Submitted:
03 November 2023
Posted:
06 November 2023
You are already at the latest version
Abstract
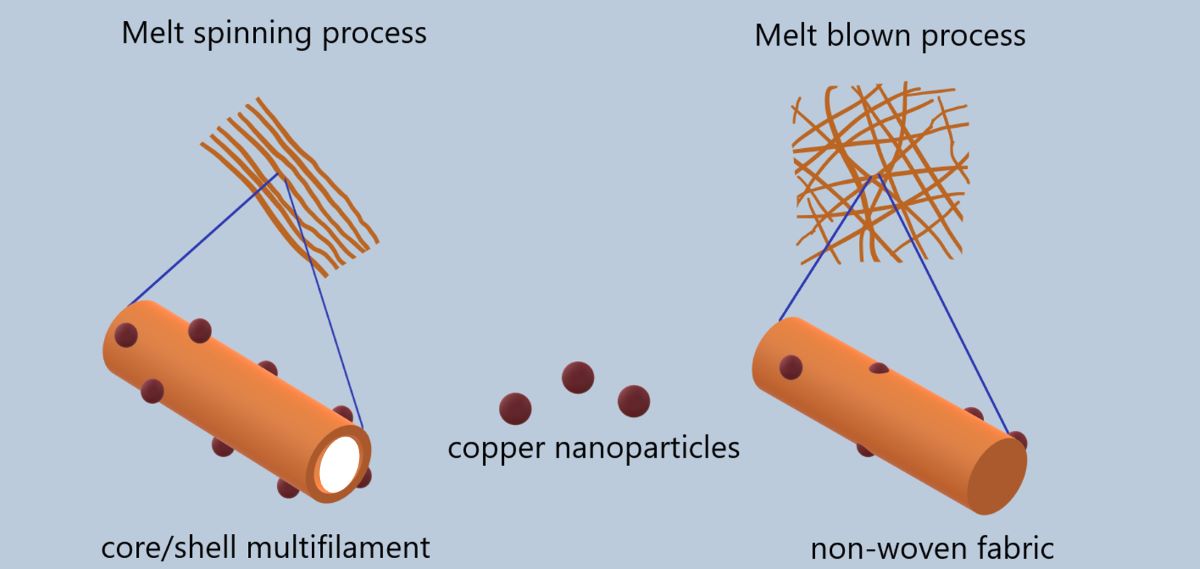
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Masterbatch Characterization
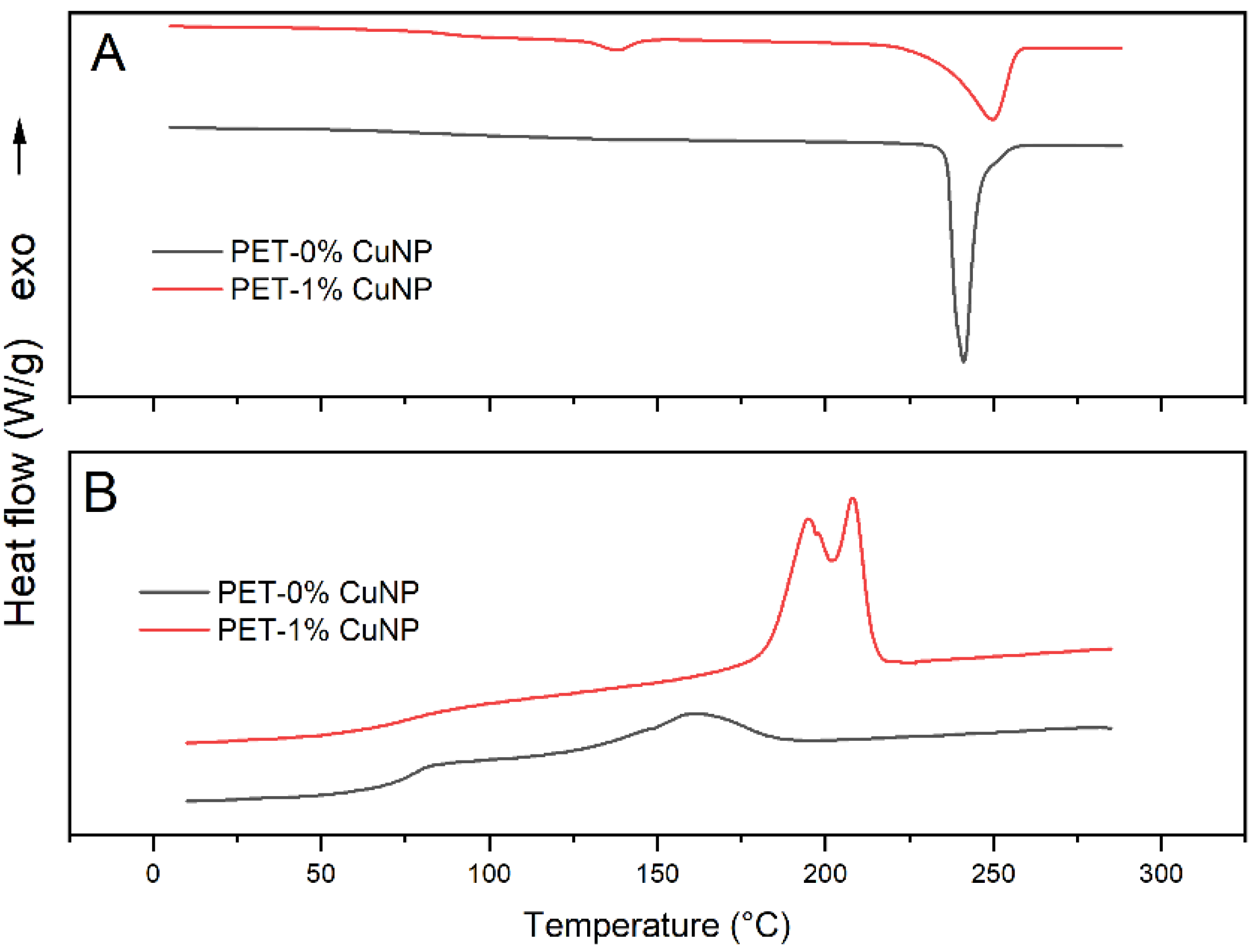
3.1.1. Differential scanning calorimetry (DSC)
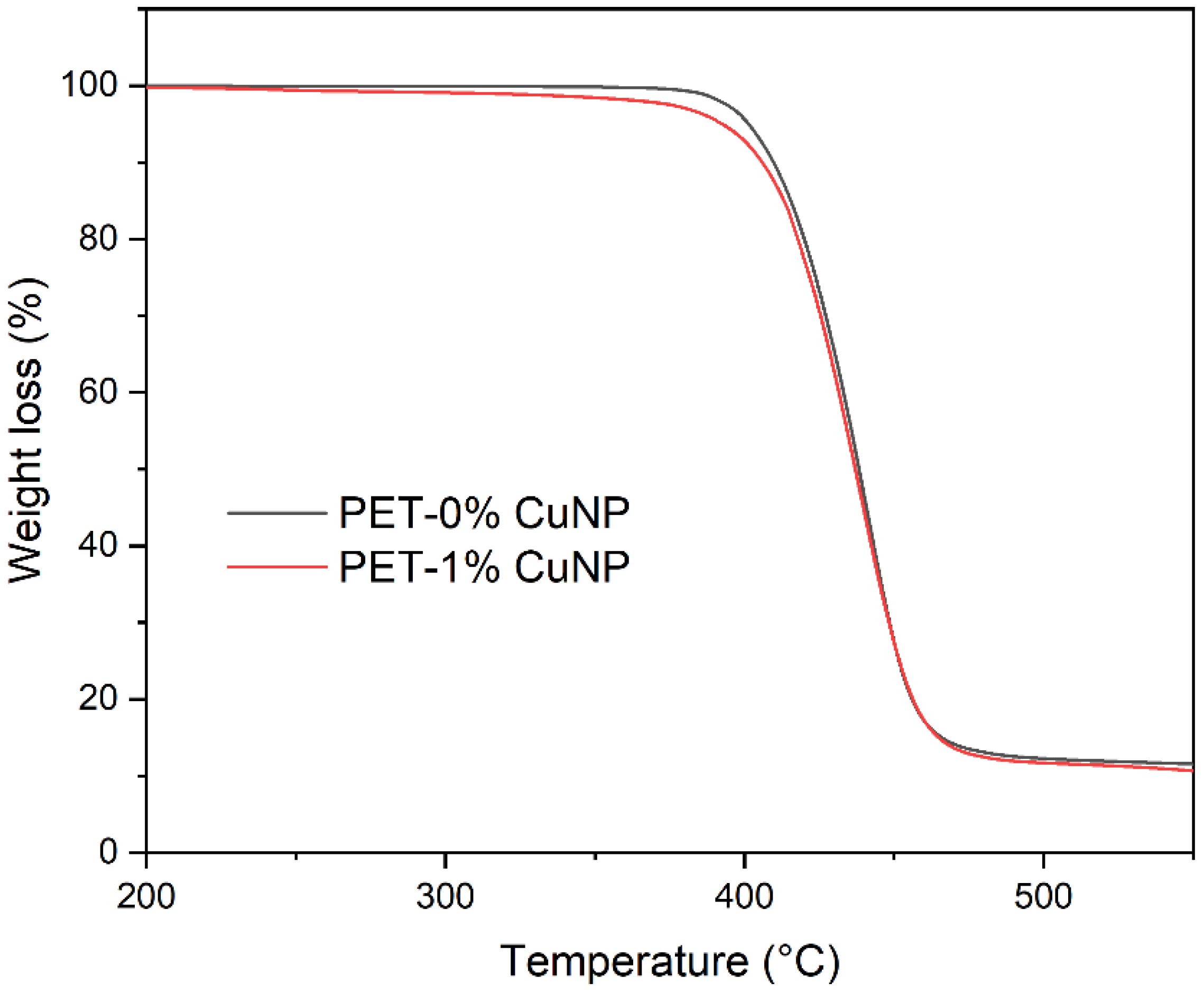
3.1.2. Thermogravimetric Analysis (TGA)
3.2. Multifilament and NWF Characterization
3.2.1. Denier and grammage
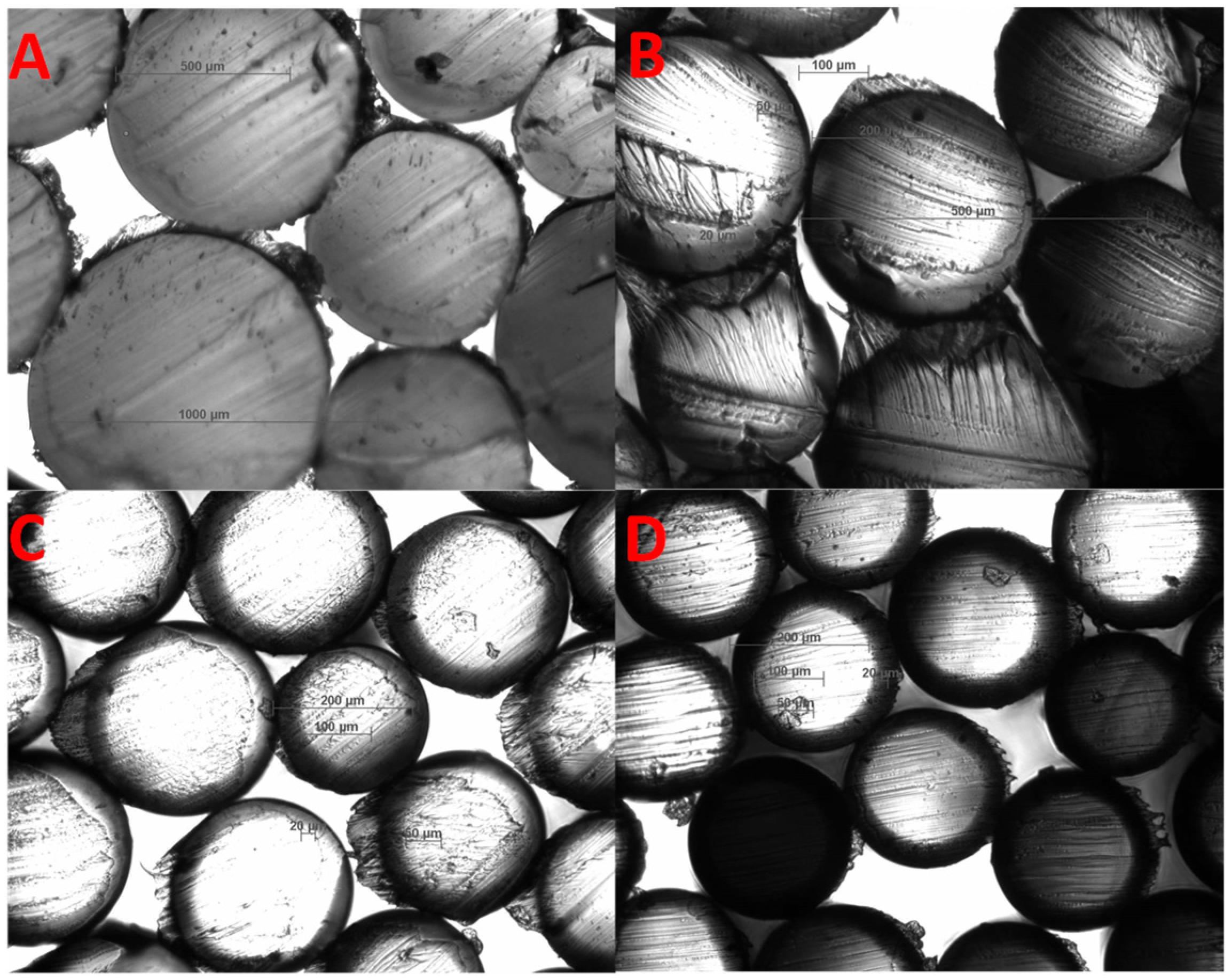
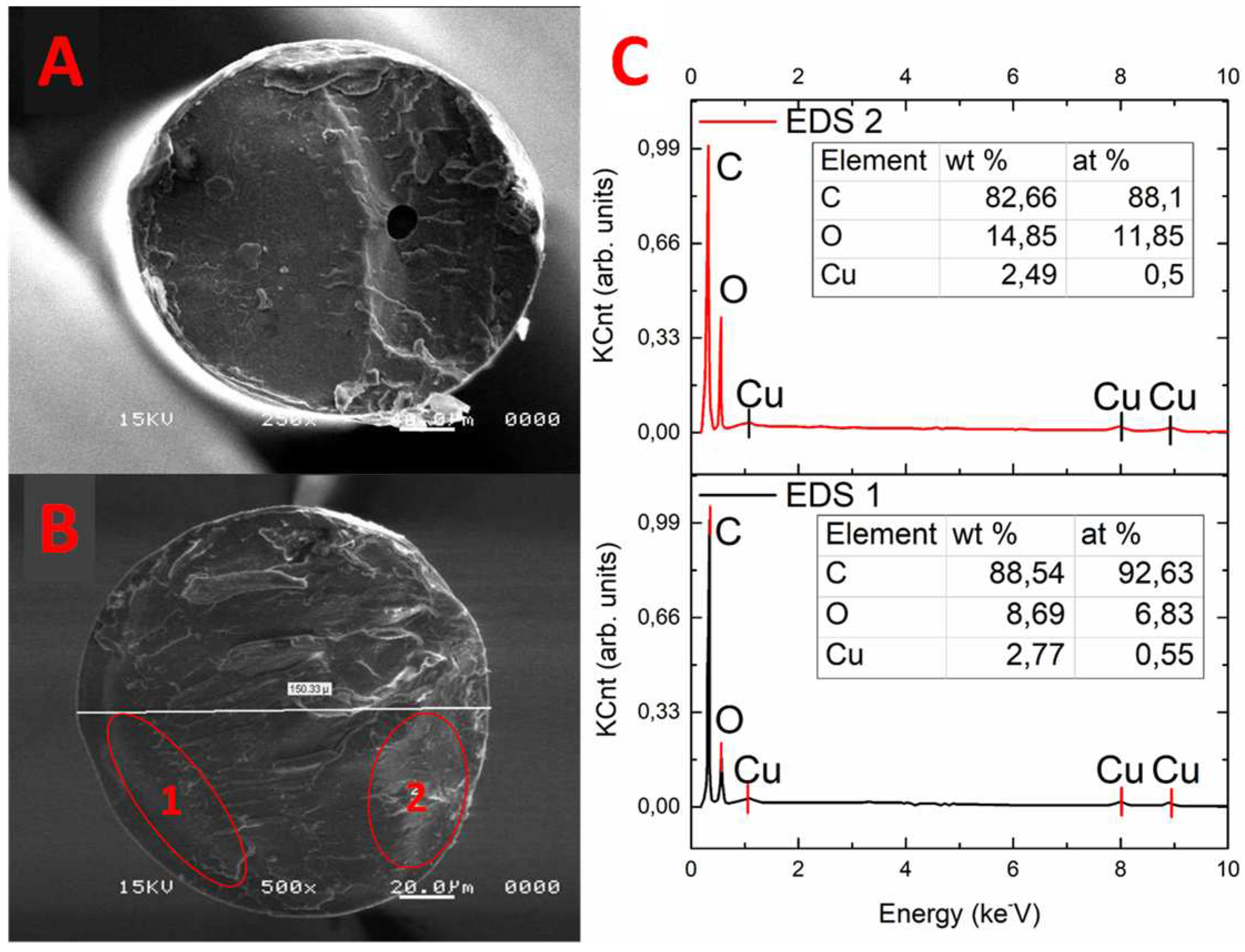
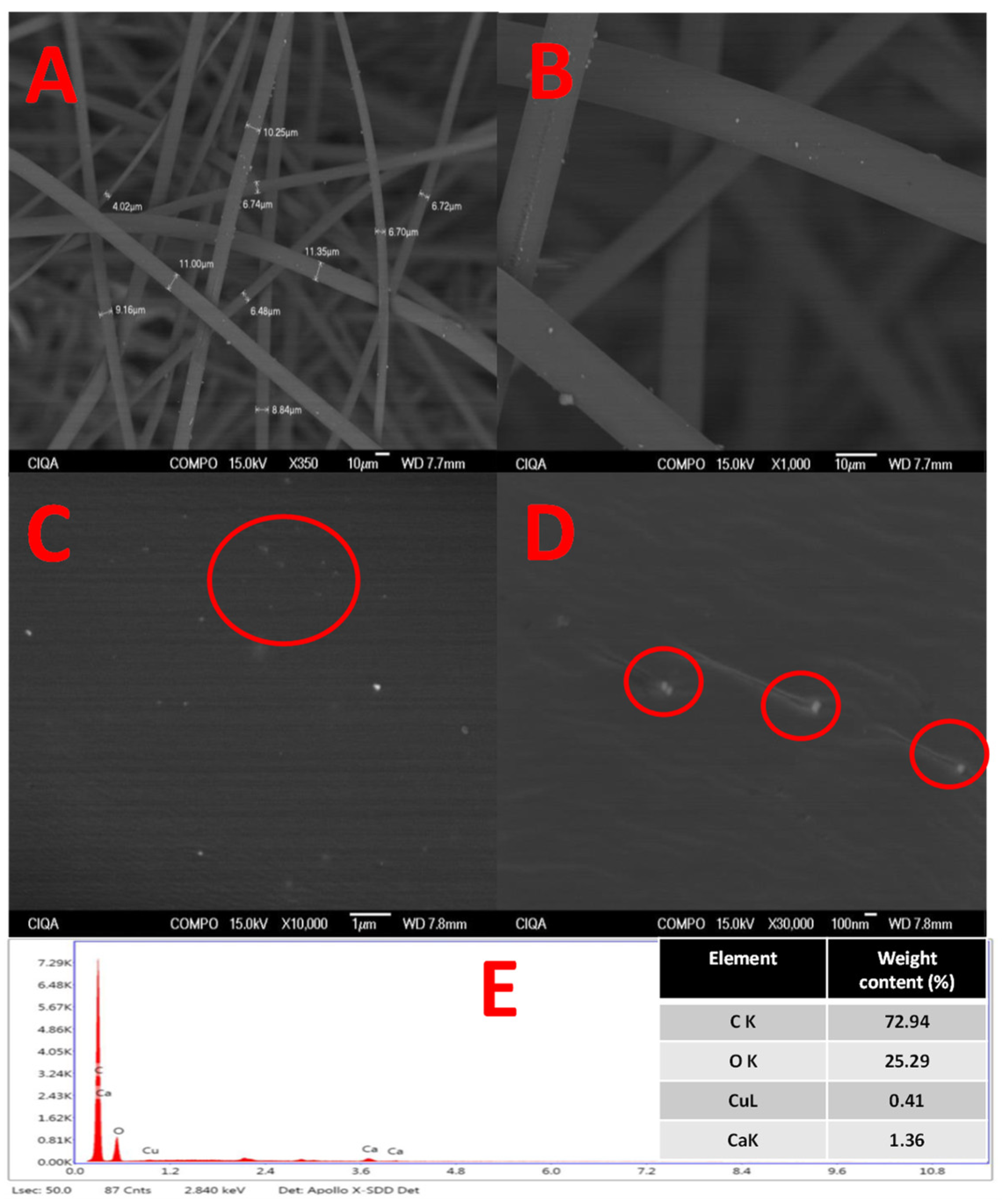
3.2.2. Multifilament Morphology
3.2.3. NWF Morphology
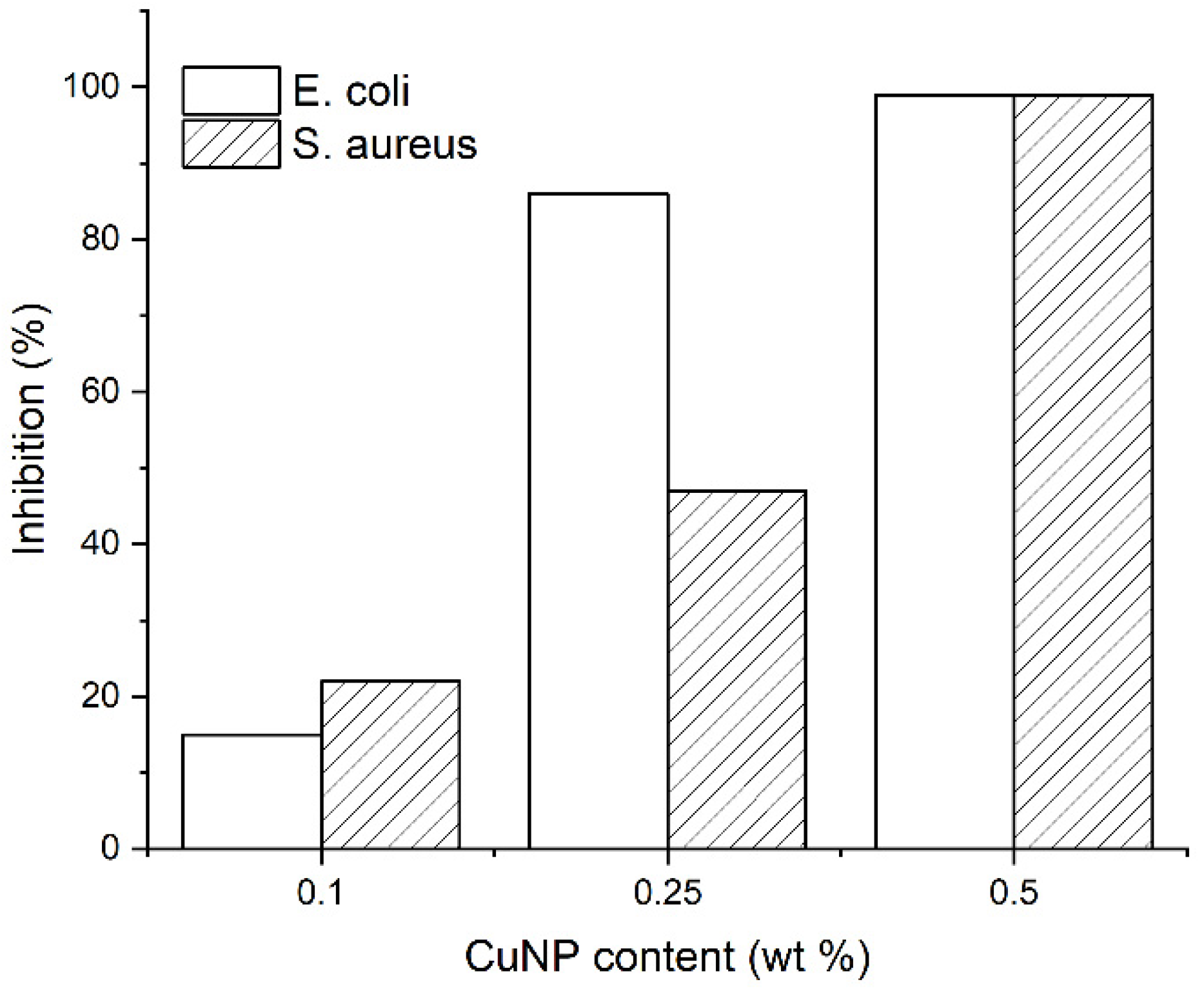
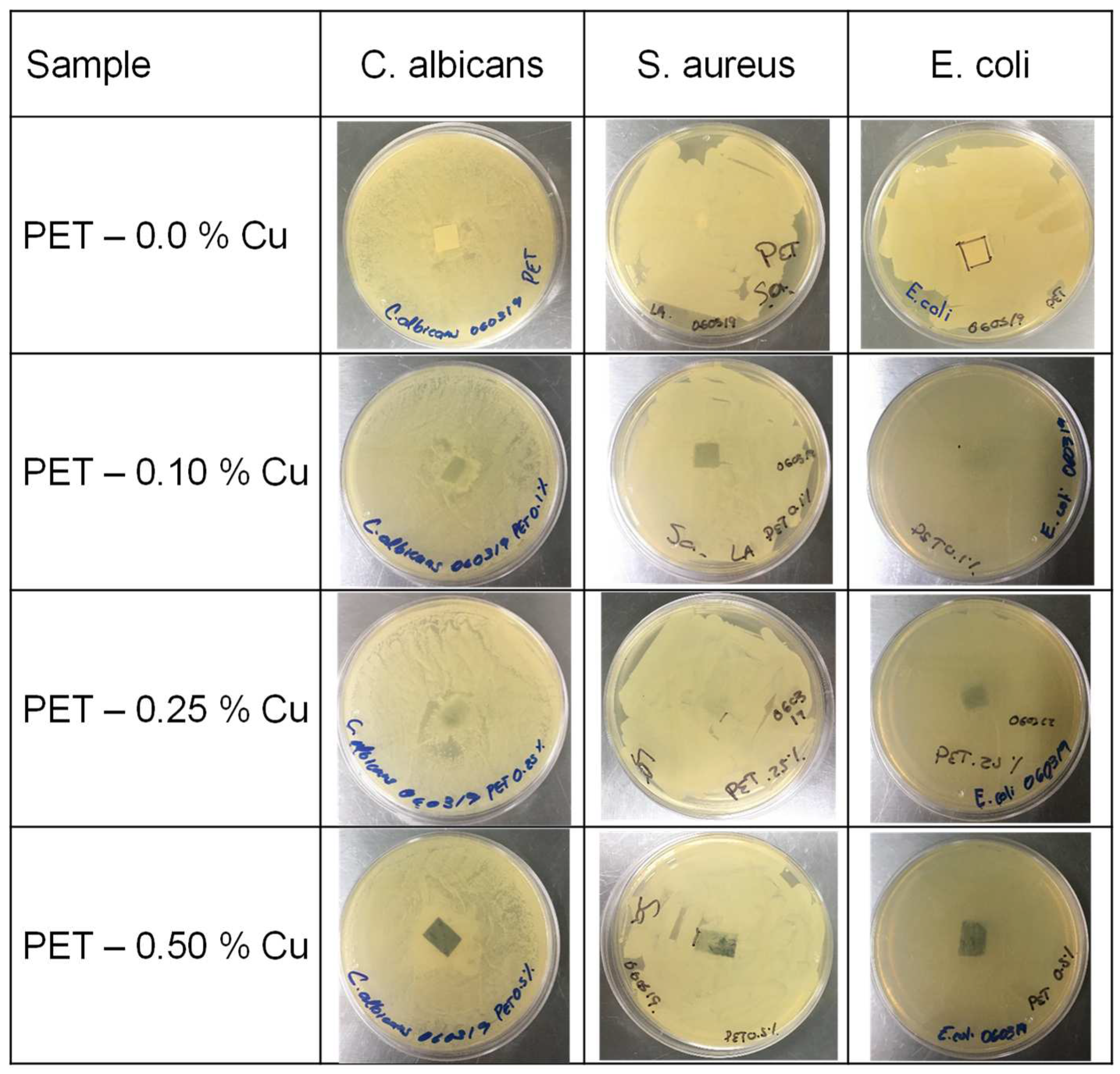
3.1.7. Antimicrobial Properties
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forrester, J.D.; Maggio, P.M.; Tennakoon, L. Cost of Health Care–Associated Infections in the United States. J. Patient Saf. 2022, 18, e477–e479. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.H.; Dryden, M. Update on the Epidemiology of Healthcare-Acquired Bacterial Infections: Focus on Complicated Skin and Skin Structure Infections. J. Antimicrob. Chemother. 2021, 76, iv2–iv8. [Google Scholar] [CrossRef] [PubMed]
- Zimlichman, E.; Henderson, D.; Tamir, O.; Franz, C.; Song, P.; Yamin, C.K.; Keohane, C.; Denham, C.R.; Bates, D.W. Health Care-Associated Infections: AMeta-Analysis of Costs and Financial Impact on the US Health Care System. JAMA Intern. Med. 2013, 173, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Tanasa, F.; Teaca, C.-A.; Nechifor, M.; Ignat, M.; Duceac, I.A.; Ignat, L. Highly Specialized Textiles with Antimicrobial Functionality—Advances and Challenges. Textiles 2023, 3, 219–245. [Google Scholar] [CrossRef]
- Gulati, R.; Sharma, S.; Sharma, R.K. Antimicrobial Textile: Recent Developments and Functional Perspective. Polym. Bull. 2022, 79, 5747–5771. [Google Scholar] [CrossRef] [PubMed]
- Bashari, A.; Shakeri, M.; Shirvan, A.R.; Najafabadi, S.A.N. Functional Finishing of Textiles via Nanomaterials. In Nanomaterials in the Wet Processing of Textiles; 2018; pp. 1–70. ISBN 9781119459804. [Google Scholar]
- Sowa-Söhle, E.N.; Schwenke, A.; Sajti, C.L.; Wagener, P.; Weiss, A.; Wiegel, H.; Haverich, A. Antimicrobial Efficacy, Cytotoxicity, and Ion Release of Mixed Metal (Ag, Cu, Zn, Mg) Nanoparticle Polymer Composite Implant Material. BioNanoMaterials 2013, 14, 217–227. [Google Scholar] [CrossRef]
- Vu, N.N.; Venne, C.; Ladhari, S.; Saidi, A.; Moskovchenko, L.; Lai, T.T.; Xiao, Y.; Barnabe, S.; Barbeau, B.; Nguyen-Tri, P. Rapid Assessment of Biological Activity of Ag-Based Antiviral Coatings for the Treatment of Textile Fabrics Used in Protective Equipment Against Coronavirus. ACS Appl. Bio Mater. 2022, 5, 3405–3417. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Kharaziha, M.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; RamaKrishna, S.; Berto, F. Antimicrobial Synthetic and Natural Polymeric Nanofibers as Wound Dressing: A Review. Adv. Eng. Mater. 2022, 24. [Google Scholar] [CrossRef]
- Su, X.; Sha, Q.; Gao, X.; Li, J.; Wu, Y.; Li, W.; Wu, W.; Han, N.; Zhang, X. Lightweight, Multifunctional Smart MXene@PET Non-Woven with Electric/Photothermal Conversion, Antibacterial and Flame Retardant Properties. Appl. Surf. Sci. 2023, 639. [Google Scholar] [CrossRef]
- Saylor, Y.; Irby, V. Metal Nanoparticles: Properties, Synthesis and Applications; 2018; ISBN 9781536141160. [Google Scholar]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of Copper Oxide Nanoparticles for Antimicrobial Applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef]
- Zhou, J.; Fei, X.; Li, C.; Yu, S.; Hu, Z.; Xiang, H.; Sun, B.; Zhu, M. Integrating Nano-Cu 2 O@ZrP into in Situ Polymerized Polyethylene Terephthalate (PET) Fibers with Enhanced Mechanical Properties and Antibacterial Activities. Polymers 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Sha, L.; Zhao, J. Preparation of Antimicrobial Fabric Using Magnesium-Based PET Masterbatch. Appl. Surf. Sci. 2017, 425, 1101–1110. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Jeong, S.H. Preparation and Characterization of Polypropylene/Silver Nanocomposite Fibers. Polym. Int. 2003, 52, 1053–1057. [Google Scholar] [CrossRef]
- Guerra, M.A.; Mariano, N.A.; Ramos, A.S.; Campos, M.G.N. Processing of Pet-Silver Nanocomposite Filaments. In Proceedings of the Materials Science Forum; 2016; Vol. 869; pp. 350–355. [Google Scholar]
- Guzman, A.; Cárcamo, H.; León, O. Elaboración y Caracterización Estructural de Fibras de Tereftalato de Polietileno (PET) Dopadas Con Nanocobre (0) Utilizando Proceso de Extrusión. Rev. Peru. Química e Ing. Química 2014, 17, 9–13. [Google Scholar]
- Abazari, M.; Badeleh, S.M.; Khaleghi, F.; Saeedi, M.; Haghi, F. Fabrication of Silver Nanoparticles-Deposited Fabrics as a Potential Candidate for the Development of Reusable Facemasks and Evaluation of Their Performance. Sci. Rep. 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.; Vale, A.C.; Pinto, A.C.; Costa, R. V.; Pais, V.; Sousa, D.; Gomes, F.; Pinto, G.; Dias, J.G.; Moreira, I.P.; et al. Comparison of Zinc Oxide Nanoparticle Integration into Non-Woven Fabrics Using Different Functionalisation Methods for Prospective Application as Active Facemasks. Polymers 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Chruściel, J.J.; Olczyk, J.; Kudzin, M.H.; Kaczmarek, P.; Król, P.; Tarzyńska, N. Antibacterial and Antifungal Properties of Polyester, Polylactide, and Cotton Nonwovens and Fabrics, by Means of Stable Aqueous Dispersions Containing Copper Silicate and Some Metal Oxides. Materials 2023, 16, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, J.; Borkow, G.; Mishal, J.; Magen, E.; Zatcoff, R.; Shemer-Avni, Y. Copper Oxide Impregnated Textiles with Potent Biocidal Activities. J. Ind. Text. 2006, 35, 323–335. [Google Scholar] [CrossRef]
- Ávila-Orta, C.A.; Quiñones-Jurado, Z. V.; Waldo-Mendoza, M.A.; Rivera-Paz, E.A.; Cruz-Delgado, V.J.; Mata-Padilla, J.M.; González-Morones, P.; Ziolo, R.F. Ultrasound-Assist Extrusion Methods for the Fabrication of Polymer Nanocomposites Based on Polypropylene/Multi-Wall Carbon Nanotubes. Materials 2015, 8, 7900–7912. [Google Scholar] [CrossRef]
- Ávila-Orta, C.; González-Morones, P.; Agüero- Valdez, D.; González-Sánchez, A. ; G. Martínez-Colunga, J.; M. Mata-Padilla, J., J. Cruz-Delgado, V. Ultrasound-Assisted Melt Extrusion of Polymer Nanocomposites. In Nanocomposites - Recent Evolutions, Sivasankaran, S., Eds.; IntechOpen: London, 2019. [Google Scholar]
- Cruz-Delgado, V.J.; Valdez-Garza, J.A.; Mata-Padilla, J.M.; Martínez-Colunga, J.G.; Ávila-Orta, C.A. Preparation and Characterization of Electrically Conductive Polymer Nanocomposites with Different Carbon Nanoparticles. In Carbon Nanotubes - Redefining the World of Electronics; IntechOpen, 2021; pp. 11–28. [Google Scholar]
- Andrade-Guel, M.; Reyes-Rodríguez, P.Y.; Cabello-Alvarado, C.J.; Cadenas-Pliego, G.; Ávila-Orta, C.A. Influence of Modified Carbon Black on Nylon 6 Nonwoven Fabric and Performance as Adsorbent Material. Nanomaterials 2022, 12. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.; Andrade-Guel, M.; Medellin-Banda, D.I.; Ávila-Orta, C.A.; Cadenas-Pliego, G.; Sáenz-Galindo, A.; Radillo-Radillo, R.; Lara-Sánchez, J.F.; Melo-Lopez, L. Non-Woven Fabrics Based on Nylon 6/Carbon Black-Graphene Nanoplatelets Obtained by Melt-Blowing for Adsorption of Urea, Uric Acid and Creatinine. Mater. Lett. 2022, 320, 132382. [Google Scholar] [CrossRef]
- España-Sánchez, B.L.; Ávila-Orta, C.A.; Padilla-Vaca, F.; Neira-Velázquez, M.G.; González-Morones, P.; Rodríguez-González, J.A.; Hernández-Hernández, E.; Rangel-Serrano, Á.; Barriga-C., E.D.; Yate, L.; et al. Enhanced Antibacterial Activity of Melt Processed Poly(Propylene) Ag and Cu Nanocomposites by Argon Plasma Treatment. Plasma Process. Polym. 2014, 11, 353–365. [Google Scholar] [CrossRef]
- Espana-Sanchez, B.L.; Avila-Orta, C.A.; Padilla-Vaca, L.F.; Barriga-Castro, E.D.; Soriano-Corral, F.; Gonzalez-Morones, P.; Ramirez-Wong, D.G.; Luna-Barcenas, G. Early Stages of Antibacterial Damage of Metallic Nanoparticles by TEM and STEM-HAADF. Curr. Nanosci. 2017, 14, 54–61. [Google Scholar] [CrossRef]
- Navarro-Rosales, M.; Ávila-Orta, C.A.; Neira-Velázquez, M.G.; Ortega-Ortiz, H.; Hernández-Hernández, E.; Solís-Rosales, S.G.; España-Sánchez, B.L.; Gõnzalez-Morones, P.; Jímenez-Barrera, R.M.; Sánchez-Valdes, S.; et al. Effect of Plasma Modification of Copper Nanoparticles on Their Antibacterial Properties. Plasma Process. Polym. 2014, 11, 685–693. [Google Scholar] [CrossRef]
- Mata-Padilla, J.M.; Ávila-Orta, C.A.; Almendárez-Camarillo, A.; Martínez-Colunga, J.G.; Hernández-Hernández, E.; Cruz-Delgado, V.J.; González-Morones, P.; Solís-Rosales, S.G.; González-Calderón, J.A. Non-Isothermal Crystallization Behavior of Isotactic Polypropylene/Copper Nanocomposites. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Tamayo, L.; Palza, H.; Bejarano, J.; Zapata, P.A. Polymer Composites With Metal Nanoparticles: Synthesis, Properties, and Applications. Synthesis, Properties, and Applications. In Polymer Composites with Functionalized Nanoparticles: Synthesis, Properties, and Applications; 2018; pp. 249–286. ISBN 978012814065. [Google Scholar]
- Demchenko, V.; Riabov, S.; Rybalchenko, N.; Shtompel’, V. Structure, Morphology, and Properties of Copper-Containing Polymer Nanocomposites. In Proceedings of the Springer Proceedings in Physics; 2017; Vol. 195; pp. 641–659. [Google Scholar]
- Tamayo, L.; Azócar, M.; Kogan, M.; Riveros, A.; Páez, M. Copper-Polymer Nanocomposites: An Excellent and Cost-Effective Biocide for Use on Antibacterial Surfaces. Mater. Sci. Eng. C 2016, 69, 1391–1409. [Google Scholar] [CrossRef]
- León-Martínez, P.A. De; Soriano-Corral, F.; Ávila-Orta, C.A.; González-Morones, P.; Hernández-Hernández, E.; Ledezma-Pérez, A.S.; Covarrubias-Gordillo, C.A.; Espinosa-López, A.C.; Gómez, R.E.D. de L. Surface Modification of NTiO2/Ag Hybrid Nanoparticles Using Microwave-Assisted Polymerization in the Presence of Bis(2-Hydroxyethyl) Terephthalate. J. Nanomater. 2017, 2017, 7079497. [Google Scholar] [CrossRef]
- Vassiliou, A.A.; Chrissafis, K.; Bikiaris, D.N. In Situ Prepared PET Nanocomposites: Effect of Organically Modified Montmorillonite and Fumed Silica Nanoparticles on PET Physical Properties and Thermal Degradation Kinetics. Thermochim. Acta 2010, 500, 21–29. [Google Scholar] [CrossRef]
- Peng, N.; Widjojo, N.; Sukitpaneenit, P.; Teoh, M.M.; Lipscomb, G.G.; Chung, T.-S.; Lai, J.-Y. Evolution of Polymeric Hollow Fibers as Sustainable Technologies: Past, Present, and Future. Prog. Polym. Sci. 2012, 37, 1401–1424. [Google Scholar] [CrossRef]
- Karaca, E.; Ozcelik, F. Influence of the Cross-Sectional Shape on the Structure and Properties of Polyester Fibers. J. Appl. Polym. Sci. 2007, 103, 2615–2621. [Google Scholar] [CrossRef]
- Huuhilo, T.; Nystrom, M. Fouling of a Polypropylene Filter Fabric with Silica. Filtr. Sep. 2001, 38, 43–47. [Google Scholar] [CrossRef]
- Naeimirad, M.; Zadhoush, A.; Kotek, R.; Esmaeely Neisiany, R.; Nouri Khorasani, S.; Ramakrishna, S. Recent Advances in Core/Shell Bicomponent Fibers and Nanofibers: A Review. J. Appl. Polym. Sci. 2018, 135, 46265. [Google Scholar] [CrossRef]
- Lim, J.C.; Park, Y.W.; Kim, H.C. Study on Manufacturing PCT/PPS Flame Retardant Fiber by Sheath/Core Conjugate Spinning. Fibers Polym. 2020, 21, 498–504. [Google Scholar] [CrossRef]
- Kara, S.; Ureyen, M.E.; Erdogan, U.H. Structural and Antibacterial Properties of PP/CuO Composite Filaments Having Different Cross Sectional Shapes. Int. Polym. Process. 2016, 31, 398–409. [Google Scholar] [CrossRef]
- Xue, B.; Jiang, Y.; Liu, D. Preparation and Characterization of a Novel Anticorrosion Material: Cu/LLDPE Nanocomposites. Mater. Trans. 2011, 52, 96–101. [Google Scholar] [CrossRef]
- Wei, Q.; Yu, L.; Wu, N.; Hong, S. Preparation and Characterization of Copper Nanocomposite Textiles. J. Ind. Text. 2008, 37, 275–283. [Google Scholar] [CrossRef]
- Fernandez, A.; Lloret, E.; Llorens, A.; Picouet, P. Metal-Based Micro and Nano-Composites as Antimicrobials in Food Packaging. In Food Packaging: Procedures, Management and Trends; 2012; pp. 79–92. ISBN 9781622573103. [Google Scholar]
- Ghosh, S.; Sarkar, B.; Thongmee, S.; Mostafavi, E. Hybrid Antibacterial, Antifungal, and Antiviral Smart Coatings. In Antiviral and Antimicrobial Smart Coatings: Fundamentals and Applications; 2023; pp. 431–431. ISBN 9780323992916. [Google Scholar]
- Odintsova, O.I.; Vladimirtseva, E.L.; Kozlova, O. V.; Smirnova, S. V.; Lipina, A.A.; Petrova, L.S.; Erzunov, K.A.; Konstantinova, Z.A.; Zimnurov, A.R.; Bykov, F.A.; et al. FINISHING TEXTILE MATERIALS WITH MICROCAPSULES AND NANOPARTICLES OF FUNCTIONAL SUBSTANCES. ChemChemTech 2023, 66, 173–184. [Google Scholar] [CrossRef]
- Pardi, G. “Determinantes de Patogenicidad de Candida Albicans” Acta Odontol. Venez. 2002, 40, 185–192. [Google Scholar]
- Dong, X.; Wang, S.; Ren, K. Application of Composite Antibacterial Nanoparticle Non-Woven Fabric in Sterilization of Hospital Infection. Prev. Med. (Baltim). 2023, 173. [Google Scholar] [CrossRef]
- García, A.; Rodríguez, B.; Giraldo, H.; Quintero, Y.; Quezada, R.; Hassan, N.; Estay, H. Copper-Modified Polymeric Membranes for Water Treatment: A Comprehensive Review. Membranes 2021, 11, 1–47. [Google Scholar] [CrossRef]

| Sample | Multifilament | NWF | |
|---|---|---|---|
| Total Denier | DFP | gsm | |
| PET | 222 | 12.3 | 67.5 |
| PET/0.10% CuNP | 345 | 19.1 | 68.3 |
| PET/0.25% CuNP | 369 | 20.5 | 68.8 |
| PET/0.50% CuNP | 391 | 21.7 | 69.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





