Submitted:
06 November 2023
Posted:
07 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
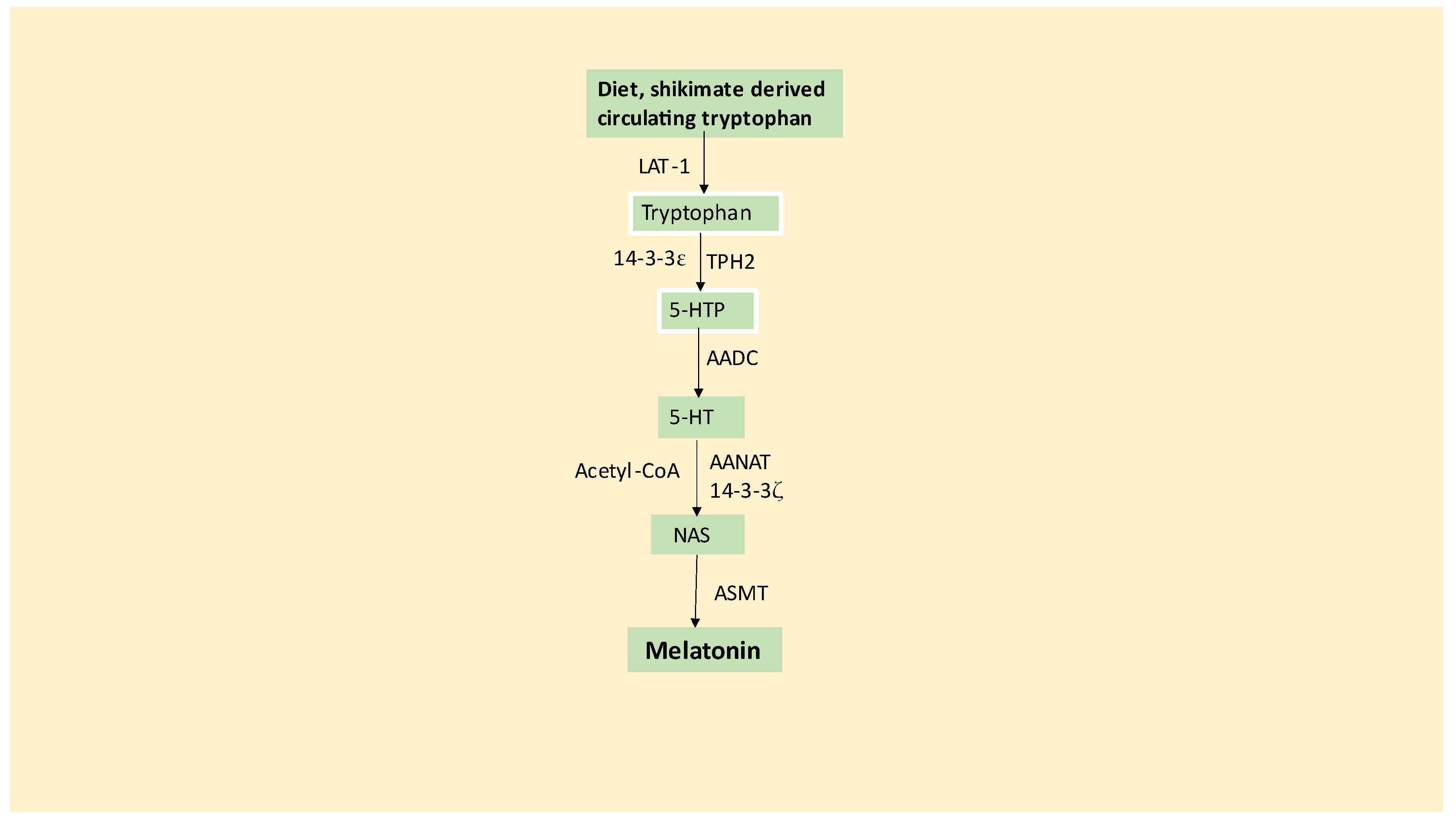
2. Tryptophan-melatonin pathway
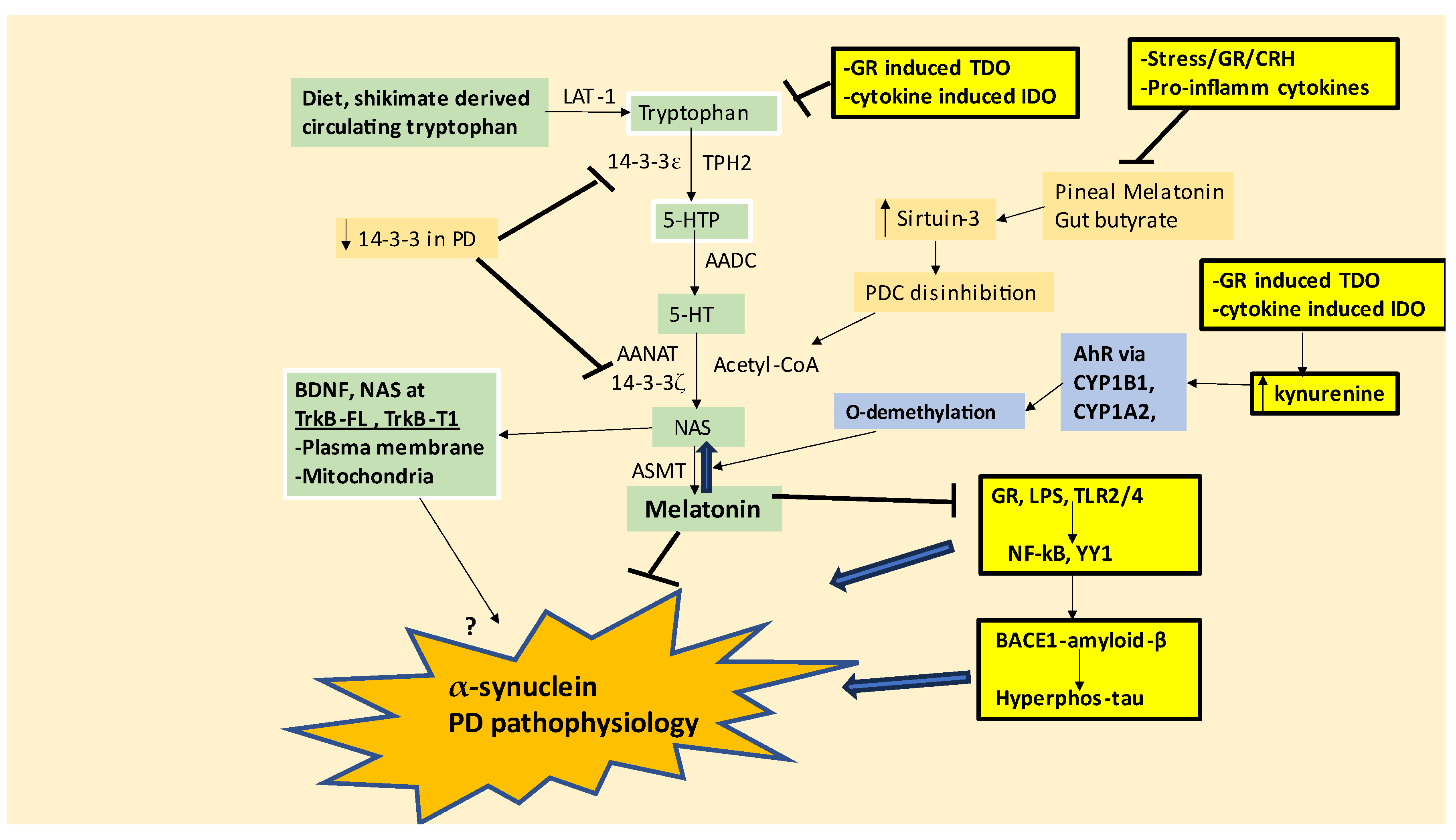
3. Integrating the tryptophan-melatonin pathway in PD pathophysiology
4. Integrating wider Parkinson’s disease pathophysiology with the tryptophan-melatonin pathway
4.1. Platelets and Erythrocytes
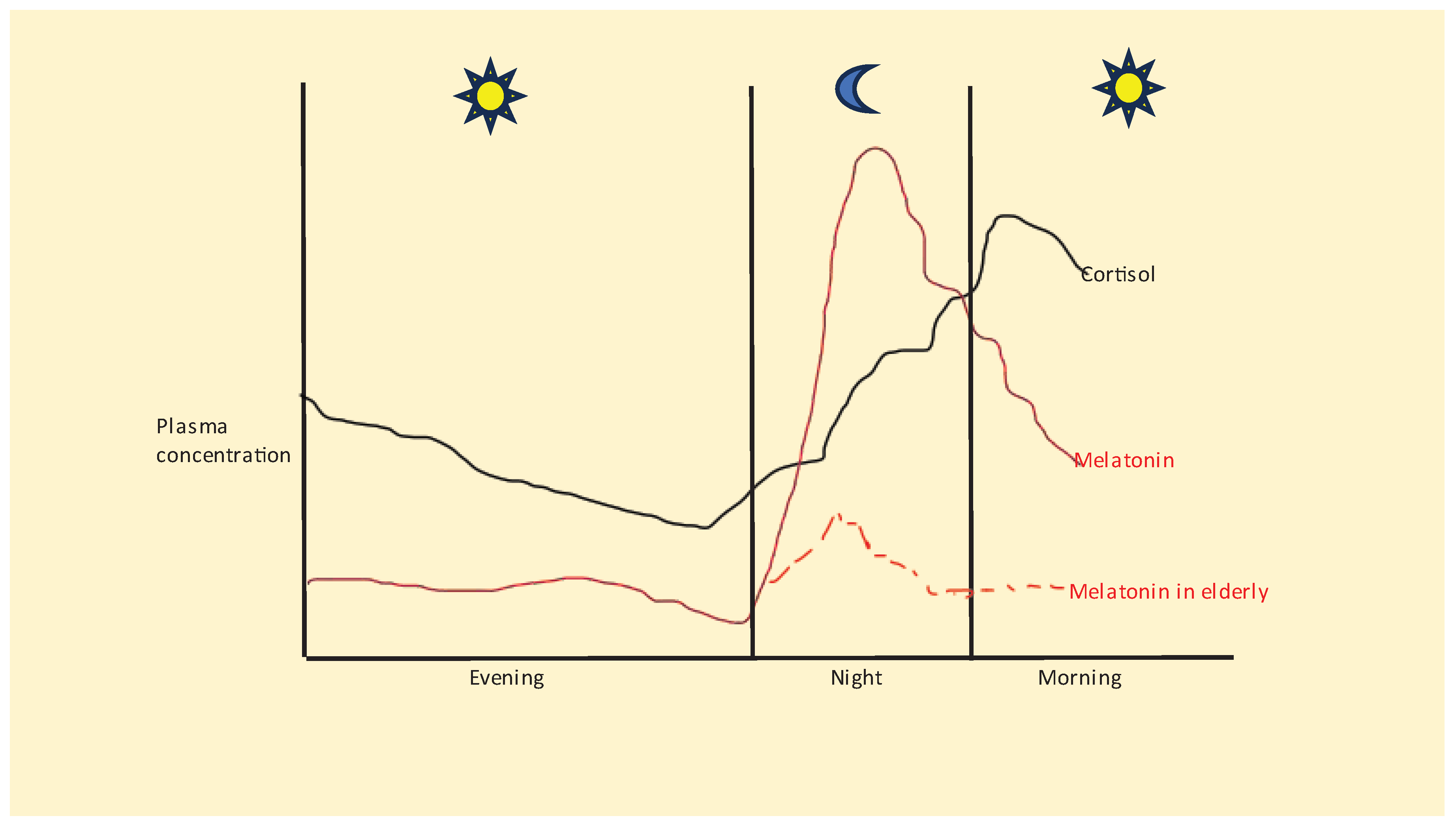
4.2. Stress, HPA axis and morning cortisol awakening response
4.3. Stress, α-synuclein, transcription factors and glutamatergic regulation
4.4. Astrocytes, α-synuclein, and neuronal mitochondrial metabolism
4.5. Melatonergic pathway regulation of amyloid-β and tau interactions with α-synuclein
5. Integrating Parkinson’s disease pathoetiology and pathophysiology
5.1. Integrating the role of herpes simplex virus-1 in Parkinson’s disease
6. Future research directions
7. Treatment implications
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AANAT | aralkylamine N-acetyltransferase |
| AhR | aryl hydrocarbon receptor |
| ASMT | acetylserotonin methytransferase |
| BACE1 | beta-site amyloid precursor protein cleaving enzyme 1 |
| BDNF | rain-derived neurotrophic factor |
| Bmal1 | brain and muscle ARNT-Like 1 |
| CYP | cytochrome P450 |
| EAAT | excitatory amino acid transporter |
| HDAC | histone deacetylase |
| HMGB | high-mobility group box |
| hsp | heat shock protein |
| IDO | indoleamine 2,3-dioxygenase |
| LPS | lipopolysaccharide |
| MHC-1 | major histocompatibility complex, class 1 |
| NAS | N-acetylserotonin |
| NF-kB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK | natural killer cell |
| NMDA | N-methyl-d-aspartate |
| OXPHOS | oxidative phosphorylation |
| PD | Parkinson’s disease |
| PDC | pyruvate dehydrogenase complex |
| PINK1 | PTEN-induced kinase 1 |
| REM | rapid eye movement |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TCA | tricarboxylic acid |
| TDO | tryptophan 2,3-dioxygenase |
| TLR | Toll-like receptor |
| TNF | tumor necrosis factor |
| TPH2 | tryptophan hydroxylase 2 |
| Treg | regulatory t cell |
| TrkB | tyrosine receptor kinase B |
References
- Anderson, G.; Seo, M.; Berk, M.; Carvalho, A.F.; Maes, M. Gut Permeability and Microbiota in Parkinson's Disease: Role of Depression, Tryptophan Catabolites, Oxidative and Nitrosative Stress and Melatonergic Pathways. Curr Pharm Des. 2016, 22(40), 6142–6151. [Google Scholar] [CrossRef]
- Anderson, G. Why Do Anti-Amyloid Beta Antibodies not work? Time to reconceptualize Dementia Pathophysiology by incorporating astrocyte melatonergic pathway desynchronization from amyloid-beta production. Braz J Psychiatry. 2023, 45(2), 89–92. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. A more holistic perspective of Alzheimer’s disease: Roles of gut microbiome, adipocytes, HPA axis, melatonergic pathway and astrocyte mitochondria in the emergence of autoimmunity. Front Biosc (Landmark), In press. 19989.
- Battis, K.; Xiang, W.; Winkler, J. The Bidirectional Interplay of α-Synuclein with Lipids in the Central Nervous System and Its Implications for the Pathogenesis of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 13270. [Google Scholar] [CrossRef]
- de Fàbregues, O.; Sellés, M.; Ramos-Vicente, D.; Roch, G.; Vila, M.; Bové, J. Relevance of tissue-resident memory CD8 T cells in the onset of Parkinson's disease and examination of its possible etiologies: infectious or autoimmune? Neurobiol Dis. 2023, 187, 106308. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.F. Alzheimer's disease as an innate autoimmune disease (AD2 ): A new molecular paradigm. Alzheimers Dement. 2022, Sep 27. [CrossRef]
- Cui, C.; Longinetti, E.; Larsson, H.; Andersson, J.; Pawitan, Y.; Piehl, F.; Fang, F. Associations between autoimmune diseases and amyotrophic lateral sclerosis: a register-based study. Amyotroph Lateral Scler Frontotemporal Degener, 2021; 22, 3-4, 211–219. [Google Scholar] [CrossRef]
- Fan, H.H.; Cui, L.; Jiang, X.X.; Song, Y.D.; Liu, S.S.; Wu, K.Y.; Dong, H.J.; Mao, M.; Ovlyakulov, B.; Wu, H.M.; et al. Autoimmune Disease Associated CLEC16A Variants Convey Risk of Parkinson's Disease in Han Chinese. Front Genet. 2022, 13, 856493. [Google Scholar] [CrossRef]
- Anderson, G.; Almulla, A.F.; Reiter, R.J.; Maes, M. Redefining Autoimmune Disorders’ Pathoetiology: Implications for Mood and Psychotic Disorders’ Association with Neurodegenerative and Classical Autoimmune Disorders. Cells 2023, 12, 1237. [Google Scholar] [CrossRef]
- Matheoud D, Cannon T, Voisin A, Penttinen AM, Ramet L, Fahmy AM, Ducrot C, Laplante A, Bourque MJ, Zhu L, Cayrol R, Le Campion A, McBride HM, Gruenheid S, Trudeau LE, Desjardins M. Intestinal infection triggers Parkinson's disease-like symptoms in Pink1-/- mice. Nature. 2019, 571(7766), 565-569. [CrossRef]
- Anderson, G. Type I Diabetes Pathoetiology and Pathophysiology: Roles of the Gut Microbiome, Pancreatic Cellular Interactions, and the 'Bystander' Activation of Memory CD8+ T Cells. Int J Mol Sci. 2023, 24(4), 3300. [Google Scholar] [CrossRef]
- Adi, N.; Mash, D.C.; Ali, Y.; Singer, C.; Shehadeh, L.; Papapetropoulos, S. Melatonin MT1 and MT2 receptor expression in Parkinson's disease. Med Sci Monit. 2010, 16(2), BR61–7. [Google Scholar] [PubMed]
- Tan, D.X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin's primary function and evolution in eukaryotes. J Pineal Res. 2013, 54(2), 127–38. [Google Scholar] [CrossRef] [PubMed]
- Nuzum, N.D.; Szymlek-Gay, E.A. Loke, S.; Dawson, S.L.; Teo, W.P.; Hendy, A.M.; Loughman, A.; Macpherson, H. Differences in the gut microbiome across typical ageing and in Parkinson's disease. Neuropharmacology, 2023, 235, 109566. [Google Scholar] [CrossRef]
- Anderson, G.; Maes, M. Gut Dysbiosis Dysregulates Central and Systemic Homeostasis via Suboptimal Mitochondrial Function: Assessment, Treatment and Classification Implications. Curr Top Med Chem. 2020, 20(7), 524–539. [Google Scholar] [CrossRef] [PubMed]
- Trinh, D.; Israwi, A.R.; Brar, H.; Villafuerte, J.E.A.; Laylo, R.; Patel, H.; Jafri, S.; Al Halabi, L.; Sinnathurai, S.; Reehal, K.; et al. Parkinson's disease pathology is directly correlated to SIRT3 in human subjects and animal models: Implications for AAV.SIRT3-myc as a disease-modifying therapy. Neurobiol Dis, 2023, 187, 106287. [Google Scholar] [CrossRef] [PubMed]
- Benigni, A.; Perico, L.; Macconi, D. Mitochondrial Dynamics Is Linked to Longevity and Protects from End-Organ Injury: The Emerging Role of Sirtuin 3. Antioxid Redox Signal. 2016, 25(4), 185–99. [Google Scholar] [CrossRef] [PubMed]
- Karasek, M.; Reiter, R.J. Melatonin and aging. Neuro Endocrinol Lett. 2002, 23(Supp1), 14–6. [Google Scholar]
- Guo, Y.L.; Wei, X.J.; Zhang, T.; Sun, T. Molecular mechanisms of melatonin-induced alleviation of synaptic dysfunction and neuroinflammation in Parkinson's disease: a review. Eur Rev Med Pharmacol Sci. 2023, 27(11), 5070–5082. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Maes, M. Local melatonin regulates inflammation resolution: a common factor in neurodegenerative, psychiatric and systemic inflammatory disorders. CNS Neurol Disord Drug Targets. 2014, 13(5), 817–27. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.R. Parkinsonism due to mutations in PINK1, parkin, and DJ-1 and oxidative stress and mitochondrial pathways. Cold Spring Harbor perspectives in medicine, 2012, 2, (9), a009415. [Google Scholar] [CrossRef]
- Cookson, M.R. alpha-Synuclein and neuronal cell death. Mol Neurodegener, 2009, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Ozkan, A.; Aytac, G.; Agar, A.; Tanriover, G. Role of melatonin in TLR4-mediated inflammatory pathway in the MTPT-induced mouse model. Neurotoxicology. 2022, 88, 168–177. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Qian, Y.; Mo, C.; Ai, P.; Yang, X.; Xiao, Q. Sodium butyrate ameliorates gut dysfunction and motor deficits in a mouse model of Parkinson's disease by regulating gut microbiota. Front Aging Neurosci. 2023, 15, 1099018. [Google Scholar] [CrossRef]
- Guedes, B.F.S; Cardoso, S.M.; Esteves, A.R. The Impact of microRNAs on Mitochondrial Function and Immunity: Relevance to Parkinson's Disease. Biomedicines. 2023, 11(5), 1349. [Google Scholar] [CrossRef]
- Seo, M.; Anderson, G. Gut-Amygdala Interactions in Autism Spectrum Disorders: Developmental Roles via regulating Mitochondria, Exosomes, Immunity and microRNAs. Curr Pharm Des. 2019, 25(41), 4344–4356. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.J.; Engstler, A.J.; Sellmann, C.; Ziegenhardt, D.; Landmann, M.; Kanuri, G.; Lounis, H.; Schröder, M.; Vetter, W.; Bergheim, I. Sodium butyrate protects mice from the development of the early signs of non-alcoholic fatty liver disease: role of melatonin and lipid peroxidation. Br J Nutr. 2016, 116(10), 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Stewart, T.; Zhang, C.; Wang, P.; Xu, Z.; Jin, J.; Huang, Y.; Liu, Z.; Lan, G.; Liang, X.; Sheng, L.; Shi, M.; Cai, Z.; Zhang, J. Erythrocytic α-Synuclein and the Gut Microbiome: Kindling of the Gut-Brain Axis in Parkinson's Disease. Mov Disord. 5. [CrossRef]
- Beura, S.K.; Panigrahi, A.R.; Yadav, P.; Singh, S.K. Role of platelet in Parkinson's disease: Insights into pathophysiology & theranostic solutions. Ageing Res Rev. 2022, 80, 101681. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. Gut Microbiome and Circadian Interactions with Platelets Across Human Diseases, including Alzheimer's Disease, Amyotrophic Lateral Sclerosis, and Cancer. Curr Top Med Chem. 2023, Oct 2. [CrossRef]
- Pépin, É.; Jalinier, T.; Lemieux, G.L.; Massicotte, G.; Cyr, M. Sphingosine-1-Phosphate Receptors Modulators Decrease Signs of Neuroinflammation and Prevent Parkinson's Disease Symptoms in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Mouse Model. Front Pharmacol. 2020, 11, 77. [Google Scholar] [CrossRef]
- Montanari, M.; Imbriani, P.; Bonsi, P.; Martella, G.; Peppe, A. Beyond the Microbiota: Understanding the Role of the Enteric Nervous System in Parkinson's Disease from Mice to Human. Biomedicines. 2023, 11(6), 1560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hao, Y.; Shabanpoor, A.; Metz, G. A. Stress and corticosterone alter synaptic plasticity in a rat model of Parkinson's disease. Neurosc Lett. 2017, 651, 79–87 doiorg/101016/jneulet201704063. [Google Scholar] [CrossRef] [PubMed]
- Soares, N. M., Pereira, G. M., Altmann, V., de Almeida, R. M. M., & Rieder, C. R. M. Cortisol levels, motor, cognitive and behavioral symptoms in Parkinson's disease: a systematic review. J Neural Transm (Vienna). 2019, 126(3), 219-232. [CrossRef]
- Anderson, G.; Maes, M. TRYCAT pathways link peripheral inflammation, nicotine, somatization and depression in the etiology and course of Parkinson's disease. CNS Neurol Disord Drug Targets. 2014, 13(1), 137–49. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Anderson, G.; Maes, M. Hypothalamic-Pituitary-Adrenal Hypofunction in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS) as a Consequence of Activated Immune-Inflammatory and Oxidative and Nitrosative Pathways. Mol Neurobiol. 2017, 54(9), 6806–6819. [Google Scholar] [CrossRef]
- Muntsant, A.; Giménez-Llort, L. Crosstalk of Alzheimer's disease-phenotype, HPA axis, splenic oxidative stress and frailty in late-stages of dementia, with special concerns on the effects of social isolation: A translational neuroscience approach. Front Aging Neurosci. 2022, 14, 969381. [Google Scholar] [CrossRef]
- Hickie, I.B.; Naismith, S.L.; Robillard, R.; Scott, E.M.; Hermens, D.F. Manipulating the sleep-wake cycle and circadian rhythms to improve clinical management of major depression. BMC Med. 2013, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Robertson-Dixon, I.; Murphy, M.J.; Crewther, S.G.; Riddell, N. The Influence of Light Wavelength on Human HPA Axis Rhythms: A Systematic Review. Life (Basel). 2023, 13(10), 1968. [Google Scholar] [CrossRef] [PubMed]
- Law, R.; Clow, A. Stress, the cortisol awakening response and cognitive function. Int Rev Neurobiol. 2020, 150, 187–217. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. Melatonin, BAG-1 and cortisol circadian interactions in tumor pathogenesis and patterned immune responses. Explor Target Antitumor Ther. 2023, 4, 962–93. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. Why are aging and stress associated with dementia, cancer, and other diverse medical conditions? Role of pineal melatonin interactions with the HPA axis in mitochondrial regulation via BAG-1. Melatonin Res. 2023, 6(3), 345–371. [Google Scholar] [CrossRef]
- Quiros, I.; Mayo, J.C.; Garcia-Suarez, O.; Hevia, D.; Martin, V.; Rodríguez, C.; Sainz, R.M. Melatonin prevents glucocorticoid inhibition of cell proliferation and toxicity in hippocampal cells by reducing glucocorticoid receptor nuclear translocation. J Steroid Biochem Mol Biol. 2008; 110, 1-2, 116–24. [Google Scholar] [CrossRef]
- Kim, M.; Lee, H.A.; Cho, H.M.; Kang, S.H.; Lee, E.; Kim, I.K. Histone deacetylase inhibition attenuates hepatic steatosis in rats with experimental Cushing's syndrome. Korean J Physiol Pharmacol. 2018, 22(1), 23–33. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Zhang, Y.; Zhang, J.; Zhou, F.; Zhang, L.; Wang, S.; Zhu, Q.; Liu, Q.; Wang, X.; Zhou, L. Acetylation of Hsp90 reverses dexamethasone-mediated inhibition of insulin secretion. Toxicol Lett. 2020, 320, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. Daytime orexin and night-time melatonin regulation of mitochondria melatonin:roles in circadian oscillations systemically and centrally in breast cancer symptomatology. Melatonin Res. 2019, 2(4) 1-8. [CrossRef]
- Reiter, R.J.; Sharma, R.; Ma, Q.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Escames, G. Inhibition of mitochondrial pyruvate dehydrogenase kinase: a proposed mechanism by which melatonin causes cancer cells to overcome cytosolic glycolysis, reduce tumor biomass and reverse insensitivity to chemotherapy. Melatonin Res. 2019, 2, 105–19. [Google Scholar] [CrossRef]
- Kermer, P.; Köhn, A.; Schnieder, M.; Lingor, P.; Bähr, M.; Liman, J.; Dohm, C.P. BAG1 is neuroprotective in in vivo and in vitro models of Parkinson's disease. J Mol Neurosci. 2015, 55(3), 587–95. [Google Scholar] [CrossRef]
- Meka, S.T.; Bojja, S.L.; Kumar, G.; Birangal, S.R.; Rao, C. M. Novel HDAC inhibitors provide neuroprotection in MPTP-induced Parkinson's disease model of rats. Eur J Pharmacol. 2023, 959, 176067. [Google Scholar] [CrossRef]
- Guttuso, T.; Jr, Shepherd, R. ; Frick, L.; Feltri, M.L.; Frerichs, V.; Ramanathan, M.; Zivadinov, R.; Bergsland, N. Lithium's effects on therapeutic targets and MRI biomarkers in Parkinson's disease: A pilot clinical trial. IBRO Neurosci Rep. 2023, 14, 429–434. [Google Scholar] [CrossRef]
- Zhou, R.; Gray, N.A.; Yuan, P.; Li, X.; Chen, J.; Chen, G.; Damschroder-Williams, P.; Du, J.; Zhang, L.; Manji, H.K. The anti-apoptotic, glucocorticoid receptor cochaperone protein BAG-1 is a long-term target for the actions of mood stabilizers. J Neurosci. 2005, 25(18), 4493–502. [Google Scholar] [CrossRef]
- Luo, S.; Hou, Y.; Zhang, Y.; Feng, L.; Hunter, R.G.; Yuan, P.; Jia, Y.; Li, H.; Wang, G.; K Manji, H.; S McEwen, B.; Xiao, C.; Bao, H.; Du, J. Bag-1 mediates glucocorticoid receptor trafficking to mitochondria after corticosterone stimulation: Potential role in regulating affective resilience. J Neurochem. 2021, 158(2), 358–372. [Google Scholar] [CrossRef]
- Németh, H.; Toldi, J.; Vécsei, L. Role of kynurenines in the central and peripheral nervous systems. Curr Neurovasc Res. 2005, 2(3), 249–60. [Google Scholar] [CrossRef]
- Qin, W.; Shi, Y.; Chen, W.; Jia, X.; Asakawa, T. Can kynurenine pathway be considered as a next-generation therapeutic target for Parkinson's disease? An update information. Biosci Trends. 2022, 16(4), 249–256. [Google Scholar] [CrossRef]
- Anderson, G.; Maes, M. Neurodegeneration in Parkinson's disease: interactions of oxidative stress, tryptophan catabolites and depression with mitochondria and sirtuins. Mol Neurobiol. 2014, 49(2), 771–83. [Google Scholar] [CrossRef]
- Chen, P.; Geng, X. Research progress on the kynurenine pathway in the prevention and treatment of Parkinson's disease. J Enzyme Inhib Med Chem. 2023, 38(1), 2225800. [Google Scholar] [CrossRef]
- Anderson, G.; Carbone, A.; Mazzoccoli, G. Aryl Hydrocarbon Receptor Role in Co-Ordinating SARS-CoV-2 Entry and Symptomatology: Linking Cytotoxicity Changes in COVID-19 and Cancers; Modulation by Racial Discrimination Stress. Biology (Basel). 2020, 9(9), 249. [Google Scholar] [CrossRef]
- Fernandes, P.A.; Tamura, E.K.; D'Argenio-Garcia, L.; Muxel, S.M.; da Silveira Cruz-Machado, S.; Marçola, M.; Carvalho-Sousa, C.E.; Cecon, E.; Ferreira, Z.S.; Markus, R.P. Dual Effect of Catecholamines and Corticosterone Crosstalk on Pineal Gland Melatonin Synthesis. Neuroendocrinology. 2017, 104(2), 126–134. [Google Scholar] [CrossRef]
- Vanuytsel, T.; van Wanrooy, S.; Vanheel, H.; Vanormelingen, C.; Verschueren, S.; Houben, E.; Salim Rasoel, S.; Tόth, J.; Holvoet, L.; Farré, R.; Van Oudenhove, L.; Boeckxstaens, G.; Verbeke, K.; Tack, J. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut, 63(8), 2014, 1293–1299. [CrossRef]
- Shukla, P.K.; Meena, A.S.; Pierre, J.F.; Rao, R. Central role of intestinal epithelial glucocorticoid receptor in alcohol- and corticosterone-induced gut permeability and systemic response. FASEB J. 2022, 36(1), e22061. [Google Scholar] [CrossRef]
- Khan, H.N.; Perlee, D.; Schoenmaker, L.; van der Meer, A.J.; Franitza, M.; Toliat, M.R.; Nürnberg, P.; Zwinderman, A.H.; van der Poll, T.; Scicluna, B. P. Leukocyte transcriptional signatures dependent on LPS dosage in human endotoxemia. J Leukoc Biol. 2019, 106(5), 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Fan, L.; Yue, B.H.; Lou, Z. Saikosaponin A mitigates the progression of Parkinson's disease via attenuating microglial neuroinflammation through TLR4/MyD88/NF-κB pathway. Eur Rev Med Pharmacol Sci. 2023, 27(15), 6956–6971. [Google Scholar] [CrossRef]
- Rannikko, E.H.; Weber, S.S.; Kahle, P.J. Exogenous α-synuclein induces toll-like receptor 4 dependent inflammatory responses in astrocytes. BMC Neurosci. 2015, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.; Kodi, T.; Satarker, S.; Gurram, P.C.; Fayaz, S.M.; Nampoothiri, M. Astrocytic transcription factors REST, YY1, and putative microRNAs in Parkinson's disease and advanced therapeutic strategies. Gene. 2023, Oct 11, 892:147898. [CrossRef]
- Karki, P.; Webb, A.; Smith, K.; Johnson, J.; Jr, Lee, K.; Son, D S.; Aschner, M.; Lee, E.Yin Yang 1 is a repressor of glutamate transporter EAAT2, and it mediates manganese-induced decrease of EAAT2 expression in astrocytes. Mol Cell Biol. 2014, 34(7), 1280-9. [CrossRef]
- Sheng, L.; Stewart, T.; Yang, D.; Thorland, E.; Soltys, D.; Aro, P.; Khrisat, T.; Xie, Z.; Li, N.; Liu, Z.; Tian, C.; Bercow, M.; Matsumoto, J.; Zabetian, C.P.; Peskind, E.; Quinn, J.F.; Shi, M.; Zhang, J. Erythrocytic α-synuclein contained in microvesicles regulates astrocytic glutamate homeostasis: a new perspective on Parkinson's disease pathogenesis. Acta Neuropathol Commun. 2020, 8(1), 102. [Google Scholar] [CrossRef] [PubMed]
- Muxel, S.M.; Pires-Lapa, M.A.; Monteiro, A.W.; Cecon, E.; Tamura, E K.; Floeter-Winter, L.M.; Markus, R.P. NF-κB drives the synthesis of melatonin in RAW 264.7 macrophages by inducing the transcription of the arylalkylamine-N-acetyltransferase (AA-NAT) gene. PLoS One. 2012, 7(12), e52010. [CrossRef]
- Bernard, M.; Voisin, P. Photoreceptor-specific expression, light-dependent localization, and transcriptional targets of the zinc-finger protein Yin Yang 1 in the chicken retina. J Neurochem. 2008, 105(3), 595–604. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhuang, J.; Zhu, H.Y.; Shen, Y.X.; Tan, Z.L.; Zhou, J.N. Cultured rat cortical astrocytes synthesize melatonin: absence of a diurnal rhythm. J Pineal Res. 2007, 43(3), 232–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Behnisch, T. The Enigmatic CA2: Exploring the Understudied Region of the Hippocampus and Its Involvement in Parkinson's Disease. Biomedicines. 2023, 11(7), 1996. [Google Scholar] [CrossRef] [PubMed]
- Melachroinou, K.; Divolis, G.; Tsafaras, G.; Karampetsou, M.; Fortis, S.; Stratoulias, Y.; Papadopoulou, G.; Kriebardis, A.G.; Samiotaki, M.; Vekrellis, K. Endogenous Alpha-Synuclein is Essential for the Transfer of Pathology by Exosome-Enriched Extracellular Vesicles, Following Inoculation with Preformed Fibrils in vivo. Aging Dis. 2023, Aug 4. [CrossRef]
- Dai, L.; Wang, J.; Zhang, X.; Yan, M.; Zhou, L.; Zhang, G.; Meng, L.; Chen, L.; Cao, X.; Zhang, Z.; Wang, G.; Zhang, Z. 27-Hydroxycholesterol Drives the Spread of α-Synuclein Pathology in Parkinson's Disease. Mov Disord. 2023, Aug 18. [CrossRef]
- Ku, H.; Kim, Y.; Kim, A.L.; Lee, G.; Choi, Y.; Kim, B. Protective Effects of Melatonin in High-Fat Diet-Induced Hepatic Steatosis via Decreased Intestinal Lipid Absorption and Hepatic Cholesterol Synthesis. Endocrinol Metab (Seoul). 2023, 38(5), 557–567. [Google Scholar] [CrossRef] [PubMed]
- Ou, T.H.; Tung, Y.T.; Yang, T.H.; Chien, Y.W. Melatonin Improves Fatty Liver Syndrome by Inhibiting the Lipogenesis Pathway in Hamsters with High-Fat Diet-Induced Hyperlipidemia. Nutrients. 2019, 11(4), 748. [Google Scholar] [CrossRef]
- Kakimoto, T.; Hosokawa, M.; Ichimura-Shimizu, M.; Ogawa, H.; Miyakami, Y.; Sumida, S.; Tsuneyama, K. Accumulation of α-synuclein in hepatocytes in nonalcoholic steatohepatitis and its usefulness in pathological diagnosis. Pathol Res Pract. 2023, 247, 154525. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, Z.; Li, Z.; Ling, H.; Song, C. Role and mechanism of action of butyrate in atherosclerotic diseases: a review. J Appl Microbiol. 2021, 131(2), 543–552. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.P.; Matzkin, M.E.; Riviere, E.; Martinez, G.; Ponzio, R.; Levalle, O.; Terradas, C.; Calandra, R.S.; Frungieri, M.B. Melatonin improves oxidative state and lactate metabolism in rodent Sertoli cells. Mol Cell Endocrinol. 2023, 576, 112034. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, P.W.; Nadarajah, C.J.; Kanan, M.F.; Patterson, J.N.; Novotny, B.; Lawrence, J.H.; King, M.W.; Brase, L.; Inman, C.E.; Yuede, C.M.; Lee, J.; Patel, T.K.; Harari, O.; Benitez, B.A.; Davis, A A.; Musiek, E.S. An astrocyte BMAL1-BAG3 axis protects against alpha-synuclein and tau pathology. Neuron. 2023, 111(15), 2383-2398.e7. [CrossRef]
- Espinoza-Vinces, C.; Villino-Rodríguez, R.; Atorrasagasti-Villar, A.; Martí-Andrés, G.; Luquin, M.R. Impact of Safinamide on Patient-Reported Outcomes in Parkinson's Disease. Patient Relat Outcome Meas. 2023, 14, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Underwood, R.; Gannon, M.; Pathak, A.; Kapa, N.; Chandra, S.; Klop, A.; Yacoubian, T.A. 14-3-3 mitigates alpha-synuclein aggregation and toxicity in the in vivo preformed fibril model. Acta Neuropathol Commun. 2021, 9(1), 13. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, R; Petit, C.M.; Yacoubian, T.A. 14-3-3 phosphorylation inhibits 14-3-3θ's ability to regulate LRRK2 kinase activity. bioRxiv [Preprint]. 2023, 2023.05.27.542591. [CrossRef]
- Vinueza-Gavilanes, R.; Bravo-González, J.J.; Basurco, L.; Boncristiani, C.; Fernández-Irigoyen, J.; Santamaría, E.; Marcilla, I.; Pérez-Mediavilla, A.; Luquin, M.R.; Vales, A.; González-Aseguinolaza, G.; Aymerich, M.S.; Aragón, T.; Arrasate, M. Stabilization of 14-3-3 protein-protein interactions with Fusicoccin-A decreases alpha-synuclein dependent cell-autonomous death in neuronal and mouse models. Neurobiol Dis. 2023, 183, 106166. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Park, J.; Hwang, E.M.; Kim, H.W.; Park, J.Y. 14-3-3γ haploinsufficiency leads to altered dopamine pathway and Parkinson's disease-like motor incoordination in mice. Mol Brain. 2023, 16(1), 2. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Thorn, D.C.; Dobson, C.M.; Meehan, S.; Jackson, S.E.; Woodcock, J.M.; Carver, J.A. The Amyloid Fibril-Forming β-Sheet Regions of Amyloid β and α-Synuclein Preferentially Interact with the Molecular Chaperone 14-3-3ζ. Molecules. 2021, 26(20), 6120. [Google Scholar] [CrossRef] [PubMed]
- Giusto, E.; Yacoubian, T.A.; Greggio, E.; Civiero, L. Pathways to Parkinson's disease: a spotlight on 14-3-3 proteins. NPJ Parkinsons Dis. 2021, 7(1), 85. [Google Scholar] [CrossRef] [PubMed]
- Pagan, C.; Goubran-Botros, H.; Delorme, R.; Benabou, M.; Lemière, N.; Murray, K.; Amsellem, F.; Callebert, J.; Chaste, P.; Jamain, S.; Fauchereau, F.; Huguet, G.; Maronde, E.; Leboyer, M.; Launay, J.M.; Bourgeron, T. Disruption of melatonin synthesis is associated with impaired 14-3-3 and miR-451 levels in patients with autism spectrum disorders. Sci Rep. 2017, 7(1), 2096. [Google Scholar] [CrossRef]
- Mokkawes, T.; De Visser, T.; Cao, Y.; De Visser, S. P. Melatonin Activation by Human Cytochrome P450 Enzymes: A Comparison between Different Isozymes. Molecules. 2023, 28(19), 6961. [Google Scholar] [CrossRef]
- Ma, X.; Idle, J.R.; Krausz, K.W.; Gonzalez, F.J. Metabolism of melatonin by human cytochromes p450. Drug Metab Dispos. 2005, 33(4), 489–94. [Google Scholar] [CrossRef] [PubMed]
- Fenner, M.E.; Achim, C.L.; Fenner, B.M. Expression of full-length and truncated trkB in human striatum and substantia nigra neurons: implications for Parkinson's disease. J Mol Histol. 2014, 45(3), 349–61. [Google Scholar] [CrossRef] [PubMed]
- Jin, W. Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson's Disease. J Clin Med. 2020, 9(1), 257. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, F.R.; Siemen, D.; Mawrin, C.; Horn, T.F.; Dietzmann, K. The neurotrophin receptor TrkB is colocalized to mitochondrial membranes. Int J Biochem Cell Biol. 2006, 38(4), 610–20. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.W. , Liu, X., Pradoldej, S., Tosini, G., Chang, Q., Iuvone, P.M., Ye, K. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc Natl Acad Sci U S A. 2010, 107(8), 3876–81. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, W.J.; Quan, W.; Qiao, C.M.; Cui, C.; Hong, H.; Shi, Y.; Niu, G.Y.; Zhao, L.P.; Shen, Y.Q. Dynamic changes of activated AHR in microglia and astrocytes in the substantia nigra-striatum system in an MPTP-induced Parkinson's disease mouse model. Brain Res Bull. 2021, 176, 174–183. [Google Scholar] [CrossRef]
- Maussion, G.; Yang, J.; Yerko, V.; Barker, P.; Mechawar, N.; Ernst, C.; Turecki, G. Regulation of a truncated form of tropomyosin-related kinase B (TrkB) by Hsa-miR-185* in frontal cortex of suicide completers. PLoS One. 2012, 7(6), e39301. [Google Scholar] [CrossRef] [PubMed]
- Tanqueiro, S.R.; Ramalho, R.M.; Rodrigues, T.M.; Lopes, L.V.; Sebastião, A.M.; Diógenes, M.J. Inhibition of NMDA Receptors Prevents the Loss of BDNF Function Induced by Amyloid β. Front Pharmacol. 2018, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Vadukul, D.M.; Papp, M.; Thrush, R.J.; Wang, J.; Jin, Y.; Arosio, P.; Aprile, F.A. α-Synuclein Aggregation Is Triggered by Oligomeric Amyloid-β 42 via Heterogeneous Primary Nucleation. J Am Chem Soc. 2023, 145(33), 18276–18285. [Google Scholar] [CrossRef] [PubMed]
- Mroczek, K.; Fernando, S.; Fisher, P.R.; Annesley, S.J. Interactions and Cytotoxicity of Human Neurodegeneration- Associated Proteins Tau and α-Synuclein in the Simple Model Dictyostelium discoideum. Front Cell Dev Biol. 2021, 9, 741662. [Google Scholar] [CrossRef]
- Sun, C. , Qiu, X., Wang, Y., Liu, J., Li, Q., Jiang, H., Li, S., & Song, C. (2020). Long-term oral melatonin alleviates memory deficits, reduces amyloid-β deposition associated with downregulation of BACE1 and mitophagy in APP/PS1 transgenic mice. Neurosci Lett. 2020, 735, 135192. [Google Scholar] [CrossRef]
- Das, R. , Balmik, A. A., & Chinnathambi, S. Melatonin Reduces GSK3β-Mediated Tau Phosphorylation, Enhances Nrf2 Nuclear Translocation and Anti-Inflammation. ASN Neuro. 2020, 12, 1759091420981204. [Google Scholar] [CrossRef]
- Shukla, M.; Htoo, H.H.; Wintachai, P.; Hernandez, J.F.; Dubois, C.; Postina, R.; Xu, H.; Checler, F.; Smith, D.R.; Govitrapong, P; Vincent, B. Melatonin stimulates the nonamyloidogenic processing of βAPP through the positive transcriptional regulation of ADAM10 and ADAM17. J Pineal Res. 2015, 58(2), 151-65. [CrossRef]
- Panmanee, J.; Nopparat, C.; Chavanich, N.; Shukla, M.; Mukda, S.; Song, W.; Vincent, B.; Govitrapong, P. Melatonin regulates the transcription of βAPP-cleaving secretases mediated through melatonin receptors in human neuroblastoma SH-SY5Y cells. J Pineal Res. 2015 Oct;59(3):308-20. [CrossRef]
- Choi, G.E.; Lee, S.J.; Lee, H.J.; Ko, S.H.; Chae, C.W.; Han, H.J. Membrane-Associated Effects of Glucocorticoid on BACE1 Upregulation and Aβ Generation: Involvement of Lipid Raft-Mediated CREB Activation. J Neurosci. 2017, 37(35), 8459–8476. [Google Scholar] [CrossRef]
- Choi, G.E.; Park, J.Y.; Park, M.R.; Yoon, J.H.; Han, H.J. Glucocorticoid enhances presenilin1-dependent Aβ production at ER's mitochondrial-associated membrane by downregulating Rer1 in neuronal cells. Redox Biol. 2023, 65, 102821. [Google Scholar] [CrossRef]
- Titze-de-Almeida, R.; Titze-de-Almeida, S.S.; Ferreira, G.G.; Brito Silva, A.P.; de Paula Brandão, P.R.; Oertel, W.H.; Schenck, C.H.; Delgado Rodrigues, R.N. microRNA signatures in prodromal REM sleep behavior disorder and early Parkinson's disease as noninvasive biomarkers. Sleep Med. 2021, 78, 160–168. [Google Scholar] [CrossRef]
- Kunz, D.; Oster, H.; Rawashdeh, O.; Neumann, W.J.; Münte, T.; Berg, D. Sleep and circadian rhythms in α-synucleinopathies-Perspectives for disease modification. Acta Physiol (Oxf). 2023, 238(1), e13966. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, K.; So, H.S.; Choi, J.H.; Yoon, I.Y.; Choi, H. REM Sleep Behavior Disorder among Veterans with and without Post-Traumatic Stress Disorder. Psychiatry Investig. 2020, 17(10), 987–995. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Garay, A. Melatonin as a Chronobiotic/Cytoprotective Agent in REM Sleep Behavior Disorder. Brain Sci. 2023, 13(5), 797. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Chau, S.W.H.; Liu, Y.; Chan, J.W.Y.; Wang, J.; Ma, S.L.; Zhang, J.; Chan, P.K.S.; Yeoh, Y.K.; Chen, Z.; Zhou, L.; Wong, S.H.; Mok, V.C.T.; To, K.F.; Lai, H.M.; Ng, S.; Trenkwalder, C.; Chan, F.K.L.; Wing, Y.K. Gut microbiome dysbiosis across early Parkinson's disease, REM sleep behavior disorder and their first-degree relatives. Nat Commun. 2023, 14(1), 2501. [Google Scholar] [CrossRef]
- Liang, S.Q.; Li, P.H.; Hu, Y.Y.; Zhao, J L. ; Shao, F.Z.; Kuang, F.; Ren, K.X.; Wei, T.X.; Fan, F.; Feng, L.; Han, H.; Qin, H.Y. Myeloid-specific blockade of notch signaling alleviates dopaminergic neurodegeneration in Parkinson's disease by dominantly regulating resident microglia activation through NF-κB signaling. Front Immunol. 2023, 14, 1193081. [Google Scholar] [CrossRef]
- Bu, X.L.; Wang, X.; Xiang, Y.; Shen, L.L.; Wang, Q.H.; Liu, Y.H.; Jiao, S.S.; Wang, Y.R.; Cao, H.Y.; Yi, X.; Liu, C.H.; Deng, B.; Yao, X.Q.; Xu, Z.Q.; Zhou, H.D.; Wang, Y.J. The association between infectious burden and Parkinson's disease: A case-control study. Parkinsonism Relat Disord. 2015, 21(8), 877–81. [Google Scholar] [CrossRef] [PubMed]
- Powell-Doherty, R.D.; Abbott, A.R.N.; Nelson, L.A.; Bertke, A.S. Amyloid-β and p-Tau Anti-Threat Response to Herpes Simplex Virus 1 Infection in Primary Adult Murine Hippocampal Neurons. J Virol. 2020, 94(9), e01874–19. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; He, Z. Concomitant protein pathogenesis in Parkinson's disease and perspective mechanisms. Front Aging Neurosci. 2023, 15, 1189809. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. Depression Pathophysiology: Astrocyte Mitochondrial Melatonergic Pathway as Crucial Hub. Int J Mol Sci. 2022, 24(1), 350. [Google Scholar] [CrossRef] [PubMed]
- Caggiu, E.; Paulus, K.; Arru, G.; Piredda, R.; Sechi, G.P.; Sechi, L.A. Humoral cross reactivity between α-synuclein and herpes simplex-1 epitope in Parkinson's disease, a triggering role in the disease? J Neuroimmunol. 2016, 291, 110–4. [Google Scholar] [CrossRef] [PubMed]
- Caggiu, E.; Paulus, K.; Galleri, G.; Arru, G.; Manetti, R.; Sechi, G.P.; Sechi, L.A. Homologous HSV1 and alpha-synuclein peptides stimulate a T cell response in Parkinson's disease. J Neuroimmunol. 2017, 310, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Garretti, F.; Agalliu, D.; Lindestam Arlehamn, C.S.; Sette, A.; Sulzer, D. Autoimmunity in Parkinson's Disease: The Role of α-Synuclein-Specific T Cells. Front Immunol. 2019, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Campos-Acuña, J.; Elgueta, D.; Pacheco, R. T-Cell-Driven Inflammation as a Mediator of the Gut-Brain Axis Involved in Parkinson's Disease. Front Immunol. 2019, 10, 239. [Google Scholar] [CrossRef]
- Joubert, M.; André, M.; Barnich, N.; Billard, E. Microbiome and Behçet's disease: a systematic review. Clin Exp Rheumatol. 2023, Jun 7. [CrossRef]
- Mesnage, R.; Antoniou, M.N. Computational modelling provides insight into the effects of glyphosate on the shikimate pathway in the human gut microbiome. Curr. Res. Toxicol. 2020, 1, 25–33. [Google Scholar] [CrossRef]
- Amorim Neto, D.P.; Bosque, B.P.; Pereira de Godoy, J.V.; Rodrigues, P.V.; Meneses, D.D.; Tostes, K.; Costa Tonoli, C.C.; Faustino de Carvalho, H.; González-Billault, C.; de Castro Fonseca, M. Akkermansia muciniphila induces mitochondrial calcium overload and α -synuclein aggregation in an enteroendocrine cell line. iScience. 2022, 25(3), 103908. [Google Scholar] [CrossRef]
- Dopkins, N.; Becker, W.; Miranda, K.; Walla, M.; Nagarkatti, P.; Nagarkatti, M. Tryptamine Attenuates Experimental Multiple Sclerosis Through Activation of Aryl Hydrocarbon Receptor. Front Pharmacol. 2021, 11, 619265. [Google Scholar] [CrossRef]
- Nunes Oda, S.; Pereira Rde, S. Regression of herpes viral infection symptoms using melatonin and SB-73: comparison with Acyclovir. J Pineal Res. 2008, 44(4), 373–8. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.l Cao, J.l Dong, Y.; Chen, Y. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome. 2023, 11(1), 17. [CrossRef]
- Goswami, P.; Ives, A.M.; Abbott, A.R.N.; Bertke, A.S. Stress Hormones Epinephrine and Corticosterone Selectively Reactivate HSV-1 and HSV-2 in Sympathetic and Sensory Neurons. Viruses. 2022, 14(5), 1115. [Google Scholar] [CrossRef]
- Harrison, K.S.; Wijesekera, N.; Robinson, A.G.J.; Santos, V.C.; Oakley, R.H.; Cidlowski, J.A.; Jones, C. Impaired glucocorticoid receptor function attenuates herpes simplex virus 1 production during explant-induced reactivation from latency in female mice. J Virol. 2023, Oct 12, e0130523. [Google Scholar] [CrossRef]
- Santos, V.C.; Ostler, J.B.; Harrison, K.S.; Jones, C. Slug, a Stress-Induced Transcription Factor, Stimulates Herpes Simplex Virus 1 Replication and Transactivates a cis-Regulatory Module within the VP16 Promoter. J Virol. 2023, 97(4), e0007323. [Google Scholar] [CrossRef]
- Ogarek, N.; Oboza, P.; Olszanecka-Glinianowicz, M.; Kocelak, P. SARS-CoV-2 infection as a potential risk factor for the development of cancer. Front Mol Biosci. 2023, 10, 1260776. [Google Scholar] [CrossRef]
- Cohen, M.; Austin, E.; Bradu, S.; Jagdeo, J. The Association Between Herpes Simplex Virus and Alzheimer's Disease: A Systematic Review. J Drugs Dermatol. 2023, 22(10), 1046–1052. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; Zale, E.; Wang, Y.; Huang, Y.; Theriault, B.; Dinner, A.R.; Musch, M.W.; Kudsk, K.A.; Prendergast, B.J.; Gilbert, J.A.; Chang, E.B. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015, 17(5), 681–9. [Google Scholar] [CrossRef]
- Park, H.; Jin, U.H.; Karki, K.; Jayaraman, A.; Allred, C.; Michelhaugh, S.K.; Mittal, S.; Chapkin, R.S.; Safe, S. Dopamine is an aryl hydrocarbon receptor agonist. Biochem J. 2020, 477(19), 3899–3910. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Slominski, R.M.; Song, Y.; Qayyum, S.; Placha, W.; Janjetovic, Z.; Kleszczyński, K.; Atigadda, V.; Song, Y.; Raman, C.; Elferink, C.J.; Hobrath, J.V.; Jetten, A.M.; Reiter, R.J. Melatonin and Its Metabolites Can Serve as Agonists on the Aryl Hydrocarbon Receptor and Peroxisome Proliferator-Activated Receptor Gamma. Int J Mol Sci. 2023, 24(20), 15496. [Google Scholar] [CrossRef]
- Milutinović, D. V., Macut, D., Božić, I., Nestorov, J., Damjanović, S., & Matić, G. (2011). Hypothalamic-pituitary-adrenocortical axis hypersensitivity and glucocorticoid receptor expression and function in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2011, 119(10), 636-43. [CrossRef]
- Keighron, C.N.; Avazzadeh, S.; Goljanek-Whysall, K.; McDonagh, B.; Howard, L.; Ritter, T.; Quinlan, L.R. Extracellular Vesicles, Cell-Penetrating Peptides and miRNAs as Future Novel Therapeutic Interventions for Parkinson's and Alzheimer's Disease. Biomedicines. 2023, 11(3), 728. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




