1. Introduction
Among older adults diagnosed with sarcopenia, the incidence of falls and the resulting injuries emerge as a paramount issue [1,2]. This risk is probably compounded by obesity, a condition marked by an excessive accumulation of adipose tissue, which imposes increased mechanical strain on the musculoskeletal system [3]. Sarcopenic obesity (SO), defined as the coexistence of muscle loss (i.e., sarcopenia) and excess body fat (i.e., obesity) among older adults, therefore presents a complex challenge, exacerbating mobility issues and affecting daily activities [4]. In this context, Clark et al. [5] emphasized that the accumulation of intramuscular fat results in a substantial and consequential reduction in muscle strength. What's particularly intriguing is that this decline surpasses the natural age-related decrease in muscle mass. These findings allude to complex underlying factors, including the potential reduction in muscle strength, which warrants further investigation.
Muscle strength serves as a critical benchmark for physical well-being, but it experiences a discernible reduction due to sarcopenia [3,6]. However, recent research has shown a burgeoning interest in rapid force markers, especially the rate of force development (RFD, denoted as Δ force/Δ time [7–9]. The RFD parameter zeroes in on the force produced during the crucial initial 200 ms when a muscle is activated [10]. Remarkably, this rate is even more sensitive to the effects of aging than conventional measures like maximal strength [8,9]. Emerging studies posit RFD as an influential predictor of certain functional attributes in older adults, particularly emphasizing its correlation with walking speed [5,8]. Furthermore, a diminished ability in swift force generation within the 100-200 ms timeframe after a misstep might be a key factor in the diminished capacity of older adults to counteract falls [9]. Evaluations of rapid torque typically encompass various contraction intervals of muscles, specifically the early (0-50 ms) and the latter (100-200 ms) phases [11]. The preliminary phase correlates with the onset of motor unit activation and their respective firing sequences, as well as intrinsic muscle characteristics like fiber composition and calcium dynamics [12]. Conversely, the latter segment largely draws influence from elements such as peak strength and the overall muscular structure [13].
Prior research has documented age-related declines in maximal voluntary isometric strength in lower limb muscles, including leg flexors [11], dorsiflexors [12], and plantar flexors [14]. Interestingly, it has been observed that the reduction in rate of force development (RFD) during aging can be more pronounced (39–64%) than the decrease in maximal isometric strength (29–46%) [9,11]. These findings are of great significance, as the capacity to swiftly generate force is crucial for various everyday activities [9]. When considering the impact of obesity on muscle strength in older adults with sarcopenia, several factors come into play, helping to elucidate the reasons behind the potential disparity in muscle strength between obese and non-obese individuals with sarcopenia. The infiltration of fat within skeletal muscles diminishes the contractile component of the overall muscle volume [15], thereby diminishing the inherent strength of the muscle as a whole[3]. Recently, our research has shown that older adults with SO possess lower relative maximal plantar flexor strength [16]. However, it remains unknown whether obesity in older adults with sarcopenia exacerbates the decline in RFD. This is particularly pertinent, as older adults with SO are at a higher risk of falls [17], and RFD is not only crucial for fall prevention but also for successfully executing various functional tasks that prioritize rapid muscle power responses over sustained strength [14].
Walking speed, a critical performance determinant, encapsulates more than just physical capabilities; it’s closely intertwined with broader aspects of health, such as autonomy, fall risk, and even longevity [15–17]. Despite its significance, there remains a paucity of exhaustive research exploring the correlation between RFD and walking speed, particularly in older adults with SO. We still lack comprehensive studies examining how RFD influences walking speed, especially in older adults with SO. Additionally, an essential question remains unanswered: does obesity impact the RFD in a discernible way? It's yet to be ascertained whether obesity exacerbates, mitigates, or remains neutral to the changes in RFD observed in older adults. Addressing this problem is crucial for targeted interventions, and to ensure that therapeutic efforts are grounded in a complete understanding of the dynamics at play.
The objectives of this study were to: i) to investigate the influence of obesity on neuromuscular markers in older adults. This objective aims to decipher whether obesity compounds the neuromuscular challenges already posed by sarcopenia. ii) to examine the relationship between neuromuscular markers and gait speed in older adults with SO. This will determine whether changes in neuromuscular health directly affect walking speed, which is a pivotal determinant of performance and overall functionality.
2. Materials and Methods
2.1. Study design
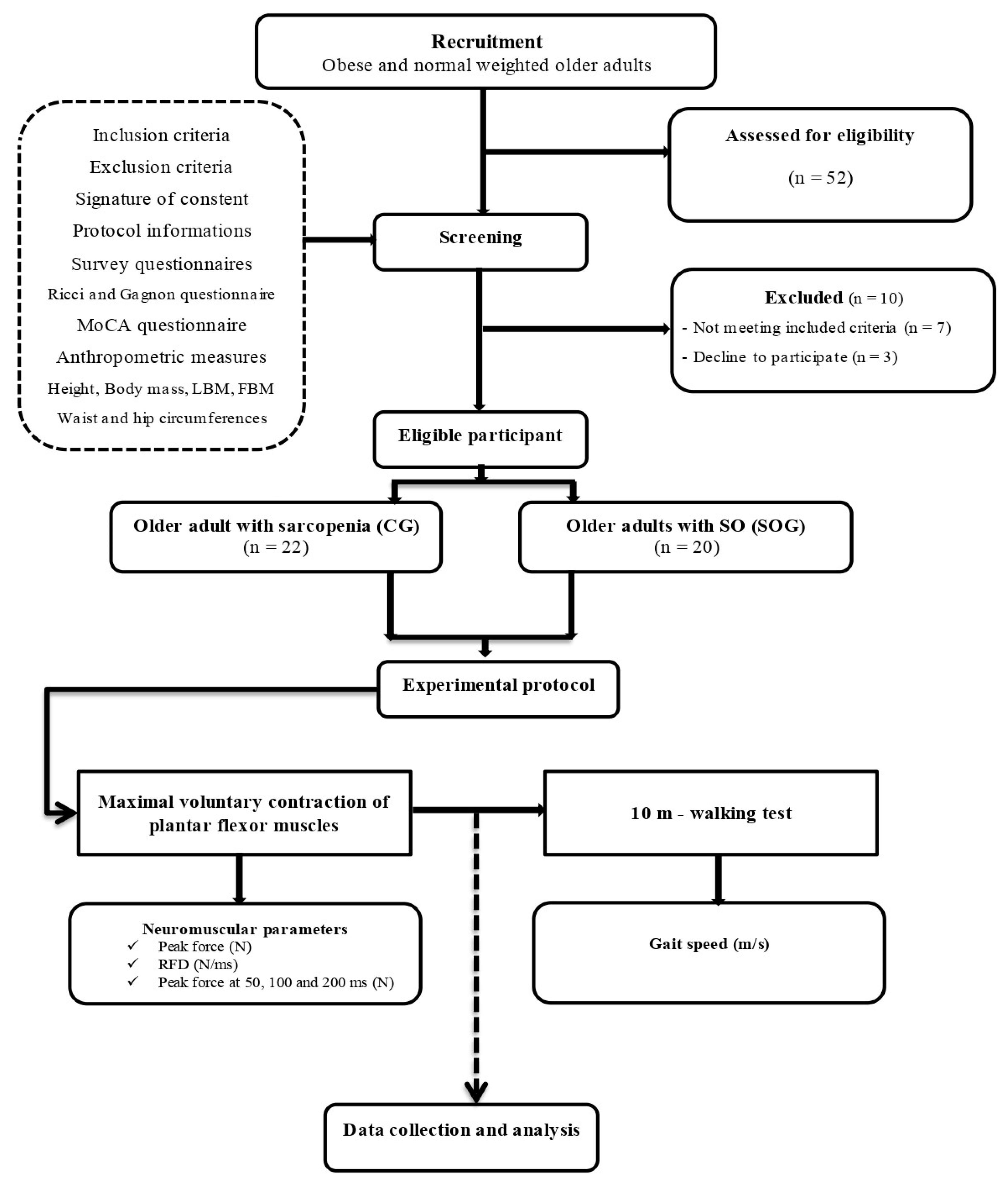
This study was conducted in alignment with the Helsinki Declaration and was approved by the Ethics Committee of South Ethics Committee for the Protection of Persons (No. 0477/2022), registered in the Pan African Clinical Trials Registry with the registration number PACTR202306912191110 and organized in compliance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines [22]. The research protocol, patient information letter, and informed consent form also received their endorsement. The study was designed following a single-blinded, analytical cross-sectional approach, as depicted in
Figure 1.
Notably, the experimenter, a physiotherapist with five years of experience, upheld single-blinding, possessing knowledge of participants' group affiliations, while the investigator remained uninformed about this. In terms of data management, information underwent thorough processing in an anonymous and consolidated manner to protect participants' identities. The study began with a 4-week recruitment period, followed by a 3-week screening phase, and concluded with a 9-week experimental testing stage. These sessions, each lasting approximately 2 hours, involved a thorough evaluation, encompassing health status questionnaires, anthropometric measurements, a 10-meter walking test, and evaluation of the neuromuscular markers of the plantar flexor muscles.
2.2. Recruitments
Participants were systematically recruited from several care centers throughout the Tunis region between 1st and 31st May 2022. Recruitment was accomplished by disseminating study participation offers in clinical centers, and subsequently, the medical staff compiled a list of volunteers to the experimenter. To be eligible, individuals needed to have a Body Mass Index (BMI) over 30 kg/m², a Handgrip force of less than 17 N [23], a gait speed of under 1.0 m/s [23], be aged above 65, be able to verbally communicate effectively with the research team and possess physical independence. Those with neurological or cognitive conditions, severe cardiovascular diseases, prominent lower limb musculoskeletal issues, other significant co-morbidities, or chronic diseases, or on medications that might affect the testing were excluded. Additionally, a Montreal Cognitive Assessment (MoCA) score below 26 was another exclusion criterion [24]. Every participant's eligibility was meticulously cross-checked using a detailed questionnaire, subsequently reviewed by medical experts from the respective facilities.
2.3. Evaluation protocol
All evaluations were conducted in a designated clinical examination room under consistent environmental conditions, overseen by a singular, trained assessor. Participants were given a standardized set of verbal instructions before the assessments to ensure familiarity with the procedures.
To begin, the MoCA test and the Ricci and Gagnon scale were employed to gauge participants' cognitive and physical activity levels. Following this, participants' body mass (BM) and fat body mass (FBM, %) were measured using an impedance-meter (Tanita; SC 240-Class III;Tanita Europe B.V., Amsterdam, The Netherlands). FBM and lean body mass (LBM) were calculated using the equations from [55]: FBM = body fat (%) × body mass; and LBM = body mass – FBM. Body height (H) were accurately measured using a digital floor scale. This data was then used to compute the Body Mass Index (BMI) as: BMI (kg/m²) = BM (kg)/H² (m)
A dynamometer (EH101, Xiangshan Inc., Guangdong, China) was used to evaluate the handgrip strength (HS, N). Participants, while seated, had their elbows flexed at 110°, wrists in a neutral position, and the interphalangeal joint of the index finger set at 90°. Both hands were tested three times, with the highest reading from either hand being chosen for subsequent analysis.
Gait speed was evaluated using a standardized 20-meter corridor walk. Participants were asked to reach their maximum walking speed. However, only the speed captured between the 5th and 15th meters was used for analysis, eliminating the acceleration and deceleration phases, to get a true representation of their consistent walking speed (m/s) [25].
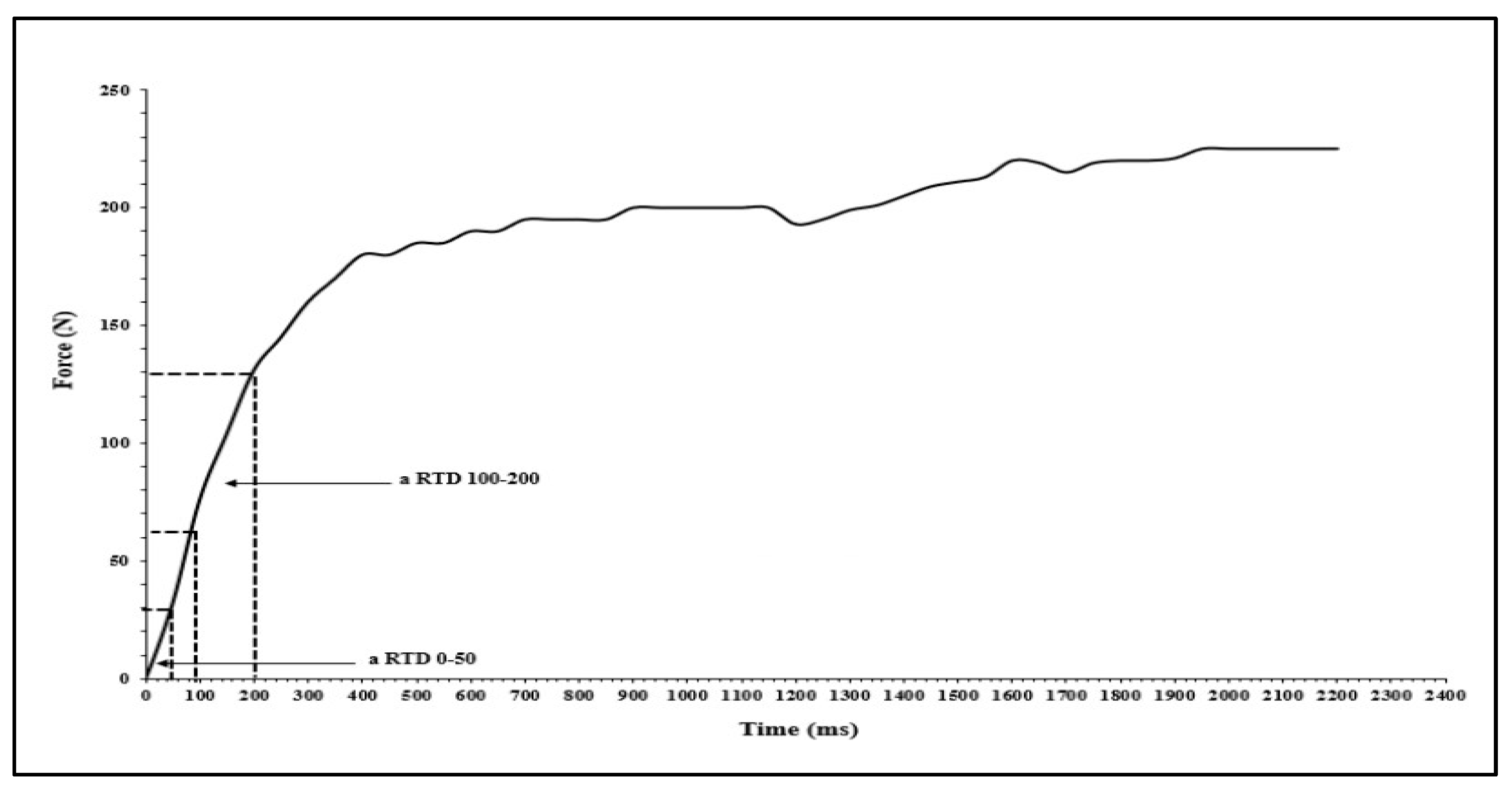
The isometric strength of the ankle plantar flexor muscles in the dominant leg was measured during maximal voluntary contractions (MVC) using a dynamometer (K-Force, Kinvent, Montpellier, France). Participants were instructed to maintain contact between their back, buttocks, and thigh with the chair while keeping their leg stretched horizontally. They were then asked to push with the tips of their foot against the dynamometer [14]. Two trials were performed with a 1-minute rest interval in between, and the peak force value from the two trials was recorded for PF (aPeak, N). The relative peak force (rPeak, N/kg) was calculated by normalizing the peak force to the participant's body mass (aPeak /BM, N/kg). The dynamometer signals were stored offline for subsequent analysis. Absolute force was recorded at 50 ms (a50), 100 ms (a100) and 200 ms (a200). Absolute forces were normalized to BM (r50, r100, r200, respectively) and to MVC (50MVC, 100MVC, 200MVC, %). Early rate of force development (RFD) was also obtained from each MVC contraction onset to 50 ms (RFD50-100). Late RFD was acquired from 100 to 200 ms (RFD100-200) (
Figure 2). All RFD was calculated from the linear slop of the force – time curve (Δ force/Δ time).
2.4. Statistical analysis
The sample size was calculated using the freeware G*Power (version 3.1.9.4) [26]. The ANOVA test was predefined for power analysis. The estimation was based on predefined control of type I error (alpha = 0.05) and Type II error (beta = 0.60), with a moderate level of estimated effect size (r = 0.35) [23]. Under these settings, 40 participants were required as the minimum sample size. For statistical analyses, we employed Jamovi (Software 2.3, Sydney, Australia). We began by implementing the Shapiro-Wilk and Levene tests to ascertain data normality and variance homogeneity, respectively. Once it was confirmed that our data adhered to these assumptions, we proceeded with the t-test for independent samples to identify variances between the groups. A Pearson correlation analysis was subsequently conducted to pinpoint parameters with a strong association to gait speed in SOGusing the heatmap correlation analysis (
Figure 4). In the subsequent steps, we introduced multiple logistic regression models, aiming to discern the model exhibiting the strongest correlation with gait speed in SOG.
3. Results
An initial group of 52 volunteers was recruited. Following a thorough application of inclusion and exclusion criteria, 45 participants met the study's qualifications. Regrettably, three individuals could not meet the study requirements, resulting in a final cohort of 42 dedicated participants who completed the study in its entirety. These participants were subsequently categorized based on their BMI. The control group, consisting of non-obese older adults with sarcopenia, comprised 22 individuals, with 10 men and 12 women. In contrast, the sarcopenic obese group (SOG) consisted of 20 individuals, evenly divided between 10 men and 10 women (
Table 1).
Table 1.
Anthropometric characteristics of groups.
Table 1.
Anthropometric characteristics of groups.
| |
95%
Confidence Interval
|
|
| |
group
|
N
|
Mean
|
Lower Limit
|
Upper Limit
|
Standard-deviation
|
Minimum
|
Maximum
|
|
Age (y)
|
|
SOG
|
|
20
|
|
77.71
|
|
76.33
|
|
79.09
|
|
2.95
|
|
72.80
|
|
83.00
|
|
| |
|
CG
|
|
22
|
|
81.13
|
|
79.35
|
|
82.91
|
|
4.02
|
|
74.90
|
|
88.00
|
|
|
H (cm)
|
|
SOG
|
|
20
|
|
162.87
|
|
159.92
|
|
165.82
|
|
6.29
|
|
152.60
|
|
173.50
|
|
| |
|
CG
|
|
22
|
|
166.03
|
|
162.69
|
|
169.37
|
|
7.54
|
|
155.80
|
|
177.20
|
|
|
BM
|
|
SOG
|
|
20
|
|
90.98
|
***
|
89.14
|
|
92.82
|
|
3.92
|
|
84.20
|
|
96.80
|
|
| |
|
CG
|
|
22
|
|
68.74
|
|
66.28
|
|
71.20
|
|
5.54
|
|
60.40
|
|
79.00
|
|
|
BMI (kg/m²)
|
|
SOG
|
|
20
|
|
34.46
|
***
|
32.94
|
|
35.98
|
|
3.25
|
|
30.11
|
|
41.31
|
|
| |
|
CG
|
|
22
|
|
25.13
|
|
23.64
|
|
26.61
|
|
3.35
|
|
19.59
|
|
30.52
|
|
|
Body fat (%)
|
|
SOG
|
|
20
|
|
35.00
|
***
|
32.03
|
|
37.97
|
|
6.34
|
|
24.30
|
|
45.60
|
|
| |
|
CG
|
|
22
|
|
17.72
|
|
16.84
|
|
18.60
|
|
1.98
|
|
14.30
|
|
20.90
|
|
|
FBM (kg)
|
|
SOG
|
|
20
|
|
32.03
|
***
|
28.78
|
|
35.27
|
|
6.93
|
|
21.75
|
|
44.14
|
|
| |
|
CG
|
|
22
|
|
12.16
|
|
11.49
|
|
12.84
|
|
1.51
|
|
9.22
|
|
14.69
|
|
|
LBM (kg)
|
|
SOG
|
|
20
|
|
58.95
|
|
57.01
|
|
60.90
|
|
4.15
|
|
52.66
|
|
67.75
|
|
| |
|
CG
|
|
22
|
|
56.58
|
|
54.36
|
|
58.79
|
|
4.99
|
|
48.44
|
|
65.91
|
|
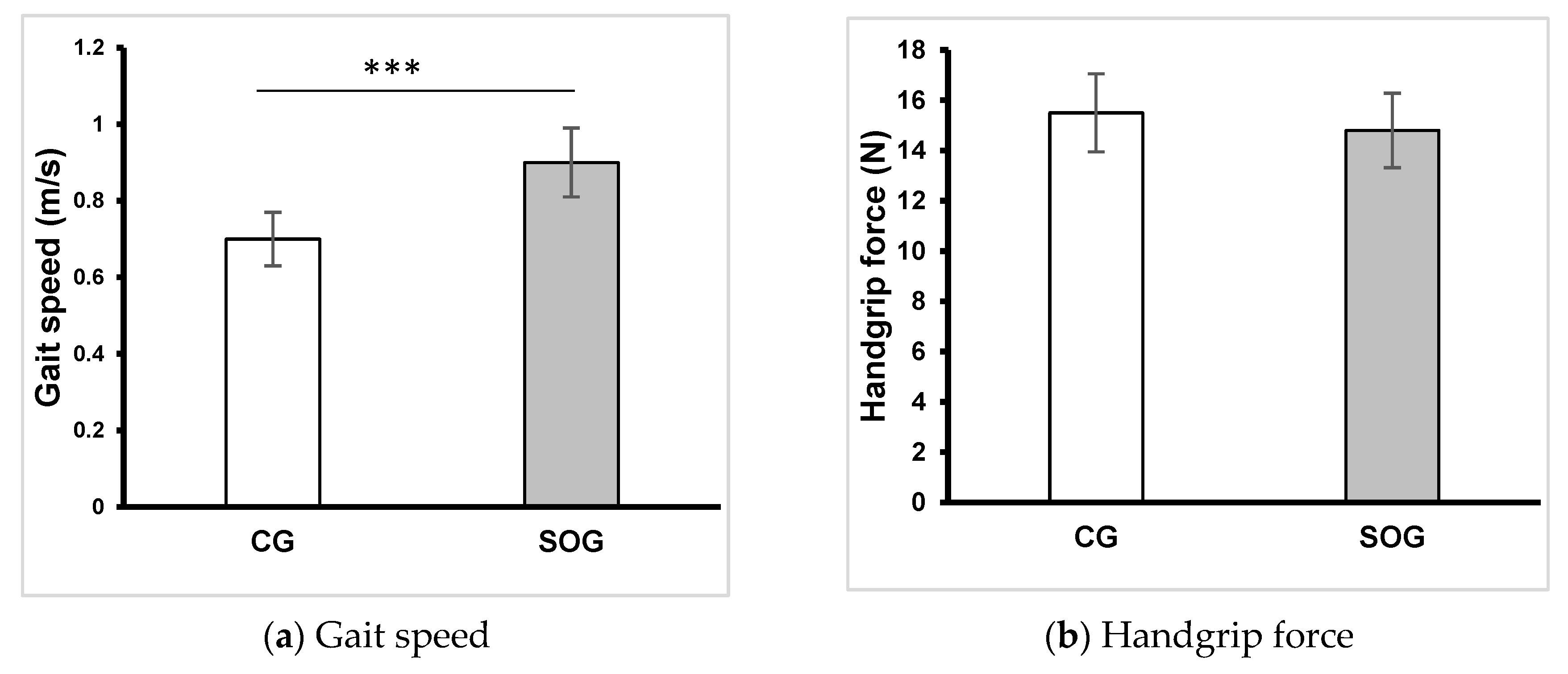
Figure 3.
Gait speed and handgrip force of groups. CG: control group, SOG: older adults with sarcopenic obesity group, *: p < .05, **: p < 0.1, ***: p < .001.
Figure 3.
Gait speed and handgrip force of groups. CG: control group, SOG: older adults with sarcopenic obesity group, *: p < .05, **: p < 0.1, ***: p < .001.
In relation to anthropometric measurements, statistical analysis showed no significant differences in age, body height and LBM. Nonetheless, BM (p <0.001; d = 4.6), BMI (p <0.001; d = 2.8), body fat (p <0.001; d = 3.7) and FBM (p <0.001; d = 4.0) were notably higher in the SOG compared to the CG. The outcomes for gait speed and handgrip force are depicted in
Figure 3. While no significant difference between the groups was observed for handgrip force, the gait speed was notably lower in the SOG compared to the CG (p <0.001; d = -1.63). The analysis of the Ricci and Gagnon questionnaire revealed that CG (10.9 ± 2.5) and SOG (9.7 ± 3.4) were inactive.
Table 2.
Neuromuscular parameters of groups.
Table 2.
Neuromuscular parameters of groups.
| |
Intervalle de confiance à 95%
|
|
| |
group
|
N
|
Mean
|
Lower Limit
|
Upper Limit
|
Standard-deviation
|
Minimum
|
Maximum
|
|
aPeak (N)
|
|
SOG
|
|
20
|
|
192.69
|
|
185.47
|
|
199.90
|
|
15.41
|
|
165.40
|
|
230.10
|
|
| |
|
CG
|
|
22
|
|
194.89
|
|
189.01
|
|
200.76
|
|
13.28
|
|
176.40
|
|
230.10
|
|
|
rPeak (N)
|
|
SOG
|
|
20
|
|
2.12
|
***
|
2.01
|
|
2.23
|
|
0.22
|
|
1.70
|
|
2.55
|
|
| |
|
CG
|
|
22
|
|
2.86
|
|
2.69
|
|
3.02
|
|
0.36
|
|
2.2
|
|
3.68
|
|
|
a50 (N)
|
|
SOG
|
|
20
|
|
33.80
|
***
|
32.56
|
|
35.04
|
|
2.65
|
|
28.94
|
|
40.26
|
|
| |
|
CG
|
|
22
|
|
31.18
|
|
30.24
|
|
32.12
|
|
2.12
|
|
28.22
|
|
36.81
|
|
|
r50 (N/kg)
|
|
SOG
|
|
20
|
|
0.37
|
***
|
0.35
|
|
0.39
|
|
0.03
|
|
0.30
|
|
0.45
|
|
| |
|
CG
|
|
22
|
|
0.45
|
|
0.43
|
|
0.48
|
|
0.05
|
|
0.40
|
|
0.58
|
|
|
a100 (N)
|
|
SOG
|
|
20
|
|
69.53
|
*
|
66.98
|
|
72.08
|
|
5.45
|
|
59.54
|
|
82.83
|
|
| |
|
CG
|
|
22
|
|
73.51
|
|
71.41
|
|
75.61
|
|
4.73
|
|
65.26
|
|
85.13
|
|
|
r100 (N/kg)
|
|
SOG
|
|
20
|
|
0.76
|
***
|
0.72
|
|
0.80
|
|
0.08
|
|
0.62
|
|
0.92
|
|
| |
|
CG
|
|
22
|
|
1.07
|
|
1.02
|
|
1.13
|
|
0.12
|
|
0.88
|
|
1.33
|
|
|
a200 (N)
|
|
SOG
|
|
20
|
|
113.96
|
***
|
109.78
|
|
118.14
|
|
8.93
|
|
97.58
|
|
135.75
|
|
| |
|
CG
|
|
22
|
|
124.73
|
|
120.97
|
|
128.49
|
|
8.48
|
|
112.89
|
|
147.26
|
|
|
r200 (N/kg)
|
|
SOG
|
|
20
|
|
1.25
|
|
1.19
|
|
1.31
|
|
0.13
|
|
1.02
|
|
1.51
|
|
| |
|
CG
|
|
22
|
|
1.82
|
|
1.731
|
|
1.92
|
|
0.21
|
|
1.48
|
|
2.35
|
|
|
50MVC (%)
|
|
SOG
|
|
20
|
|
17.60
|
|
16.87
|
|
18.33
|
|
1.56
|
|
15.84
|
|
20.40
|
|
| |
|
CG
|
|
22
|
|
16.02
|
|
15.60
|
|
16.45
|
|
0.96
|
|
14.66
|
|
18.08
|
|
|
100MVC (%)
|
|
SOG
|
|
20
|
|
36.14
|
|
35.20
|
|
37.08
|
|
2.01
|
|
29.88
|
|
39.18
|
|
| |
|
CG
|
|
22
|
|
37.81
|
|
36.67
|
|
38.95
|
|
2.57
|
|
32.89
|
|
41.53
|
|
|
200MVC (%)
|
|
SOG
|
|
20
|
|
59.40
|
*
|
56.76
|
|
62.05
|
|
5.64
|
|
48.38
|
|
69.17
|
|
| |
|
CG
|
|
22
|
|
64.16
|
|
62.06
|
|
66.25
|
|
4.72
|
|
56.50
|
|
74.70
|
|
|
RFD 50/100 (N/ms)
|
|
SOG
|
|
20
|
|
676.06
|
***
|
651.25
|
|
700.86
|
|
53.00
|
|
578.90
|
|
805.35
|
|
| |
|
CG
|
|
22
|
|
708.19
|
|
691.25
|
|
725.14
|
|
38.21
|
|
644.48
|
|
816.32
|
|
|
RFD 100/200 (N/ms)
|
|
SOG
|
|
20
|
|
444.26
|
***
|
406.11
|
|
482.41
|
|
81.50
|
|
284.97
|
|
616.35
|
|
| |
|
CG
|
|
22
|
|
512.11
|
|
477.47
|
|
546.75
|
|
78.13
|
|
356.31
|
|
641.55
|
|
Table 3.
Correlation analysis between neuromuscular parameters and gait speed.
Table 3.
Correlation analysis between neuromuscular parameters and gait speed.
| |
|
RFD50/100 |
RFD100/200 |
r50 |
r100 |
r200 |
rPeak |
r50/MVC |
r100/MVC |
r200/MVC |
| Gait speed |
r |
0,84 |
0,83 |
0,77 |
0,66 |
0,75 |
0,6 |
0,32 |
0,26 |
0,35 |
| p |
<.001 |
<.001 |
<.001 |
,001 |
<.001 |
,005 |
,164 |
,263 |
,129 |
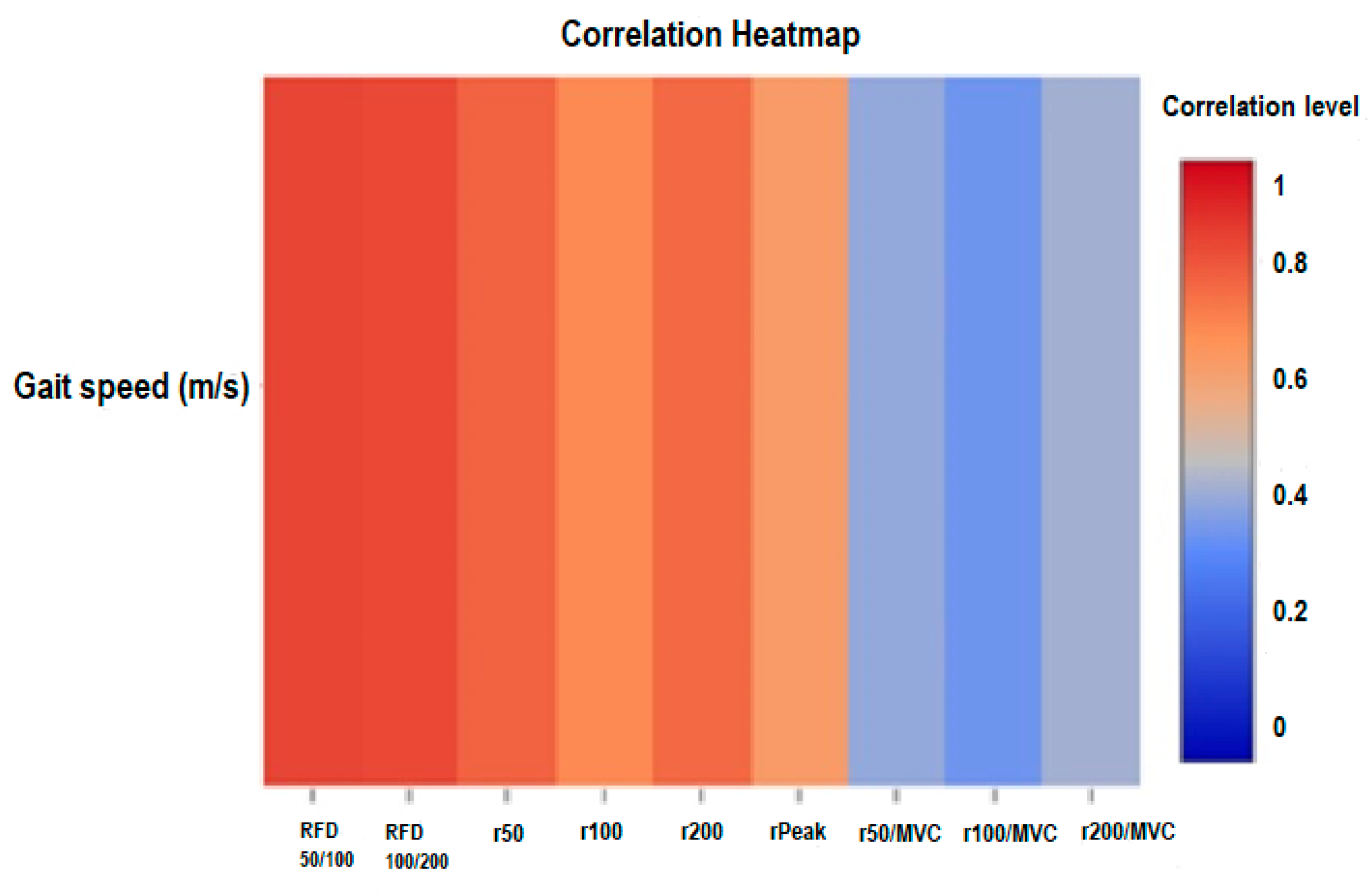
Figure 4.
Correlation heatmap analysis of gait speed and neuromuscular paramaeters of older adults with sarcopenic obesity. RFD: rate of force development, MVC: maximal voluntary contraction, a50, a100, a200: absolute peak force at 50, 100 and 200ms, r50, r100, r200: relative peak force at 50, 100 and 200 ms, aPeak: absolute peak force, rPeak: relative peak force.
Figure 4.
Correlation heatmap analysis of gait speed and neuromuscular paramaeters of older adults with sarcopenic obesity. RFD: rate of force development, MVC: maximal voluntary contraction, a50, a100, a200: absolute peak force at 50, 100 and 200ms, r50, r100, r200: relative peak force at 50, 100 and 200 ms, aPeak: absolute peak force, rPeak: relative peak force.
The logistic regression analysis assessed the impact of various neuromuscular parameters on gait speed. Multiple models were analyzed to deduce the combination of variables that best predicted gait speed (
Table 4).
Neuromuscular parameters of the plantar muscle during MVC are detailed in
Table 2. While there was no significant difference between the groups in terms of aPeak, the measures for a50 (p <0.001; d = -1.09), a100 (p = 0.015; d = -0.78), and a200 (p <0.001; d = -1.24) were elevated in the CG relative to the SOG. When normalized for BM, the metrics r50 (p <0.001; d = -1.80), r100 (p <0.001; d = -2.89), and r200 (p <0.001; d = -3.13) were also heightened in the CG in comparison to the SOG. Conclusively, RFD50/100 and RFD100/200 values were diminished in the SOG compared to the CG (p <0.001; d = -2.31 and p <0.001; d = -1.10, respectively).
Gait speed demonstrated significant correlations with several neuromuscular markers (
Table 3). The strongest correlation was observed with RFD
50/100 (r = 0.84, p <0.001)
Figure 4. Similarly, the correlation with RFD
100/200 was also notably high with an (r = 0.83, p <0.001). Further associations included r50 (r = 0.77, p <0.001), r100 (r = 0.66, p = 0.001), r200 (r = 0.75, p <0.001). However, when considering normalized values to MVC, correlations between gait speed and r50/MVC (r = 0.32, p = 0.164), r100/MVC (r = 0.26, p = 0.263), and r200/MVC (r = 0.35, p = 0.129) were less pronounced and not statistically significant.
Table 4.
Logistic regression analysis assessed the impact of various neuromuscular parameters on gait speed in older adults with sarcopenic obesity .
Table 4.
Logistic regression analysis assessed the impact of various neuromuscular parameters on gait speed in older adults with sarcopenic obesity .
| |
|
|
Unstandardized
Coefficients
|
Standardized
Coefficients
|
|
|
|
95% confidence interval for B |
Model summary |
| |
|
Model |
B |
Beta |
Standard error |
t |
p |
lower bound |
upper bound |
R |
R² |
Adjusted R² |
Standard error |
| Gait speed (m/s) |
1 |
(Constant) |
-0.46 |
|
0.18 |
-2.58 |
0.019 |
-0.830 |
-0.08 |
0,84 |
0,7 |
0,69 |
0,06 |
| RFD50/100 (N/ms) |
0 |
0.84 |
0 |
6.55 |
<0.001 |
|
0 |
| 2 |
(Constant) |
-0,28 |
|
0,15 |
-1,89 |
,076 |
-0,6 |
0,03 |
0,91 |
0,82 |
0,8 |
0,05 |
| RFD50/100 (N/ms) |
0 |
0,51 |
0 |
3,61 |
,002 |
0 |
0 |
| RFD100/200 (N/ms) |
0 |
0,48 |
0 |
3,39 |
,004 |
0 |
0 |
| 3 |
(Constant) |
-0,51 |
|
0,14 |
-3,55 |
,003 |
-0,81 |
-0,2 |
0,94 |
0,89 |
0,87 |
0,04 |
| RFD50/100 (N/ms) |
0 |
0,51 |
0 |
4,45 |
<.001 |
0 |
0 |
| RFD100/200 (N/ms) |
0 |
0,35 |
0 |
2,8 |
,013 |
0 |
0 |
| rPeak (N) |
0,14 |
0,29 |
0,05 |
3,06 |
,008 |
0,04 |
0,24 |
| 4 |
(Constant) |
-0,47 |
|
0,16 |
-3,01 |
,009 |
-0,81 |
-0,14 |
0,95 |
0,89 |
0,86 |
0,04 |
| RFD50/100 (N/ms) |
0 |
0,46 |
0 |
3,19 |
,006 |
0 |
0 |
| RFD100/200 (N/ms) |
0 |
0,38 |
0 |
2,58 |
,022 |
0 |
0 |
| r50 (N) |
0,3 |
0,1 |
0,42 |
0,71 |
,487 |
-0,61 |
1,21 |
| r100 (N) |
-0,15 |
-0,1 |
0,25 |
-0,57 |
,575 |
-0,69 |
0,4 |
| 5 |
(Constant) |
-0,5 |
|
0,14 |
-3,52 |
,003 |
-0,81 |
-0,2 |
0,95 |
0,89 |
0,87 |
0,04 |
| RFD50/100 (N/ms) |
0 |
0,43 |
0 |
3,04 |
,008 |
0 |
0 |
| RFD100/200 (N/ms) |
0 |
0,34 |
0 |
2,77 |
,014 |
0 |
0 |
| r200 (N) |
0,12 |
0,14 |
0,11 |
1,07 |
,302 |
-0,12 |
0,35 |
| rPeak (N) |
0,13 |
0,26 |
0,05 |
2,64 |
,019 |
0,02 |
0,23 |
In the first model, with RFD50/100 as the sole predictor, the equation was: Gait speed = - 0.46 + 0.84 RFD50/100 with R² = 0.7, indicating that approximately 70% of the variance in gait speed could be explained by RFD50/100 alone (p <0.001). The second model combined RFD50/100 and RFD100/200. In this model, Gait speed = - 0.28 + 0.51 RFD50/100+ 0.48 RFD100/200, had an R² = 0.82, showing an improved explanatory power over the first model. Both predictors were statistically significant (p <0.001). In the third model, the equation was: Gait Speed = - 0.51 + 0.51 RFD50/100 + 0.35 RFD100/200 + 0.29 rPeak. This model, with an R² = 0.89, included RFD50/100, RFD100/200, with all three variables having a significant influence on gait speed (p <0.001). The fourth model was more comprehensive and included r50 and r100 alongside RFD50/100 and RFD100/200. However, while RFD50/100 and RFD100/200 remained significant (p <0.001), r50 and r100 did not significantly influence gait speed in this model. The final model considered was: Gait Speed = - 0.5 + 0.43 RFD50/100 + 0.34 RFD100/200 + 0.26 rPeak + 0.14 r200. With an R² = 0.90, this model, which incorporated RFD50/100, RFD100/200, rPeak, and r200, explained 90% of the variability in gait speed. However, only RFD50/100, RFD100/200, and rPeak were significant predictors in in this configuration. Among the assessed models, the third, which integrated RFD50/100, RFD100/200, and rPeak, showcased the most compelling adjusted R² = 0.89. In this configuration, RFD50/100 emerged as the predominant influencer on gait speed, accounting for 51% of its variance. Concurrently, RFD100/200 and rPeak contributed to 35% and 29% of the gait speed variation in the SOG group, respectively.
4. Discussion
The primary aim was to discern the impact of obesity on neuromuscular markers of plantar flexors in older adults. The findings underscored the significant influence of obesity on neuromuscular parameters, particularly the RFD and relative maximal and submaximal force. Our secondary objective delved into the relationship between these neuromuscular markers and gait speed. Notably, RFD stood out as the predominant factor affecting gait speed, contributing to a notable 51% of its variability, which notably surpassed the impact of relative maximal force.
Our study found that obesity had no significant impact on the absolute peak force generated by the plantar flexor muscles, a finding consistent with the results of Maktouf et al. (2018), who also observed no differences between older adults with normal weight and their obese counterparts [27]. However, when forces were normalized to body mass, both maximal and submaximal peak forces in plantar flexors were notably lower. This is in alignment with multiple studies examining the effects of obesity on young adults [28–30]. This phenomenon may be partially explained by the observations of [31], who noted that the most significant impact of combined aging and adiposity was evident in the rate of muscle volume loss. One plausible mechanism underlying this accelerated muscle loss is that obesity exacerbates the challenges posed by sarcopenia [3,25]. It does so by exerting additional mechanical stress on the musculoskeletal system, particularly due to the need to support elevated adipose tissue weight [32]. Beyond serving as mere energy storage, adipose tissue is a dynamic endocrine organ that secretes an array of hormones and pro-inflammatory cytokines, thereby amplifying biochemical stress in the body [33]. Chronic adiposity results in elevated levels of circulating pro-inflammatory cytokines such as TNF-α, IL-1α, IL-6, and CRP, which contribute to both acute and chronic systemic inflammation [34]. These inflammatory agents negatively impact skeletal muscle by promoting protein degradation over synthesis, ultimately leading to muscle wasting or atrophy [35]. Further complicating the scenario is the association between obesity and a decline in anabolic hormones, specifically insulin-like growth factor-1 (IGF-1), which plays a crucial role in muscle repair and growth [36].
The innovative aspect of this study centers on the assessment of the RFD, which we identify as a crucial parameter for evaluating mobility in vulnerable populations. Our findings reveal a marked influence of obesity on RFD metrics. Specifically, the values for RFD50/100 and RFD100/200 decreased by 5% and 13%, respectively, in the SOG when compared to the CG. In a related vein, Olmos et al. (2020) highlighted that late rapid force parameters are disproportionately compromised in older adults without obesity. Based on these observations, we propose that the detrimental effects on early rapid force are likely obesity-specific in older adults with sarcopenia, while the impact on late rapid force seems to exacerbate existing sarcopenia-related impairments. The impact of obesity on both early and late rapid force in older adults with sarcopenia can be elucidated by examining multiple factors, such as motor unit recruitment [37], intrinsic muscle properties [6,31], and systemic inflammation [3,35]. Early rapid force is predominantly associated with initial motor unit recruitment and firing rates, as well as intrinsic muscle attributes such as fiber type composition and calcium kinetics [12]. Obesity might specifically affect these early markers due to the increased mechanical stress it places on the musculoskeletal system, thereby affecting the efficiency of motor unit recruitment and the contractile properties of muscle fibers. Moreover, adipose tissue in obese individuals functions as a dynamic endocrine organ, secreting an array of hormones and pro-inflammatory cytokines, like TNF-a, IL-1a, IL-6, and CRP [35]. This heightened state of systemic inflammation could adversely affect early rapid force by causing protein degradation to outpace synthesis, leading to muscle atrophy and reduced contractile capabilities. On the other hand, late rapid force measures are more strongly influenced by factors such as maximal strength, muscle size, tendon stiffness, and pennation angle [13]. Obesity can exacerbate the loss of muscle mass—often referred to as sarcopenia—which is already compromised in older adults with sarcopenia. The compounded effect of obesity and aging leads to a decline in maximal strength and muscle size, which in turn, significantly impacts late rapid force measures. The accelerated muscle loss may be further exacerbated by chronic inflammation and a decline in anabolic hormones like insulin-like growth factor-1 (IGF-1), which are crucial for muscle repair and growth [31,35].
Our study brings forth compelling evidence that the RFD is an invaluable predictor of gait speed, especially among older adults with SO. When integrating variables such as RFD50/100, RFD100/200, and rPeak, we obtained an impressively high adjusted R² value of 0.89. Strikingly, the early rapid RFD, represented by RFD50/100, was the dominant determinant, accounting for 51% of the variation in gait speed within this vulnerable population. Additionally, RFD100/200 and rPeak contributed to the explanatory power by 35% and 29%, respectively. These results are in harmony with earlier studies on frail populations, which have demonstrated RFD to be an independent predictor of an array of physical functions [6,38]. These include abilities as varied as rising from a chair, completing timed "up and go" tests, and achieving both casual and maximal walking speeds in older adults with regular weight [8]. Moreover, our findings echo other research that emphasizes the significant role of muscle power, rather than mere muscle strength, in affecting walking velocity and, by extension, susceptibility to falls [7,8].
A critical question that warrants discussion is why RFD is a more salient predictor of gait speed than maximal force. One plausible explanation is that quick force generation could constrain an individual's capacity to engage in rapid movements, especially in activities requiring sequential agonist and antagonist muscle contractions, such as walking [9]. Many daily activities necessitate the rapid application of force over a short duration—high RFD capacity, in other words. For instance, a noticeable surge in force within approximately 200 milliseconds is essential when an older adult stands up from a seated position [7]. Furthermore, the ability to avert a fall is not solely reliant on the production of maximal force but is also contingent on the speed of motor response [39]. This underscores the functional significance of the rate at which sub-maximal force can be generated, i.e., RFD. Hence, RFD serves a dual role: it is not only a performance determinant in functional tasks that demand more power than force but also a pivotal metric for assessing fall risk [8,9].
4.1. Limitations and perspectives
Our study has several limitations that warrant attention. First and foremost, the small cohort size restricts the generalizability of our findings, particularly given the heterogeneity often observed in older adults and obese individuals. These populations can sometimes present a complex interplay of factors, resulting in characteristics that may not be wholly reflective of those in our study sample. Another key limitation lies in the non-utilization of electromyography (EMG), which could have provided further insights into the neurological mechanisms involved in force development and muscle function. The exclusion of this analytical tool leaves certain questions unanswered and calls for additional research to elucidate these mechanisms more comprehensively. These limitations pave the way for future studies aimed at addressing these gaps. Larger, more diverse cohorts should be examined to confirm our findings and extend their applicability to broader populations. Incorporating EMG and other neurophysiological measures in subsequent research could also offer a more nuanced understanding of the interactions between obesity, aging, and neuromuscular function. This would contribute to a more holistic view of how these factors influence mobility and fall risk among older adults.
4.2. Practical recommendations
Considering our study's findings, which emphasize the critical role of the RFD in physical function, clinicians are urged to integrate RFD measurements into their standard neuromuscular assessments for a more nuanced understanding, particularly in vulnerable populations like older adults with SO. Based on this comprehensive evaluation, tailored intervention programs should be designed to improve RFD and thereby enhance essential functional capacities, such as walking speed. Instead of solely focusing on maximal force, exercise regimens should prioritize muscle power and RFD, incorporating high-intensity, explosive movements that mimic real-world scenarios requiring rapid force generation. This approach is particularly pertinent for fall prevention programs, as RFD is a significant predictor of fall risk.
5. Conclusions
This study provides an in-depth exploration of the complex relationship between obesity, neuromuscular performance indicators, and functional mobility in older adults with sarcopenia. Our findings reveal that obesity has a pronounced negative impact on the RFD. Specifically, the adverse effects on early rapid force appear to be obesity-specific in this older population, while also exacerbating the existing impairments related to sarcopenia in late rapid force. Significantly, RFD emerges as the dominant factor influencing gait speed, accounting for an impressive 51% of its variability, far surpassing the impact of relative peak force. This underscores the critical diagnostic and prognostic role that RFD could play in the management of this vulnerable population. furthermore, rather than concentrating exclusively on improving maximal force, physical exercise regimens should prioritize boosting muscle power to better address the nuanced challenges faced by older adults with sarcopenia and obesity.
Author Contributions
Conceptualization, H.F. and E.M.; methodology, W.M.; software, A.A.; validation, S.D., S.G.S and W.M.; formal analysis, H.F.; investigation, E.M.; resources, A.A.; data curation, H.F. and E.M.; writing—original draft preparation, H.F. and E.M.; writing—review and editing, W.M., S.B., B.B;; visualization, S.B., and B. B.; supervision, W.M.; project administration, S.D.; funding acquisition, S.G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of SOUTHERN ETHICS COMMITTEE in OF TUNISIA (C.P.P. SOUTH /No. 0477/2022, granted on 22/02/2022). Moreover, the study has been duly registered with the Pan African Clinical Trials Registry (PACTR202306912191110).
Informed Consent Statement
informed consent was obtained from all the individuals who participated in the study.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
We would like to extend our sincere appreciation to the medical staff and directors of the institutions where we recruited the participants for their valuable support and facilitation throughout this study. We would also like to express to express our thanks to all the participants involved in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Frontera, W.R. Physiologic Changes of the Musculoskeletal System with Aging: A Brief Review. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 705–711. [CrossRef]
- Benton, M.J.; Spicher, J.M.; Silva-Smith, A.L. : : A Comparison of Two Widely Used DynamometersValidity and Reliability of Handgrip Dynamometry in Older Adults. PLoS One 2022, 17, 1–15. [CrossRef]
- Tomlinson, D.J.; Erskine, R.M.; Morse, C.I.; Winwood, K.; Onambélé-Pearson, G. The Impact of Obesity on Skeletal Muscle Strength and Structure through Adolescence to Old Age. Biogerontology 2016, 17, 467–483. [CrossRef]
- Handrigan, G.A.; Maltais, N.; Gagné, M.; Lamontagne, P.; Hamel, D.; Teasdale, N.; Hue, O.; Corbeil, P.; Brown, J.P.; Jean, S. Sex-Specific Association between Obesity and Self-Reported Falls and Injuries among Community-Dwelling Canadians Aged 65 Years and Older. Osteoporos. Int. 2017, 28. [CrossRef]
- Clark, D.J.; Manini, T.M.; Fielding, R.A.; Patten, C.; Manuscript, A. Neuromuscular Determinants of Maximum Walking Speed in Wellfunctioning Older Adults. Exp Gerontol. 2013, 48, 358–363. [CrossRef]
- Maffiuletti, N.A.; Aagaard, P.; Blazevich, A.J.; Folland, J.; Tillin, N.; Duchateau, J. Rate of Force Development: Physiological and Methodological Considerations. Eur. J. Appl. Physiol. 2016, 116, 1091–1116. [CrossRef]
- Olmos, A.A.; Stratton, M.T.; Ha, P.L.; Dalton, B.E.; VanDusseldorp, T.A.; Mangine, G.T.; Feito, Y.; Poisal, M.J.; Jones, J.A.; Smith, T.M.; et al. Early and Late Rapid Torque Characteristics and Select Physiological Correlates in Middle-Aged and Older Males. PLoS One 2020, 15. [CrossRef]
- Hester, G.M.; Ha, P.L.; Dalton, B.E.; Vandusseldorp, T.A.; Olmos, A.A.; Stratton, M.T.; Bailly, A.R.; Vroman, T.M. Rate of Force Development as a Predictor of Mobility in Community-Dwelling Older Adults. J. Geriatr. Phys. Ther. 2021, 44, 74–81. [CrossRef]
- Gerstner, G.R.; Thompson, B.J.; Rosenberg, J.G.; Sobolewski, E.J.; Scharville, M.J.; Ryan, E.D. Neural and Muscular Contributions to the Age-Related Reductions in Rapid Strength. Med. Sci. Sports Exerc. 2017, 49, 1331–1339. [CrossRef]
- Aagaard, P.; Simonsen, E.B.; Andersen, J.L.; Magnusson, P.; Dyhre-Poulsen, P. Neural Adaptation to Resistance Training: Changes in Evoked V-Wave and H-Reflex Responses. J. Appl. Physiol. 2002, 92, 2309–2318. [CrossRef]
- Thompson, B.J.; Ryan, E.D.; Herda, T.J.; Costa, P.B.; Herda, A.A.; Cramer, J.T. Age-Related Changes in the Rate of Muscle Activation and Rapid Force Characteristics. Age (Omaha). 2014, 36, 839–849. [CrossRef]
- Klass, M.; Baudry, S.; Duchateau, J. Age-Related Decline in Rate of Torque Development Is Accompanied by Lower Maximal Motor Unit Discharge Frequency during Fast Contractions. J. Appl. Physiol. 2008, 104, 739–746. [CrossRef]
- Andersen, L.L.; Aagaard, P. Influence of Maximal Muscle Strength and Intrinsic Muscle Contractile Properties on Contractile Rate of Force Development. Eur. J. Appl. Physiol. 2006, 96, 46–52. [CrossRef]
- Maktouf, W.; Durand, S.; Boyas, S.; Pouliquen, C.; Beaune, B. Combined Effects of Aging and Obesity on Postural Control, Muscle Activity and Maximal Voluntary Force of Muscles Mobilizing Ankle Joint. J. Biomech. 2018, 79, 198–206. [CrossRef]
- Hilton, T.N.; Tuttle, L.J.; Bohnert, K.L.; Mueller, M.J.; Sinacore, D.R. Excessive Adipose Tissue Infiltration in Skeletal Muscle in Individuals With Obesity, Diabetes Mellitus, and Peripheral Neuropathy: Association With Performance and Function. Phys. Ther. 2008, 88, 1336–1344. [CrossRef]
- Maktouf, W.; Durand, S.; Beaune, B.; Boyas, S. Influence of Obesity and Impact of a Physical Activity Program on Postural Control and Functional and Physical Capacities in Institutionalized Older Adults: A Pilot Study. J. Phys. Act. Heal. 2020, 17, 169–176. [CrossRef]
- Handrigan, G.A.; Maltais, N.; Gagné, M.; Lamontagne, P.; Hamel, D.; Teasdale, N.; Hue, O.; Corbeil, P.; Brown, J.P.; Jean, S. Sex-Specific Association between Obesity and Self-Reported Falls and Injuries among Community-Dwelling Canadians Aged 65 Years and Older. Osteoporos. Int. 2017, 28, 483–494. [CrossRef]
- JBean, J.F., Kiely, D.K., Herman, S., Leveille, S.G., Mizer, K., Frontera, W.A., & F.; R.A. The Relationship between Leg Power and Physical Performance in Mobility-Limited Older People. J. Am. Geriatr. Soc. 2002, 50, 461–467. [CrossRef]
- Sayers, S.P. High Velocity Power Training in Older Adults. Curr. Aging Sci. 2010, 1, 62–67. [CrossRef]
- Ko, M.; Hughes, L.; Lewis, H. Walking Speed and Peak Plantar Pressure Distribution during Barefoot Walking in Persons with Diabetes. Physiother. Res. Int. 2012, 17, 29–35. [CrossRef]
- Holmes, S.J.; Mudge, A.J.; Wojciechowski, E.A.; Axt, M.W.; Burns, J. Impact of Multilevel Joint Contractures of the Hips, Knees and Ankles on the Gait Profile Score in Children with Cerebral Palsy. Clin. Biomech. 2018. [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; Group, C. CONSORT 2010 Statement : Updated Guidelines for Reporting Parallel Group Randomised Trials. 2010, 152, 26–32. [CrossRef]
- Ferhi Hamza, Gaied Chortane Sabri, Durand Sylvain, Beaune Bruno, B.S. and M.W. Effects of Physical Activity Program on Body Composition , Physical Performance , and Neuromuscular Strategies during Walking in Older Adults with Sarcopenic Obesity : Randomized Controlled Trial. 2023, 11. [CrossRef]
- Maktouf, W.; Durand, S.; Beaune, B.; Boyas, S. Influence of Obesity and Impact of a Physical Activity Program on Postural Control and Functional and Physical Capacities in Institutionalized Older Adults: A Pilot Study. J. Phys. Act. Heal. 2019, 17, 169–176. [CrossRef]
- Maktouf, W.; Durand, S.; Boyas, S.; Pouliquen, C.; Beaune, B. Interactions among Obesity and Age-Related Effects on the Gait Pattern and Muscle Activity across the Ankle Joint. Exp. Gerontol. 2020, 140, 111054. [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. In Proceedings of the Behavior Research Methods; 2007. [CrossRef]
- Maktouf, W.; Durand, S.; Boyas, S.; Pouliquen, C.; Beaune, B. Combined Effects of Aging and Obesity on Postural Control, Muscle Activity and Maximal Voluntary Force of Muscles Mobilizing Ankle Joint. J. Biomech. 2018. [CrossRef]
- Maffiuletti, N.A.; Jubeau, M.; Munzinger, U.; Bizzini, M.; Agosti, F.; De Col, A.; Lafortuna, C.L.; Sartorio, A. Differences in Quadriceps Muscle Strength and Fatigue between Lean and Obese Subjects. Eur J Appl Physiol 2007, 101, 51–59. [CrossRef]
- Lafortuna, C.L.; Maffiuletti, N. a; Agosti, F.; Sartorio, a Gender Variations of Body Composition, Muscle Strength and Power Output in Morbid Obesity. Int. J. Obes. (Lond). 2005, 29, 833–841. [CrossRef]
- Abdelmoula, A.; Martin, V.; Bouchant, A.; Walrand, S.; Lavet, C.; Taillardat, M.; Maffiuletti, N.A.; Boisseau, N.; Duché, P.; Ratel, S. Knee Extension Strength in Obese and Nonobese Male Adolescents. Appl. Physiol. Nutr. Metab. 2012, 37, 269–275. [CrossRef]
- Tomlinson, D.J.; Erskine, R.M.; Morse, C.I.; Winwood, K.; Onambélé-Pearson, G.L. Combined Effects of Body Composition and Ageing on Joint Torque, Muscle Activation and Co-Contraction in Sedentary Women. Age (Dordr). 2014, 36, 9652. [CrossRef]
- Maffiuletti, N.A.; Jubeau, M.; Agosti, F.; Col, A.; Sartorio, A. Quadriceps Muscle Function Characteristics in Severely Obese and Nonobese Adolescents. Eur. J. Appl. Physiol. 2008, 103, 481–484. [CrossRef]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased Adipose Tissue Expression of Tumor Necrosis Factor-Alpha in Human Obesity and Insulin Resistance. J. Clin. Invest. 1995, 95, 2409–2415. [CrossRef]
- Park, H.S.; Park, J.Y.; Yu, R. Relationship of Obesity and Visceral Adiposity with Serum Concentrations of CRP, TNF-α and IL-6. Diabetes Res. Clin. Pract. 2005, 69, 29–35. [CrossRef]
- Erskine, R.M.; Tomlinson, D.J.; Morse, C.I.; Winwood, K.; Hampson, P.; Lord, J.M.; Onambélé, G.L. The Individual and Combined Effects of Obesity- and Ageing-Induced Systemic Inflammation on Human Skeletal Muscle Properties. Int. J. Obes. 2017, 41, 102–111. [CrossRef]
- Galli, G.; Pinchera, A.; Piaggi, P.; Fierabracci, P.; Giannetti, M.; Querci, G.; Scartabelli, G.; Manetti, L.; Ceccarini, G.; Martinelli, S.; et al. Serum Insulin-like Growth Factor-1 Concentrations Are Reduced in Severely Obese Women and Raise after Weight Loss Induced by Laparoscopic Adjustable Gastric Banding. Obes. Surg. 2012, 22, 1276–1280. [CrossRef]
- Hammond, K.G.; Pfeiffer, R.F.; LeDoux, M.S.; Schilling, B.K. Neuromuscular Rate of Force Development Deficit in Parkinson Disease. Clin. Biomech. 2017, 45, 14–18. [CrossRef]
- Mathern, R.M.; Anhorn, M.; Uygur, M. A Novel Method to Assess Rate of Force Relaxation: Reliability and Comparisons with Rate of Force Development across Various Muscles. Eur. J. Appl. Physiol. 2019, 119, 291–300. [CrossRef]
- Bellumori, M.; Jaric, S.; Knight, C.A. Age-Related Decline in the Rate of Force Development Scaling Factor. Motor Control 2013, 17, 370–381. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).