1. Introduction
Numerous peptides with cosmetic potential have been developed in response to frequent and not fully satisfied market needs for the formulation of innovative and effective cosmetic products with noticeable, immediate and long–lasting effects [
1,
2]. The potential benefits of topically applied peptides have been identified through research in the area of wound healing, as these compounds may have a positive effect on skin components, particularly collagen synthesis [
3]. In general, peptides intended for topical use can be divided into four main categories based on their mechanism of action: signal peptides (stimulate matrix proteins production, cell growth and other cell metabolic functions); carrier peptides (facilitate the transport of trace elements within the cell); neurotransmitter–inhibiting peptides (inhibit the release of acetylcholine and thus reduce the contraction of facial muscles and the expression of lines and wrinkles) and enzyme-inhibiting peptides (decrease the activity of enzymes related to skin ageing) [
3,
4,
5]. In addition, the application of cosmetic formulations containing peptides showed some immediate effects, including a significant increase in the water content of the stratum corneum layer, an increase in skin elasticity and an improvement in microrelief after a single application [
6].
Biopeptides are a new generation of active ingredients from natural sources, developed as a response to the growing consumer awareness of environmental protection and sustainability [
2,
4,
7]. Researchers are particularly interested in food–derived bioactive peptides produced by hydrolysis of food proteins (animal, marine and plant origin) because they are naturally derived and considered "green" and potentially have few side effects [
4,
8]. Milk proteins (from cow, sheep, goat, etc.) are considered the most important source of bioactive peptides and an increasing number of bioactive peptides have been identified in milk protein hydrolysates and fermented milk products [
9]. The main function of milk in infancy is to serve as a source of protein and nitrogen for young mammals [
10]. A considerable part of the health benefits of milk is associated with its proteins, not only for their nutritional value but also for their biological properties. Although proteins from plant sources are attracting increasing interest, plant foods generally provide less complete protein nutrition than animal proteins due to lower digestibility and source–specific deficiencies in essential amino acids [
11]. Protein hydrolysates containing bioactive peptides are classified as "Generally Recognized as Safe" (GRAS) by the U.S. Food and Drug Administration (FDA) [
5].
Bioactive peptides are defined as specific regions of proteins whose activity is based on their inherent amino acid composition and sequence. Active sequences can have two to twenty amino acid residues [
7,
9]. Food peptides have no effect unless they are released from the precursor protein in which they are encoded [
9,
12,
13]. Various methods have been developed to obtain biopeptides. The characteristics of an optimal method include low cost, large scale production, reproducibility and biocompatibility [
10]. The most efficient methods for producing functional hydrolysates or peptides are enzymatic hydrolysis of precursor proteins by digestive enzymes, fermentation with proteolytic starter cultures and proteolysis by enzymes from microorganisms or plants [
14]. Biological activity of peptides depends on the protein source, the enzyme used and the processing conditions, such as substrate concentration, enzyme/substrate ratio, incubation time, degree of hydrolysis (number of broken peptide bonds relative to the original protein) and physicochemical conditions during the process (pH and temperature) [
8,
15]. Therefore, to ensure high activity of the peptides formed, it is necessary to strictly control the degree of enzymatic hydrolysis [4].
It is known that many peptides have single or multifunctional biological properties (antioxidant, antimicrobial, anti-inflammatory and anti-ageing) with potential topical applications. In particular, antioxidant peptides have become the active materials of interest in various fields [
16]. There is growing evidence that oxidative stress caused by disruption of redox homeostasis in living organisms is involved in ageing, as well as the onset and development of numerous chronic diseases such as cancer, cardiovascular disease, weakened immune system, liver disease, inflammation, kidney failure, brain dysfunction, cataracts and diabetes. Reactive oxygen species (ROS) are a class of free radical species produced primarily by the mitochondrial respiratory chain and are associated with oxidative stress signaling in normal cells. However, when the accumulation of ROS exceeds the ability of the cellular system to scavenge free radicals, these reactive species trigger uncontrolled reactions with non-target biomolecules (lipids, proteins and DNA) and mediate the subsequent activation of pro-inflammatory or pro-apoptotic signaling pathways. This condition necessitates additional supplementation to balance antioxidants and oxidants in biological tissues [
17,
18]. Antioxidants can stabilise or deactivate free radicals before they attack cells [
18]. Bioactive peptides with antioxidant properties can be used in cosmetics to prevent the harmful effects of oxidative stress caused by the excessive production of ROS in the skin. The antioxidant activity depends on the structural properties of the peptides, such as molecular weight, hydrophobicity and the presence of certain amino acids, as well as on the amino acid sequence. It occurs through several mechanisms: hydrogen atom transfer (HAT), single electron transfer (SET) and chelating (prooxidant) transition metals [
4,
7,
12]. The hydrophobic character of peptides plays an important role in enhancing antioxidant properties by increasing the accessibility of the peptide to hydrophobic cellular targets such as polyunsaturated fatty acids in biological membranes [
7].
Antimicrobial peptides (AMPs) serve as the first line of defense of the innate immune system and are secreted by keratinocytes and other skin cells such as eccrine gland cells, mast cells and sebocytes. AMPs play an essential role in protecting the skin and body from invading pathogens and exhibit broad-spectrum antimicrobial activity against a range of bacteria (both Gram-negative and Gram-positive), fungi, and viruses [
19]. AMPs are primarily a family of small peptides (less than 100 amino acids) that are generally cationic and amphipathic. AMPs are water soluble and their amphipathic structure allows them to bind both hydrophilic phospholipid head groups and hydrophobic tail groups that form the lipid bilayers of microbial cells. The positive charge allows AMPs to adsorb to the anionic phospholipids of bacterial cell membranes, effectively disrupting and destabilizing them, leading to leakage of cell contents and eventual death of the microbial cell [
19,
20]. AMPs can be produced from a variety of protein sources such as milk by enzymatic hydrolysis or microbial fermentation. AMPs are more advantageous compared to conventional antibiotics because they bypass common resistance mechanisms and limit microbial resistance [
20].
The main concern and challenge in the use of bioactive peptides in cosmetic formulations is the maintenance of their physicochemical stability and biological activity during the manufacture and storage of the product, since their properties can be affected by several variables [7, 21]. Chemical instability of peptides corresponds to the formation or degradation of covalent bonds; physical instability is mainly caused by denaturation, aggregation and surface adsorption. Biological instability is caused by the hydrolytic action of cellular enzymes leading to degradation or inactivation of the active molecule and consequent loss of biological activity. All these degradation processes shorten the shelf life of peptides and limit their use in different formulations [
21]. It is therefore necessary to evaluate the quality of a cosmetic product using different techniques.
The aim of the present study was to explore the use of two different cow's milk protein hydrolysates in the development of safe, stable and efficacious fluid O/W emulsion with good sustainability profile (also defined as “green”). In addition to the hydrolysates, the formulations contained two novel emollients from the class of C15-C19 alkanes, promoted as biodegradable alternatives to silicone oils and an innovative naturally-derived glycolipid mixed emulsifier (lauryl glucoside/myristyl glucoside/polyglyceryl–6 laurate). This emulsifier allowed the use of cold emulsification process, contributing to the green credentials of the proposed formulation.
The formulations with and without active ingredients were extensively studied for their rheological behavior, physical stability and textural profile, while bioactivity and safety were assessed through in vitro evaluation of antioxidant activity, antimicrobial activity and effects on cell viability.
2. Materials and Methods
2.1. Materials
The active ingredients, milk protein hydrolysates named Nutrilan® Milk EP (NM hydrolysate) and Milk Protein COS (MPC hydrolysate) were kindly provided by BASF (Monheim, Germany) and Lipoid (Duisburg, Germany), respectively. A key emulsion stabilizer (mixed emulsifier consisting of lauryl glucoside, myristyl glucoside and polyglyceryl−6 laurate) named Fluidifeel® Easy (FFE) was generously provided by Seppic (Paris, France), as were the biocompatible emollients Emogreen® L15 and Emogreen® L19 (both with the same International Nomenclature of Cosmetic Ingredients (INCI) name, C15–19 alkane). Glycerin was purchased from Fagron (Trikala, Greece). A preservative mixture of Ethylhexylglycerol and Phenoxyethanol (Sharomix® EG 10) was purchased from Sharon Laboratories (Ashdod, Israel), while xanthan gum (Safic Care® T XGC 80) was from Safic-Alcan (Milan, Italy). 2,2−diphenyl−1−picrylhydrazyl (DPPH) was from Merck (Darmstadt, Germany) and 2,2′−azino−bis–(3−ethylbenzothiazoline−6−sulfonic) acid (ABTS) from Alfa Aesar (Massachusetts, USA). Potassium persulfate was purchased from Lechner (Neratovice, Czech Republic), while 96% ethanol was from Zorka Pharma (Šabac, Serbia). Pharmaceutical grade purified water was obtained internally using a Gen Pure Ultrapure device (Thermo Fisher Scientific GmbH, Munich, Germany).
2.2. Preparation of the Samples
All emulsions (see
Table 1 for exact composition), differing in the type of emollients and in the presence and concentration of milk protein hydrolysates, were prepared by “cold” emulsification procedure (at room temperature). In liquid form, the nonionic O/W emulsifier Fluidifeel
® Easy (lauryl glucoside/myristyl glucoside/polyglyceryl–6 laurate mixture) at 2% (w/w) was added to the aqueous phase and then stirred at 6000 rpm for 40 seconds using a rotor–stator homogenizer (Ultra-Turrax T25, IKA, Staufen, Germany). Subsequently, the oil phase consisting of 30% (w/w) of a single emollient (either Emogreen
® L15 - EG L15 or Emogreen
® L19 - EG L19) was slowly added to the aqueous phase while stirring at 4400 rpm. The obtained emulsion was then homogenized at 8000 rpm for 5 minutes. In the last minute of homogenization, 1% (w/w) of the preservative was added. The emulsion was then transferred to a laboratory stirrer with a propeller agitator (Heidolph RZR 2020, Heidolph, Schwabach, Germany) and homogenization was continued with the addition of the rheology modifier (xanthan gum dispersed in glycerol) at 650 rpm for 6 minutes. Finally, in the preparation of the “active” emulsions, the liquid form of the active ingredient was added at reduced mixing speed (200rpm) and homogenization continued for 5 minutes.
2.3. Determination of the antioxidative activity of milk protein hydrolysates
The antioxidant activity of milk protein hydrolysates per se was evaluated by two spectrophotometric SET methods: 2,2′−azino−bis−(3-ethylbenzothiazoline−6−sulfonic) acid (ABTS) and 2,2−Diphenyl−1−picrylhydrazyl (DPPH) radical scavenging test.
Before performing the antioxidant tests, the peptide content in both commercial hydrolysates was determined using the Lowry method [
22] (data not shown). The total peptide content was calculated from the linear equation of a standard curve prepared with bovine serum albumin.
2.3.1. DPPH radical scavenging activity
The modified method of Li et al. [
23] was used to determine the DPPH radical scavenging activity of the milk protein hydrolysates: 0.5 mL of the hydrolysate sample (each sample with different peptide concentrations, from 1 to 10 mg/mL) was mixed with 1.5 mL of 0.1 mM DPPH solution in 96% ethanol. The control was prepared in the same way, except that distilled water was used instead of the sample. The mixture obtained was shaken vigorously before incubation for 30 minutes under exclusion of light and at room temperature. The sample was again vigorously shaken and the absorbance at 517 nm was measured using a UV-VIS spectrophotometer (Ultrospec
® 3300 Amersham Biescens, UK). A lower absorbance of the reaction mixture indicates higher scavenging activity for DPPH radical. DPPH radical scavenging was calculated using the following equation:
In the formula,
Ac is the absorbance of the control, while
As is the absorbance of the sample. The activity is expressed as inhibitory concentration IC50, which is the amount of antioxidant required to reduce the initial DPPH activity by 50%. The lower the IC50, the higher the efficacy against radicals [
18]. All measurements were performed in triplicate and data are presented as the mean of three values.
2.3.2. ABTS radical scavenging activity
The antioxidant activity of the milk protein hydrolysates was determined according to the method described by Re et al. [
24]. ABTS radical cation (ABTS
•+) was prepared by reacting 7 mM ABTS stock solution with 2.45 mM potassium persulfate (final concentration) and the mixture was kept in the dark at the room temperature for 12-16 hours before use. The solution was then diluted in 0.1 M phosphate-buffered saline (PBS, pH 7.4) to an absorbance of 0.70 ± 0.02 at 734 nm. 10 µL of the sample (each sample with different peptide concentration, from 1 to 10 mg/mL) was added to 1 mL of diluted ABTS
•+ solution and incubated for 6 minutes at room temperature in the dark. The control was prepared in the same manner, except that PBS was used instead of the sample. The reduction of the blue-green ABTS
•+ by hydrogen-donating antioxidant was measured spectrophotometrically by observing the suppression of the characteristic long-wavelength absorption spectrum [
18,
25]
. The ABTS scavenging effect was calculated using the following equation:
where
Ac is the absorbance of the control and
As is the absorbance of the sample. The activity is expressed as inhibitory concentration IC50, which is the amount of antioxidant required to reduce the initial ABTS activity by 50%. All measurements were performed in triplicate and data are presented as the mean of three values.
2.4. Rheological analysis
The rheological characteristics of the formulated model emulsions were explored with the MCR 302 air-bearing rheometer (Anton Paar, Austria), equipped with coaxial cylinders system CC27/C-PTD 180/AIR (including appropriate evaporation blocker system) and navigated by RheoCompass
® software (Anton Paar). To obtain flow curves and viscosity curves of the emulsion samples, continuous rotational tests were conducted at 20 °C in the shear rate range of 0.1–100 s
-1 and
vice versa, for the upward and downward curves, respectively. The parameters obtained from continuous rotational testing were apparent viscosity (interpolated at shear rate of 50 s
-1) and hysteresis area. To characterize the viscoelastic properties of the developed emulsions, isothermal oscillatory tests (dynamical-mechanical analysis) including amplitude and frequency sweeps were performed. First, the amplitude sweeps were conducted at a radial frequency (ω) value of 10 rad s
-1, in the deformation range of 0.01–200%. Afterwards, the frequency sweeps were carried out in the linear viscoelastic region (LVER), at the deformation value equal to the deformation limit (γ
L) acquired from previously performed amplitude sweeps [
26], while ω was varied from 0.01 to 100 rad s
-1. The parameters gained from the amplitude sweeps were: yield point (yield stress), determined as the shear stress at the upper limit of LVER where non-destructive, reversible, elastic deformations still exist; flow point, determined as the shear stress where G' = G", i.e., above which the internal structure sufficiently breaks down and the system flows (G" > G'); storage (elastic) modulus G'; loss (viscous) modulus G"; and loss factor (tan δ = G"/G'). From the frequency sweeps, the G' value at ω = 0.01 rad s
-1 was acquired, as a parameter useful for assessing emulsion behavior at rest.
2.5. Stability study by Dynamical-mechanical thermoanalysis (DMTA)
DMTA test, recently explored as a rapid, rheology-based alternative to the conventional freeze-thaw test, was applied to monitor the change in maximal G' value during each successive temperature oscillation cycle (G'
max, n, n = 1–6) relative to the initial G' value obtained before temperature oscillation (G’
max, t0) [
27,
28]. The test was performed by using an air-bearing rheometer (MCR 302), in the LVER, at deformation γ
L for respective model formulations, while ω was fixed at 10 rad s
-1. The test started by setting the sample temperature to 20 °C, and once the thermal equilibrium was reached, G' was measured and G'
max, t0 was determined. Afterwards, the temperature was increased and then cycled from the selected high temperature (45 °C), through the chosen low temperature (4 °C), back to room temperature (20 °C), constituting a single freeze-thaw cycle (20 °C to 45 °C/ 45 °C to 4 °C/ 4 °C to 20 °C). At each cycle, G' measurements were taken and the subsequent G'
max analysis at thermal equilibrium (20 °C) was conducted, allowing for the calculation of the Delta (Δ) value (Δ
n = G'
max, n/ G'
max, t0, n = 1–6), which serves as an indicator of the structural change in the tested emulsion formulation. Afterwards, an absolute value of Delta difference was calculated (Δ
dif = |Δ
n – Δ
t0|, n = 1–6) [
28]. Temperature oscillations were programed only once and repeated for six times using internal loop in the RheoCompass
® software [
27,
28].
2.6. pH value and electrical conductivity measurements
The pH value and electrical conductivity of all emulsion samples were determined by direct immersion of the pH meter glass electrode (Eutech pH 700; Eutech Instruments Pte Ltd., Singapore) and conductivity meter electrode (Eutech CON 700; Eutech Instruments), respectively, into the investigated samples. The measurements were performed in triplicate, at 20 °C, before and after conducting the DMTA test for stability assessment purposes.
2.7. Texture Analysis
The prepared emulsions were analyzed with the EZ-LX Texture analyzer (Shimadzu,Kyoto, Japan). The immersion/deimmersion test (2-cycle compression analysis) wascarried out with two different measuring tools: cylindrical acrylic press jig and a conical acrylic press jig. The test speed was set to 1 mm/s and the immersion distance to 30 mm. All measurements were performed in triplicate at a constant temperature of 20 ± 2 °C. The built-in Trapezium-X Single software was used for data analysis.
2.8. Antimicrobial activity determination
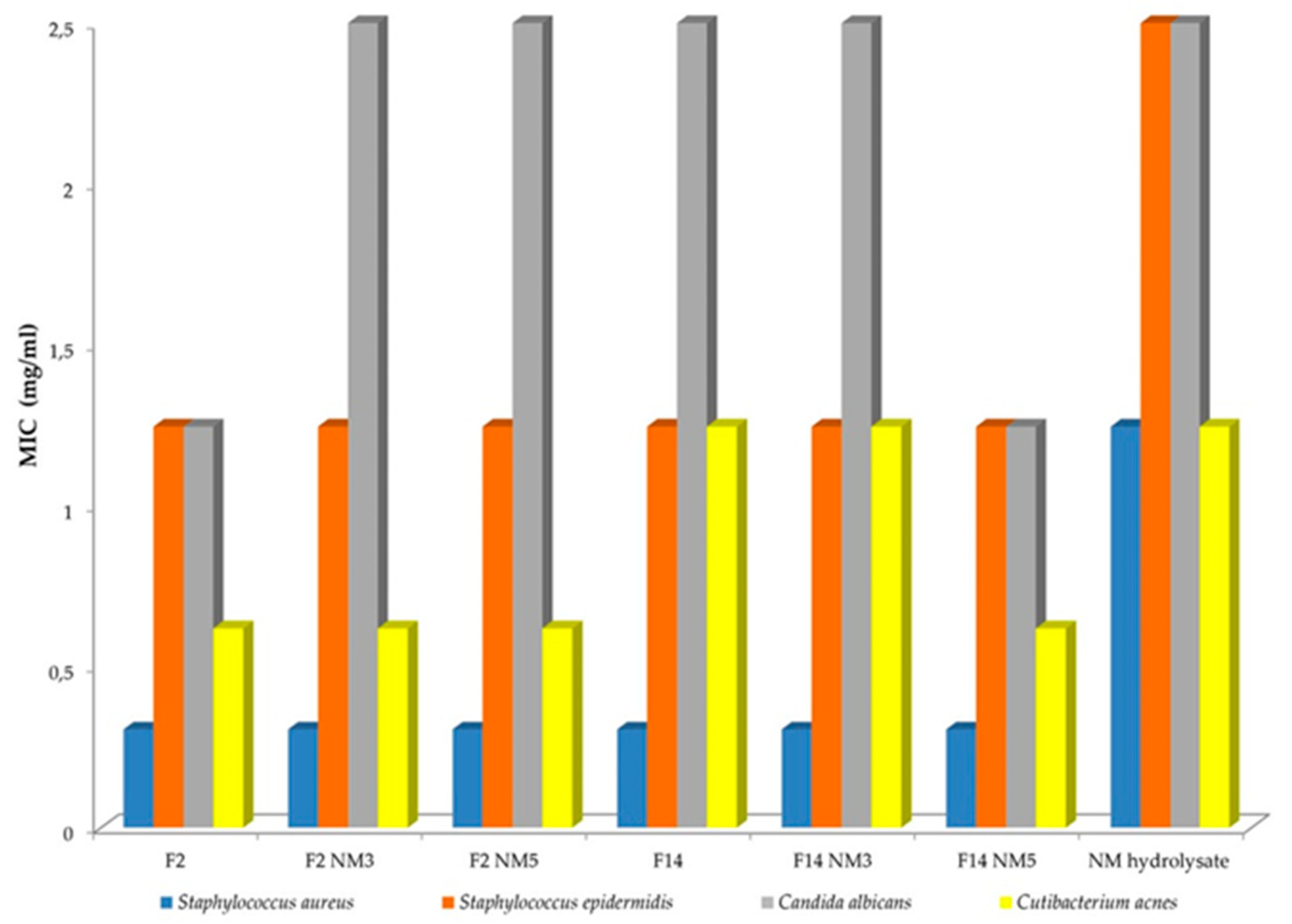
The antimicrobial activity of blank formulations (F2, F14), “active” emulsions containing different emollients and different concentrations of milk protein hydrolysate (F2NM3, F2NM5, F14NM3, F14NM5) and pure NM hydrolysate were tested against four ATCC laboratory control strains of microorganisms (KWIK-STIK®, Microbiologics, USA): three Gram-positive bacteria - Staphylococcus aureus subsp. aureus Rosenbach ATCC 6538, Staphylococcus epidermidis ATCC 12228 and Cutibacterium acnes ATCC 29399 and a yeast Candida albicans ATCC 24433. The lyophilized microorganism pellet was hydrated before experiments; staphylococci were inoculated on Triptic Soy Agar/Broth (TSA/TSB, Oxoid Ltd., Basingstoke, Hampshire, UK) and Mueller-Hinton broth (MHB, Oxoid), C. acnes on blood agar (anaerobic agar + 5% defibrinated horse blood) and thioglycollate broth (TG, Merck-Millipore, Darmstadt, Germany), and C. albicans on Sabouraud dextrose agar/broth (SDA/SDB, Oxoid). Staphylococci and C. albicans were cultured under aerobic conditions at 35±1 °C for 24-48 hours and C. acnes were cultured under anaerobic conditions (GasPack® systems, Becton, Dickinson and Company, Sparks, MD, USA) at 35-37 °C for 48-72 hours. The suspension of microorganisms used as inoculum was prepared from fresh cultures in a saline solution with a density of 0.5 per McFarland standard (Bio-Merieux, France).
Minimum inhibitory concentrations (MICs) were determined by broth microdilution assay in 96-well flat-bottomed microtiter plates according to the European Committee on Antimicrobial Susceptibility Testing guidelines (EUCAST 2020, 2023). Samples F2, F14, F2NM3, F2NM5, F14NM3, F14NM5, and NM hydrolysate were prepared in fresh MHB, TG or SDB at concentrations of 0.08-10 mg/mL; each concentration was prepared in duplicate and inoculated with 5x10
5 CFU/mL microorganisms. To detect cell growth and metabolism, the broths were spiked with the redox indicator resazurin (7-hydroxy-3H-phenoxazin-3-one 10-oxide) (Sigma-Aldrich, USA), a blue non-fluorescent dye that is reduced to the pink metabolite resorufin (7-hydroxy-3H-phenoxazin-3-one) when the microorganisms are viable. The MIC of the samples was determined to be the lowest concentration that inhibited the growth of the microorganisms after incubation at 35 °C for 20 to 48 hours under aerobic/anaerobic conditions (depending on the bacterial species). Positive controls (microorganisms in the medium) and negative controls (medium with samples only) were included in the experiments. Each test was repeated three times (The European Committee on Antimicrobial Susceptibility Testing; breakpoint tables for interpretation of MICs and zone diameters. Version V.13.1; June 2023,
http://www.eucast.org).
2.9. Cytotoxicity MTT assay
2.9.1. Preparation of stock solutions
Stock solutions of samples (F2, F14, F2NM3, F2NM5, F14NM3, F14NM5 and NM hydrolysate) were prepared in dimethyl sulfoxide (DMSO) at the concentration of 600 µg/mL. All samples were diluted to the appropriate working concentrations for use in the culture medium. For all cells used, the culture medium was RPMI 1640 (Sigma, USA) supplemented to final concentration with L-glutamine (3 mM), streptomycin (100 mg/mL) and penicillin (100 IU/mL), fetal bovine serum (10%; FBS; 56 °C heat-inactivated due to inactivation of cholinesterases and system complement and HEPES (25 mM)) and adjusted to pH 7.2 (bicarbonate solution). For cell survival determination, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide - MTT (Sigma, USA) was dissolved in phosphate buffered saline (PBS).
2.9.2. Cell lines
The cell lines of human lung fibroblast (MRC-5) and human keratinocytes (HaCaT) were maintained in complete nutrient medium RPMI-1640 at 37 °C in humidified atmosphere with 5% CO2. All cell lines were obtained from American Type Culture Collection (Manassas, VA, USA).
2.9.3. Cell sensitivity analysis
MRC-5 (5x103 cells/100 µl per well) and HaCaT (7x103 cells/100 µL per well) cells were seeded in 96-well microtiter plates and 20 hours later, after cell attachment, five different concentrations of test compounds were added to the wells. The final test compound concentrations ranged from 12.5 μg/mL to 200 μg/mL. Only culture medium was added to the cells in the control wells.
All experiments were performed in triplicate. Nutrient medium with the corresponding concentrations of the test substances but without cells was used as blank.
2.9.4. Determination of Target-Cell Survival
Cell survival was determined using the MTT assay according to the method of Mosmann [
29] and van Meerloo [
30] 48 hours after addition of the compounds. Briefly, 20 µL of MTT solution (5 mg/mL in PBS) was added to each well. Samples were incubated for an additional 4 hours at 37 °C, 5% CO2, and in a humidified atmosphere. Then, 100 µL of 10% sodium dodecyl sulphate - SDS (Sigma, USA) was added to each of the wells, and the absorbance of the cell medium from each well was measured at 490 nm the next day. The measurements were performed using the Multiskan
® FC Microplate Photometer (Thermo Scientific, USA).
To calculate the cell survival rate (%), the absorbance at 490 nm of each sample containing cells grown in the presence of the test compounds was divided by the absorbance of the control sample (the absorbance of cells grown in the culture medium only) after subtracting the absorbance of the blank sample. The IC50 was determined from the graph as the concentration of the compound that reduced the survival of treated cells by 50%. The IC50 values for each compound were determined by numerical analysis of the data obtained.
2.10. Statistical analysis
The effect of formulation composition, i.e., the presence of two different types of milk proteins at two different concentrations and of two types of emollients, on the critical rheological properties of the developed model emulsions was investigated using a model fitting/regression procedure created with the Design–Expert® software platform (version 11.1.0; Stat-Ease Inc., Minnesota, USA). The main effects and the interaction effects of the studied formulation variables on the apparent viscosity, hysteresis area, yield point, storage modulus, loss factor, and structural change of the emulsions as response variables were statistically evaluated by backward analysis using analysis of variance (One –way ANOVA).
In addition, the statistical differences between the electrical conductivity values and pH values of the emulsions measured before and after performing the DMTA test (two matched groups) were determined with the paired samples t-test using IBM SPSS® Statistics software (version 26.0; IBM, New York, USA). The significance level was set at p < 0.05 with 95% confidence interval.
4. Discussion
In cosmetics, emulsion systems are the most commonly used formulations. The reasons for the widespread use of low-viscosity O/W emulsions (lotions) in skin care products are their high water content (which means lower cost), their light, non-greasy feel, and their good spreadability on the skin. Emulsions must meet numerous requirements and expectations in terms of stability, safety, efficacy (both actual and perceived), and appropriate aesthetic and sensory properties [
34]. Bioactive peptides have attracted considerable attention in the cosmetic industry due to their potential to improve skin health and appearance. These small protein fragments are shown to have biological activities, such as antioxidant, anti-ageing, anti-inflammatory, and antimicrobial, making them potential active ingredients for cosmetic formulations [
40]. The major challenge in the use of bioactive peptides in cosmetic formulations is the maintenance of their physicochemical stability and biological activity during manufacturing and storage of the product. Therefore, it is necessary to evaluate the quality of a developed cosmetic product using various methods.
Peptides and protein hydrolysates derived from the proteolysis of various dietary sources (including cow milk) are believed to have antioxidant activity. Antioxidant mechanisms include radical scavenging activity (both the ability to donate hydrogen and to suppress free radicals), inhibition of lipid peroxidation, chelation of metal ions, or a combination of these properties. Antioxidant activities could protect biological systems from damage associated with oxidative stress [
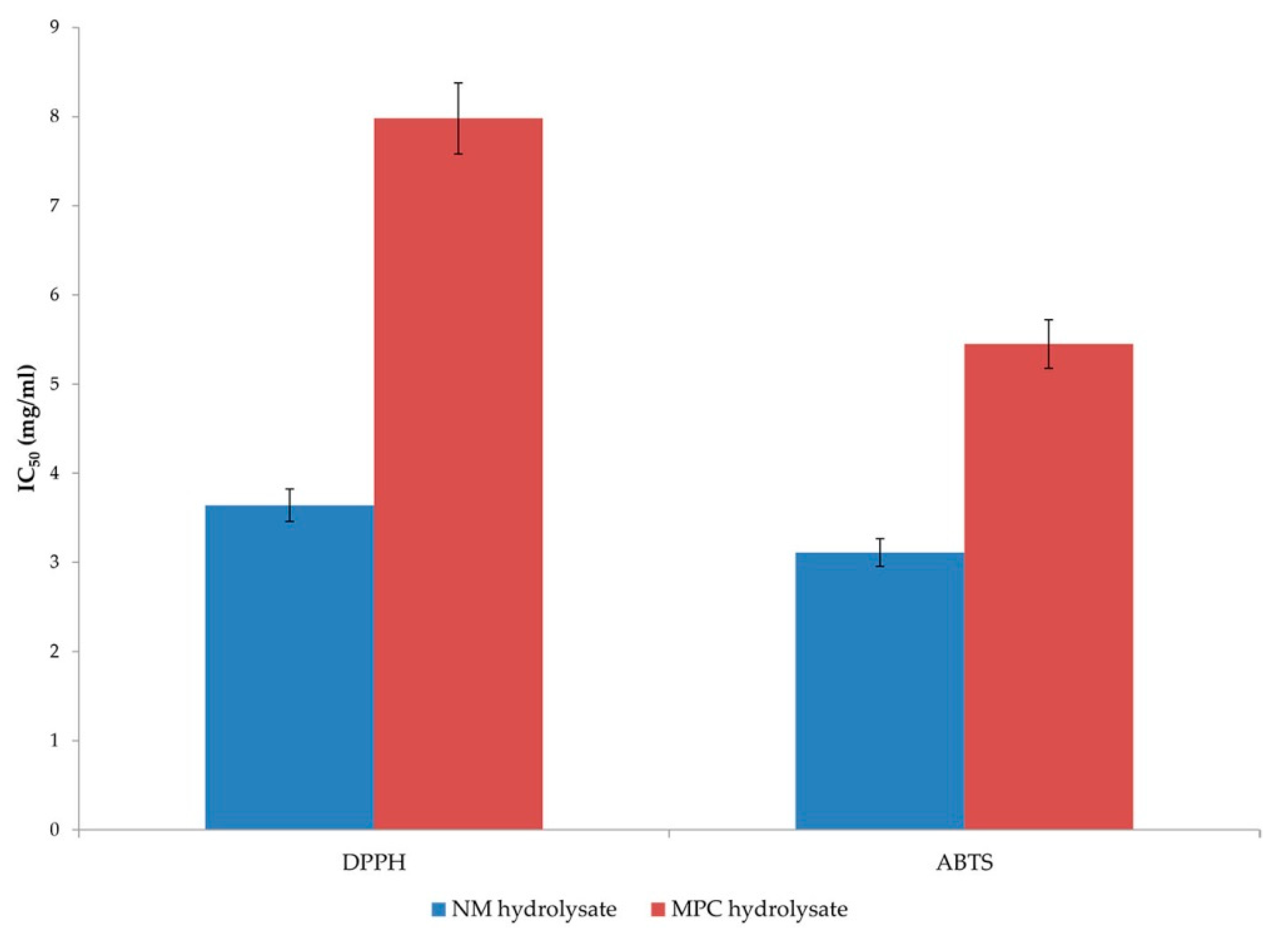
16]. The peptides in both commercial hydrolysates tested in this study showed concentration-dependent antioxidant activity (
Figure 1). The differences in antioxidant ability of the peptides in two hydrolysates are probably due to different peptide properties (molecular mass and amino acid composition). The difference in the relative radical scavenging ability of the protein hydrolysates with two test radicals could be due in part to the different solubility of these radicals and their ability to diffuse into the reaction medium. DPPH can only be dissolved in organic solvents (especially alcohol) and not in aqueous solution. Therefore, it poses a significant limitation in determining the potential of hydrophilic antioxidants. The opaqueness caused by the presence of peptides in alcoholic solvents negatively affects the DPPH assay [
25]. In addition, the steric accessibility of the DPPH radical is an important factor in the reaction, since small molecules that have better access to the radical site have a relatively higher antioxidant capacity. Many large antioxidant compounds that react rapidly with peroxyl radicals may react slowly or even be inert in this assay [
18]. In contrast, the ABTS method is more sensitive because ABTS radicals can be dissolved in aqueous and organic media, which allows the measurement of radical scavenging activity of both hydrophilic and lipophilic compounds [
25].
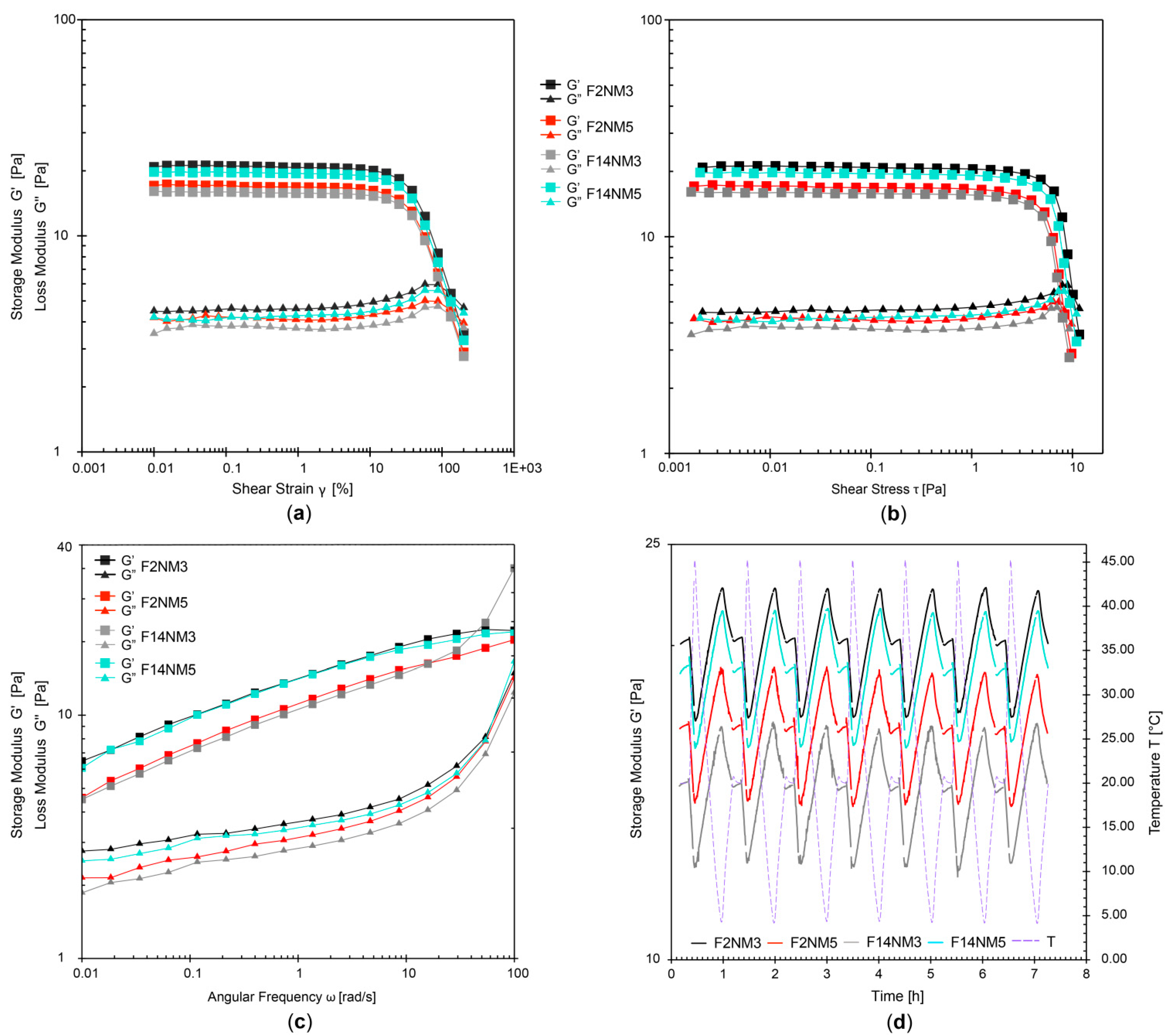
Combining all rheological results, it has been revealed that all developed emulsions exhibited favorable shear-thinning flow behavior (with thixotropy, yield point and flow point) and viscoelastic character with dominant elastic properties. Importantly, the DMTA test have shown a very small extent of structural change Δ (0.02%–1.24%). Together with small changes in pH and electrical conductivity, this indicated that all test emulsions had satisfactory stability profiles.
The concentration of hydrolysed milk protein was found to have a notable effect on the rheological properties (apparent viscosity, hysteresis area, yield point, G', loss factor) as critical quality characteristics of the developed emulsions (
Table 2). In addition, the interactions between the independent formulation factors (concentration and type of hydrolysed milk protein, type of emollient) were found to considerably affect the hysteresis area, loss factor and structural change of the developed emulsions (
Table 2). However, the addition of hydrolysates to the model emulsions, regardless of their type and concentration, does not adversely affect the rheological properties and stability of the emulsions.
It should be noted that MPC hydrolysate has a low protein content (17.5 mg/mL) and, according to the manufacturer, contains about 80% glycerin, so it can be assumed that the influence on the rheological properties of emulsions with this active ingredient comes from the glycerin and not from the peptides. A much higher protein content of NM hydrolysate (234 mg/mL) has caused a larger hysteresis area in comparison to emulsions loaded with MPC hydrolysate, and on the electrical conductivity values of the emulsions. Due to its higher peptide content and potentially better cosmetic effect, NM hydrolysate is preferred as an active ingredient.
It is of interest to note that the DMTA test has proven to be a useful method for accelerated stability testing of the developed naturally based emulsions loaded with hydrolysed milk protein. It was capable of distinguishing the structural changes and thus predicting the stability of different formulations in a much shorter period of time. The DMTA test required about 7 hours per formulation, compared to at least 12 days required for the conventional freeze-thaw test.
Texture analysis, a technique widely used in food research, is used to evaluate the mechanical properties of emulsion systems. It is becoming a standard method for the evaluation of emulsion stability, but it is even more useful in the analysis of sensory properties [
41]. The success of a product often depends on its sensory properties, of which many are related to its texture [
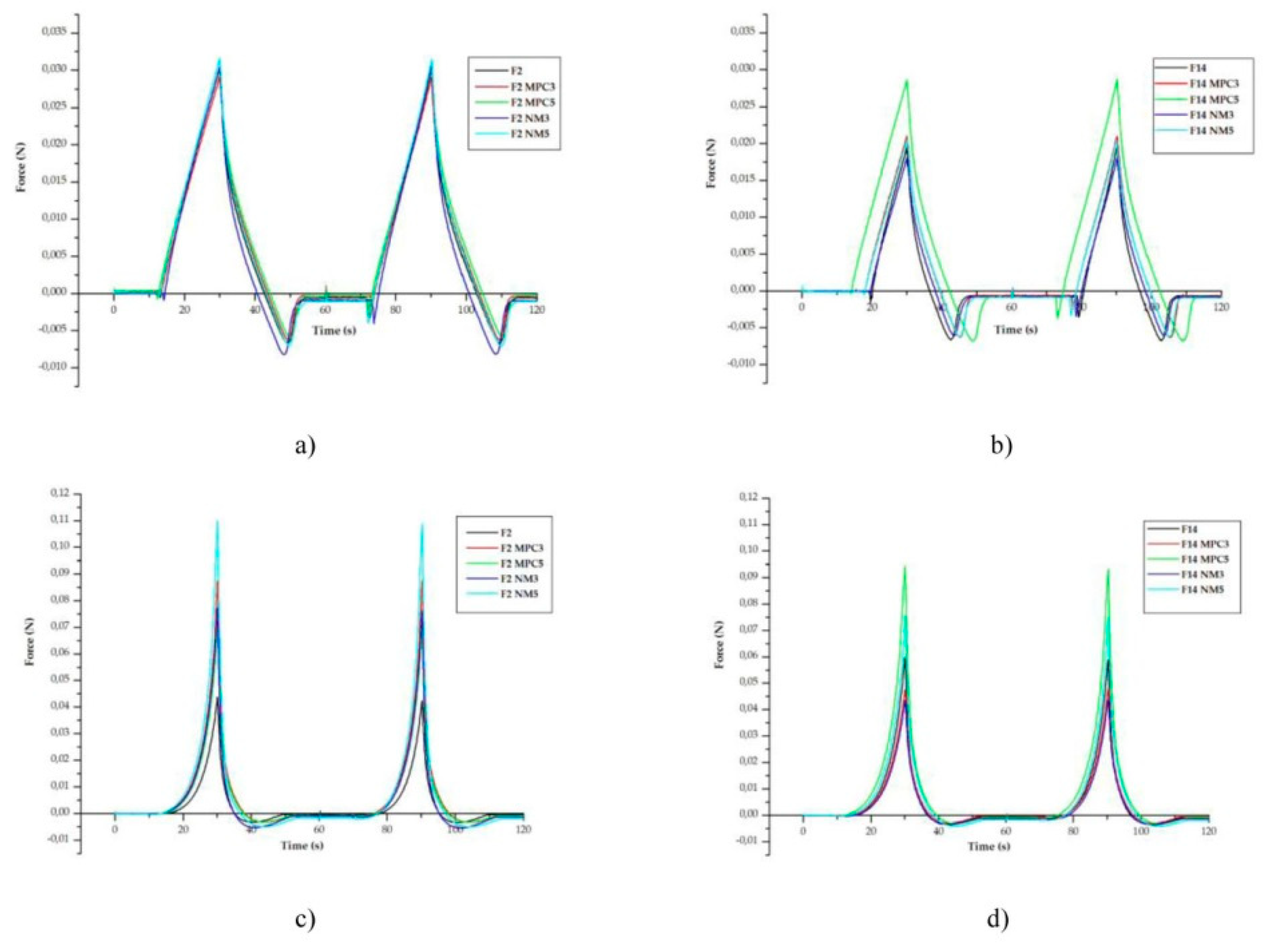
42]. All emulsions developed in this study have exhibited good texture profiles, i.e., satisfactory spreadability and adhesiveness (
Figure 3). The presence and concentration of milk protein hydrolysates has had an effect on the emulsions’ sensory properties. This is reflected in the increase of firmness with increasing hydrolysate concentration (exception F14NM3 - both cylindrical and conical probe and F14MPC3 - conical probe) and in the change in adhesiveness compared to blank emulsions. In general, adhesiveness was higher in emulsions with NM hydrolysate and lower in emulsions with MPC hydrolysate, compared to the blank emulsions. It is possible that the effect is due to the presence of peptides, since emulsions with NM hydrolysate have a higher peptide content.
Cow milk is a rich source of antimicrobial peptides released during hydrolysis of milk proteins. The antimicrobial activity of the peptides depends on the cations bound (peptides rich in arginine, lysine, and histidine) and the composition of the hydrophobic amino acids. Cationic peptides interact electrostatically with the negatively charged surfaces of bacterial cell membranes, leading to their destabilization. Highly hydrophobic peptides can also easily penetrate the lipid bilayer of cell membranes [
43] According to Najafian and Babji [
44], antimicrobial peptides usually have less than 50 amino acids, of which almost 50% are hydrophobic and have a molecular weight below 10 kDa. Commercial protein hydrolysates investigated in this showed relatively low antimicrobial activity against several selected laboratory microorganisms (Fig. 4). It is worth noting that NM hydrolysate is itself preserved with ethylhexylglycerol and phenoxyethanol. The antimicrobial activity of the placebo emulsions can also be attributed to the presence of preservatives. Therefore, it is assumed that the concentration of antimicrobial peptides present in protein hydrolysates was quite low.
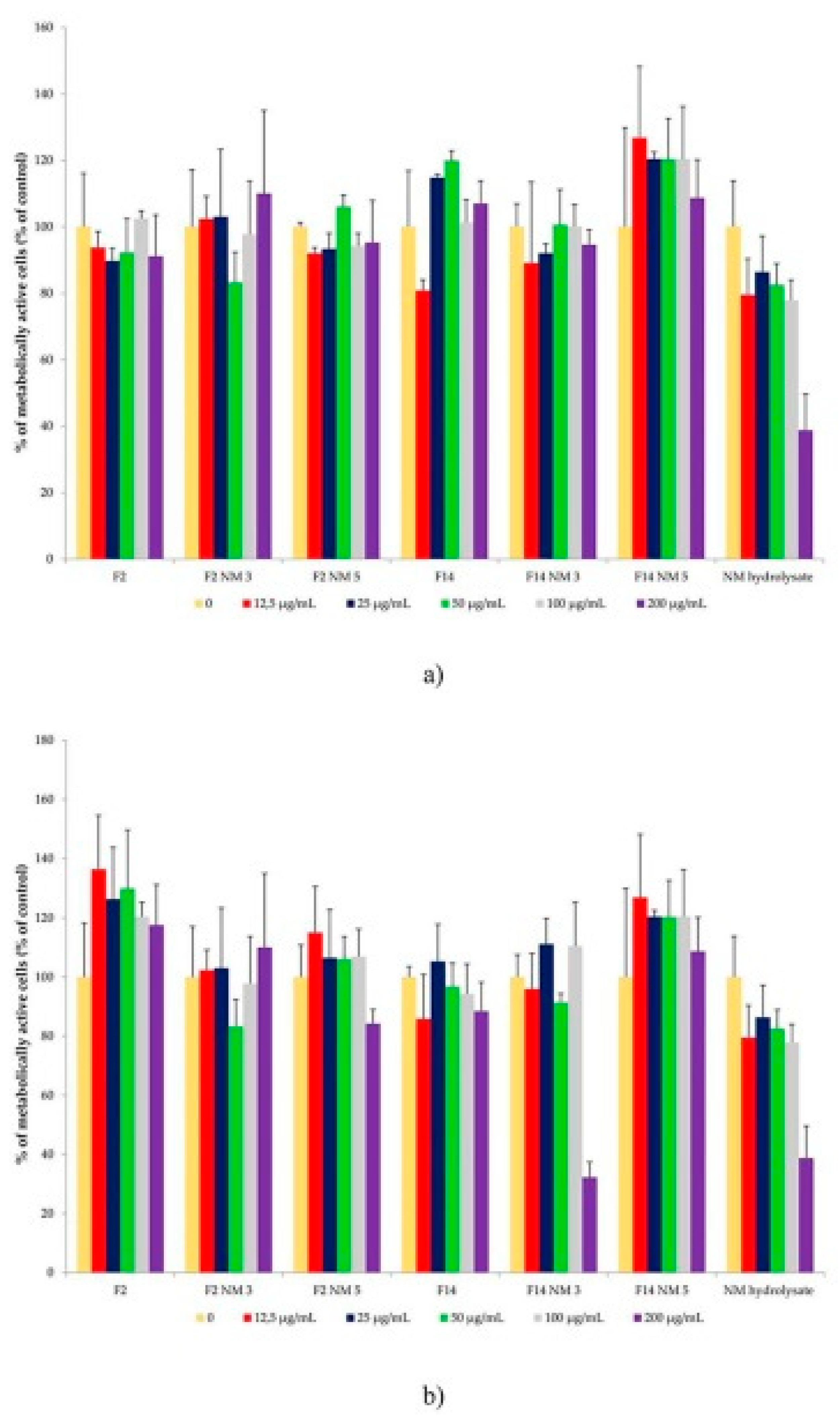
The results of the cytotoxicity assay have shown that milk protein hydrolysates have no cytotoxic effects on skin cells. In fact, it was shown that those hydrolysates can even promote cell proliferation (
Figure 5)
Author Contributions
Conceptualization, S.D.S., N.C., S.T.; methodology, I.P., S.S., J.A.S., D.B., S.S.; validation, N.C.; formal analysis, M.V. M.V.S, A.T.; investigation, M.V.; resources, S.D.S. and N.C.; writing—original draft preparation, M.V., S.S. and I.P.; writing—review and editing, S.D.S.; N.C. and S.T.; supervision, S.D.S. and S.T. All authors have read and agreed to the published version of the manuscript.