1. Introduction
Obesity is a chronic inflammatory disorder characterized by an increase in body weight relative to height. White adipose tissue may become dysfunctional and not expand properly to store the excess energy in obesity. Enlarged visceral adipose tissue is infiltrated with pro-inflammatory immune cells (e.g., M1 macrophages and cytotoxic T cells), and secretes excessive amounts of pro-inflammatory adipokines (e.g., adiponectin, leptin, visfatin, resistin, chemerin) and cytokines (e.g., tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and IL-6) [
1]. These adverse inflammatory signals induce a state of chronic systemic inflammation and increased levels of C-reactive protein (CRP) in blood. [
2].
CRP is an acute inflammatory protein and an important marker of systemic inflammation [
3]. CRP is primarily synthesized in liver hepatocytes. In response to the increased levels of inflammatory cytokines, in particular interleukin 6, transcriptional activation of CRP gene predominantly occurs in hepatic hepatocytes. Other cells including macrophages, endothelial cells, lymphocytes, and adipocytes can also synthesize CRP [
3]. CRP is produced as a homopentameric protein, termed native CRP (nCRP), which at sites of inflammation and infection irreversibly dissociates into five separate monomers, termed monomeric CRP (mCRP) [
3]. CRP was named for its reaction with the capsular (C)-polysaccharide of Pneumococcus [
4]. CRP is not only a marker of infection and inflammation. CRP also has a protective role against bacterial infections through the activation of complement, opsonization of pathogens, and induction of inflammatory cytokines [
3]. CRP exhibits elevated expression in many inflammatory conditions including obesity [
2].
Periodontitis is a chronic inflammatory oral disease characterized by gingival inflammation and destruction of alveolar bone. Disproportionate host responses and bacterial dysbiosis are the two main etiological factors [
5]. A failure in resolution of gingival inflammation leads to destructive periodontal disease. In the pathogenesis of periodontitis, monocytes/macrophages play an important role in tissue destruction and repair. Their cytokine secretions contribute significantly to the inflammatory burden in the gingival tissues, they bridge innate with adaptive immunity, and finally they provide for resolution of inflammation [
6]. Major inflammatory cytokines involved with periodontitis include IL-1β [
7,
8], TNFα [
9,
10], IL-6 [
11,
12], IL-8 [
13,
14], and anti-inflammatory IL-10 [
15,
16].
Periodontal disease is prevalent among obese individuals [
17,
18], and response to periodontal therapy is compromised [
19]. Previously it was shown that CRP levels are elevated in gingival fluid of obese individuals [
20]. Since CRP is a proinflammatory protein, we hypothesized that increased levels of CRP in gingival tissues of obese individuals would promote gingival inflammation and may explain the increased susceptibility to periodontitis in obesity. Previous studies mainly focused on periodontally diseased individuals. The effect of obesity on the periodontium without the confounding effect of periodontitis is not well understood. Obesity-related increased levels of CRP in gingival tissues may offer valuable insights on the impact of obesity on periodontal health.
The objectives of this study were to examine the effect of obesity (clinical measures and peripheral inflammatory cytokines) on the relationship between peripheral and gingival CRP levels and examine the association of gingival CRP levels with clinical periodontal measures and gingival fluid inflammatory cytokines in periodontally healthy obese individuals.
2. Materials and Methods
We recently identified a unique cohort of periodontitis-resistant elderly (>60 yrs.) individuals [
21,
22]. Subject recruitment/enrollment were previously described [
21,
22].
Subject Recruitment, Screening and Enrollment: One hundred thirty-six individuals were successfully interviewed by phone to assess eligibility. Fifty-four subjects agreed to participate, and 39 subjects fulfilled periodontal health criteria (pocket depth ≤3mm and bleeding on probing <10%). Written informed consent was obtained from all subjects. Exclusion criteria included history of medical conditions known to impact the periodontium (e.g., diabetes), fewer than 15 teeth present, pocket depth >3mm and bleeding on probing ≥10%, antibiotic treatment in the past 3 months, dental cleaning in the past 3 months, current use of tobacco products, inability to comprehend or follow instructions in English, and physical limitations interfering with dental examination.
For all subjects, a comprehensive medical history interview was performed. Height and weight measurements were recorded, and body mass index (BMI) was calculated. Waist circumference was recorded with a tape measure placed around the abdomen at the top of the hip bone and at the level of the belly button. All subjects answered a questionnaire related to dietary habits, education, income, dental hygiene practice, dental care, and history of cigarette smoking [
21,
22].
Clinical Assessments and Sample Collection:
A single experienced dental examiner (LL) performed all dental exams. The examiner had been calibrated and standardized in the use of the clinical evaluation measures that were employed in the study [
21,
22]. The examiner recorded the plaque index (PI) and the gingival index (GI) around all teeth present [
23]. Third molars, partially erupted teeth and surfaces with large restorations and teeth with crowns were not scored. Caries and missing teeth were also recorded.
A conventional periodontal probe (Michigan-O Probe with Williams markings, Hu-Friedy, Chicago, IL) was used for all probing measurements. Probing depth (PD) was recorded on six sites per tooth, mesiobuccal, buccal, distobuccal, mesiolingual, lingual and distolingual. The position of the gingival margin (GM) to the cementoenamel junction was recorded at the same six sites per tooth. Periodontal attachment level (AL) was calculated per the formula AL = PD + GM. Based on periodontal charting, periodontally diseased subjects with PD > 3mm and BOP >10% were excluded [
24].
Blood Samples: A blood sample was collected by standard vein puncture techniques into serum-separating (red/black-topped) Vacutainer tubes at the scheduled clinic visit. Blood was allowed to clot at room temperature for 30 min. The clot was removed by centrifuging at 1,000–2,000 x g for 15 min. Serum was collected and aliquoted into 8 x 0.6 ml microcentrifuge tubes. Serum samples were frozen at -80 oC until analyzed.
Gingival crevicular fluid (GCF) was collected by filter paper strips (Oraflow Inc., Smithtown, NY). For the purposes of GCF sampling, non-diseased sites were defined as having PD ≤ 3 mm, no bleeding on probing and no loss of attachment; and diseased sites as having PD ≥ 4 mm with equivalent or greater attachment loss. In periodontitis subjects, three interproximal diseased sites and three interproximal non-diseased sites were selected. In non-periodontitis subjects, three interproximal non-diseased sites were selected. All selected sites were chosen at random from teeth in separate quadrants.
The selected sites were isolated, supragingival plaque was removed and the tooth surface air-dried prior to sample collection. The strip was gently inserted into the orifice of the gingival crevice 1 to 2 mm and left for 30 seconds. Strips showing blood contamination were discarded. Sample volume was assessed with Periotron 8000 (Oraflow Inc., Smithtown, NY). The three non-diseased samples collected from each subject were pooled together in one microcentrifuge tube; and the 3 diseased samples collected from each periodontitis subject were also pooled together in a second microcentrifuge tube. Samples visibly stained with blood were discarded. Following sample collection, the tubes were sealed, placed on ice and subsequently frozen at -80 oC until testing was done. All clinical assessments and sample collections were done on the same day.
Laboratory Analyses:
GCF and serum samples were thawed and processed for analysis prior to running cytokine assays as per standard protocol. GCF and serum samples were analyzed for IL-1β, IL-6, IL-8, IL-10, TNF-α and CRP.
GCF elution: At the time of analysis, the three strips in each vial were immersed in 1 ml of elution buffer composed of PBS containing 0.1% Triton X-100 and 0.1% bovine serum albumin (BSA) [
25]. The elution process was carried out overnight under refrigeration [
25]. A set of controls for each biomarker (in which a known concentration of each biomarker was added to the filter papers) was used to mimic the elution of the investigated biological factors from the filter paper strips to calculate the percent recovery. The percentage recovery for each of the biomarkers was determined to be at least 98%. The standard curves were generated in the same elution buffer, thus maintaining matrix integrity.
The concentrations of CRP were determined in duplicate samples using an enzyme-linked immunosorbent assay (ELISA) kits from AssayPro (St. Charles, MO). Each sample was assayed in duplicate in accordance with the manufacturers’ instructions [
26] and data expressed in ng/ml. The minimal detectable concentration was 100 pg/ml. The levels of IL-1β, IL-6, IL-8, IL-10, IL-17A and TNF-α were determined simultaneously using a human cytokine/chemokine Milliplex Luminex multiplexed panel with magnetic beads (EMD Millipore, Billerica, Massachusetts) coated with specific antibodies (Milliplex
® MAP Kit) and expressed in pg/ml. Samples were run in duplicate, according to the manufacturer’s instructions. Fluorescence was read using a Luminex 200 instrument (Millipore). Analytes were normalized to total protein concentration, which was determined by Bradford assay (using elution buffer as a baseline), and data expressed in pg/ml.
Statistical Analysis:
This is a convenience sample of a previously described periodontitis resistant cohort [
21,
22]. Twenty subjects were lean (BMI < 25), 11 subjects were overweight (BMI 25-<30), and 8 subjects were obese (BMI ≥ 30). For data analysis purposes, overweight and obese subjects were combined into a single group. The distributions of clinical periodontal measures, CRP and cytokine levels were positively skewed. We used non-parametric tests for data analyses. Significant differences among the groups for continuous measures were determined using the Wilcoxon Rank-Sum test and for categorical variables using Pearson’s Chi-squared test.
Zero-order correlations between blood CRP, GCF CRP, periodontal measures and GCF inflammatory cytokines were determined with Pearson correlation analyses. We used rank regression analyses adjusted for age, gender, and race to examine the associations of measures of obesity (BMI and waist size) with peripheral CRP levels, peripheral CRP levels with GCF CRP levels, GCF CRP with clinical measures of gingival health and GCF inflammatory cytokines. A type 1 error rate of 5% was used.
3. Results
Twenty subjects had BMI scores <25 and were categorized as lean, 19 subjects had BMI scores ≥25 and were categorized as overweight/obese. All subjects were non-smokers and systemically healthy.
Table 1 summarizes the demographic and clinical data by BMI status. Age, sex, race, blood pressure measures, number of missing teeth, PI, GI, BOP, PD and AL were statistically comparable between the two groups. As would be expected, weight, waist size and BMI scores were significantly higher in the obese group. Subjects in both groups exhibited minimal gingival inflammation and shallow pockets, thus conforming with periodontal health criteria. PI, albeit statistically comparable between the two groups, tended to be higher in obese individuals. All subjects reported practicing oral hygiene at least once per day and receiving preventive dental care on average twice a year. Blood pressure measures were within normal range for the age of the subjects. Obese individuals tended to show slightly higher systolic blood pressure.
In
Table 2 we compare blood and GCF CRP and inflammatory cytokine levels between lean and obese individuals. For GCF measures (
Table 2.a), the univariate analyses showed that the two groups were statistically comparable on all measures except GCF CRP, levels were significantly higher in obese than lean individuals. Despite lack of statistical significance, IL-6 and TNF-α levels tended to be higher in lean than obese individuals; IL-8 tended to be higher in obese than lean individuals. GCF IL-17A was detected at low levels in only two subjects (one lean and one obese) and was excluded from analysis. The lack of detectible GCF IL-17A in most subjects suggests that IL-17A activity is absent in healthy gingival tissues. For blood measures (
Table 2.b), both CRP and inflammatory cytokine levels were statistically comparable between the obese and lean subjects.
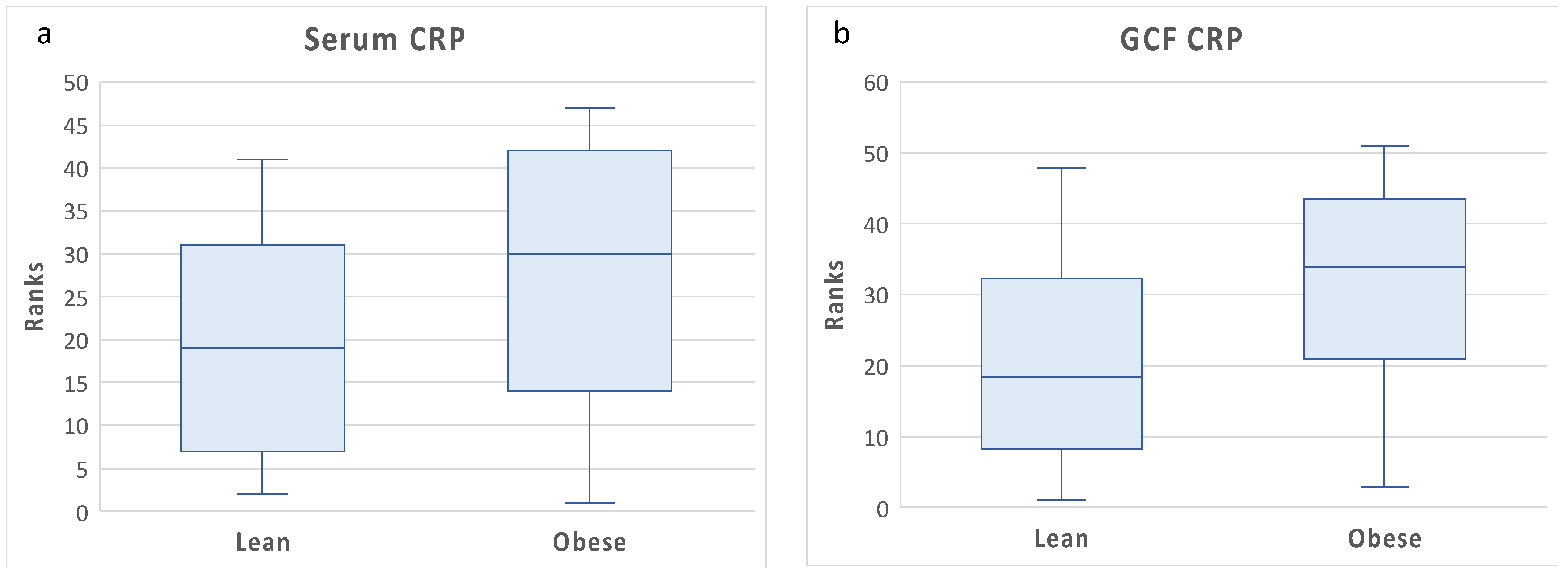
The differences in GCF and blood CRP levels between lean and obese are illustrated in
Figure 1. Although blood CRP levels tended to be higher in obese than lean individuals, the differences were not statistically significant. Blood CRP levels showed high variance among obese individuals, which may explain the lack of statistical significance between the two groups. By contrast, GCF levels were significantly higher in obese than lean individuals. This suggests the possibility of local CRP production in gingival tissues of obese individuals.
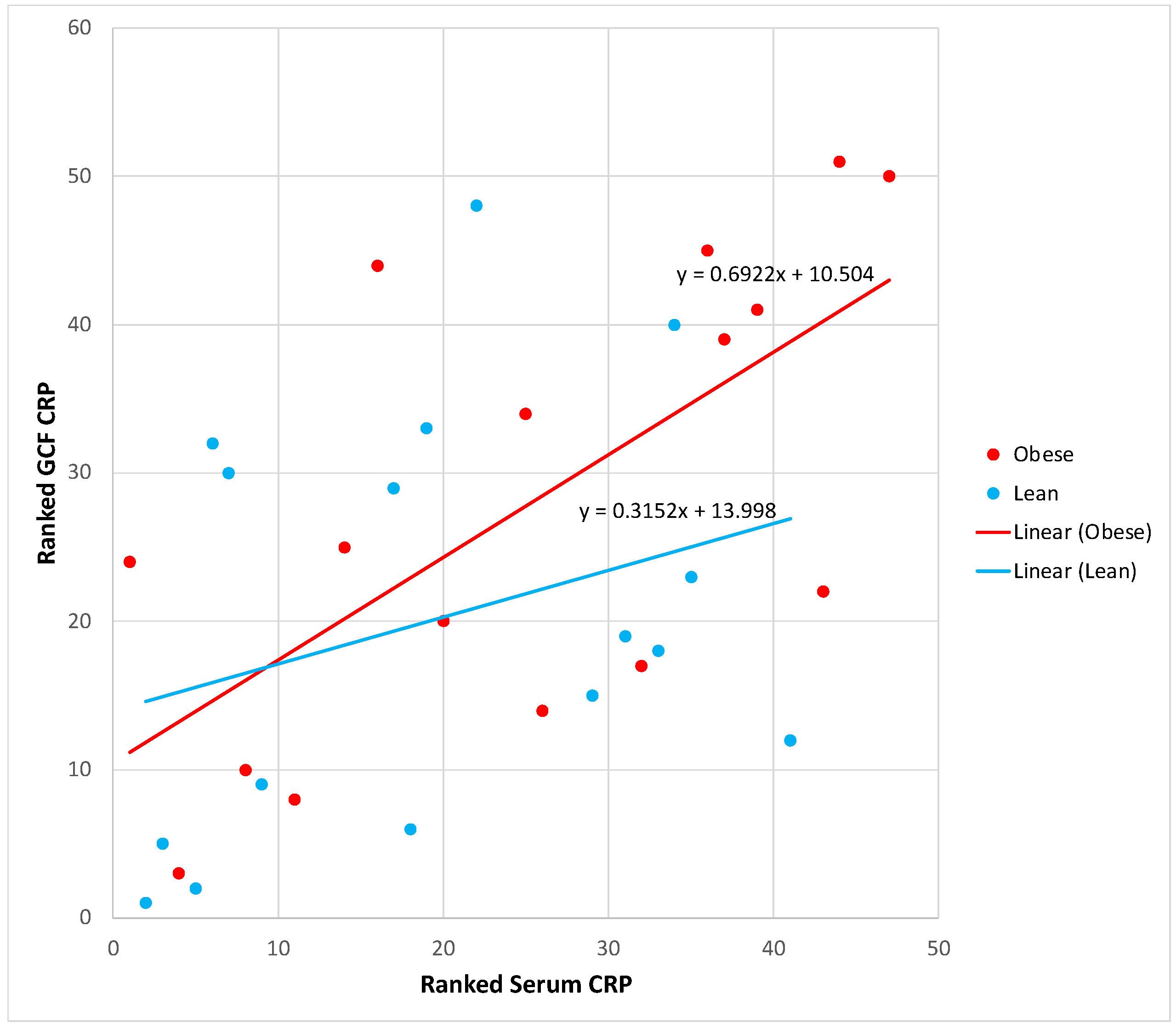
We then examined the association between blood and gingival CRP levels. Pearson correlation analysis indicated a significant correlation between blood and GCF CRP levels in all subjects combined, r=0.52, p=0.001.
Figure 2 presents the regression line slopes of the association of blood with GCF CRP by obesity status. Although the obese group line slope was steeper than the lean group, the difference was not statistically significant. The significant association between blood and GCF CRP suggests that peripheral CRP levels significantly contribute to gingival CRP levels in both obese and lean subjects.
Blood cytokines measures showed no statistically significant associations with obesity measures (BMI and waist size), blood pressure measures and GCF cytokine measures (data not presented).
Next, we investigated the association of GCF CRP levels and oral hygiene (PI scores) with measures of gingival health and GCF inflammatory cytokines. Zero-order correlation analyses showed significant positive associations between GCF CRP levels with multiple measures of gingival health and GCF inflammatory cytokines in both lean and obese individuals (
Table 3.a). Correlation coefficients for IL-1β, IL-6 and TNF-α tended to be stronger in lean individuals; by contrast correlation coefficient for IL-8 tended to be stronger in obese individuals (
Table 3.a). These data suggest that GCF CRP levels may be involved in the promotion of inflammation in gingival tissues. IL-10 showed no association with GCF CRP in both subject groups and GCF IL-1β association with GCF CRP in obese subjects lacked statistical significance (
Table 3.a). In
Table 3.b, we compared the association of PI scores with measures of gingival health and GCF inflammatory cytokines between lean and obese individuals. In both groups PI significantly associated with BOP, the association was weaker in the obese. No statistically significant correlations were noted between PI and inflammatory cytokines. The Pearson correlation coefficients between PI and inflammatory cytokines were poor in both groups and tended to be even weaker in obese than lean individuals.
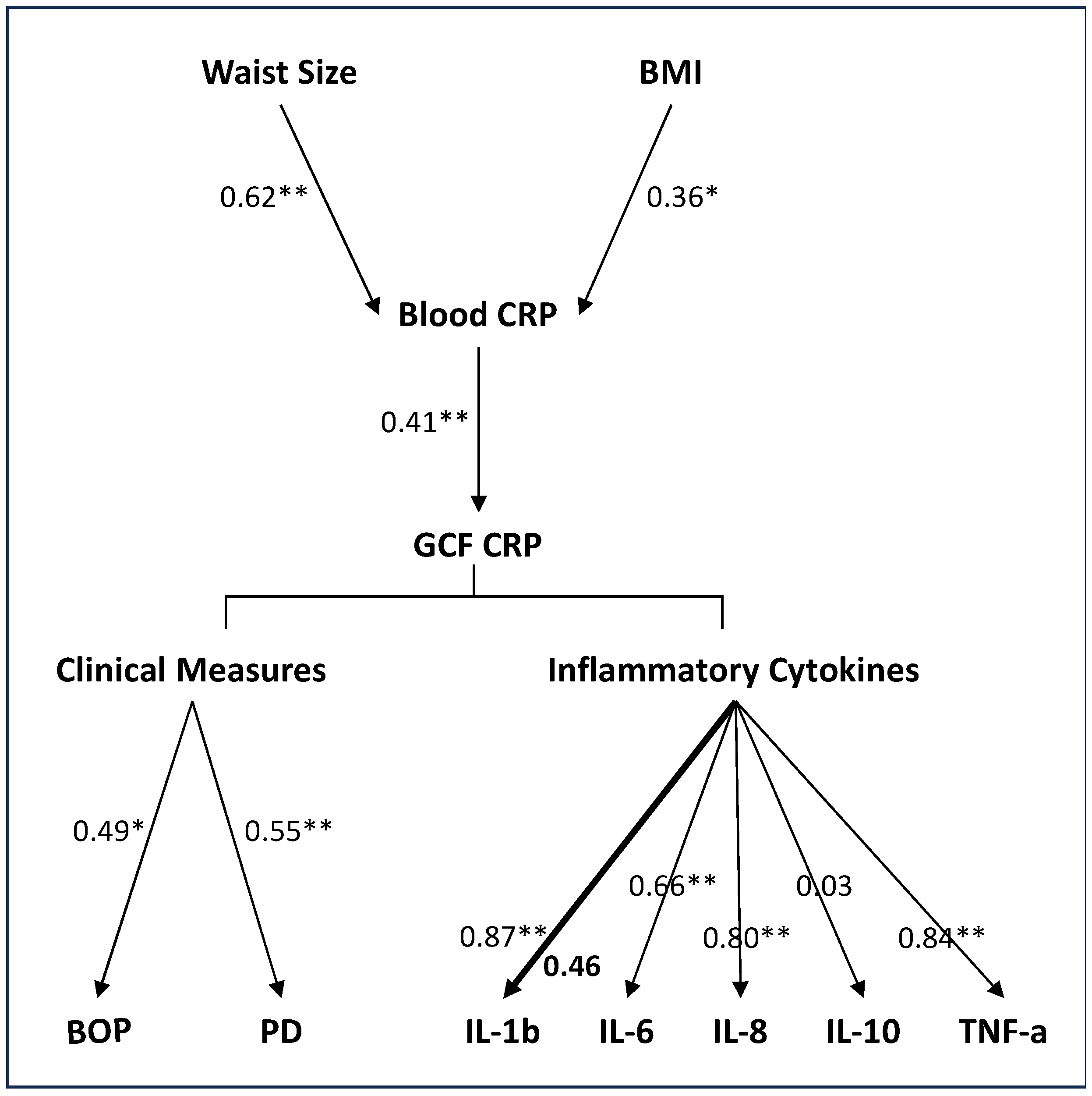
Finally, in a series of rank regression models adjusted for age, race, and gender, we further examined the association of measures of obesity (BMI and waist size) with peripheral CRP levels, peripheral CRP levels with GCF CRP levels, GCF CRP with clinical measures of gingival health and GCF inflammatory cytokines.
Figure 3 outlines the noted associations between the various factors assessed in the multivariate analyses. As would be expected, both waist size and BMI measures positively associated with serum CRP levels. Waist size measures showed a stronger association with serum CRP levels than BMI measures.
In the adjusted models, the association between blood and GCF CRP levels remained significant. Again, the strength of the association was statistically comparable between the lean and the obese. The multivariate analyses also confirmed the positive associations of GCF CRP with clinical measures of gingival health (BOP and PD) and inflammatory cytokines IL-6, IL-8, and TNF-α, the strength of the association for these measures was statistically comparable between the two groups. The association of GCF CRP with IL-1β showed a group interaction, the association was significant only in lean individuals. The non-significant association between GCF CRP and IL-1β in obese individuals confirms the univariate findings and suggests a dysregulated inflammatory cytokine response in obesity.
Additional multivariate analyses adjusting for oral hygiene (PI scores) did not alter the previously noted associations between GCF CRP with clinical measures of gingival health and inflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α (data not presented). PI showed an association with BOP (standardized beta=0.52, p=0.0006) independent from GCF CRP levels. In addition, the multivariate analyses also showed that IL-6 and TNF-α levels were significantly lower in obese than lean individuals (
Figure 4). These data further suggest a depressed gingival inflammatory cytokine response in obese subjects.
4. Discussion
The aims of this study were to examine the effect of obesity on the association between peripheral blood with GCF CRP levels and to examine the association between GCF CRP with GCF inflammatory cytokines in periodontally healthy obese individuals without the confounding effect of periodontal disease. The data showed that blood CRP levels positively correlated with GCF CRP levels in all subjects, the association tended to be stronger in obese individuals. GCF CRP levels were significantly higher in obese than lean individuals. Although GCF CRP levels positively associated with multiple GCF inflammatory cytokines (e.g., IL-1β, IL-6, TNF-α) in all subjects, the associations tended to be weaker in obese individuals. Furthermore, the levels of GCF inflammatory cytokines IL-6 and TNF-α were depressed in obese individuals. Collectively, these findings suggest a dysregulated inflammatory cytokine response to increased levels of CRP in the gingival tissues of obese individuals.
Subjects in this study were recruited from the Adventist Health Study-2 (AHS-2), a cohort of approximately 96,000 Seventh-day Adventists in the US and Canada [
27,
28]. Seventh-day Adventists are health-conscious individuals who expand on positive behaviors by avoiding meat, focusing instead on a plant-based diet filled with legumes, nuts, and whole grains. In this study, overweight and obese subjects were combined in a single group to improve power and boost the small number of available subjects. The obese subjects in this study had low-level obesity (average BMI = 32) and their clinical, CRP and cytokines profiles were statistically comparable with the overweight subjects. The health-conscious behavior and good systemic health of this cohort may explain the lack of differences in systemic inflammatory biomarker levels between the lean and obese individuals.
CRP, a proinflammatory protein, is primarily produced in the liver in response to inflammatory stimuli [
3]. CRP levels in blood reflect presence of inflammation [
3]. Our data showed a significant correlation between blood and GCF CRP levels in all subjects, suggesting that blood CRP contributes to GCF CRP levels. Contrary to our findings, a previous study reported lack of correlation between blood and GCF levels of CRP in periodontitis subjects [
29]. It is possible that gingival inflammation in periodontitis may mask the association between blood and GCF CRP levels. Our data also showed that obesity has an unfavorable influence on the association between blood and GCF CRP levels; the association is more pronounced in the obese. Despite the variability in blood CRP levels in this cohort, obese individuals tended to have higher blood CRP levels. This may explain the stronger association between blood and CRP levels in obese individuals. However, the difference in the strength of the association was not statistically significant. Again, the variance in blood CRP levels may also explain the lack of statistical significance.
In the current study, GCF CRP levels were significantly elevated in obese individuals. These data support previous studies showing elevated GCF CRP in obese individuals [
20,
30] and expands on previous reports to include periodontitis resistant elderly individuals. GCF is a transudate of serum and thus includes both serum constituents as well as locally produced molecules. Blood is the primary contributor of CRP in GCF [
31]. In this cohort, there was variance in blood CRP levels among obese subjects. Yet, despite the blood CRP variance, gingival fluid demonstrated significantly elevated levels of CRP in obese individuals. These findings suggest the possibility of local CRP production in the gingival tissues of obese individuals. Although a previous study did not show CRP mRNA in the gingival tissues [
31], gingival CRP production was not examined in obesity. Possible local cells that may contribute to gingival CRP production may include lymphocytes [
32], macrophages [
33] and epithelial cells [
34].
CRP is not only a marker of inflammation but also plays an active role in the inflammatory process [
3]. Our data showed significant associations between GCF CRP levels with IL-1β, IL-6, IL-8 and TNF-α cytokine measures.
IL-1β is a proinflammatory cytokine with angiogenic activity that contributes to blood vessel formation in inflamed tissues [
35]. IL-1β levels tend to be elevated with gingival inflammation and increased probing depth [
36]. Gingival epithelial cells have been shown to produce IL-1β in response to microbial pathogens [
37]. TNF-α is an important pro-inflammatory cytokine that is produced by activated macrophages and other inflammatory cells [
10]. TNFα is elevated in GCF of individuals with gingival inflammation [
36,
38] and facilitates the advancement of the inflammatory front deeper into the gingival connective tissue. IL-6 is a multifunctional pro-inflammatory cytokine primarily produced by T cells. Its main role is the terminal differentiation of B-lymphocytes to plasma cells, which are associated with advanced periodontal disease [
12].
Previous research showed that elevated CRP levels in atheroma induce production of IL-1β, IL-6, and TNF-α by macrophages [
39]. A similar effect may be operating in gingival tissues, where increased levels of CRP would promote inflammatory cytokine production from local immune cells. Conversely, increased production of inflammatory cytokines in the gingival tissues may induce local transcription of CRP [
40,
41,
42].
Our data showed that the association of GCF CRP with IL-1β was lacking in obese individuals. Furthermore, the multivariate analyses showed that GCF IL-6 and TNF-α levels were depressed in obese individuals. These data suggest a dysregulated inflammatory cytokine response to increased levels of CRP in the gingival tissues of obese individuals. The main contributor of inflammatory cytokines in the gingiva are macrophages [
43]. In the animal model, infiltration of macrophages is significantly decreased in gingival tissues of obese mice [
44]. Furthermore, obese mice with periodontitis developed a macrophage blunted inflammatory response with reduced expression of NLRP3 signal pathway [
44]. These obesity-related alterations in macrophage number and function in the gingival tissues would lead to innate immune dysfunction and adversely affect cytokine production. Human studies examining gingival macrophage activity in obesity are lacking.
IL-8 is a chemokine primarily released by monocytes/macrophages. Its main function is the recruitment and activation of neutrophils [
14]. Neutrophils and monocytes recruited by IL-8 release copious inflammatory cytokines such as IL-1β and TNF-α. CRP plays a role in atherosclerosis via enhanced IL-8 production and increased expression of IL-8 mRNA [
45]. CRP promotes IL-8 production via the activation of the ERK, p38 MAPK, and JNK pathways [
45]. In our study, IL-8 was the only cytokine that tended to show a strong association with GCF CRP in obese subjects, suggesting that IL-8 production in gingival tissues is not weakened by obesity. Unlike IL-1β, IL-6 and TNF-α which are primarily produced by cells of the immune system such as monocytes, macrophages and T helper cells, IL-8 is produced by numerous other cell types including inflammatory cells, as well as keratinocytes, fibroblasts, and endothelial cells. Although infiltration and function of immune cells in the gingival tissues of obese individuals may be compromised, other cells may compensate for immune cell deficiency and produce IL-8 in gingival tissues of obese individuals.
IL-10 is an anti-inflammatory cytokine that inhibits activation and effector function of immune cells. IL-10 levels tend to inversely associate with pocket depth [
46]. Our data showed no association between GCF IL-10 with GCF CRP. CRP decreases IL-10 production in immune cells via inhibition of cyclic AMP production [
47]. The combination of obesity-related monocyte dysfunction and the suppressing effect of CRP on IL-10 secretion may explain the noted lack of association between GCF CRP and IL-10 in our results.
Although study limitations include the small number of subjects and the low-grade obesity status of the participants, relevant associations were noted between obesity related increase in GCF CRP levels with measures of periodontal health and GCF inflammatory cytokines which further our understanding of how obesity may impact periodontal health. Future studies correlating gingival immune cell changes in obesity with measures of local cytokine production could enhance our understanding of how obesity associates with periodontal disease development, progression, and response to therapy.
5. Conclusions
Obesity unfavorably influences the relationship between blood and GCF CRP levels and promotes increased CRP levels in GCF. Our findings were contrary to our hypothesis. Despite obesity related increase in GCF CRP levels, clinical measures of gingival inflammation and GCF inflammatory cytokine levels were not significantly increased. The association between GCF CRP with GCF IL-1β levels in obese individuals lacked statistical significance. Furthermore, the levels of GCF inflammatory cytokines IL-6 and TNF-α were depressed in obese individuals. Collectively, these findings suggest a dysregulated inflammatory cytokine response in the gingival tissues of obese individuals.
Author Contributions
Conceptualization, A.K. and G.F.; methodology, A.K. and D.B.; formal analysis, A.K.; investigation, A.K., L.L. and C.I.; writing—original draft preparation, A.K.; writing—review and editing, A.K., D.B. and G.F; supervision, A.K.; project administration, A.K.; funding acquisition, A.K. and G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by GRASP small project grant, Loma Linda University to Dr. Khocht; and NIH grant # U01CA152939 to Dr. Fraser (NIH support for parent cohort Adventist Health Study-2).
Institutional Review Board Statement
The study protocol and consent forms were approved by Loma Linda University Institutional Review Board, approval #5160077.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data published in this study are available upon reasonable request from the first author.
Conflicts of Interest
The authors declare that they have no conflicts of interest or financial affiliations to disclose.
References
- Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:6184-223. [CrossRef]
- Park, H.S.; Park, J.Y.; Yu, R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res. Clin. Pract. 2005, 69, 29–35. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Tillett, W.S.; Francis, T. SEROLOGICAL REACTIONS IN PNEUMONIA WITH A NON-PROTEIN SOMATIC FRACTION OF PNEUMOCOCCUS. J. Exp. Med. 1930, 52, 561–571. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, N.; Arce, R.M. Periodontal inflammation: Integrating genes and dysbiosis. Periodontology 2000 2020, 82, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S. Mapping the Pathogenesis of Periodontitis: A New Look. J. Periodontol. 2008, 79, 1560–1568. [Google Scholar] [CrossRef]
- Prince LR, Allen L, Jones EC, Hellewell PG, Dower SK, Whyte MK, et al. The role of interleukin-1beta in direct and toll-like receptor 4-mediated neutrophil activation and survival. Am J Pathol. 2004;165:1819-26. [CrossRef]
- Tamura M, Sebastian S, Yang S, Gurates B, Fang Z, Bulun SE. Interleukin-1beta elevates cyclooxygenase-2 protein level and enzyme activity via increasing its mRNA stability in human endometrial stromal cells: an effect mediated by extracellularly regulated kinases 1 and 2. J Clin Endocrinol Metab. 2002;87:3263-73. [CrossRef]
- Sainson, R.C.A.; Johnston, D.A.; Chu, H.C.; Holderfield, M.T.; Nakatsu, M.N.; Crampton, S.P.; Davis, J.; Conn, E.; Hughes, C.C.W. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood 2008, 111, 4997–5007. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Heulsmann, A.; Tondravi, M.M.; Mukherjee, A.; Abu-Amer, Y. Tumor Necrosis Factor-α (TNF) Stimulates RANKL-induced Osteoclastogenesis via Coupling of TNF Type 1 Receptor and RANK Signaling Pathways. J. Biol. Chem. 2001, 276, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.C.; Anderson, M.E.; Moots, R.J. The Many Faces of Interleukin-6: The Role of IL-6 in Inflammation, Vasculopathy, and Fibrosis in Systemic Sclerosis. Int. J. Rheumatol. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Giannopoulou, C.; Kamma, J.J.; Mombelli, A. Effect of inflammation, smoking and stress on gingival crevicular fluid cytokine level. J. Clin. Periodontol. 2003, 30, 145–153. [Google Scholar] [CrossRef]
- Senturk, T.; Kozaci, L.D.; Kok, F.; Kadikoylu, G.; Bolaman, Z. Proinflammatory cytokine levels in hyperthyroidism. . 2003, 26, 58–63. [Google Scholar]
- Wilson ME, Zambon JJ, Suzuki JB, Genco RJ. Generalized juvenile periodontitis, defective neutrophil chemotaxis and Bacteroides gingivalis in a 13-year-old female. A case report. J Periodontol. 1985;56:457-63. [CrossRef]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683-765. [CrossRef]
- Arboleda, S.; Vargas, M.; Losada, S.; Pinto, A. Review of obesity and periodontitis: an epidemiological view. Br. Dent. J. 2019, 227, 235–239. [Google Scholar] [CrossRef]
- Martinez-Herrera, M.; Silvestre-Rangil, J.; Silvestre, F.J. Association between obesity and periodontal disease. A systematic review of epidemiological studies and controlled clinical trials. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e708–e715. [Google Scholar] [CrossRef]
- Akram, Z.; Safii, S.H.; Vaithilingam, R.D.; Baharuddin, N.A.; Javed, F.; Vohra, F. Efficacy of non-surgical periodontal therapy in the management of chronic periodontitis among obese and non-obese patients: a systematic review and meta-analysis. Clin. Oral Investig. 2016, 20, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Abduljabbar, T.; Abu Hassan, M.I.; Javed, F.; Vohra, F. Cytokine Profile in Chronic Periodontitis Patients with and without Obesity: A Systematic Review and Meta-Analysis. Dis. Markers 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Khocht, A.; Orlich, M.; Paster, B.; Bellinger, D.; Lenoir, L.; Irani, C.; Fraser, G. Cross-sectional comparisons of subgingival microbiome and gingival fluid inflammatory cytokines in periodontally healthy vegetarians versus non-vegetarians. J. Periodontal Res. 2021, 56, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Khocht, A.; Paster, B.; Lenoir, L.; Irani, C.; Fraser, G. Metabolomic profiles of obesity and subgingival microbiome in periodontally healthy individuals: A cross-sectional study. J. Clin. Periodontol. 2023, 50, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Loe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967;38:Suppl:610-6. [CrossRef]
- Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, et al. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Periodontol. 2018;89 Suppl 1:S1-S8. [CrossRef]
- Aboyoussef, H.; Carter, C.; Jandinski, J.J.; Panagakos, F.S. Detection of prostaglandin E2 and matrix metalloproteinases in implant crevicular fluid. . 1998, 13, 689–96. [Google Scholar]
- Dos Santos H, Raphael JC, Reis WP, Gaio J, Berk LS, Bellinger DL, et al. The Association between Affect and Biomarkers of Stress and Inflammation in Healthy Seventh-Day Adventists. Adv Mind Body Med. 2019;33:12-20.
- Fraser, G.; Katuli, S.; Anousheh, R.; Knutsen, S.; Herring, P.; Fan, J. Vegetarian diets and cardiovascular risk factors in black members of the Adventist Health Study-2. Public Health Nutr 2014, 18, 537–545. [Google Scholar] [CrossRef]
- E Fraser, G.; Jaceldo-Siegl, K.; Henning, S.M.; Fan, J.; Knutsen, S.F.; Haddad, E.H.; Sabaté, J.; Beeson, W.L.; Bennett, H. Biomarkers of Dietary Intake Are Correlated with Corresponding Measures from Repeated Dietary Recalls and Food-Frequency Questionnaires in the Adventist Health Study-2. J. Nutr. 2016, 146, 586–594. [Google Scholar] [CrossRef]
- Baser U, Oztekin G, Ademoglu E, Isik G, Yalcin F. Is the severity of periodontitis related to gingival crevicular fluid and serum high-sensitivity C-reactive protein concentrations? Clin Lab. 2014;60:1653-8. [CrossRef]
- Pradeep, A.R.; Priyanka, N.; Prasad, M.V.R.; Kalra, N.; Kumari, M. Association of Progranulin and High Sensitivity CRP Concentrations in Gingival Crevicular Fluid and Serum in Chronic Periodontitis Subjects with and without Obesity. Dis. Markers 2012, 33, 207–213. [Google Scholar] [CrossRef]
- Megson, E.; Fitzsimmons, T.; Dharmapatni, K.; Bartold, P.M. C-reactive protein in gingival crevicular fluid may be indicative of systemic inflammation. J. Clin. Periodontol. 2010, 37, 797–804. [Google Scholar] [CrossRef]
- E Kuta, A.; Baum, L.L. C-reactive protein is produced by a small number of normal human peripheral blood lymphocytes. . J. Exp. Med. 1986, 164, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Yasojima, K.; Schwab, C.; McGeer, E.G.; McGeer, P.L. Generation of C-Reactive Protein and Complement Components in Atherosclerotic Plaques. Am. J. Pathol. 2001, 158, 1039–1051. [Google Scholar] [CrossRef]
- Gould, J.M.; Weiser, J.N. Expression of C-Reactive Protein in the Human Respiratory Tract. Infect. Immun. 2001, 69, 1747–54. [Google Scholar] [CrossRef] [PubMed]
- Carmi Y, Dotan S, Rider P, Kaplanov I, White MR, Baron R, et al. The role of IL-1beta in the early tumor cell-induced angiogenic response. J Immunol. 2013;190:3500-9. [CrossRef]
- Baser U, Cekici A, Tanrikulu-Kucuk S, Kantarci A, Ademoglu E, Yalcin F. Gingival inflammation and interleukin-1 beta and tumor necrosis factor-alpha levels in gingival crevicular fluid during the menstrual cycle. J Periodontol. 2009;80:1983-90. [CrossRef]
- Uchida Y, Shiba H, Komatsuzawa H, Takemoto T, Sakata M, Fujita T, et al. Expression of IL-1 beta and IL-8 by human gingival epithelial cells in response to Actinobacillus actinomycetemcomitans. Cytokine. 2001;14:152-61. [CrossRef]
- Ertugrul AS, Sahin H, Dikilitas A, Alpaslan N, Bozoglan A. Comparison of CCL28, interleukin-8, interleukin-1beta and tumor necrosis factor-alpha in subjects with gingivitis, chronic periodontitis and generalized aggressive periodontitis. J Periodontal Res. 2013;48:44-51. [CrossRef]
- Han, K.H.; Hong, K.-H.; Park, J.-H.; Ko, J.; Kang, D.-H.; Choi, K.-J.; Hong, M.-K.; Park, S.-W.; Park, S.-J. C-Reactive Protein Promotes Monocyte Chemoattractant Protein-1—Mediated Chemotaxis Through Upregulating CC Chemokine Receptor 2 Expression in Human Monocytes. Circulation 2004, 109, 2566–2571. [Google Scholar] [CrossRef]
- Szalai, A.J.; van Ginkel, F.W.; Dalrymple, S.A.; Murray, R.; McGhee, J.R.; Volanakis, J.E. Testosterone and IL-6 Requirements for Human C-Reactive Protein Gene Expression in Transgenic Mice. J. Immunol. 1998, 160, 5294–5299. [Google Scholar] [CrossRef]
- Weinhold B, Bader A, Poli V, Ruther U. Interleukin-6 is necessary, but not sufficient, for induction of the humanC-reactive protein gene in vivo. Biochem J. 1997;325 ( Pt 3):617-21.
- Zhang, D.; Sun, M.; Samols, D.; Kushner, I. STAT3 Participates in Transcriptional Activation of the C-reactive Protein Gene by Interleukin-6. J. Biol. Chem. 1996, 271, 9503–9509. [Google Scholar] [CrossRef]
- Fageeh HI, Fageeh HN, Patil S. Monocyte Differentiation into Destructive Macrophages on In Vitro Administration of Gingival Crevicular Fluid from Periodontitis Patients. J Pers Med. 2021;11. [CrossRef]
- Huang, X.; Yu, T.; Ma, C.; Wang, Y.; Xie, B.; Xuan, D.; Zhang, J. Macrophages Play a Key Role in the Obesity-Induced Periodontal Innate Immune Dysfunction via Nucleotide-Binding Oligomerization Domain-Like Receptor Protein 3 Pathway. J. Periodontol. 2016, 87, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Kibayashi, E.; Urakaze, M.; Kobashi, C.; Kishida, M.; Takata, M.; Sato, A.; Yamazaki, K.; Kobayashi, M. Inhibitory effect of pitavastatin (NK-104) on the C-reactive-protein-induced interleukin-8 production in human aortic endothelial cells. Clin. Sci. 2005, 108, 515–521. [Google Scholar] [CrossRef]
- Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A. Levels of interleukin-1 beta, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol. 2000;71:1535-45. [CrossRef]
- Singh, U.; Devaraj, S.; Dasu, M.R.; Ciobanu, D.; Reusch, J.; Jialal, I.; S, D.; I, J.; S, E.; J, H.; et al. C-Reactive Protein Decreases Interleukin-10 Secretion in Activated Human Monocyte-Derived Macrophages via Inhibition of Cyclic AMP Production. Arter. Thromb. Vasc. Biol. 2006, 26, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Blood and gingival fluid CRP levels by obesity status. Box plots of rank transformed CRP levels by obesity status. The bottom and top sides of each box represent the lower and upper quartiles, respectively. The line inside the box represents the median. The bottom and top whiskers represent the minimum and maximum values, respectively. (a) Blood CRP: Wilcoxon rank-sum test (untransformed actual data) showed no statistically significant difference between the two groups (Z = 1.18, p< 0.23). ANCOVA (rank transformed data) adjusting for age, gender, and race confirmed the univariate analysis, F(4,32)=2.06,p=0.16. (b) GCF CRP: Wilcoxon rank sum test (untransformed actual data) showed that GCF CRP levels were significantly higher in obese than lean individuals (Z = -2.02, p< 0.04). ANCOVA (rank transformed data) adjusting for age, gender, and race confirmed the univariate analysis, F(4,37)=4.77,p=0.03.
Figure 1.
Blood and gingival fluid CRP levels by obesity status. Box plots of rank transformed CRP levels by obesity status. The bottom and top sides of each box represent the lower and upper quartiles, respectively. The line inside the box represents the median. The bottom and top whiskers represent the minimum and maximum values, respectively. (a) Blood CRP: Wilcoxon rank-sum test (untransformed actual data) showed no statistically significant difference between the two groups (Z = 1.18, p< 0.23). ANCOVA (rank transformed data) adjusting for age, gender, and race confirmed the univariate analysis, F(4,32)=2.06,p=0.16. (b) GCF CRP: Wilcoxon rank sum test (untransformed actual data) showed that GCF CRP levels were significantly higher in obese than lean individuals (Z = -2.02, p< 0.04). ANCOVA (rank transformed data) adjusting for age, gender, and race confirmed the univariate analysis, F(4,37)=4.77,p=0.03.
Figure 2.
Scatter plots and regression lines of serum CRP (independent variable) versus GCF CRP (dependent variable) by group, unadjusted rank transformed data. Slope equations are indicated. Pairwise slope difference test: Difference (obese - lean) = 0.37, p = 0.27. Pearson correlation coefficients for obese: r=0.63, p=0.008, and lean: r=0.40, p=0.12.
Figure 2.
Scatter plots and regression lines of serum CRP (independent variable) versus GCF CRP (dependent variable) by group, unadjusted rank transformed data. Slope equations are indicated. Pairwise slope difference test: Difference (obese - lean) = 0.37, p = 0.27. Pearson correlation coefficients for obese: r=0.63, p=0.008, and lean: r=0.40, p=0.12.
Figure 3.
Flow diagram outlining the hypothesized association sequence of measures of obesity (BMI and waist size) with peripheral CRP levels, peripheral CRP levels with GCF CRP levels, GCF CRP with clinical measures of gingival health and GCF inflammatory cytokines. Rank regression models adjusted for age, race, and gender were used for prediction of dependent variables. Data are presented as standardized beta weight. When the strength of association was comparable between the two groups, a light arrow with a single value is presented. When the strength of association differed between the two groups, a heavy arrow with two values is presented, obese value is bolded. P<0.05*, P<0.01**.
Figure 3.
Flow diagram outlining the hypothesized association sequence of measures of obesity (BMI and waist size) with peripheral CRP levels, peripheral CRP levels with GCF CRP levels, GCF CRP with clinical measures of gingival health and GCF inflammatory cytokines. Rank regression models adjusted for age, race, and gender were used for prediction of dependent variables. Data are presented as standardized beta weight. When the strength of association was comparable between the two groups, a light arrow with a single value is presented. When the strength of association differed between the two groups, a heavy arrow with two values is presented, obese value is bolded. P<0.05*, P<0.01**.
Figure 4.
GCF IL-6 and TNF-α levels by obesity status. Box plots of rank transformed cytokine levels by obesity status. The bottom and top sides of each box represent the lower and upper quartiles, respectively. The line inside the box represents the median. The bottom and top whiskers represent the minimum and maximum values, respectively.IL-6: (a) Wilcoxon rank-sum test (untransformed actual data) showed no statistically significant difference between the two groups (Z = -0.93, p< 0.35). (b) ANCOVA of rank transformed data adjusting for PI and GCF CRP levels indicated that GCF IL-6 levels in lean individuals were significantly higher than obese individuals, F(3,34)=10.68,p=0.002. TNF-α: (c) Wilcoxon rank-sum test (untransformed actual data) showed no statistically significant difference between the two groups (Z = -0.16, p< 0.86). (d) ANCOVA of rank transformed data adjusting for PI and GCF CRP levels indicated that GCF TNF-α levels in lean individuals were significantly higher than obese individuals, F(3,34)=8.45,p=0.006.
Figure 4.
GCF IL-6 and TNF-α levels by obesity status. Box plots of rank transformed cytokine levels by obesity status. The bottom and top sides of each box represent the lower and upper quartiles, respectively. The line inside the box represents the median. The bottom and top whiskers represent the minimum and maximum values, respectively.IL-6: (a) Wilcoxon rank-sum test (untransformed actual data) showed no statistically significant difference between the two groups (Z = -0.93, p< 0.35). (b) ANCOVA of rank transformed data adjusting for PI and GCF CRP levels indicated that GCF IL-6 levels in lean individuals were significantly higher than obese individuals, F(3,34)=10.68,p=0.002. TNF-α: (c) Wilcoxon rank-sum test (untransformed actual data) showed no statistically significant difference between the two groups (Z = -0.16, p< 0.86). (d) ANCOVA of rank transformed data adjusting for PI and GCF CRP levels indicated that GCF TNF-α levels in lean individuals were significantly higher than obese individuals, F(3,34)=8.45,p=0.006.
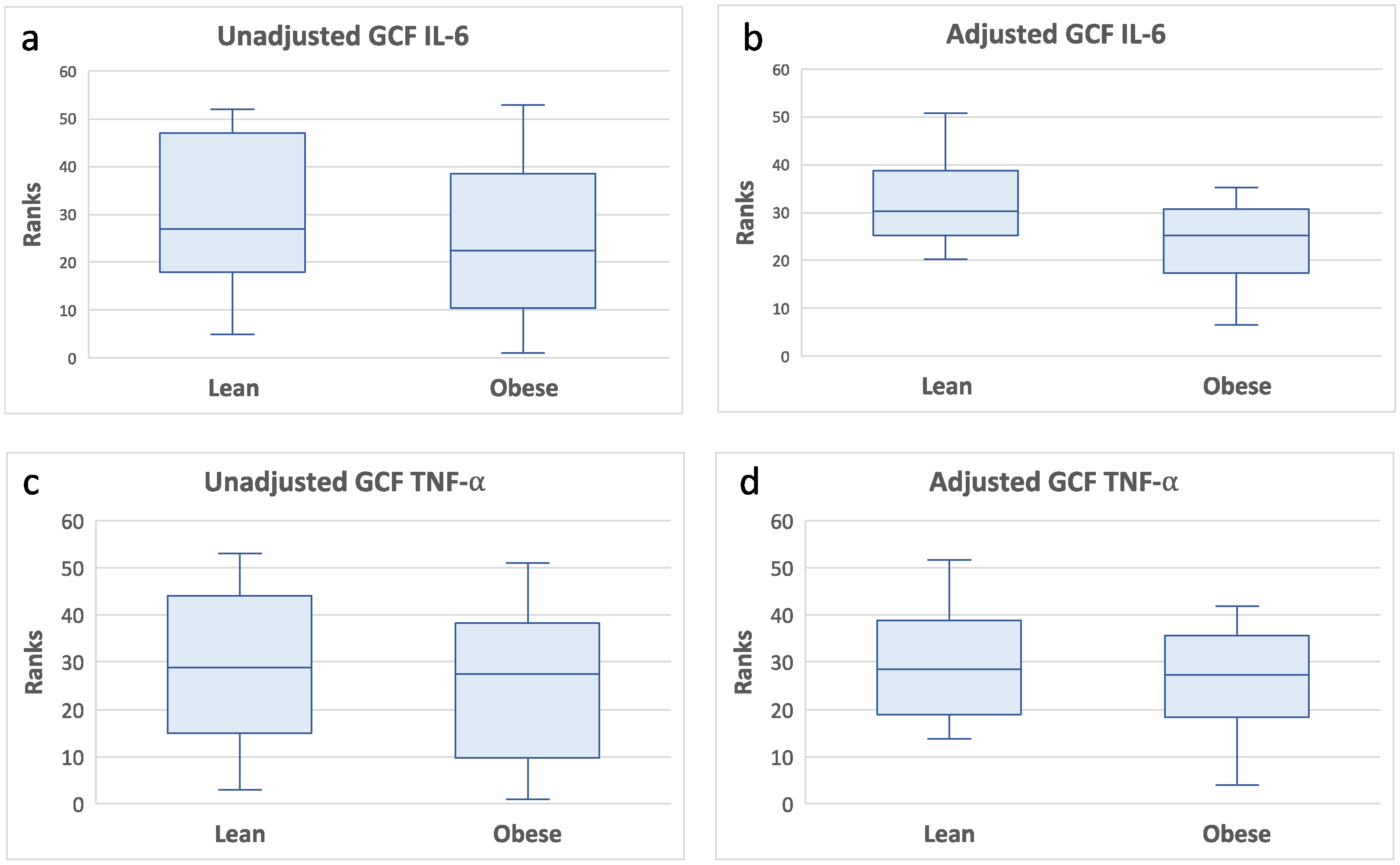
Table 1.
Demographics and clinical measures by obesity status
Table 1.
Demographics and clinical measures by obesity status
| Variable |
Lean (BMI < 25) |
Obese (BMI ≥ 25) |
p-value |
| a. Demographics |
|
|
|
| Age (years) |
69.1 (2.18) |
71.42 (1.71) |
0.55 |
| % Male |
45 |
47 |
0.77 |
| % Black |
30 |
37 |
0.65 |
| b. Clinical Measures |
|
|
|
| Weight (lbs.) |
153.2 (5.49) |
183.3 (8.18) |
0.009 |
| Waist (cm) |
87.91 (83.54) |
102.5 (96.94) |
0.0003 |
| BMI |
22.26 (0.35) |
29.65 (0.73) |
0.0001 |
| Missing teeth |
1.95 (0.58) |
2.52 (0.60) |
0.51 |
| PI |
0.44 (0.06) |
0.63 (0.08) |
0.06 |
| GI |
0.58 (0.06) |
0.58 (0.06) |
0.83 |
| GCF volume |
2.06 (0.23) |
1.93 (0.26) |
0.59 |
| BOP% |
4.28 (1.14) |
5.84 (1.30) |
0.32 |
| PD mm |
1.85 (0.06) |
1.98 (0.04) |
0.16 |
| AL mm |
1.32 (0.21) |
1.34 (0.19) |
0.63 |
Table 2.
CRP and cytokine measures by obesity status
Table 2.
CRP and cytokine measures by obesity status
| Variable |
Lean (BMI < 25) |
Obese (BMI ≥ 25) |
p-value |
| a. Gingival Fluid |
|
|
|
| GCF CRP (ng/ml) |
0.03 (0-0.08) |
0.07 (0.03-0.54) |
0.04 |
| IL-1β (pg/ml) |
1.41 (0.27-9.99) |
2.58 (1.54-5.7) |
0.17 |
| IL-6 (pg/ml) |
0 (0-1.91) |
0 (0-0.57) |
0.34 |
| IL-8 (pg/ml) |
57.59 (22.24-307.80) |
103.68 (58.78-282.08) |
0.16 |
| IL-10 (pg/ml) |
5 (0-5) |
0 (0-5) |
0.21 |
| TNF-α (pg/ml) |
0 (0-1.49) |
0 (0-0.68) |
0.85 |
| b. Blood (serum) |
|
|
|
| CRP (µg/ml) |
7.6 (2.4-18.2) |
9.8 (4.42-29.5) |
0.22 |
| IL-1β (pg/ml) |
1.84 (1.26-2.14) |
1.2 (0.78-3.64) |
0.52 |
| IL-6 (pg/ml) |
5 (2.86-8.67) |
5.06 (2.92-11.9) |
1 |
| IL-8 (pg/ml) |
10.56 (5.9-21.62) |
10.6 (6.52-13.54) |
0.93 |
| IL-10 (pg/ml) |
16.2 (5.12-23.36) |
11.98 (5.38-37.14) |
0.89 |
| IL-17A (pg/ml) |
12.47 (9.07-25.84) |
11.96 (8.8-27.52) |
1 |
| TNF-α (pg/ml) |
9.7 (7.98-11.29) |
10.38 (8.2-14.02) |
0.27 |
Table 3.
Pearson’s correlation analyses examining the associations of GCF CRP and PI with clinical measures of gingival health and GCF inflammatory cytokines by obesity status.
Table 3.
Pearson’s correlation analyses examining the associations of GCF CRP and PI with clinical measures of gingival health and GCF inflammatory cytokines by obesity status.
| |
Lean |
Obese |
| Variable |
r |
p-value |
r |
p-value |
| a. GCF CRP correlations |
|
|
|
|
| BOP% |
0.45 |
0.06 |
0.56 |
0.01 |
| PD (average) |
0.57 |
0.01 |
0.32 |
0.2 |
| IL-1β (pg/ml) |
0.64 |
0.003 |
0.36 |
0.1 |
| IL-6 (pg/ml) |
0.80 |
0.0001 |
0.52 |
0.02 |
| IL-8 (pg/ml) |
0.48 |
0.03 |
0.82 |
0.0001 |
| IL-10 (pg/ml) |
0.09 |
0.7 |
0.09 |
0.7 |
| TNF-α (pg/ml) |
0.83 |
0.0001 |
0.73 |
0.0004 |
| b. PI correlations |
|
|
|
|
| BOP% |
0.59 |
0.005 |
0.48 |
0.03 |
| PD-ave |
0.17 |
0.45 |
0.20 |
0.40 |
| IL-1β (pg/ml) |
0.28 |
0.23 |
0.06 |
0.79 |
| IL-6 (pg/ml) |
0.15 |
0.51 |
-0.01 |
0.94 |
| IL-8 (pg/ml) |
0.15 |
0.50 |
-0.16 |
0.49 |
| IL-10 (pg/ml) |
0.12 |
0.60 |
0.01 |
0.95 |
| TNFα (pg/ml) |
0.18 |
0.43 |
-0.12 |
0.61 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).