of the hair follicle, replaces it with fibrous scar tissue, due to irreversible hair loss [
2]. In contrast, non-scarring alopecia is more common than scarring alopecia affecting both men and women with a prevalence about 3% to 7% [
3]. This condition doesn’t lead to destruction of hair follicle bulbs and although the hair cycle is altered, follicles are preserved with the ability to regenerate, allowing hair regrowth if the condition is treated early in the disease course [
3]. Non-scarring alopecia include male and female pattern hair loss, also known as androgenetic alopecia, alopecia areata and other less common conditions [
3]. Androgenetic alopecia (AGA), especially male pattern baldness, is one of the most prevalent forms of hair loss whose prevalence is based on age: up to 50% of white men by 50 years and 80% by 70 years will have AGA and we know that Caucasians are largely affected. The occurrence and the hair loss trend depend on the interaction of endocrine factors (testosterone and dihydrotestosterone (DHT) are the most important regulators) and genetic predisposition. Abnormal levels of testosterone and DHT alter the cycle of certain hair follicles, hindering the hair growth process. In men, the action of these hormones involves depletion of the frontal hairline with hair loss at the top of the scalp and causes the hair to become increasingly thin leading to vellus transformation of terminal hair. Based on the knowledge of the hair follicle cycle that is divided into three phases, anagen (the active phase in which the hair follicle works to produce hair fiber determining hair length), catagen (the transition phase characterized by hair follicle regression) and telogen (the resting phase in which the hair shaft growth does not occur), androgenetic alopecia results from an alteration of this cycle: the anagen phase duration decreases while that of telogen phase increases [
4,
5].
In addition, it is well known that the hair follicle is a complicated biological system finely regulated by the action of various growth factors, involved in the proper communication between epithelial cells and mesenchymal stem cells, translating in the correct progression of the hair growth cycle. Abnormal levels of testosterone and DHT determine, in fact, the inappropriate activation of pathways that lead to the abnormal release of factors such as transforming growth factor (TGF - ß), insuline-like growth factor (IGF – 1), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF) [
6,
7]. IGF - 1 and bFGF are crucial factors for follicle survival and growth in the anagen phase, while their expression is inhibited during the catagen phase [
8,
9]. IGF-1 has been shown to affect follicular proliferation, tissue remodeling, and the hair growth cycle, as well as follicular differentiation, identifying the IGF-1 signaling as an important mitogenic and morphogenetic regulator in hair follicle biology [
10]. During the phase of growth (anagen) the dermal papilla cells (fibroblasts) produce IGF-1 that promotes cell division of the hair matrix cells, to growth hair and to promote hair maintenance. In the anagen phase, IGF - 1 is supported by other factors such as HGF and VEGF, which get activated in dermal papilla cells, affecting the follicular keratinocytes and melanocytes by paracrine mechanism. EGF promotes the growth of the outer sheaths (ORS) of follicle in anagen phase and enhances the proliferation and migration of ORS cells during the initial phase of hair follicle growth. TGF - β (1-3), on the other hand, is mainly involved in the apoptotic process that characterizes the catagen phase, accompanied by the removal of the hair shaft from the dermal papilla. Finally, IGF-1 and EGF combination promote the transition of the hair cycle from telogen to anagen and stimulate the growth of hair shaft. All the growth factors are closely interrelated and affect each other in both regulating and controlling manner [
10].
In recent years, the emphasis of research has focused on the study and identification of biocompatible carrier vesicles capable of transporting bioactive molecules, including growth factors, facilitating cell communication and the correct activation of pathways underlying hair follicle regeneration. In this context, exosomes have held a dominant role.
Exosomes are a subset of extracellular microvesicles, with an average diameter of 30-200 nanometers, involved in intercellular communication and cellular trafficking. For example, they play an important role in many aspects of human health, including development, immunity, and tissue homeostasis. They are produced by various animal cells such as red blood cells, lymphocytes, dendritic cells, as well as being concentrated in various biological fluids such as milk, urine, and blood plasma [
11]. More recently, vesicle like-exosomes have also been isolated in plants [
12,
13]. Exosomes are formed from inward budding of endosomes resulting in membrane-surrounded multivesicular bodies (MVBs), which are secreted by fusion of the MVBs with the cell membrane. Once internalized, they may fuse with the membrane of endosomes thus enabling the transfer of their content to the cytoplasm of target cells. Their functional components include proteins (e.g., tetraspanins and heat shock proteins), lipids (exosomes are enriched in phosphatidylserine, cholesterol, prostaglandins), amino acids and different patterns of RNAs (microRNAs, mRNAs, rRNAs). Due to their high biocompatibility, exosomes are utilized in many biomedical and therapeutic applications from drug delivery in various tumour types (pancreatic, liver, gastric) [
14,
15], to the uptake of pathogenic molecules involved in the etiogenesis of neurodegenerative diseases such as Parkinson's and Alzheimer's [
16] to tissue stimulation in the regenerative medicine field [
13,
17,
18].
The aim of the present study was to evaluate the action on hair elongation in vitro of several compounds and compare their efficacy. We tested exosomes from different matrices such as plants, human cord blood stem cells (CBSC) and colostrum. Furthermore, we also evaluated the effectiveness of a product, called AMPLEX PLUS technology, a mixture of exosomes and 20 growth factors both derived from colostrum.
2. Materials and Methods
2.1. Cord blood stem cells (CBSCs)-derived exosomes
Exosomes derived from human cord blood stem cells (CBSCs) were a gift from our collaborators in University of Bradford. All the samples were collected from healthy mothers after obtaining consent in Tehran's Taleghani Hospital and received from Tissue Bank at the University of Bradford (Ref: ET-17-088). The morphology of exosomes was evaluated by transmission electron microscopy [
19].
2.2. Plant-Derived NanoVesicles (PDNVs)
We used lyophilized commercial nanovescicles derived to
Citrus bergamia. The PDNVs, obtained according to [
20], are measured through Nanoparticle Tracking Analysis to analyze number and size; the expression of TET8 (Tetraspanin-8) was evaluated.
2.3. Isolation and characterization of colostrum-derived exosomes
In this study, the colostrum was obtained from healthy cows. Exosomes from colostrum were concentrated through ultracentrifuge (Sorvall WX Ultra 100, Thermo Scientific) with successive centrifuges at ultrahigh speeds up to 100,000 x g. All centrifuges were performed at 4 °C. Briefly, colostrum was centrifuged for 10 min at 2,000 x g. The upper layer of fat globules and the pellet of dead cells were discarded, only the supernatant was collected. Thereafter, the supernatant was ultracentrifuged at 10,000 x g for 30 min to remove cell debris, and as in the previous step, pellet (cell debris) was discarded while the supernatant was collected and used for the following step. The supernatant was then subjected to two consecutive ultracentrifugations at 100,000 x g for 70 min to purify exosomes. After the first of the two 100,000 x g ultracentrifugations, pellet containing exosomes and contaminating proteins was collected, and the supernatant was discarded. The resultant pellet was resuspended in a large volume of filtered PBS to thrown away the contaminating proteins and centrifuged at least for 70 min at the same high speed to wash the exosome pellet.
The characterization of exosomes was performed with a light scattering according to [
21,
22]. Measurements were performed with a homemade apparatus using a quartz scattering cell, confocal collecting optics, a Hamamatzu photomultiplier mounted on a rotating arm, a BI-9100AT hardware correlator (Brookhaven Instruments Corporation) and illuminating the sample with a 660 nm laser. The power ranged between 5 and 15mW. Low power intensity was used to avoid convective motions due to local heating.
2.4. Colostrum- derived exosomes imaging with Scanning Electron Microscopy
Pellets containing exosomes isolated from colostrum were vortexed and resuspended in 2.5% EMS-quality glutaraldehyde in PBS for 15 min at 4 °C. Subsequently, 500 μl of sample was resuspended in 1.5 ml of PBS and brought to volume to be ultracentrifuged at 100,000 xg (23,200 rpm) for 1h at 4°C. After the samples were dehydrated with alcohol at 50° and 100° (always after ultracentrifugation at 100,000 xg (23,200 rpm) for 1h at 4°C). After collecting the pellets left to air dry overnight on specific carbon fiber-covered stubs and observed by SEM (Coxem EM-30 plus).
2.5. GF20 preparation and detection of bioactive factors by ELISA
The sample was processed according to the procedure described by [
23]. The concentrations of factors of GF20 (
Table 1) were determined using commercially available ELISA tests specific for human molecules. Since the samples used came from non-human matrices, specifically from bovine, it was necessary to validate the ELISA tests used. We evaluated the use of the commercial immunometric methods QUANTIKINE human IGF-1 Immunoassay (DG100B) and QUANTIKINE human TGF-β1 Immunoassay (DB100B) (S1, S2). This validation is possible due to degree of analogy sequential between the human-derived molecule and the bovine-derived molecule (95% for IGF-1 and 94% for TGF- β). All procedures were performed according to the instructions of the manufacturer.
2.6. AMPLEX PLUS technology preparation
To obtain AMPLEX PLUS technology in 5 mL of water were added 20 billion exosomes previously isolated from colostrum and 200 mg of GF20.
2.7. In vitro culture of human hair follicle
Human follicles were micro dissected and isolated from the occipital scalp during hair transplantation procedures of 10 healthy male individuals. All individuals signed informed consent, according to the Helsinki Declaration (2001). Intact and anagen phase VI follicles, observed and selected by stereomicroscopy, were cultured in 24-well plates (1 per well) with 500 µl Williams E medium (Gibco BRL, Rockville, MD, USA), with insulin 10 µg ⁄ ml, hydrocortisone 10 ng ⁄ ml, streptomycin 10 µg ⁄ ml and penicillin 100 U ⁄ ml (Wuppertal, Germany), at 37°C and 5% CO2. A total of 72 follicles were distributed in different plates to test a different matrices previously dissolved in Williams E. medium. The growth parameters of the follicles were analyzed every 3 days for 18 days. Hair length was assessed using an inverted microscope (LEICA DM IRB) and the images obtained were measured using ImageJ. Three replicates were performed.
2.8. Identification and analysis of hair follicle derma papilla cells
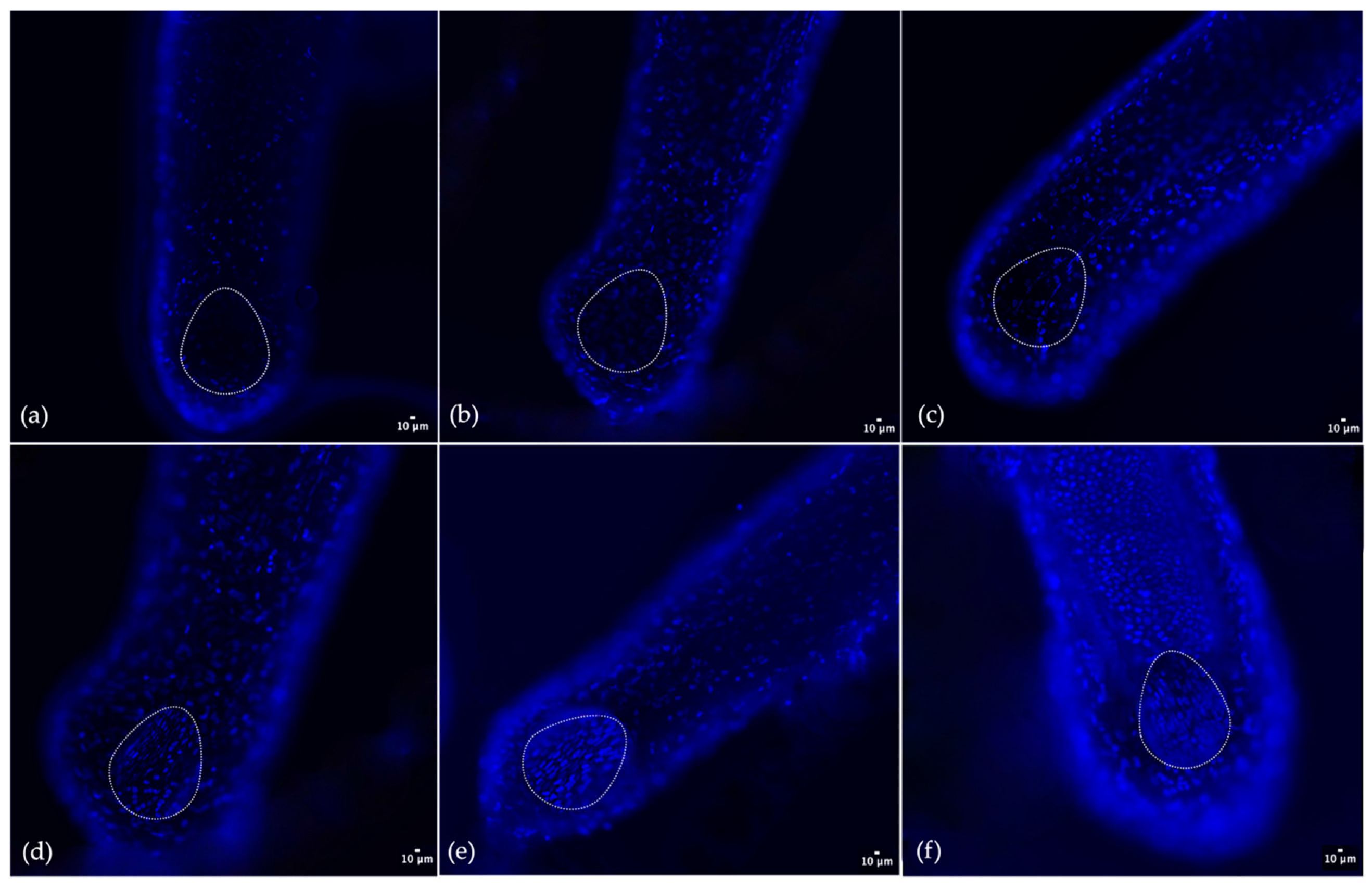
After 18 days of exposure, hair follicles were stained with DAPI to highlight the nuclei in blue and allowed them to be identified. We used a fast-staining method of hair follicles that required to place the samples directly on microscope slides, upon which a drop of DAPI (Sigma-Aldrich) was added. A coverslip glass was applied, and the hair follicles were immediately visualized under the fluorescence microscope (Nikon Eclipse Ci) with a magnification of 10x.
3. Results
3.1. Characterization of Plant-Derived NanoVesicles
Plant-Derived NanoVesicles of C. bergamia within the separated and purified suspension showed a range of 182.5 ± 1.3 nm. The PDNVs concentration were 2x1010 mL-1.
3.2. Characterization of CBSCs-derived exosomes
The isolated CBSCs-derived exosomes showed a spherical morphology and a diameter between 30 and 120 nm and a concentration of 3.4 × 109 particles/ml.
3.3. Characterization of colostrum-derived exosomes
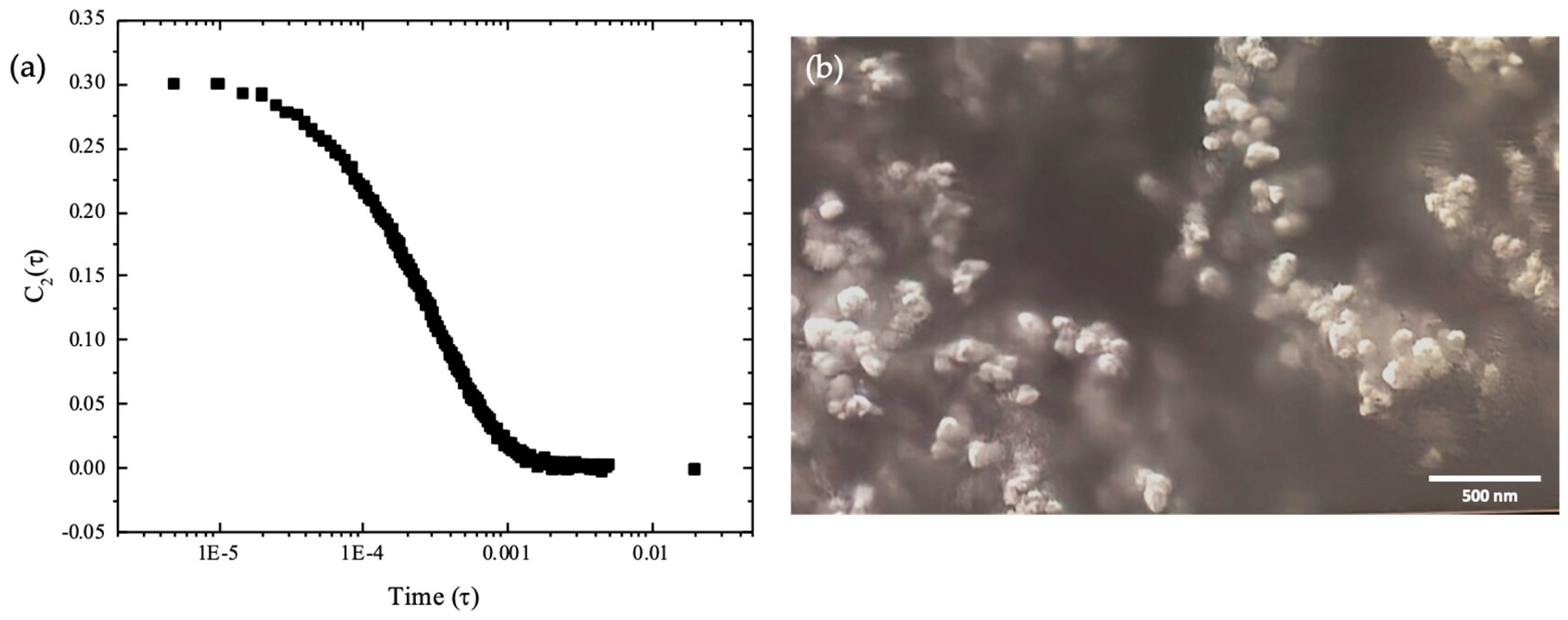
Exosomes were obtained from bovine colostrum collected in the first 6 h. The particle size was verified by dynamic light scattering (DLS) and morphology verified by scansion electron microscopy (SEM) (
Figure 1). The average diameter size was 90-120 nm and exosomes presented a spherical morphology. The concentration mean was 4.2 x 10
12 particles/mL.
3.4. Bioactive components of colostrum
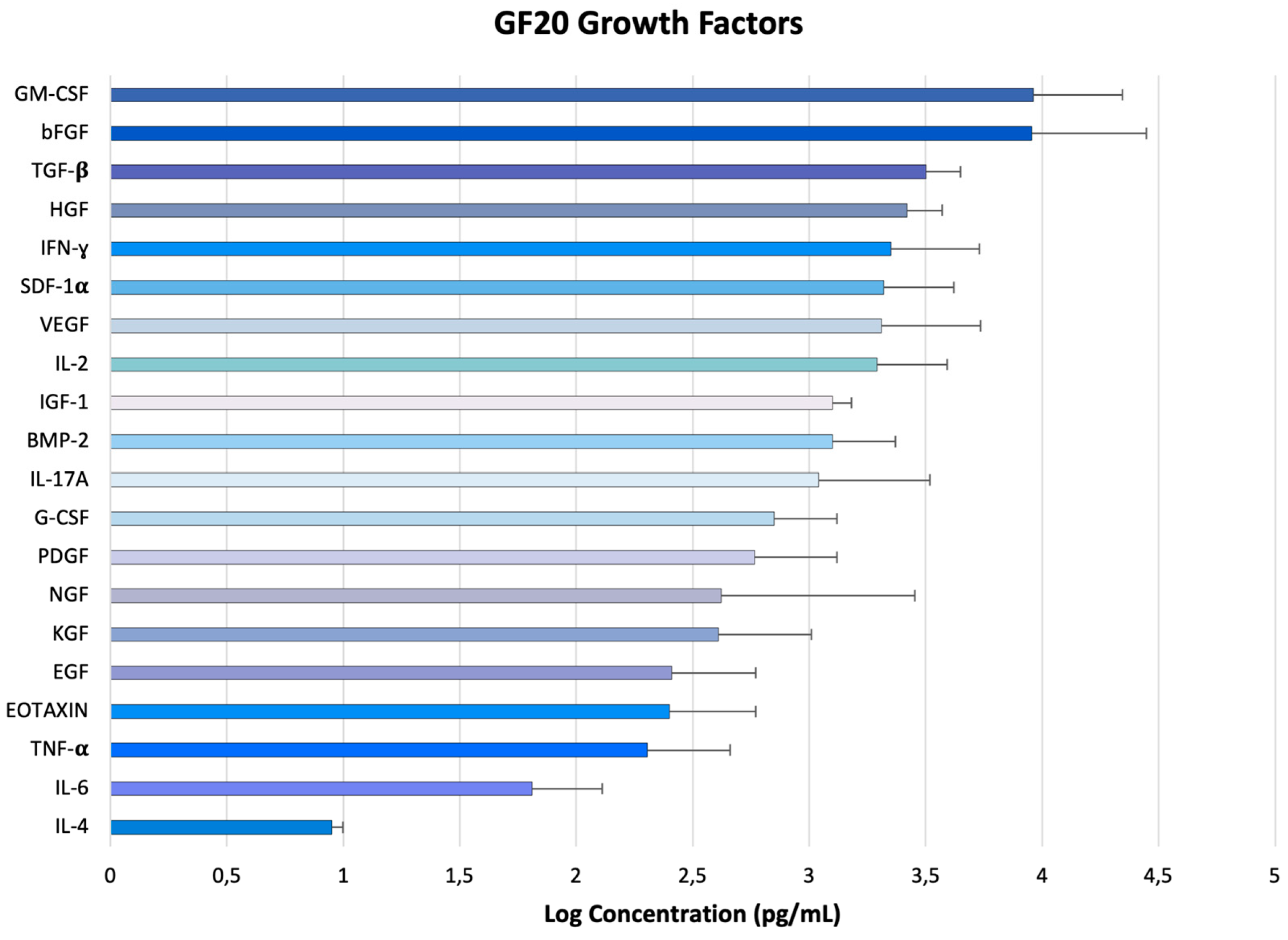
All bioactive factors were measured in colostrum after lyophilization. In
Figure 2 are reported the concentration (pg/mL) of main bioactive substances such as cytokines and growth factors, isolated from GF20. The graph showed that all components found in colostrum had an elevated concentration, especially GM-CSF, bFGF, TGF-ß, for which the concentrations, expressed in logarithmic scale corresponded to 3.963 ± 0.38, 3.955 ± 0.492, 3.5 ±0.15, respectively. All measurements obtained are shown in Table 2S.
3.5. In vitro culture of human hair follicle
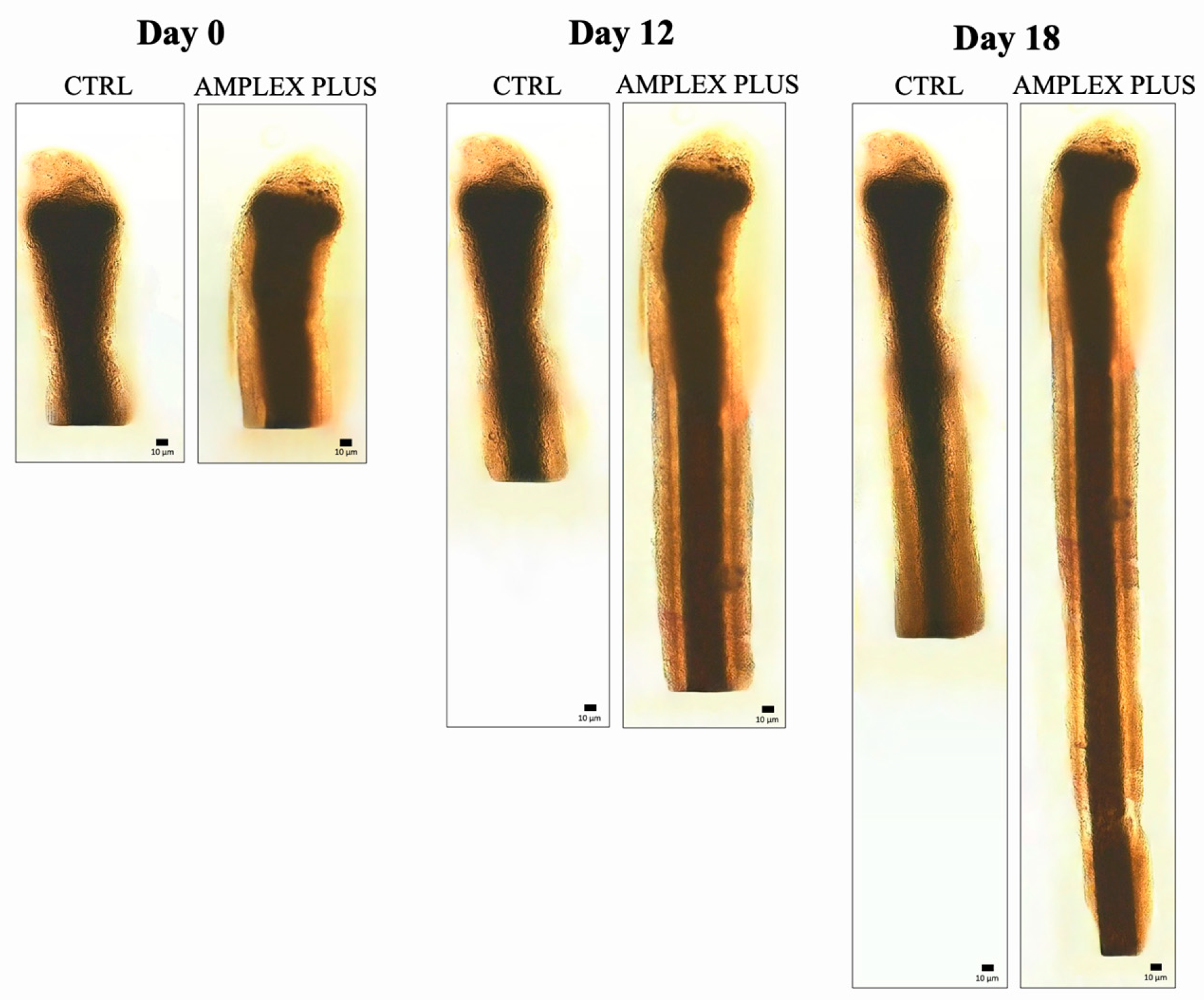
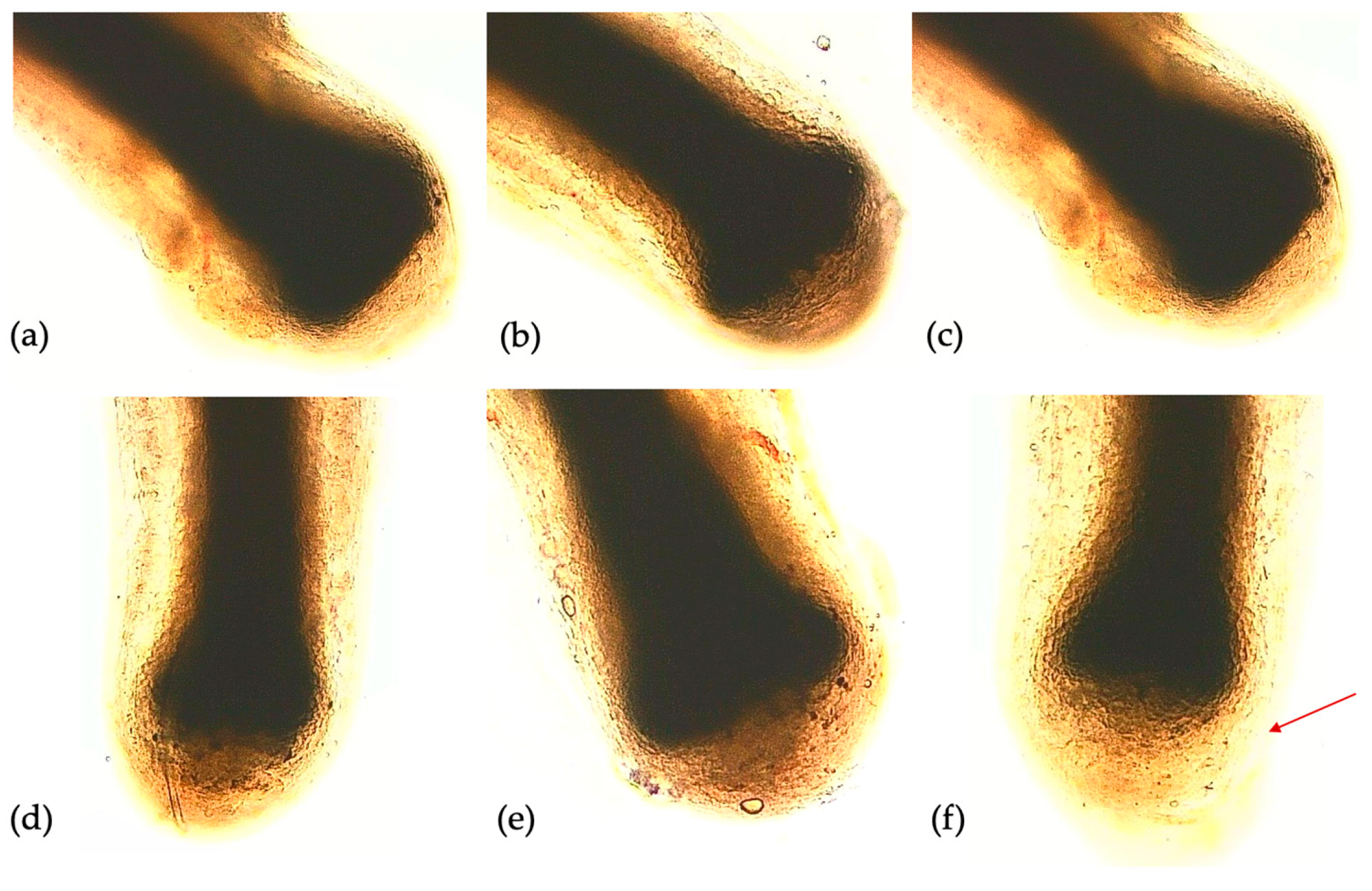
The biological model used for this test is represented by hair follicles micro-dissected from fragments of human skin. The purpose of the test is to verify the growth of the hair follicle in the presence or absence of the substances to be tested: the hair follicles were grown in the William culture medium without adding other compositions (negative control) and in the same culture medium with the addition of all tested exosomes. However, samples exposed to exosomes from various matrices showed a significant difference in the width of germinative growth area of the hair bulb compared to the negative control. As shown in the
Figure 3, the higher growth was highlighted in the samples treated with, primarily, AMPLEX PLUS, followed by GF20. Minor germinative area expansion was noted in bulbs treated with colostrum exosomes applied alone, followed by CBSCs-derived exosomes and, finally, Plant-Derived Nanovesicles. This evidence was also confirmed by analysis of the nuclei in the dermal papilla detected by DAPI. As shown in
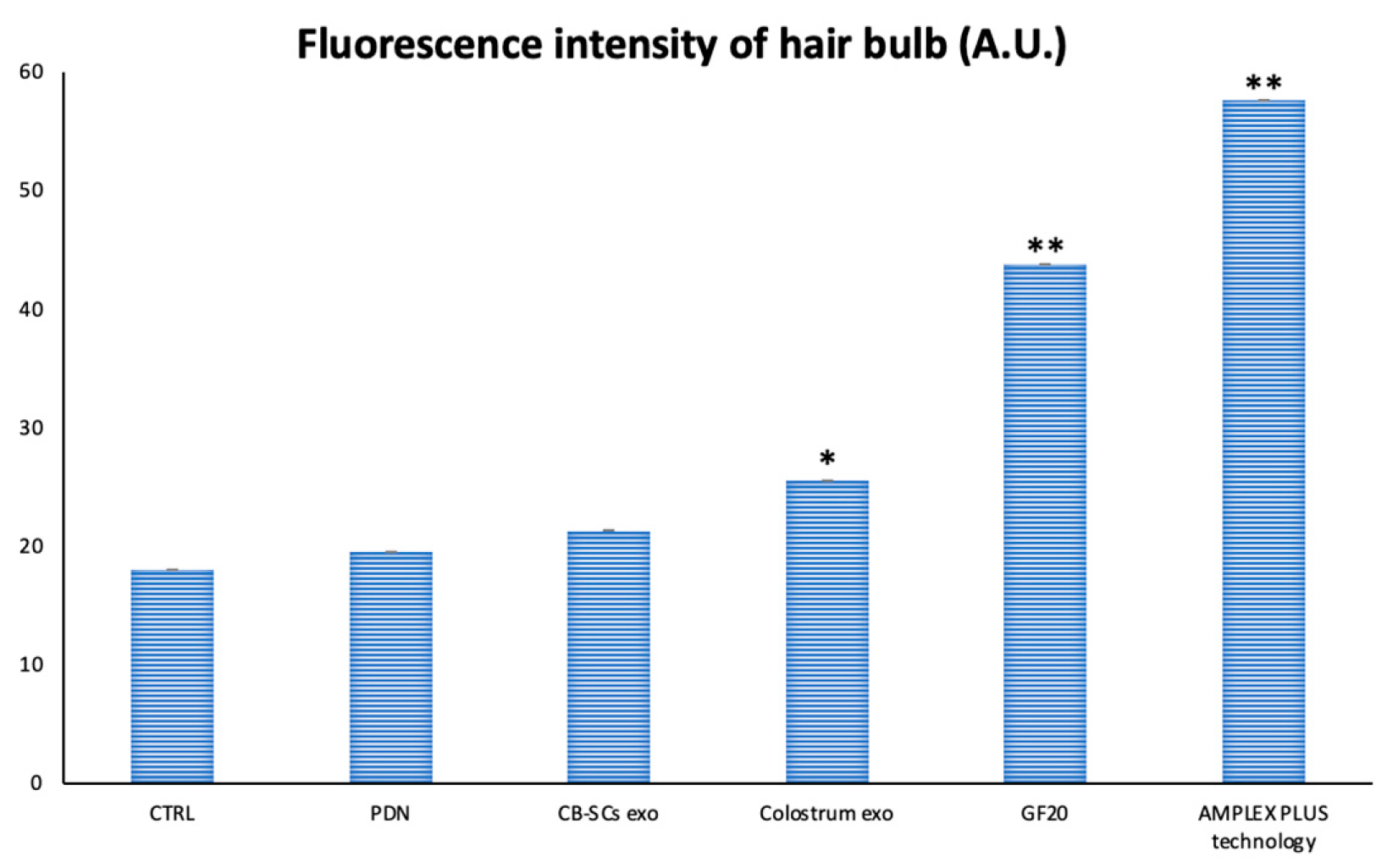
Figure 4, a progressive increase in the number of cells was found in samples treated with colostrum exosomes, GF20 and AMPLEX PLUS. The images obtained were analyzed using ImageJ software, which provided the fluorescence intensity. The graph in
Figure 5, showed a significant increase in the measured parameter indirectly related to the number of cells, in samples treated with colostrum exosomes (25.477 ± 0.02, * p < 0.05), GF20 (43.766 ± 0.02, ** p < 0.01) and AMPLEX PLUS (57.632 ± 0.02, ** p < 0.01) compared to CTRL (17.984 ± 0.02), plant derived nanovesicles (19.463 ± 0.03) and CB-SCs exosomes (21.244 ± 0.04).
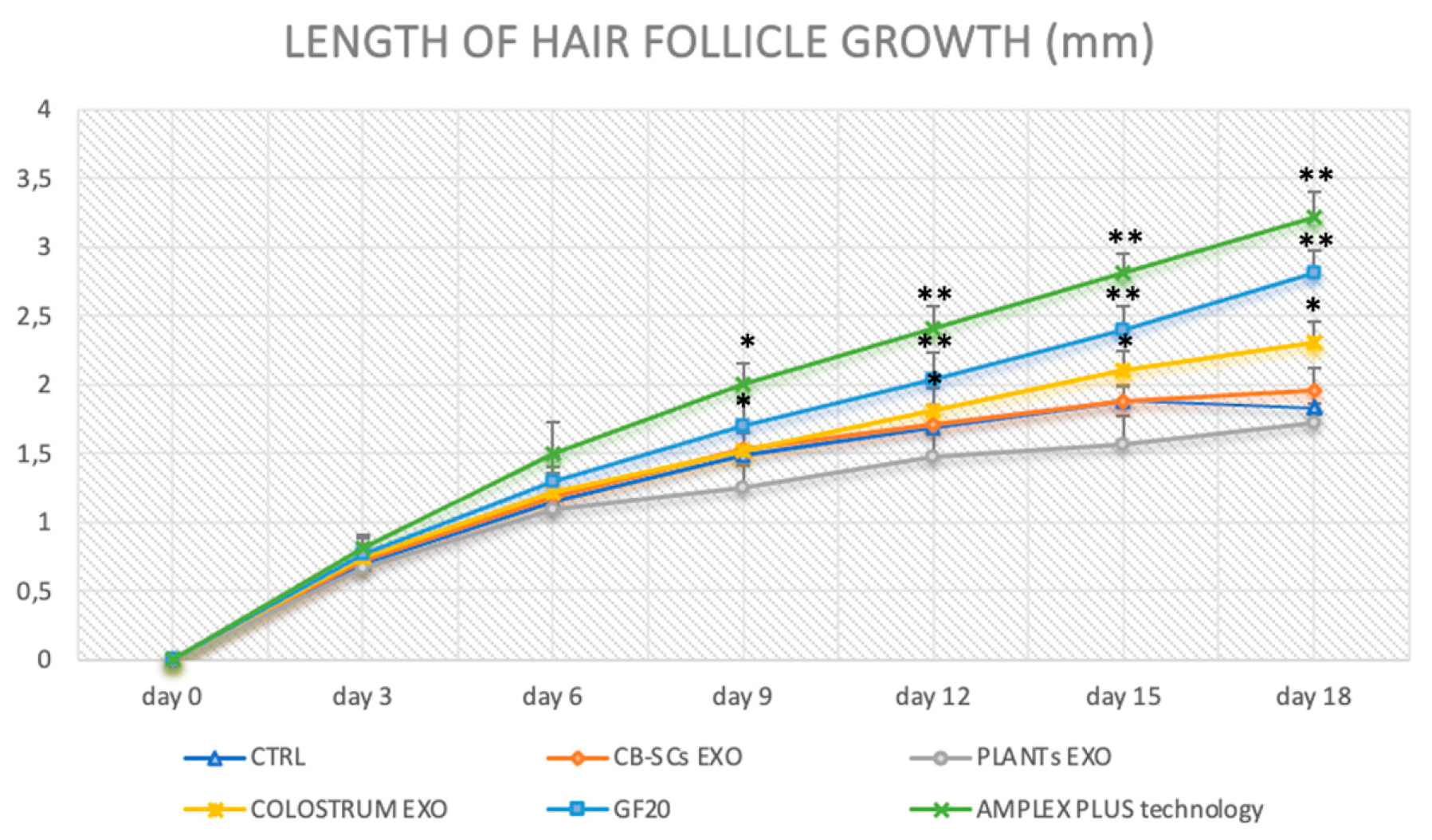
The growth of the hair follicle was followed for 18 days, measuring its length at regular intervals (every 3 days). The results demonstrated the effectiveness of the AMPLEX PLUS and GF20 blend compared to the other compounds tested (
Figure 4). Specifically, at the end of treatment, the hair follicle exposed to AMPLEX PLUS measured 3.22 ± 0.18 mm, while those exposed to the new GF20 showed a length of 2.81 ± 0.16 mm. Lower growth was evidenced following treatment with: colostrum exosomes used alone in which the length corresponded to 2.31 ± 0.15 mm, plant derived nanovesicles with a length of 1.72 ± 0.14 mm, CB-SCs exosomes with a length corresponding to 1.96 ± 0.16 mm. Finally, the negative control exhibited a value of 1.83 ± 0.13 mm. It can be seen from the graph that highly significant values (** p < 0.01) were obtained following AMPLEX PLUS and GF20 treatment from day 12. Treatment with colostrum exosomes also showed a significant (* p < 0.05) increase in the measured parameter, but lower than the two previously mentioned compounds. In contrast, no significant difference was highlighted in follicle growth in the samples treated with CBSCs exosomes and PDNVs, compared with the untreated negative control.
Figure 7.
Observation of hair bulb length in CTRL and in samples exposed to AMPLEX PLUS at day 0 - 12 -18.
Figure 7.
Observation of hair bulb length in CTRL and in samples exposed to AMPLEX PLUS at day 0 - 12 -18.
4. Discussion
Alopecia is a highly prevalent in society and beyond its sociological meaning, it has become a very important part of self-identity, causing a negative impact on individual’s life [
24]. In recent years, research in this field has focused on developing possible treatments that could stimulate hair follicle growth in the anagenic phase. Particular attention was reserved for the use of exosomes, which are known to act as cargoes for the transport of bioactive molecules, some of which involved in regenerative mechanisms [
25]. The purpose of the present study was to investigate the potential hair growth stimulatory effects of exosomes or nanovesicles isolated from various matrices, including plants, embryonic stem cells, and colostrum. Plant nanovesicles and stem cell exosomes have already been extensively studied and characterized in previous years by several Authors [
26,
27]. Plant derived nanovesicles had already demonstrated their regenerative function, particularly, in the context of skin repair mechanisms, and their action as scavengers in reducing oxidative stress [
28,
29]; PDNVs biological properties include also anti-proliferative and pro- apoptotic effects which account for their anticancer activities [
30,
31,
32]. Anti-cancerous and anti-inflammatory properties have also been investigated for stem cells [
33,
34,
35,
36]. Their possible role in the dynamics of hair follicle growth, however, has not been discussed. Exosomes from these matrices have not yet been investigated regarding their possible effects on hair regeneration, whereas interesting results have been achieved with exosomes and factors present in colostrum, using mouse models [
37,
38].
The preliminary part of our study was focused to the isolation and characterization of exosomes derived from colostrum, whose size and concentration corresponded to 90-120 and 4.2 x 10
12 particles/mL, respectively. Compared with those obtained from plants nanovesicles and exosomes from stem cells, colostrum derived exosomes appear to have higher concentrations (particles/mL), while the average size was lower than in
C. Bergamia, but higher than CBSCs exosomes. However, exosomes derived from the matrices in comparison exhibited a similar and regular spherical morphology. Following isolation and characterization, colostrum exosomes known for their high biocompatibility [
39,
40,
41], were combined with bioactive molecules present in GF20, a mixture isolated from colostrum and purified from potentially allergenic components, e.g., immunoglobulins [
42,
43,
44] and molecules not involved in tissue regeneration mechanisms. Thus, GF20, as described in our study by ELISA test, is composed of a high variety of growth factors, cytokines and other protein molecules known to act synergistically during the hair follicle growth process. bFGF and TGF-ß showed, among the characterized factors, showed the highest concentrations. These growth factors are crucial in providing a substantial help in regenerating hair tissue, and each of them is engaged in an explicit biomolecular activity. bFGF promotes hair growth by inducing the anagen phase stimulating the proliferation of papilla cells, leading to an increase in the size of the hair follicle [
45]. TGF- ß plays a crucial role in regulating hair growth: in hair follicles it is involved in the development of the dermal papilla and hair matrix during the anagen phase, but also in the apoptotic process that characterizes the catagen phase, accompanied by the removal of the hair shaft from the dermal papilla [
46].
The final goal of the present work was to evaluate the effects a new technology called AMPLEX PLUS that combines the action of colostrum-derived exosomes with the growth factors and cytokines of GF20. The stimulatory effect on hair growth was evaluated through an in vitro test involving treatment for 18 days. At the end of the exposure, AMPLEX PLUS and GF20 resulted not only in an extension of follicle growth compared to all other compounds tested, but also in an increase in the number of dermal papilla cells. The positive results observed could be related partly to the action of the exosomes and partly to that of the free factors. The exosomes, in fact, act as carriers of the same free factors present in GF20 and, by fusing with the plastic membranes of the target cells, release their contents. This caused more factors to recognize specific receptors, triggering the enzymatic cascades that ultimately leaded to the stimulation of hair growth.
5. Conclusions
Based on the results obtained, exosomes from plants and stem cells had no significant effect on the stimulation of hair growth, unlike exosomes from colostrum. In contrast, these exosomes combined with bioactive molecules with regenerative potential in the final product AMPLEX PLUS resulted in a significant increase in bulb growth and regeneration of the dermal papilla. This evidence could represent the key to implement current treatments solving the problem of hair loss, with consequent positive impact on the quality of life and psychological sphere of subjects suffering from alopecia or other conditions characterized by limited follicular growth.
Author Contributions
Conceptualization, M.V.B.; methodology, M.V.B.; software, G.F., M.C. and M.Z.; validation, G.F. and M.C.; formal analysis, G.F., M.C. and M.Z.; investigation, G.F. and M.C.; resources, G.F. and M.C.; data curation, G.F. and M.C.; writing—original draft preparation, M.V.B.; writing—review and editing, G.F. and M.C.; visualization, G.F. and M.C.; supervision, M.V.B.; funding acquisition, M.V.B. All authors have read and agreed to the published version of the manuscript.
Funding
Open access funding provided by Università degli Studi di Catania within the CRUI-CARE Agreement.
Institutional Review Board Statement
This study was performed in line with the principles of the Declaration of Helsinki and does not require approval by the Ethics Committee of University of Catania.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
G.F. thanks PhD program FSE Notice 1/2021. Authors acknowledge the PON project Bio-nanotech Research and Innovation Tower (BRIT), financed by the Italian Ministry for Education, University and Research (MIUR) (Grant no. PONa3_00136).
Conflicts of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- Thadanipon, K.; Suchonwanit, P. Measuring Patient Quality of Life Following Treatment for Alopecia. Patient Preference Adherence 2021, ume 15, 1601–1610. [Google Scholar] [CrossRef]
- Lin, J.; Saknite, I.; Valdebran, M.; Balu, M.; Lentsch, G.; Williams, J.N.; Koenig, K.; Tromberg, B.J.; Mesinkovska, N.A. Feature characterization of scarring and non-scarring types of alopecia by multiphoton microscopy. Lasers Surg. Med. 2018, 51, 95–103. [Google Scholar] [CrossRef]
- Gurusamy, U.; Venkataswamy, C. Hair Loss in Paediatric and Adolescent Age Group: A Clinico-Pathological Analysis in a Tertiary Health Care Centre. J. Clin. Diagn. Res. 2017. [Google Scholar] [CrossRef]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef]
- Piraccini BM, Alessandrini A. Androgenetic alopecia. G Ital Dermatol Venereol. 2014, 149, 15–24.
- Hibino, T.; Nishiyama, T. Role of TGF-β2 in the human hair cycle. J. Dermatol. Sci. 2004, 35, 9–18. [Google Scholar] [CrossRef]
- Shin, S.H.; Koh, Y.G.; Lee, W.G.; Seok, J.; Park, K.Y. The use of epidermal growth factor in dermatological practice. Int. Wound J. 2022, 20, 2414–2423. [Google Scholar] [CrossRef]
- Ahn, S.-Y.; Pi, L.-Q.; Hwang, S.T.; Lee, W.-S. Effect of IGF-I on Hair Growth Is Related to the Anti-Apoptotic Effect of IGF-I and Up-Regulation of PDGF-A and PDGF-B. Ann. Dermatol. 2012, 24, 26–31. [Google Scholar] [CrossRef]
- Woo, J.; Suh, W.; Sung, J.-H. Hair Growth Regulation by Fibroblast Growth Factor 12 (FGF12). Int. J. Mol. Sci. 2022, 23, 9467. [Google Scholar] [CrossRef]
- Trüeb, R.M. Further Clinical Evidence for the Effect of IGF-1 on Hair Growth and Alopecia. Ski. Appendage Disord. 2017, 4, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Akuma P, Okagu OD and Udenigwe CC. Naturally Occurring Exosome Vesicles as Potential Delivery Vehicle for Bioactive Compounds. Front. Sustain. Food Syst. 2019, 3. [CrossRef]
- Kim, Y.; Pérez-González, R.; Miller, C.; Kurz, M.; D’acunzo, P.; Goulbourne, C.N.; Levy, E. Sex Differentially Alters Secretion of Brain Extracellular Vesicles During Aging: A Potential Mechanism for Maintaining Brain Homeostasis. Neurochem. Res. 2022, 47, 3428–3439. [Google Scholar] [CrossRef] [PubMed]
- Sarasati, A.; Syahruddin, M.H.; Nuryanti, A.; Ana, I.D.; Barlian, A.; Wijaya, C.H.; Ratnadewi, D.; Wungu, T.D.K.; Takemori, H. Plant-Derived Exosome-like Nanoparticles for Biomedical Applications and Regenerative Therapy. Biomedicines 2023, 11, 1053. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Shin, K.J.; Chae, Y.C. Protocol for evaluation of tumor-derived exosome-induced cancer cell metastasis in a mouse model. STAR Protoc. 2023, 4, 102444. [Google Scholar] [CrossRef] [PubMed]
- Akad, F.; Mocanu, V.; Peiu, S.N.; Scripcariu, V.; Filip, B.; Timofte, D.; Zugun-Eloae, F.; Cuciureanu, M.; Hancianu, M.; Oboroceanu, T.; et al. Mesenchymal Stem Cell-Derived Exosomes Modulate Angiogenesis in Gastric Cancer. Biomedicines 2023, 11, 1031. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, D.J. Potential for Therapeutic-Loaded Exosomes to Ameliorate the Pathogenic Effects of α-Synuclein in Parkinson’s Disease. Biomedicines 2023, 11, 1187. [Google Scholar] [CrossRef]
- Zeng, Y.; Qiu, Y.; Jiang, W.; Shen, J.; Yao, X.; He, X.; Li, L.; Fu, B.; Liu, X. Biological Features of Extracellular Vesicles and Challenges. Front. Cell Dev. Biol. 2022, 10, 816698. [Google Scholar] [CrossRef]
- Schepici, G.; Silvestro, S.; Mazzon, E. Regenerative Effects of Exosomes-Derived MSCs: An Overview on Spinal Cord Injury Experimental Studies. Biomedicines 2023, 11, 201. [Google Scholar] [CrossRef]
- Naeem, P.; Baumgartner, A.; Ghaderi, N.; Sefat, F.; Alhawamdeh, M.; Heidari, S.; Shahzad, F.; Swaminathan, K.; Akhbari, P.; Isreb, M.; et al. Anticarcinogenic impact of extracellular vesicles (exosomes) from cord blood stem cells in malignant melanoma: A potential biological treatment. J. Cell. Mol. Med. 2022, 27, 222–231. [Google Scholar] [CrossRef]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. Nanovesicles from Organic Agriculture-Derived Fruits and Vegetables: Characterization and Functional Antioxidant Content. Int. J. Mol. Sci. 2021, 22, 8170. [Google Scholar] [CrossRef]
- Zimbone, M.; Musumeci, P.; Baeri, P.; Messina, E.; Boninelli, S.; Compagnini, G.; Calcagno, L. Rotational dynamics of gold nanoparticle chains in water solution. J. Nanoparticle Res. 2012, 14. [Google Scholar] [CrossRef]
- Zimbone, M.; Baeri, P.; Calcagno, L.; Musumeci, P.; Contino, A.; Barcellona, M.L.; Bonaventura, G. Dynamic Light Scattering on Bioconjugated Laser Generated Gold Nanoparticles. PLoS ONE 2014, 9, e89048. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Mussano, F.; Franchi, S.; Panerai, A.; Bussolati, G.; Carossa, S.; Bartorelli, A.; Bussolati, B. Biological components in a standardized derivative of bovine colostrum. J. Dairy Sci. 2013, 96, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Dhami, L. Psychology of Hair Loss Patients and Importance of Counseling. Indian J. Plast. Surg. 2021, 54, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Wu, D.; Li, G.; Yang, J.; Zhang, Y.S. Exosomes targeted towards applications in regenerative medicine. Nano Sel. 2021, 2, 880–908. [Google Scholar] [CrossRef]
- Yaghoubi, Y.; Movassaghpour, A.; Zamani, M.; Talebi, M.; Mehdizadeh, A.; Yousefi, M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019, 233, 116733. [Google Scholar] [CrossRef] [PubMed]
- Shkryl, Y.; Tsydeneshieva, Z.; Degtyarenko, A.; Yugay, Y.; Balabanova, L.; Rusapetova, T.; Bulgakov, V. Plant Exosomal Vesicles: Perspective Information Nanocarriers in Biomedicine. Appl. Sci. 2022, 12, 8262. [Google Scholar] [CrossRef]
- Lian, M.Q.; Chng, W.H.; Liang, J.; Yeo, H.Q.; Lee, C.K.; Belaid, M.; Tollemeto, M.; Wacker, M.G.; Czarny, B.; Pastorin, G. Plant-derived extracellular vesicles: Recent advancements and current challenges on their use for biomedical applications. J. Extracell. Vesicles 2022, 11, e12283. [Google Scholar] [CrossRef]
- Sarasati, A.; Syahruddin, M.H.; Nuryanti, A.; Ana, I.D.; Barlian, A.; Wijaya, C.H.; Ratnadewi, D.; Wungu, T.D.K.; Takemori, H. Plant-Derived Exosome-like Nanoparticles for Biomedical Applications and Regenerative Therapy. Biomedicines 2023, 11, 1053. [Google Scholar] [CrossRef]
- Di Giulio, S.; Carata, E.; Mariano, S.; Panzarini, E. Plant Extracellular Vesicles: Investigating Their Utilization as Beneficial Nutrients in Diet. Appl. Sci. 2023, 13, 6656. [Google Scholar] [CrossRef]
- Di Gioia, Sante, Hossain, Niamat Mt and Conese M. "Biological properties and therapeutic effects of plant-derived nanovesicles", Open Medicine. 2020.
- Liu, Y.; Wu, S.; Koo, Y.; Yang, A.; Dai, Y.; Khant, H.; Osman, S.R.; Chowdhury, M.; Wei, H.; Li, Y.; et al. Characterization of and isolation methods for plant leaf nanovesicles and small extracellular vesicles. Nanomedicine: Nanotechnology, Biol. Med. 2020, 29, 102271. [Google Scholar] [CrossRef]
- Naeem, P.; Baumgartner, A.; Ghaderi, N.; Sefat, F.; Alhawamdeh, M.; Heidari, S.; Shahzad, F.; Swaminathan, K.; Akhbari, P.; Isreb, M.; et al. Anticarcinogenic impact of extracellular vesicles (exosomes) from cord blood stem cells in malignant melanoma: A potential biological treatment. J. Cell. Mol. Med. 2022, 27, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.M.S.; Rodrigues, S.C.; Gomes, C.F.; Duarte, F.V.; Romao, M.; Leal, E.C.; Freire, P.C.; Neves, R.; Simões-Correia, J. Development of an optimized and scalable method for isolation of umbilical cord blood-derived small extracellular vesicles for future clinical use. STEM CELLS Transl. Med. 2021, 10, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Hu, Y.; Rao, S.-S.; Wang, Z.-X.; Cao, J.; Tan, Y.-J.; Luo, J.; Li, H.-M.; Zhang, W.-S.; Chen, C.-Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Wang, T.; Rapaport, J.A. Systematic review of exosome treatment in hair restoration: Preliminary evidence, safety, and future directions. J. Cosmet. Dermatol. 2023, 22, 2424–2433. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, Y.; Kim, E.H.; Jang, H.; Cho, H.; Han, G.; Song, H.K.; Kim, S.H.; Yang, Y. Potential of Colostrum-Derived Exosomes for Promoting Hair Regeneration Through the Transition From Telogen to Anagen Phase. Front. Cell Dev. Biol. 2022, 10, 815205. [Google Scholar] [CrossRef]
- Somiya, M.; Yoshioka, Y.; Ochiya, T. Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1440132. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef]
- Zhong, J.; Xia, B.; Shan, S.; Zheng, A.; Zhang, S.; Chen, J.; Liang, X.-J. High-quality milk exosomes as oral drug delivery system. Biomaterials 2021, 277, 121126. [Google Scholar] [CrossRef]
- Wheeler, T.T.; Hodgkinson, A.J.; Prosser, C.G.; Davis, S.R. Immune Components of Colostrum and Milk—A Historical Perspective. J. Mammary Gland. Biol. Neoplasia 2007, 12, 237–247. [Google Scholar] [CrossRef] [PubMed]
- El-Agamy, E.I. The challenge of cow milk protein allergy. Small Rumin. Res. 2007, 68, 64–72. [Google Scholar] [CrossRef]
- Ulfman, L.H.; Leusen, J.H.W.; Savelkoul, H.F.J.; Warner, J.O.; van Neerven, R.J.J. Effects of Bovine Immunoglobulins on Immune Function, Allergy, and Infection. Front. Nutr. 2018, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Lin WH, Xiang LJ, Shi HX, Zhang J, Jiang LP, Cai PT, Lin ZL, Lin BB, Huang Y, Zhang HL, Fu XB, Guo DJ, Li XK, Wang XJ, Xiao J. Fibroblast growth factors stimulate hair growth through β-catenin and Shh expression in C57BL/6 mice. Biomed Res Int. 2015, 2015, 730139. [CrossRef]
- Rishikaysh, P.; Dev, K.; Diaz, D.; Qureshi, W.M.S.; Filip, S.; Mokry, J. Signaling Involved in Hair Follicle Morphogenesis and Development. Int. J. Mol. Sci. 2014, 15, 1647–1670. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).