Submitted:
03 November 2023
Posted:
03 November 2023
You are already at the latest version
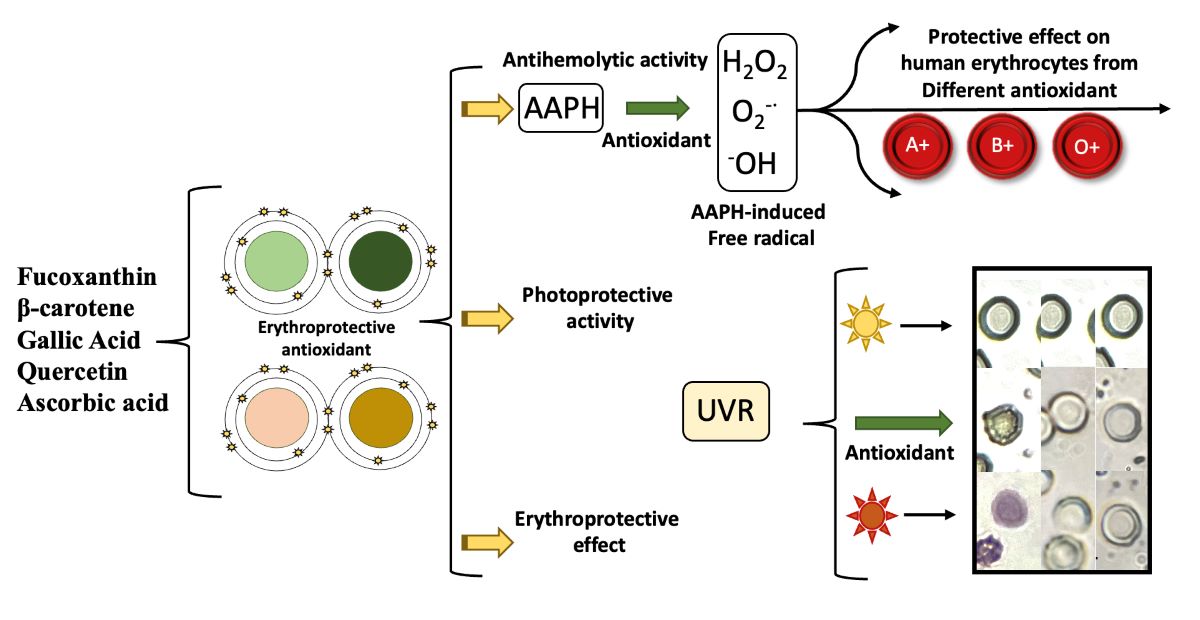
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Biological Material
2.3. Preparation of Samples
2.4. Determination of Antioxidant Capacity
2.5. Erythroprotective Potential
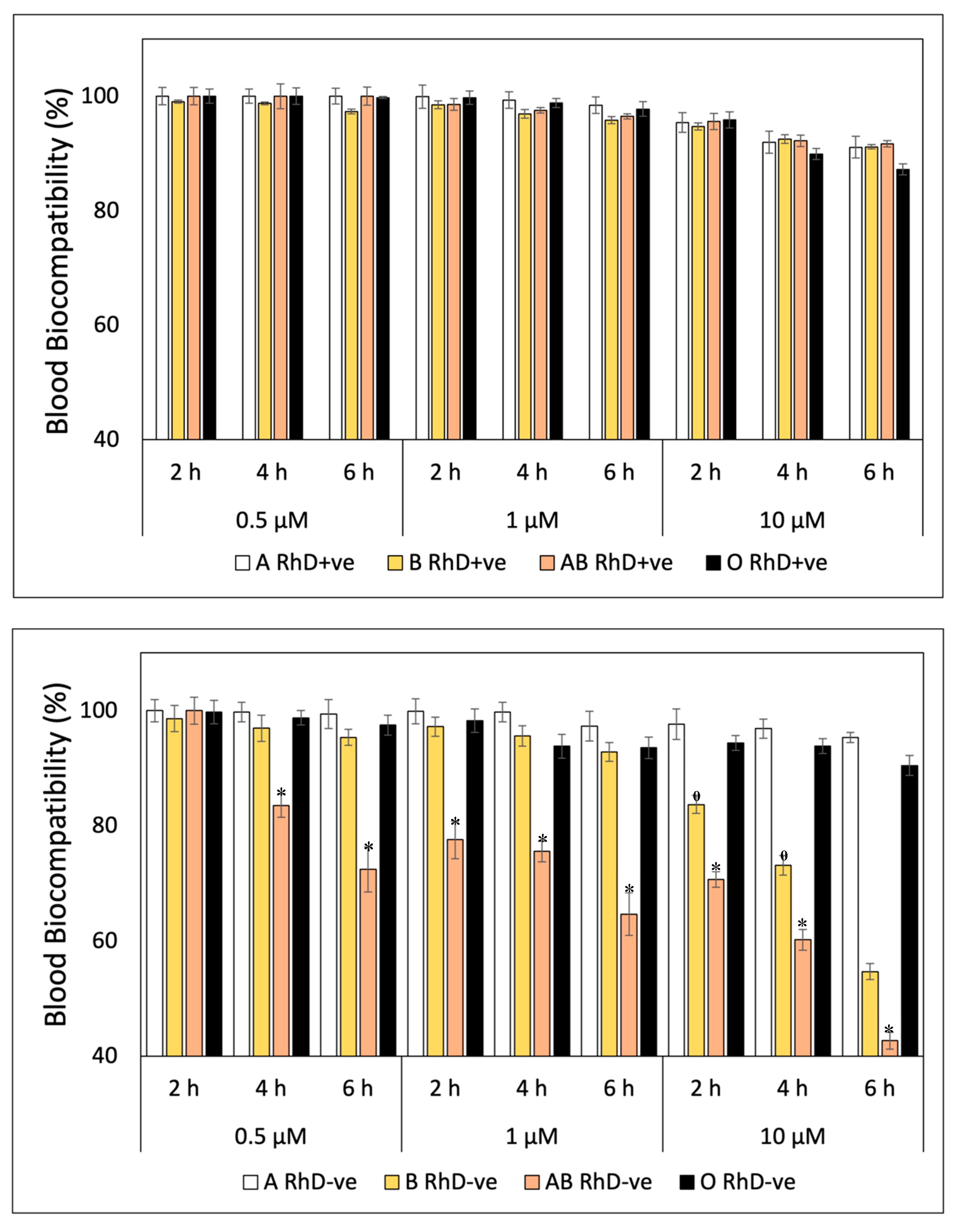
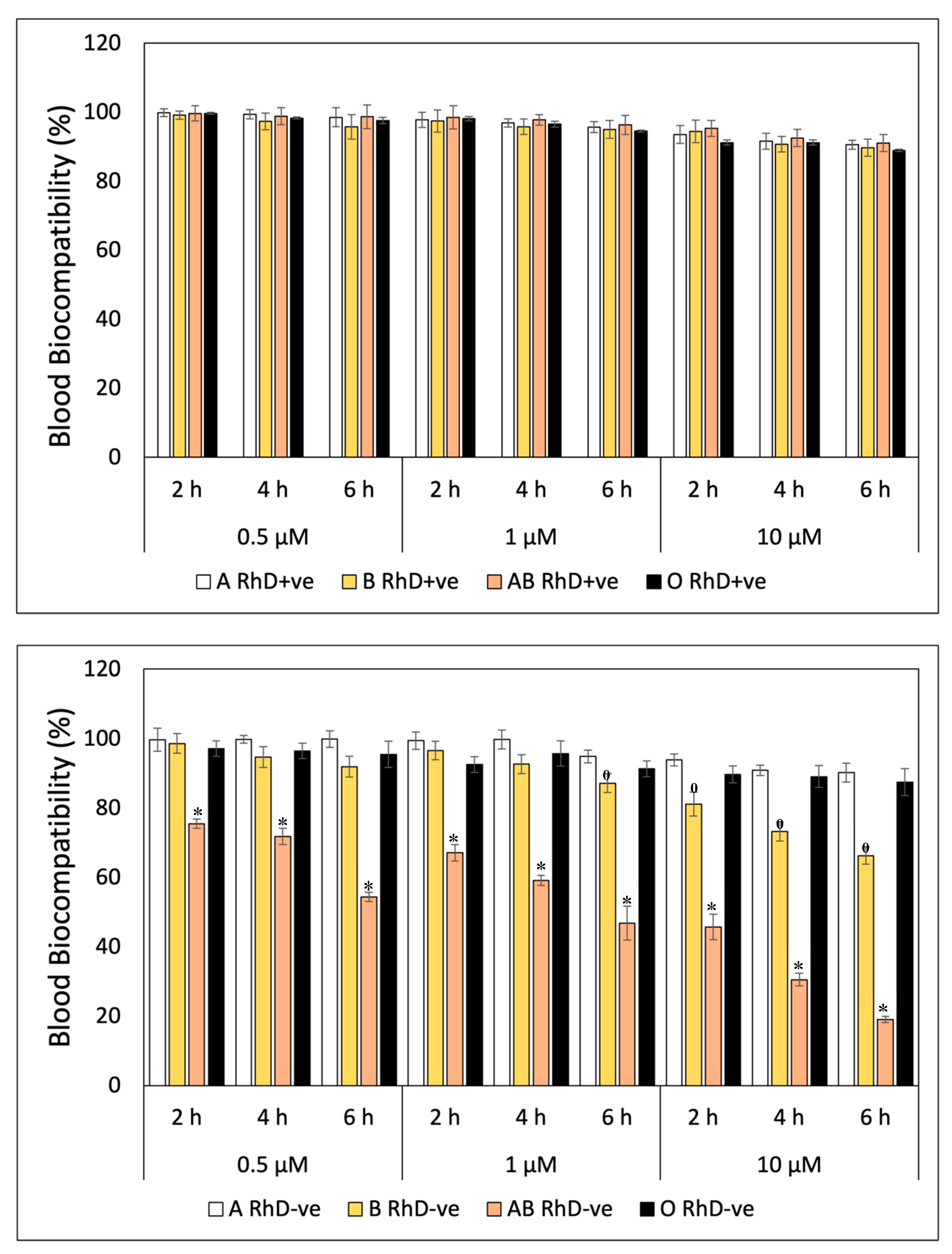
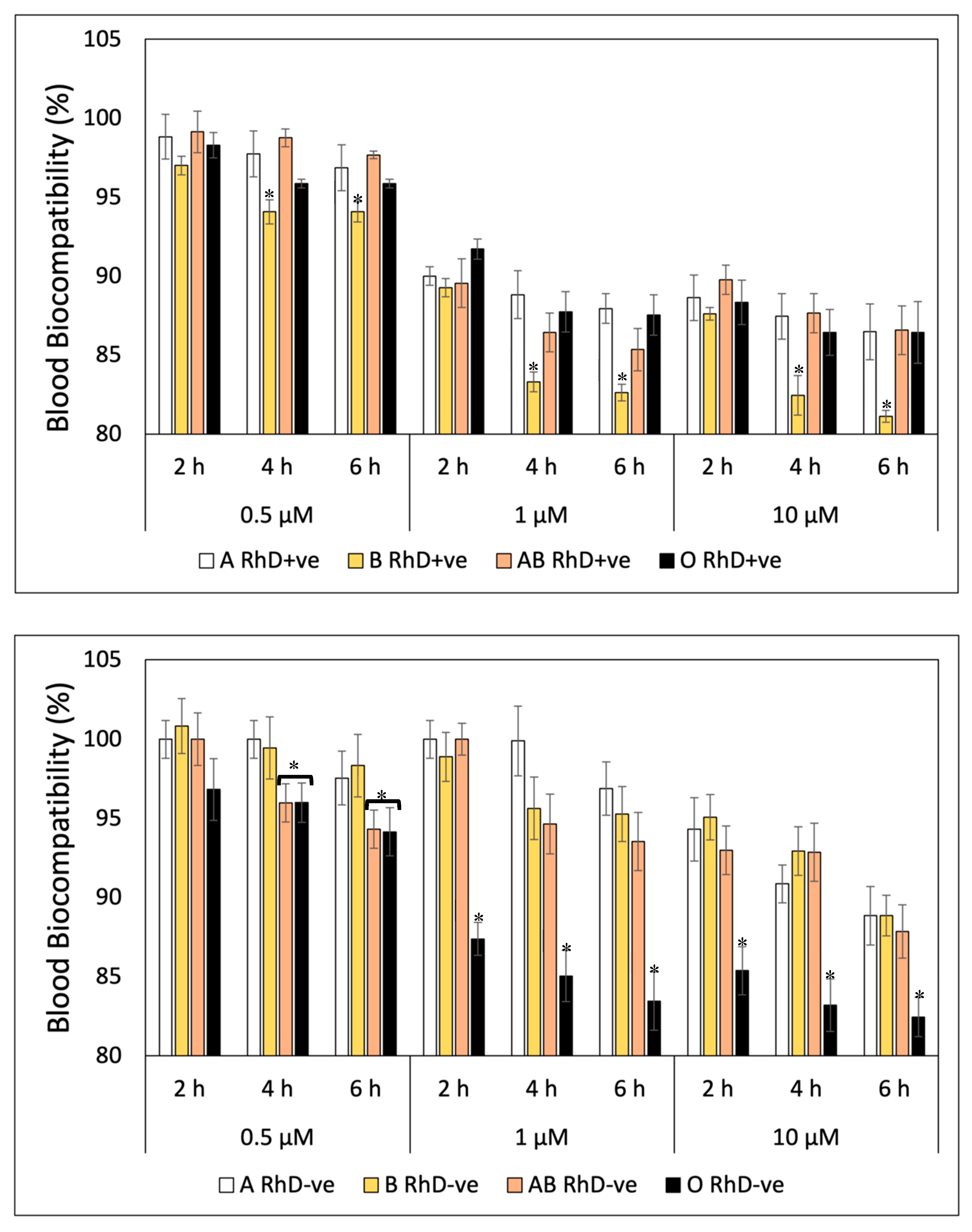
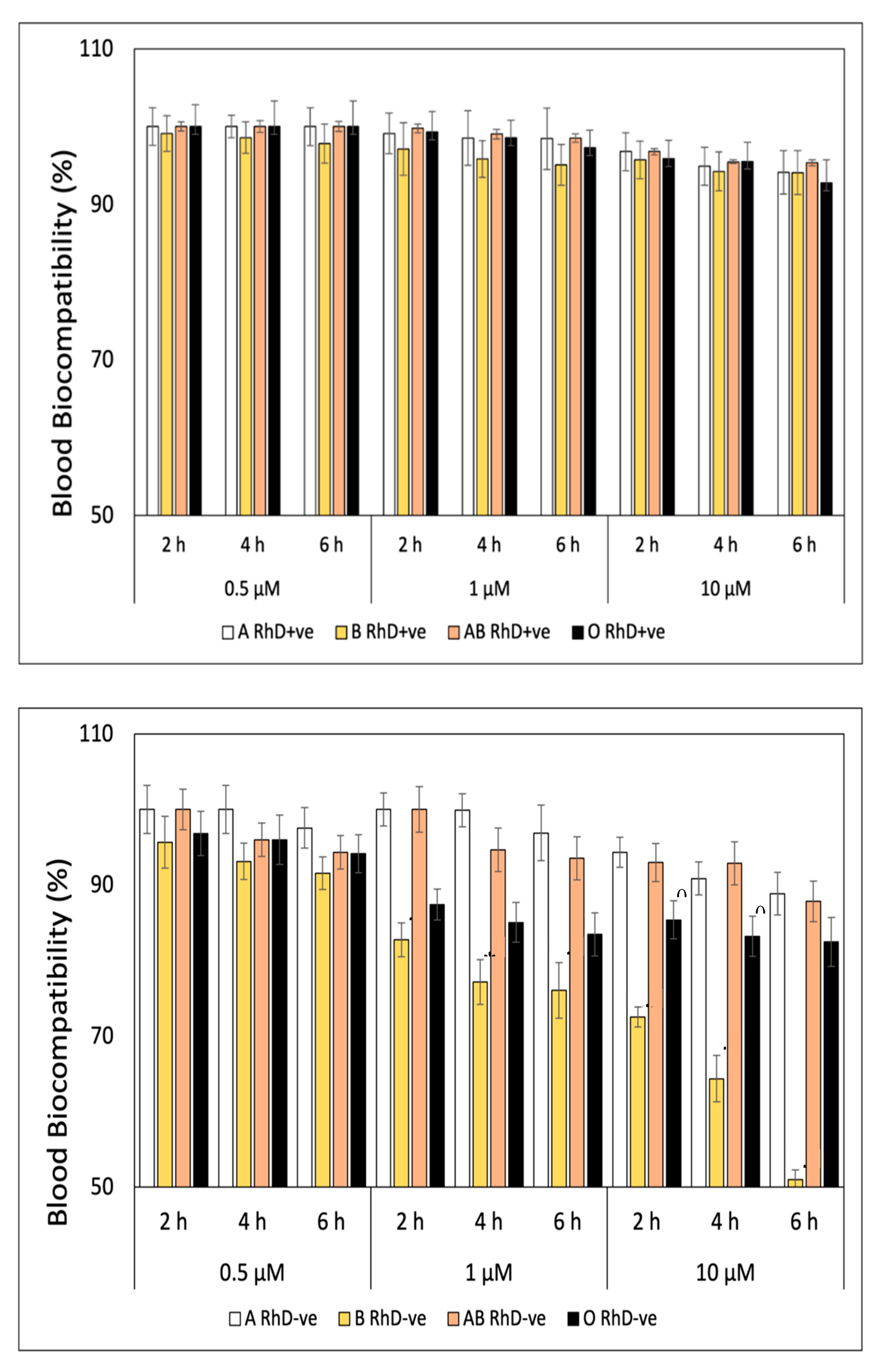
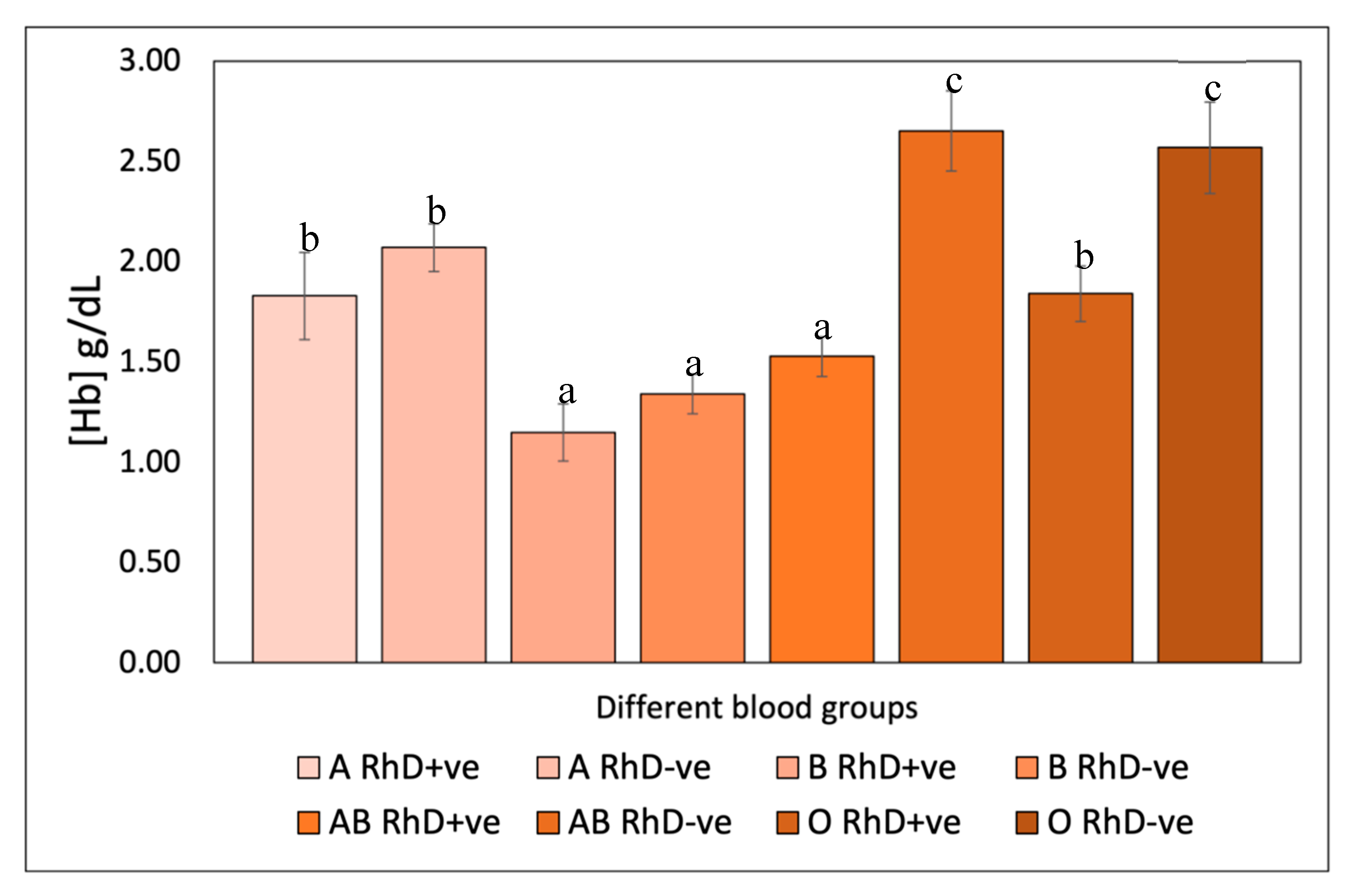
2.5.1. Blood Biocompatibility
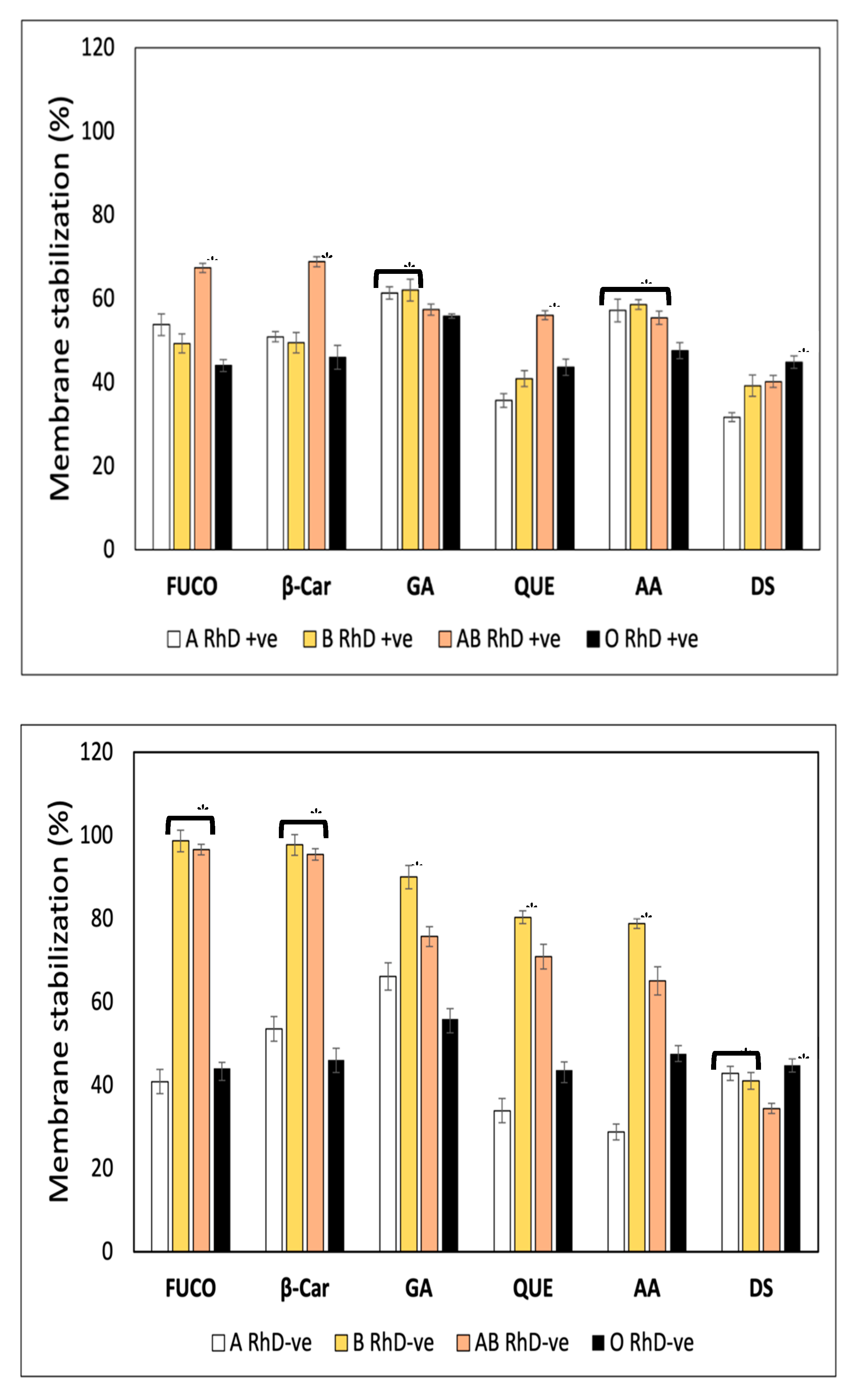
2.5.2. Membrane Stabilization Assay
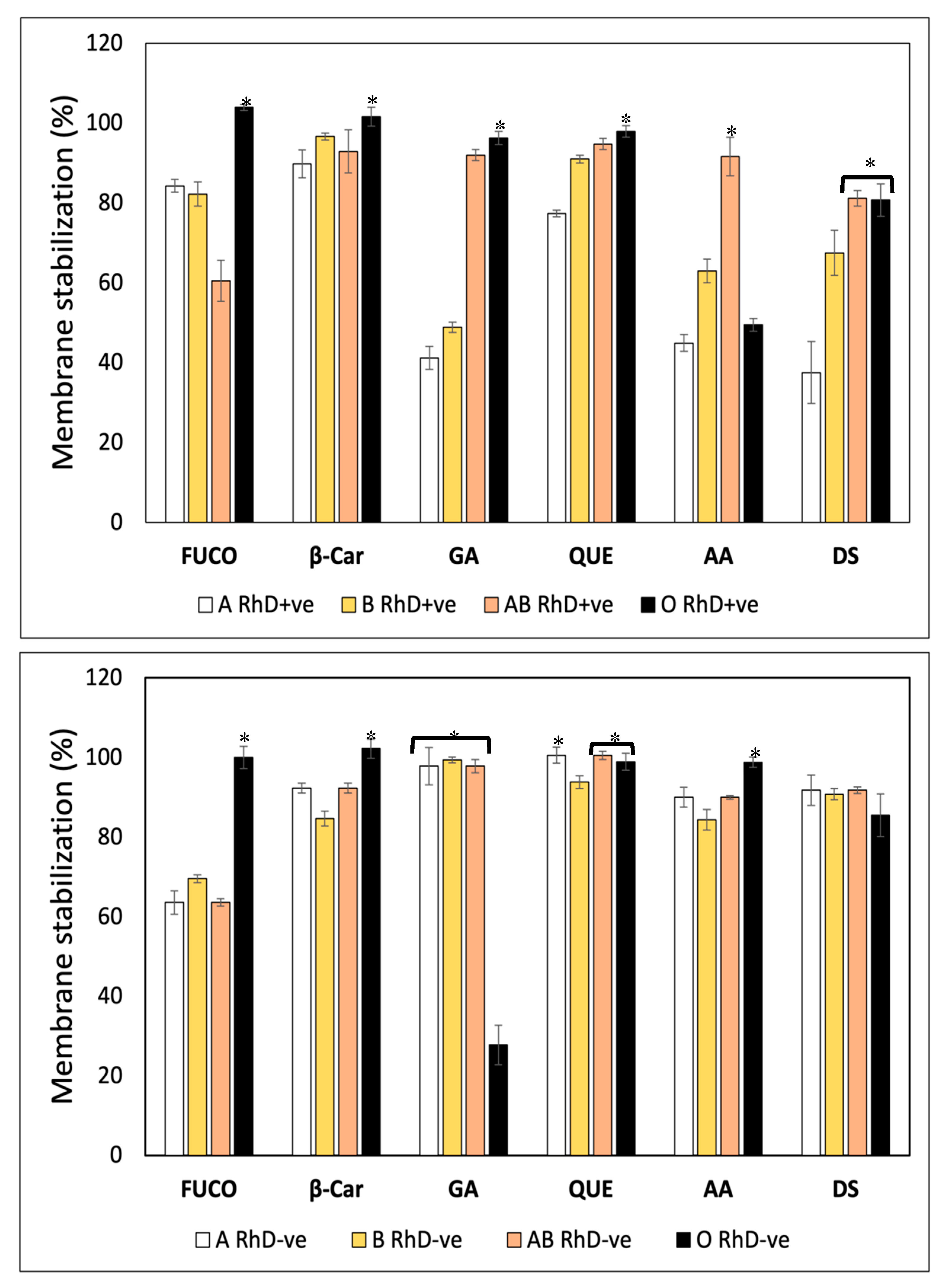
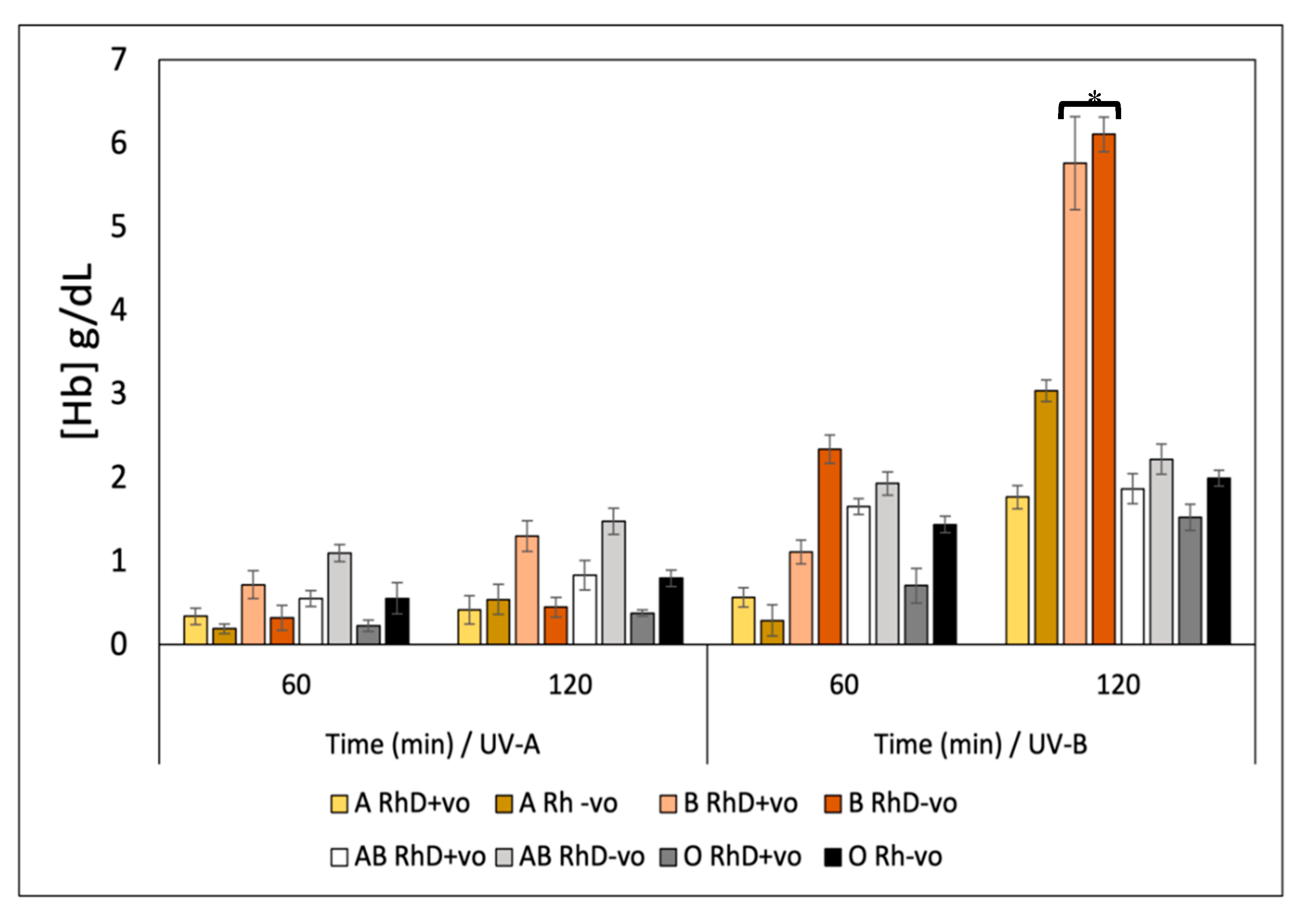
2.5.3. Blood Susceptibility Test against Oxidative Stress
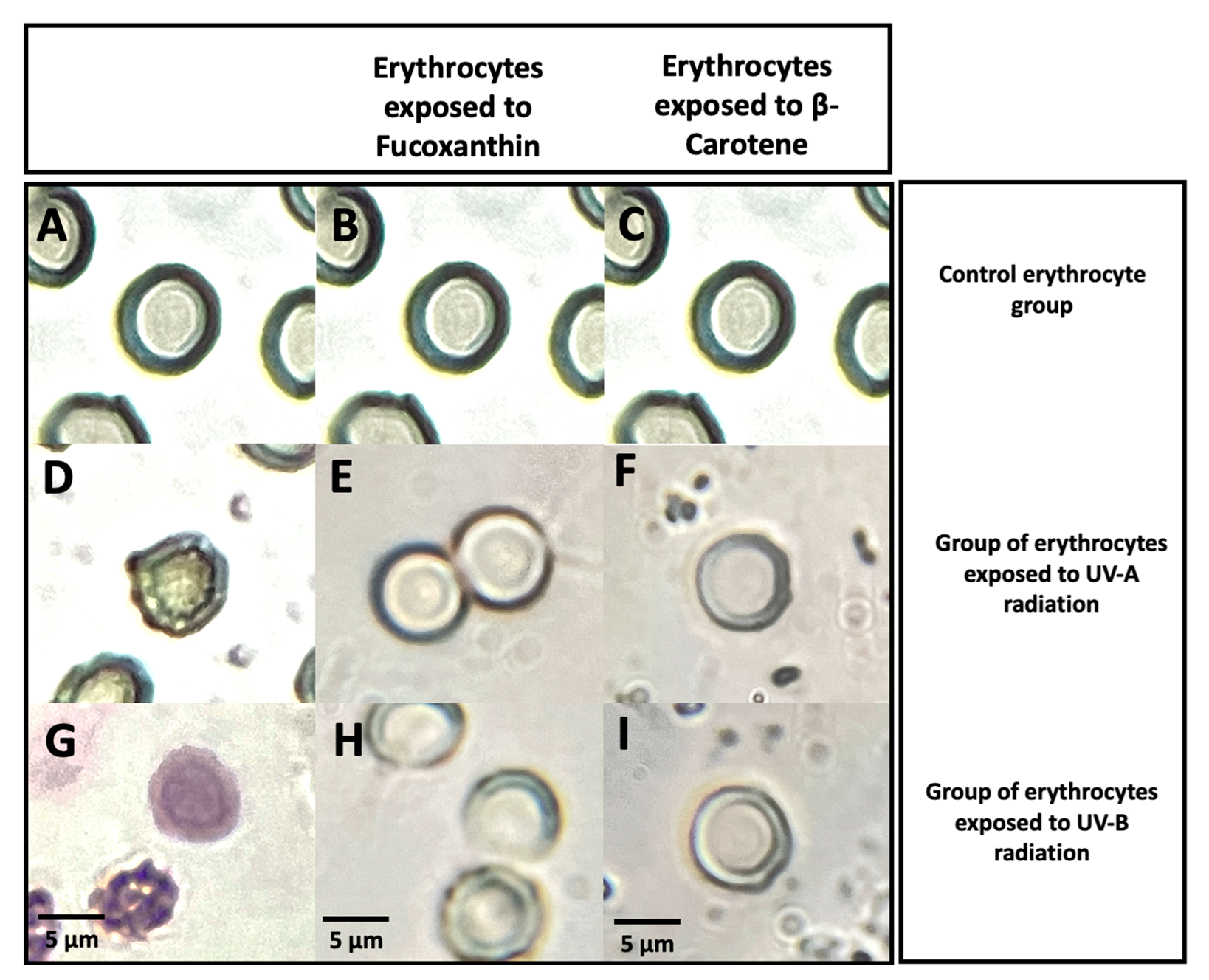
2.5.4. In Vitro Photostability Studies
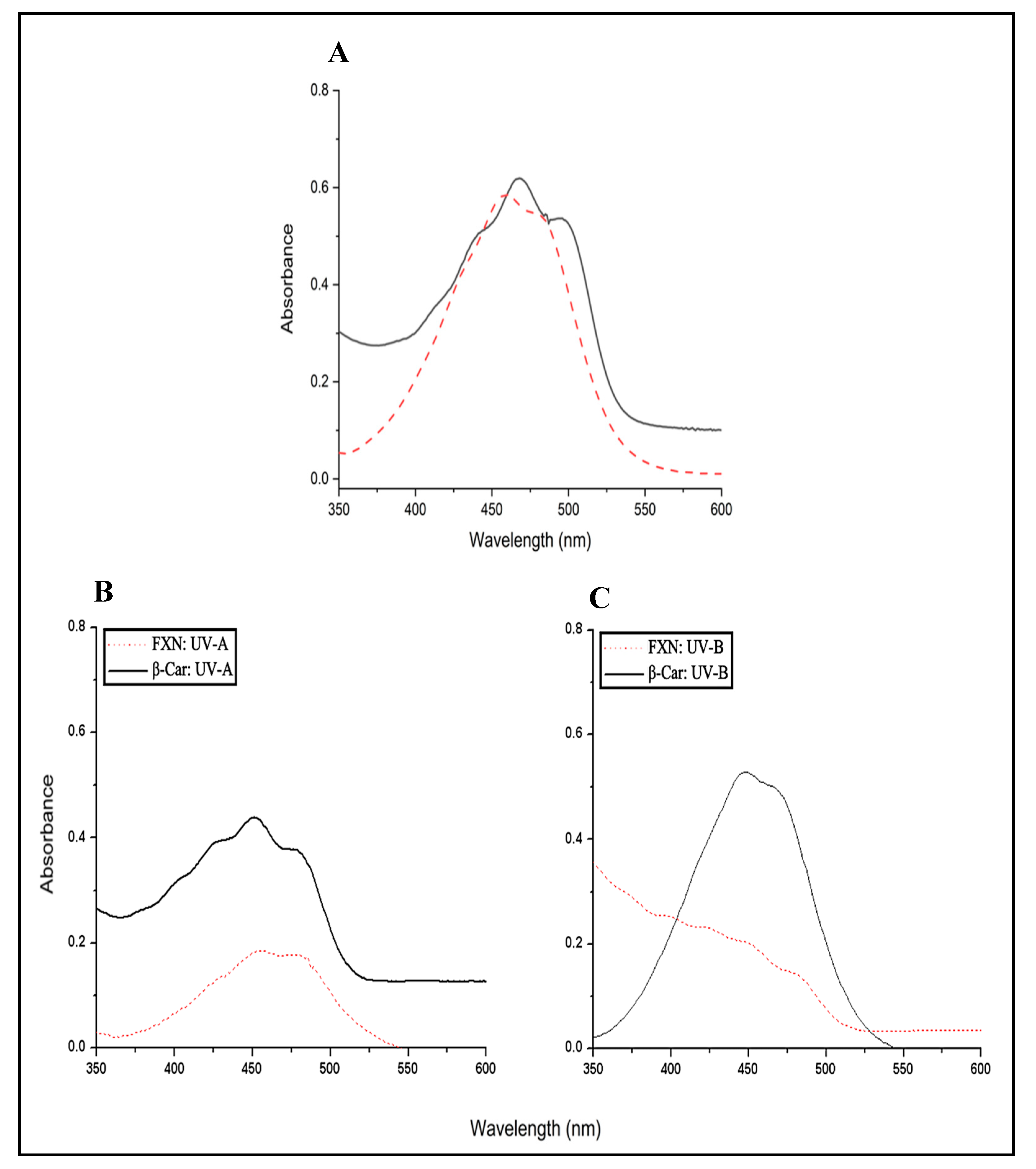
Ultraviolet-Visible (UV-Vis) Spectrophotometric Analysis
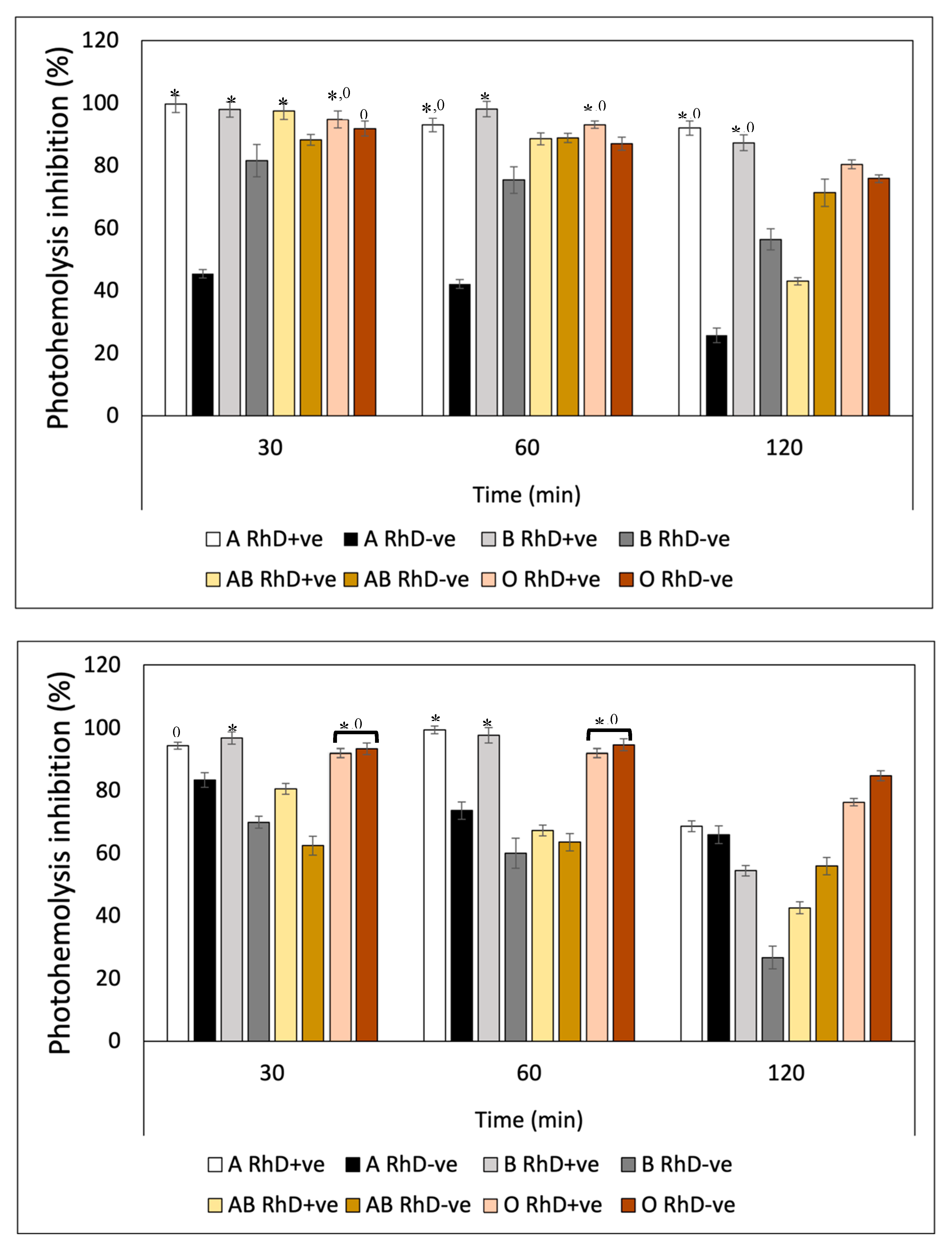
Photoprotector Assay
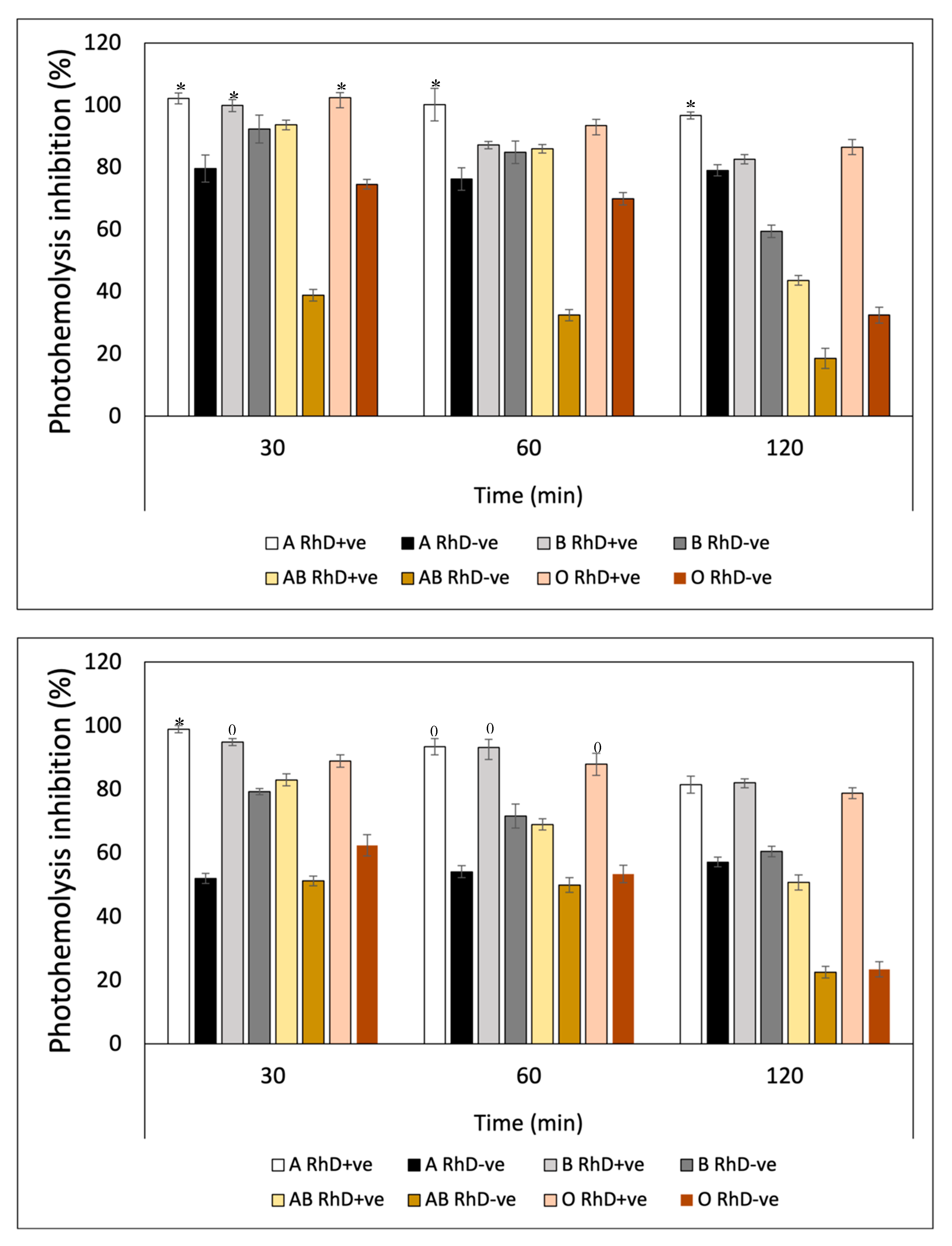
2.5.5. Antihemolytic Activity Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Capacity Assay
3.2. Erythroprotective Potential
3.2.1. Blood Biocompatibility
3.2.2. Membrane Stabilization Assay
3.2.3. Blood Susceptibility Test Against Oxidative Stress
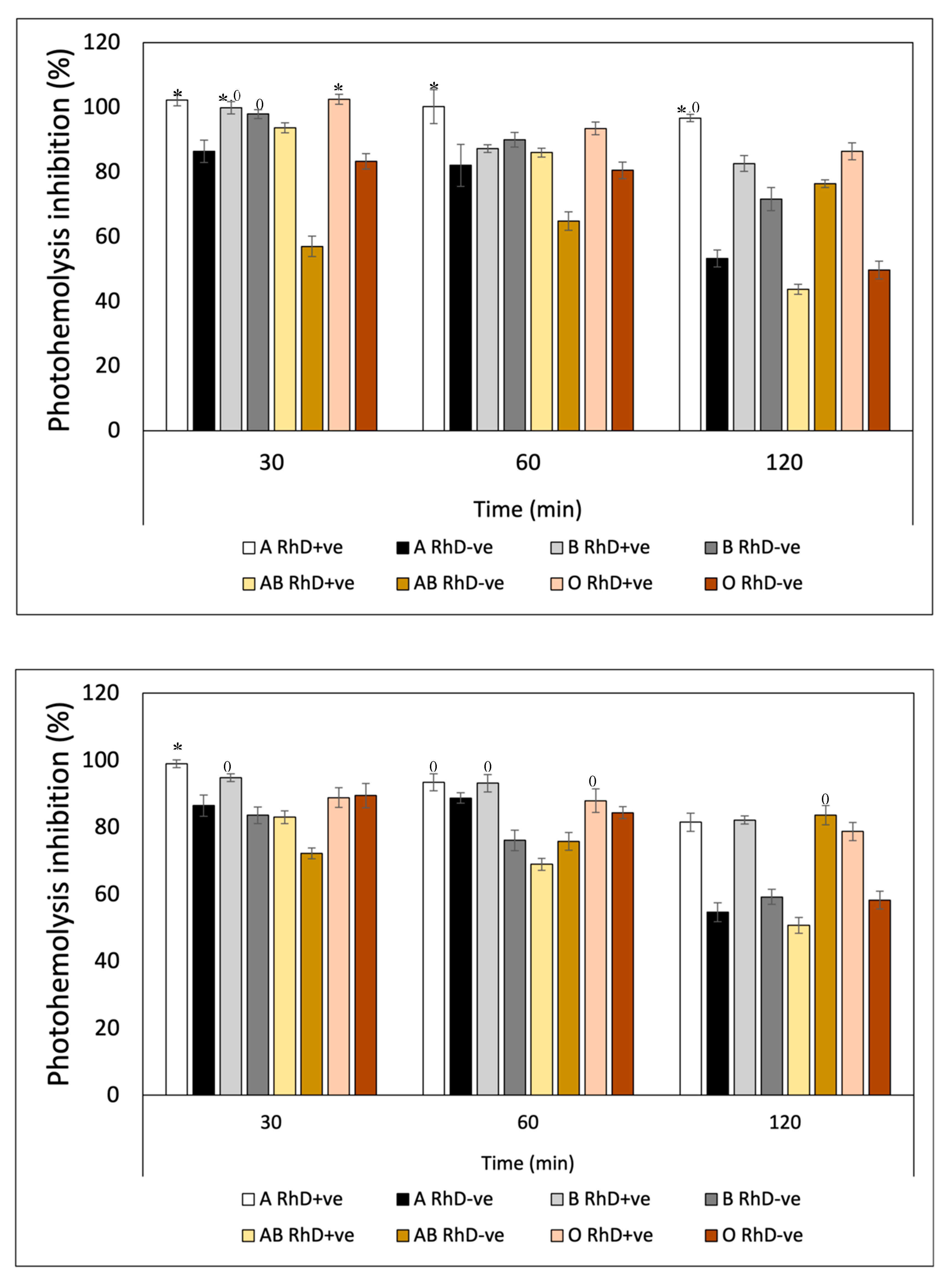
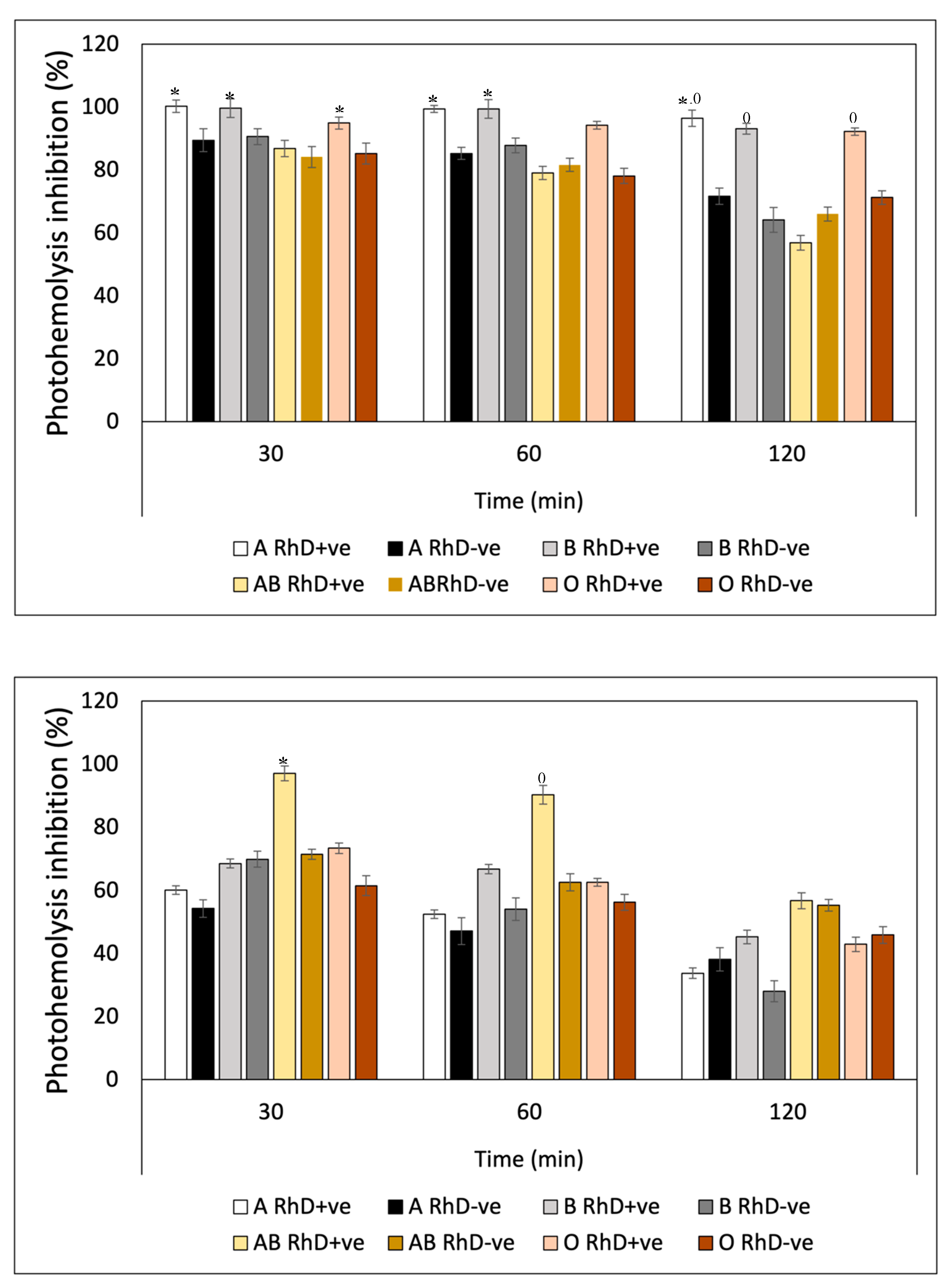
3.2.4. In vitro Photostability Studies
Ultraviolet Spectrum of Fucoxanthin before UVR Exposure
In Vitro Photoprotection Efficacy
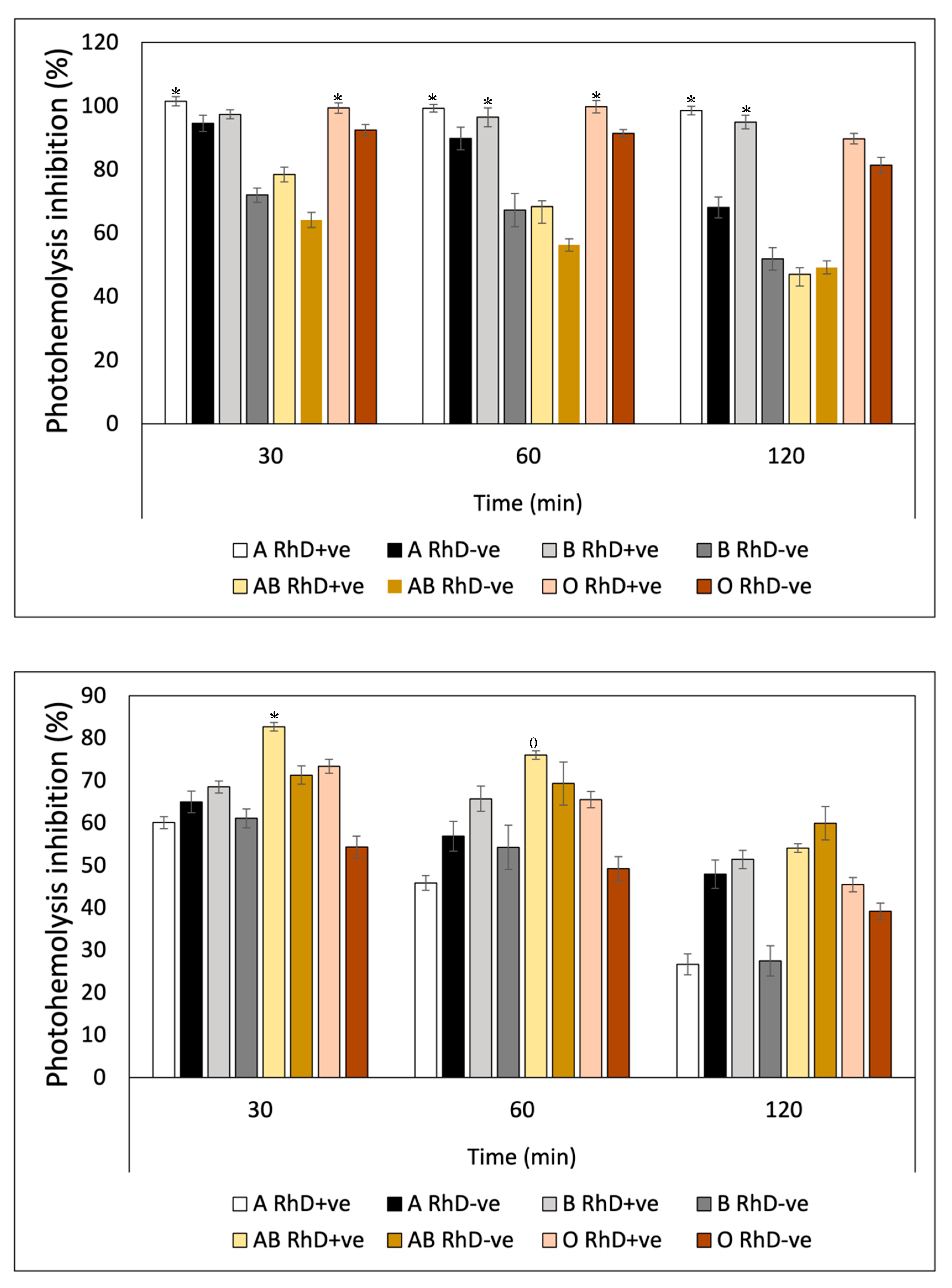
3.2.5. Antihemolytic Activity Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
References
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress, and the biology of ageing. Nature. 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants, and animals. Comparative Biochemistry and Physiology Part C. Toxicol. Pharmacol. 2010, 153(2), 175–190. [Google Scholar]
- Goiris, K.; Van Colen, W.; Wilches, I.; Tamariz, F.; De Cooman, L.; Muylaert, K. Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Research. 2015, 7, 51–57. [Google Scholar] [CrossRef]
- Achar,A.;Myers,R.;Ghosh, C. Drug Delivery Challenges in Brain Disorders across the Blood–Brain Barrier: Novel Methods and Future Considerations for Improved Therapy. Biomedicines. 2021, 9, 1834. [CrossRef]
- Biela, M.; Rimarčík, J.; Senajová, E.; Kleinová, A.; Klein, E. Antioxidant action of deprotonated flavonoids: Thermodynamics of sequential proton-loss electron-transfer. [CrossRef]
- Xue, Y.; Liu, Y.; Luo, Q.; Wang, H.; Chen, R.; Liu, Y.; Li, Y. Antiradical activity and mechanism of coumarin-chalcone hybrids: theoretical insights. J. Phys. Chem. 2018, 122, 8520–8529. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Z.; Chen, D.-F.; Deng, G.; Guo, R.; Lai, R.-C. The influence of C2=C3 double bond on the antiradical activity of flavonoid: different mechanisms analysis. Phytochem. 2019, 157, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zemlicka, L.; Fodran, P.; Lukes, V.; Vaganek, A.; Slovakova, M.; Stasko, A.; Dubaj, T.; Liptaj, T.; Karabín, M., Bírosova, L., Rapta, P. Physicochemical and biological properties of luteolin-7-O-β-d-glucoside (cynaroside) isolated from Anthriscus sylvestris (L.) Hoffm. Mon. 2014, 145, 1307–1318. [CrossRef]
- Alghazwi, M.; Smid, S.; Musgrave, I.: Zhang, W. In vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta toxicity and aggregation. Neurochem. Int. 2019, 124, 215-224. [CrossRef]
- González-Vega, R.I.; Cárdenas-López, J.C.; López-Elías, J.A.; Ruiz-Cruz, S.; Reyes-Díaz, A.; Perez-Perez, L.M.; Cinco-Moroyoqui, F.J.; Robles-Zepeda, R.E.; Borboa-Flores, J.; Del-Toro-Sánchez, C.L. Optimization of growing conditions for pigments production from microalga Navicula incerta using response surface methodology and its antioxidant capacity. Saudi J. Biol. Sci. 2021, 28; 1401–1416. [CrossRef]
- Ruiz-Cruz, S.; González-Vega, R.I.; Robles-Zepeda, R.E.; Reyes-Díaz, A.; López-Elías, J.A.; Alvarez-Ainza, M.L.; Cinco-Moroyoqui, F.J.; Moreno-Corral, R.A.; Wong-Corral, F.J.; Borboa-Flores, J.; et al. Association of Different ABO and Rh Blood Groups with the Erythroprotective Effect of Extracts from Navicula Incerta and Their Anti-Inflammatory and Antiproliferative Properties. Metabolites 2022, 12, 1203. [Google Scholar] [CrossRef] [PubMed]
- Peña-Medina, R.L.; Fimbres-Olivarría, D.; Enríquez-Ocaña, L.F.; Martínez-Córdova, L.R.; Del-Toro-Sánchez, C.L.; López-Elías, J.A.; González-Vega, R.I. Erythroprotective potential of phycobiliproteins extracted from Porphyridium cruentum. Metabolites. 2023, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Yan-jun, C.; Jia, Q.; Min-min, Z.; Tian-liang, Z.; Min, C.; Shun-mei, L.; Guangce, W. Fucoxanthin Exerts Cytoprotective Effects against Hydrogen Peroxide-induced Oxidative Damage in L02 Cells. BioMed Res. Int. 2018, x(x), 1–11. [Google Scholar] [CrossRef]
- Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N. Engl J Med. 2020, 1–13. [CrossRef]
- Celic, D.; Lipozencic, J.; Kolaric, B.; Ferencak, G.; Kanižaj Rajkovic, J.; Borlinic, T. Association between Blood Group and Nonmelanoma Skin Cancers (Basal Cell Carcinoma and Squamous Cell Carcinoma). Int. J. Environ. Res. Public Health. 2019, 16, 2267; [Google Scholar] [CrossRef]
- Zhang, J.; Hou, X.; Ahmad, H.; Zhang, H.; Zhang, L.; Wang, T. Assessment of free radicals scavenging activity of seven natural pigments and protective effects in AAPH-challenged chicken erythrocytes. Food Chem. 2014, 145, 57–75. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Reza, Falsafi, S.; Mahdi, Jafari, S. Nanoencapsulation of carotenoids within lipid-based nanocarriers. JCR 2019, 298, 38–67. [Google Scholar] [CrossRef] [PubMed]
- González-Vega, R.I.; Del-Toro-Sánchez, C.L.; Moreno-Corral, R.A.; López-Elías, J.A.; Reyes-Díaz, A.; García-Lagunas, N.; Carvajal-Millán, E.; Fimbres-Olivarría, D. Sulfated polysaccharide-rich extract from Navicula incerta: physicochemical characteristics, antioxidant activity, and anti- hemolytic property. AIMS Bioengineering, 9(4), 364–382. [CrossRef]
- Rodríguez-Roque, M.J.; Del-Toro-Sánchez, C.L.; Chávez-Ayala, J.M.; González-Vega, R.I.; Pérez-Pérez, L.M.; Sánchez-Chávez, E.; Salas-Salazar, N.A.; Soto-Parra, J.M; Iturralde-García, R.D.; Flores-Córdova, M.A. Digestibility, Antioxidant and Anti-Inflammatory Activities of Pecan Nutshell (Carya illioinensis) Extracts. Journal of Renewable Materials, 2022. Impact Factor: 2.115. 2570-2580. [CrossRef]
- Perez-Perez, L.M.; Huerta-Ocampo, J.A.; Ruiz-Cruz, S.; Cinco-Moroyoqui, F.J.; Wong-Corral, F.J.; Rascón-Valenzuela, L.A.; Robles-García, M.A.; González-Vega, R.I; Rosas-Burgos, E.C.; Corella-Madueño, M.A.G.; et al. Evaluation of Quality, Antioxidant Capacity, and Digestibility of Chickpea (Cicer arietinum L. cv Blanoro) Stored under N2 and CO2 Atmospheres. Molecules, 2021. Impact Factor: 4.927. 26:2773. [CrossRef]
- Reyna-Reyna, L.Y.; Montaño-Leyva, B.; Valencia-Rivera, D.E.; Cinco-Moroyoqui, F.J.; González-Vega, R.I.; Bernal-Mercado, A.T.; Ballesteros-Monrrea, M.G.; Méndez-Encinas, M.A.; Del-Toro-Sánchez, C.L. Antioxidant, antibacterial, anti-inflammatory, and antiproliferative activity of sorghum lignin (Sorghum bicolor) treated with ultrasonic pulses. “Metabolites” 2023. [CrossRef]
- Alghazwi, M.; Smid, S.; Musgrave, I.: Zhang, W. In vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta toxicity and aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Shanmugam, V.K. Anti-inflammatory activity screening of Kalanchoe pinnata methanol extract T and its validation using a computational simulation approach. Inform. Med. Unlocked. 2019, 14, 6–14. [Google Scholar] [CrossRef]
- Takebayashi, J.; Chen, J.; Tai, A. A method for evaluation of antioxidant activity based on inhibition of free radical-induced erythrocyte hemolysis. Methods Mol. Biol. 2010, 594, 287–296. [Google Scholar]
- Rizvi, S.I.; Jha, R.; Pandey, K.B. Activation of erythrocyte plasma membrane redox system provides a useful method to evaluate anti-oxidant potential of plant polyphenols. Methods Mol. Biol. 2010, 594, 341–348. [Google Scholar]
- Dere, S.; Gűnes, T.; Sivaci, R. Spectrophotometric determination of chlorophyll - A, B and total carotenoid contents of some algae species using different solvents. Turkish J. Botany. 1998, 22, 13–17. [Google Scholar]
- Simioni, C.; Schmidt, E.; Felix, M.R.L.; Polo, L.K.; Rover, T.; Kreusch, M.; Pereira, D.T.; Chow, F.; Ramlov, F.; Maraschin, M.; Bouzon, Z.L. Effects of Ultraviolet Radiation (UVA+UVB) on Young Gametophytes of Gelidium floridanum: Growth Rate, Photosynthetic Pigments, Carotenoids, Photosynthetic Performance, and Ultrastructur. Photochem. and Photobiol. 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ruiz, K.L.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Gassos-Ortega, L.E.; Ornelas-Paz, J.J.; Del-Toro-Sánchez, C.L.; Márquez-Ríos, E.; López-Mata, M.A.; Rodríguez-Félix, F. Evaluation of Antioxidant Capacity, Protective Effect on Human Erythrocytes and Phenolic Compound Identification in Two Varieties of Plum Fruit (Spondias spp.) by UPLC-MS. Moelcyles. 2018, 23, 3200; [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Carpena, M.; Pereira, A.G.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181. [Google Scholar] [CrossRef]
- Chuyen, V.H.; Eun, J.-B. Marine carotenoids: bioactivities and potential benefits to human health. Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Miyashita, T.; Nishikawa, S.; Emi, S.; Tsukui, T.; Beppu, F.; Okada, T.; Miyashita, K. Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-Ay mice. Arch. Biochem. Biophys. 2010, 504, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Jian-Ping, Y.; Chou-Fei, W.; Jiang-Hai, W. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs. 2011, 9:1806-1828. [CrossRef]
- Peng, C.H.; Chang, C.H.; Peng, R.Y.; Chyau, C.C. Improved membrane transport of astaxanthin by liposomal encapsulation. Eur. J. Pharm. Biopharm. 2010, 75, 154–161. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, N.; Liao, L.; Yu, K.; Shu, X.-O.; Zheng, W.; Yuan, J.-M.; Koh, W.-P.; Qiao, Y.-L.; Fan, J.-H.; et al. ABO Genotypes and the Risk of Esophageal and Gastric Cancers. BMC Cancer 2021, 21, 589. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotecnol. Adv. 2016, 34:1396-1412. [CrossRef]
- Zheng, Y.-Z.; Chen, D.-F.; Deng, G.; Guo, R.; Lai, R.-C. The influence of C2=C3 double bond on the antiradical activity of flavonoid: different mechanisms analysis. Phytochem. 2019, 157, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Masek, A., Latos, M., Piotrowska, M., Zaborski, M. 2018. The potential of quercetin as an effective natural antioxidant and indicator for packaging materials. Food Packaging and Shelf Life, 16, 51-58. [CrossRef]
- Miltonprabu, S., Tomczyk, M., Skalicka-Woźniak, K., Rastrelli, L., Daglia, M., NabavI, S.F., Alavian, S.M., Nabavi, S.M. 2017. Hepatoprotective effect of quercetin: From chemistry to medicine. Food and Chemical Toxicology, 108, 365-374. [CrossRef]
- Mu, Y., Fu, Y., Li, J., Yu, X., Li, Y., Wang, Y., Wu, X., Zhang, K., Kong, M., Feng, C., Chen, X. 2019. Multifunctional quercetin conjugated chitosan nano-micelles with P-gp inhibition and permeation enhancement of anticancer drug. Carbohydrate Polymers, 203, 10-18. [CrossRef]
- Chisté, R.C.; Freitas, M.; Mercadante, A.Z.; Fernandes, E. Carotenoids are Effective Inhibitors of in vitro Hemolysis of Human Erythrocytes, as Determined by a Practical and Optimized Cellular Antioxidant Assay. J. Food Sci. 2014, 79, 9. [Google Scholar] [CrossRef]
- Miyashita, K.; Maeda, H.; Okada, T.; Abe, M.; Hosokawa, M. Anti-obesity, and anti-diabetic effects of allenic carotenoid, fucoxanthin. Agro. Food Ind. Hi Tech 2010, 21, 24–27. [Google Scholar]
- Niki, E.; Komuro, E.; Takahashi, M.; Urano, S.; Ito, E.; Terao, K. Oxidative hemolysis of erythrocytes and its inhibition by free radical scavengers. J. Biol.Chem. 1988, 263, 19809–19814. [Google Scholar] [CrossRef] [PubMed]
- Parentini, I.; Bergamini, E.; Cecchi, L.; Cavallini, G.; Donati, A.; Maccheroni, M.; Tamburini, I.; Gori, Z. The effect of carbon tetrachloride and ultraviolet radiation on dolichol levels in liver cells isolated from 3- and 24-month-old male Sprague–Dawley rats. Biogerontology 2003, 4, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Ananthi, S.; Raghavendran, H.R.B.; Sunil, A.G.; Gayathri, V.; Ramakhrisnan, G.; Vasanthi, H.R. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornate (Marine Brown Alga). Food Chem. Toxicol. 2010, 48, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Moualek, I.; Iratni Aiche, G.; Mestar Guechaoui, N.; Lahcene, S.; Houali, K. Antioxidant and anti-inflammatory activities of Arbutus unedo aqueous extract. Asian Pac. J. Trop. Biomed. 2016, 6, 937–944. [Google Scholar] [CrossRef]
- Fernández-Botrán, R. Association of ABO blood group with COVID-19 susceptibility. Ciencia, Tecnología y Salud. 2020, 7(3), 325–332. [Google Scholar] [CrossRef]
- Medithi, S., Jonnalagadda, P.R.; Jee, B. Predominant role of antioxidants in ameliorating the oxidative stress induced by pesticides. Arch. Environ. Occup. Health. 2021, 76(2):61-74. [CrossRef]
- Fuchs, J. Potentials and limitations of the natural antioxidants RRR-alpha-tocopherol, L-ascorbic acid, and beta-carotene in cutaneous photoprotection. Free Radic. Biol. Med. 1998, 25, 848–873. [Google Scholar] [CrossRef] [PubMed]
- Steenvoorden, D.P.; van Henegouwen, G.M. The use of endogenous antioxidants to improve photoprotection. J. Photochem. Photobiol. B. 1997, 41, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Medithi, S., Jonnalagadda, P.R.; Jee, B. Predominant role of antioxidants in ameliorating the oxidative stress induced by pesticides. Arch. Environ. Occup. Health. 2021, 76(2):61-74. [CrossRef]
- Benítez-Leite, S.; Ml, M.; Fernández, V.; Franco, D.; Ea, F.; Cuevas, M.A.; Alfonso, J.; Sales, L. Daño celular en una población infantil potencialmente expuesta a pesticidas Cell Damage in a Pediatric Population Potentially Exposed to Pesticides.
- Pattison, D.I.; Davies, M.J. Actions of ultraviolet light on cellular structures. Cancer: Cell Structures, Carcinogens and Genomic Instability. 2006, 131-157. [CrossRef]
- Cadet, J.; Sage, E.; Douki, T. Ultraviolet radiation-mediated damage to cellular DNA. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2005, 571(1-2), 3-17. [CrossRef]
- Jacobson, M.K.; Kim, H. Coyle, W. R. et al. Effect of myristyl nicotinate on retinoic acid therapy for facial photodamage. Exp. Dermatol. 2007, 16, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Greul, A.K.; Grundmann, J.U.; Heinrich, F.; et al. Photoprotection of UV-irradiated human skin: an antioxidative combination of vitamins E and C, carotenoids, selenium and proanthocyanidins. Skin Pharmacol. Appl. Skin. Physiol. 2002; 15, 307–315. [Google Scholar]
- Greul, A.-K.; Grundmann, J.-U.; Heinrich, F.; Pfitzner, I.; Bernhardt, J.; Ambach, A.; Biesalski, H.-K.; Gollnick, H. Photoprotection of UV-Irradiated Human Skin: An Antioxidative Combination of Vitamins E and C, Carotenoids, Selenium and Proanthocyanidins. Skin Pharmacol. Appl Skin Physiol. 2002, 15, 307–315. [Google Scholar] [CrossRef]
- Chen, L.; Judy Y. Hu, J.J.; Steven Q. Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67:1013-24. [CrossRef]
- Simioni, C.; Schmidt, E.C. Felix, M.R.L.; Polo, L.P.; Rover, T.; Kreusch, M.; Pereira, D.T.; Chow, F.; Ramlov, F.; Maraschin, M.; Bouzon, A.L. Effects of Ultraviolet Radiation (UVA+UVB) on Young Gametophytes of Gelidium floridanum: Growth Rate, Photosynthetic Pigments, Carotenoids, Photosynthetic Performance, and Ultrastructure. Photochemistry and Photobiology. 2014. [CrossRef]
- Greul, A.-K.; Grundmann, J.-U.; Heinrich, F.; Pfitzner, I.; Bernhardt, J.; Ambach, A.; Biesalski, H.-K.; Gollnick, H. Photoprotection of UV-Irradiated Human Skin: An Antioxidative Combination of Vitamins E and C, Carotenoids, Selenium and Proanthocyanidins. Skin Pharmacol. Appl. Skin Physiol. 2002, 15, 307–315. [Google Scholar] [CrossRef]
- Nichols, J.A., Katiyar, S.K. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant, and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302: 71–83 (2010). [CrossRef]
- Kumar, S.; Devi, S.; Misra, P. ; Priyanka. Solar and artificial ultraviolet-B induced erythrocytes hemolysis with photosensitizers. Ind. J. Experimen. Biol. 2009, 47, 906–910. [Google Scholar]
- Rostamabadi, H.; Reza, Falsafi, S.; Mahdi, Jafari, S. Nanoencapsulation of carotenoids within lipid-based nanocarriers. JCR 2019, 298, 38–67. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Verkempinck, S.; Rijal, SK.; Van Loey, A.; Grauwet, T.; Hendrickx, M. Lipid nanoparticles with fats or oils containing β-carotene: Storage stability and in vitro digestibility kinetics. Food Chem. 2019, 278(1), 396–405. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam R.; Ki, J-S.A Review of the Biological Activities of Microalgal Carotenoids and Their Potential Use in Healthcare and Cosmetic Industries. Mar Drugs. 2018, 16(1):26. [CrossRef]
- Zmijewski, C.M. Inmunohematology. Eherd Edition; Appleton Century Crojts: New York, NY, USA, 1978. [Google Scholar]
- Hou, J.; Cui, H.L. In Vitro Antioxidant, Antihemolytic, and Anticancer Activity of the Carotenoids from Halophilic Archaea. Curr. Microbiol. 2018, 75, 266–271. [Google Scholar] [CrossRef]
- Sathasivam, R.; Ki, J.S. A Review of the Biological Activities of Microalgal Carotenoids and Their Potential Use in Healthcare and Cosmetic Industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Carotenoids and Flavonoids Contribute to Nutritional Protection against Skin Damage from Sunlight. Mol. Biotechnol. 2007, 37:26–30. [CrossRef]
- Carini, M.; G. Aldini, G.; Bombardelli, E.; Morazzon, P.; R. Maffei Facino, R.M. UVB-induced hemolysis of rat erythrocytes: Protective effect of procyanidins from grape seeds. Life Sciences. 2000, 67, 1799–1814. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. b-Carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012, 96. [Google Scholar] [CrossRef] [PubMed]
- Camera, E.; Mastrofrancesco, A.; Fabbri, C.; Daubrawa, F.; Picardo, M.; Sies, H.; Stahl, W. Astaxanthin, canthaxanthin and b-carotene differently affect UVA-induced oxidative damage and expression of oxidative stress-responsive enzymes. Exp. Dermatol. 2009, 18: 222–231. [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Carpena, M.; Pereira, A.G.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of Natural Deep Eutectic Solvents for Extraction of Hydrophilic and Lipophilic Compounds from Fucus vesiculosus. Molecules 2021, 26, 4198. [Google Scholar] [CrossRef]
- Akhavan, S.; Assadpour, E.; Katouzian, I.; Jafari, S.M. Lipid nanoscale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trends Food Sci. Technol. 2018, 74, 132–146. [Google Scholar] [CrossRef]
- Aly, R.; Yousef, A.; Elbably, O. Association of ABO blood group and risk of breast cancer. J. Blood Disord. Transfus. 2014, 5: 241. [CrossRef]
- Abella, A.; Messaoudi, C.; Laurent, D.; Marot, D.; Chalas, J.; Breux, J.; Claise, C.; Lindenbaum, A. A method for simultaneous determination of plasma and erythrocyte antioxidant status. Evaluation of the antioxidant activity of vitamin E in healthy volunteers. Br. J. Clin. Pharmacol. 1996, 42, 737–741. [Google Scholar] [CrossRef] [PubMed]

| µmol TE/g ± SD | |||
|---|---|---|---|
| Compounds | FRAP | ABTS | DPPH |
| FXN | 2687.03 b ±173.26 | 2638.53c ±244.87 | 303.15 c ±42.66 |
| β - Car | 2780.87 b ±92.23 | 2371.99 c ±26.79 | 348.12 c ±38.49 |
| GA | 5970.45 a ±230.07 | 3124.72 b ±133.22 | 614.74 b ±99.77 |
| QUE | 896.70 c ±19.06 | 2037.62 d ±80.62 | 3980.61 a ±222.58 |
| AA | 2684.54 b ±104.13 | 3412.22 a ±21.94 | 4190.9 a ±155.16 |
| Inhibition of Hemolysis (%) | ||||
|---|---|---|---|---|
| Different blood groups (RhD+ ve) | ||||
| Compounds | A | B | AB | O |
| FXN | 100.70 Aa ±0.71 | 79.57 Bd ±1.13 | 97.30 Bb ±1.37 | 91.15 Bc ±1.04 |
| β - Car | 101.05 Aa ±3.78 | 84.82 Ac ±2.56 | 98.87 ABb ±0.49 | 97.05 Ab ±0.31 |
| GA | 94.42 Bb ±1.08 | 77.16 Bd ±0.49 | 99.43 Aa ±1.37 | 81.94 Dc ±1.08 |
| QUE | 95.61 Bb ±0.41 | 70.78 Cc ±0.64 | 100.85 Aa ±1.43 | 83.51 Cb ±0.61 |
| AA | 93.12 Bb ±3.55 | 62.84 Dd ±3.08 | 99.29 Aa ±1.07 | 83.33 CDc ±3.26 |
| Inhibition of Hemolysis (%) | ||||
|---|---|---|---|---|
| Different blood groups (RhD-ve) | ||||
| Compounds | A | B | AB | O |
| FXN | 79.93 Ab ±3.28 | 92.41 Aa ±1.34 | 81.28 Bb ±1.28 | 91.15 Ba ±1.43 |
| β - Car | 73.72 Bd ±1.47 | 90.86 Ab ±2.63 | 78.30 Bc ±1.53 | 97.05 Aa ±2.43 |
| GA | 75.30 Bd ±2.34 | 89.76 Ab ±1.18 | 95.60 Aa ±1.30 | 81.94 Cc ±2.66 |
| QUE | 50.85 Cc ±2.56 | 82.44 Ba ±2.45 | 62.55 Db ±4.60 | 83.51 Ca ±1.65 |
| AA | 80.66 Ab ±3.39 | 91.02 Aa ±1.11 | 74.75 Cc ±1.30 | 83.33 Cb ±4.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





