Submitted:
06 November 2023
Posted:
06 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Experimental design
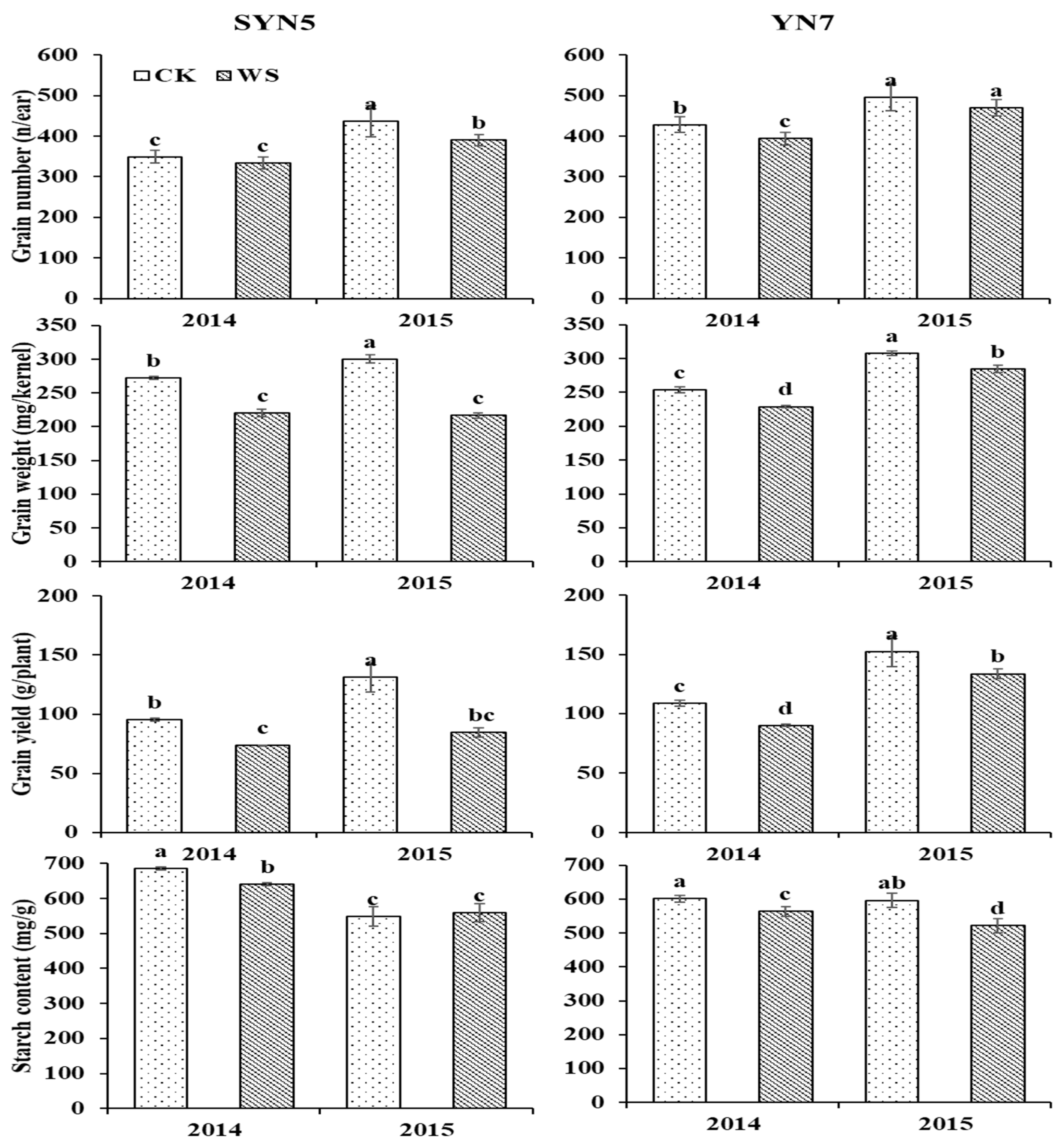
2.2. Grain yield
2.3. Starch content
2.4. Starch isolation
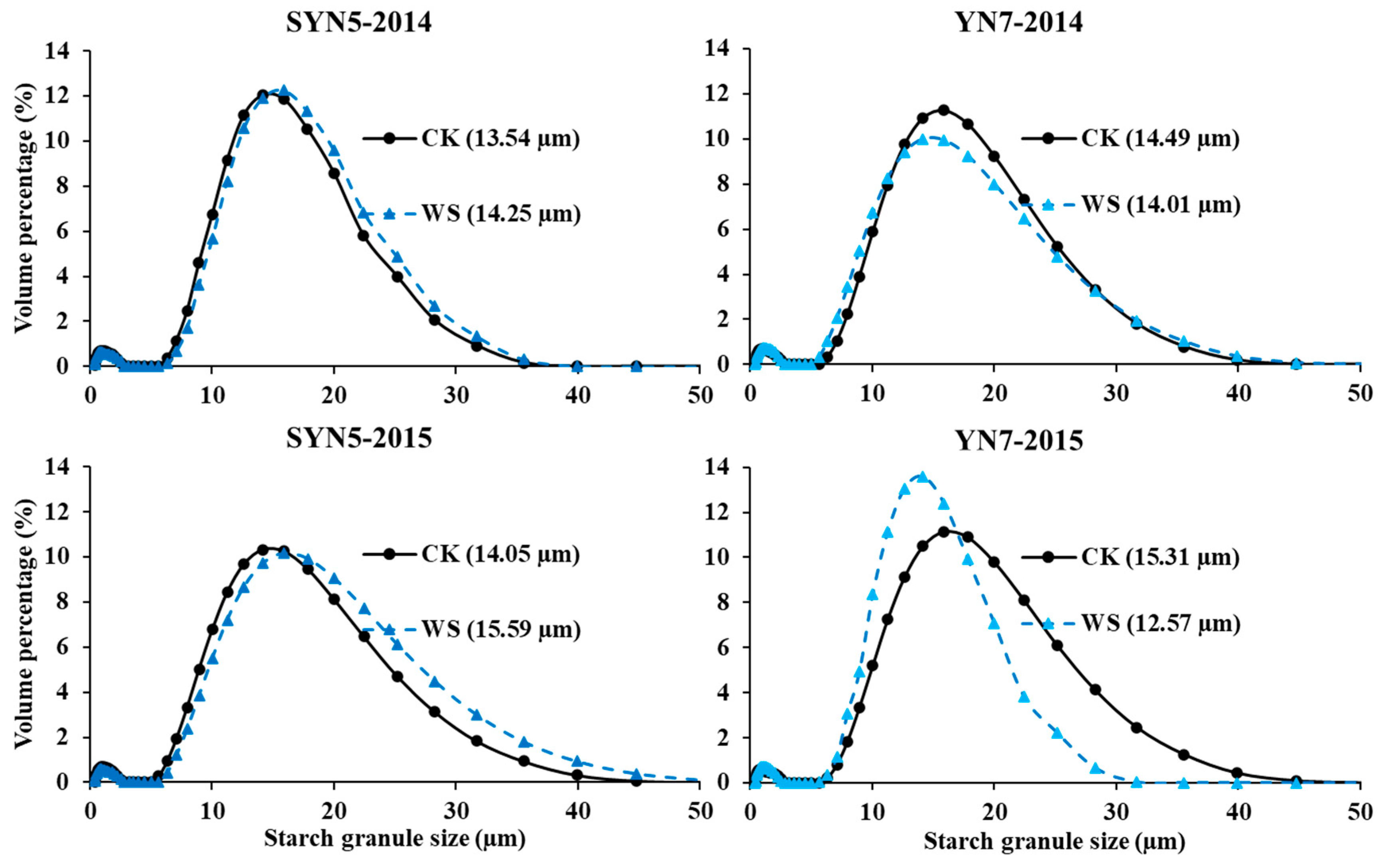
2.5. Starch granule size
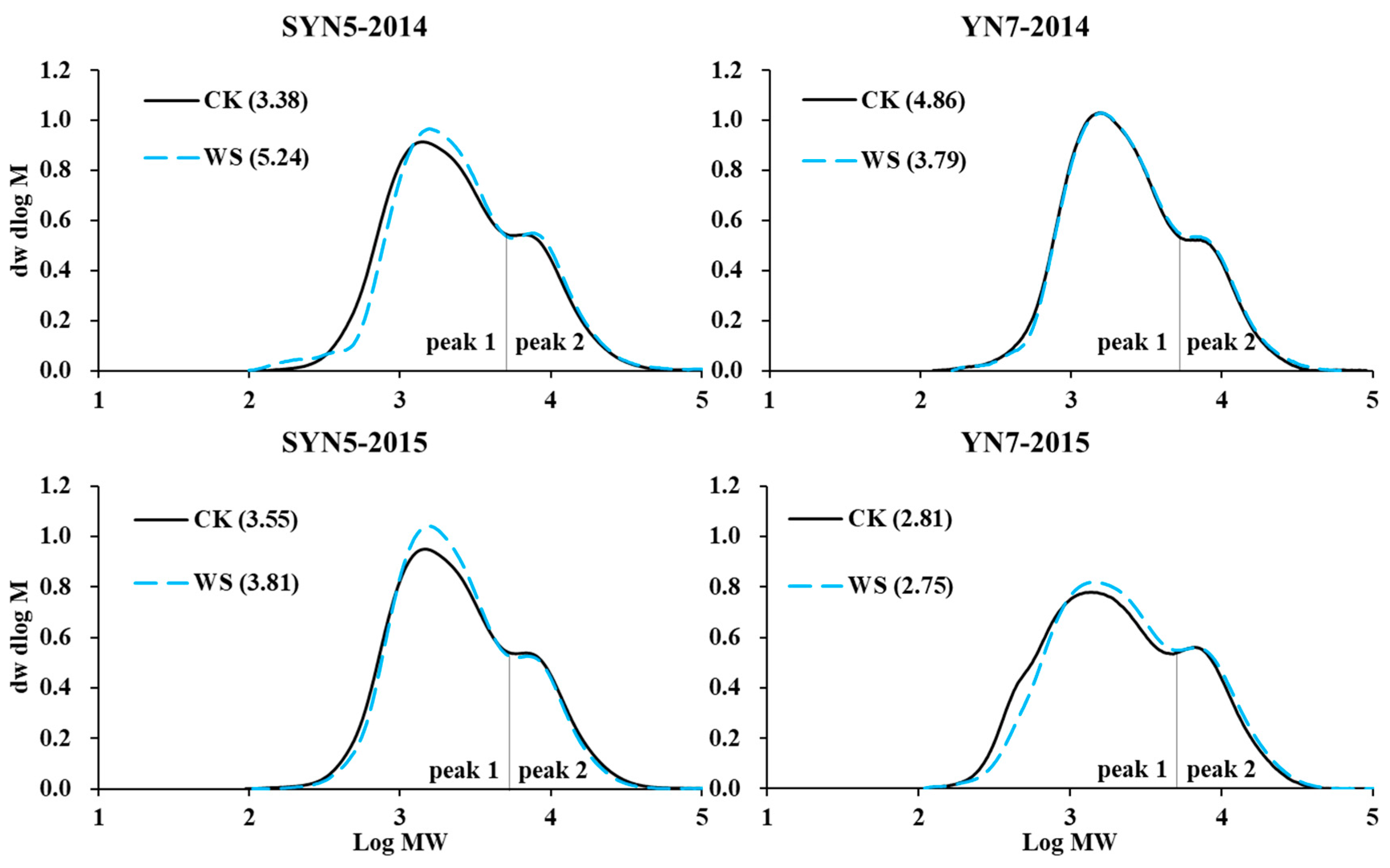
2.6. Starch molecular weight
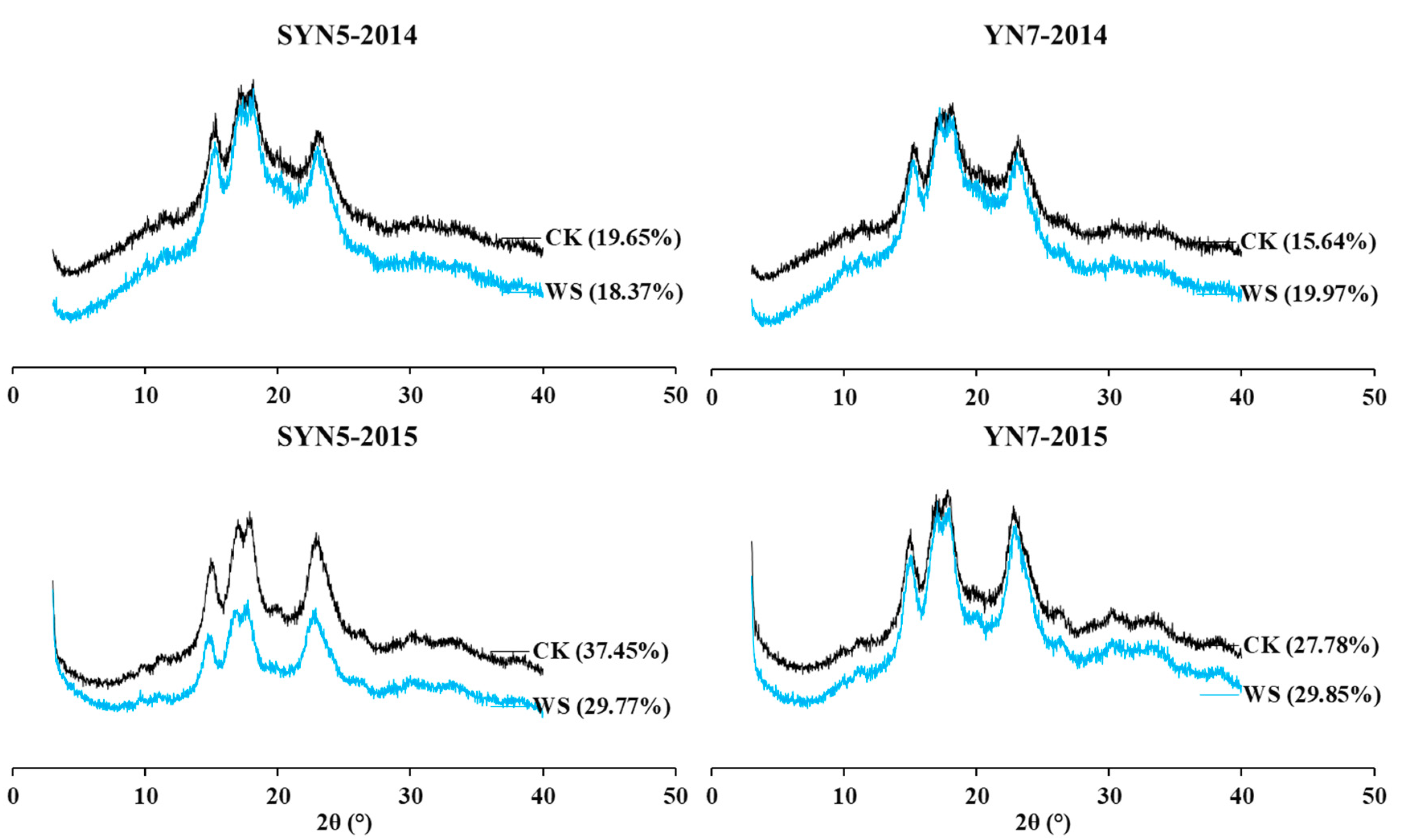
2.7. X-ray diffraction
2.8. Pasting property
2.9. Thermal property
2.10. Statistical design
3. Results and discussion
3.1. Grain yield
3.2. Starch content
3.3. Starch granule size
3.4. Starch molecular weight distribution
3.5. Starch X-ray diffraction
3.6. Pasting property
3.7. Thermal property
| Year | Hybrid | water | ΔHgel (J/g) |
To (℃) |
Tp (℃) |
Tc (℃) |
ΔHret (J/g) |
%R (%) |
|---|---|---|---|---|---|---|---|---|
| 2014 | SYN5 | CK | 8.84±0.24abc | 69.6±0.1d | 75.6±0.0de | 82.4±0.1c | 3.5±0.0a | 39.7±0.9a |
| WS | 8.74±0.22bcd | 68.5±0.1e | 74.8±0.0f | 81.9±0.1c | 2.9±0.1b | 33.0±0.3b | ||
| YN7 | CK | 8.53±0.12cd | 70.2±0.0cd | 75.5±0.0e | 82.3±0.0c | 2.7±0.2b | 31.9±1.5b | |
| WS | 8.23±0.07d | 68.3±0.1e | 74.6±0.0f | 83.1±0.1b | 2.1±0.2c | 24.9±1.9c | ||
| 2015 | SYN5 | CK | 8.93±0.07abc | 72.5±0.0a | 77.8±0.2a | 84.7±0.0a | 3.6±0.1a | 40.8±1.8a |
| WS | 9.36±0.11a | 70.4±0.1cd | 76.2±0.1b | 83.3±0.2b | 4.1±0.2a | 43.2±2.2a | ||
| YN7 | CK | 8.83±0.11abcd | 71.4±0.6b | 76.0±0.0c | 82.9±0.1b | 3.6±0.1a | 40.6±1.1a | |
| WS | 9.16±0.30ab | 70.9±0.2bc | 75.8±0.2cd | 83.0±0.2b | 3.7±0.2a | 40.0±0.7a |
4. Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mustroph, A. Improving Flooding Tolerance of Crop Plants. Agronomy-Basel 2018, 8. [Google Scholar] [CrossRef]
- Kaur, G.; Vikal, Y.; Kaur, L.; Kalia, A.; Mittal, A.; Kaur, D.; Yadav, I. Elucidating the morpho-physiological adaptations and molecular responses under long-term waterlogging stress in maize through gene expression analysis. Plant Sci 2021, 304. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.X.; Bi, W.S.; Ren, X.S.; Li, W.L.; Sun, L.; Li, J. Flooding has more adverse effects on the stem structure and yield of spring maize (Zea mays L.) than waterlogging in Northeast China. Eur J Agron 2020, 117. [Google Scholar] [CrossRef]
- Dash, S.S.; Lenka, D.; Sahoo, J.P.; Tripathy, S.K.; Samal, K.C.; Lenka, D.; Panda, R.K. Biochemical characterization of maize (Zea mays L.) hybrids under excessive soil moisture stress. Cereal Res Commun 2022. [Google Scholar] [CrossRef]
- Otie, V.; Ping, A.; Udo, I.; Eneji, E. Brassinolide effects on maize (Zea mays L.) growth and yield under waterlogged conditions. J Plant Nutr 2019, 42, 954–969. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B. Responses of carbon metabolism and antioxidant system of summer maize to waterlogging at different stages. J Agron Crop Sci 2018, 204, 505–514. [Google Scholar] [CrossRef]
- Tian, L.X.; Li, J.; Bi, W.S.; Zuo, S.Y.; Li, L.J.; Li, W.L.; Sun, L. Effects of waterlogging stress at different growth stages on the photosynthetic characteristics and grain yield of spring maize (Zea mays L.) Under field conditions. Agr Water Manage 2019, 218, 250–258. [Google Scholar] [CrossRef]
- Ren, B.Z.; Dong, S.T.; Zhao, B.; Liu, P.; Zhang, J.W. Responses of Nitrogen Metabolism, Uptake and Translocation of Maize to Waterlogging at Different Growth Stages. Front Plant Sci 2017, 8. [Google Scholar] [CrossRef]
- Fan, H.Y.; Zhou, Z.Q.; Yang, C.N.; Jiang, Z.; Li, J.T.; Cheng, X.X.; Guo, Y.J. Effects of waterlogging on amyloplasts and programmed cell death in endosperm cells of Triticum aestivum L. Protoplasma 2013, 250, 1091–1103. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, X.J.; Xin, L.; Jiang, H.D.; Wang, X.; Cai, J.; Jiang, D. Waterlogging and simulated acid rain after anthesis deteriorate starch quality in wheat grain. Plant Growth Regul 2018, 85, 257–265. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, M.; Huang, X.; Liu, J.; Wang, X.; Cai, J.; Dai, T.B.; Cao, W.X.; Jiang, D. Effect of post-anthesis waterlogging on biosynthesis and granule size distribution of starch in wheat grains. Plant Physiol Bioch 2018, 132, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Arata, A.F.; Dinolfo, M.I.; Martinez, M.; Lazaro, L. Effects of Waterlogging during Grain Filling on Yield Components, Nitrogen Uptake and Grain Quality in Bread Wheat. Cereal Res Commun 2019, 47, 42–52. [Google Scholar] [CrossRef]
- Li, H.W.; Wang, Z.S.; Zhuo, Q.C.; Zhang, B.; Wang, F.H.; Jiang, D. Starch Granule Size Distribution and Pasting Characteristic Response to Post-Anthesis Combined Stress of Waterlogging and Shading. Agriculture-Basel 2020, 10. [Google Scholar] [CrossRef]
- Chen, Z.K.; Du, Y.F.; Mao, Z.L.; Zhang, Z.J.; Li, P.; Cao, C.G. Grain starch, fatty acids, and amino acids determine the pasting properties in dry cultivation plus rice cultivars. Food Chem 2022, 373. [Google Scholar] [CrossRef]
- Xiong, R.Y.; Xie, J.X.; Chen, L.M.; Yang, T.T.; Tan, X.M.; Zhou, Y.J.; Pan, X.H.; Zeng, Y.J.; Shi, Q.H.; Zhang, J.; Zeng, Y.H. Water irrigation management affects starch structure and physicochemical properties of indica rice with different grain quality. Food Chem 2021, 347. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Chen, T.; Zhang, H.; Cao, J.; Li, X.; Wang, X.; Wang, Y.; Yao, S.; Gao, Y.; Chen, Y.; Zhang, L. Effect of waterlogging stress on grain nutritional quality and pod yield of peanut (Arachis hypogaea L.). J Agron Crop Sci 2023, 209, 286–299. [Google Scholar] [CrossRef]
- Yu, X.R.; Yu, H.; Zhang, J.; Shao, S.S.; Xiong, F.; Wang, Z. Endosperm Structure and Physicochemical Properties of Starches from Normal, Waxy, and Super-Sweet Maize. Int J Food Prop 2015, 18, 2825–2839. [Google Scholar] [CrossRef]
- Yang, H.; Wen, Z.R.; Huang, T.Q.; Lu, W.P.; Lu, D.L. Effects of waterlogging at grain formation stage on starch structure and functionality of waxy maize. Food Chem 2019, 294, 187–193. [Google Scholar] [CrossRef]
- Lu, D.L.; Cai, X.M.; Shi, Y.X.; Zhao, J.R.; Lu, W.P. Effects of waterlogging after pollination on the physicochemical properties of starch from waxy maize. Food Chem 2015, 179, 232–238. [Google Scholar] [CrossRef]
- Lu, D.L.; Cai, X.M.; Lu, W.P. Effects of water deficit during grain filling on the physicochemical properties of waxy maize starch. Starch-Starke 2015, 67, 692–700. [Google Scholar] [CrossRef]
- Hansen, J.; Møller, I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem 1975, 68, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.S.; Guo, D.W.; Zhao, L.X.; Zhang, X.D.; Wang, J.; Zhang, F.M.; Wei, C.X. Comparative structure of starches from high-amylose maize inbred lines and their hybrids. Food Hydrocolloid 2016, 52, 19–28. [Google Scholar] [CrossRef]
- Cai, C.H.; Lin, L.S.; Man, J.M.; Zhao, L.X.; Wang, Z.F.; Wei, C.X. Different Structural Properties of High-Amylose Maize Starch Fractions Varying in Granule Size. J Agr Food Chem 2014, 62, 11711–11721. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.L.; Lu, W.P. Effects of protein removal on the physicochemical properties of waxy maize flours. Starch-Starke 2012, 64, 874–881. [Google Scholar] [CrossRef]
- Jiang, D.; Fan, X.M.; Dai, T.B.; Cao, W.X. Nitrogen fertiliser rate and post-anthesis waterlogging effects on carbohydrate and nitrogen dynamics in wheat. Plant Soil 2008, 304, 301–314. [Google Scholar] [CrossRef]
- Yang, H.; Huang, T.Q.; Ding, M.Q.; Lu, D.L.; Lu, W.P. Effects of Waterlogging Around Flowering Stage on the Grain Yield and Eating Properties of Fresh Waxy Maize. Cereal Chemistry 2016, 93, 605–611. [Google Scholar] [CrossRef]
- Zheng, C.F.; Jiang, D.; Dai, T.B.; Jing, Q.; Cao, W.X. Effects of salt and waterlogging stress at post-anthesis stage on wheat grain yield and quality. Chinese Journal of Applied Ecology 2009, 20, 2391–2398. [Google Scholar] [PubMed]
- Zeng, R.E.; Chen, T.T.; Wang, X.Y.; Cao, J.; Li, X.; Xu, X.Y.; Chen, L.; Xia, Q.; Dong, Y.L.; Huang, L.P. , et al. Physiological and Expressional Regulation on Photosynthesis, Starch and Sucrose Metabolism Response to Waterlogging Stress in Peanut. Front Plant Sci 2021, 12. [Google Scholar] [CrossRef]
- Yang, H.; Shen, X.; Ding, M.Q.; Lu, D.L.; Lu, W.P. Effects of High Temperature after Pollination on Grain Development and Endogenous Hormone Contents of Waxy Maize. Journal of Maize Sciences 2017, 25, 55–60, 67. [Google Scholar] [CrossRef]
- Hsieh, C.F.; Liu, W.C.; Whaley, J.K.; Shi, Y.C. Structure and functional properties of waxy starches. Food Hydrocolloid 2019, 94, 238–254. [Google Scholar] [CrossRef]
- Wu, A.C.; Gilbert, R.G. Molecular Weight Distributions of Starch Branches Reveal Genetic Constraints on Biosynthesis. Biomacromolecules 2010, 11, 3539–3547. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Q.; Xu, X.; Qi, L.; Dong, Z.; Luo, Z.; Lu, X.; Peng, X. Structural changes of waxy and normal maize starches modified by heat moisture treatment and their relationship with starch digestibility. Carbohyd Polym 2017, 177, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.L.; Guo, H.F.; Dong, C.; Lu, W.P. Starch Granule Size Distribution and Thermal Properties of Waxy Maize Cultivars in Growing Seasons. ACTA AGRONOMICA SINICA 2010, 36, 1998–2003. [Google Scholar]
- Lu, D.L.; Sun, X.L.; Yan, F.B.; Wang, X.; Xu, R.C.; Lu, W.P. Effects of high temperature during grain filling under control conditions on the physicochemical properties of waxy maize flour. Carbohyd Polym 2013, 98, 302–310. [Google Scholar] [CrossRef] [PubMed]
| Year | Hybrid | water | PV (mPa.s) |
TV (mPa.s) |
BD (mPa.s) |
FV (mPa.s) |
SB (mPa.s) |
Ptemp (℃) |
|---|---|---|---|---|---|---|---|---|
| 2014 | SYN5 | CK | 1384±1d | 1256±6a | 128±7d | 1660±20a | 404±14a | 76.1±0.4bc |
| WS | 998±26e | 927±24e | 71±2e | 1181±32e | 254±8de | 75.3±0.4c | ||
| YN7 | CK | 1378±42d | 1275±34a | 103±8d | 1692±52a | 417±18a | 76.1±0.4bc | |
| WS | 1016±2e | 983±3de | 33±1f | 1299±12cd | 316±9b | 75.3±0.4c | ||
| 2015 | SYN5 | CK | 1586±20b | 1134±4b | 452±16c | 1427±6b | 293±2bc | 77.9±0.4a |
| WS | 1477±29c | 1022±22cd | 455±7c | 1246±22de | 224±0e | 76.3±0.4bc | ||
| YN7 | CK | 1791±4a | 1075±5bc | 717±9a | 1344±1bc | 270±6cd | 76.3±0.5bc | |
| WS | 1659±13b | 1061±5c | 599±9b | 1354±9bc | 293±4bc | 77.1±0.4ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





