Submitted:
06 November 2023
Posted:
06 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
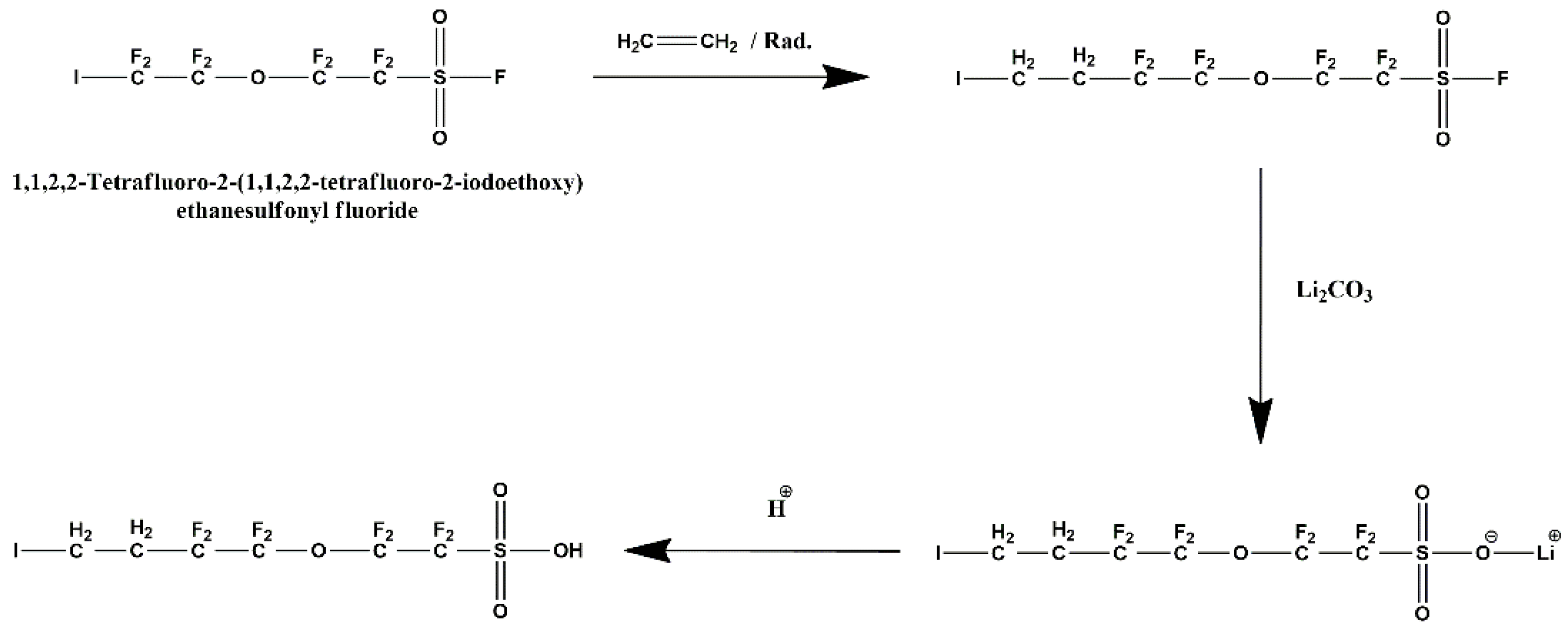
2. Materials and Methods
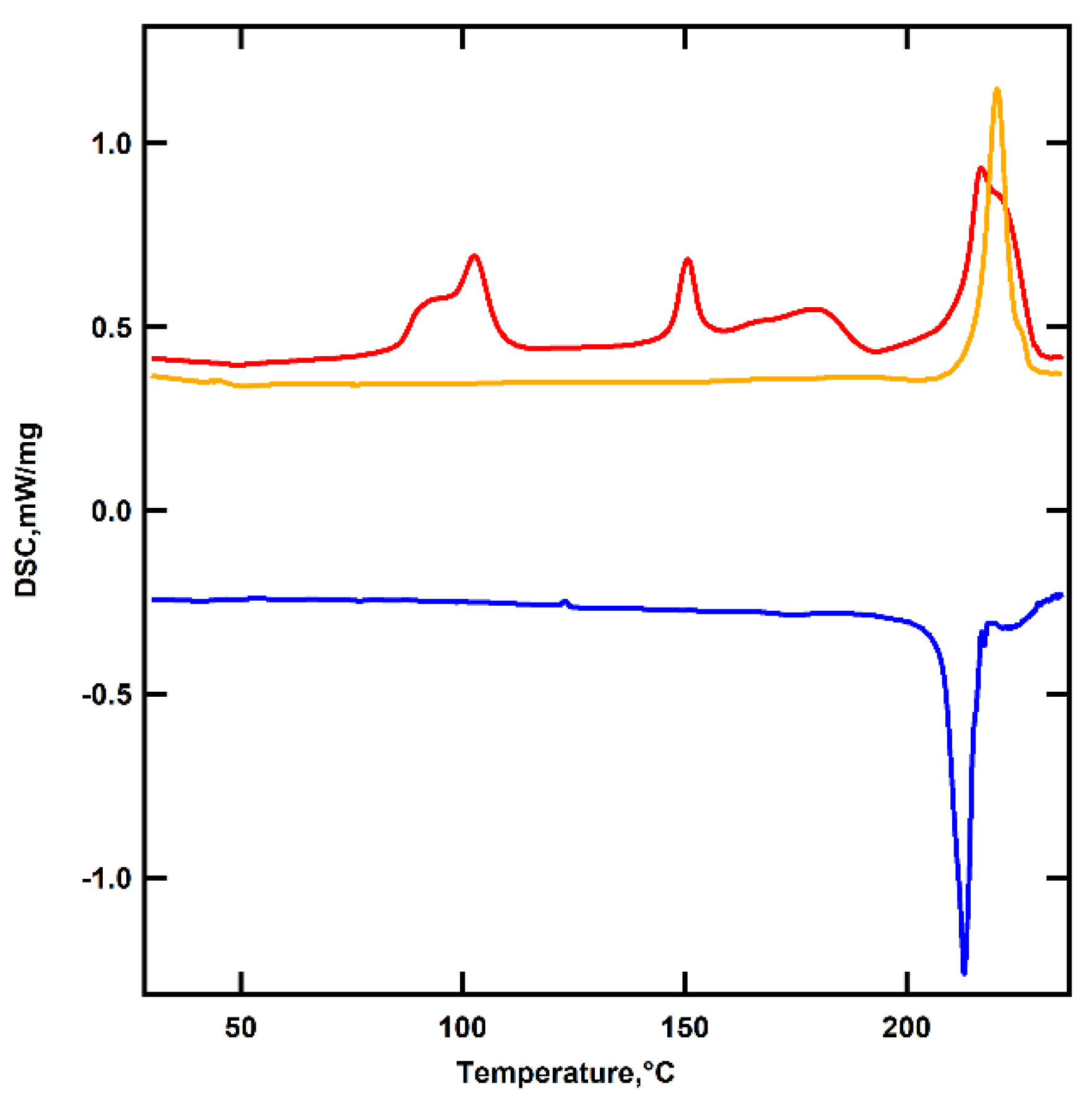
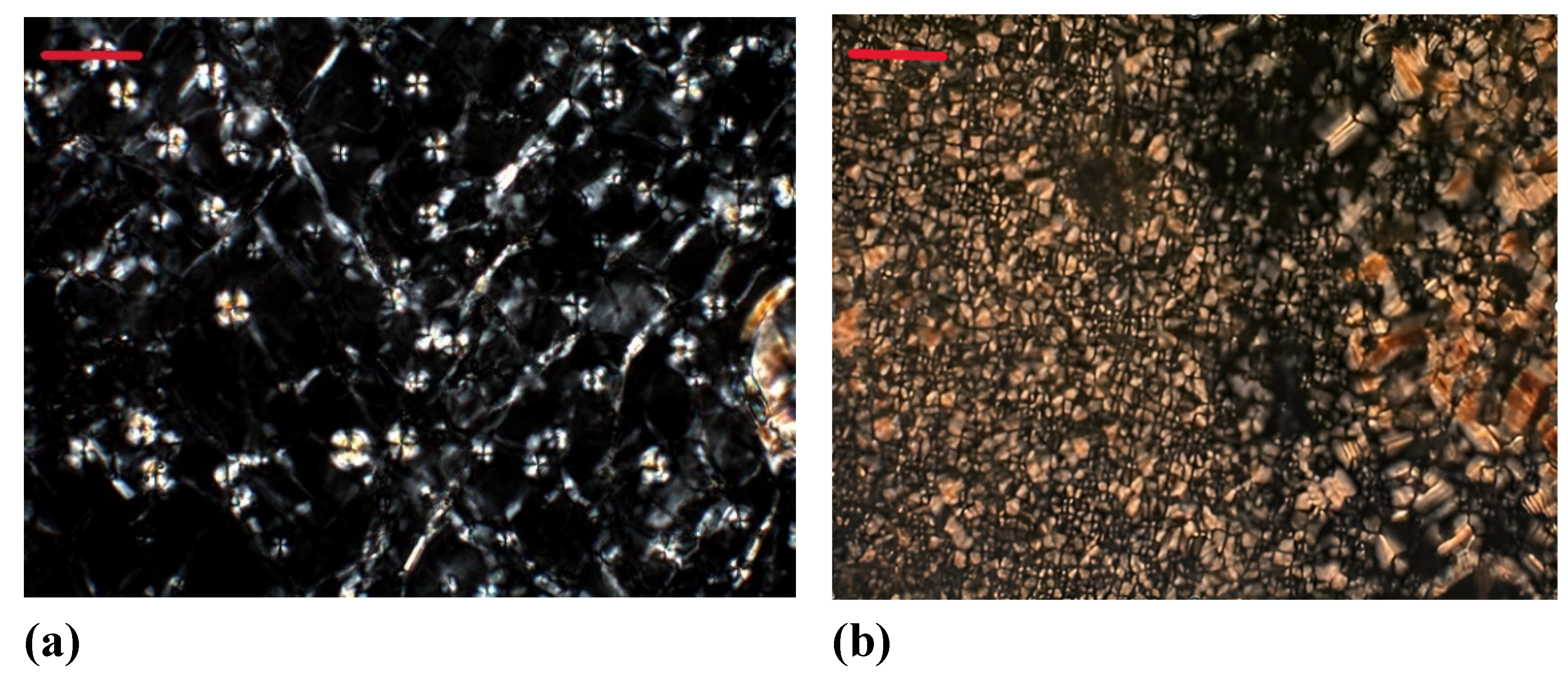
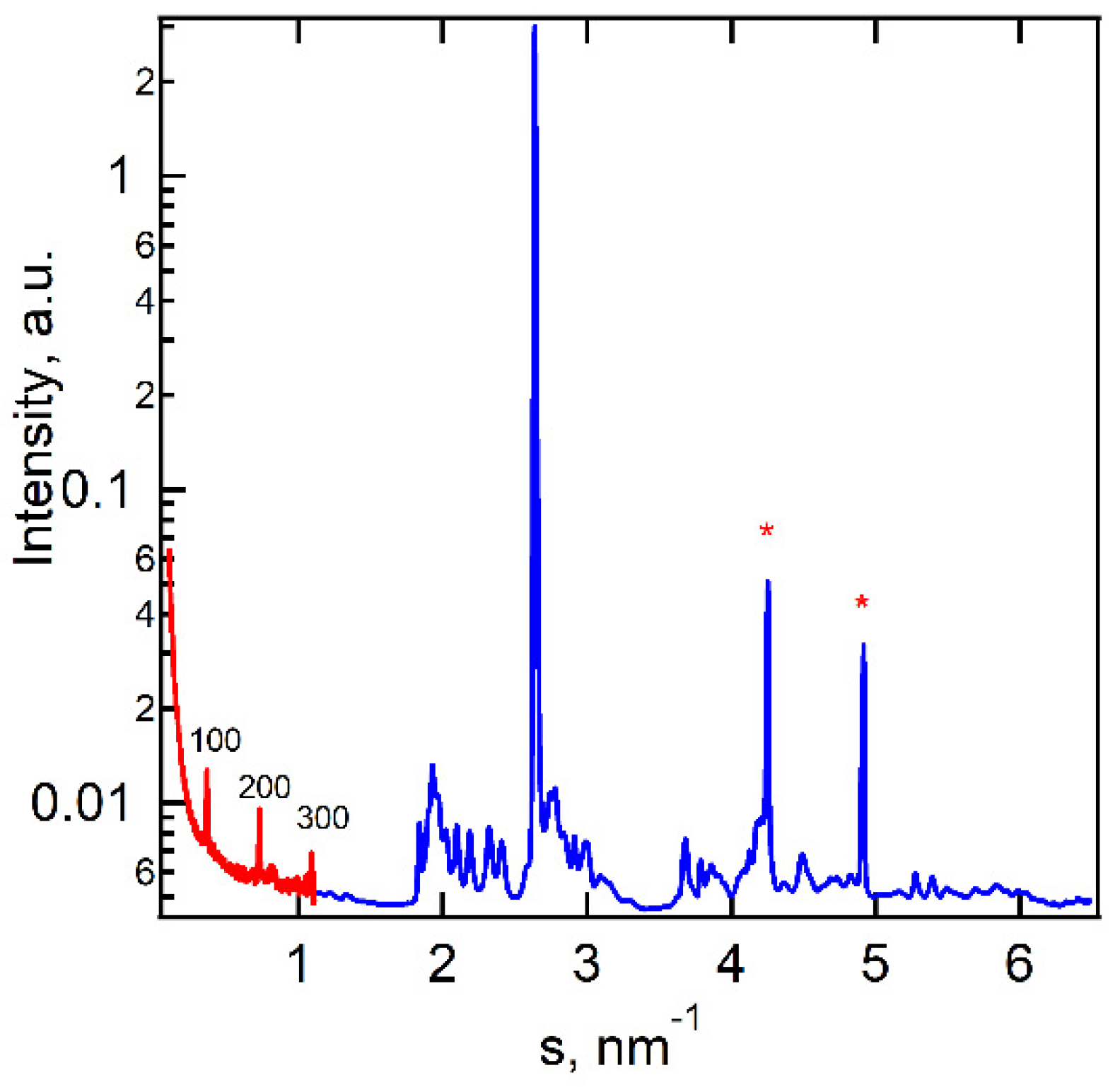
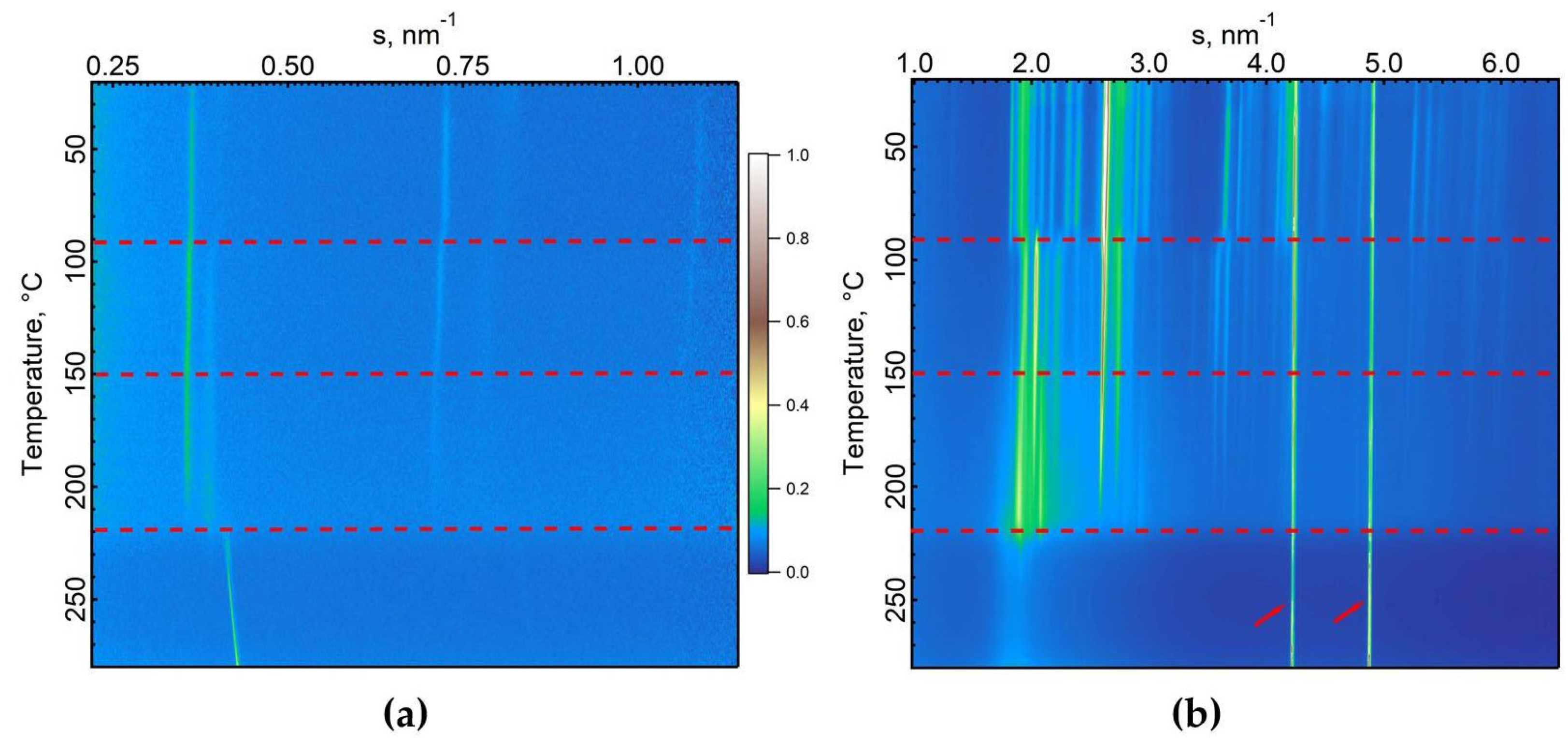
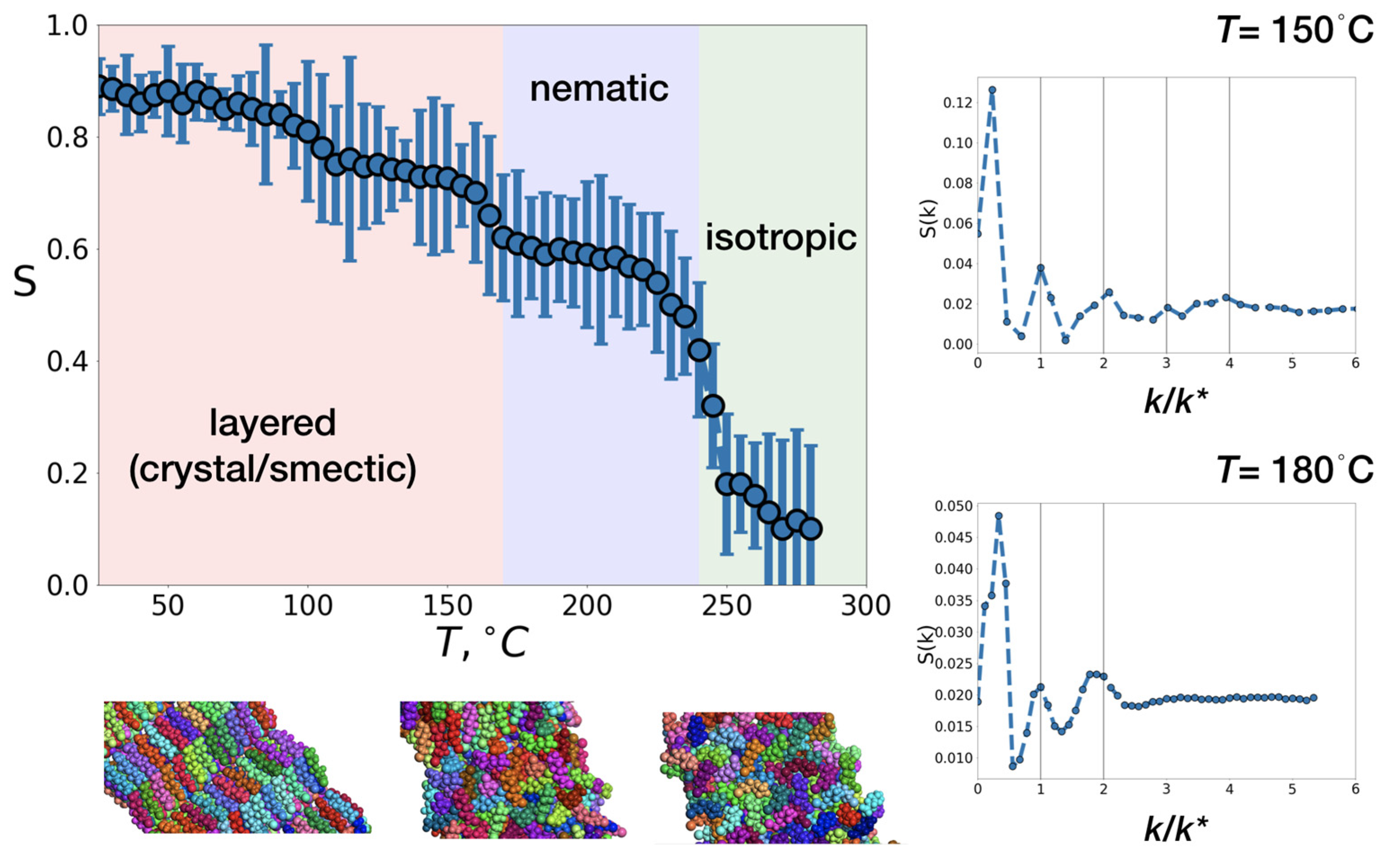
3. Results and discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- D. Gielen, F. Boshell, D. Saygin, M. D. Bazilian, N. Wagner, R. Gorini, The Role of Renewable Energy in the Global Energy Transformation, Energy Strategy Reviews, 2019, 24, 38-50. [CrossRef]
- M. Z. Jacobson, The Health and Climate Impacts of Carbon Capture and Direct Air Capture, Energy & Environmental Science, 2019, 12, 3567-3574. [CrossRef]
- D. Lew, N. Miller, K. Clark, G. Jordan, Z. Gao, Impact of High Solar Penetration in the Western Interconnection, National Renewable Energy Agency, 2010, 1-9.
- Inganäs O. Organic Photovoltaics over Three Decades. Adv Mater. 2018,30(35), 1800388. [CrossRef]
- G. Zhang, F. R. Lin, F. Qi, T. Heumüller, A. Distler, H. J. Egelhaaf, N. Li, P. C. Y. Chow, C. J. Brabec, A. K. Jen, H. L. Yip, Renewed Prospects for Organic Photovoltaics, Chem Rev., 2022, 122(18), 14180-14274. [CrossRef]
- M. Armand, J.-M. Tarascon, Building better batteries, Nature, 2008, 451, 652–657. [CrossRef]
- P. K. Sharma, D. A. Kumar, P. William, D. Obulesu, P. M. Pandian, T. K. H. Khan, G. Manikandan, Energy Storage System Based on Hybrid Wind and Photovoltaic Technologies, Measurement: Sensors, 2023, 30, 100915, ISSN 2665-9174. [CrossRef]
- Okonkwo, Paul C.; Belgacem, I.B.; Emori, W.; Uzoma, P.C., Nafion degradation mechanisms in proton exchange membrane fuel cell (PEMFC) system: A review; Intern. J. Hydrogen Energy, 2021, 46, 27956-27973. [CrossRef]
- A. Kusoglu, A. Z. Weber, New Insights into Perfluorinated Sulfonic-Acid Ionomers, Chemical Reviews, 2017, 117, 987-1104. [CrossRef]
- L. Fan, Z. Tu, S. H. Chan, Recent development of hydrogen and fuel cell technologies: A review, Energy Reports, 2021, 7, 8421-8446. [CrossRef]
- Y. Tanaka, Development of the MIRAI Fuel Cell vehicle, In Hydrogen Energy Engineering, A Japanese Perspective; K. Sasaki, H. W. Li, A. Hayashi, J. Yamabe, T. Ogura, S. M. Lyth, Eds; Springer: Japan, Tokyo, 2016, 34, 461-476. [CrossRef]
- Jinnouchi, R.; Kudo, K.; Kodama, K.; Kitano, N.; Suzuki, T.; Minami, S.; Shinozaki, K.; Hasegawa, N., Shinohara, A., The role of oxygen-permeable ionomer for polymer electrolyte fuel cells, Nature Comm.; 2021, 12, 4956. [CrossRef]
- T. Suzuki, S. Tsushima, S. Hirai, Effects of Nafion® Ionomer and Carbon Particles on Structure Formation in a Proton-Exchange Membrane Fuel Cell Catalyst Layer Fabricated by the Decal-Transfer Method, International Journal of Hydrogen Energy, 2011, 36, 19, 12361–12369. [CrossRef]
- S. Liu, R. Lin, J. Lu, Y. Wang, X. Cai, The Impact of Different Side Chain Ionomer on Membrane Electrode Assembly Performance and Durability, Chemical Engineering Journal, 2023, 472, 145050. [CrossRef]
- X. Yan, Z. Xu, S. Yuan, A. Han, Y. Shen, X. Cheng, Y. Liang, S. Shen, J. Zhang, Structural and Transport Properties of Ultrathin Perfluorosulfonic Acid Ionomer Film in Proton Exchange Membrane Fuel Cell Catalyst Layer: A Review, Journal of Power Sources, 2022, 536, 231523. [CrossRef]
- I. Antipin, M. Alémov, V. Arslanov, V. Burilov, S. Vatsadze, Y. Voloshin, K. Volcho, V. Gorbatchuk, Y. Gorbunova, S. Gromov, et al., Functional supramolecular systems: Design and applications, Russ. Chem. Rev. 2021, 90, 895–1107. [CrossRef]
- A. Alfutimie, R. Curtis, G. J. T. Tiddy, Lyotropic Surfactant Liquid Crystals: Micellar Systems, In Handbook of Liquid Crystals, 2nd ed.; J. Goodby, P. Collings, T. Kato, C. Tschierske, H. Gleeson, P. Raynes, V. Vill, Eds.; Wiley-VCH: Weinheim, Germany, 2014; Volume 6. [CrossRef]
- N. Illy, D. Urayeneza, A.V. Maryasevskaya, L. Michely, S. Boileau, B. Brissault, E.A. Bersenev, D.V. Anokhin, D.A. Ivanov, J. Penelle. Synthesis and Solid-State Properties of PolyC3 (Co)polymers Containing (CH2-CH2-C(COOR)2) Repeat Units with Densely Packed Fluorocarbon Lateral Chains, Macromolecules, 2019, 52(23), 9199-9207. [CrossRef]
- Q. Berrod, S. Lyonnard, A. Guillermo, J. Ollivier, B. Frick, A. Manseri, B. Ameduri, G. Gebel, Nanostructure and transport properties of proton conducting self-assembled perfluorinated surfactants: a bottom-up approach towards PFSA fuel cell membranes, Macromolecules, 2015, 48, 6166–6176. [CrossRef]
- Y. Wang, J. Liu, S. Yang, Multi-functional liquid crystal elastomer composites, Applied Physics Reviews, 2022, 9(1). [CrossRef]
- A. Doostmohammadi, B. Ladoux, Physics of liquid crystals in cell biology, Trends in cell biology, 2022, 32(2), 140-150. [CrossRef]
- N. Kapernaum, A. Lange, M. Ebert, M. A. Grunwald, C. Haege, S. Marino, A. Zens, A. Taubert, F. Giesselmann, S. Laschat, Current topics in ionic liquid crystals, ChemPlusChem, 2022, 87(1), e202100397. [CrossRef]
- A. V. Komolkin, A. Laaksonen, A. Maliniak, Molecular dynamics simulation of a nematic liquid crystal, The Journal of chemical physics, 1994, 101(5), 4103-4116. [CrossRef]
- F. Affouard, M. Kröger, S. Hess, Molecular dynamics of model liquid crystals composed of semiflexible molecules, Physical Review E, 1996, 54(5), 5178. [CrossRef]
- M. R. Wilson, Molecular simulation of liquid crystals: progress towards a better understanding of bulk structure and the prediction of material properties, Chem. Soc. Rev., 2007,36, 1881-1888. [CrossRef]
- X. Wei, J. B. Hooper, D. Bedrov, Influence of electrostatic interactions on the properties of cyanobiphenyl liquid crystals predicted from atomistic molecular dynamics simulations, Liquid Crystals, 2017, 44(2), 332-347. [CrossRef]
- P. Ahmadpour-Samani, P. Zahedi, An investigation on nematic-isotropic phase transition, viscosity, and diffusion coefficient of liquid crystalline elastomers at different temperatures using molecular dynamics simulation, Journal of Molecular Liquids, 2022, 367, 120403. [CrossRef]
- Sun, H., Ren, P., & Fried, J. R. The COMPASS force field: parameterization and validation for phosphazenes. Computational and Theoretical Polymer Science, 1998, 8(1-2), 229-246. [CrossRef]
- Beckers, J. V. L., Lowe, C. P., & De Leeuw, S. W. An iterative PPPM method for simulating Coulombic systems on distributed memory parallel computers. Molecular simulation, 1998, 20(6), 369-383. [CrossRef]
- Berendsen, H. J. C., Postma,J. P. M., van Gunsteren, W. F., DiNola, A., Haak, J. R. Molecular dynamics with coupling to an external bath. Journal of Chemical Physics , 1984, 81, 3684-3690. [CrossRef]
- Shuichi N. Constant temperature molecular dynamics methods, Progress of Theoretical Physics Supplement, 1991, 103, 1-46. [CrossRef]
- Ball, J. M., Majumdar, A. Nematic liquid crystals: from Maier-Saupe to a continuum theory. Molecular crystals and liquid crystals, 2010, 525, 1-11. [CrossRef]
- A. Manseri, B. Ameduri, B. Boutevin, G. Caporiccio, M. Kotora, M. Hajek, Synthesis of Telechelic Dienes from Fluorinated α,ω-Diiodoperfluoroalcanes. Part I. Divinyl and Diallyl Derivatives from Model I(C2F4)nI Compounds, J. Fluorine Chem., 1995, 73, 151-158. [CrossRef]
- L. Sauguet, B. Ameduri, B. Boutevin, Fluorinated Copolymers and Terpolymers Based on Vinylidene Fluoride and Bearing Sulfonic Acid Side-Group, J. Polym. Sci. Part A: Polym. Chem., 2007, 45, 1814-1834. [CrossRef]
- J. W. Goodby. Materials and Phase Structures of Calamitic and Discotic Liquid Crystals. Handbook of Visual Display Technology, Springer, Berlin, Heidelberg, 2012, 1243–1287. [CrossRef]
- J.-M. Corpart, S. Girault, D. Juhué, Structure and Surface Properties of Liquid Crystalline Fluoroalkyl Polyacrylates: Role of the Spacer, Langmuir, 2001, 17, 7237–7244. [CrossRef]
- F. Vivien, N. Wicker, Minimal enclosing parallelepiped in 3D, Comput. Geom, 2004, 29, 177–190. [CrossRef]
- A.A. Glagoleva, V.V. Vasilevskaya, A.R. Khokhlov, Microphase separation in the melts of diblock copolymers composed of linear and amphiphilic blocks, Polymer Science Series A, 2010, 52, 182-190. [CrossRef]
- I. Dierking, S. Al-Zangana, Lyotropic liquid crystal phases from anisotropic nanomaterials, Nanomaterials, 2017, 7(10), 305. [CrossRef]
- M.R. Wilson, G. Yu, T.D. Potter, M. Walker, S. J. Gray, J. Li, N. J. Boyd, Molecular simulation approaches to the study of thermotropic and lyotropic liquid crystals. Crystals, 2022, 12(5), 685. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




