Submitted:
03 November 2023
Posted:
06 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
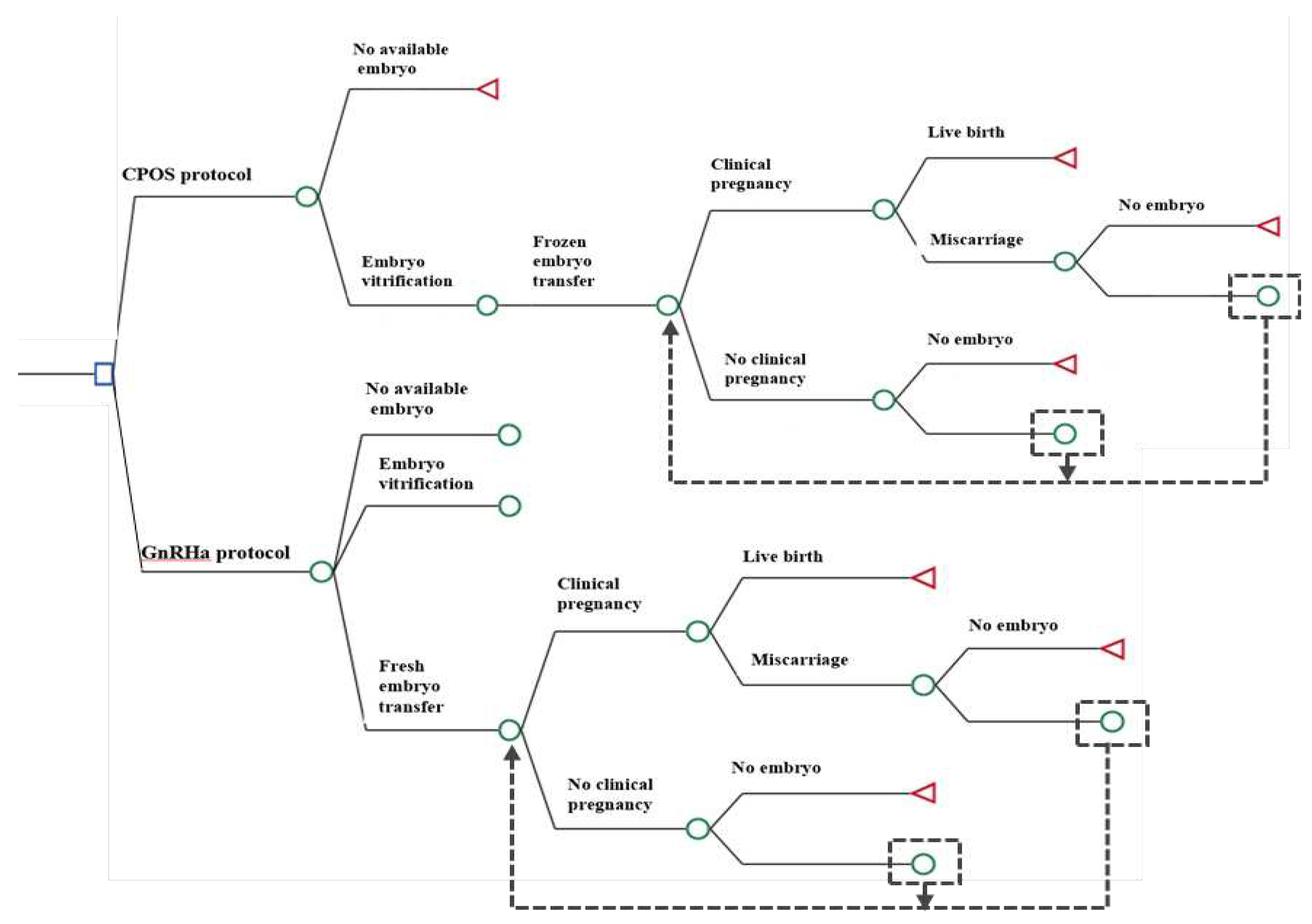
Study Design
Ovarian Stimulation
- GnRHa protocol: Tripreilin acetate was injected 0.05mg/d (Ferring Pharmaceuticals, SaintPrex, Switzerland) at the middle luteal stage of the previous cycle for pituitary down-regulation. When the down-regulation standard was reached (serum luteinizing hormone (LH) < 5IU/L, serum estradiol (E2) < 50pg/ml, endometrial thickness <10mm, no functional ovarian cyst, urine follicle-stimulating hormone (uFSH) (Zhuhai Lizon Pharmaceutical, Zhuhai, China) or recombinant follicle-stimulating hormone (rFSH) (Merck Serono, Buchs, Switzerland) was initiated with 225-300U, and the adjustment was made according to the ovarian response.
- CPOS protocol: From the second or third day of menstruation until the trigger day, 100mg of CC (Fertila, Codal Synto Ltd., Cyprus) was administered orally; 225-300IU of uFSH were also administered daily, with the dosage being adjusted based on ovarian response.When bilateral ovaries had ≥2~3 follicles with diameter >18mm, 10000 IU human chorionic gonadotropin (hCG) (Lizhu Pharmaceutical Trading Co., China) or 250mg Ovidrel (Merck Serono S.p.A., Modugno, Italy) was administered to trigger the final maturation of the oocytes. Oocytes were retrieved 34~36h later. Transvaginal oocyte retrieval was performed 34–36h after hCG administration. Supplementary Figure S1.
Fertilization and Embryo Evaluation
Fresh and Frozen Embryo Transfer
Luteal Support
Outcome Measures
Cost-Effectiveness Analysis
Sensitivity Analysis
Statistics Analyses
3. Results
3.1. Comparisons of Baseline Characteristics between the Two Groups
3.2. Comparisons of Ovarian Stimulation and Embryonic Laboratory Outcomes
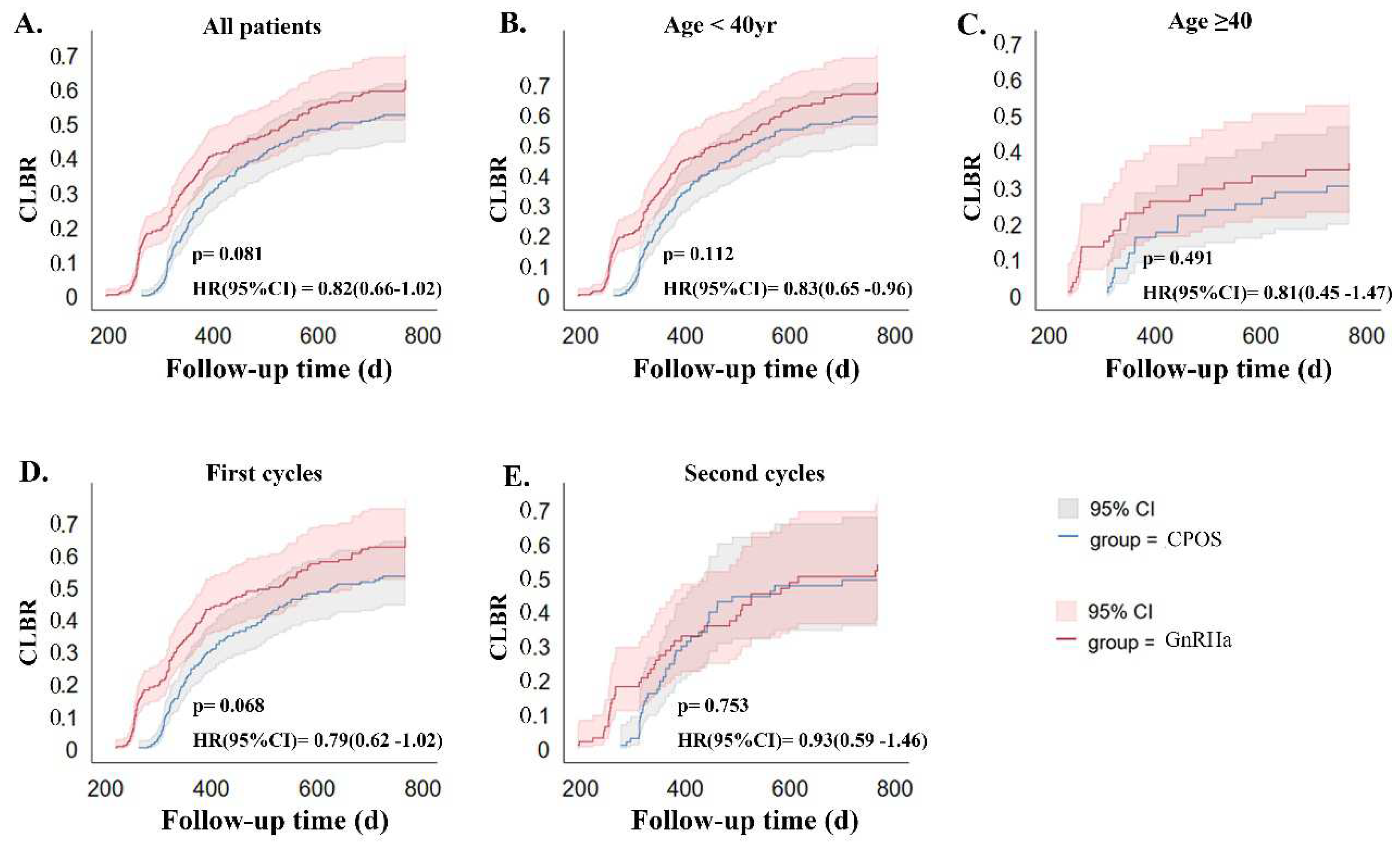
3.3. Comparisons of Clinical Outcomes between the Two Groups
3.4. Subgroup Analysis
3.5. Cost-Effectiveness Analysis
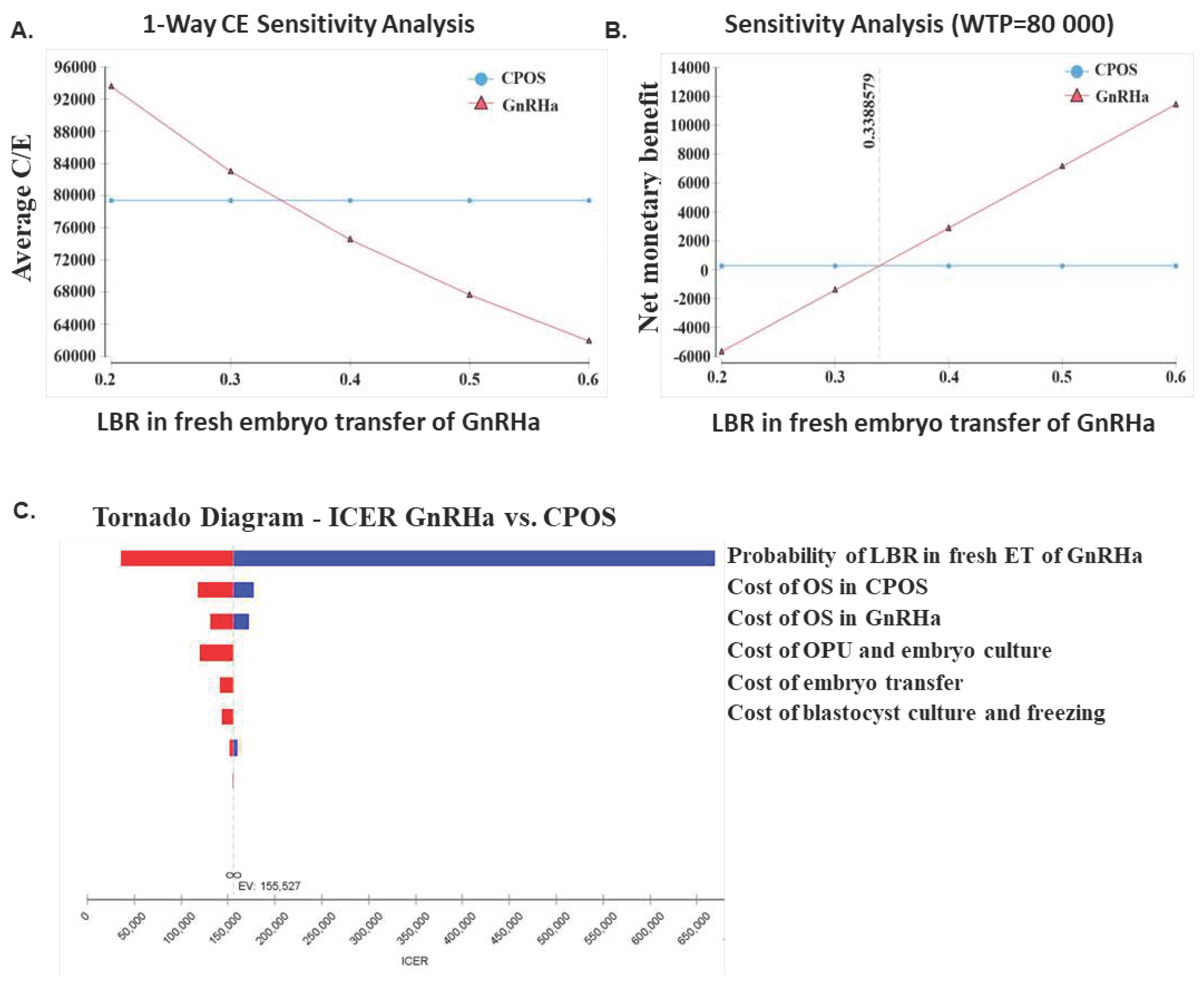
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vollset SE, Goren E, Yuan CW, Cao J, Smith AE, Hsiao T, Bisignano C, Azhar GS, Castro E, Chalek J et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285-1306. [CrossRef]
- Zhou Z, Zheng D, Wu H, Li R, Xu S, Kang Y, Cao Y, Chen X, Zhu Y, Xu S et al. Epidemiology of infertility in China: a population-based study. Bjog. 2018;125:432-441. [CrossRef]
- Kushnir VA, Smith GD, Adashi EY. The Future of IVF: The New Normal in Human Reproduction. Reprod Sci. 2022;29:849-856. [CrossRef]
- Chua SJ, Danhof NA, Mochtar MH, van Wely M, McLernon DJ, Custers I, Lee E, Dreyer K, Cahill DJ, Gillett WR et al. Age-related natural fertility outcomes in women over 35 years: a systematic review and individual participant data meta-analysis. Hum Reprod. 2020;35:1808-1820. [CrossRef]
- Kouvidi E, Zachaki S, Tsarouha H, Pantou A, Manola KN, Kanavakis E, Mavrou A. Female Reproductive Ageing and Chromosomal Abnormalities in a Large Series of Women Undergoing IVF. Cytogenet Genome Res. 2021;161:551-555. [CrossRef]
- Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, Rienzi L. Impact of Maternal Age on Oocyte and Embryo Competence. Front Endocrinol (Lausanne). 2018;9:327. [CrossRef]
- Pathare ADS, Loid M, Saare M, Gidlöf SB, Zamani Esteki M, Acharya G, Peters M, Salumets A. Endometrial receptivity in women of advanced age: an underrated factor in infertility. Human Reproduction Update. 2023. Online ahead of print. [CrossRef]
- Ubaldi FM, Cimadomo D, Vaiarelli A, Fabozzi G, Venturella R, Maggiulli R, Mazzilli R, Ferrero S, Palagiano A, Rienzi L. Advanced Maternal Age in IVF: Still a Challenge? The Present and the Future of Its Treatment. Front Endocrinol (Lausanne). 2019;10:94. [CrossRef]
- Pai AH, Sung YJ, Li CJ, Lin CY, Chang CL. Progestin Primed Ovarian Stimulation (PPOS) protocol yields lower euploidy rate in older patients undergoing IVF. Reprod Biol Endocrinol. 2023;21:72. [CrossRef]
- Sovino H, Sir-Petermann T, Devoto L. Clomiphene citrate and ovulation induction. Reproductive biomedicine online. 2002;4:303-310.
- Ovarian Stimulation TEGGO, Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, Kolibianakis E, Kunicki M, La Marca A, Lainas G et al. ESHRE guideline: ovarian stimulation for IVF/ICSI(†). Hum Reprod Open. 2020;2020:hoaa009. [CrossRef]
- Bechtejew TN, Nadai MN, Nastri CO, Martins WP. Clomiphene citrate and letrozole to reduce follicle-stimulating hormone consumption during ovarian stimulation: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;50:315-323. [CrossRef]
- Lin HT, Wu MH, Tsai LC, Chen TS, Ou HT. Co-Administration of Clomiphene Citrate and Letrozole in Mild Ovarian Stimulation Versus Conventional Controlled Ovarian Stimulation Among POSEIDON Group 4 Patients. Front Endocrinol (Lausanne). 2021;12:780392. [CrossRef]
- Revelli A, Chiadò A, Dalmasso P, Stabile V, Evangelista F, Basso G, Benedetto C. "Mild" vs. "long" protocol for controlled ovarian hyperstimulation in patients with expected poor ovarian responsiveness undergoing in vitro fertilization (IVF): a large prospective randomized trial. J Assist Reprod Genet. 2014;31:809-815. [CrossRef]
- Gleicher N, Weghofer A, Barad DH. A case-control pilot study of low-intensity IVF in good-prognosis patients. Reprod Biomed Online. 2012;24:396-402. [CrossRef]
- Le H, Nguyen DD, Cao AT, Nguyen HTL, Tham DC, Le TD, Hugues JN. Comparative Effectiveness of Mild or Conventional GnRH-Antagonist Protocols for Ovarian Stimulation in Poor Responders (Poseidon Group 4). Front Reprod Health. 2020;2:606036. [CrossRef]
- Bhor SA, Nakayama K, Ono H, Iwashita T, Kinoshita K. Effects of controlled ovarian stimulation regimens on top-quality blastocyst development and perinatal outcomes with the freeze-all strategy: A retrospective comparative study. Clin Exp Reprod Med. 2023;50:132-140. [CrossRef]
- Singh A, Bhandari S, Agrawal P, Gupta N, Munaganuru N. Use of clomiphene-based stimulation protocol in oocyte donors: A comparative study. J Hum Reprod Sci. 2016;9:159-163. [CrossRef]
- Veeck LL. Oocyte assessment and biological performance. Ann N Y Acad Sci. 1988;541:259-274. [CrossRef]
- Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155-1158. [CrossRef]
- Liu L, Li YH, Ding XF, Geng YH, Chen CY, Gao Y. Influence of blastocysts morphological score on pregnancy outcomes in frozen-thawed blastocyst transfers: a retrospective study of 741 cycles. J Huazhong Univ Sci Technolog Med Sci. 2014;34:750-754. [CrossRef]
- Payne K, Gavan SP, Wright SJ, Thompson AJ. Cost-effectiveness analyses of genetic and genomic diagnostic tests. Nat Rev Genet. 2018;19:235-246. [CrossRef]
- Liu M, Zhao X, Peng Y, Zheng J, Guo K, Fan Y, Jiang L, Yang A, Cui N, Hao G. Outcomes After a Single Ovarian Stimulation Cycle in Women of Advanced Reproductive Age: A Retrospective Analysis. Frontiers in Endocrinology. 2022;13:792159.
- Cozzolino M, Cecchino GN, Bosch E, Garcia-Velasco JA, Garrido N. Minimal ovarian stimulation is an alternative to conventional protocols for older women according to Poseidon’s stratification: a retrospective multicenter cohort study. Journal of Assisted Reproduction and Genetics. 2021;38:1799-1807.
- Drakopoulos P, Romito A, Errázuriz J, Santos-Ribeiro S, Popovic-Todorovic B, Racca A, Tournaye H, De Vos M, Blockeel C. Modified natural cycle IVF versus conventional stimulation in advanced-age Bologna poor responders. Reproductive biomedicine online. 2019;39:698-703.
- Haahr T, Dosouto C, Alviggi C, Esteves SC, Humaidan P. Management Strategies for POSEIDON Groups 3 and 4. Front Endocrinol (Lausanne). 2019;10:614. [CrossRef]
- Zhu F, Yin S, Yang B, Li S, Feng X, Wang T, Che D. TEAS, DHEA, CoQ10, and GH for poor ovarian response undergoing IVF-ET: a systematic review and network meta-analysis. Reprod Biol Endocrinol. 2023;21:64. [CrossRef]
- Ghasemian F, Esmaeilnezhad S. Metformin, clomiphene citrate and flutamide effects on oocyte ultrastructure status and quality in PCOS mouse model. Reprod Biomed Online. 2022;45:191-201. [CrossRef]
- Hernandez-Nieto C, Lee J, Alkon-Meadows T, Soto-Cossio L, Sandler B, Mukherjee T, Copperman A. Recent clomiphene citrate exposure does not impact subsequent clinical outcomes in single euploid frozen embryo transfer cycles. Hum Reprod. 2023. [CrossRef]
- Chambers GM, Adamson GD, Eijkemans MJ. Acceptable cost for the patient and society. Fertility and sterility. 2013;100:319-327.
- Chambers GM, Sullivan EA, Ishihara O, Chapman MG, Adamson GD. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertility and sterility. 2009;91:2281-2294.
- Verberg M, Eijkemans M, Heijnen E, Broekmans F, de Klerk C, Fauser B, Macklon N. Why do couples drop-out from IVF treatment? A prospective cohort study. Human reproduction. 2008;23:2050-2055.
- Dewi AK, Wicaksana AL, Lutfi M, Dewanto A. The barriers of joining in vitro fertilization programs among infertile couples in developing countries: A scoping review. Asian Pacific Journal of Reproduction. 2023;12:147-154.
- Liu Y, Su R, Wu Y. Cumulative live birth rate and cost-effectiveness analysis of gonadotropin releasing hormone-antagonist protocol and multiple minimal ovarian stimulation in poor responders. Frontiers in Endocrinology. 2021;11:605939.
- Zech NH, Zech M, Baldauf S, Comploj G, Murtinger M, Spitzer D, Hradecký L, Ajayi R, Schuff M, Zech H. Ovarian stimulation in ART - Unwinding pressing issues. Minerva Ginecol. 2015;67:127-147.
- Ochin H, Ma X, Wang L, Li X, Song J, Meng Y, Shen J, Cui YG, Liu J. Low dose clomiphene citrate as a mild stimulation protocol in women with unsuspected poor in vitro fertilization result can generate more oocytes with optimal cumulative pregnancy rate. J Ovarian Res. 2018;11:37. [CrossRef]
- Triantafyllidou O, Sigalos G, Gkoles L, Kastora S, Vakas P, Batsiou E, Vlahos N. The addition of clomiphene citrate to ovarian stimulation protocols for poor responders. Eur J Obstet Gynecol Reprod Biol. 2020;251:136-140. [CrossRef]
- Schuster NA, Rijnhart JJM, Bosman LC, Twisk JWR, Klausch T, Heymans MW. Misspecification of confounder-exposure and confounder-outcome associations leads to bias in effect estimates. BMC Med Res Methodol. 2023;23:11. [CrossRef]
- Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399-424. [CrossRef]
| Before Propensity Score Matching | Before Propensity Score Matching | |||||
|---|---|---|---|---|---|---|
| Characteristic | GnRHa group (n=1,074) |
CPOS group (n=1,797) |
P value | GnRHa group (n=375) |
CPOS group (n=375) |
P Value |
| Age of women (y) | 36(35, 38) | 39(37,42) | <0.001 | 37(36, 39) | 37(36, 39) | 0.410 |
| Infertility duration (y) | 3(2, 7) | 4(2, 8) | 0.023 | 4(2, 8) | 3(2, 7) | 0.268 |
| Primary infertility, n (%) | 349(32,50) | 381(21.20) | <0.001 | 110(29.33) | 99(26.40) | 0.415 |
| Cycle number, n (%) | <0.001 | 1 | ||||
| First cycle | 909(84.64) | 1116(62.10) | 278(74.10) | 279(74.40) | ||
| Repeated cycles | 165(15.36) | 681(37.90) | 97(25.90) | 96(25.60) | ||
| Basal FSH level (IU/L) | 6.70(5.68, 7.96) | 8.36(6.77, 10.92) | <0.001 | 7.24(6.13, 8.21) | 7.12(6.01, 9.07) | 0.376 |
| Basal LH level (IU/L) | 4.75(3.50, 6.56) | 4.51(3.36, 6.15) | 0.001 | 5.15(3.65, 6.45) | 5.09(3.47, 6.42) | 0.340 |
| AMH (ng/ml) | 3.43(2.22, 5.23) | 1.09(0.63, 1.74) | <0.001 | 1.35(1.01,2.55) | 1.45(0.94, 2.13) | 0.270 |
| Body mass index (kg/m2) | 22.71(20.80, 25.03) | 22.60(20.80, 24.70) | 0.189 | 22.88(21.01, 24.93) | 22.91(21.13, 25.12) | 0.237 |
| Casue of infertility, n(%) | <0.001 | 0.956 | ||||
| Tubal | 376(35.01) | 810(45.08) | 133(35.47) | 145(38.67) | ||
| Male factor | 106(9.87) | 251(13.97) | 35(9.33) | 29(7.73) |
0.230 |
|
| Anovulatory | 39(3.63) | 57(3.17) | 42(11.20) | 35(9.33) | ||
| Unexplained | 327(30.45) | 294(16.36) | 104(27.73) | 98(26.13) | ||
| Diminished ovarian reserve | 181(16.85) | 304(16.92) | 39(10.40) | 40(10.67) | ||
| Endometriosis | 40(3.72) | 66(3.67) | 16(4.27 | 22(5.87) | ||
| Mixed factors | 5(0.47) | 15(0.83) | 6(1.60) | 6(1.60) | ||
| Insemination method, n(%) | <0.001 | |||||
| IVF | 714(66.48) | 916(50.97) | 250(66.66) | 229(61.07) | ||
| ICSI | 320(29.80) | 818(45.52) | 111(29.60) | 133(35.47) | ||
| IVF+RICSI | 40(3.72) | 63(3.51) | 14(3.73) | 13(3.47) | ||
| Characteristic | CPOS Group | GnRHa Group | p Value | Subgroup Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| CPOS <40yr | GnRHa <40yr | P Value | CPOS ≥40yr | GnRHa ≥40yr | P Value | ||||
| Patients, n | 375 | 375 | - | 296 | 298 | - | 79 | 77 | - |
| Failure of Ovarian stimulation | 4(1.07) | 3(0.80) | 1.000 | 3(1.01) | 1(0.34) | 0.372 | 1(1.27) | 2(2.60) | 0.618 |
| Premature LH surge, n (%) | 23(6.13) | 2(0.53) | <0.001 | 17(5.74) | 0(0) | <0.001 | 7(8.86) | 2(2.60) | 0.167 |
| Premature ovulation | 9(2.40) | 3(0.80) | 0.143 | 5(1.69) | 2(0.67) | 0.285 | 4(5.06) | 1(1.30) | 0.367 |
| No oocyte retrieved cycles during OPU, n (%) | 9(2.40) | 4(1.06) | 0.263 | 5(1.69) | 2(0.67) | 0.285 | 4(5.06) | 3(3.90) | 1.000 |
| No. of cycles without available embryo after culture, n (%) | 23(6.13) | 9(2.4) | 0.018 | 15(5.07) | 5(1.68) | 0.024 | 8(10.13) | 4(5.19) | 0.369 |
| Total no. of cycle cancellations, n (%) | 49(13.07) | 30(8.00) | 0.032 | 32(10.81) | 19(6.38) | 0.06 | 17(21.52) | 11(14.29) | 0.298 |
| Not undergo embryo transfer, n (%) | 5(1.33) | 9(2.40) | 0.420 | 4(1.35) | 8(2.68) | 0.383 | 1(1.27) | 1(1.30) | 1.000 |
| LH on trigger day (mIU/mL) | 7.83(4.92, 10.49) | 2.81(2.29, 3.48) | <0.001 | 2.81(2.36, 3.53) | 7.62(4.65, 10.41) | <0.001 | 5.36(2.03, 11.37) | 2.42(1.90, 3.44) | <0.001 |
| E2 on trigger day (pg/ml) | 1447(884.2, 2268) | 1609(936, 2516) | 0.18 | 1665(883.4, 2351) | 1884(1014, 2809) | 0.09 | 1193(881.4, 1986) | 1248(824, 1989) | 0.453 |
| Progesterone on trigger day (ng/ml) | 0.97(0.64, 1.38) | 0.95(0.70, 1.24) | 0.728 | 1.10(0.68, 1.45) | 0.92(0.69, 1.22) | 0.170 | 0.83(0.56, 1.15) | 0.92(0.71, 1.24) | 0.581 |
| Gn dosage (IU) | 2495(2025, 3075) | 2400(2000, 3088) | 0.356 | 2362(2025, 3000) | 2400(2000, 2925) | 0.545 | 3000(2700, 3675) | 3000(2700, 3450) | 0.773 |
| Gn duration, d | 10(9, 12) | 10(9, 12) | 0.071 | 10(9, 12) | 10(9, 12) | 0.108 | 10(9, 12) | 11(10, 12) | 0.301 |
| Moderate or severe OHSS n (%) | 7(1.86) | 9(2.40) | 0.801 | 7(2.36) | 8(2.68) | 1.000 | 0 | 1(1.30) | 0.494 |
| Average no. of oocytes retrieved | 6(4, 10) | 11(7, 11) | <0.001 | 7(5, 13) | 11(8, 14) | 0.006 | 5(3, 9) | 7(4, 10) | 0.007 |
| Mature oocyte rate, % | 88.9(75.0, 100) | 85.7(72.7, 100) | 0.001 | 87.5(75.0, 100) | 85.7(72.8, 96.4) | 0.008 | 97.1(78.1, 100) | 87.5(71.4, 100) | 0.058 |
| Normal fertilization rate, % | 75.0(60.0, 100) | 75.0(57.3, 87.4) | 0.255 | 75.0(59.6, 100) | 75.0(60.0, 87.5) | 0.575 | 75.0(60, 100) | 71.43(50.0, 83.3) | 0.190 |
| Cleavage rate, % | 100(100, 100) | 100(100, 100) | 0.147 | 100(100, 100) | 100(100, 100) | 0.069 | 100(100,100) | 100(100,100) | 0.665 |
| High quality embryo rate, % | 37.5(0, 60.0) | 33.33(1.11, 50.0) | 0.092 | 37.50(0, 60.0) | 33.33(10.4, 50.0) | 0.084 | 38.10(0, 66.7) | 33.33(11.1, 60.0) | 0.811 |
| Blastocyst formation rate, % | 70.1(50.0, 91.9) | 71.3(50.0, 92.3) | 0.156 | 71.0(50.0, 92.2) | 71.8(50.0, 93.0) | 0.124 | 70.0(50.0, 90.9) | 70.2(50.0, 91.7) | 0.255 |
| Characteristic | CPOS Group | Gnrha Group | p Value | Subgroup Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| CPOS <40yr | GnRHa <40yr | P Value | CPOS ≥40yr | GnRHa ≥40yr | P Value | ||||
| Patients, n | 375 | 375 | - | 296 | 298 | - | 79 | 77 | - |
| Cycle type of embryo transfer, n (%) | |||||||||
| Fresh embryo transfer | / | 217 | - | / | 174 | - | / | 43 | - |
| Frozen embryo transfer | 445 | 348 | - | 346 | 269 | - | 99 | 79 | - |
| Implantation rate in fresh embryo transfer, %(n) | / | 27.39(103/376) | - | / | 27.96(85/304) | - | / | 25.00(18/72) | - |
| Clinical pregnancy rate in fresh embryo transfer n (%) | / | 87(40.09) | - | / | 75(43.10) | - | / | 12(27.90) | - |
| Live birth rate in Fresh embryo transfer n (%) | 60(28.04) | - | / | 51(29.31) | - | / | 10(23.26) | - | |
| No. of patients underwent embryo transfer | 321 | 336 | - | 260 | 271 | - | 61 | 65 | - |
| Implantation rate per transfer, %(n) | 45.42(263/579) | 39.46(324/821) | 0.028 | 48.25(221/458) | 41.71(269/645) | 0.032 | 34.71(42/121) | 30.68(54/176) | 0.528 |
| Clinical pregnancy rate per transfer cycle, n (%) | 233/445(52.36) | 260/565(46.02) | 0.049 | 198/346(57.23) | 223/443(50.34) | 0.062 | 35/99(35.35) | 37/122(30.33) | 0.472 |
| Multiple birth rate per transfer cycle, n (%) | 40/233(17.17) | 44/260(16.92) | 1.000 | 38/198(19.19) | 38/223(17.04) | 0.612 | 2/35(5.71) | 6/37(16.22) | 0.262 |
| Miscarriage rate per transfer cycle, n (%) | 69/233(29.61) | 88/260(33.85) | 0.334 | 58/198(29.29) | 74/223(33.18) | 0.402 | 11/35(31.42) | 14/37(37.84) | 0.626 |
| Ectopic pregnancy rate per transfer cycle, n (%) | 1/233(0.43) | 2/260(0.77) | 1.000 | 1/198(0.51) | 2/223(0.90) | 1.000 | 0 | 0 | 1.000 |
| Cumulative biochemical pregnancy rate per initial cycle | 225(60.00) | 258(68.80) | 0.015 | 191(64.53) | 214(71.81) | 0.06 | 34(43.04) | 44(57.14) | 0.109 |
| Cumulative clinical pregnancy rate per initial cycle, n (%) | 194(51.73) | 226(60.27) | 0.023 | 167(56.42) | 194(65.10) | 0.036 | 27(34.18) | 32(41.56) | 0.41 |
| Miscarriage rates per initial cycle, n (%) | 39(20.10) | 54(23.89) | 0.409 | 33(19.76) | 45(23.20) | 0.445 | 6(22.22) | 9(28.13) | 0.766 |
| CLBR per initial cycle | 154(41.07) | 170(45.33) | 0.269 | 133(44.93) | 147(49.33) | 0.287 | 21(26.58) | 23(29.87) | 0.723 |
| Preterm birth rate, n(%) | 13(8.44) | 25(14.71) | 0.086 | 12(9.02) | 23(15.65) | 0.106 | 1(4.76) | 2(8.70) | 1.00 |
| Average birth weight | 3300(2973, 3550) | 3280(2650, 3600) | 0.143 | 3300(2950, 3550) | 3250(2750, 3600) | 0.427 | 3200(3000, 3500) | 3265(2563, 3550) | 0.448 |
| Average gestational age at birth | 38(38, 39) | 38(37, 39) | 0.005 | 38(38, 39) | 38(37, 39) | 0.052 | 38(38, 39) | 37.5(36, 39) | 0.351 |
| Congenital malformation rates | 0 | 0 | 1.000 | 0 | 0 | 1.000 | 0 | 0 | 1.000 |
| TTLB | 360(315.8, 445) | 326.5(257.5, 436) | <0.001 | 357(313, 445) | 325(257, 418) | 0.0002 | 361(322.5, 468) | 313(255, 389) | 0.012 |
| Factors to Predict a Cumulative Live Birth |
Coefficient(B) | S.E. | Wald(χ2) | P Value | Hazard Ratio (95% CI) |
|---|---|---|---|---|---|
| Age of women | -0.135 | 0.039 | 11.877 | 0.001 | 0.874(0.809-0.943) |
| Type of infertility | |||||
| primary infertility | 1 | ||||
| secondary infertility | -0.052 | 0.198 | 0.069 | 0.794 | 0.949(0.644-1.400) |
| Infertility duration | 0.000 | 0.022 | 0.000 | 0.988 | 1.000(0.958-1.043) |
| Cycle number | |||||
| The first cycle | 1 | ||||
| The second cycle | 0.084 | 0.200 | 0.175 | 0.676 | 1.087(0.734-1.610) |
| Treatment protocol | |||||
| GnRHa | 1 | ||||
| CPOS | 0.388 | 0.201 | 3.725 | 0.084 | 1.475(0.994-2.187) |
| Body mass index | 0.000 | 0.000 | 0.160 | 0.689 | 1.000(1.00-1.00) |
| Basal FSH | 0.026 | 0.035 | 0.545 | 0.461 | 1.026(0.958-1.098) |
| AMH | 0.116 | 0.041 | 18.77 | <0.001 | 1.112(1.017-1.431) |
| Total Gn dosage | 0.000 | 0.000 | 0.570 | 0.450 | 1.000(1.00-1.00) |
| Days of stimulation | -0.106 | 0.073 | 2.102 | 0.147 | .900(0.780-1.038 |
| No. of oocytes retrieved | 0.023 | 0.020 | 1.378 | 0.240 | 1.023(0.985-1.063) |
| No. of available embryos | 0.352 | 0.050 | 50.517 | <0.001 | 1.422(1.290-1.567) |
| Cause of infertility | |||||
| Tubal | 1 | ||||
| Male factor | 0.439 | 0.307 | 2.043 | 0.153 | 1.551(0.850-2.830) |
| Anovulatory | -0.460 | 0.456 | 1.017 | 0.313 | 0.631(0.258-1.543) |
| Unexplained | 0.087 | 0.210 | 0.173 | 0.678 | 1.091(0.723-1.657) |
| Diminished ovarian reserve | 0.055 | 0.308 | 0.032 | 0.857 | 1.057(0.578-1.933) |
| Endometriosis | 0.371 | 0.420 | 0.782 | 0.376 | 1.449(0.637-3.298) |
| Mixed | 0.689 | 0.741 | 0.865 | 0.352 | 1.991(0.466-8.506) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




