Background
Overview of Elaeagnus angustifolia (Russian olive) and its medicinal properties
Elaeagnus angustifolia (EA), commonly known as Russian olive, is a deciduous tree native to regions in Asia, including Iran, Kazakhstan, and parts of China [
1]. It has also been introduced to North America, Europe, and other parts of the world. Russian Olive is well-known for its medicinal properties and has been used in traditional medicine for various health conditions [
2].
EA is rich in antioxidants, such as flavonoids, phenolic compounds, and vitamins C and E. These antioxidants help neutralize free radicals in the body, reducing oxidative stress and protecting cells from damage [
3]. The tree's extracts have demonstrated anti-inflammatory properties, which can help reduce inflammation and provide relief from conditions such as arthritis and other inflammatory disorders [
4].
There are 50 species in the three genera that make up the family
Elaeagnaceae. Russian olives
Elaeagnus angustifolia and
Elaeagnus. pungens have some adaptation to central Asia.
Shepherdia canadensis is a native of Canada and the United States. In Europe,
Hippophae rhamnoides is endemic [
2]. In this family,
EA is well known for its therapeutic properties. The autumnal fruit
EA is a member of the genus
Elaeagnus and the family
Elaeagnaceae.
EA is an attractive plant in Europe and the Middle East [
5]. In traditional medicine, the flowers and leaves were utilized as antipyretic and diuretic medicines. Due to the presence of phenolic and flavonoids, they guard against oxidative cell damage, and their fruits are utilized as appetizers [
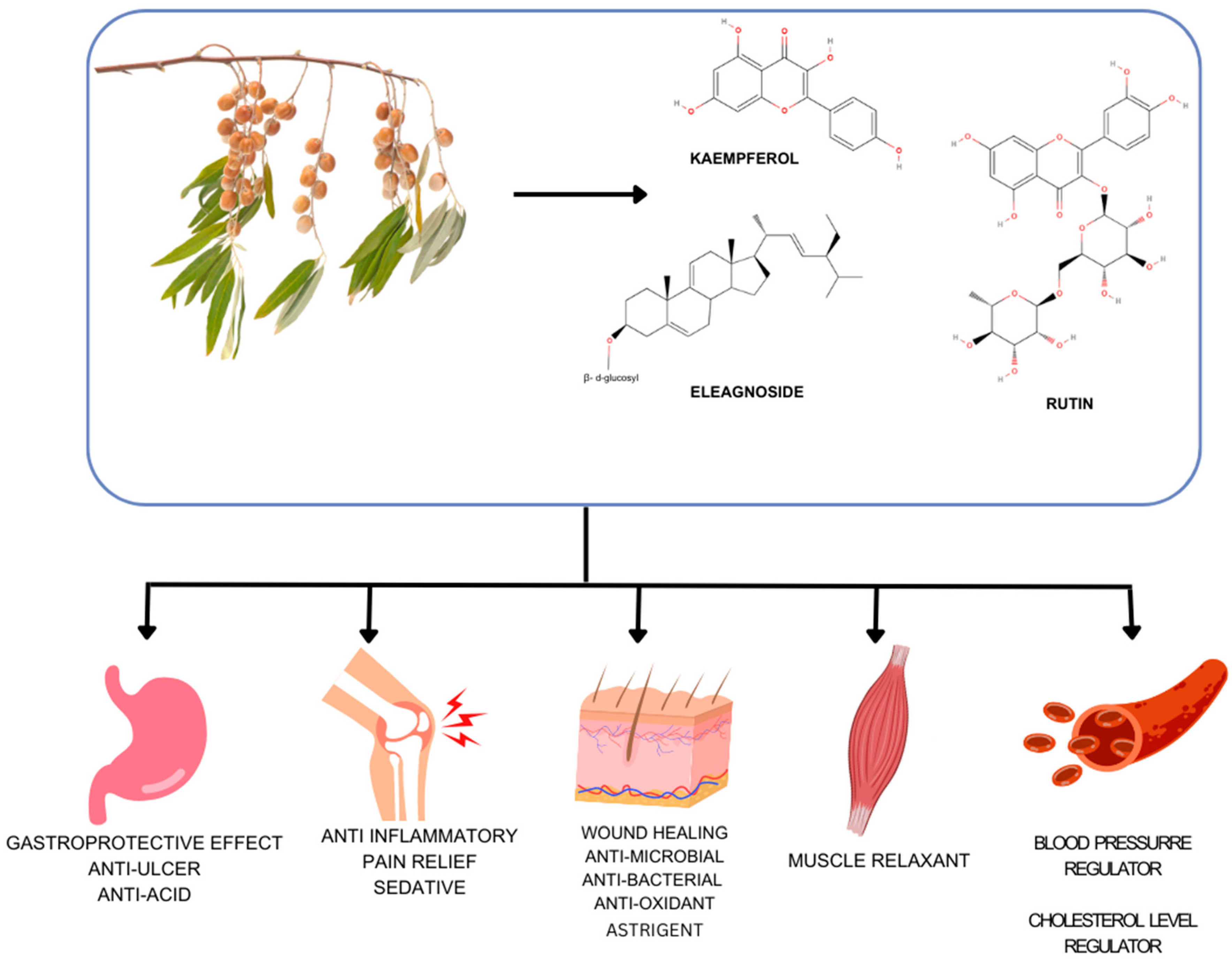
6]. The fruit ripens in September and is elliptical, reddish to brown, and approximately 12 mm long and 10 mm broad. Both fresh and dried fruit are typically consumed for health advantages.
EA is a tiny tree or shrub that can reach 35 feet (10 meters). While the elder branches are dark, the younger branches are silvery. They may have thorns and are scale-covered. The leaves are lanceolate to oblong-lanceolate, alternating, and straightforward. They have silver scales on both sides and are 1-4 in. (3-10 cm) long. The fragrant blooms have a silvery exterior and a golden inside, and they are 0.5–0.6 in. (1.2–1.5 cm) broad. The leaf axils have one to three blooms. They show up from May to June. The fruit is golden, 0.4 in. (1 cm) long, and nearly entirely coated in thick silver scales. One substantial seed, up to 0.4 inches (1 cm) long, is inside each fruit. They have abundant chemical constituents, nutritive value, protein, total soluble carbohydrates, fat, dietary fibre, and total polyphenols in their fruit, giving them antioxidants and other pharmacological potential [
7]. As a sedative, digestive aid, and expectorant, the herbal tea "Zhourat," a concoction of herbs and
EA blossoms, is used [
8]. Ice cream contains
EA flower to increase the product's sensory appeal and antioxidant potential [
9]. The aerial parts of this plant have reportedly been used in traditional Turkish medicine as tonics, antipyretics, diuretics, antidiarrheal, anti-inflammatory, antinociceptives, analgesics, and remedies to treat dysentery, liver disease, tetanus, fever, bronchitis, rheumatoid arthritis, as well as antimicrobial and anticancer properties (
Figure 1) [
10].
Consuming fruits regularly can help avoid conditions including cataracts, neoplastic diseases, rheumatoid arthritis, major non-communicable diseases like Alzheimer's and Parkinson's, cardiovascular diseases, and certain malignancies [
11]. Fruits are high in micronutrients and dietary fibre, which helps people change their eating habits, especially those who frequently consume meals high in fats and sugar [
12]. Additionally, the fruit seed of
EA has demonstrated astringent, antipyretic, anti-ulcerogenic, wound-healing, and muscle-relaxing qualities [
13], among other features [
14]. The leaves of
EA have been applied topically to wounds to promote healing, and its anti-inflammatory and antimicrobial properties may contribute to this beneficial effect [
3,
15]. One study showed that
EA aqueous extract accelerated cutaneous wound healing in rats by increasing epidermal regeneration and collagen deposition at wound site, leading to faster recovery [
16]. Extracts from
EA have also shown immunomodulatory effects, meaning they can influence the immune system. This may enhance the immune response, making
EA potentially helpful in boosting the body's defence against infections and diseases [
3]. Additionally, the tree's extracts have been used traditionally to improve digestive health and alleviate symptoms of indigestion, constipation, and other gastrointestinal issues [
3].
Some studies have suggested that
EA extracts may have cardioprotective properties, including potential effects on blood pressure regulation and cholesterol levels [
3,
4,
15]. Moreover,
EA extracts have demonstrated antimicrobial activity against various pathogens, indicating potential use in treating infections [
3,
17,
18].
In recent years, there has been interest in the potential anticancer properties of
EA [
19]. Preliminary research suggests that its extracts may have the ability to induce apoptosis (programmed cell death) and inhibit cancer cell proliferation [
20,
21,
22]. However, further research, including human clinical trials, is necessary to fully understand its potential anticancer effects and determine safe and effective dosages for specific types of cancer.
Research has also shown that
EA may enhance the efficacy of certain chemotherapy drugs, such as cisplatin, and reduce their side effects [
22,
23]. Overall, while more research is needed,
EA shows promise as a potential therapeutic agent in cancer treatment.
Potential therapeutic applications of Elaeagnus angustifolia
The rationale for exploring the potential therapeutic applications
of EA in cancer treatment stems from its reported anti-cancer properties and its long history of traditional use in herbal medicine [20-22].
EA extracts have shown promising anti-cancer properties in preclinical studies, including
in vitro and animal models [
24]. These studies have demonstrated its ability to induce apoptosis (programmed cell death) in cancer cells, inhibit cancer cell proliferation, and modulate critical cellular signalling pathways in cancer development and progression [
20].
Moreover
, EA is a natural source of bioactive compounds, including flavonoids, phenolic compounds, and antioxidants. Natural products like these have been an important source of potential anticancer agents, inspiring scientific interest in their therapeutic potential [
3]. Unlike conventional cancer treatments that can cause collateral damage to healthy cells,
EA reported anti-cancer effects appear selective for cancer cells, sparing normal cells from harm [21, 25]. This targeted approach may lead to fewer side effects and improved quality of life for cancer patients.
Another potential benefit of EA in cancer treatment is its immunomodulatory effects [1, 26-28]. By modulating the immune system, the plant may enhance the body's ability to recognize and target cancer cells, potentially aiding the immune response against the disease [1, 26-28]. This could open up new avenues for combination therapies, where EA reported anticancer effects could complement existing cancer treatments, such as chemotherapy, radiation, and immunotherapy.
The traditional use of EA in herbal medicine for various health conditions, including cancer, provides a basis for further investigation. Traditional knowledge can guide modern scientific research to explore its potential applications. Moreover, the reported modulation of multiple cellular pathways involved in cancer development and progression suggests that EA may have a multi-targeted approach in cancer treatment, potentially addressing different aspects of the disease.
Elaeagnus angustifolia effects in breast cancer
To date, evidence regarding the effect of
EA on breast cancer cells is still minimal, and only two
in vitro studies investigated this relationship [20, 21]. Jabeen et al. (2020) studied the effect of
EA on HER2-positive breast cancer cells at varying concentrations (25, 50, 75, 100, 150, and 200 μL/mL) for 48 hours [
21]. Several tests revealed that
EA inhibits cell proliferation dose-dependently, induces apoptosis by dysregulating cell-cycle progression, downgrades cell invasion and metastasis, suppresses colony formation, and induces morphological changes. An analysis of the underlying molecular pathways showed that
EA extract might exhibit its activity by blocking HER2 and JNK1/2/3 activities. This potentially leads to higher expression of the proteins E-cadherin and β-catenin and lower expression of the proteins vimentin and fascin. These effects of
EA are crucial in preventing the development and progression of cancer [
21].
Another study by Fouzat et al. (2021) showed a similar effect of
EA on triple-negative breast cancer cells [
20]. At the concentrations of 100 and 200 μL/mL,
EA was able to suppress cell proliferation and induce cell apoptosis by altering cell cycle regulation, upregulating Bax and cleaved caspase-8, and downregulating Bcl-2, which are pro- and anti-apoptotic markers, respectively. Similar to its effect on HER2-positive breast cancer cells, triple-negative breast cancer cells exposed to
EA significantly lost their colony-forming ability [
20].
Notably, both studies used a control cell line (Human normal mammary epithelial cells and non-tumorigenic epithelial cell line (MCF 10A)), showing that EA extract has a very minimal or no toxic effects to normal body cells [20, 21]. This enhances the strength of evidence obtained from these studies, with a need for additional evidence confirming these effects and exploring the possibility of becoming a promising approach to overcome therapy resistance.
Elaeagnus angustifolia effects in cervical cancer
Cervical cancer happens to females its is caused low immune response towards continuous infections of human-papillomavirus (HPV) in the cervix [
29]. A study by Erdogan et al. (2001) used silver nanoparticles loaded with the aqueous extract of
EA fruit and tested its effect on HELA human cervical cancer and PC3 human prostate cancer [
30]. Their results showed a dose-dependent cytotoxic effect on both cancer types. Morphological examinations also showed that silver nanoparticles exhibit apoptotic markers such as cell size reduction and bubbling in the plasma membrane. Although there is limited research on the effects of
EA on cervical cancer specifically, some studies suggest that the plant may have potential therapeutic applications in cervical cancer treatment [
30]. These findings suggest that
EA may have potential as a complementary therapy in treating cervical cancer. However, more research is needed to determine the plant extracts' optimal dosing and safety profiles and their efficacy in clinical trials.
Elaeagnus angustifolia effects in hepatocellular carcinoma
Hepatocellular Carcinoma (HCC) is a deadly type of liver cancer caused by inflammation and oxidative stress in the liver [31, 32]. It is responsible for severe fatality rates [
33]. Over half a million people die yearly from HCC [
34]. The stage of malignanancy determines HCC treatment in patients. Radical treatment can be introduced to treat patients with early stages of HCC [
35]. However, patients with late stages of HCC have limited treatment options [
36]. Given the antioxidant, anti-inflammatory and anti-mutagenic effects of
EA, a study was conducted by Amereh et al. (2017) to examine the chemo-preventive effects of aqueous
EA fruit extract against diethylnitrosamine (DEN)-induced HCC in male-Sprague rats
in vivo [
34].
EA was collected from the Ardabil province in Iran. Segments of the
EA fruit, including the flesh, peel and seed, were pulverized and then finely ground before it was combined with distilled water to create the aqueous extract. Oleaster-treated rats were given an oral pretreatment of
EA in escalating doses of (200,400,600 mg/kg body weight) two weeks before initiating HCC in rats until the trial ended. The results showed that liver damage biomarkers such as Alfa-fetoprotein (AFP), gamma-glutamyl transpeptidase (GGT), alanine transaminase (ALT), and aspartate transaminase (AST) were significantly lower in the
EA treated rats compared to the HCC rats especially when
EA were orally administered with a dose of 400 mg/kg [
34].
Additionally,
EA reduced lipid peroxidation in the liver tissue to prevent oxidative stress and raised Glutathione levels (GSH) in
EA treated rats, which is significant because GSH regulates carcinogenic mechanisms, the death of cancer cells, and the metabolism of free radicals and carcinogenic compounds. Moreover, rats treated with
EA before HCC initiation had less liver mass than HCC and DEN-induced rats. These outcomes conclude that the aqueous solution of the
EA plant could have potential chemo-prevention effects in patients [
34].
Effects of Elaeagnus angustifolia on oral cancer
To test the effects of
EA on oral cancer was experimented with by using two oral cancer cell lines called FaDu and SCC25
in vivo [
37]. The analysis showed that both FaDu and SCC25 cancer cells suppressed cell proliferation, induced apoptosis, inhibited colony formation and caused deregulation of cell cyle in G1/G2 phase and S phase.
EA prevented cell invasion by mesenchymal-to-epithelial (MET) activity suppression in the oral cells; these results were found using two methods, Matrigel and wound healing assays. In addition, to compare E-cadherin regulation in treated cell lines with
EA extract 100 ml/ml for 48 hours versus untreated controls using the western blot method showed E-cadherin overexpression. The phosphorylation of Erk1/Erk2 signalling pathways stopped with the presence of the
EA extract, which may be the cause of the upregulation of E-cadherin, the prevention of angiogenesis, and the morphological changes found in FaDu and SCC25 that were observed [
37]. This research confirms that
EA extract assists in the therapeutic prevention of oral cancer cells by reducing blood vessel development and tumour cell migration to arrest cell progression. There is limited research on the effects of
EA on oral cancer specifically, but some studies suggest that the plant may have potential therapeutic applications in treating oral cancer. It is important to note that these studies have been done
in vitro or animal models, and further clinical trials in humans are necessary to determine the effectiveness of
EA in oral cancer treatment.
Effects of Elaeagnus angustifolia on colorectal cancer
Colorectal cancer (CRC), the second deadliest cancer, takes place in the colon or rectum and is caused by the rapid increase of glandular epithelial cells in the colon [
38]. A study by Fouzat et al. (2021) looked at the effects of
EA on colorectal cancer (CRC) [
39]. Two CRC KRAS cell lines known as HCT-166 and LoVo were used
in vitro, and transgenic Drosophila melanogaster was used as a model
in vivo for the KRAS cell lines. Their
in vitro analysis showed that
EA flower extract stopped cell motility, invasion and colony formation. CRC cell invasion ihibition was caused by the suppression of Epithelial-mesenchymal (EMT) activity which induced the downregution of vimetin and increased E-cadherin. In contrast, the effects of
EA in vivo increased the chances of survival of KRAS mutations in the Drosophila melanogaster model. This study shows that
EA has chemo-preventative effects on CRC [
39].
The table below summarizes the different cell lines,
EA plant applications and the type of process discussed in this paper.
EA has pontinoal therapeutic applications in breast cancer, cervical cancer, hepatocellular carcinoma, oral cancer, and colorectal cancer. Further studies are needed to be done to explore the therapeutic applications of
EA.
Table 1.
Overview of different cell lines, EA extract, and processes for each study.
Table 1.
Overview of different cell lines, EA extract, and processes for each study.
| Cell line |
EA plant |
Processes performed |
Chemo-preventive |
References |
| Breast cancer |
EA plant extract |
In vitro |
Yes |
[20,21] |
| Cervical cancer |
EA fruit extract |
In vivo |
Yes |
[30] |
| Hepatocellular carcinoma |
EA fruit extract |
In vivo |
Yes |
[34] |
| Oral cancer |
EA flower extract |
In vivo |
Yes |
[37] |
| Colorectal cancer |
EA flower extract |
In vitro and In vivo
|
Yes |
[39] |
Molecular mechanisms underlying Elaeagnus angustifolia's anti-cancer effects
Although the exact molecular mechanisms are not fully elucidated, several proposed pathways contribute to its anti-cancer effects. The plant extracts have been shown to induce apoptosis in cancer cells, leading to their elimination and inhibition of tumour growth [20, 26, 40]. Additionally, EA extracts cause cell cycle arrest in certain cancer cell lines, preventing uncontrolled proliferation [21, 41]. The plant's significant antioxidant activity may also reduce oxidative stress, thus contributing to cancer prevention [2, 34]. Furthermore, EA may exhibit anti-angiogenic effects by inhibiting the formation of new blood vessels, which are crucial for tumour growth [4, 15, 37]. The plant's potential to modulate specific signalling pathways involved in cancer development and progression further supports its anti-cancer properties [21, 22]. Nevertheless, more research, including molecular studies and clinical trials, is required to comprehensively understand EA’s anti-cancer potential and its suitability for human cancer treatment.
The cellular and molecular interactions of Elaeagnus angustifolia with cancer cells have not been fully elucidated, and research in this area is still in its early stages. However, available studies and existing knowledge have identified several potential cellular and molecular interactions.
The plant's interactions with cancer cells include the modulation of various signalling pathways that are dysregulated in cancer. Notably,
EA has been shown to affect pathways such as PI3K/AKT [
42], MAPK/ERK [
42], and NF-κB [
43], which play critical roles in cancer development and progression [44-46].
In addition,
EA extracts have demonstrated anti-angiogenic effects by inhibiting the production of pro-angiogenic factors, such as VEGF [
37]. This action restricts the formation of new blood vessels that supply nutrients to tumours, thereby limiting tumour growth and potential metastasis.
Furthermore, the plant's potential impact on epigenetic changes is noteworthy [
19].
EA extracts may influence epigenetic modifications, such as DNA methylation and histone modifications, leading to changes in gene expression patterns that influence cancer cell behaviour [
19].
It is essential to consider that these cellular and molecular interactions of Elaeagnus angustifolia with cancer cells can vary depending on factors such as the type of cancer, cancer stage, dosage, and specific components of the plant extract used in the studies. As research progresses, a deeper understanding of the mechanisms underlying EA’s effects on cancer cells may lead to the development of novel and targeted therapies for cancer patients. Nevertheless, caution should be exercised in interpreting these findings until more comprehensive evidence from clinical trials becomes available.
Targeted pathways and potential therapeutic implications
EA has been reported to interact with various cellular pathways, many of which have implications for potential therapeutic applications.
Table 2 summarises targeted pathways and their potential therapeutic implications of
EA:
It is important to note that while the interactions of EA with these pathways show promise in preclinical studies, including in vitro and animal models, further research is needed to fully understand the therapeutic implications and potential clinical applications. Human clinical trials are necessary to validate these findings and determine the safety and efficacy of Elaeagnus angustifolia in targeting specific pathways for cancer treatment.
Clinical trials and case studies involving Elaeagnus angustifolia
Few studies investigated the potential use of
EA in cancer patients. One clinical trial aimed to assess the effects of
EA extract as an adjuvant therapy in women with breast cancer [
47]. Additionally, A recent trial reported positive effects of combining ginger and
EA. fruit extracts, with significant improvements in pain intensity and occurrence observed in patients taking 200 mg of the combined extracts for eight weeks [
15]. In some cases, healthcare professionals have reported using
EA in cancer patients as part of complementary or alternative treatments [
15]. These case studies provide preliminary insights into the potential effects and safety of the plant in real-world clinical settings.
Nevertheless, it is crucial to interpret the findings from these studies with caution, as they may have limitations such as small sample sizes, lack of control groups, and variations in dosage and treatment protocols.
Synergistic enhancement of cancer therapy using Elaeagnus angustifolia
The potential synergies of
EA with standard cancer treatments, such as chemotherapy and radiation, are an area of interest in cancer research. Synergy refers to the combined effects of two or more substances that result in a more significant therapeutic benefit than the sum of their individual effects [
48]. While more research is needed, there are some theoretical mechanisms by which
EA may enhance the efficacy of standard cancer treatments.
Firstly,
EA is known for its potent antioxidant properties [
4]. Chemotherapy and radiation treatments generate reactive oxygen species (ROS) [
49], which can cause damage to healthy cells. The antioxidant effects of
EA may help reduce oxidative stress and protect normal cells from damage, potentially leading to improved treatment tolerance [
4].
Secondly, some studies suggest that
EA extracts can modulate the immune system, enhancing the body's ability to recognize and target cancer cells [
24]. This immune-modulating effect may complement standard cancer treatments, such as immunotherapy, by boosting the immune response against cancer cells [
28].
Moreover, EA has been reported to exhibit anti-angiogenic properties, inhibiting the formation of new blood vessels that supply nutrients to tumours [2, 37]. Combining this effect with chemotherapy and radiation may help restrict tumour growth and reduce the potential for metastasis.
Furthermore, preclinical studies have suggested that certain natural compounds, including some found in
EA, can sensitize cancer cells to the effects of chemotherapy [50-52]. Similarly,
EA extracts may potentially sensitize cancer cells to the effects of radiation therapy [
47]. Combined with radiation therapy, this could improve cancer cell killing and better treatment outcomes. Despite these promising mechanisms, it is crucial to acknowledge that the potential synergies of
EA with standard cancer treatments are still largely theoretical. Clinical trials are necessary to validate these findings and determine the safety and efficacy of combining
EA with chemotherapy or radiation therapy in human cancer patients.
The implications of combining EA with standard cancer treatments or other therapeutic agents are promising. It may enhance therapeutic effects, reduce treatment resistance with single agents, and potentially result in synergistic effects for better treatment outcomes. The plant's reported hepatoprotective and anti-inflammatory properties may contribute to better treatment tolerability and reduced side effects of other treatments.
However, individual patient responses and potential interactions need to be carefully monitored and considered to ensure safety and efficacy.
Assessment of the safety profile of Elaeagnus angustifolia
The experimental evidence from animal studies suggests that EA has a decent safety profile, with no significant adverse effects reported [2, 53, 54]. However, it is essential to interpret these findings cautiously, as preclinical studies may not always accurately predict human safety and efficacy. The safety profile of EA in cancer patients is not fully established due to limited clinical data.
Some aspects of its safety profile explored in preclinical and limited clinical studies include general safety, mild adverse effects like gastrointestinal discomfort and allergic reactions in plant allergy patients, and potential drug interactions. Long-term safety data on cancer patients are also lacking. Considering the limited evidence, cancer patients should cautiously approach Elaeagnus angustifolia or other complementary or alternative treatments. Patients must discuss these treatments with their healthcare providers to assess potential risks and benefits and to ensure a comprehensive and informed approach to cancer management.
Challenges and Future Perspectives
One of the primary limitations of using EA in cancer treatment is the lack of extensive clinical data. While preclinical studies show promise, clinical trials in humans are essential to establish its safety, efficacy, and optimal dosing in cancer patients. There is a need for standardized preparations of EA extracts to ensure consistency in the composition and concentration of bioactive compounds. Standardization would help improve the reproducibility and comparability of research findings.
The combined usage of EA with conventional cancer treatments and other medications needs further investigation. Drug interactions could impact treatment outcomes and patient safety, necessitating careful monitoring and consideration. While EA is generally considered safe, its side effect profile in cancer patients must be better understood, especially when combined with standard therapies. Systematic monitoring of adverse events is critical to ensure patient safety.
Identifying the most effective and safe combinations of EA with standard cancer treatments remains challenging. Determining which types and stages of cancer may benefit the most from combination therapies is an area that requires exploration.
Further research is needed to uncover the specific cellular and molecular mechanisms underlying its anti-cancer effects, which can provide valuable insights into potential therapeutic targets. Identifying biomarkers to predict patient response could help personalize cancer care and optimize treatment strategies. Investigating the synergistic effects of EA with standard cancer treatments like chemotherapy, radiation, and immunotherapy may offer new opportunities for improving cancer management.
Conclusion
EA has shown promising therapeutic applications in cancer treatment based on preclinical studies, including in vitro and animal models. The plant extracts have demonstrated various anti-cancer properties, such as inducing apoptosis, cell cycle arrest, and anti-angiogenic effects, suggesting potential benefits in cancer therapy. Additionally, EA has been reported to interact with critical cellular pathways involved in cancer development and progression, including the PI3K/AKT, MAPK/ERK, and NF-κB pathways. These molecular interactions and the plant's immunomodulatory, anti-inflammatory, and antioxidant properties hold promise for enhancing the body's natural defence mechanisms against cancer. While these reasons provide a compelling rationale for exploring EA's potential in cancer treatment, it is essential to emphasize that further research, including well-designed clinical trials in human cancer patients, is essential to validate its safety and efficacy. A rigorous scientific investigation is needed to fully understand its mechanisms of action, optimal dosage, potential side effects, and how it can be effectively integrated into cancer management strategies. Until more evidence becomes available, cautious consideration and collaboration with healthcare professionals are crucial when exploring the use of EA in cancer care.
Authorship contribution statement
Conceptualization, Z.Z. and M.A.; writing – original draft, Y.A., N.A. and S.S.; writing – review & editing, Z. Z.. H.B and M.A.; Supervision, Z.Z and M.A.; funding acquisition, M.A. All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
List of abbreviations
EA, HCC, CRC, EMT, DEN, MET, HPV
Funding
This work was funded by grants from Qatar University (QUCP-CHS-2022-483).
References
- Nirmala, C., et al., Promising underutilized wild plants of cold desert Ladakh, India for nutritional security and health benefits. Applied Food Research, 2022. 2(2): p. 100145.
- Tehranizadeh, Z.A., A. Baratian, and H. Hosseinzadeh, Russian olive (Elaeagnus angustifolia) as a herbal healer. BioImpacts: BI, 2016. 6(3): p. 155.
- Amiri Tehranizadeh, Z., A. Baratian, and H. Hosseinzadeh, Russian olive (Elaeagnus angustifolia) as a herbal healer. Bioimpacts, 2016. 6(3): p. 155-167.
- Hamidpour, R., et al., Russian olive (Elaeagnus angustifolia L.): From a variety of traditional medicinal applications to its novel roles as active antioxidant, anti-inflammatory, anti-mutagenic and analgesic agent. Journal of traditional and complementary medicine, 2017. 7(1): p. 24-29.
- Sastre, J., et al., Allergenicity and cross-reactivity of Russian olive pollen (Eleagnus angustifolia). Allergy, 2004. 59(11): p. 1181-1186.
- Saboonchian, F., R. Jamei, and S.H. Sarghein, Phenolic and flavonoid content of Elaeagnus angustifolia L.(leaf and flower). Avicenna journal of phytomedicine, 2014. 4(4): p. 231.
- Cansev, A., et al., Chemical properties and antioxidant capacity of Elaeagnus angustifolia L. fruits. Asian Journal of Chemistry, 2011. 23(6): p. 2661-2665.
- Obón, C., et al., Beverage and culture.“Zhourat”, a multivariate analysis of the globalization of a herbal tea from the Middle East. Appetite, 2014. 79: p. 1-10.
- Çakmakçı, S., et al., Antioxidant capacity and functionality of oleaster (E laeagnus angustifolia L.) flour and crust in a new kind of fruity ice cream. International Journal of Food Science & Technology, 2015. 50(2): p. 472-481.
- Erdemoglu, N., E. Küpeli, and E. Yeşilada, Anti-inflammatory and antinociceptive activity assessment of plants used as remedy in Turkish folk medicine. J Ethnopharmacol, 2003. 89(1): p. 123-9.
- Wallace, T.C., et al., Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit Rev Food Sci Nutr, 2020. 60(13): p. 2174-2211.
- Kohlmeier, L., et al., Lycopene and myocardial infarction risk in the EURAMIC Study. American Journal of Epidemiology, 1997. 146(8): p. 618-626.
- Gürbüz, I., et al., Anti-ulcerogenic activity of some plants used as folk remedy in Turkey. Journal of ethnopharmacology, 2003. 88(1): p. 93-97.
- Hosseinzadeh, H., M. Ramezani, and N. Namjo, Muscle relaxant activity of Elaeagnus angustifolia L. fruit seeds in mice. Journal of ethnopharmacology, 2003. 84(2-3): p. 275-278.
- Hamidpour, R., et al., Russian olive (Elaeagnus angustifolia L.): From a variety of traditional medicinal applications to its novel roles as active antioxidant, anti-inflammatory, anti-mutagenic and analgesic agent. J Tradit Complement Med, 2017. 7(1): p. 24-29.
- Natanzi, M.M., et al., Effect of aqueous extract of Elaeagnus angustifolia fruit on experimental cutaneous wound healing in rats. Acta Medica Iranica, 2012: p. 589-596.
- Farzaei, M.H., et al., A comprehensive review on phytochemical and pharmacological aspects of Elaeagnus angustifolia L. Journal of Pharmacy and Pharmacology, 2015. 67(11): p. 1467-1480.
- Khan, S.U., et al., Antimicrobial potentials of medicinal plant’s extract and their derived silver nanoparticles: A focus on honey bee pathogen. Saudi journal of biological sciences, 2019. 26(7): p. 1815-1834.
- Ghanghareh, M. and M. Zare, Cytotoxic Effects of Hydro-Alcoholic Extract of the Leaf of Elaeagnus angustifolia in Hepatocellular Carcinoma Cell Line (HepG2). Jentashapir Journal of Cellular and Molecular Biology, 2020. 11(3).
- Fouzat, A., et al., Elaeagnus angustifolia Plant Extract Induces Apoptosis via P53 and Signal Transducer and Activator of Transcription 3 Signaling Pathways in Triple-Negative Breast Cancer Cells. Front Nutr, 2022. 9: p. 871667.
- Jabeen, A., et al., Elaeagnus angustifolia plant extract inhibits epithelial-mesenchymal transition and induces apoptosis via HER2 inactivation and JNK pathway in HER2-positive breast cancer cells. Molecules, 2020. 25(18): p. 4240.
- Alharbi, K.S., et al., Role of Medicinal plant-derived Nutraceuticals as a potential target for the treatment of breast cancer. Journal of Food Biochemistry, 2022. 46(12): p. e14387.
- Forouzanfar, F. and H. Hosseinzadeh, Medicinal herbs in the treatment of neuropathic pain: a review. Iranian journal of basic medical sciences, 2018. 21(4): p. 347.
- Gupta, M., et al., Herbal bioactives in treatment of inflammation: An overview. South African Journal of Botany, 2021. 143: p. 205-225.
- Kaur, B. and P. Singh, Inflammation: Biochemistry, cellular targets, anti-inflammatory agents and challenges with special emphasis on cyclooxygenase-2. Bioorganic Chemistry, 2022. 121: p. 105663.
- Amirghofran, Z., Medicinal plants as immunosuppressive agents in traditional Iranian medicine. Iran J Immunol, 2010. 7(2): p. 65-73.
- Zhao, Y., et al., Natural polysaccharides with immunomodulatory activities. Mini reviews in medicinal chemistry, 2020. 20(2): p. 96-106.
- Sun, N.-x., et al., Immunological activities of polysaccharide extracted from Elaeagnus angustifolia L. CyTA-Journal of Food, 2018. 16(1): p. 995-1002.
- Castle, P.E., M.H. Einstein, and V.V. Sahasrabuddhe, Cervical cancer prevention and control in women living with human immunodeficiency virus. CA Cancer J Clin, 2021. 71(6): p. 505-526.
- ERDOĞAN, Ö., P. Salih, and O. Cevik, Green Synthesis and Characterization of Anticancer Effected Silver Nanoparticles with Silverberry (Elaeagnus angustifolia) Fruit Aqueous Extract. International Journal of Pure and Applied Sciences, 2021. 7(3): p. 391-400.
- Buendia, M.A. and C. Neuveut, Hepatocellular carcinoma. Cold Spring Harb Perspect Med, 2015. 5(2): p. a021444.
- Llovet, J.M., et al., Hepatocellular carcinoma. Nat Rev Dis Primers, 2021. 7(1): p. 6.
- El Dika, I., I. Makki, and G.K. Abou-Alfa, Hepatocellular carcinoma, novel therapies on the horizon. Chinese clinical oncology, 2021. 10(1): p. 12.
- Amereh, Z., et al., Cancer chemoprevention by oleaster (Elaeagnus angustifoli L.) fruit extract in a model of hepatocellular carcinoma induced by diethylnitrosamine in rats. Excli j, 2017. 16: p. 1046-1056.
- Luo, P., et al., A Large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma. Hepatology, 2018. 67(2): p. 662-67.
- Sim, H.-W. and J. Knox, Hepatocellular carcinoma in the era of immunotherapy. Current Problems in Cancer, 2018. 42(1): p. 40-48.
- Saleh, A.I., et al., Elaeagnus angustifolia Plant Extract Inhibits Angiogenesis and Downgrades Cell Invasion of Human Oral Cancer Cells via Erk1/Erk2 Inactivation. Nutr Cancer, 2018. 70(2): p. 297-305.
- Hossain, M.S., et al., Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers (Basel), 2022. 14(7).
- Fouzat, A., Elaeagnus Angustifolia extract inhabits cell invasion of human colorectal cancer cells and increases the survival rate of the Drosophila colon cancer model. 2021. 103-103.
- Patel, S., Plant genus Elaeagnus: underutilized lycopene and linoleic acid reserve with permaculture potential. Fruits, 2015. 70(4): p. 191-199.
- Arab, S., et al., Effects of Whole Fruit Extract of Elaeagnus angustifolia L. on Glioblastoma Cell Lines. Journal of microbiology, biotechnology and food sciences, 2022. 11(5): p. e4314-e4314.
- Zhang, H.-W., et al., Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Scientific Reports, 2018. 8(1): p. 11255.
- Mamashli, M., et al., Anti-inflammatory effects of N-Acetylcysteine and Elaeagnus angustifolia extract on acute lung injury induced by λ-carrageenan in rat. Inflammopharmacology, 2022. 30(5): p. 1759-1768.
- Bai, D., L. Ueno, and P.K. Vogt, Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int J Cancer, 2009. 125(12): p. 2863-70.
- He, Y., et al., Targeting PI3K/Akt signal transduction for cancer therapy. Signal transduction and targeted therapy, 2021. 6(1): p. 425.
- Han, S.-S., et al., NF-κB/STAT3/PI3K signaling crosstalk in iMycEμ B lymphoma. Molecular Cancer, 2010. 9(1): p. 97.
- Salamzadeh, J., et al., The effect of elaeagnus angustifolia L. Cream on radiation-induced skin reactions in women with breast cancer; A preliminary clinical trial. Iranian Journal of Pharmaceutical Sciences, 2017: p. 25-36.
- Pezzani, R., et al., Synergistic Effects of Plant Derivatives and Conventional Chemotherapeutic Agents: An Update on the Cancer Perspective. Medicina (Kaunas), 2019. 55(4).
- Yang, H., et al., The role of cellular reactive oxygen species in cancer chemotherapy. J Exp Clin Cancer Res, 2018. 37(1): p. 266.
- de Oliveira Júnior, R.G., et al., Sensitization of tumor cells to chemotherapy by natural products: A systematic review of preclinical data and molecular mechanisms. Fitoterapia, 2018. 129: p. 383-400.
- Ji, A. and J. Xu, Neuropathic pain: biomolecular intervention and imaging via targeting microglia activation. Biomolecules, 2021. 11(9): p. 1343.
- Carradori, S., et al., Phytocomplex characterization and biological evaluation of powdered fruits and leaves from Elaeagnus angustifolia. Molecules, 2020. 25(9): p. 2021.
- Panahi, Y., et al., Efficacy of Elaeagnus Angustifolia extract in the treatment of knee osteoarthritis: a randomized controlled trial. Excli j, 2016. 15: p. 203-10.
- Mahboubi, M., Elaeagnus angustifolia and its therapeutic applications in osteoarthritis. Industrial crops and products, 2018. 121: p. 36-45.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).