Submitted:
03 November 2023
Posted:
06 November 2023
You are already at the latest version
Abstract
Keywords:
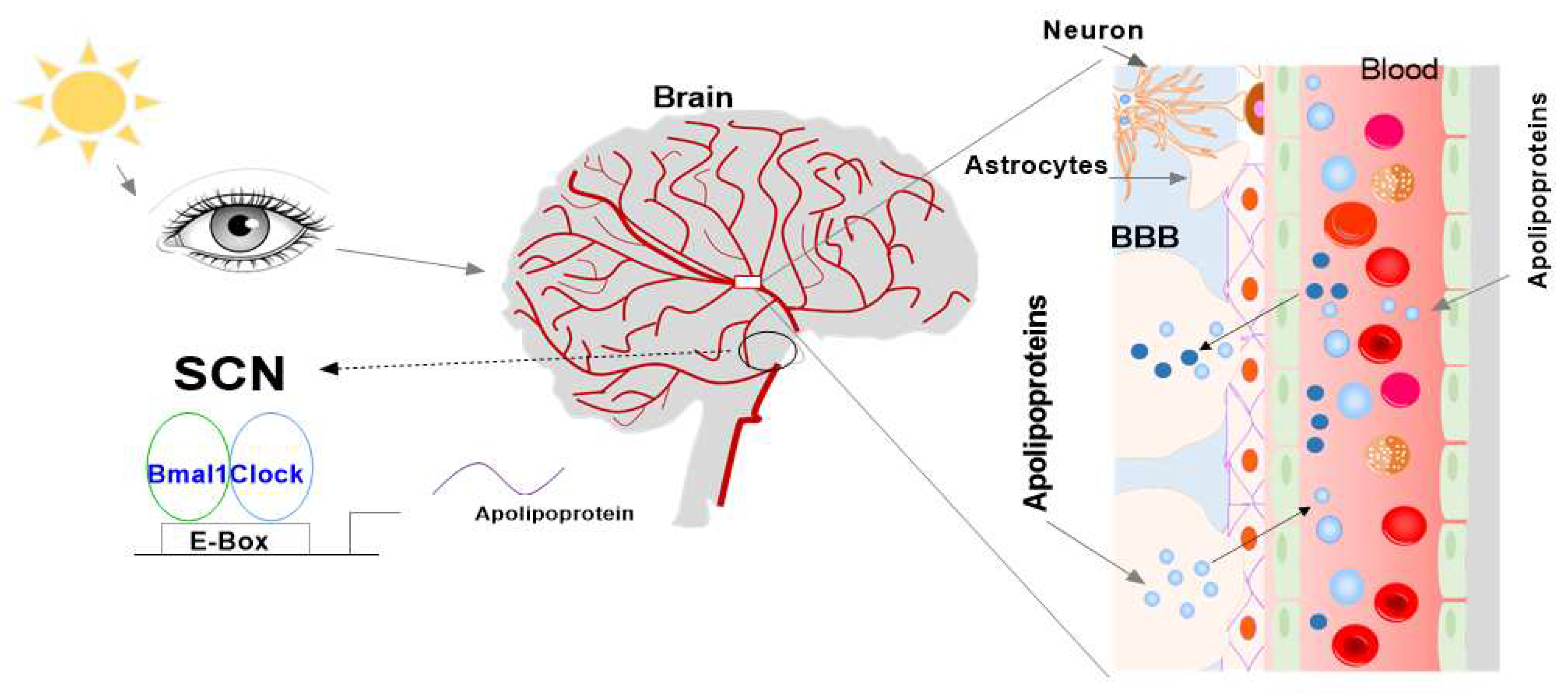
1. Introduction
2. SCN Systemic
2.1. SCN
2.2. Neurons and Hormone in SCN
2.3. Immune Factors in SCN
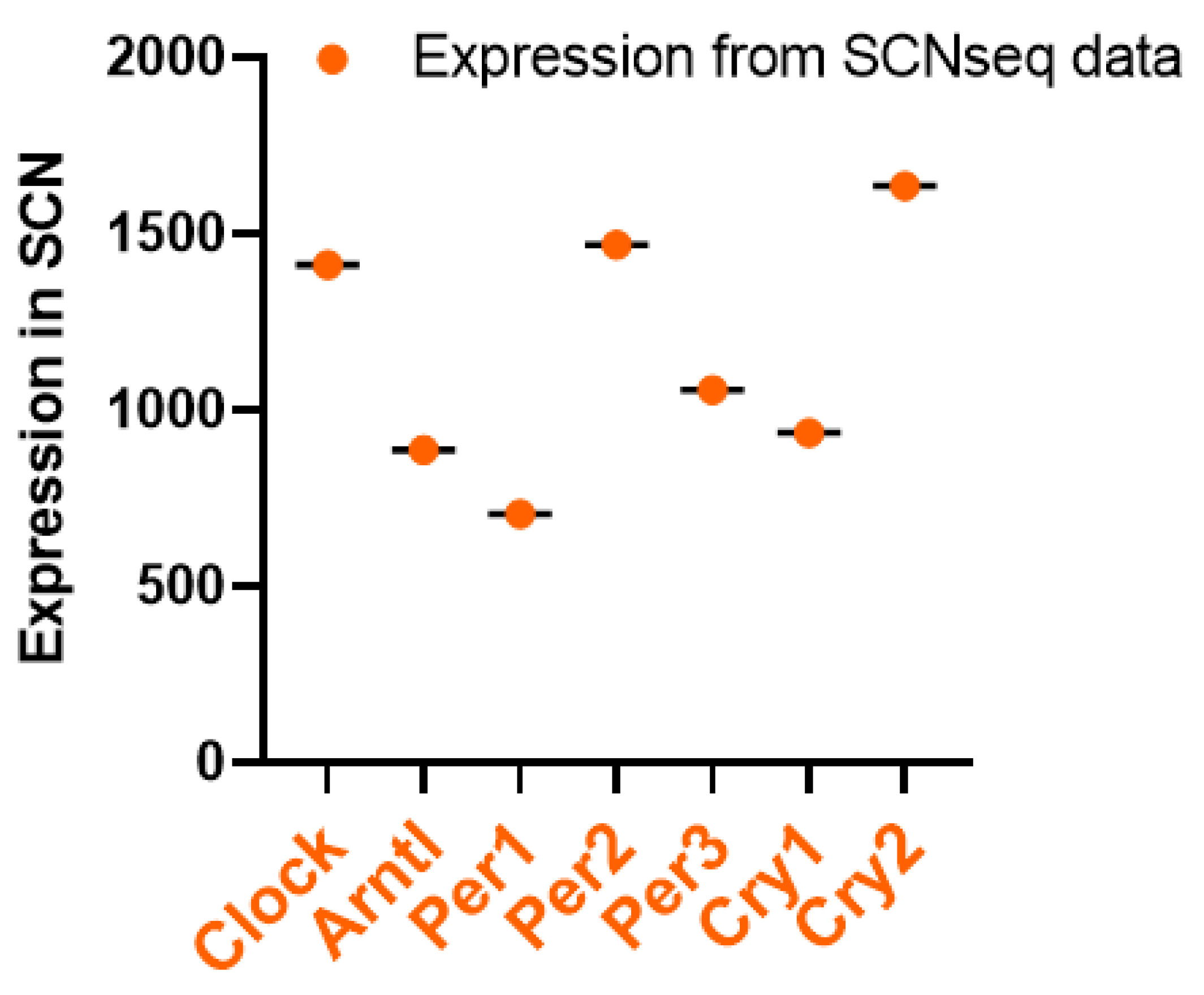
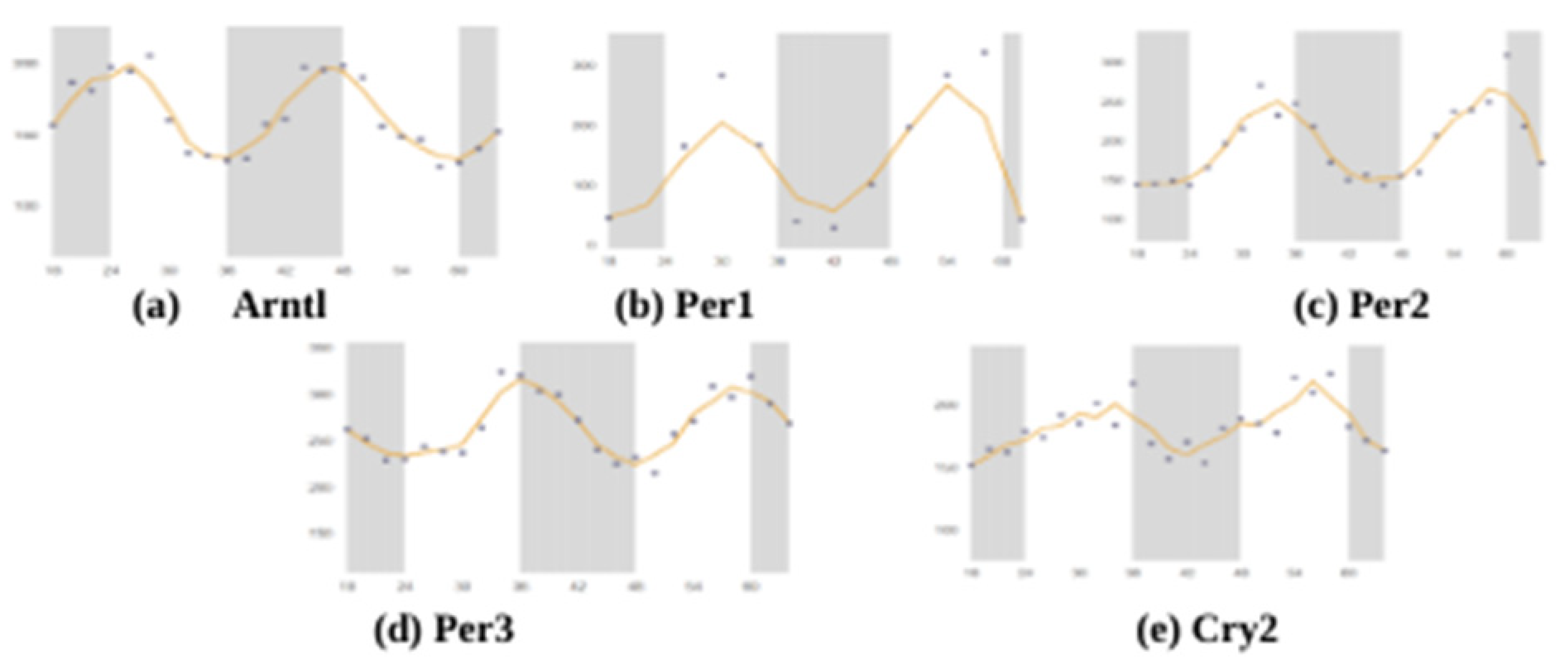
3. Circadian Rhythm of Clock Genes in SCN
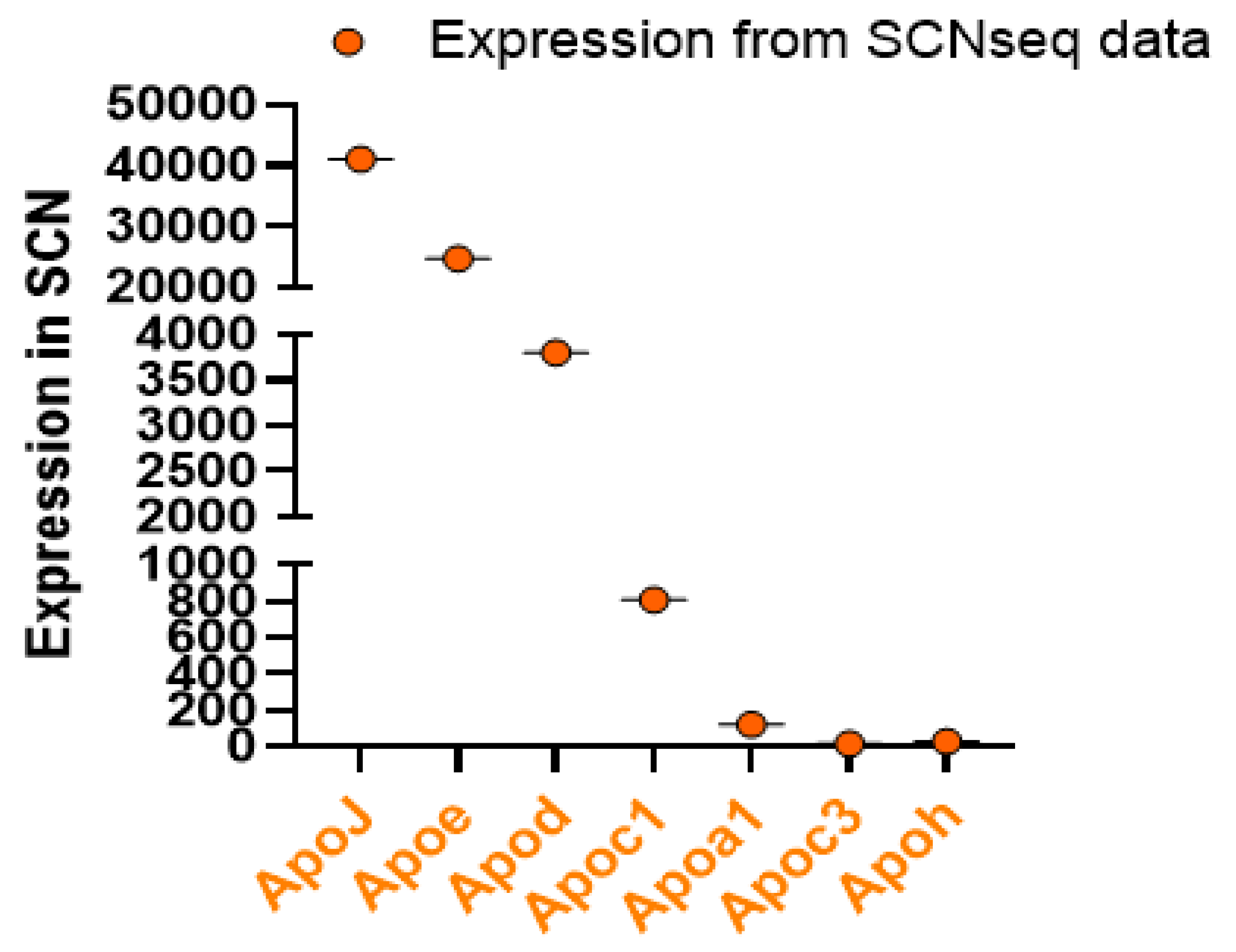
4. Circadian Rhythm of Apolipoprotein Genes in SCN
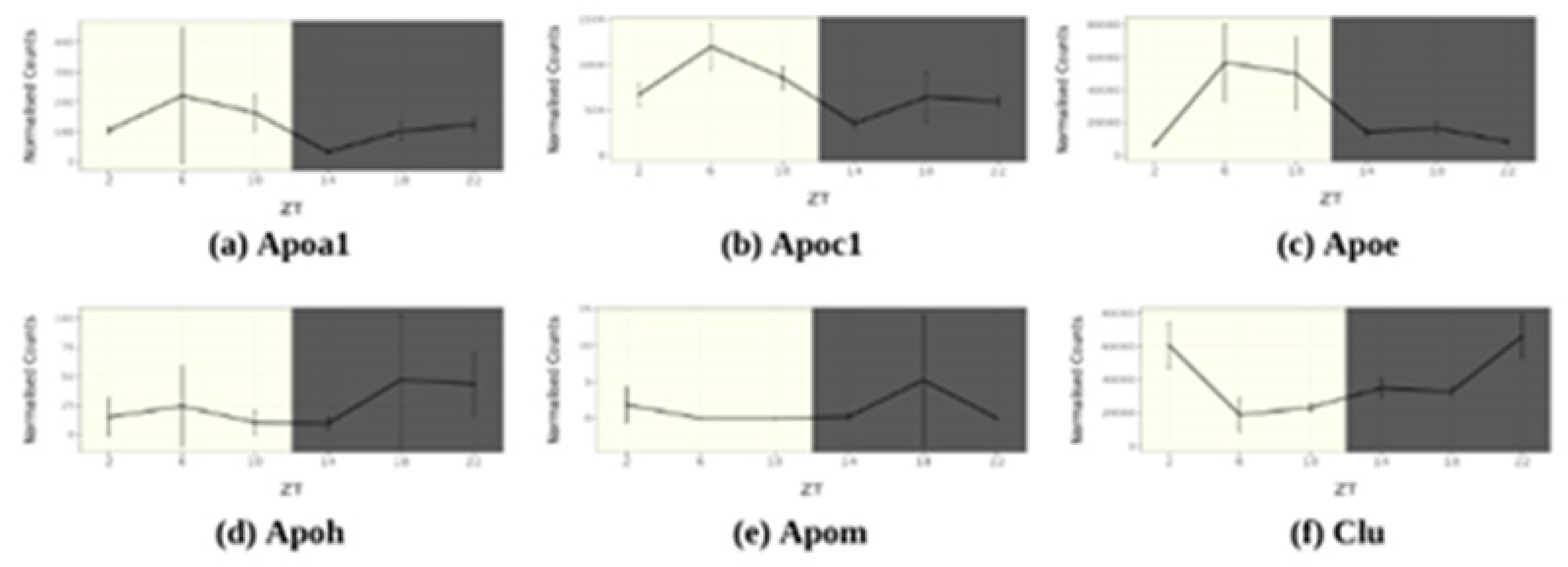
4.1. ApoA1
4.2. ApoA4
4.3. ApoB
4.4. ApoD
4.5. ApoE
4.6. ApoC1
4.7. ApoH
4.8. ApoJ
4.9. ApoM
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Roenneberg, T.; Merrow, M. The Circadian Clock and Human Health. Curr. Biol. 2016, 26, R432–R443. [CrossRef]
- Green, C.B.; Takahashi, J.S.; Bass, J. The Meter of Metabolism. Cell 2008, 134, 728–742. [CrossRef]
- Bass, J.T. The circadian clock system’s influence in health and disease. Genome Med. 2017, 9, 1–5. [CrossRef]
- Lananna, B.V.; Musiek, E.S. The wrinkling of time: Aging, inflammation, oxidative stress, and the circadian clock in neurodegeneration. Neurobiol. Dis. 2020, 139, 104832–104832. [CrossRef]
- Prasai MJ, George JT, and Scott EM. Molecular clocks, type 2 diabetes and cardiovascular disease. Diab Vasc Dis Res. 2008;5(2):89-95. [CrossRef]
- Maury E, Ramsey KM, and Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res. 2010;106(3):447-62. [CrossRef]
- Firsov, D.; Bonny, O. Circadian rhythms and the kidney. Nat. Rev. Nephrol. 2018, 14, 626–635. [CrossRef]
- Pan X, Mota S, and Zhang B. Circadian Clock Regulation on Lipid Metabolism and Metabolic Diseases. Adv Exp Med Biol. 2020;1276:53-66. [CrossRef]
- Nassan, M.; Videnovic, A. Circadian rhythms in neurodegenerative disorders. Nat. Rev. Neurol. 2021, 18, 7–24. [CrossRef]
- Zhang, D.; Pollock, D.M. Circadian regulation of kidney function: finding a role for Bmal1. Am. J. Physiol. Physiol. 2018, 314, F675–F678. [CrossRef]
- Levi, F.; Schibler, U. Circadian Rhythms: Mechanisms and Therapeutic Implications. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 593–628. [CrossRef]
- Gooley, J.J. Circadian regulation of lipid metabolism. Proc. Nutr. Soc. 2016, 75, 440–450. [CrossRef]
- Muenchhoff, J.; Song, F.; Poljak, A.; Crawford, J.D.; Mather, K.A.; Kochan, N.A.; Yang, Z.; Trollor, J.N.; Reppermund, S.; Maston, K.; et al. Plasma apolipoproteins and physical and cognitive health in very old individuals. Neurobiol. Aging 2017, 55, 49–60. [CrossRef]
- Pan, X.; Zhang, Y.; Wang, L.; Hussain, M.M. Diurnal Regulation of MTP and Plasma Triglyceride by CLOCK Is Mediated by SHP. Cell Metab. 2010, 12, 174–186. [CrossRef]
- Pan, X.; Jiang, X.-C.; Hussain, M.M. Impaired Cholesterol Metabolism and Enhanced Atherosclerosis in Clock Mutant Mice. Circ. 2013, 128, 1758–1769. [CrossRef]
- Pan, X.; Bradfield, C.A.; Hussain, M.M. Global and hepatocyte-specific ablation of Bmal1 induces hyperlipidaemia and enhances atherosclerosis. Nat. Commun. 2016, 7, 13011–13011. [CrossRef]
- Pan X, and Hussain MM. Bmal1 regulates production of larger lipoproteins by modulating cAMP-responsive element-binding protein H and apolipoprotein AIV. Hepatology. 2022;76(1):78-93. [CrossRef]
- Pan, X.; Munshi, M.K.; Iqbal, J.; Queiroz, J.; Sirwi, A.A.; Shah, S.; Younus, A.; Hussain, M.M. Circadian Regulation of Intestinal Lipid Absorption by Apolipoprotein AIV Involves Forkhead Transcription Factors A2 and O1 and Microsomal Triglyceride Transfer Protein. J. Biol. Chem. 2013, 288, 20464–20476. [CrossRef]
- Martins IJ, Hone E, Foster JK, Sunram-Lea SI, Gnjec A, Fuller SJ, et al. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol Psychiatry. 2006;11(8):721-36. [CrossRef]
- Dufouil C, Richard F, Fievet N, Dartigues JF, Ritchie K, Tzourio C, et al. APOE genotype, cholesterol level, lipid-lowering treatment, and dementia: the Three-City Study. Neurology. 2005;64(9):1531-8. [CrossRef]
- Sparks DL, Sabbagh MN, Connor DJ, Lopez J, Launer LJ, Browne P, et al. Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch Neurol. 2005;62(5):753-7. [CrossRef]
- Reppert, S.M.; Weaver, D.R. Molecular Analysis of Mammalian Circadian Rhythms. Annu. Rev. Physiol. 2001, 63, 647–676. [CrossRef]
- Hussain, M.M.; Pan, X. Clock genes, intestinal transport and plasma lipid homeostasis. Trends Endocrinol. Metab. 2009, 20, 177–185. [CrossRef]
- Froy O. The circadian clock and metabolism. Clin Sci (Lond). 2011;120(2):65-72. [CrossRef]
- Douris, N.; Kojima, S.; Pan, X.; Lerch-Gaggl, A.F.; Duong, S.Q.; Hussain, M.M.; Green, C.B. Nocturnin Regulates Circadian Trafficking of Dietary Lipid in Intestinal Enterocytes. Curr. Biol. 2011, 21, 1347–1355. [CrossRef]
- Schnell A, Chappuis S, Schmutz I, Brai E, Ripperger JA, Schaad O, et al. The nuclear receptor REV-ERBalpha regulates Fabp7 and modulates adult hippocampal neurogenesis. PLoS One. 2014;9(6):e99883. [CrossRef]
- Landgraf, D.; Long, J.E.; Proulx, C.D.; Barandas, R.; Malinow, R.; Welsh, D.K. Genetic Disruption of Circadian Rhythms in the Suprachiasmatic Nucleus Causes Helplessness, Behavioral Despair, and Anxiety-like Behavior in Mice. Biol. Psychiatry 2016, 80, 827–835. [CrossRef]
- Wolff SEC, Wang XL, Jiao H, Sun J, Kalsbeek A, Yi CX, et al. The Effect of Rev-erbalpha Agonist SR9011 on the Immune Response and Cell Metabolism of Microglia. Front Immunol. 2020;11:550145. [CrossRef]
- McKee, C.A.; Lee, J.; Cai, Y.; Saito, T.; Saido, T.; Musiek, E.S. Astrocytes deficient in circadian clock gene Bmal1 show enhanced activation responses to amyloid-beta pathology without changing plaque burden. Sci. Rep. 2022, 12, 1796. [CrossRef]
- Yang, G.; Zhang, J.; Jiang, T.; Monslow, J.; Tang, S.Y.; Todd, L.; Puré, E.; Chen, L.; FitzGerald, G.A. Bmal1 Deletion in Myeloid Cells Attenuates Atherosclerotic Lesion Development and Restrains Abdominal Aortic Aneurysm Formation in Hyperlipidemic Mice. Arter. Thromb. Vasc. Biol. 2020, 40, 1523–1532. [CrossRef]
- Lee, J.; Kim, D.E.; Griffin, P.; Sheehan, P.W.; Kim, D.H.; Musiek, E.S.; Yoon, S.Y. Inhibition of REV-ERBs stimulates microglial amyloid-beta clearance and reduces amyloid plaque deposition in the 5XFAD mouse model of Alzheimer’s disease. Aging Cell 2019, 19, e13078. [CrossRef]
- Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349(9046):151-4. [CrossRef]
- Wingo, T.S.; Cutler, D.J.; Wingo, A.P.; Le, N.-A.; Rabinovici, G.D.; Miller, B.L.; Lah, J.J.; Levey, A.I. Association of Early-Onset Alzheimer Disease With Elevated Low-Density Lipoprotein Cholesterol Levels and Rare Genetic Coding Variants of APOB. JAMA Neurol. 2019, 76, 809–817. [CrossRef]
- Marchant NL, Reed BR, Sanossian N, Madison CM, Kriger S, Dhada R, et al. The aging brain and cognition: contribution of vascular injury and abeta to mild cognitive dysfunction. JAMA Neurol. 2013;70(4):488-95. [CrossRef]
- Power, M.C.; Rawlings, A.; Sharrett, A.R.; Bandeen-Roche, K.; Coresh, J.; Ballantyne, C.M.; Pokharel, Y.; Michos, E.D.; Penman, A.; Alonso, A.; et al. Association of midlife lipids with 20-year cognitive change: A cohort study. Alzheimer’s Dement. 2017, 14, 167–177. [CrossRef]
- Blue, M.L.; Williams, D.L.; Zucker, S.; A Khan, S.; Blum, C.B. Apolipoprotein E synthesis in human kidney, adrenal gland, and liver.. Proc. Natl. Acad. Sci. 1983, 80, 283–287. [CrossRef]
- Wang H, and Eckel RH. What are lipoproteins doing in the brain? Trends Endocrinol Metab. 2014;25(1):8-14. [CrossRef]
- Fuior, E.V.; Gafencu, A.V. Apolipoprotein C1: Its Pleiotropic Effects in Lipid Metabolism and Beyond. Int. J. Mol. Sci. 2019, 20, 5939. [CrossRef]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2016, 18, 164–179. [CrossRef]
- Earnest, D.J.; A Olschowka, J. Circadian regulation of c-fos expression in the suprachiasmatic pacemaker by light.. 1993, S65–71.
- Brancaccio, M.; Enoki, R.; Mazuski, C.N.; Jones, J.; Evans, J.A.; Azzi, A. Network-Mediated Encoding of Circadian Time: The Suprachiasmatic Nucleus (SCN) from Genes to Neurons to Circuits, and Back. J. Neurosci. 2014, 34, 15192–15199. [CrossRef]
- Wen, S.; Ma, D.; Zhao, M.; Xie, L.; Wu, Q.; Gou, L.; Zhu, C.; Fan, Y.; Wang, H.; Yan, J. Spatiotemporal single-cell analysis of gene expression in the mouse suprachiasmatic nucleus. Nat. Neurosci. 2020, 23, 456–467. [CrossRef]
- Barca-Mayo, O.; Pons-Espinal, M.; Follert, P.; Armirotti, A.; Berdondini, L.; Tonelli, D.D.P. Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nat. Commun. 2017, 8, 14336. [CrossRef]
- Tso, C.F.; Simon, T.; Greenlaw, A.C.; Puri, T.; Mieda, M.; Herzog, E.D. Astrocytes Regulate Daily Rhythms in the Suprachiasmatic Nucleus and Behavior. Curr. Biol. 2017, 27, 1055–1061. [CrossRef]
- Brancaccio, M.; Patton, A.P.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Astrocytes Control Circadian Timekeeping in the Suprachiasmatic Nucleus via Glutamatergic Signaling. Neuron 2017, 93, 1420–1435.e5. [CrossRef]
- Brancaccio, M.; Edwards, M.D.; Patton, A.P.; Smyllie, N.J.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science 2019, 363, 187–192. [CrossRef]
- Granados-Fuentes, D.; Prolo, L.M.; Abraham, U.; Herzog, E.D. The Suprachiasmatic Nucleus Entrains, But Does Not Sustain, Circadian Rhythmicity in the Olfactory Bulb. J. Neurosci. 2004, 24, 615–619. [CrossRef]
- Ralph, M.R.; Foster, R.G.; Davis, F.C.; Menaker, M. Transplanted Suprachiasmatic Nucleus Determines Circadian Period. Science 1990, 247, 975–978. [CrossRef]
- Lehman, M.; Silver, R.; Gladstone, W.; Kahn, R.; Gibson, M.; Bittman, E. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J. Neurosci. 1987, 7, 1626–1638. [CrossRef]
- Harmar, A.J.; Marston, H.M.; Shen, S.; Spratt, C.; West, K.M.; Sheward, W.; Morrison, C.F.; Dorin, J.R.; Piggins, H.D.; Reubi, J.-C.; et al. The VPAC2 Receptor Is Essential for Circadian Function in the Mouse Suprachiasmatic Nuclei. Cell 2002, 109, 497–508. [CrossRef]
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic Nucleus: Cell Autonomy and Network Properties. Annu. Rev. Physiol. 2010, 72, 551–577. [CrossRef]
- Pittman, Q.J. Vasopressin and central control of the cardiovascular system: A 40-year retrospective. J. Neuroendocr. 2021, 33, e13011. [CrossRef]
- Smith, M.C.; Dunn, M.J. The Role of Prostaglandins in Human Hypertension. Am. J. Kidney Dis. 1985, 5, A32–A39. [CrossRef]
- Reghunandanan, V. Vasopressin in circadian function of SCN. J. Biosci. 2020, 45, 1–11. [CrossRef]
- Olszewski PK, Wirth MM, Shaw TJ, Grace MK, and Levine AS. Peptides that regulate food intake: effect of peptide histidine isoleucine on consummatory behavior in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284(6):R1445-53. [CrossRef]
- Shinohara, K.; Funabashi, T.; Mitushima, D.; Kimura, F. Effects of gap junction blocker on vasopressin and vasoactive intestinal polypeptide rhythms in the rat suprachiasmatic nucleus in vitro. Neurosci. Res. 2000, 38, 43–47. [CrossRef]
- Kruijver, F.P.; Swaab, D.F. Sex Hormone Receptors Are Present in the Human Suprachiasmatic Nucleus. Neuroendocrinology 2002, 75, 296–305. [CrossRef]
- Kruijver FP, Zhou JN, Pool CW, Hofman MA, Gooren LJ, and Swaab DF. Male-to-female transsexuals have female neuron numbers in a limbic nucleus. J Clin Endocrinol Metab. 2000;85(5):2034-41. [CrossRef]
- O’Brien, E.R.; Howarth, C.; Sibson, N.R. The role of astrocytes in CNS tumors: pre-clinical models and novel imaging approaches. Front. Cell. Neurosci. 2013, 7, 40. [CrossRef]
- Brierley, D.I.; Holt, M.K.; Singh, A.; de Araujo, A.; McDougle, M.; Vergara, M.; Afaghani, M.H.; Lee, S.J.; Scott, K.; Maske, C.; et al. Central and peripheral GLP-1 systems independently suppress eating. Nat. Metab. 2021, 3, 258–273. [CrossRef]
- Rowlands, J.; Heng, J.; Newsholme, P.; Carlessi, R. Pleiotropic Effects of GLP-1 and Analogs on Cell Signaling, Metabolism, and Function. Front. Endocrinol. 2018, 9, 672. [CrossRef]
- Nauck MA, and Meier JJ. GLP-1 receptor agonists and SGLT2 inhibitors: a couple at last? Lancet Diabetes Endocrinol. 2016;4(12):963-4. [CrossRef]
- Hussein H, Zaccardi F, Khunti K, Davies MJ, Patsko E, Dhalwani NN, et al. Efficacy and tolerability of sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists: A systematic review and network meta-analysis. Diabetes Obes Metab. 2020;22(7):1035-46. [CrossRef]
- Cheng, D.; Yang, S.; Zhao, X.; Wang, G. The Role of Glucagon-Like Peptide-1 Receptor Agonists (GLP-1 RA) in Diabetes-Related Neurodegenerative Diseases. Drug Des. Dev. Ther. 2022, ume 16, 665–684. [CrossRef]
- Leone, M.J.; Marpegan, L.; Bekinschtein, T.A.; Costas, M.A.; Golombek, D.A. Suprachiasmatic astrocytes as an interface for immune-circadian signalling. J. Neurosci. Res. 2006, 84, 1521–1527. [CrossRef]
- Hainich, E.C.; Pizzio, G.A.; Golombek, D.A. Constitutive activation of the ERK-MAPK pathway in the suprachiasmatic nuclei inhibits circadian resetting. FEBS Lett. 2006, 580, 6665–6668. [CrossRef]
- Liu, X.; Quan, N. Microglia and CNS Interleukin-1: Beyond Immunological Concepts. Front. Neurol. 2018, 9, 8. [CrossRef]
- Lundkvist, G.; Hill, R.; Kristensson, K. Disruption of Circadian Rhythms in Synaptic Activity of the Suprachiasmatic Nuclei by African Trypanosomes and Cytokines. Neurobiol. Dis. 2002, 11, 20–27. [CrossRef]
- Gekakis, N.; Staknis, D.; Nguyen, H.B.; Davis, F.C.; Wilsbacher, L.D.; King, D.P.; Takahashi, J.S.; Weitz, C.J. Role of the CLOCK protein in the mammalian circadian mechanism. Science 1998, 280, 1564–1569. [CrossRef]
- Chua, E.C.-P.; Shui, G.; Lee, I.T.-G.; Lau, P.; Tan, L.-C.; Yeo, S.-C.; Lam, B.D.; Bulchand, S.; Summers, S.A.; Puvanendran, K.; et al. Extensive diversity in circadian regulation of plasma lipids and evidence for different circadian metabolic phenotypes in humans. Proc. Natl. Acad. Sci. 2013, 110, 14468–14473. [CrossRef]
- Spišská, V.; Pačesová, D.; Míková, H.; Pohanová, P.; Telenský, P.; Novotný, J.; Bendová, Z. Prenatal exposure to lipopolysaccharide induces changes in the circadian clock in the SCN and AA-NAT activity in the pineal gland. Brain Res. 2020, 1743, 146952. [CrossRef]
- Huo, M.; Huang, Y.; Qu, D.; Zhang, H.; Wong, W.T.; Chawla, A.; Huang, Y.; Tian, X.Y. Myeloid Bmal1 deletion increases monocyte recruitment and worsens atherosclerosis. FASEB J. 2016, 31, 1097–1106. [CrossRef]
- Ma, Z.; Jiang, W.; Zhang, E.E. Orexin signaling regulates both the hippocampal clock and the circadian oscillation of Alzheimer’s disease-risk genes. Sci. Rep. 2016, 6, 36035. [CrossRef]
- Chen, Q.; Peng, X.; Huang, C.; Hu, X.; Zhang, X. Association between ARNTL (BMAL1) rs2278749 polymorphism T >C and susceptibility to Alzheimer disease in a Chinese population. Evolution 2015, 14, 18515–18522. [CrossRef]
- Shimba, S.; Ishii, N.; Ohta, Y.; Ohno, T.; Watabe, Y.; Hayashi, M.; Wada, T.; Aoyagi, T.; Tezuka, M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc. Natl. Acad. Sci. 2005, 102, 12071–12076. [CrossRef]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and Metabolic Syndrome in Circadian Clock Mutant Mice. Science 2005, 308, 1043–1045. [CrossRef]
- Pan, X.; Hussain, M.M. Clock is important for food and circadian regulation of macronutrient absorption in mice. J. Lipid Res. 2009, 50, 1800–1813. [CrossRef]
- Shimba, S.; Ogawa, T.; Hitosugi, S.; Ichihashi, Y.; Nakadaira, Y.; Kobayashi, M.; Tezuka, M.; Kosuge, Y.; Ishige, K.; Ito, Y.; et al. Deficient of a Clock Gene, Brain and Muscle Arnt-Like Protein-1 (BMAL1), Induces Dyslipidemia and Ectopic Fat Formation. PLOS ONE 2011, 6, e25231. [CrossRef]
- Nikolaeva, S.; Ansermet, C.; Centeno, G.; Pradervand, S.; Bize, V.; Mordasini, D.; Henry, H.; Koesters, R.; Maillard, M.; Bonny, O.; et al. Nephron-Specific Deletion of Circadian Clock Gene Bmal1 Alters the Plasma and Renal Metabolome and Impairs Drug Disposition. J. Am. Soc. Nephrol. 2016, 27, 2997–3004. [CrossRef]
- Kress GJ, Liao F, Dimitry J, Cedeno MR, FitzGerald GA, Holtzman DM, et al. Regulation of amyloid-beta dynamics and pathology by the circadian clock. J Exp Med. 2018;215(4):1059-68.
- Vitali, C.; Wellington, C.L.; Calabresi, L. HDL and cholesterol handling in the brain. Cardiovasc. Res. 2014, 103, 405–413. [CrossRef]
- Feingold KR. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al. eds. Endotext. South Dartmouth (MA); 2000.
- Koch, S.; Donarski, N.; Goetze, K.; Kreckel, M.; Stuerenburg, H.-J.; Buhmann, C.; Beisiegel, U. Characterization of four lipoprotein classes in human cerebrospinal fluid. J. Lipid Res. 2001, 42, 1143–1151. [CrossRef]
- Borghini, I.; Barja, F.; Pometta, D.; James, R.W. Characterization of subpopulations of lipoprotein particles isolated from human cerebrospinal fluid. Biochim. et Biophys. Acta (BBA)–Lipids Lipid Metab. 1995, 1255, 192–200. [CrossRef]
- Rhea, E.M.; Banks, W.A. Interactions of Lipids, Lipoproteins, and Apolipoproteins with the Blood-Brain Barrier. Pharm. Res. 2021, 38, 1469–1475. [CrossRef]
- Button, E.B.; Robert, J.; Caffrey, T.M.; Fan, J.; Zhao, W.; Wellington, C.L. HDL from an Alzheimer’s disease perspective. Curr. Opin. Infect. Dis. 2019, 30, 224–234. [CrossRef]
- Hartmann, H.; Ho, W.Y.; Chang, J.; Ling, S. Cholesterol dyshomeostasis in amyotrophic lateral sclerosis: cause, consequence, or epiphenomenon?. FEBS J. 2021, 289, 7688–7709. [CrossRef]
- Colardo, M.; Petraroia, M.; Lerza, L.; Pensabene, D.; Martella, N.; Pallottini, V.; Segatto, M. NGF Modulates Cholesterol Metabolism and Stimulates ApoE Secretion in Glial Cells Conferring Neuroprotection against Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 4842. [CrossRef]
- A Elliott, D.; Weickert, C.S.; Garner, B. Apolipoproteins in the brain: implications for neurological and psychiatric disorders. Clin. Lipidol. 2010, 5, 555–573. [CrossRef]
- Koch, M.; Aroner, S.A.; Fitzpatrick, A.L.; Longstreth, W.; Furtado, J.D.; Mukamal, K.J.; Jensen, M.K. HDL (High-Density Lipoprotein) Subspecies, Prevalent Covert Brain Infarcts, and Incident Overt Ischemic Stroke: Cardiovascular Health Study. Stroke 2021, 53, 1292–1300. [CrossRef]
- Delk, S.C.; Chattopadhyay, A.; Escola-Gil, J.C.; Fogelman, A.M.; Reddy, S.T. Apolipoprotein mimetics in cancer. Semin. Cancer Biol. 2021, 73, 158–168. [CrossRef]
- Möckel, B.; Zinke, H.; Flach, R.; Weiß, B.; Gassen, H.G.; Weiler-Güttler, H. Expression of Apolipoprotein A-I in Porcine Brain Endothelium In Vitro. J. Neurochem. 1994, 62, 788–798. [CrossRef]
- Weiler-Güttler, H.; Sommerfeldt, M.; Papandrikopoulou, A.; Mischek, U.; Bonitz, D.; Frey, A.; Grupe, M.; Scheerer, J.; Gassen, H.G. Synthesis of Apolipoprotein A-1 in Pig Brain Microvascular Endothelial Cells. J. Neurochem. 1990, 54, 444–450. [CrossRef]
- Endres K. Apolipoprotein A1, the neglected relative of Apolipoprotein E and its potential role in Alzheimer’s disease. Neural Regen Res. 2021;16(11):2141-8. [CrossRef]
- Smach, M.A.; Edziri, H.; Charfeddine, B.; Ben Othman, L.; Lammouchi, T.; Ltaief, A.; Nafati, S.; Dridi, H.; Bennamou, S.; Limem, K. Polymorphism in apoA1 Influences High-Density Lipoprotein Cholesterol Levels but Is Not a Major Risk Factor of Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. Extra 2011, 1, 249–257. [CrossRef]
- Vollbach, H.; Heun, R.; Morris, C.M.; Edwardson, J.A.; McKeith, I.G.; Jessen, F.; Schulz, A.; Maier, W.; Kölsch, H. APOA1 polymorphism influences risk for early-onset nonfamiliar AD. Ann. Neurol. 2005, 58, 436–441. [CrossRef]
- Swanson, C.R.; Li, K.; Unger, T.L.; Gallagher, M.D.; Van Deerlin, V.M.; Agarwal, P.; Leverenz, J.; Roberts, J.; Samii, A.; Gross, R.G.; et al. Lower plasma apolipoprotein A1 levels are found in Parkinson’s disease and associate with apolipoprotein A1 genotype. Mov. Disord. 2014, 30, 805–812. [CrossRef]
- Wang, F.; Kohan, A.B.; Lo, C.-M.; Liu, M.; Howles, P.; Tso, P. Apolipoprotein A-IV: a protein intimately involved in metabolism. J. Lipid Res. 2015, 56, 1403–1418. [CrossRef]
- Shen, L.; Pearson, K.J.; Xiong, Y.; Lo, C.-M.; Tso, P.; Woods, S.C.; Davidson, W.S.; Liu, M. Characterization of apolipoprotein A-IV in brain areas involved in energy homeostasis. Physiol. Behav. 2008, 95, 161–167. [CrossRef]
- Liu, M.; Doi, T.; Shen, L.; Woods, S.C.; Seeley, R.J.; Zheng, S.; Jackman, A.; Tso, P.; Weng, J.; Lou, D.; et al. Intestinal satiety protein apolipoprotein AIV is synthesized and regulated in rat hypothalamus. Am. J. Physiol. Integr. Comp. Physiol. 2001, 280, R1382–R1387. [CrossRef]
- Fagan, A.M.; Holtzman, D.M.; Munson, G.; Mathur, T.; Schneider, D.; Chang, L.K.; Getz, G.S.; Reardon, C.A.; Lukens, J.; Shah, J.A.; et al. Unique Lipoproteins Secreted by Primary Astrocytes From Wild Type, apoE (−/−), and Human apoE Transgenic Mice. J. Biol. Chem. 1999, 274, 30001–30007. [CrossRef]
- Okumura, T.; Fukagawa, K.; Tso, P.; Taylor, I.L.; Pappas, T.N. Intracisternal injection of apolipoprotein A-IV inhibits gastric secretion in pylorus-ligated conscious rats. Gastroenterology 1994, 107, 1861–1864. [CrossRef]
- Tso, P.; Chen, Q.; Fujimoto, K.; Fukagawa, K.; Sakata, T. Apolipoprotein A-IV: A Circulating Satiety Signal Produced by the Small Intestine. Obes. Res. 1995, 3, 689S–695S. [CrossRef]
- Roula D, Theiler A, Luschnig P, Sturm GJ, Tomazic PV, Marsche G, et al. Apolipoprotein A-IV acts as an endogenous anti-inflammatory protein and is reduced in treatment-naive allergic patients and allergen-challenged mice. Allergy. 2020;75(2):392-402. [CrossRef]
- Császár, A.; Kálmán, J.; Szalai, C.; Janka, Z.; Romics, L. Association of the apolipoprotein A-IV codon 360 mutation in patients with Alzheimer’s disease. Neurosci. Lett. 1997, 230, 151–154. [CrossRef]
- Cui, Y.; Huang, M.; He, Y.; Zhang, S.; Luo, Y. Genetic Ablation of Apolipoprotein A-IV Accelerates Alzheimer’s Disease Pathogenesis in a Mouse Model. Am. J. Pathol. 2011, 178, 1298–1308. [CrossRef]
- Fan, Y.; Gao, J.; Li, Y.; Chen, X.; Zhang, T.; You, W.; Xue, Y.; Shen, C. The Variants at APOA1 and APOA4 Contribute to the Susceptibility of Schizophrenia With Inhibiting mRNA Expression in Peripheral Blood Leukocytes. Front. Mol. Biosci. 2021, 8. [CrossRef]
- Deng, X.; Walker, R.G.; Morris, J.; Davidson, W.S.; Thompson, T.B. Role of Conserved Proline Residues in Human Apolipoprotein A-IV Structure and Function. J. Biol. Chem. 2015, 290, 10689–10702. [CrossRef]
- Haas, M.E.; Attie, A.D.; Biddinger, S.B. The regulation of ApoB metabolism by insulin. Trends Endocrinol. Metab. 2013, 24, 391–397. [CrossRef]
- Picard, C.; Nilsson, N.; Labonté, A.; Auld, D.; Rosa-Neto, P.; Ashton, N.J.; Zetterberg, H.; Blennow, K.; Breitner, J.C.; Villeneuve, S.; et al. Apolipoprotein B is a novel marker for early tau pathology in Alzheimer’s disease. Alzheimer’s Dement. 2021, 18, 875–887. [CrossRef]
- Dassati, S.; Waldner, A.; Schweigreiter, R. Apolipoprotein D takes center stage in the stress response of the aging and degenerative brain. Neurobiol. Aging 2014, 35, 1632–1642. [CrossRef]
- Sanchez, D.; Ganfornina, M.D. The Lipocalin Apolipoprotein D Functional Portrait: A Systematic Review. Front. Physiol. 2021, 12. [CrossRef]
- Belloir, B.; Kövari, E.; Surini-Demiri, M.; Savioz, A. Altered apolipoprotein D expression in the brain of patients with Alzheimer disease. J. Neurosci. Res. 2001, 64, 61–69. [CrossRef]
- Kalman, K.; McConathy, W.; Araoz, C.; Kasa, P.; Lacko, A.G. Apolipoprotein D in the aging brain and in Alzheimer’s dementia. Neurol. Res. 2000, 22, 330–336. [CrossRef]
- A Thomas, E.; Laws, S.M.; Sutcliffe, J.; Harper, C.; Dean, B.; McClean, C.; Masters, C.; Lautenschlager, N.; E Gandy, S.; Martins, R.N. Apolipoprotein D levels are elevated in prefrontal cortex of subjects with Alzheimer’s disease: no relation to apolipoprotein E expression or genotype. Biol. Psychiatry 2003, 54, 136–141. [CrossRef]
- Terrisse, L.; Poirier, J.; Bertrand, P.; Merched, A.; Visvikis, S.; Siest, G.; Milne, R.; Rassart, . Increased Levels of Apolipoprotein D in Cerebrospinal Fluid and Hippocampus of Alzheimer’s Patients. J. Neurochem. 1998, 71, 1643–1650. [CrossRef]
- Paasila PJ, Aramideh JA, Sutherland GT, and Graeber MB. Synapses, Microglia, and Lipids in Alzheimer’s Disease. Front Neurosci. 2021;15:778822. [CrossRef]
- Li J, Luo J, Liu L, Fu H, and Tang L. The genetic association between apolipoprotein E gene polymorphism and Parkinson disease: A meta-Analysis of 47 studies. Medicine (Baltimore). 2018;97(43):e12884. [CrossRef]
- Jung JH, Jeon S, Baik K, Lee YH, Chung SJ, Yoo HS, et al. Apolipoprotein E4, amyloid, and cognition in Alzheimer’s and Lewy body disease. Neurobiol Aging. 2021;106:45-54. [CrossRef]
- Zhao J, Lu W, Ren Y, Fu Y, Martens YA, Shue F, et al. Apolipoprotein E regulates lipid metabolism and alpha-synuclein pathology in human iPSC-derived cerebral organoids. Acta Neuropathol. 2021;142(5):807-25. [CrossRef]
- Dickson DW, Heckman MG, Murray ME, Soto AI, Walton RL, Diehl NN, et al. APOE epsilon4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology. 2018;91(12):e1182-e95. [CrossRef]
- Pohlkamp T, Xian X, Wong CH, Durakoglugil MS, Werthmann GC, Saido TC, et al. NHE6 depletion corrects ApoE4-mediated synaptic impairments and reduces amyloid plaque load. Elife. 2021;10. [CrossRef]
- Montagne, A.; Nation, D.A.; Sagare, A.P.; Barisano, G.; Sweeney, M.D.; Chakhoyan, A.; Pachicano, M.; Joe, E.; Nelson, A.R.; D’Orazio, L.M.; et al. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature 2020, 581, 71–76. [CrossRef]
- Kotredes KP, Oblak A, Pandey RS, Lin PB, Garceau D, Williams H, et al. Uncovering Disease Mechanisms in a Novel Mouse Model Expressing Humanized APOEepsilon4 and Trem2*R47H. Front Aging Neurosci. 2021;13:735524. [CrossRef]
- Yu, T.-S.; Tensaouti, Y.; Stephanz, E.P.; Chintamen, S.; Rafikian, E.E.; Yang, M.; Kernie, S.G. Astrocytic ApoE underlies maturation of hippocampal neurons and cognitive recovery after traumatic brain injury in mice. Commun. Biol. 2021, 4, 1–12. [CrossRef]
- Vervoordt, S.M.; Arnett, P.; Engeland, C.; Rabinowitz, A.R.; Hillary, F.G. Depression associated with APOE status and hippocampal volume but not cognitive decline in older adults aging with traumatic brain injury.. Neuropsychology 2021, 35, 863–875. [CrossRef]
- Main, B.S.; Villapol, S.; Sloley, S.S.; Barton, D.J.; Parsadanian, M.; Agbaegbu, C.; Stefos, K.; McCann, M.S.; Washington, P.M.; Rodriguez, O.C.; et al. Apolipoprotein E4 impairs spontaneous blood brain barrier repair following traumatic brain injury. Mol. Neurodegener. 2018, 13, 1–18. [CrossRef]
- Kundu, P.; Zimmerman, B.; Perez, R.; Whitlow, C.T.; Cline, J.M.; Olson, J.D.; Andrews, R.N.; Raber, J. Apolipoprotein E levels in the amygdala and prefrontal cortex predict relative regional brain volumes in irradiated Rhesus macaques. Sci. Rep. 2021, 11, 1–14. [CrossRef]
- Kloske, C.M.; Wilcock, D.M. The Important Interface Between Apolipoprotein E and Neuroinflammation in Alzheimer’s Disease. Front. Immunol. 2020, 11, 754. [CrossRef]
- Jackson, R.J.; Meltzer, J.C.; Nguyen, H.; Commins, C.; E Bennett, R.; Hudry, E.; Hyman, B.T. APOE4 derived from astrocytes leads to blood–brain barrier impairment. Brain 2021, 145, 3582–3593. [CrossRef]
- den Hoedt S, Crivelli SM, Leijten FPJ, Losen M, Stevens JAA, Mane-Damas M, et al. Effects of Sex, Age, and Apolipoprotein E Genotype on Brain Ceramides and Sphingosine-1-Phosphate in Alzheimer’s Disease and Control Mice. Front Aging Neurosci. 2021;13:765252. [CrossRef]
- Konings, S.C.; Torres-Garcia, L.; Martinsson, I.; Gouras, G.K. Astrocytic and Neuronal Apolipoprotein E Isoforms Differentially Affect Neuronal Excitability. Front. Neurosci. 2021, 15. [CrossRef]
- Jones, N.S.; Watson, K.Q.; Rebeck, G.W. High-fat diet increases gliosis and immediate early gene expression in APOE3 mice, but not APOE4 mice. J. Neuroinflammation 2021, 18, 1–13. [CrossRef]
- Asaro, A.; Sinha, R.; Bakun, M.; Kalnytska, O.; Carlo-Spiewok, A.-S.; Rubel, T.; Rozeboom, A.; Dadlez, M.; Kaminska, B.; Aronica, E.; et al. ApoE4 disrupts interaction of sortilin with fatty acid-binding protein 7 essential to promote lipid signaling. J. Cell Sci. 2021, 134. [CrossRef]
- Pankiewicz, J.E.; Lizińczyk, A.M.; Franco, L.A.; Diaz, J.R.; Martá-Ariza, M.; Sadowski, M.J. Absence of Apolipoprotein E is associated with exacerbation of prion pathology and promotes microglial neurodegenerative phenotype. Acta Neuropathol. Commun. 2021, 9, 1–30. [CrossRef]
- Zhang, S.H.; Reddick, R.L.; Piedrahita, J.A.; Maeda, N. Spontaneous Hypercholesterolemia and Arterial Lesions in Mice Lacking Apolipoprotein E. Science 1992, 258, 468–471. [CrossRef]
- Wang, H.; Kulas, J.A.; Wang, C.; Holtzman, D.M.; Ferris, H.A.; Hansen, S.B. Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc. Natl. Acad. Sci. 2021, 118. [CrossRef]
- Weisgraber, K.H.; Mahley, R.W.; Kowal, R.C.; Herz, J.; Goldstein, J.L.; Brown, M.S. Apolipoprotein C-I modulates the interaction of apolipoprotein E with beta-migrating very low density lipoproteins (beta-VLDL) and inhibits binding of beta-VLDL to low density lipoprotein receptor-related protein.. J. Biol. Chem. 1990, 265, 22453–22459. [CrossRef]
- Kowal, R.C.; Herz, J.; Weisgraber, K.H.; Mahley, R.W.; Brown, M.S.; Goldstein, J.L. Opposing effects of apolipoproteins E and C on lipoprotein binding to low density lipoprotein receptor-related protein. J. Biol. Chem. 1990, 265, 10771–10779. [CrossRef]
- Sehayek, E.; Eisenberg, S. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway.. J. Biol. Chem. 1991, 266, 18259–18267. [CrossRef]
- Petit-Turcotte, C.; Stohl, S.M.; Beffert, U.; Cohn, J.S.; Aumontb, N.; Tremblayd, M.; Deab, D.; Yangc, L.; Poirierab, J.; Shachter, N.S. Apolipoprotein C-I Expression in the Brain in Alzheimer’s Disease. Neurobiol. Dis. 2001, 8, 953–963. [CrossRef]
- Drigalenko, E.; Poduslo, S.; Elston, R. Interaction of the apolipoprotein E and CI loci in predisposing to late-onset Alzheimer’s disease. Neurology 1998, 51, 131–135. [CrossRef]
- Jong MC, Hofker MH, and Havekes LM. Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler Thromb Vasc Biol. 1999;19(3):472-84. [CrossRef]
- van der Hoogt CC, Berbee JF, Espirito Santo SM, Gerritsen G, Krom YD, van der Zee A, et al. Apolipoprotein CI causes hypertriglyceridemia independent of the very-low-density lipoprotein receptor and apolipoprotein CIII in mice. Biochim Biophys Acta. 2006;1761(2):213-20. [CrossRef]
- Jong, M.C.; E Dahlmans, V.; van Gorp, P.J.; van Dijk, K.W.; Breuer, M.L.; Hofker, M.H.; Havekes, L.M. In the absence of the low density lipoprotein receptor, human apolipoprotein C1 overexpression in transgenic mice inhibits the hepatic uptake of very low density lipoproteins via a receptor-associated protein-sensitive pathway.. J. Clin. Investig. 1996, 98, 2259–2267. [CrossRef]
- Abildayeva, K.; Berbée, J.F.P.; Blokland, A.; Jansen, P.J.; Hoek, F.J.; Meijer, O.; Lütjohann, D.; Gautier, T.; Pillot, T.; De Vente, J.; et al. Human apolipoprotein C-I expression in mice impairs learning and memory functions. J. Lipid Res. 2008, 49, 856–869. [CrossRef]
- Cudaback, E.; Li, X.; Yang, Y.; Yoo, T.; Montine, K.S.; Craft, S.; Montine, T.J.; Keene, C.D. Apolipoprotein C-I is an APOE genotype-dependent suppressor of glial activation. J. Neuroinflammation 2012, 9, 192–192. [CrossRef]
- Song, F.; Poljak, A.; Crawford, J.; Kochan, N.A.; Wen, W.; Cameron, B.; Lux, O.; Brodaty, H.; Mather, K.; Smythe, G.A.; et al. Plasma Apolipoprotein Levels Are Associated with Cognitive Status and Decline in a Community Cohort of Older Individuals. PLOS ONE 2012, 7, e34078. [CrossRef]
- Mather, K.A.; Thalamuthu, A.; Oldmeadow, C.; Song, F.; Armstrong, N.J.; Poljak, A.; Holliday, E.G.; McEvoy, M.; Kwok, J.B.; Assareh, A.A.; et al. Genome-wide significant results identified for plasma apolipoprotein H levels in middle-aged and older adults. Sci. Rep. 2016, 6, 23675–23675. [CrossRef]
- Hoekstra M, Chen HY, Rong J, Dufresne L, Yao J, Guo X, et al. Genome-Wide Association Study Highlights APOH as a Novel Locus for Lipoprotein(a) Levels-Brief Report. Arterioscler Thromb Vasc Biol. 2021;41(1):458-64. [CrossRef]
- Vogrinc, Ž.; Trbojević-Čepe, M.; Coen, D.; Vitale, K.; Stavljenić-Rukavina, A. Apolipoprotein H (apoH)-dependent autoantibodies and apoH protein polymorphism in selected patients showing lupus anticoagulant activity. cclm 2005, 43, 17–21. [CrossRef]
- Gelissen IC, Hochgrebe T, Wilson MR, Easterbrook-Smith SB, Jessup W, Dean RT, et al. Apolipoprotein J (clusterin) induces cholesterol export from macrophage-foam cells: a potential anti-atherogenic function? Biochem J. 1998;331 (Pt 1)(Pt 1):231-7. [CrossRef]
- Wyatt, A.R.; Yerbury, J.J.; Wilson, M.R. Structural Characterization of Clusterin-Chaperone Client Protein Complexes. J. Biol. Chem. 2009, 284, 21920–21927. [CrossRef]
- Giannakopoulos, P.; Kövari, E.; French, L.E.; Viard, I.; Hof, P.R.; Bouras, C. Possible neuroprotective role of clusterin in Alzheimer’s disease: a quantitative immunocytochemical study. Acta Neuropathol. 1998, 95, 387–394. [CrossRef]
- DeMattos RB, Cirrito JR, Parsadanian M, May PC, O’Dell MA, Taylor JW, et al. ApoE and clusterin cooperatively suppress Abeta levels and deposition: evidence that ApoE regulates extracellular Abeta metabolism in vivo. Neuron. 2004;41(2):193-202. [CrossRef]
- Freixes, M.; Puig, B.; Blanco, R.; Ferrer, I. Clusterin solubility and aggregation in Creutzfeldt-Jakob disease. Acta Neuropathol. 2004, 108, 295–301. [CrossRef]
- Sasaki, K.; Doh-Ura, K.; Ironside, J.W.; Iwaki, T. Increased clusterin (apolipoprotein J) expression in human and mouse brains infected with transmissible spongiform encephalopathies. Acta Neuropathol. 2001, 103, 199–208. [CrossRef]
- Pan, X. The Roles of Fatty Acids and Apolipoproteins in the Kidneys. Metabolites 2022, 12, 462. [CrossRef]
- Liu, D.; Pan, J.-M.; Pei, X.; Li, J.-S. Interaction Between Apolipoprotein M Gene Single-Nucleotide Polymorphisms and Obesity and its Effect on Type 2 Diabetes Mellitus Susceptibility. Sci. Rep. 2020, 10, 1–6. [CrossRef]
- Katz, P.; Pedro, S.; Trupin, L.; Yelin, E.; Michaud, K. The Impact of Asthma and Chronic Obstructive Pulmonary Disease (COPD) on Patient-Reported Outcomes in Systemic Lupus Erythematosus (SLE). ACR Open Rheumatol. 2021, 3, 221–230. [CrossRef]
- Du, W.; Shen, T.; Li, H.; Liu, Y.; He, L.; Tan, L.; Hu, M.; Ren, Y. Low apolipoprotein M serum levels correlate with Systemic lupus erythematosus disease activity and apolipoprotein M gene polymorphisms with Lupus. Lipids Heal. Dis. 2017, 16, 1–6. [CrossRef]
- Liu, M.; Frej, C.; Langefeld, C.D.; Divers, J.; Bowden, D.W.; Carr, J.J.; Gebre, A.K.; Xu, J.; Larsson, B.; Dahlbäck, B.; et al. Plasma apoM and S1P levels are inversely associated with mortality in African Americans with type 2 diabetes mellitus. J. Lipid Res. 2019, 60, 1425–1431. [CrossRef]
- Christoffersen C. Apolipoprotein M-A Marker or an Active Player in Type II Diabetes? Front Endocrinol (Lausanne). 2021;12:665393. [CrossRef]
- Christoffersen, C.; Jauhiainen, M.; Moser, M.; Porse, B.; Ehnholm, C.; Boesl, M.; Dahlbäck, B.; Nielsen, L.B. Effect of Apolipoprotein M on High Density Lipoprotein Metabolism and Atherosclerosis in Low Density Lipoprotein Receptor Knock-out Mice. J. Biol. Chem. 2008, 283, 1839–1847. [CrossRef]
- Mathiesen Janiurek M, Soylu-Kucharz R, Christoffersen C, Kucharz K, and Lauritzen M. Apolipoprotein M-bound sphingosine-1-phosphate regulates blood-brain barrier paracellular permeability and transcytosis. Elife. 2019;8. [CrossRef]
- Raulin, A.-C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.-C. ApoE in Alzheimer’s disease: pathophysiology and therapeutic strategies. Mol. Neurodegener. 2022, 17, 1–26. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




