1. Introduction
The prevalence and outbreak of infectious diseases is always an important issue affecting the national economy and people’s livelihood [
1]. Researches on the transmission mechanism and dynamics of infectious diseases have attracted the attention of many scholars. Specifically, mathematical models have been a potent tool for forecasting the trajectory of infectious diseases and evaluating different prevention and control measures [
2,
3]. Through the application of optimal control theory and qualitative assessment of mathematical models, the effective management, reduction, and potential eradication of an infectious disease can be achieved. [
4,
5,
6,
7,
8]. Based on different mechanisms of disease transmission, Kermack and McKendrick introduced the SIS and SIR epidemic models [
9,
10,
11]. Subsequent to their work, a variety of epidemic models have emerged to shed light on the progression of various diseases and to offer insightful control measures.
Any research progress on the dynamics and control of epidemic transmission may have a significant impact on the prevention and control of infectious diseases, which has attracted widespread attention from scientific research [
12,
13]. During the transmission of infectious diseases, the infected transmission mainly occurs through the contact between susceptible and infected individuals, then quarantine has become a commonly employed strategy for controlling disease. For example, Hethcote et al. studied SIQS and SIQR epidemiology models with different incidence [
14]. The Refs. [
15,
16,
17] have also conducted qualitative analysis of mathematical models with infected isolation on the assumption of uniformly mixing population. Compared with the classics propagation models, heterogeneous networks own a better reflection with the statistical properties of real social networks. Li et al. introduced an SIQRS epidemic model based on scale-free networks, and their theoretical results indicate that the epidemic threshold is closely linked to the network’s structural characteristics [
18]. Kang et al. explored how the quarantine based on node degree, as well as constant rates of birth and death, affect disease dynamics on scale-free networks by utilizing a novel deterministic model [
19]. Chen et al. formulated SVIQR and SVIQS models with heterogeneous quarantine rate and immunity rate to investigate the effects of intervention methods on the behavior of infectious diseases within heterogeneous networks [
12]. Cheng et al. considered a network-based SIQS model with non-monotone incidence rate and studied the global stability of equilibria [
20]. Zhao et al. developed a stochastic SIQS epidemic model on scale-free networks, taking into account random fluctuations in infection rates [
21]. However, those models either ignored the demographics or assumed that the recruitment of population is constant.
Consistently implementing control measures can effectively reduce the scale of infection, however, if considering the economic factors, relying solely on continuous control measures may not be sufficient to achieve optimal control goals when the disease prevalence is substantial. Therefore, A viable approach is to employ time-varying control within certain bounds to strike a balance between control objectives and the associated costs via optimal control theory. Optimal control applications in epidemic dynamics primarily concentrated on homogeneous contact networks [
22,
23,
24,
25,
26,
27]. Recently, many intriguing works on the optimal control of epidemics on heterogeneous networks have emerged [
18,
28,
29,
30,
31,
32,
33,
34,
35]. Li et al. presented a nonlinear SIQS epidemic model on networks and explored the issue of optimal quarantine control to minimize the cost of control measures [
32]. Zhang et al. examined the optimal control of an SIQRS model that includes vaccination on a network, and studied the effects of different control strategies [
34]. Yang et al. investigated the stability and optimal control on treatment for SIS epidemic systems combining in directed networks [
35]. However, the above results only considered the optimal control strategy for isolation and did not compare it with other forms of heterogeneous isolation strategies.
The above works provide strong theoretical supports for the prevention of infectious diseases through isolation measures. However, appropriate vaccination for susceptible individuals is also crucial for preventing and controlling infection. On the basis of above consideration, we study an SIS epidemic model with heterogeneous immune for susceptible individuals and quarantine for infected one, which also consider the demographics of individuals on heterogeneous networks. The organization of this paper is as follows. In Sect.2, we introduce an SIQS model with heterogeneous control strategies
1 of individuals on complex networks and provide some description and assumptions. In Sect.3, we obtain the equilibria and basic reproduction number. The main results are showed in Sect.4, we establish uniform persistence and analyze the global stability of the disease-free and endemic equilibria of system by different mathematical methods. The optimal time-varying quarantine control for the model is considered in Sect.5. In Sect.6, some numerical simulations are conducted to illustrate and supplement the analysis results. Finally, a brief conclusion is given in Sect.7.
2. Description and formation of epidemic models
Consider a population situated on a complex network
N, in which each node of
N represents either an individual or an unoccupied state. Every node has three optional states: susceptible state(S), quarantined state(Q), infected state(I) [
21]. We divided the population into
n groups, let
,
,
denote the densities of susceptible, infected and quarantine nodes (individuals) with connectivity (degree)
k at time
t, respectively. The mechanism of our model mainly involves three factors. The schematic diagram of the model is shown in
Figure 1, the meaning of parameters is listed in
Table 1.
(1) Birth and Death: Each vacant node i in the network randomly selects a neighbor. If the neighbor is a vacant node, the state of i remains unchanged. If the neighbor is a non-vacant node, the vacant node i will be activated to generate a new susceptible nodes at the birth rate b. All non-vacant nodes become vacant nodes at a natural death rate d per unit time. We assume that each non-vacant node has the same birth contact ability A (where ) due to physiological constraints.
(2) Immunization and Quarantine (): At each time step, susceptible individuals with degree k are immunized with the immune rate . The infected nodes with degree k will be quarantined with rate . The quarantined individuals will recover to susceptible node with rate . Nodes with the same degree have identical quarantine (immunization) strategies, while those with different degrees have different strategies.
(3) SIS epidemic framework:
Infection : At the initial moment, randomly select some nodes as infected nodes. At each time step, the possibility that each infected node
i connect to its neighboring nodes is
, where
represents the infectivity of infected nodes with degree
k, and
[
36,
37,
38],
=
A [
39],
=
[
40],
=
[
41]. If an infected node
i interacts with a susceptible node
j along a connecting edge, node
j has a possibility of being infected by
i at a transmission rate
. For the node with degree
k, the overall transmission rate is
.
Recovery : Each infected node reverts to being susceptible at recovery rate .
According to above assumption, the model is as follows,
with initial conditions
, where
stands for the probability that the infected contact through a link which originates from a node with degree
k and points to the infected node. The
denotes the probability that a node of degree
k has a reproductive contact with an individual neighbor, the
denotes the probability that an individual neighbor of vacant node with degree
i. On uncorrelated networks, the likelihood is deemed to be independent of the connectedness of the originating node of the link. Thus, we note
,
. The average density of susceptible, infected and quarantined nodes is
,
,
.
Let
denotes the density of individuals of degree
k at time
t, and
We develop the equation for
as,
According to Ref. [
42], we get
when
, no other dynamic patterns are present; when
,
, where
,
.
Given that the original system and the limiting system exhibit identical long-term dynamic characteristics. In order to better analyze the stability of the model, we can only consider the limit system of (
1) under the condition of
and
based on the analysis of
. The corresponding limiting systems of model (
1) are formulated as follows:
with initial conditions
.
The following proposition shows that the state space of the solutions of system (
3) is positively invariant.
Proposition 1. If represents a solution to the system (3) that meets the initial conditions, then , for any , i.e., Γ is positively invariant.
Proof. ∀
. Thus,
, we have
.
We obtain that
. By
and system (
2), the evolution of
is derived by the following equation:
We have
. Then, from system (
2), we also acquire
Obviously,
, and
Then, we verify
for any
. If not, because
, there is exist a
and
satisfy
It is evident that
for any
,
, we have
Because of
, we obtain
. On the other hand, for any
,
, we attain
That is to say
. When
,
Then, we acquire
. It is contradictory, hence,
for any
k and
. We also can get
, and
. That completes the proof. □
5. The Optimal Control for SIQS Model
In this section, we study the optimal control for system (
1). To meet the control objective and minimize control expenses at the same time, a viable approach to target epidemic outbreaks is by implementing time-varying control using optimal control theory for various infectious diseases, for example, the results in Culshaw et al. [
44], Chen et al. [
45] and Abboubakar et al. [
46]. The aim of this section is to identify the optimal quarantine measure for controlling the transmission of diseases.
First, we introduce an time-varying control rate
represents the percentage of infected individuals with degree
k being quarantined. Hence, system (
3) can be written as
We note the quarantine function
are bounded, Lebesgue integrable functions. Since our aim is to reduce both the scale of infection and control strengths, we consider the following objective functional
associated with model in the control set
,
denotes a positive weight parameter. The objective (cost) functional given by
The Lagrange function for optimal control problems (
18) and (
19) is defined as
In order to solve the optimal control problem, the existence of an optimal control must be assured. We firstly establish the existence of a solution for the control system (
18) by applying the method in Refs. [
45,
46].
Theorem 5. For the objective functional associated with model (18) defined in Ω, there exists an optimal quarantine strategy minimizing .
Proof. (i) Clearly, for any
, the
is an nonempty set of Lebesgue integrable functions. (ii) It is obviously that the solutions are bounded ensuring the boundedness and convexity of admissible control set. (iii) Model (
18) can be written as
where
is the right side of system (
18), and
,
. It follows that
For any
, since
and the boundedness of solution, there exists a constant
satisfied that
. Then, There exists a positive constant
M to guarantee the inequality
is satisfied. (iv) Let
and
, we get
. (v) There exist
,
,
such that
.
By utilizing the results of Refs. [
45,
46], we get that there exists a optimal control
minimizing
. The proof is completed. □
The solution to the optimal control problem will be determined through Pontryagin’s minimum principle [
47]. We note the Hamiltonian
H as
If
is an optimal solution of the optimal control problem, then there exists a non-trivial vector function
,
satisfying the following equalities:
It follows from the derivation above
Now the necessary conditions are implemented on the Hamiltonian
H in (
21) and we obtain the following results.
Theorem 6.
For a given optimal control solution and the corresponding system solution , , there is an adjacency function that satisfies the following conditions
with conditions ===0. Furthermore, the optimal control is given by
Proof. We apply for the necessary conditions in the Pontryagin maximum principle with Hamiltonian function (
21) to derive the adjoint variables is determined by the following equations.
By the optimal conditions, we have
It follows that
. Using the property of the control space, we obtain
Then, we have the optimal control
in compact notation,
That completes the proof. □
6. Simulations
In this section, we conducted some numerical simulations to validate and supplement our theoretical results, and to study the impact of parameters on the dynamics of disease, in order to find better control strategies. The numerical simulations include three parts, the first part is to verify the main theoretical results including the epidemic threshold and global stability. We will discuss and make some simulations to show the effectiveness of different immunity and quarantine strategies in second part. The numerical simulation in the third part demonstrates the effectiveness of the optimal control method that has been proved in
Section 5. We constructed a BA (Barab
si-Albert) scale-free network [
48], which satisfies a power-law degree distribution
, the network size
N is set as 1000,
=80. This network evolved from the initial network with size of
and during each time step, new nodes with
edges are added to the network. Without loss of generality, we note
.
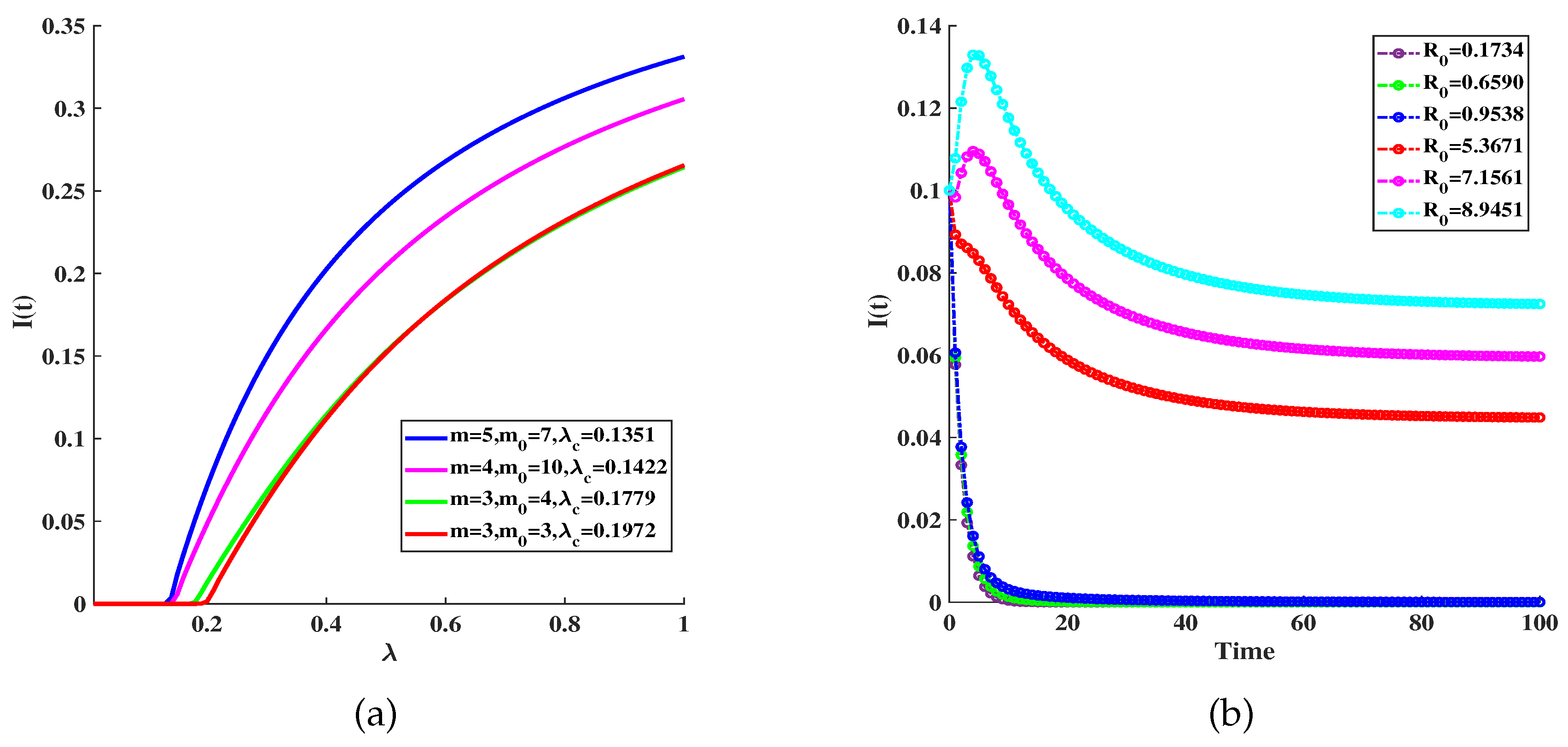
(I) Firstly, we perform some simulations in
Figure 2 to verify the important results of global stability of equilibria. We denote
Figure 2(a) shows the relationship between
and infectious rate
on some different networks. Based on the basic reproduction number
, we can acquire the epidemic threshold of the disease is
, where
,
,
,
,
,
. One can see that the epidemic threshold is consistent with our theoretical results very well.
Figure 2(b) depicts that the disease-free equilibrium cannot undergo a Hopf bifurcation, i.e., if
, the disease free equilibrium is global stability, and if
, then the endemic equilibrium is persist and global stability, where
,
,
,
,
,
=0.01, 0.038, 0.055, 0.21, 0.28, 0.35 and
=0.36, 0.36, 0.36, 0.18, 0.18, 0.18.
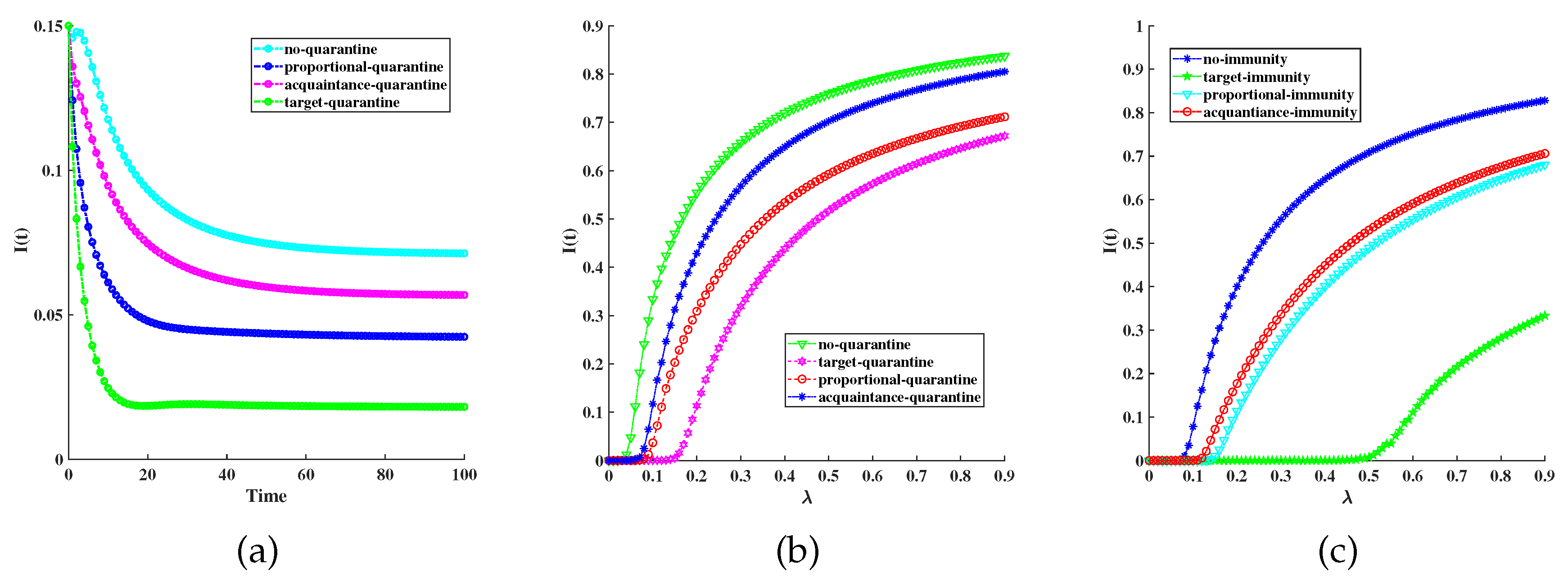
(II) Secondly, we depicts the effectiveness of the immune and quarantining control strategies in
Figure 3 to
Figure 4. In this part, we also discuss the SIQS model (
1) on the network with various immunization (proportional immune strategy
, target immune strategy
, acquaintance immune strategy
) schemes based on [
49] and define quarantine schemes of infected individual (proportional quarantine strategy
, target quarantine strategy
, acquaintance quarantining strategy
) according to various immunization schemes. Then, we define the heterogeneous quarantine rate
as follows,
Proportional quarantine strategy: Denote the average quarantine rate of proportional quarantine , . Randomly selecting one infected node for isolation, the quarantine rate is independent of the degree of node, which is also a situation discussed in many papers.
Target quarantine strategy: we can devise a quarantine strategy for the infected nodes according to the definition of immunization [
49]. Introduce an upper threshold
, such that all infected nodes with connectivity
are prioritize quarantined, i.e., we defined the quarantine rate
by
where
, and
, where
is the average quarantine rate of target quarantine.
Acquaintance quarantining strategy: Select a random portion p from the N nodes. The likelihood of quarantining a infected node with degree k is given by , therefore . We note that denote the average quarantine rate of acquaintance quarantine, where .
Figure 3(a) shows the
under different quarantining control strategies, the target quarantine strategy is the most effective in controlling disease.
Figure 3(b) compares the effectiveness of different quarantine strategies. We get that the target quarantine strategy has better effectiveness than others.
Figure 3(c) shows the effectiveness of different immunity strategies, it shows that all three immunization schemes are effective compared to the case without any immunization; the targeted immunization scheme is more efficient than the proportional scheme discussed.
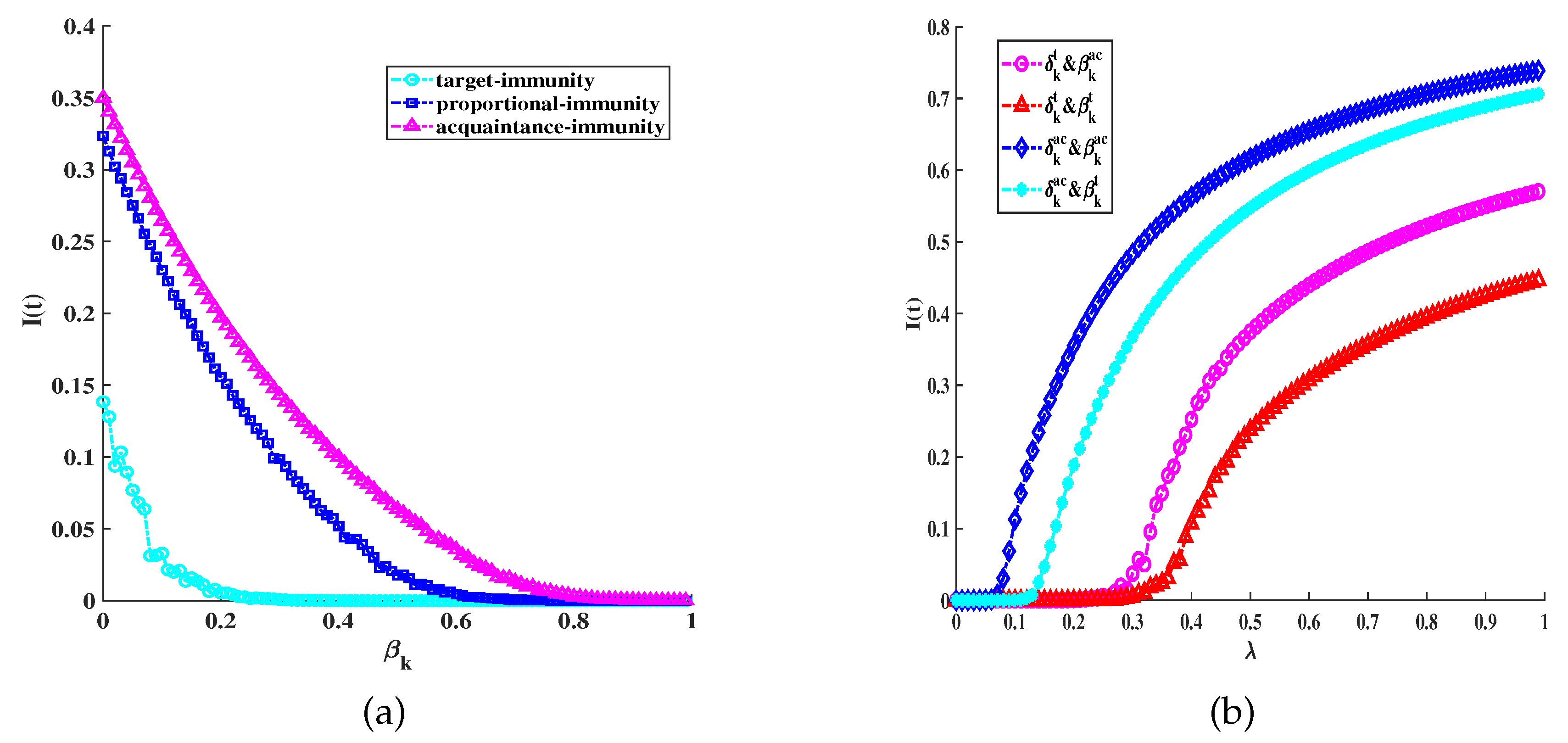
Figure 4(a) depicts the average infectious density with respect to quarantine rate under different immunity strategies. It also shows that the target strategy is better than proportional and acquaintance. We further show the average infectious density with respect to infected rate for the immunization schemes and quarantine strategies targeted, and acquaintance in
Figure 4(b). We get that the targeted immunity and the target quarantine strategy have better effectiveness than others.
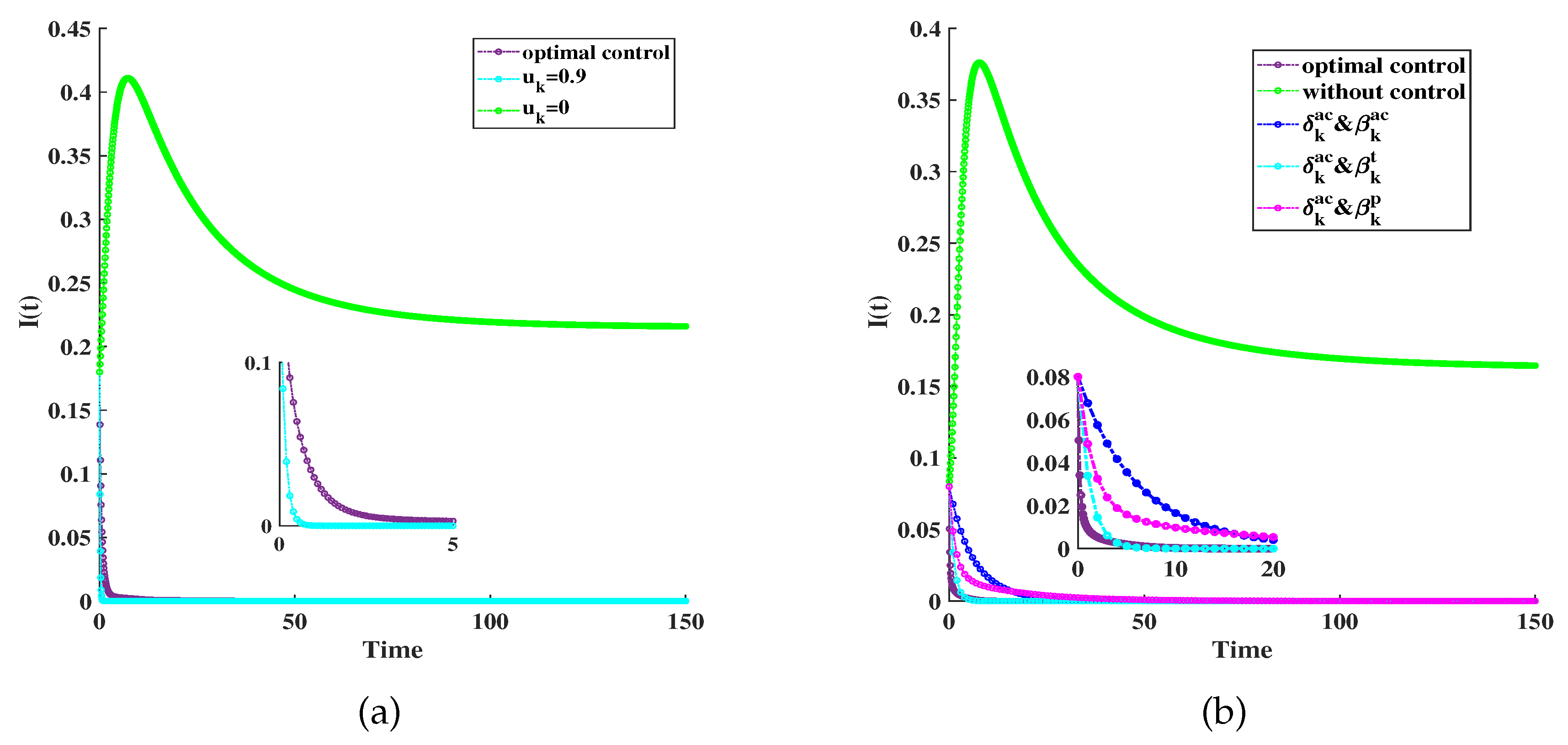
(III) Thirdly, we analyze and illustrate the optimal control and various quarantine schemes by numerical simulations in
Figure 5 to
Figure 6. In
Figure 5, we depict
under different control strategies. In order to provide a clearer representation of the findings in
Figure 5(a), we additionally computed the objective function
values for diverse control approaches, as shown in
Table 2. In the absence of any control strategy (i.e.,
), the infection ultimately breaks out and reaches a stable level of infection. We also set a fixed control strategy compared with the optimal control strategy, which can find that if we want to obtain better results than the optimal control strategy, it will usually lead to a significant increase in control costs (i.e.,
). In
Figure 5(b), we demonstrate the comparative effects of optimal control, without control, and acquaintance immunity combining with multiple quarantine strategies on infectious disease control. Compared with other strategies, the optimal control strategy has the best control effect.
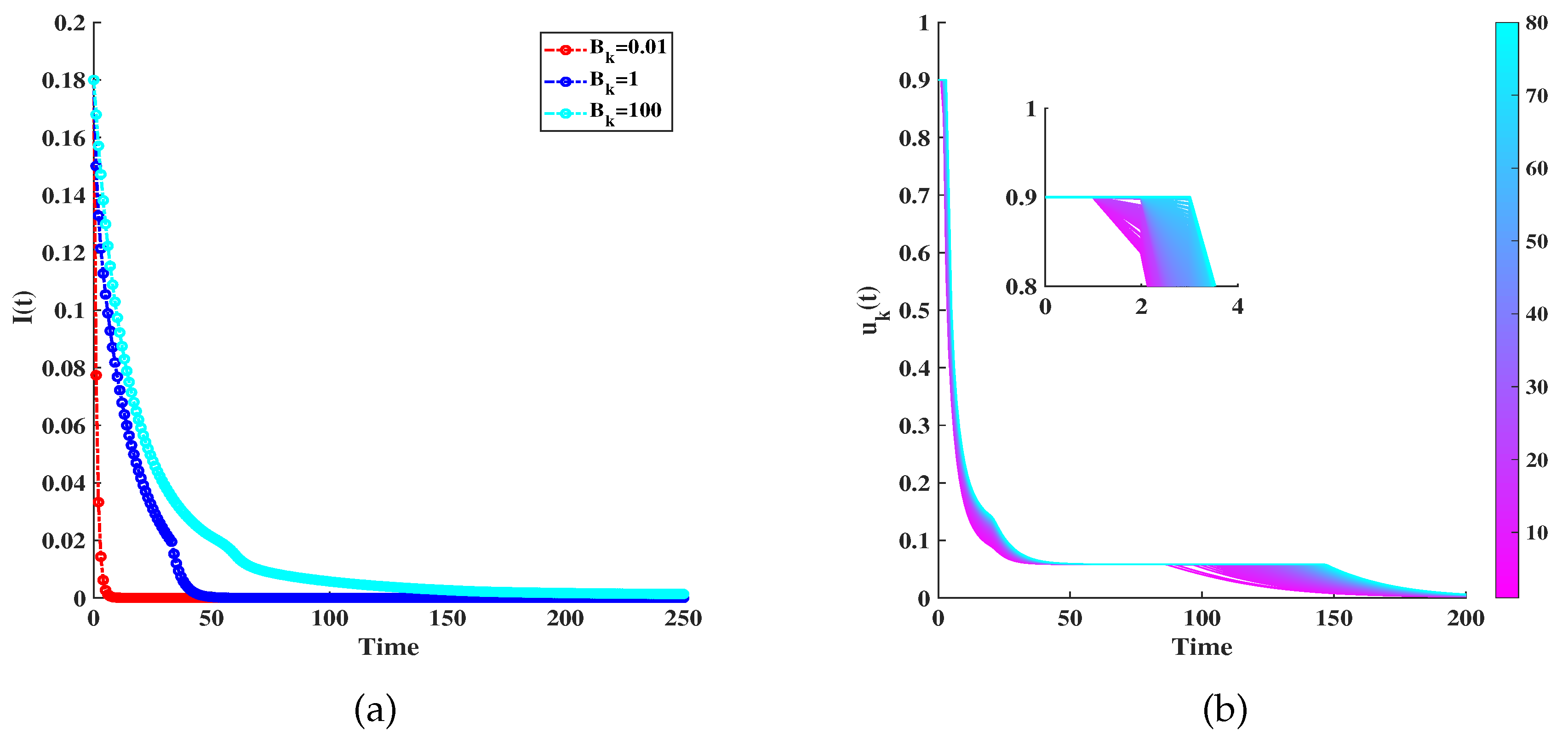
Figure 6(a) illustrates the
under optimal control under three cases: Low-cost at
, moderate-cost at
, and high-cost at
. Even under high costs, as seen in the third scenario, the scale of infection can be notably curtailed through optimal control.
Figure 6(b) displays the fluctuation of
across varying degrees under optimal regulation. It is evident that
tends to escalate as the degree rises. This indicates a higher level of infection, resulting in a higher value of
.
7. Conclusion
We propose an SIQS epidemic model with heterogeneous immune and quarantine measures on heterogeneous networks. We have performed a detailed mathematical analysis of our system, which deduces that the basic reproduction number
, is collectively determined by the infection rate and the effectiveness of disease control measures. Specifically, when
, the disease-free equilibrium
exhibits global asymptotic stability. When
, the endemic equilibrium
demonstrates both local and global asymptotic stability. At the same time, we conducted an in-depth theoretical examination on system (
1) to tackle the optimal quarantine control issue, confirming the presence of optimal solutions. We define different forms of quarantine measures and numerically simulated the effectiveness of a combination of different quarantine strategies and immune schemes in controlling diseases. Our findings indicate that the implementation of any control strategy can have a favorable impact on the magnitude of an epidemic outbreak, with the optimal control being particularly effective. Among them, the effect of target quarantine scheme can be comparable to the effect of optimal control. However, target isolation requires knowing the degree of all nodes in the network, which is a significant cost and workload.
A variety of control strategies, such as quarantine and immune schemes, can be employed for the effective management, mitigation, and potential eradication of infectious diseases. We expect that this paper can contributes to a understanding of the dynamics of infectious disease with the control measures. However, our paper is not based on a specific infectious disease, so there are still certain limitations in providing a specific basis for public health prevention measures. Studying the control measures for a specific disease based on infectious disease data will be a very meaningful work.
Figure 1.
Flow chart of the SIQS model. Here S, I, Q represent susceptible, infected and quarantined state.
Figure 1.
Flow chart of the SIQS model. Here S, I, Q represent susceptible, infected and quarantined state.
Figure 2.
(a) The with respect to and the structure of network with different parameters m and . denotes the theoretical epidemic threshold. Other parameter settings are as follows: , , , , ; (b) The average infected density under different parameters: 0.01,0.038,0.055,0.21,0.28,0.3, =0.36,0.36,0.36,0.18,0.18,0.18, , , , , , which are corresponding to =0.1734, 0.6590, 0.9538<1; =5.3671,7.1561,8.9451> 1.
Figure 2.
(a) The with respect to and the structure of network with different parameters m and . denotes the theoretical epidemic threshold. Other parameter settings are as follows: , , , , ; (b) The average infected density under different parameters: 0.01,0.038,0.055,0.21,0.28,0.3, =0.36,0.36,0.36,0.18,0.18,0.18, , , , , , which are corresponding to =0.1734, 0.6590, 0.9538<1; =5.3671,7.1561,8.9451> 1.
Figure 3.
(a) The under different quarantining control strategies, no-quarantine(cyan line), acquaintance-quarantine(prune line), proportional-quarantine(blue line), target-quarantine(green line): , , , , , (b) The epidemic threshold under different quarantine control strategies, no-quarantine(green line); target-quarantine(prune line); acquaintance-quarantine(blue line); proportional-quarantine(red line): , , , , , , , . (c) The epidemic threshold under different immunize strategies, no-immunity(blue line); target-immunity(green line); proportional-immunity(cyan line); acquaintance-immunity(red line):, , , , , , , , .
Figure 3.
(a) The under different quarantining control strategies, no-quarantine(cyan line), acquaintance-quarantine(prune line), proportional-quarantine(blue line), target-quarantine(green line): , , , , , (b) The epidemic threshold under different quarantine control strategies, no-quarantine(green line); target-quarantine(prune line); acquaintance-quarantine(blue line); proportional-quarantine(red line): , , , , , , , . (c) The epidemic threshold under different immunize strategies, no-immunity(blue line); target-immunity(green line); proportional-immunity(cyan line); acquaintance-immunity(red line):, , , , , , , , .
Figure 4.
The effectiveness of combination immune and quarantining control strategies: , , , , , . (a) Different immunity strategies with respect to quarantine rate: , , , acquaintance-immunity (prune line), proportional-immunity (blue line), target-immunity(cyan line). (b) Comparison of different combination control strategies: , , , , , , target-immunity and target-quarantine(red line), target-immunity and acquaintance-quarantine(prune line), acquaintance-immunity and target-quarantine(cyan line), acquaintance-immunity and acquaintance-quarantine(blue line).
Figure 4.
The effectiveness of combination immune and quarantining control strategies: , , , , , . (a) Different immunity strategies with respect to quarantine rate: , , , acquaintance-immunity (prune line), proportional-immunity (blue line), target-immunity(cyan line). (b) Comparison of different combination control strategies: , , , , , , target-immunity and target-quarantine(red line), target-immunity and acquaintance-quarantine(prune line), acquaintance-immunity and target-quarantine(cyan line), acquaintance-immunity and acquaintance-quarantine(blue line).
Figure 5.
(a) The average density under different control strategies, the lines with different colors corresponding to different optimal control: optimal control(purple line); (cyan line); (green line). Other parameter settings are as follows: , , ,, , ; (b) Comparison of optimal control and combined heterogeneous control strategies, without control (green line); acquaintance immunization and acquaintance quarantine(blue line); acquaintance immunization and proportional quarantine(prune line); acquaintance immunization and target quarantine(cyan line); optimal control (purple line):, , ,, , , , , .
Figure 5.
(a) The average density under different control strategies, the lines with different colors corresponding to different optimal control: optimal control(purple line); (cyan line); (green line). Other parameter settings are as follows: , , ,, , ; (b) Comparison of optimal control and combined heterogeneous control strategies, without control (green line); acquaintance immunization and acquaintance quarantine(blue line); acquaintance immunization and proportional quarantine(prune line); acquaintance immunization and target quarantine(cyan line); optimal control (purple line):, , ,, , , , , .
Figure 6.
(a) The under optimal control with varying weights. Low costs: (red line); Moderate costs: (blue line); High costs: (cyan line). Other parameters are fixed as , , ,, , . (b) The quarantine rates for any degree. Other parameters are fixed as , , ,, , .
Figure 6.
(a) The under optimal control with varying weights. Low costs: (red line); Moderate costs: (blue line); High costs: (cyan line). Other parameters are fixed as , , ,, , . (b) The quarantine rates for any degree. Other parameters are fixed as , , ,, , .
Table 1.
Symbols Employed in Models.
Table 1.
Symbols Employed in Models.
| Symbols |
Description |
|
Proportion of nodes with degree k. |
|
Average degree . |
| n |
The max degree. |
| b |
Birth rate. |
| d |
Natural death rate. |
|
The fertile contact probability between a node with degree k and its neighbors. |
|
Transmission rate of infected nodes with degree k. |
|
Vaccination rate of susceptible nodes with degree k. |
|
Quarantine rate of infected nodes with degree k. |
|
Recovery rate of infected nodes. |
|
Recovery rate of quarantined nodes. |
Table 2.
The values of under different control strategies.
Table 2.
The values of under different control strategies.
| Optimal control |
Max control |
No control |
|
|
|