Submitted:
03 November 2023
Posted:
07 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethics and consents
2.2. Preparation of cells free-filtrates (CFFs)
2.3. CFFs biochemical characterization
2.4. Animals
2.5. Administration protocols
2.6. Autopsy, tissue processing, and immunohistochemistry
2.7. Statistical processing
3. Results
3.1. I-Cells-free filtrates descriptive biochemical characterization
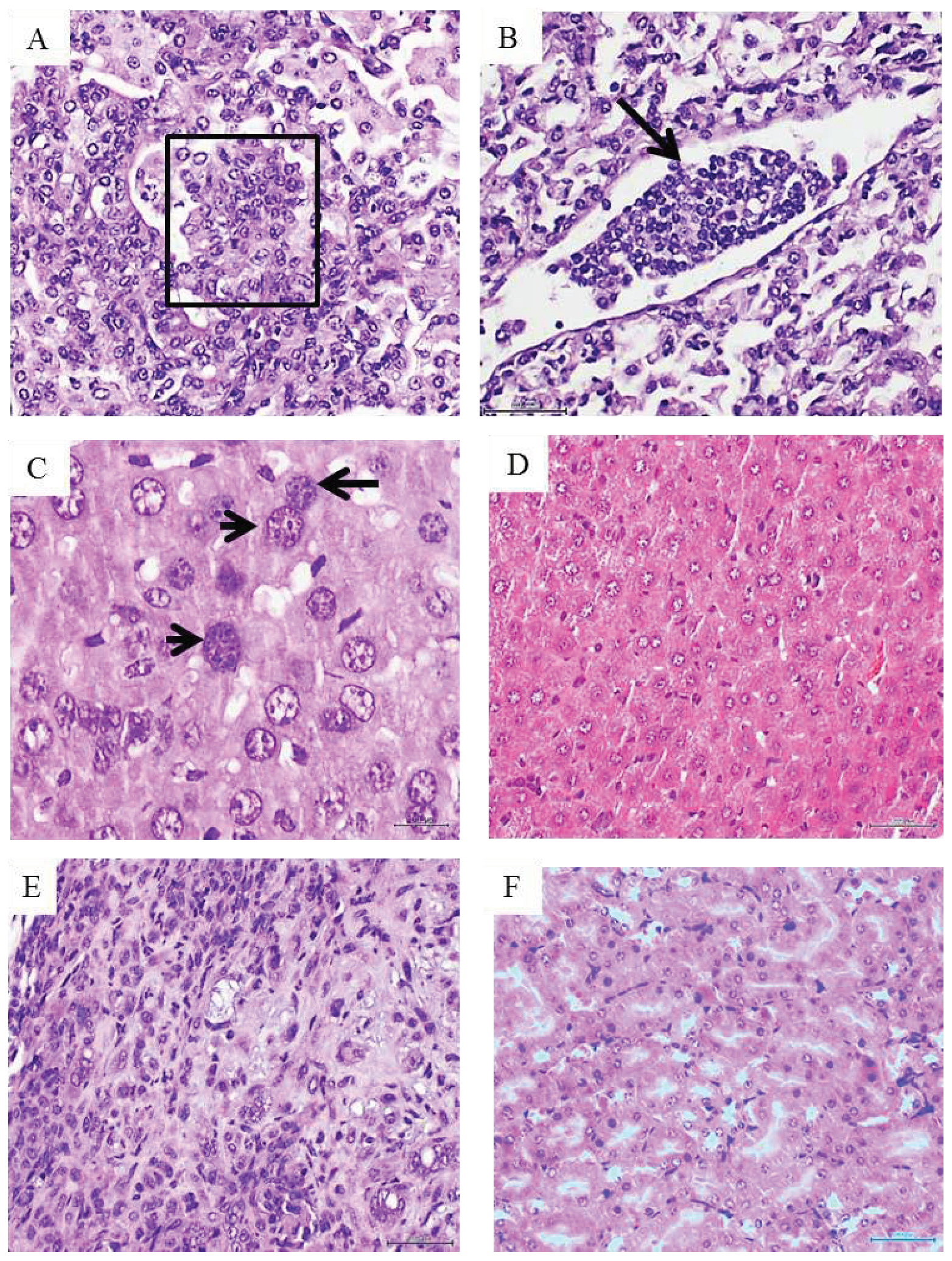
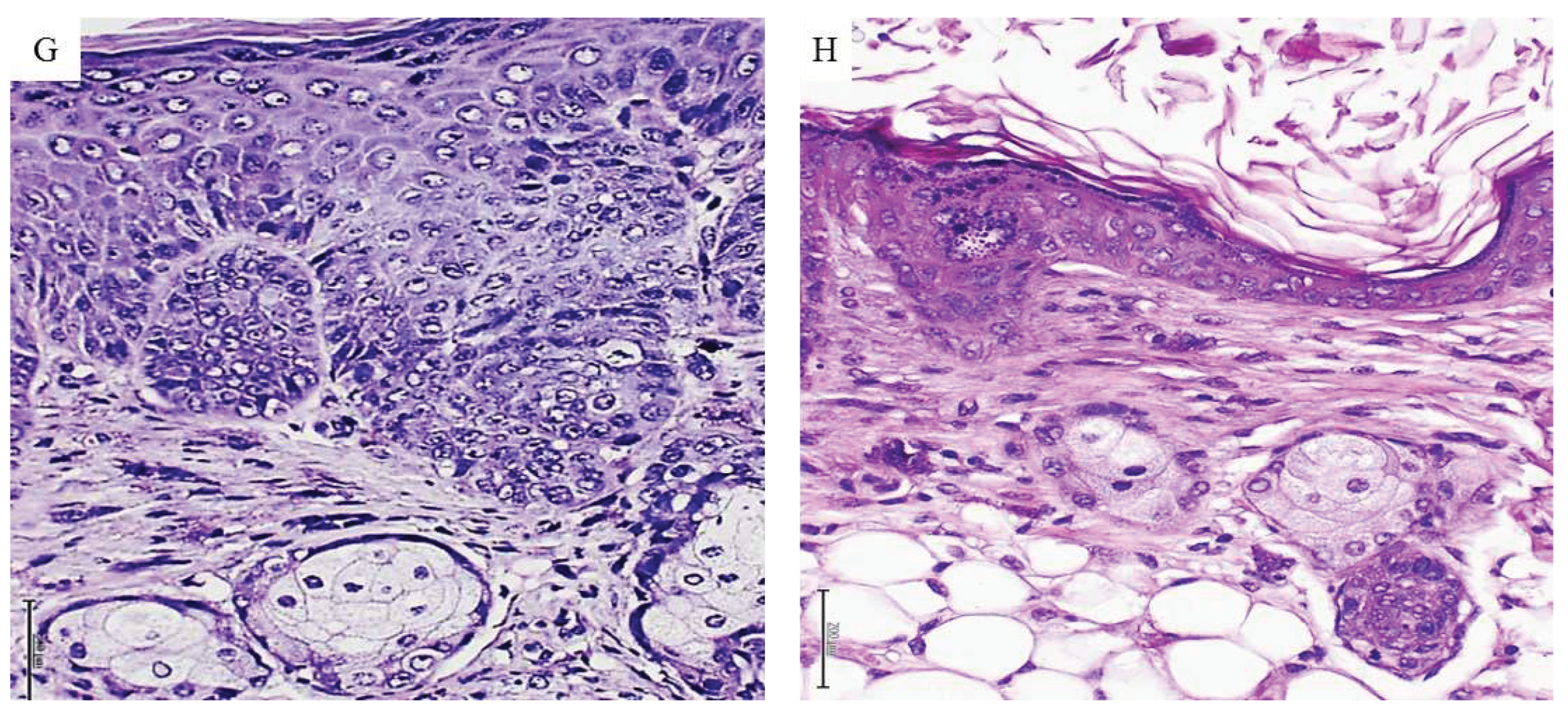
3.2. I-Protocol 1. Breast tumors CFF administration
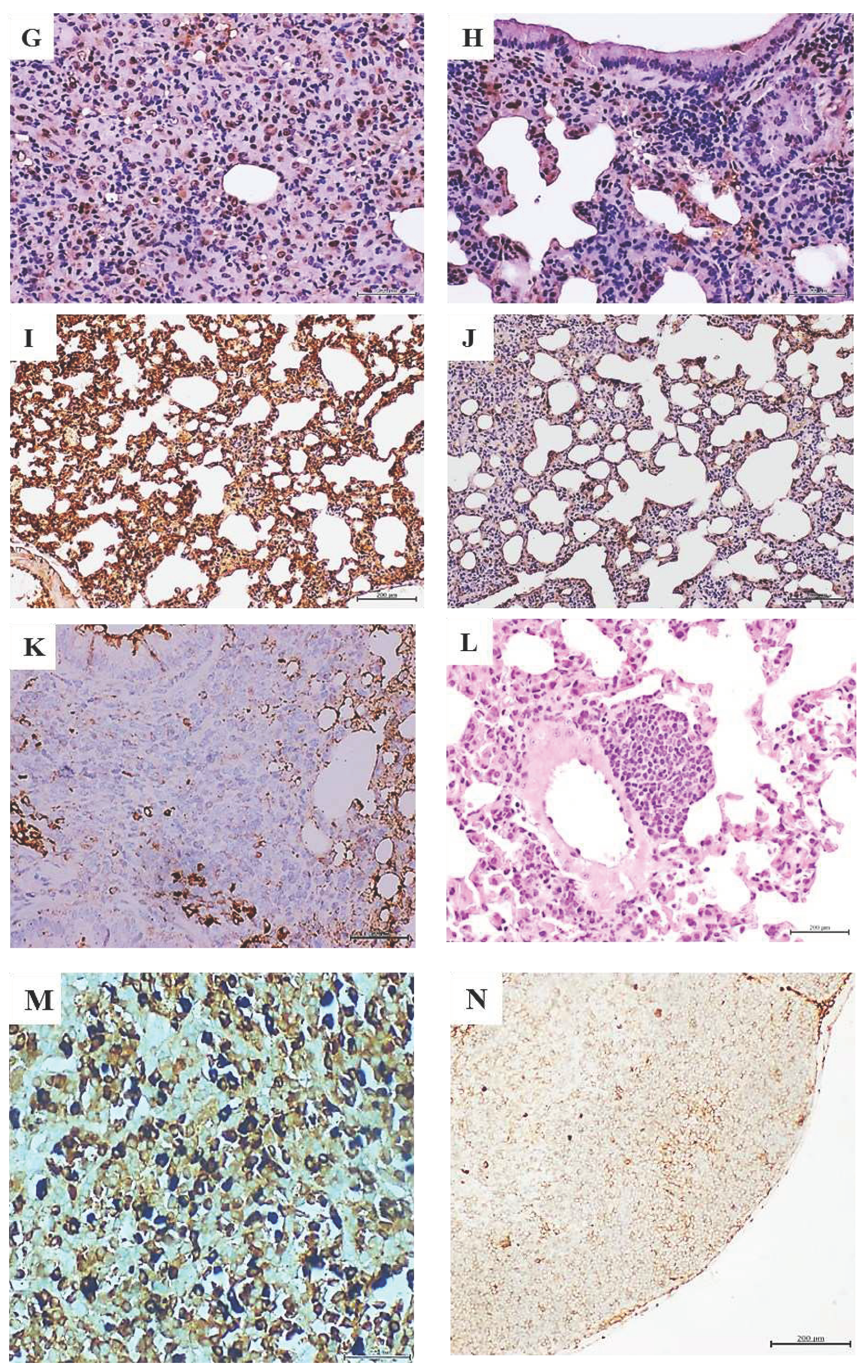
3.3. Protocol 2. Pancreatic ductal adenocarcinoma CFF administration
3.4. Protocol 3. Metastatic melanoma CFF administration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest/Disclaimer
References
- Bordonaro, M. Quantum biology and human carcinogenesis. Biosystems 2019, 178, 16–24. [Google Scholar] [CrossRef]
- NCI. What Is Cancer? 2021 [cited 2023; National Cancer Institute]. Available from: https://www.cancer.gov/about-cancer/understanding/what-is-cancer.
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Cataisson, C.; Lee, A.J.; Zhang, A.M.; Mizes, A.; Korkmaz, S.; Carofino, B.L.; Meyer, T.J.; Michalowski, A.M.; Li, L.; Yuspa, S.H. RAS oncogene signal strength regulates matrisomal gene expression and tumorigenicity of mouse keratinocytes. Carcinog. 2022, 43, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.Y.; Ko, Y.C.; Chen, Y.L.; Lin, S.F. The Way to Malignant Transformation: Can Epigenetic Alterations Be Used to Diagnose Early-Stage Head and Neck Cancer? Biomedicines 2023, 11, 1717. [Google Scholar] [CrossRef]
- Nijman, S.M. Perturbation-Driven Entropy as a Source of Cancer Cell Heterogeneity. Trends Cancer 2020, 6, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, S.; Ramirez, C.N.; Kuo, H.-C.D.; Chou, P.; Wu, R.; Sargsyan, D.; Yang, Y.; Shannar, A.; Peter, R.M.; Yin, R.; et al. The environmental carcinogen benzo[a]pyrene regulates epigenetic reprogramming and metabolic rewiring in a two-stage mouse skin carcinogenesis model. Carcinog. 2023, 44, 436–449. [Google Scholar] [CrossRef]
- Grunt, T.W.; Heller, G. A critical appraisal of the relative contribution of tissue architecture, genetics, epigenetics and cell metabolism to carcinogenesis. Prog. Biophys. Mol. Biol. 2023, 182, 26–33. [Google Scholar] [CrossRef]
- Kariagina, A.; Lunt, S.Y.; McCormick, J.J. Genomic and metabolomic analysis of step-wise malignant transformation in human skin fibroblasts. Carcinog. 2019, 41, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Missiroli, S.; Perrone, M.; Genovese, I.; Pinton, P.; Giorgi, C. Cancer metabolism and mitochondria: Finding novel mechanisms to fight tumours. EBioMedicine 2020, 59, 102943. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; Croucher, P.I. The dormant cancer cell life cycle. Nat. Rev. Cancer 2020, 20, 398–411. [Google Scholar] [CrossRef]
- Torres, A.J.F.; Duryea, J.; McDonald, O.G. Pancreatic cancer epigenetics: Adaptive metabolism reprograms starving primary tumors for widespread metastatic outgrowth. Cancer Metastasis Rev. 2023, 42, 389–407. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Abbott, M.; Ustoyev, Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin. Oncol. Nurs. 2019, 35, 150923. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef]
- Han, Y.; Wang, D.; Peng, L.; Huang, T.; He, X.; Wang, J.; Ou, C. Single-cell sequencing: A promising approach for uncovering the mechanisms of tumor metastasis. J. Hematol. Oncol. 2022, 15, 1–19. [Google Scholar] [CrossRef]
- Weiss, F.; Lauffenburger, D.; Friedl, P. Towards targeting of shared mechanisms of cancer metastasis and therapy resistance. Nat. Rev. Cancer 2022, 22, 157–173. [Google Scholar] [CrossRef]
- Liu, K.; Dou, R.; Yang, C.; Di, Z.; Shi, D.; Zhang, C.; Song, J.; Fang, Y.; Huang, S.; Xiang, Z.; et al. Exosome-transmitted miR-29a induces colorectal cancer metastasis by destroying the vascular endothelial barrier. Carcinog. 2023, 44, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Pardini, B.; Sabo, A.A.; Birolo, G.; Calin, G.A. Noncoding RNAs in Extracellular Fluids as Cancer Biomarkers: The New Frontier of Liquid Biopsies. Cancers 2019, 11, 1170. [Google Scholar] [CrossRef]
- Elemento, O. The road from Rous sarcoma virus to precision medicine. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Fernandez-Mayola, M.; Mendoza-Mari, Y.; Garcia-Ojalvo, A.; Martinez-Jimenez, I.; Rodriguez-Rodriguez, N.; Herrera, D.G.d.B.; Guillén-Nieto, G. Cell-Free Filtrates (CFF) as Vectors of a Transmissible Pathologic Tissue Memory Code: A Hypothetical and Narrative Review. Int. J. Mol. Sci. 2022, 23, 11575. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Fernández-Mayola, M.; Mendoza-Marí, Y.; García-Ojalvo, A.; Martinez-Jimenez, I.; Rodriguez-Rodriguez, N.; Playford, R.J.; Reyes-Acosta, O.; Lopez-Marín, L.; Guillén-Nieto, G. Intralesional Infiltrations of Arteriosclerotic Tissue Cells-Free Filtrate Reproduce Vascular Pathology in Healthy Recipient Rats. Int. J. Mol. Sci. 2022, 23, 1511. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Fernández-Mayola, M.; Mendoza-Marí, Y.; García-Ojalvo, A.; Playford, R.J.; Guillen-Nieto, G. Intralesional Infiltrations of Cell-Free Filtrates Derived from Human Diabetic Tissues Delay the Healing Process and Recreate Diabetes Histopathological Changes in Healthy Rats. Front. Clin. Diabetes Heal. 2021, 2. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Acosta, J.; Mendoza-Mari, Y.; Martinez-Jimenez, I.; Suarez-Alba, J.; Rodriguez-Rodriguez, N.; Garcia Ojalvo, A. Induction of Premalignant and Malignant Changes in Nude Mice by Human Tumors-Derived Cell-Free Filtrates. Ann. Hematol. Oncol. 2022, 9, 1387. [Google Scholar]
- Scudamore, C.L.; Busk, N.; Vowell, K. A simplified necropsy technique for mice: Making the most of unscheduled deaths. Lab. Anim. 2014, 48, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, A.Y.; Alcaraz, A.; Anver, M.R.; Bronson, R.T.; Cardiff, R.D.; Dixon, D.; Fraire, A.E.; Gabrielson, E.W.; Gunning, W.T.; Haines, D.C.; Kaufman, M.H.; Linnoila, R.I.; Maronpot, R.R.; Rabson, A.S.; Reddick, R.L.; Rehm, S.; Rozengurt, N.; Schuller, H.M.; Shmidt, E.N.; Travis, W.D.; Ward, J.M.; Jacks, T. Classification of proliferative pulmonary lesions of the mouse: Recommendations of the mouse models of human cancers consortium. Cancer Res. 2004, 64, 2307–2316. [Google Scholar] [CrossRef]
- NTP. Nonneoplastic Lesion Atlas. 2023 [cited 2023; National Toxicology Program]. Available from: https://ntp.niehs.nih.gov/atlas/nnl/respiratory-system/lung.
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Schroder, R.; Illert, A.L.; Erbes, T.; Flotho, C.; Lubbert, M.; Duque-Afonso, J. The epigenetics of breast cancer - Opportunities for diagnostics, risk stratification and therapy. Epigenetics 2022, 17, 612–624. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Yeo, D.; Giardina, C.; Saxena, P.; Rasko, J.E. The next wave of cellular immunotherapies in pancreatic cancer. Mol. Ther. - Oncolytics 2022, 24, 561–576. [Google Scholar] [CrossRef]
- Alekseenko, I.; Kondratyeva, L.; Chernov, I.; Sverdlov, E. From the Catastrophic Objective Irreproducibility of Cancer Research and Unavoidable Failures of Molecular Targeted Therapies to the Sparkling Hope of Supramolecular Targeted Strategies. Int. J. Mol. Sci. 2023, 24, 2796. [Google Scholar] [CrossRef]
- Yang, N.; Xiao, W.; Song, X.; Wang, W.; Dong, X. Recent Advances in Tumor Microenvironment Hydrogen Peroxide-Responsive Materials for Cancer Photodynamic Therapy. Nano-Micro Lett. 2020, 12, 1–27. [Google Scholar] [CrossRef]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodriguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef]
- Costa-Machado, L.F.; Fernandez-Marcos, P.J. The sirtuin family in cancer. Cell Cycle 2019, 18, 2164–2196. [Google Scholar] [CrossRef]
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef]
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic inflammation, cancer development and immunotherapy. Front. Pharmacol. 2022, 13, 1040163. [Google Scholar] [CrossRef]
- Luna, H.G.C.; Imasa, M.S.; Juat, N.; Hernandez, K.V.; Sayo, T.M.; Cristal-Luna, G.; Asur-Galang, S.M.; Bellengan, M.; Duga, K.J.; Buenaobra, B.B.; et al. Expression landscapes in non-small cell lung cancer shaped by the thyroid transcription factor 1. Lung Cancer 2022, 176, 121–131. [Google Scholar] [CrossRef]
- Wang, X.; Langer, E.M.; Daniel, C.J.; Janghorban, M.; Wu, V.; Wang, X.-J.; Sears, R.C. Altering MYC phosphorylation in the epidermis increases the stem cell population and contributes to the development, progression, and metastasis of squamous cell carcinoma. Oncogenesis 2020, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Xu, D. Telomerase Reverse Transcriptase (TERT) in Action: Cross-Talking with Epigenetics. Int. J. Mol. Sci. 2019, 20, 3338. [Google Scholar] [CrossRef] [PubMed]
- Hamad, S.H.; Montgomery, S.A.; Simon, J.M.; Bowman, B.M.; Spainhower, K.B.; Murphy, R.M.; Knudsen, E.S.; Fenton, S.E.; Randell, S.H.; Holt, J.R.; Hayes, D.N.; Witkiewicz, A.K.; Oliver, T.G.; Major, M.B.; Weissman, B.E. TP53, CDKN2A/P16, and NFE2L2/NRF2 regulate the incidence of pure- and combined-small cell lung cancer in mice. Oncogene 2022, 41, 3423–3432. [Google Scholar] [CrossRef] [PubMed]
- Janker, F.; Weder, W.; Jang, J.-H.; Jungraithmayr, W. Preclinical, non-genetic models of lung adenocarcinoma: A comparative survey. Oncotarget 2018, 9, 30527–30538. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Curtis, C. Looking backward in time to define the chronology of metastasis. Nat. Commun. 2020, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. Br. J. Cancer 2020, 124, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Compton, C. Cancer Initiation, Promotion, and Progression and the Acquisition of Key Behavioral Traits. In Cancer: The Enemy from Within; Springer Science and Business Media LLC: Cham, Switzerland, 2020; pp. 25–48. [Google Scholar]


| Samples | MDA (μM) |
H2O2 (pmol/μL) |
CRP (ng/mL) |
IL-6 (pg/mL) |
IL-1β (pg/mL) |
TNFα (pg/mL) |
Sirtuin 1 (ng/mL) |
|---|---|---|---|---|---|---|---|
| Control healthy donor skin | 3.47 | 17.39 | 21.95 | ND | 25.80 | ND | ND |
| Mammary ductal carcinoma | 3.89 | 25.88 | 152.39 | 30.15 | 116.18 | 72.89 | 17.21 |
| Pancreatic ductal adenocarcinoma | 3.05 | 21.66 | 529.75 | 151.39 | 99.70 | 27.25 | 5.30 |
| Metastatic Melanoma | 4.20 | 108.43 | 18.35 | 262.40 | 807.24 | 127.18 | 23.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





