1. Introduction
Portal vein thrombosis (PVT) is a common complication of hepatocellular carcinoma (HCC), occurring in approximately 23-31% of patients at the time of diagnosis [
1,
2] and in 44% of them at autopsy [
3]. The occurrence of PVT negatively impacts patients’ prognosis, largely because it limits their therapeutic options: indeed, in the Barcelona Clinic Liver Cancer (BCLC) Staging System, HCC complicated by PVT is classified as stage C (advanced), no more suitable to surgical resection because of the high rate of tumor recurrence [
4,
5].
Several risk factors are associated with the development of PVT in HCC (advanced stage, major vessel involvement, higher Child-Pugh class, higher MELD score, low serum albumin, high serum bilirubin, elevated INR, and high α-fetoprotein)[
2], but to date no diagnostic biomarker has been identified to facilitate early diagnosis.
HMGB-1 (High Mobility Group Box-1) is a non-histone DNA-binding protein expressed in all nucleated cells and in platelets[
6]. Following platelet activation, HMGB-1 is initially exposed on the cell membrane, and then released into the extracellular space, becoming crucial in thrombus formation and neutrophil activation [
7]. In an animal model of venous thrombosis, disulfide HMGB-1 released by platelets promotes neutrophil recruitment and formation of Neutrophil Extracellular Traps (NET), thus contributing to venous thrombosis as a central mediator of the sterile inflammation process [
8]. In addition to platelet activation and NET formation, HMGB-1 exerts a prothrombotic effect by upregulating tissue factor (TF) expression in monocytes, inhibiting protein C activation, and inducing the formation of plasminogen activator inhibitor-1/tissue plasminogen activator (PAI-1/tPA) complexes [
9,
10]. Several studies suggest the important role of HMGB-1 in many thrombosis-related disorders [
11], such as acute coronary syndrome [
12], ischemic stroke [
13], peripheral arterial disease[
14] and disseminated intravascular coagulation [
15,
16].
HMGB-1 is also known to be implicated in the pathogenesis of cancer, enhancing the mechanisms of cell proliferation and migration, and promoting angiogenesis [
17]. HMGB-1 is overexpressed in many types of cancer, among which HCC [
18], and in clinical studies HMGB-1 correlates with clinical outcome and prognosis of patients with HCC [
19].
Despite these premises, to date no study has investigated the role of HMGB-1 in PVT complicating HCC. Based on the pathobiological background outlined above, we aimed to explore the hypothesis that HMGB-1 could qualify as a candidate biomarker of PVT in HCC.
2. Materials and Methods
2.1. Patients.
We performed a cross-sectional study based on prospectively recruited patients who attended the liver clinic of two academic hospitals in northern Italy from March 2021 to March 2022. The study protocol was approved by the institutional ethical committee (Ethics Committee of A.O.U. Città della Salute e della Scienza di Torino - A.O. Ordine Mauriziano - A.S.L. Città di Torino, approval code: 2CEI-452) and was conducted in strict accordance with the principles of the Declaration of Helsinki; all patients gave an informed consent in writing to their participation to the study. We included 100 consecutive patients willing to participate, with a new diagnosis of HCC of any etiology (HBV, HCV, alcoholic, dysmetabolic, autoimmune), in any stage of BCLC classification. Data collected included sex, age, body mass index (BMI), liver disease etiology (categorized as due to hepatitis B, HBV, hepatitis C, HCV, alcoholic, dysmetabolic, and other), anti-HCV therapy, follow-up time, Child Pugh-Turcotte (CPT) and model-for-endstage-liver disease (MELD) score, cirrhosis, Eastern Cooperative Oncology Group (ECOG) score, tumor burden, presence or absence of PVT, and BCLC staging. The laboratory data included complete blood count, albumin, total bilirubin, INR, creatinine, as well as plasma and serum HMGB-1 levels. The latter values were compared to those of a group of 34 healthy control subjects.
2.2. Laboratory studies.
Serum HMGB-1 concentration was measured using the kit HMGB-1 ELISA IBL International GmbH (Hamburg, Germany).
2.3. Statistical analysis.
Statistical analysis was conducted using the Stata statistical software package, version 13.1 (StataCorp LP, College Station, TX, USA). Continuous variables are presented as medians (interquartile ranges) and categorical variables as frequencies (percentages). For the continuous variables the differences between the groups were analyzed with the Wilcoxon signed rank test, the Mann–Whitney test, and the Kruskal-Wallis test. For post-hoc comparison, the Dunn test was used, while the analysis of the trend to increase / decrease of a continuous variable as a function of belonging to groups listed in an ordinal manner was performed using the variant of the Wilcoxon test proposed by Cuzick. In the case of categorical variables, the existence of a significant association was explored with Fisher’s exact test or Pearson’s chi-square test, as appropriate. Finally, to identify independent predictors of portal thrombosis, a logistic regression model was built. For all the tests used, the value chosen to indicate the statistical significance threshold was 0.05 (two-tailed).
3. Results
3.1. HMGB-1 levels and PVT.
Table 1 presents the main features of the study population, as well as the comparison between HCC patients with and without PVT.
As shown, the presence of PVT is associated with older age (p=0.036), larger major HCC node diameter (p<0.001), and lower HMGB-1 serum level (p=0.012). On multivariate analysis (
Table 2), the only independent predictors of PVT were major node diameter and serum HMGB-1 level. Only one out of 22 patients with PVT had a serum HMGB-1 concentration in the highest quartile (≥10.5 ng/ml), whereas 7/22 patients with PVT had a major tumor diameter in the highest quartile (≥45 mm). No patient with serum HMGB-1 concentration <10.5 ng/ml and major tumor diameter <45 mm had PVT at presentation.
3.2. Comparison between HCC patients and healthy controls.
To offer a pathophysiological explanation of the inverse association between HMGB-1 concentration and PVT, we compared the following three groups: group A (N=78), patients with HCC without PVT; group B (N=22), patients with HCC and PVT; group C (N=34), healthy controls.
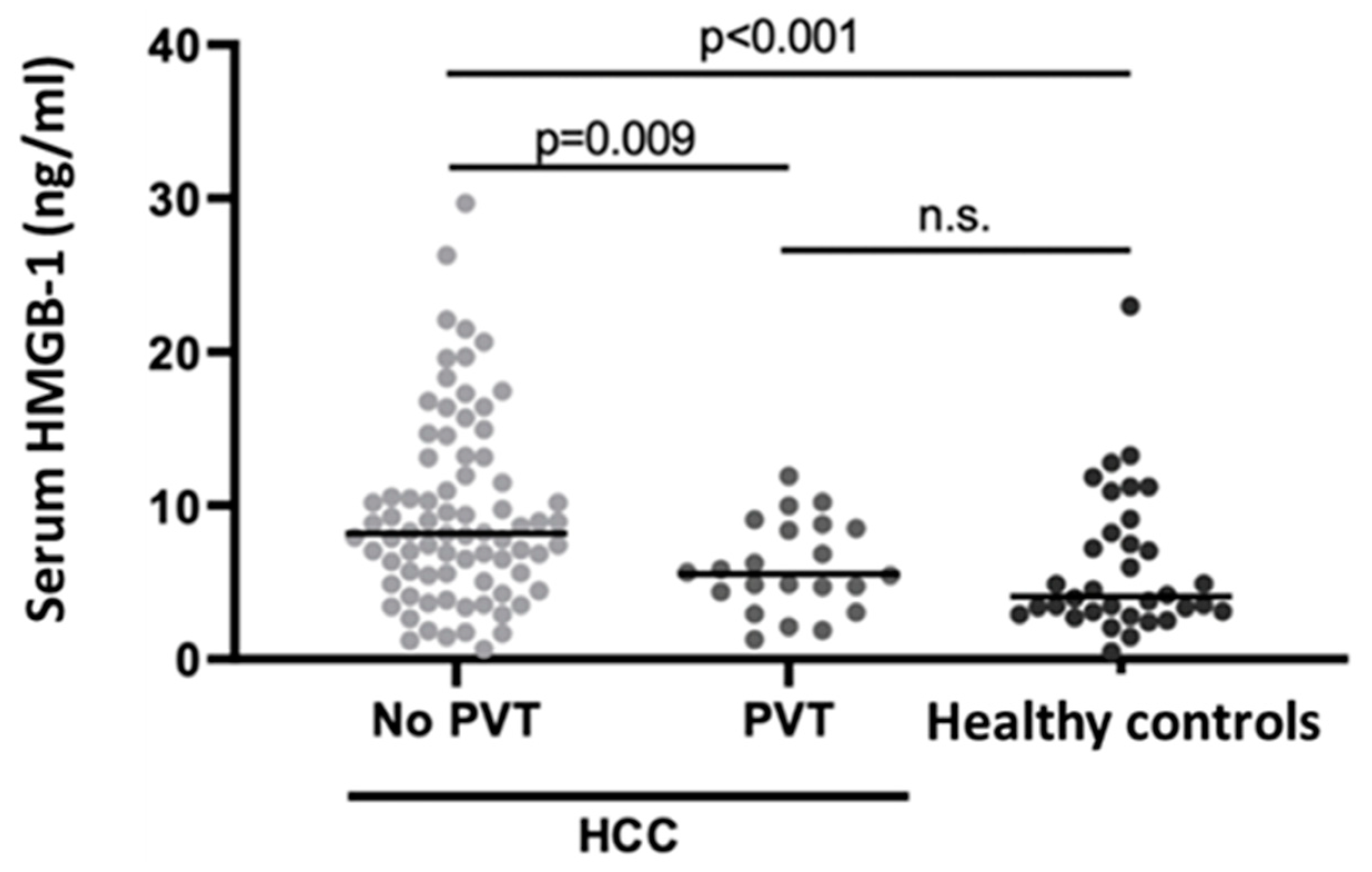
Figure 1.
Scatterplot of individual serum HMGB-1 levels in the study population, with and without PVT, compared to healthy controls. The horizontal lines indicate the median values for each group. Abbreviations: HMGB-1, High Mobility Group Box-1; HCC, Hepatocellular Carcinoma; PVT, Portal vein thrombosis.
Figure 1.
Scatterplot of individual serum HMGB-1 levels in the study population, with and without PVT, compared to healthy controls. The horizontal lines indicate the median values for each group. Abbreviations: HMGB-1, High Mobility Group Box-1; HCC, Hepatocellular Carcinoma; PVT, Portal vein thrombosis.
HMGB-1 serum levels were significantly different between group A and groups B-C (p=0.009 and p<0.001 with Dunn’s test, respectively), while there was no statistically significant difference between group B and group C (p=0.354). Moreover, serum HMGB-1 levels decreased progressively from group A to group B and C (p<0.001 at Cuzick’s test).
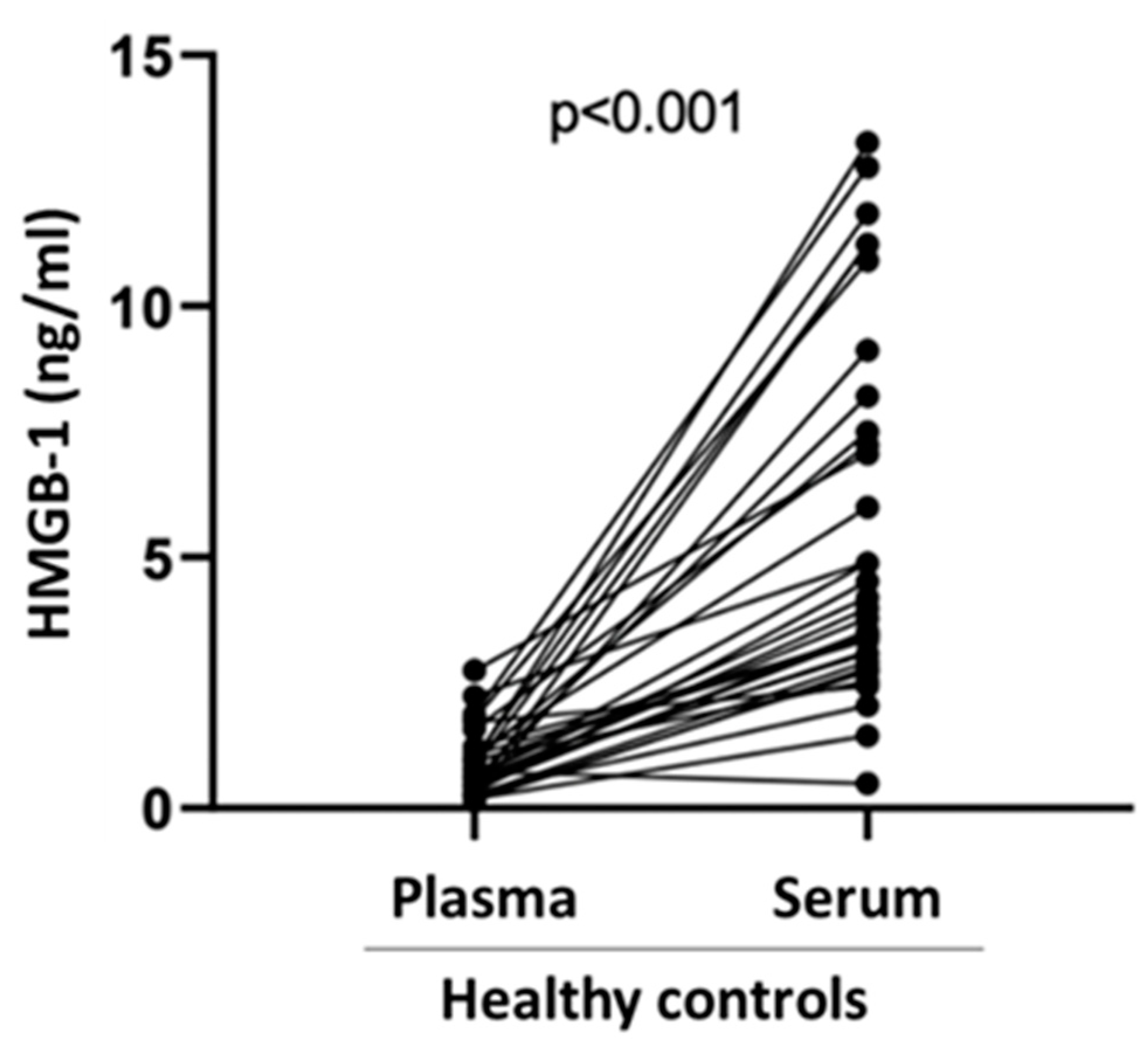
Finally, we compared serum vs. plasma HMGB-1 levels in specimens obtained from N=34 healthy subjects (21 males, 13 females) with a median age of 44 years (30-53). As shown in
Figure 2, plasma HMGB-1 levels were significantly lower than serum HMGB-1 levels (0.65 ng/ml vs 4.09 ng/ml, p<0.001.
4. Discussion
In the present study, we confirm previous observations showing that serum HMGB-1 concentrations are significantly higher among patients with HCC when compared to healthy subjects; however, to our knowledge, we are the first to show that among HCC patients with PVT serum HMGB-1 concentration is lower than that observed among HCC patients without PVT, in fact not being different from that observed among healthy subjects. Since HMGB-1 is a critical mediator of thrombosis [
7], the inverse association with PVT was somewhat unexpected. We would like to offer a plausible explanation for these findings, which stems from what is known about the pathophysiologic role of HMGB-1 in inflammatory thrombosis. By doing so, we hope to stimulate further research in this area.
The proteins collectively called the “high mobility group” have pleiotropic functions, related to factors such as cell type, subcellular location, and redox state. Specifically, it has been shown that in resting platelets, which lack a nucleus, HMGB-1 is located in the cytoplasm [
20]. Upon platelet activation, HMGB-1 is translocated to the cell surface and its disulfide isoform is abundantly released to coordinate venous thrombosis [
8]. In turn, disulfide HMGB-1 facilitates the formation of prothrombotic NETs. One proposed model of HMGB-1 release into the extracellular space states that HMGB-1 secretion is mediated by intracellular vesicles (including lysosomes) inside which the molecule is packaged, been extruded after lysosome fusion with the plasma membrane [
21]. Following its release into the extracellular space, HMGB-1 acts as a prototypical damage-associated molecular pattern (DAMP) signal, promoting NETosis and perpetuating inflammatory responses. As other soluble mediators of inflammation, HMGB-1 is subjected to regulation to limit its pro-inflammatory activities. Among the control mechanisms, it is worth mentioning degradation by thrombomodulin, an anticoagulant whose plasma levels are higher in patients with HCC compared to those of patients with benign focal liver lesions and normal controls [
22]. Interestingly, these Authors also showed that plasma thrombomodulin levels of patients with single HCC or HCC not complicated by PVT were significantly higher than those of those patients with multiple HCC or HCC complicated by PVT [
22].
In agreement with others, in the present paper serum HMGB-1 concentrations were significantly higher in HCC patients than in healthy controls: HMGB-1 expression correlates with peritumoral macrophage infiltration [
23] and appears to predict a poor prognosis for patients with HCC after durative hepatectomy [
24]. Moreover, there is a trend towards increasing HMGB-1 levels from healthy to chronic hepatitis patients and, among the latter in those with cirrhosis and HCC [
19]. In this sense, our paper adds little to the existing literature. Surprisingly for what has been called the venous clot coordinator, however, there have been no reports linking HMGB-1 levels to PVT.
To confirm that serum HMGB-1 is overwhelmingly derived from activated platelets, we compared serum and plasma concentrations of HMGB-1 in samples simultaneously obtained from healthy donors: the plasma level was on average 6 times lower than the serum one. Indeed, it has been shown in a transgenic mouse model that platelets are the main source of HMGB-1 in the context of thrombus [
7]. Mice with HMGB-1-deprived platelets have an increased bleeding time, reduced thrombus formation, platelet aggregation, inflammation, and organ damage in the context of trauma and hemorrhagic shock [
7]. HMGB-1 is believed to be paramount to thrombus formation in humans [
25]. Based on these premises, platelets derived from patients with PVT might be relatively depleted of HMGB-1, as part of control mechanisms that counter-off the pro-inflammatory effects of this molecule [
26]. Alternatively, a physical phenomenon of local consumption in the portal vein may lead to the detection of low serum levels of HMGB-1 in peripheral blood. Finally, one could speculate that this finding might be due to a laboratory interference: a different form of HMGB-1 in the PVT may not have been recognized by the ELISA test, being complexed with other molecules, like thrombomodulin and/or proinflammatory molecules. The formation of HMGB-1/IL-1b complexes has been described by others: for example, the presence of these heterocomplexes in human plasma has been identified in the context of burn damage[
27]. Indeed, via the interaction with cytokines such as IL-1b, HMGB-1 can enhance its proinflammatory activity [
26]. This hypothesis is fully compatible with the pathophysiology of PVT, a condition associated with increased levels of systemic inflammatory markers [
28]. In any case, given the independent roles of tumor size and serum HMGB-1 concentration in predicting PVT at multivariate analysis, these two factors could be explored for use in combination, for example, to guide clinicians in offering PVT prophylaxis with anticoagulants.
The present study has several limitations: first of all, the relatively small sample size. The study population was typical of HCC in Western countries, made as it was by aged Caucasians with viral and/or alcoholic liver disease [
29], thus these results may not apply to HCC patients in other regions of the world. Being cross-sectional, the present study is unfit by design to test the mechanism(s) through which the putative HMGB-1 depletion might occur in patients with HCC complicated by PVT. We acknowledge that the explanations we offer for this phenomenon remain, at present, speculative.
5. Conclusions
The present work identifies serum HMGB-1 as a promising candidate biomarker of PVT in HCC and confirms the involvement of this molecule in the local and systemic tumor-related inflammatory reaction. Being HMGB-1 overexpressed in advanced liver disease, measuring serum and plasma HMGB-1 concentrations serially both in animal models and in clinical settings may allow verification of the hypothesis that serum HMGB-1 depletion may mark PVT development.
Author Contributions
For research articles with several authors, a short paragraph specifying their contributions must be provided. The following statements should be used “Conceptualization, M.Pi.; methodology, M.Pe., M.N.B., G.P.C., S.G.; formal analysis, M.Pi.; investigation, R.P, E.R., P.C., M.E.B.; data curation, M.B., R.M.; writing—original draft preparation, M. Pe., R.P.; writing—review and editing, M.B., S.T., G.F.M., R.M.; supervision, M.Pi. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding”.
Institutional Review Board Statement
The study was conducted by the Declaration of Helsinki and approved by the Ethics Committee of A.O.U. Città della Salute e della Scienza di Torino - A.O. Ordine Mauriziano - A.S.L. Città di Torino, approval code: 2CEI-452.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
This research was supported by the Italian Ministry of Education, University and Research (MIUR) program “Departments of Excellence 2023–2027”, AGING Project—Department of Translational Medicine, Università del Piemonte Orientale.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schöniger-Hekele, M.; Müller, C.; Kutilek, M.; Oesterreicher, C.; Ferenci, P.; Gangl, A. Hepatocellular Carcinoma in Central Europe: Prognostic Features and Survival. Gut 2001, 48, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Connolly, G.C.; Chen, R.; Hyrien, O.; Mantry, P.; Bozorgzadeh, A.; Abt, P.; Khorana, A.A. Incidence, Risk Factors and Consequences of Portal Vein and Systemic Thromboses in Hepatocellular Carcinoma. Thromb. Res. 2008, 122, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Pirisi, M.; Avellini, C.; Fabris, C.; Scott, C.; Bardus, P.; Soardo, G.; Beltrami, C.A.; Bartoli, E. Portal Vein Thrombosis in Hepatocellular Carcinoma: Age and Sex Distribution in an Autopsy Study. J. Cancer Res. Clin. Oncol. 1998, 124, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Quirk, M.; Kim, Y.H.; Saab, S.; Lee, E.W. Management of Hepatocellular Carcinoma with Portal Vein Thrombosis. World J. Gastroenterol. 2015, 21, 3462–3471. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.E.; Rodriguez De Lope, C.; Bruix, J. Current Strategy for Staging and Treatment: The BCLC Update and Future Prospects. Semin. Liver Dis. 2010, 30, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Yang, H.; Harris, H. High-Mobility Group Box 1 Protein (HMGB1) Operates as an Alarmin Outside as Well as Inside Cells. Semin. Immunol. 2018, 38, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Bodenstein, R.; Chen, Q.; Feil, S.; Feil, R.; Rheinlaender, J.; Schäffer, T.E.; Bohn, E.; Frick, J.S.; Borst, O.; et al. Platelet-Derived HMGB1 Is a Critical Mediator of Thrombosis. J. Clin. Invest. 2015, 125, 4638–4654. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.; Philippi, V.; Stockhausen, S.; Busse, J.; Antonelli, A.; Miller, M.; Schubert, I.; Hoseinpour, P.; Chandraratne, S.; Von Bruhl, M.L.; et al. Disulfide HMGB1 Derived from Platelets Coordinates Venous Thrombosis in Mice. Blood 2016, 128, 2435–2449. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kawahara, K.; Nakamura, T.; Yamada, S.; Nakamura, T.; Abeyama, K.; Hashiguchi, T.; Maruyama, I. High-Mobility Group Box 1 Protein Promotes Development of Microvascular Thrombosis in Rats. J. Thromb. Haemost. 2007, 5, 109–116. [Google Scholar] [CrossRef]
- Bongoni, A.K.; Klymiuk, N.; Wolf, E.; Ayares, D.; Rieben, R.; Cowan, P.J. Transgenic Expression of Human Thrombomodulin Inhibits HMGB1-Induced Porcine Aortic Endothelial Cell Activation. Transplantation 2016, 100, 1871–1879. [Google Scholar] [CrossRef]
- Wu, H.; Li, R.; Pei, L.G.; Wei, Z.H.; Kang, L.N.; Wang, L.; Xie, J.; Xu, B. Emerging Role of High Mobility Group Box-1 in Thrombosis-Related Diseases. Cell. Physiol. Biochem. 2018, 47, 1319–1337. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M. V.; Pedersen, S.; Møgelvang, R.; Skov-Jensen, J.; Flyvbjerg, A. Plasma High-Mobility Group Box 1 Levels Predict Mortality after ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Interv. 2011, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Schulze, J.; Zierath, D.; Tanzi, P.; Cain, K.; Shibata, D.; Dressel, A.; Becker, K. Severe Stroke Induces Long-Lasting Alterations of High-Mobility Group Box 1. Stroke 2013, 44, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Oozawa, S.; Sanoa, S.; Nishibori, M. Usefulness of High Mobility Group Box 1 Protein as a Plasma Biomarker in Patient with Peripheral Artery Disease. Acta Med. Okayama 2014, 68, 157–162. [Google Scholar]
- Wang, M.; Mei, H.; Kou, H.; Deng, J.; Wang, H.; Guo, T.; Hu, Y. Role of Plasma High Mobility Group Box-1 in Disseminated Intravascular Coagulation with Leukemia. Thromb. Res. 2015, 136, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Eskici, Z.M.; Açikgöz, Ş.; Pişkin, N.; Mungan, G.; Can, M.; Güven, B.; Köktürk, F. High Mobility Group B1 Levels in Sepsis and Disseminated Intravascular Coagulation. Acta Biochim. Pol. 2012, 59, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.R.; Kuang, X.Y.; Huang, Y.; Li, N.; Zou, M.X.; Tang, D.L.; Fan, X.G. Potential Role of High Mobility Group Box 1 in Hepatocellular Carcinoma. Cell Adhes. Migr. 2014, 8, 493–498. [Google Scholar] [CrossRef]
- Kostova, N.; Zlateva, S.; Ugrinova, I.; Pasheva, E. The Expression of HMGB1 Protein and Its Receptor RAGE in Human Malignant Tumors. Mol. Cell. Biochem. 2010, 337, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.Q.; Jia, C.Q.; Liu, C.T.; Lu, X.F.; Zhong, N.; Zhang, Z.L.; Fan, W.; Li, Y.Q. Serum High Mobility Group Box Chromosomal Protein 1 Is Associated with Clinicopathologic Features in Patients with Hepatocellular Carcinoma. Dig. Liver Dis. 2008, 40, 446–452. [Google Scholar] [CrossRef]
- Rouhiainen, A.; Imai, S.; Rauvala, H.; Parkkinen, J. Occurrence of Amphoterin (HMG1) as an Endogenous Protein of Human Platelets That Is Exported to the Cell Surface upon Platelet Activation. Thromb. Haemost. 2000, 84, 1087–1094. [Google Scholar] [CrossRef]
- Chen, R.; Kang, R.; Tang, D. The Mechanism of HMGB1 Secretion and Release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tang, Z.Y.; Fan, J.; Wu, Z.Q.; Ji, Y.; Ye, S.L. The Potential of Plasma Thrombomodulin as a Biomarker of Portal Vein Tumor Thrombus in Hepatocellular Carcinoma. J. Cancer Res. Clin. Oncol. 2001, 127, 559–564. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Jia, Q. an; Wang, H.; Hu, C.X.; Sun, D.; Jiang, R. De; Zhang, Z.L. High-Mobility Group Protein Box1 Expression Correlates with Peritumoral Macrophage Infiltration and Unfavorable Prognosis in Patients with Hepatocellular Carcinoma and Cirrhosis. BMC Cancer 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.S.; Zhao, G.; Yu, H.F.; Chen, K.; Dong, J.H.; Tan, J.W. High Expression of AP-4 Predicts Poor Prognosis for Hepatocellular Carcinoma after Curative Hepatectomy. Tumor Biol. 2013, 34, 271–276. [Google Scholar] [CrossRef]
- Maugeri, N.; Campana, L.; Gavina, M.; Covino, C.; De Metrio, M.; Panciroli, C.; Maiuri, L.; Maseri, A.; D’Angelo, A.; Bianchi, M.E.; et al. Activated Platelets Present High Mobility Group Box 1 to Neutrophils, Inducing Autophagy and Promoting the Extrusion of Neutrophil Extracellular Traps. J. Thromb. Haemost. 2014, 12, 2074–2088. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Zmijewski, J.; Xu, Z.; Abraham, E. HMGB1 Develops Enhanced Proinflammatory Activity by Binding to Cytokines. J. Immunol. 2008, 180, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Coleman, L.G.; Maile, R.; Jones, S.W.; Cairns, B.A.; Crews, F.T. HMGB1/IL-1β Complexes in Plasma Microvesicles Modulate Immune Responses to Burn Injury. PLoS One 2018, 13, 1–23. [Google Scholar] [CrossRef]
- Han, J.B.; Shu, Q.H.; Zhang, Y.F.; Yi, Y.X. Predictive Value of Inflammation Biomarkers in Patients with Portal Vein Thrombosis. J. Clin. Transl. Hepatol. 2021, 9, 384–391. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD Guidelines for the Treatment of Hepatocellular Carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).