1. Introduction
S-adenosylmethionine (SAM) is a crucial intermediate metabolite resulting from the catalytic processes of methionine and ATP by methionine adenosyltransferase [
1]. Although SAM was initially synthesized in 1940 [
2], it gained significant attention only after the chemical structure was described in 1953 [
3]. Over the past 50 years, extensive research has been conducted, which suggests SAM as a potential tool for the management of various disorders. SAM is currently marketed in the United States as a dietary supplement and a viable alternative for treating depression [
4], osteoarthritis [
5], and liver diseases [
6].
Clinical trials have indicated that SAM may be an effective option for addressing osteoarthritis [
7,
8,
9,
10]. Specifically, it showed promising results in stimulating proteoglycan synthesis and secretion in cultured human chondrocytes [
11,
12]. SAM is recognized as the primary methyl donor in various biological processes, including DNA methylation, polyamine synthesis, and transsulfuration [
13]. Despite the impressive safety and potential benefits of SAM [
5], the exact mechanism underlying its effectiveness in the management of osteoarthritis remains incompletely understood.
Polyamines are small organic compounds containing multiple amines and are known to be essential for cell growth, differentiation, and apoptosis [
14]. In mammalian cells, three types of polyamines, putrescine, spermine, and spermidine, are synthesized. The first polyamine putrescine is converted from L-ornithine by ornithine decarboxylase (ODC), a rate-limiting enzyme of polyamine synthesis. Spermidine and spermine are then synthesized by sequential addition of aminopropyl groups of decarboxylated SAM (dcSAM) to each end of the putrescine [
15]. Interestingly, spermidine and spermine produced with SAM by transaminopropylation, have been reported to enhance chondrogenesis, increase type II collagen deposition, and stimulate chondrocyte terminal differentiation [
16]. We previously reported that induction of ODC followed by an increase in polyamine levels in primary cultured rabbit growth cartilage cells stimulated by differentiation factors such as parathyroid hormone (PTH and) cAMP derivatives precedes the increased expression of the differentiated phenotype of chondrocytes [
17,
18], and that the exposure of the growth chondrocytes to exogenous polyamine increases Glycosaminoglycan (GAG) production, a marker of chondrocyte differentiation [
19]. However, whether SAM is clinically effective against OA through regulation of polyamine synthesis has not been investigated.
In addition to the involvement of polyamine in SAM’s mechanism of action, this study also focused on the gene expression of chondrocyte markers and chondrocyte differentiation factors, including cellular communication network factor 2 (CCN2). CCN2, a classical member of CCN family of matricellular proteins, also known as CTGF, recently renamed as cellular communication network factor 2, was found to have a huge influence on chondrocytes. Having a special structure constructed by four distinct modules (insulin-like growth factor binding protein-like (IGFBP), von Willebrand factor type C repeat (VWC), thrombospondin 1 type 1 repeat (TSP1), and C-terminal cystine knot (CT) modules) [
20,
21], CCN2 possesses a vast binding capacity to an array of molecules. These binding counterparts include aggrecan core protein [
22], heparan sulfate proteoglycans [
23], cell-surface receptors, and growth factors [
24]. This multiple interactions confer the massive influence of CCN2 in a variety of chondrocyte-related signaling pathways [
25]. More importantly, CCN2 has been shown to stimulate the proliferation and differentiation of chondrocytes towards the terminal state in the growth plate, but did not promote chondrocyte hypertrophic differentiation in articular chondrocytes [
26]. Such evidence unequivocally demonstrates that CCN2 must play the role of orchestra conductor in the mechanisms that directly drive chondrocytes toward their distinct destinations with different functionalities in the in articular cartilage and growth plates.
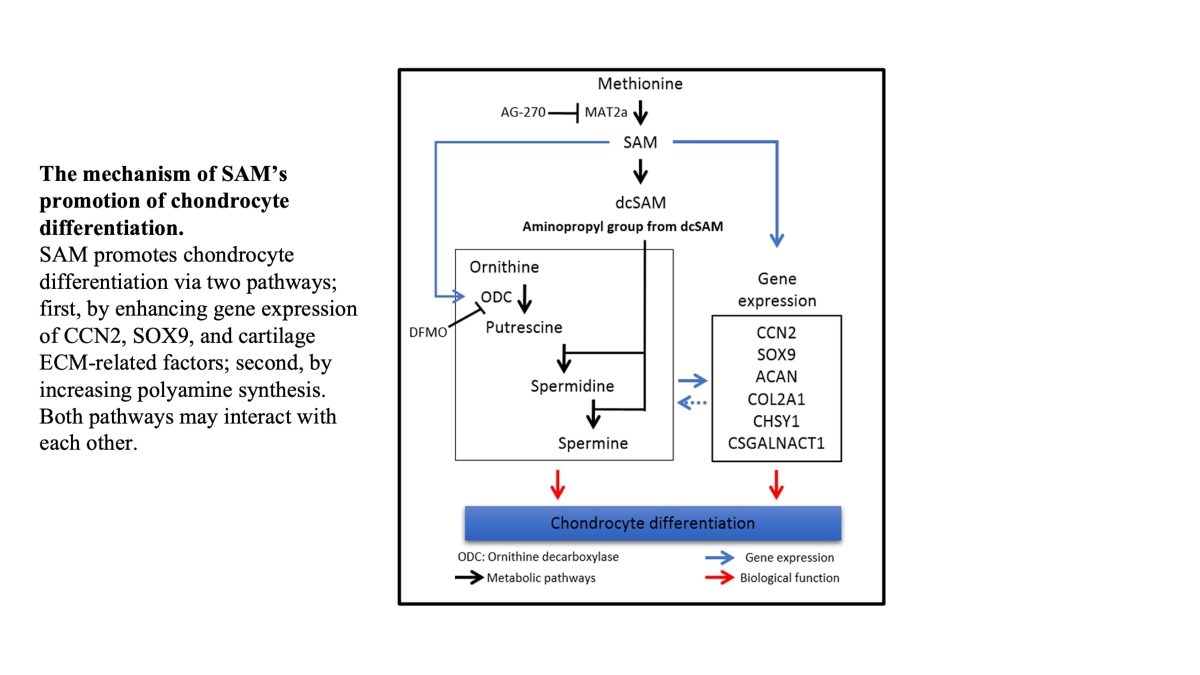
The mechanism of SAM’s effects on chondrocytes are not fully understood. We hypothesized that the metabolic pathways regulated by SAM may induce CCN2 and regulate polyamine synthesis in chondrocytes, thereby promoting chondrocyte differentiation. We herein report that SAM positively regulates chondrocyte differentiation and plays an important role in cartilage protection via the polyamine synthesis pathway and the gene expression of several chondrogenic markers and growth factors.
3. Discussion
In this study, we provide insights into the effects of SAM on chondrocyte differentiation. Previously, Harmand et al. reported that treatment with SAM at a concentration of 10 μg/ml resulted in a significant increase in cartilage-specific proteoglycan (now called aggrecan) determined via
3H-serine and
35S-sulfate incorporation, as well as hexuronic acid in human articular osteoarthritis chondrocytes, but did not reveal its mechanisms [
11]. In this study, Alcian blue staining demonstrated that SAM enhanced sulfated aggrecan accumulation, a typical component of cartilaginous proteoglycan [
28] in HCS-2/8 cells, with the most significant improvement occurring at a concentration of 10 μg/ml (
Figure 1a-c). This finding is consistent with the results of the aforementioned study. We also confirmed our results by conducting the same experiments with RCS cells, and a comparable result was observed: the administration of SAM at 100 μg/ml significantly enhanced the aggrecan production (Supporting
Figure 1a, b). In addition, we found that administering SAM externally resulted in a positive outcome regarding the gene expression of aggrecan core protein, type II collagen, two major markers of cartilaginous ECM, and the chondrogenesis marker SOX9 in HCS-2/8 cells (
Figure 2a-c) and RCS cells (Supporting
Figure 2a-c). These findings indicated that SAM has the potential to enhance proteoglycan production and chondrocyte differentiation.
Our current study indicates that SAM stimulated proteoglycan production at high concentrations (50 or 100 μg/ml for RCS and 5 or 10 μg/ml for HCS-2/8 cells). However, the administration of SAM at these concentrations had an inhibitory effect on cell proliferation in both HCS-2/8 (
Figure 1d, e) and RCS cells (Supporting Figure. 1c, d) without any appreciable damage during culture in the presence of SAM. These phenomena are consistent with a few reports indicating that reported that SAM at high concentrations had a negative effect on cell proliferation [
29,
30,
31]. Taken together, these reports and our results suggest that cell cycle arrest generally occurs as the first step in the differentiation process [
32]. The contrasting effects of SAM in terms of cell proliferation and aggrecan accumulation imply that the enhancing effect of SAM on proteoglycan production is independent from cell proliferation.
Notably, GAG is an important structure for the function of the ECM as a cushion, and chondroitin sulfate (CS) constitutes approximately 80% of GAGs found in adult articular cartilage [
33]. These facts prompted us to evaluated whether SAM has an impact on the gene expression of CS synthesis enzymes in chondrocytes, similar to that of aggrecan core protein. Interestingly, quantitative RT-PCR revealed that the addition of exogenous SAM enhanced the expression of glycosyltransferases;
CHSY1, CHSY3, CSGALNACT1, and
CSGALNACT2 in HCS-2/8 cells (
Figure 2d-g). Considering the positive effect of SAM observed in Alcian blue staining together, these findings indicate that SAM has a positive effect on ECM production via the gene induction of, not only the aggrecan core protein but also the enzymes responsible for the synthesis of chondroitin sulfate, especially
CSGALNACT1 which has been suggested to mainly regulate CS synthesis in cartilage [
34,
35].
Endogenous SAM is described as a natural compound present that occurs in all living cells, participates in diverse biochemical reactions, and involved in an extensive network over many crucial processes [
36]. Therefore, we assumed that endogenous SAM might play a role in the regulation of the cartilage-specific phenotype. In the current study, we observed that the inhibition of intracellular SAM biosynthesis by AG-270 led to a significant drop in aggrecan production and the expression of cartilage-associated genes in HCS-2/8 (
Figure 3c, 4a-e) and RCS cells (Supporting Figure3b-c, 4a-f). These experiments were designed and carried out to evaluate the impact of exogenous SAM, as well as to confirm the role of endogenous SAM in regulating chondrocyte function. Collectively, these results were not merely conformable with the increase in proteoglycan synthesis observed through Alcian blue staining, but could also elucidate the chondroprotective effect that was mentioned in a previous
in vivo study [
37].
Our study showed that exogenous SAM enhanced the gene expression and the protein level of CCN2 in HCS-2/8 cells (
Figure 5a-c). These findings suggest that CCN2 may participate in the mechanism by which SAM stimulates the chondrocyte differentiation. This was also supported by the finding that MAT2A siRNA suppressed CCN2 gene expression (Fig 5e). Nevertheless, AG-270 did not decrease, but rather unexpectedly increased the gene expression of
CCN2 (
Figure 5d). Previous studies have reported that the expression of CCN2 is well correlated with tissue repair [
38,
39,
40] including cartilage regeneration [
41,
42]. This suggests that high concentrations of AG-270 may cause cell damage, and that the increase in the
CCN2 induction may have been triggered as a response to counteract the impairment.
Along with its main function as a common methyl donor, SAM is also involved in the polyamine synthesis pathway as an aminopropyl-group donor. In the early stages, ornithine is decarboxylated by ODC enzyme to produce putrescine, whereas SAM is decarboxylated to dcSAM by the adenosylmethionine decarboxylase 1 (AMD1) enzyme. Putrescine uses an aminopropyl group from dcSAM to produce spermidine and spermine. Therefore, it is quite convincing that a high concentration of polyamines was detected using PolyamineRED™ staining in SAM-treated HCS-2/8 cells (
Figure 6a-b) and RCS cells (Supporting
Figure 4a-b). An HPLC analysis showed that spermine and spermidine levels were respectively upregulated in SAM-treated cells compared to those in non-treated cells (
Figure 6d-e, Supporting
Figure 4d-e). Interestingly, SAM also promoted the gene expression of the rate-limiting enzyme ODC for polyamine synthesis in both HCS-2/8 (Fig 6c) and RCS cells (Supporting
Figure 4c). These data revealed a new insight that SAM plays dual roles in the polyamine synthesis; one is a dcSAM supplier and the other is a promoter of ODC gene expression.
Next, to investigate the contribution of polyamine synthesis to the mechanism underlying the functions of SAM, we used DFMO to completely inhibit polyamine synthesis by interfering with ODC, the rate-limiting enzyme of polyamine synthesis. The results showed that DFMO suppressed aggrecan production in chondrocytes as well as reduced SAM-induced aggrecan accumulation (
Figure 7c). This result indicates that polyamine synthesis plays an important role in the SAM-induced ECM production.
As shown in Figure. 8, the presence of DFMO did not result in a decrease in the expression of the genes of interest in HCS-2/8. This indicates that the expression of these genes at basal levels do not depend on polyamine synthesis. Interestingly, DFMO abolished the positive effect of additional SAM on the expression of all genes in HCS-2/8, which demonstrates that the stimulation of these gene expressions by additional SAM is dependent on polyamine synthesis.
Overall, the endogenous SAM and exogenous SAM showed partially similar behavior, but some results indicated differences in their effects. This is possibly due to the fact that the concentration of SAM in serum is reported to be in the hundreds of nanograms per milliliter [
43], which is considerably lower than the concentration of SAM that was added in this study. Since the concentration of SAM administered as a supplement or drug may be close to the concentration used in the experiments conducted here, the results of this study may help to understand the conditions that may occur
in vivo when these drugs are taken.
Although we provided preliminary results on the effects of SAM on the chondrocytic phenotype, more in vivo experiments are necessary to obtain definitive conclusions. In this study, we identified two pathways as candidates that mediate the effects of SAM on chondrocytes; enhancement of polyamine production and stimulation of the gene expression of the cartilage regeneration factor CCN2. However, the responses of RCS and HCS-2/8 cells to the experimental conditions were different each other. This means that more experimental models must be created to further confirm our hypotheses.
In conclusion, this study elucidated the mechanism of the positive effect of SAM on chondrocytes, that is, stimulation of the gene expression of CCN2, SOX9, and cartilage ECM-related factors and an increase in polyamine synthesis, both of which may interact with each other.
4. Materials and Methods
4.1. Materials
Dulbecco’s modified Eagle’s medium (DMEM), and fetal bovine serum (FBS) were purchased from Nissui Pharmaceutical Co. Ltd. (Tokyo, Japan) and Nichirei Bioscience, Inc. (Tokyo, Japan), respectively. S-adenosylmethionine (SAM), alpha-difluoromethylornithine (DFMO), and Protease Inhibitor Cocktail (#P8340) were purchased from Sigma-Aldrich (Tokyo, Japan). AG-270 was purchased from Selleck Chemicals (Houston, TX).
4.2. Cell culture
Human chondrosarcoma-derived chondrocytic cell line (HCS-2/8) [
44] and rat chondrosarcoma-derived chondrocytic cell line (RCS) [
45] were grown in DMEM supplemented with 10% FBS, 50 μg/ml streptomycin, and 100 units/ml penicillin G and 5% FBS. All cells were maintained in a humidified incubator (5% CO
2 atmosphere) at 37°C until they reached confluence. The culture medium was changed every 3 days.
4.3. Proliferation assay
For cell counting assay, chondrocytes were seeded in 96-well plates and exposed to SAM. After 2 days, the cell proliferation kit-8 (Dojindo, Tokyo, Japan) was used to measure cell growth according to the manufacturer’s instructions. Cell proliferation was measured by measuring the optical absorbance at a wavelength of 450 nm. For counting the number of cells, the cells were cultured as same as the cell counting assay, and cell nuclear were stained with 1 μg/mL 4,6-diamidino-2-phenylindole (DAPI; Sigma, Tokyo, Japan) for 30 min. The number of nuclear was counted as the number of cells by using the Cellomics Array ScanTM High Content Screening System (Thermo Fischer Scientific, Waltham, MA, USA).
4.4. Alcian blue staining
Alcian blue staining was performed to measure the accumulation of aggrecan. Briefly, the cells were fixed with 3% cold acetic acid and immediately stained with 1% Alcian blue 8GX (Sigma, Tokyo, Japan) for 30 min at room temperature. The plates were then washed three times with distilled water to remove excess dye. Guanidine chloride (6 M) was added to extract bound Alcian blue dye and incubated overnight at room temperature. Absorbance at a wavelength of 620 nm was measured using a plate reader.
4.5. Reverse transcription - quantitative PCR
Total RNA was isolated from the cells using the RNeasy Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Isolated total RNA was reverse-transcribed to cDNA using a Takara RNA PCR kit (AMV), version 3.0 (Takara Shuzo. Tokyo, Japan). Next, PCR with cDNA was performed in duplicate using SYBR
® Green Real-time PCR Master Mix (Toyobo, Tokyo, Japan) and StepOne
TM (Applied Biosystems, Foster City, CA, USA). For all quantitative PCR measurements, the total amount of cDNA was standardized based on the expression level of Glyceraldeyde-3-phostphate dehydrogenase (GAPDH). The nucleotide sequences of the primers used in this study are listed in
Table 1 and Supporting
Table S1.
4.6. Western blotting
Cells in each well were harvested by using a scraper and radio immunoprecipitation assay (RIPA) buffer containing Protease Inhibitor Cocktail. After sonication for 10 s and centrifugation at 12,000 ×
g for 10 min, protein solutions were collected as supernatants. The protein concentration in each sample was detected using a Pierce BCA Protein Assay Kit (Thermo Fischer Scientific). The protein solution adjusted to the same concentration was applied to 10% SDS-PAGE and then transferred to a polyvinylidene difluoride membrane (Bio-Rad). The experimental protocol has been previously described [
46]. The proteins of CCN2 and MAT2A were detected with anti-CCN2 antibody (ab6992) (Abcam PLC, Cambridge, England), anti-MAT2A (NB110-94158) (Novus Biologicals LLC, Centennial、CO). To detect the β-actin protein, mouse anti-beta Actin, Monoclonal Antibody (010-27841) (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) and Alexa Fluor
TM 680 goat anti-mouse IgG were used. The images were acquired and calculated by ChemiDoc Imaging system (Bio-Rad). The expression levels of each of the proteins are represented as a value relative to that of β-actin.
4.7. Knockdown of mRNA using MAT2A siRNA
Cells were seeded in 6-well plates at the indicated concentrations and allowed to adhere onto the plate for 24 h. Then, the cells were transfected with 80 pmol of siRNA targeting MAT2A (sc-149292) (Santa Cruz) using Lipofectamine RNAiMAX (Thermo Fischer Scientific) following the manufacturer’s instructions. Scrambled siRNA (sc-37007) was used as control. After 24 hours, the cells were then harvested and subjected to subsequent experiments. The knockdown efficiency of MAT2A siRNA was evaluated by western blotting and RT-qPCR.
4.8. Detection of polyamine level by PolyamineRED staining
Chondrocytes were seeded at the indicated concentrations in 96-well plates and exposed to SAM for three days. At the time of staining, the cells were treated with 30 μM Polyamine RED dye for 1 h. After incubation, the cells were washed three times with phosphate-buffered saline (PBS), followed by DAPI staining and formalin fixation. Fluorescence images and quantitative analysis were performed with the Cellomics Array ScanTM High Content Screening System.
4.9. Determination of SAM level by ELISA
The cells were harvested by a scraper and sonicated on ice in 500 μL of PBS. After centrifugation at 12,000 × g for 10 min at 4°C, the supernatant was removed for ELISA. The SAM ELISA was performed using a SAM ELISA kit (Cell Biolabs, Japan) in accordance with the manufacturer’s instructions.
4.10. Determination of polyamine level by high performance liquid chromatography
Cells were seeded at a density of 60x10
4 cells/well in 6-well plates and treated with SAM for indicated time. Then, the cells were harvested by using a scraper with cold PBS. Cells were homogenized with sonication and centrifuged at 10,000 × g at 4°C. Supernatants were collected and stored at –30°C until use. Polyamines were quantified by dansylation and HPLC as reported previously [
47,
48]. Briefly, supernatant was incubated with 150 nM 1,8-diaminooctane as an internal standard and 4.3% PCA for 30 minutes on ice. After centrifugation at 12,000 ×
g for 15 minutes at 4°C, the resulting supernatant was incubated with 600 µL of 10 mg/mL dansyl chloride and 600 µL of saturated Na
2CO
3 for 30 minutes at 60°C, then incubated with 100 µL of 100 mg/mL
l-proline for 30 minutes at 60°C. Dansyl derivatives were extracted with 1.5 mL of toluene and evaporated in a centrifugal vacuum concentrator, Speedvac Plus (Savant Instruments, Inc., Holbrook, NY). The resulting pellet was resuspended with 100 µL of solvent (acetonitrile:water = 7:3). A HPLC technique using the Chromaster system (Hitachi High-Tech Co., Tokyo, Japan) was used to detect the polyamine content.
4.11. Statistical analysis
All experiments were repeated at least twice, and similar results were obtained. All data are expressed as the mean ± standard deviation (SD). Mean values were compared using Welch’s t-test, one-way ANOVA, or two-way ANOVA, and post hoc comparisons were performed if necessary.
Abbreviations
ACAN, Aggrecan; AMD1, Adenosylmethionine Decarboxylase 1; CCN2, Cellular communication network factor 2; CTGF, Connective tissue growth factor; COL2A1, Collagen type 2a1; DFMO, Difluoromethylornithine; CHSY, Chondroitin sulfate synthase; CSGALNACT, Chondroitin sulfate N-acetylgalactosaminyltransferase; dcSAM, decarboxylated SAM; DMEM, Dulbecco’s modified Eagle’s medium; ECM, Extracellular matrix; ELISA, Enzyme-linked immunosorbent assay; FBS, Fetal bovine serum; GAG, Glycosaminoglycan; GAPDH, Glyceraldeyde-3-phostphate dehydrogenase; HCS-2/8, Human chondrocyte-like cell line-2/8; HPLC, High Performance Liquid Chromatography; MAT2A, Methionine adenosyltransferase 2A; MMP13, Matrix metalloproteinase 13; ODC, Ornithine decarboxylase; PBS, phosphate-buffered saline; RCS, Rat chondrosarcoma derived cell line; SAM, S-adenosylmethionine; siRNA, Small interfering RNA; SOX9, Sry-Box transcription factor 9
Figure 1.
SAM enhanced aggrecan production in chondrocytes, but negatively regulated cell proliferation in chondrocytes. Human chondrocyte-like cell line-2/8 (HCS-2/8) cells (1×105 cells/well) were inoculated in 24-well plates and allowed to adhere for 24 hours. Subsequently, S-adenosylmethionine (SAM) was added to culture at the indicated concentrations and kept incubated for 7 or 14 days. (a) Alcian blue staining revealed an increase of aggrecan accumulation in SAM-treated groups. (b-c) Quantitative measurement of Alcian blue staining on HCS-2/8 cells cultured with 7-day (b) and 14-day (c) of SAM stimulation showed that the addition of SAM significantly enhanced aggrecan production in HCS-2/8 cells (values are represent the fold-changes relative to a non-treated group, one-way ANOVA, Dunnett, n=12). (d) Cell counting assay in cultures with several concentrations of SAM. HCS-2/8 cells (3,000 cells/well) were inoculated in 96-well plates and allowed to adhere for 24 hours. Cells were treated with the indicated concentrations of SAM for 2 days, and cell viability was measured with a WST-8 assay kit, as described in the Materials and Methods. The Y axis shows the relative ratio of absorbance to control that was obtained at a wavelength of 450 nm (ratio =1) (one-way ANOVA, Dunnett, * * *p<0.005, n=3). (e) Counting the number of cells cultured with SAM at several time points. Cells were treated with 10 μg/ml SAM for the indicated days and stained with DAPI. The number of nuclei was counted as the number of the cells using a Cellomics Array ScanTM High Content Screening System. (The numbers of cells in the SAM-treated group and control group at each time point were compared using Welch’s t test, * *p<0.01, * * *p<0.005, n=10).
Figure 1.
SAM enhanced aggrecan production in chondrocytes, but negatively regulated cell proliferation in chondrocytes. Human chondrocyte-like cell line-2/8 (HCS-2/8) cells (1×105 cells/well) were inoculated in 24-well plates and allowed to adhere for 24 hours. Subsequently, S-adenosylmethionine (SAM) was added to culture at the indicated concentrations and kept incubated for 7 or 14 days. (a) Alcian blue staining revealed an increase of aggrecan accumulation in SAM-treated groups. (b-c) Quantitative measurement of Alcian blue staining on HCS-2/8 cells cultured with 7-day (b) and 14-day (c) of SAM stimulation showed that the addition of SAM significantly enhanced aggrecan production in HCS-2/8 cells (values are represent the fold-changes relative to a non-treated group, one-way ANOVA, Dunnett, n=12). (d) Cell counting assay in cultures with several concentrations of SAM. HCS-2/8 cells (3,000 cells/well) were inoculated in 96-well plates and allowed to adhere for 24 hours. Cells were treated with the indicated concentrations of SAM for 2 days, and cell viability was measured with a WST-8 assay kit, as described in the Materials and Methods. The Y axis shows the relative ratio of absorbance to control that was obtained at a wavelength of 450 nm (ratio =1) (one-way ANOVA, Dunnett, * * *p<0.005, n=3). (e) Counting the number of cells cultured with SAM at several time points. Cells were treated with 10 μg/ml SAM for the indicated days and stained with DAPI. The number of nuclei was counted as the number of the cells using a Cellomics Array ScanTM High Content Screening System. (The numbers of cells in the SAM-treated group and control group at each time point were compared using Welch’s t test, * *p<0.01, * * *p<0.005, n=10).
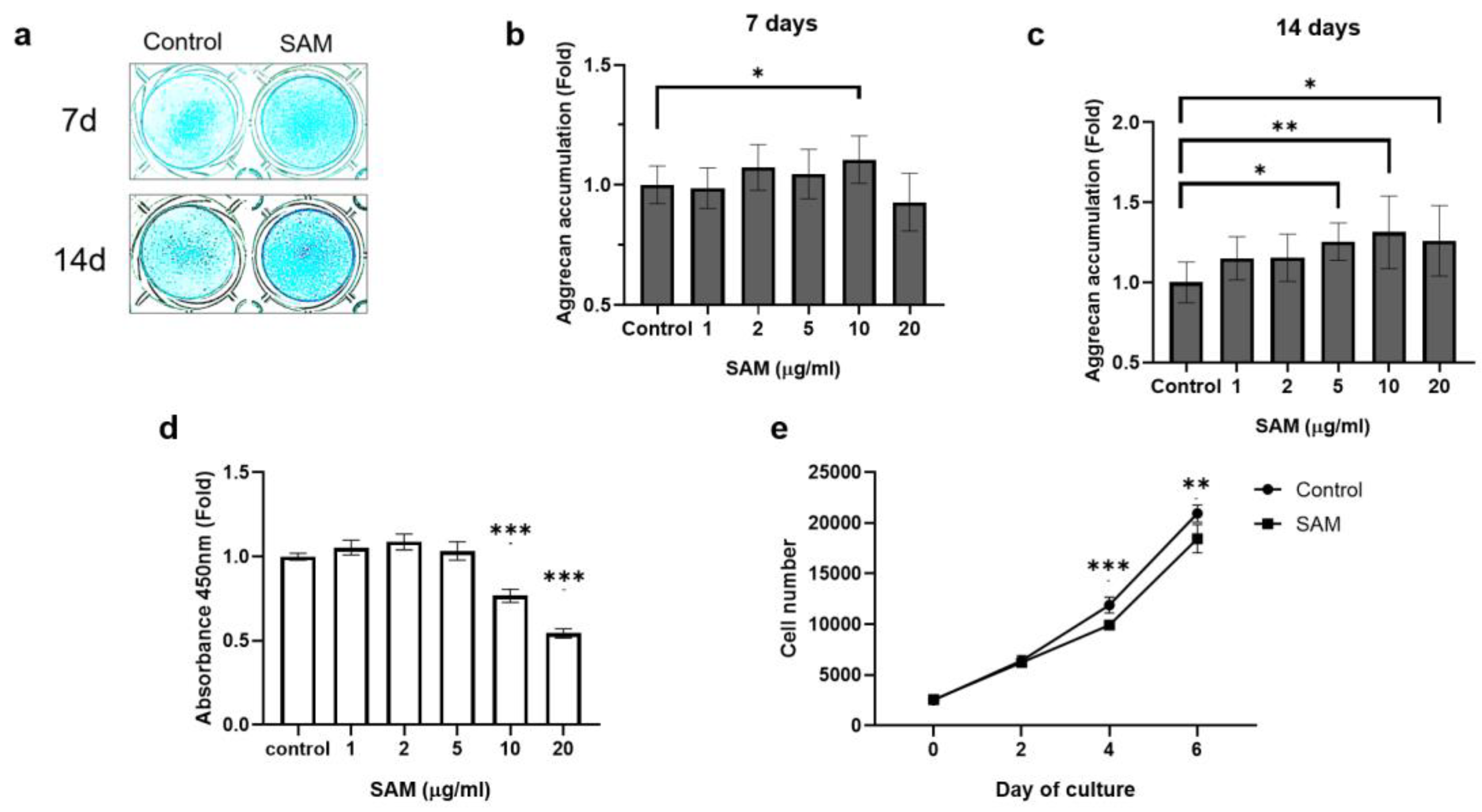
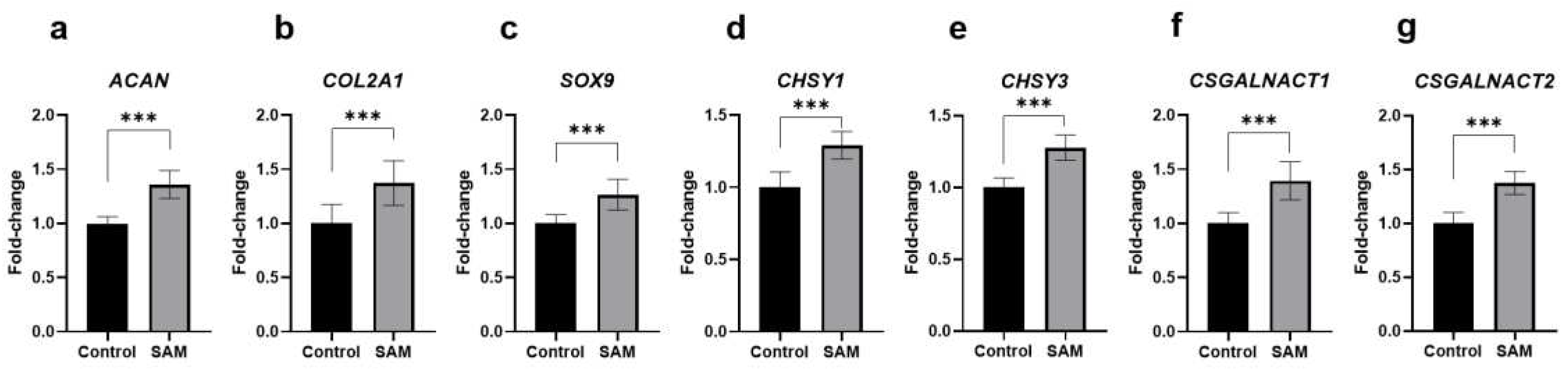
Figure 2.
SAM enhanced the gene expression of cartilage-specific markers, chondrogenesis associated factors and enzymes involved in chondroitin sulfate synthesis in chondrocytes. HCS-2/8 cells were placed in 6-well plates at a density of 6×105 cells/well and incubated with SAM at a concentration of 10 μg/ml. Three days later, total RNAs were extracted from the cells and subjected to RT-qPCR to detect the effects of SAM stimulation on the gene expression. The results demonstrated that the addition of SAM enhanced the gene expression of cartilage-specific markers: ACAN (a), COL2A1 (b); a chondrogenesis-associated factor: SOX9 and enzymes involved in chondroitin sulfate synthesis: (c); CHSY1 (d); CHSY3 (e); CSGALNACT1 (f) CSGALNACT2 (g) (values represent the fold-change relative to a non-treated group as control, Welch’s t test, * * *p<0.005, n=9).
Figure 2.
SAM enhanced the gene expression of cartilage-specific markers, chondrogenesis associated factors and enzymes involved in chondroitin sulfate synthesis in chondrocytes. HCS-2/8 cells were placed in 6-well plates at a density of 6×105 cells/well and incubated with SAM at a concentration of 10 μg/ml. Three days later, total RNAs were extracted from the cells and subjected to RT-qPCR to detect the effects of SAM stimulation on the gene expression. The results demonstrated that the addition of SAM enhanced the gene expression of cartilage-specific markers: ACAN (a), COL2A1 (b); a chondrogenesis-associated factor: SOX9 and enzymes involved in chondroitin sulfate synthesis: (c); CHSY1 (d); CHSY3 (e); CSGALNACT1 (f) CSGALNACT2 (g) (values represent the fold-change relative to a non-treated group as control, Welch’s t test, * * *p<0.005, n=9).
Figure 3.
The inhibition of intracellular SAM synthesis impedes aggrecan accumulation in HCS-2/8 chondrocytes. (a) A diagram of AG-270 inhibiting methionine adenosyltransferase 2A (MAT2A), an enzyme catalyzing the production of SAM from methionine and ATP. (b) AG-270 pretreatment decreased intracellular SAM levels in HCS-2/8 cells. Cells were treated with AG-270 at the indicated concentrations for 3 days and the cell lysates were subjected to an ELISA (one-way ANOVA, Dunnett, n=3). (c) AG-270 pretreatment suppressed aggrecan production in HCS-2/8 cells. Cells were treated with AG-270 at the indicated concentrations for 14 days and then cell layers were stained by Alcian blue (one-way ANOVA, Dunnett, ***p<0.005, n=6). The images show the representative result. (d) The addition of SAM aggravated negative effect of AG-270 on aggrecan production. HCS-2/8 cells were cultured with AG-270/vehicle at the indicated concentration with/without SAM (10 μg/ml) for 14 days and then cell layers were stained with Alcian blue (two-way ANOVA, Tukey, **p<0.01, n=6). The images show the representative result. In all experiments, values represent the fold-change relative to non-treated groups (ratio=1).
Figure 3.
The inhibition of intracellular SAM synthesis impedes aggrecan accumulation in HCS-2/8 chondrocytes. (a) A diagram of AG-270 inhibiting methionine adenosyltransferase 2A (MAT2A), an enzyme catalyzing the production of SAM from methionine and ATP. (b) AG-270 pretreatment decreased intracellular SAM levels in HCS-2/8 cells. Cells were treated with AG-270 at the indicated concentrations for 3 days and the cell lysates were subjected to an ELISA (one-way ANOVA, Dunnett, n=3). (c) AG-270 pretreatment suppressed aggrecan production in HCS-2/8 cells. Cells were treated with AG-270 at the indicated concentrations for 14 days and then cell layers were stained by Alcian blue (one-way ANOVA, Dunnett, ***p<0.005, n=6). The images show the representative result. (d) The addition of SAM aggravated negative effect of AG-270 on aggrecan production. HCS-2/8 cells were cultured with AG-270/vehicle at the indicated concentration with/without SAM (10 μg/ml) for 14 days and then cell layers were stained with Alcian blue (two-way ANOVA, Tukey, **p<0.01, n=6). The images show the representative result. In all experiments, values represent the fold-change relative to non-treated groups (ratio=1).
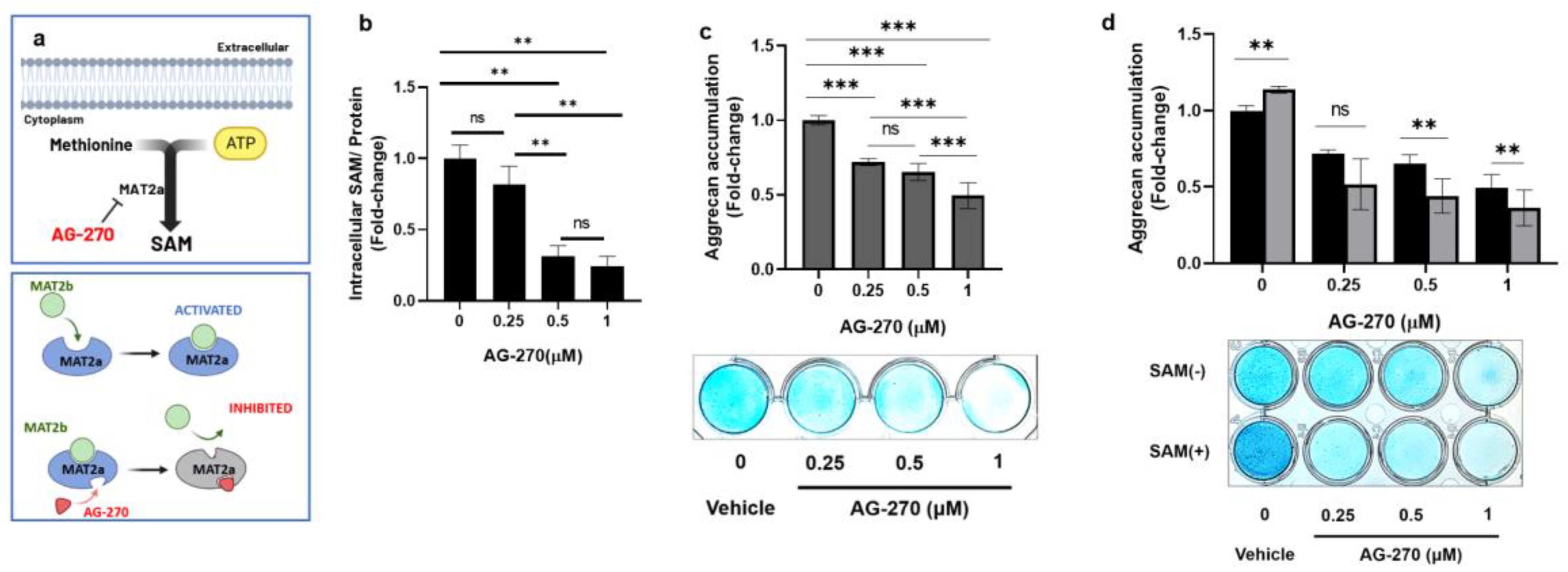
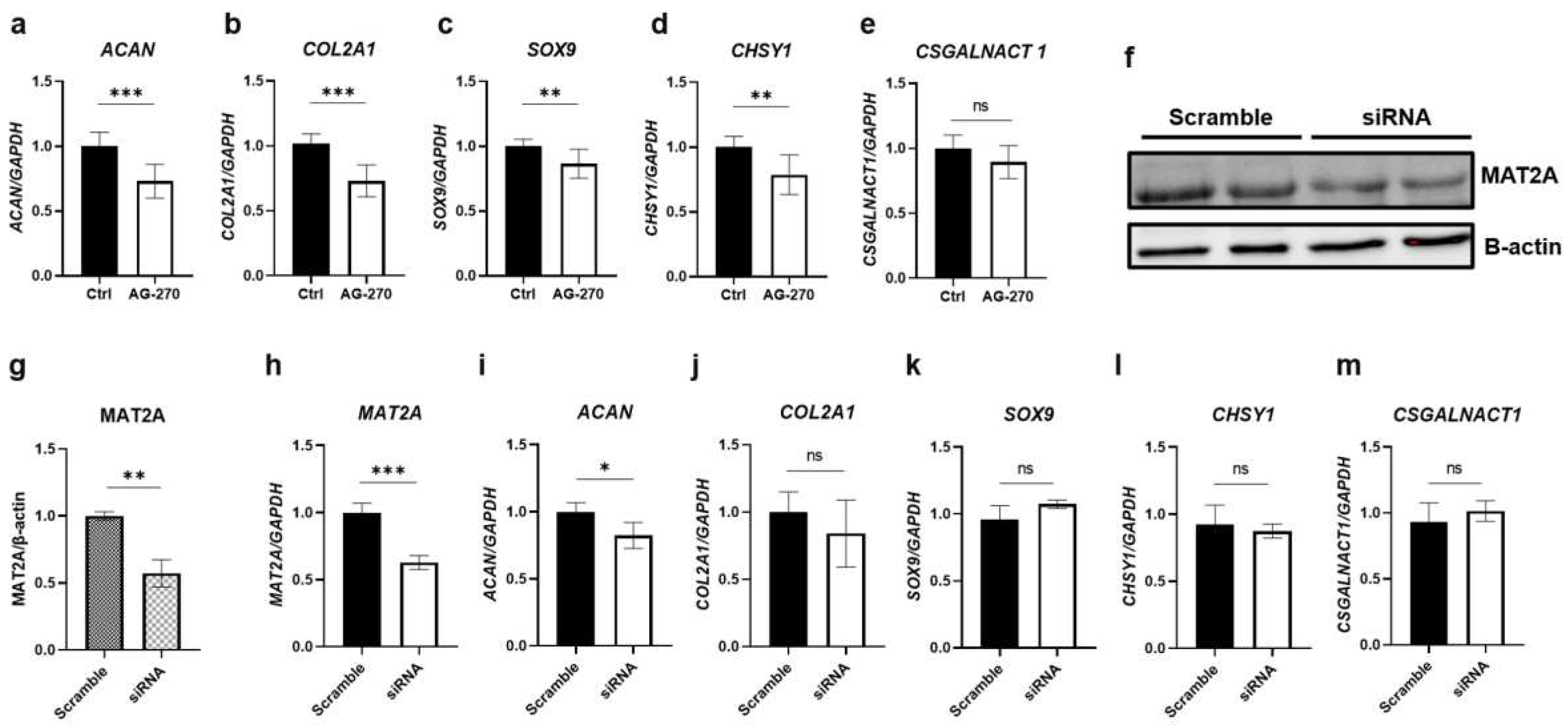
Figure 4.
The inhibition of intracellular SAM synthesis decreases the gene expression of cartilage-related factors. HCS-2/8 cells were seeded in 6-well plates at 6 ×105 cells/well and allowed to adhere for 24 h prior to incubation with inhibitor or siRNA, as described in “Materials and Methods.” (a-e) The effect of AG-270 on the gene expression of the following cartilage-related factors were analyzed by RT-qPCR: (a) ACAN, (b) COL2A1, (c) SOX9, (d) CHSY1, (e) CSGALNACT1 (n=9). (f-g) Western blotting and RT-qPCR (h) confirmed the efficiency of knockdown of siRNA targeting MAT2A (n=5). (i-m) The gene expression of cartilage-related factors under the effect of MAT2A knockdown: (i) ACAN, (j) COL2A1, (k) SOX9, (l) CHSY1, and (m) CSGALNACT1 (n=5). All data are presented as the mean ± SD of fold-change relative the control group (Welch’s t-test, *p<0.05, **p<0.01, ***p<0.005).
Figure 4.
The inhibition of intracellular SAM synthesis decreases the gene expression of cartilage-related factors. HCS-2/8 cells were seeded in 6-well plates at 6 ×105 cells/well and allowed to adhere for 24 h prior to incubation with inhibitor or siRNA, as described in “Materials and Methods.” (a-e) The effect of AG-270 on the gene expression of the following cartilage-related factors were analyzed by RT-qPCR: (a) ACAN, (b) COL2A1, (c) SOX9, (d) CHSY1, (e) CSGALNACT1 (n=9). (f-g) Western blotting and RT-qPCR (h) confirmed the efficiency of knockdown of siRNA targeting MAT2A (n=5). (i-m) The gene expression of cartilage-related factors under the effect of MAT2A knockdown: (i) ACAN, (j) COL2A1, (k) SOX9, (l) CHSY1, and (m) CSGALNACT1 (n=5). All data are presented as the mean ± SD of fold-change relative the control group (Welch’s t-test, *p<0.05, **p<0.01, ***p<0.005).
Figure 5.
Effect of SAM on the gene expression and protein level of CCN2 in HCS-2/8 cells. HCS-2/8 cells were placed in 6-well plates at a density of 6 ×105 cells/well and allowed to settle for 24 hours prior to the subsequent experiments. (a) Exogenous SAM enhanced the gene expression of CCN2 (n=9). Cells were treated with SAM (10 μg/ml) for 3 days then total RNAs were extracted and subjected to RT-qPCR. (b-c) The addition of SAM enhanced the protein level of CCN2. Cells were cultured with SAM (10 μg/ml) for 6 days then cell lysates were prepared and analyzed by western blotting (c) with an antibody specific to CCN2. (b) Quantitative values of western blots were calculated from the density of the CCN2 signals and normalized to the signals of β-actin (n=5). (d) Effect of AG-270 on the gene expression of CCN2 in HCS-2/8 cells. Cells were treated with AG-270 (1 μM) or DSMO, for 3 days and RT-qPCR showed that AG-270 significantly increased the gene expression of CCN2 (n=9). (e) The effect of siRNA against MAT2A on the gene expression of CCN2 in HCS-2/8 cells. Cells were transfected with siRNA against MAT2A (80 pmol) or scramble siRNA by lipofectamine for 24 hours and subjected to RNA extraction and RT-qPCR. The result shows that MAT2A knockdown significantly suppressed the gene expression of CCN2 (n=5). In these graphs, the Y axis presents the fold-change relative to the control group set up at ratio=1 (Welch’s t test, *p<0.05, **p<0.01, ***p<0.005).
Figure 5.
Effect of SAM on the gene expression and protein level of CCN2 in HCS-2/8 cells. HCS-2/8 cells were placed in 6-well plates at a density of 6 ×105 cells/well and allowed to settle for 24 hours prior to the subsequent experiments. (a) Exogenous SAM enhanced the gene expression of CCN2 (n=9). Cells were treated with SAM (10 μg/ml) for 3 days then total RNAs were extracted and subjected to RT-qPCR. (b-c) The addition of SAM enhanced the protein level of CCN2. Cells were cultured with SAM (10 μg/ml) for 6 days then cell lysates were prepared and analyzed by western blotting (c) with an antibody specific to CCN2. (b) Quantitative values of western blots were calculated from the density of the CCN2 signals and normalized to the signals of β-actin (n=5). (d) Effect of AG-270 on the gene expression of CCN2 in HCS-2/8 cells. Cells were treated with AG-270 (1 μM) or DSMO, for 3 days and RT-qPCR showed that AG-270 significantly increased the gene expression of CCN2 (n=9). (e) The effect of siRNA against MAT2A on the gene expression of CCN2 in HCS-2/8 cells. Cells were transfected with siRNA against MAT2A (80 pmol) or scramble siRNA by lipofectamine for 24 hours and subjected to RNA extraction and RT-qPCR. The result shows that MAT2A knockdown significantly suppressed the gene expression of CCN2 (n=5). In these graphs, the Y axis presents the fold-change relative to the control group set up at ratio=1 (Welch’s t test, *p<0.05, **p<0.01, ***p<0.005).
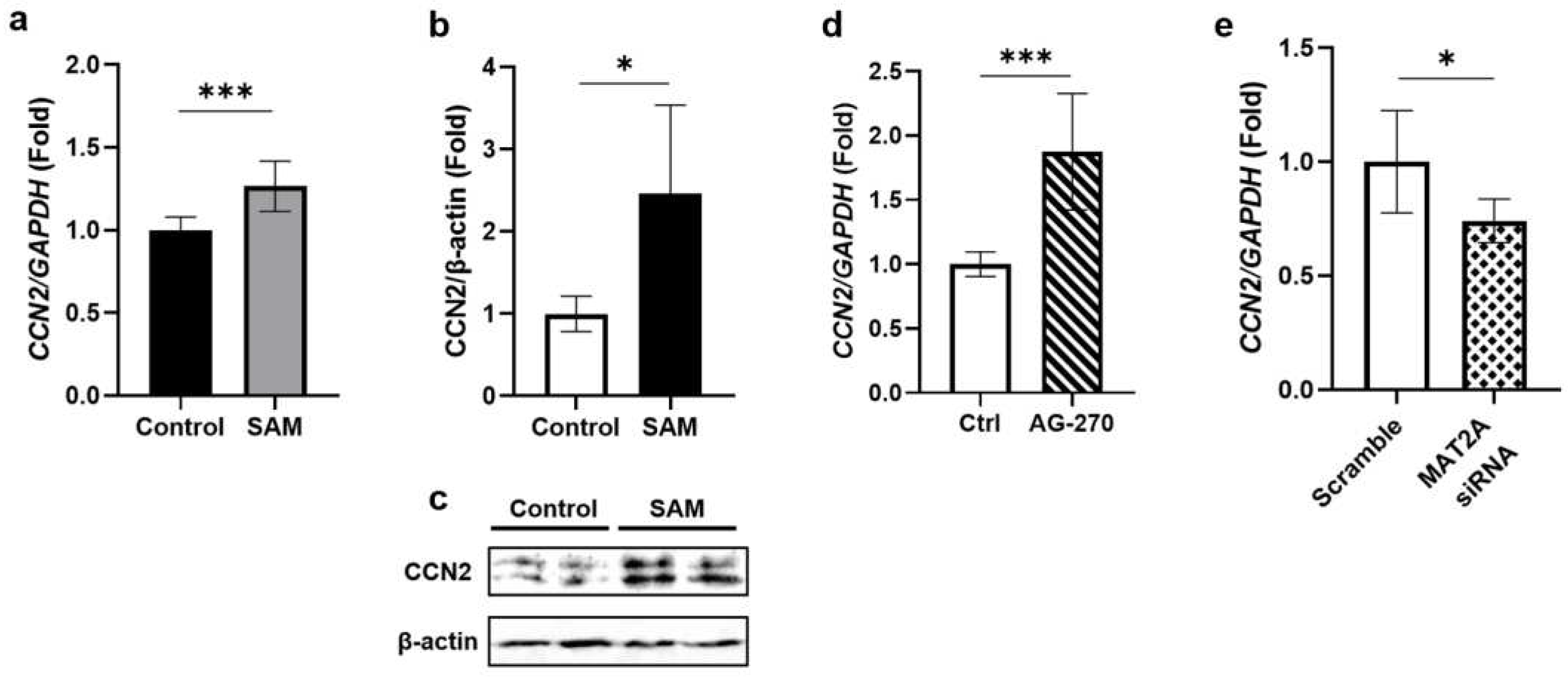
Figure 6.
Additional SAM promoted polyamine production in chondrocytes. (a) PolyamineRED staining indicated polyamine levels enhanced by additional SAM in HCS-2/8 cells. HCS-2/8 cells were seeded in 96-well plates at a density of 3000 cells/well and cultured in the presence of SAM (10 μg/ml) for 3 days. Then the cells were stained with PolyamineRED as described in Materials and Methods. The scale bar indicates 500 μm. (b) The histogram of polyamineRED intensity from each cell (X-axis) versus cell numbers (Y-axis). (c) SAM enhanced the expression of ornithine decarboxylase (ODC). HCS-2/8 cells (6×105 cells/well) were seeded in 6-well plates and incubated with SAM (10 μg/ml) for 3 days. Total RNAs were extracted from cells and subjected to RT-qPCR. The quantitation was normalized to GAPDH and the level set in control=1 (Welch’s t-test, *p<0.05, n=9). (d, e) An HPLC analysis of spermidine and spermine in HCS-2/8 cells. Cells (6×105 cells/well) were seeded in 6-well plates and allowed to adhere for 24 hours. Then, SAM was added at the concentrations of 10 μg/ml and incubated for indicated time. Cell lysates were collected at the time indicated and subjected to HPLC as described in Materials and Methods. The levels of spermidine (d) and spermine (e) were normalized to total protein levels. (Welch’s t test, *p<0.05,**p<0.01, n=3).
Figure 6.
Additional SAM promoted polyamine production in chondrocytes. (a) PolyamineRED staining indicated polyamine levels enhanced by additional SAM in HCS-2/8 cells. HCS-2/8 cells were seeded in 96-well plates at a density of 3000 cells/well and cultured in the presence of SAM (10 μg/ml) for 3 days. Then the cells were stained with PolyamineRED as described in Materials and Methods. The scale bar indicates 500 μm. (b) The histogram of polyamineRED intensity from each cell (X-axis) versus cell numbers (Y-axis). (c) SAM enhanced the expression of ornithine decarboxylase (ODC). HCS-2/8 cells (6×105 cells/well) were seeded in 6-well plates and incubated with SAM (10 μg/ml) for 3 days. Total RNAs were extracted from cells and subjected to RT-qPCR. The quantitation was normalized to GAPDH and the level set in control=1 (Welch’s t-test, *p<0.05, n=9). (d, e) An HPLC analysis of spermidine and spermine in HCS-2/8 cells. Cells (6×105 cells/well) were seeded in 6-well plates and allowed to adhere for 24 hours. Then, SAM was added at the concentrations of 10 μg/ml and incubated for indicated time. Cell lysates were collected at the time indicated and subjected to HPLC as described in Materials and Methods. The levels of spermidine (d) and spermine (e) were normalized to total protein levels. (Welch’s t test, *p<0.05,**p<0.01, n=3).
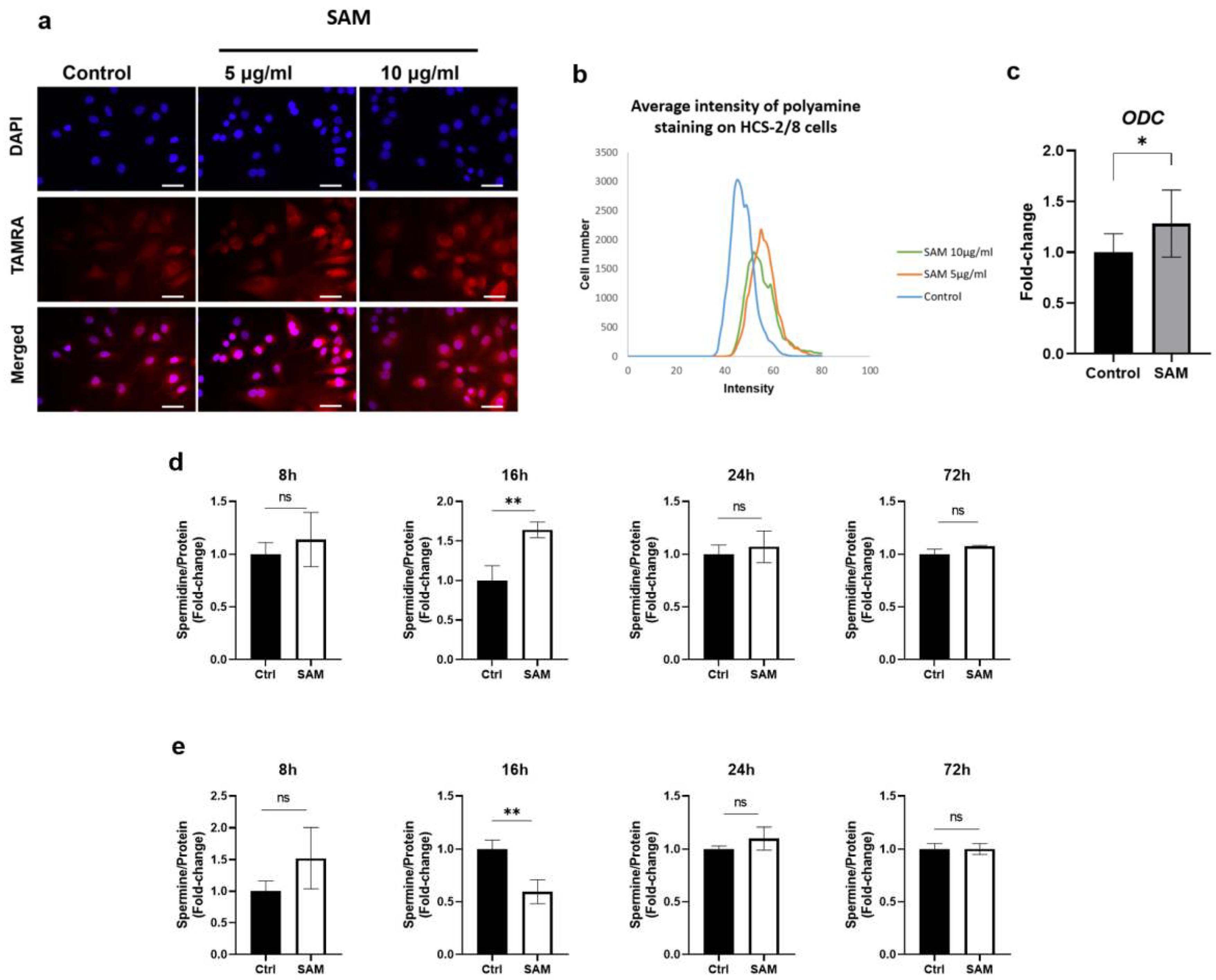
Figure 7.
DFMO inhibited the SAM-induced aggrecan accumulation in chondrocytes. (a) The position inhibited by difluoromethylornithine (DFMO) in polyamine biosynthesis pathway. (b) Aggrecan accumulation was drastically reduced by DFMO. HCS-2/8 cells (1 ×105cells/well) were seeded in 24-well plates and allowed to adhere for 24 hours. Then cells were cultured with DFMO at the indicated concentrations for 14 days. Alcian blue staining was performed to measure the aggrecan accumulation. (***p<0.005, One-way ANOVA, Dunnett, n=6). (c) The aggrecan enhancement that occurred with the addition of SAM was suppressed by DFMO. HCS-2/8 cells (10×104 cells/well) were seeded in 24-well plates and allowed to adhere for 24 hours. Then cells were cultured with/without SAM (10 μg/ml) in the presence of DFMO for 14 days. Alcian blue staining was performed to measure the aggrecan accumulation. (**p<0.01, ***p<0.005, two-way ANOVA, Tukey, n=6). These values represent the relative ratio to the DFMO(-) SAM(-) group as the control (ratio=1.0).
Figure 7.
DFMO inhibited the SAM-induced aggrecan accumulation in chondrocytes. (a) The position inhibited by difluoromethylornithine (DFMO) in polyamine biosynthesis pathway. (b) Aggrecan accumulation was drastically reduced by DFMO. HCS-2/8 cells (1 ×105cells/well) were seeded in 24-well plates and allowed to adhere for 24 hours. Then cells were cultured with DFMO at the indicated concentrations for 14 days. Alcian blue staining was performed to measure the aggrecan accumulation. (***p<0.005, One-way ANOVA, Dunnett, n=6). (c) The aggrecan enhancement that occurred with the addition of SAM was suppressed by DFMO. HCS-2/8 cells (10×104 cells/well) were seeded in 24-well plates and allowed to adhere for 24 hours. Then cells were cultured with/without SAM (10 μg/ml) in the presence of DFMO for 14 days. Alcian blue staining was performed to measure the aggrecan accumulation. (**p<0.01, ***p<0.005, two-way ANOVA, Tukey, n=6). These values represent the relative ratio to the DFMO(-) SAM(-) group as the control (ratio=1.0).
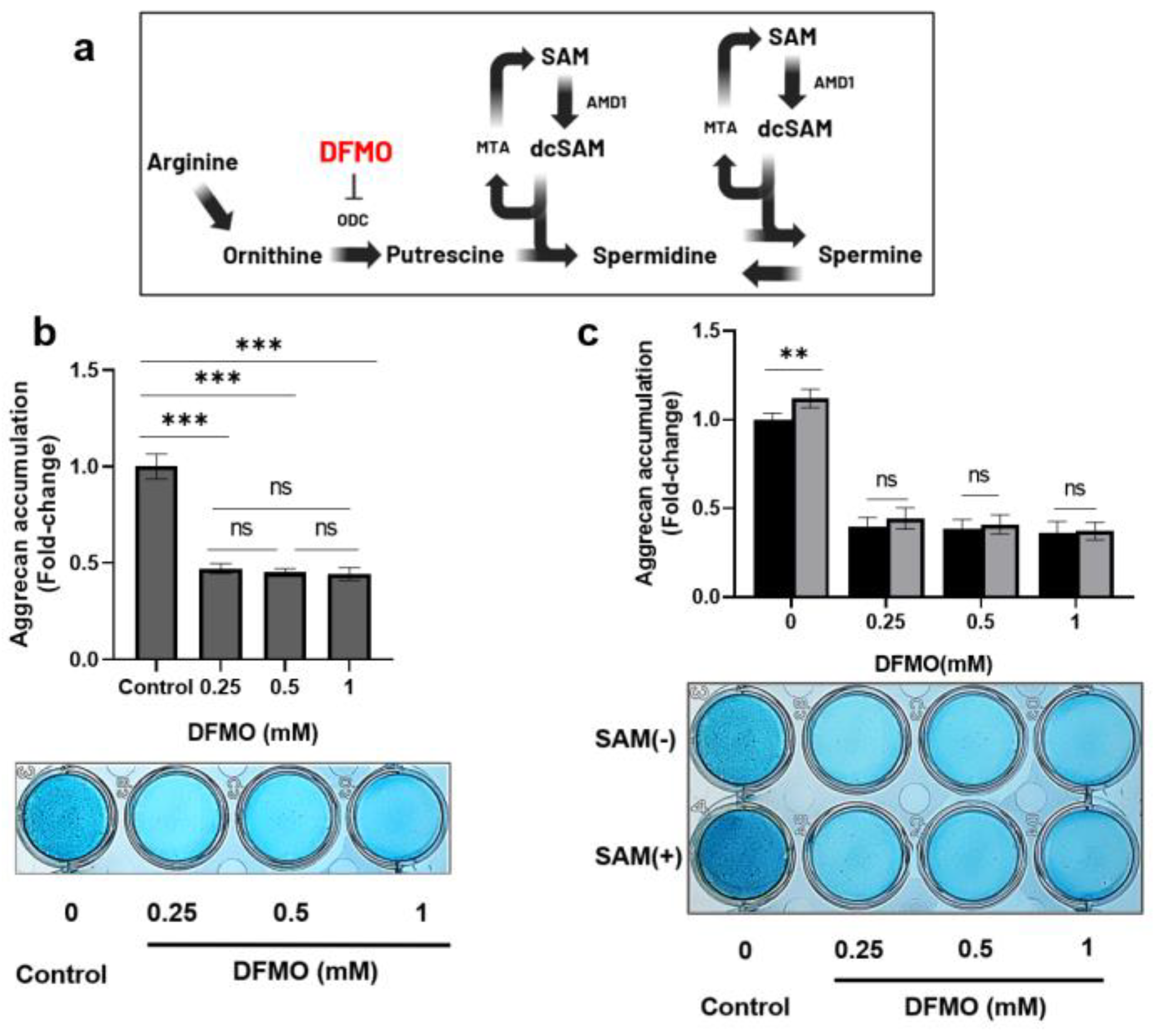
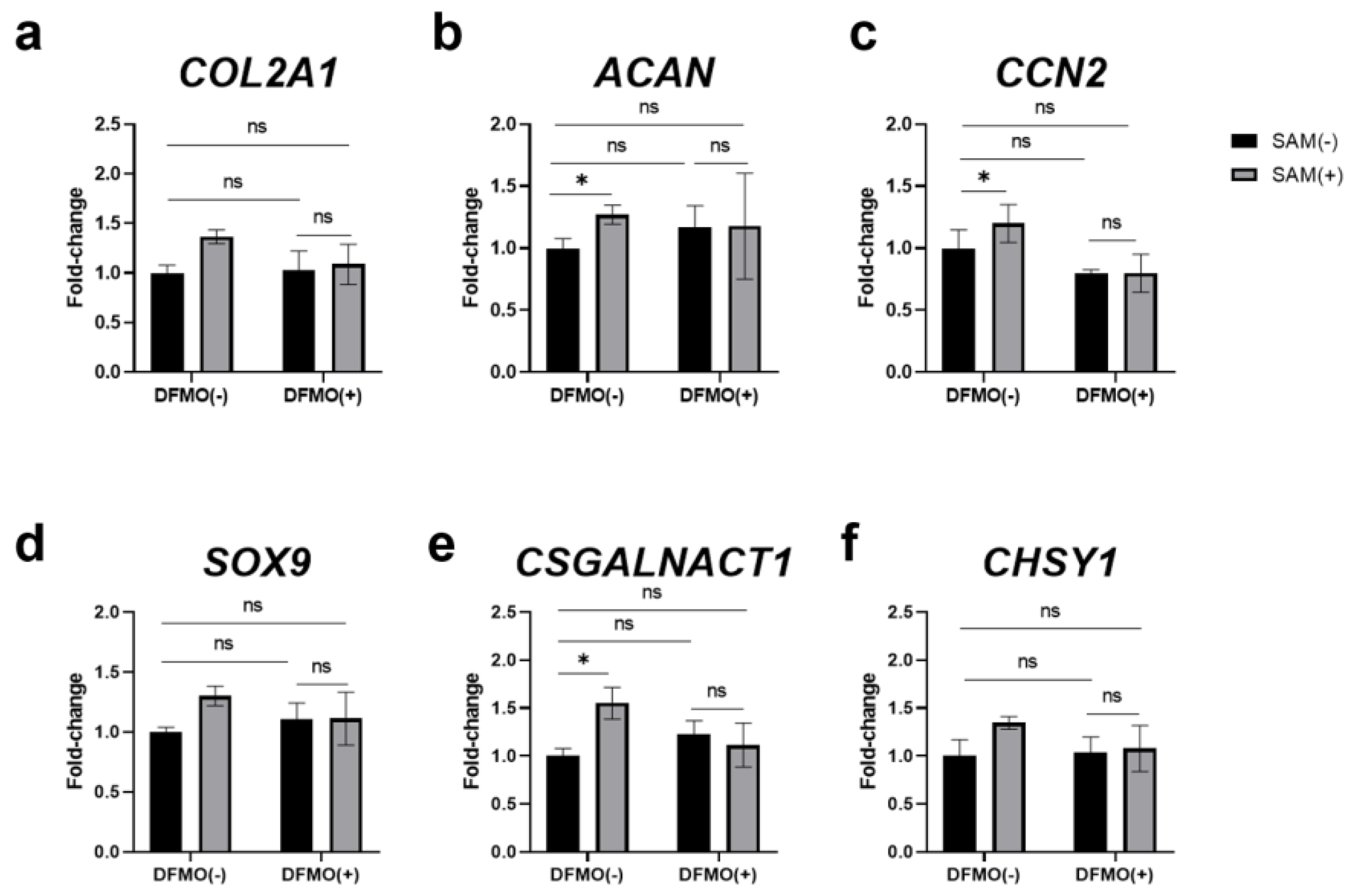
Figure 8.
DFMO inhibited the SAM-induced enhancement of the gene expression in HCS-2/8 cells. HCS-2/8 cells (6×105 cells/well) were seeded in 24-well plates and allowed to adhere for 24 hours. Then cells were treated with DFMO (0.5 mM) with/without SAM (10 μg/ml) for the next 3 days. Subsequently, total RNAs were extracted from the cells and the expression of (a) COL2A1, (b) ACAN, (c) CCN2, (d) SOX9, (e) CSGALNACT1, and (f) CHSY1 was analyzed by RT-qRCR. In these experiments, SAM(-) DFMO(-) was used as control. In these experiments, SAM(-) DFMO(-) was used as control (*p<0.05, **p<0.01, two-way ANOVA, Tukey, n=3).
Figure 8.
DFMO inhibited the SAM-induced enhancement of the gene expression in HCS-2/8 cells. HCS-2/8 cells (6×105 cells/well) were seeded in 24-well plates and allowed to adhere for 24 hours. Then cells were treated with DFMO (0.5 mM) with/without SAM (10 μg/ml) for the next 3 days. Subsequently, total RNAs were extracted from the cells and the expression of (a) COL2A1, (b) ACAN, (c) CCN2, (d) SOX9, (e) CSGALNACT1, and (f) CHSY1 was analyzed by RT-qRCR. In these experiments, SAM(-) DFMO(-) was used as control. In these experiments, SAM(-) DFMO(-) was used as control (*p<0.05, **p<0.01, two-way ANOVA, Tukey, n=3).
Table 1.
Nucleotide sequences of primers for HCS-2/8.
Table 1.
Nucleotide sequences of primers for HCS-2/8.
| Gene |
Sequences |
species |
| CCN2 |
F- GCAGGCTAGAGAAGCAGAGC
R-ATGTCTTCATGCTGGTGCAG
|
Human |
| ACAN |
F-TTCGGGCAGAAGAAGGAC
R-CGTGAGCTCCGCTTCTGT
|
Human |
| COL2a1 |
F- ATGGCTTCCAGAGGCC GAC
R- TTGCTGCATGAGTTGCCACGCA
|
Human |
| ODC |
F- ATGGCTTCCAGAGGCC GAC
R- TTGCTGCATGAGTTGCCACGCA
|
Human |
| SOX9 |
F- AGGCAAGCAAAGGAGATGAA
R- TGGTGTTCTGAGAGGCACAG
|
Human |
| MMP13 |
F-ACTTCACGATGGCATTGCTG
R-CATAATTTGGCCCAGGAGGA
|
Human |
| GAPDH |
F- GCCAAAAGGGTCATCATCTC
R- GTCTTCTGGGTGGCAGTGAT
|
Human |
| CHSY1 |
F-CGACAGGAACTTTCTCTTCGTGG
R-GGTACAGATGTGTCAGAACCCTC
|
Human |
| CHSY3 |
F-AGTTGGAGCGGGCTTACAGTGA
R-CAGCACCTCAAAGCGAGAGTGT
|
Human |
| CSGALNACT1 |
F-GGATGACGTGTCAGTATCGGTC
R-CCGTACCACTATGAGGTTGCTG
|
Human |
| CSGALNACT2 |
F-TCATCTCACAGTGGTGTATTTTGG
R-GCACCCACATTTAGTCCTCGTC
|
Human |