1. Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide, accounting for approximately 6% of total deaths worldwide according to the 2019 WHO report [
1]. Clinical management of patients with high levels of complexity and multiple chronic conditions associated with COPD appears particularly challenging. A meta-analysis by Putcha et al. showed that approximately 86% to 98% of COPD-patients have at least one comorbidity (average number of comorbidities per individual = 1.2 - 4) [
2]. The overlap between high levels of comorbidity and COPD is associated with a poor clinical and prognostic outcome: poorer quality of life, increased risk of exacerbations, hospitalization and mortality [
3,
4,
5,
6,
7]. Although the underlying pathophysiological mechanisms linking COPD and comorbidities are not fully clarified, evidence suggests that a putative trait d'union might be represented by the chronic low-grade systemic inflammatory status frequently observed in these patients [
8]. In this context, an important paradigm shift for patients may be a patient-centered approach with holistic and integrated management, rather than an approach focused on forced expiratory volume in one second (FEV1)/dyspnea [
9,
10]. Adherence to guidelines designed for the management of a single disease may be inappropriate for patients with multimorbidity, and there is a growing need for targeted tools for the management of highly complex COPD patients [
11]. In recent years, several multidimensional scales such as the “Charlson Comorbidity Index” (CCI), the “Comorbidity Test” (COTE) and the “Comorbidities in chronic obstructive lung disease” (COMCOLD) have emerged to be predictors of mortality, hospitalization, exacerbation, degree of dyspnea, providing physicians with new tools beyond lung function indices (for example, FEV1) [
12,
13]. Their prognostic validation in COPD patients is well established [
13], however, they are poorly suited to explore the severity of the comorbidity burden. The Cumulative Illness Rating Scale (CIRS) is a well validated multidimensional test commonly used as part of the Comprehensive Geriatric Assessment. The CIRS examines 14 organ systems based on a rating from 0 to 4. The main objective of this longitudinal prospective study was to determine the effectiveness of the CIRS score in predicting the risk of acute exacerbations in COPD patients; we hypothesized that a high severity index (CIRS-SI) and a high comorbidity index (CIRS-CI) would be associated with higher clinical severity of COPD, worse spirometric lung function and/or higher incidence of acute exacerbations of COPD (AECOPD).
2. Materials and Methods
We recruited 105 participants with COPD who were referred to the “Internal Medicine and Stroke Care” Unit and Cardiovascular Risk” outpatient Unit of the Department of Promoting Health, Maternal-Infant. Excellence and Internal and Medicine (Promise) of the Policlinico Paolo Giaccone of the University of Palermo from 01/09/2021 to 01/09/2022. This ad interim analysis belongs to the ongoing MACH (
Multidimensional
Approach for
COPD and
High complexity) Trial, registered on
ClinicalTrial.gov Platform (NCT04986332) and was approved by Institutional review board (Comitato Etico Palermo 1;
Approval Ref N. 04/2021). When completed, in 2026, the MACH study will have enrolled 300 subjects. The total follow-up period will be 36 months.
To be eligible to participate in this study, an individual had to meet all the following criteria:
Provide a signed and dated informed consent form;
Be available for the duration of the study;
Male or female aged >18 years;
Ascertained COPD diagnosis according to the “Global Initiative for Chronic Obstructive Lung Disease” [
14] or subjects who had never performed a spirometry with risk factors such as history or active tobacco smoke, occupational dust, vapor, fumes, gases and other chemicals and clinical indicators such as chronic dyspnea and/or cough, recurrent wheezing, shortness of breath on exertion;
History of ≥2 moderate exacerbations or ≥1 leading to hospitalization;
Exclusion criteria were the following:
Solid or haematological neoplasia under active (at the time of enrolment) or recent (completed less than 6 months earlier) chemo-radiotherapy treatment at the time of enrolment;
Pregnancy;
Ongoing SARS-CoV-2 infection;
Each subject included in this analysis underwent a comprehensive medical history with particular attention to the assessment of COPD and co-morbidities, pharmacological history, complete physical examination, and assessment of body mass index (BMI), calculated as the individual's body weight divided by the square of height.
Each participant underwent a 12-month follow-up, as follows: a reassessment was performed after 3, 6 and 12 months of discharge referring the patients at the “COPD and Cardiovascular Risk” outpatient Unit; each follow-up visit included a comprehensive clinical and therapeutic reassessment of the patients. During the control visits, information was collected on both moderate and severe acute exacerbations of COPD leading to hospitalisation.
2.1. COPD evaluation and outcomes
The diagnosis of COPD was made according to the current GOLD report “Global Strategy for Prevention, Diagnosis and Management of COPD” [
14]. For participants with previously diagnosed COPD, only spirometry tests performed within six months from enrolment were collected. Participants who met all inclusion criteria and had never performed a pulmonary function test, spirometry was performed in the outpatient clinic as soon as participants were considered clinically stable and free from a respiratory tract infection. Spirometric measurements was performed using the POXY FX desktop spirometer (COSMED Srl, Rome, Italy). The procedures for Forced Vital Capacity (FVC) manoeuvres were performed according to the statement “Standardization of Spirometry 2019 Update of American Thoracic Society/European Respiratory Society” [
15] and for the comparison of measured values to the healthy population were used the Global Lung Initiative (GLI) reference equation for spirometry [
16]. Assessment of symptoms through the Modified British Medical Research Council (mMRC) and COPD Assessment Test (CAT™) were performed under stable COPD conditions. The primary outcome of the study was a composite of moderate or severe COPD exacerbation during the 12-months follow-up.
2.2. Administration of questionnaires
One specifically trained research assistant administered the Cumulative Illness Rating Scale (CIRS). CIRS score was originally developed by Linn et al. and offers a comprehensive disease assessment for 14 organ systems, based on a rating scale ranging from 0 to 4 [
17]. The scale was later revised by Miller et al. which standardize the scoring system through concrete examples listed in the CIRS-G manual [
18]. Three indices were derived from CIRS: the total score (CIRS-TS) or the total scores of the 14 system scores; the severity index (CIRS-SI) or mean of the scores of the first 13 categories (excluding the category of psychiatric/behavioral pathologies); the comorbidity index (CIRS-CI) or the number of categories in which a score greater than or equal to 3 is obtained (excluding the category of psychiatric/behavioral pathologies) [
18]. CIRS score is a valid indicator of health status among residents of frail older institutions [
18,
19];
2.3. Statistical analysis
Statistical analysis of quantitative and qualitative data, including descriptive statistics, was performed for all data collected. Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables are expressed as frequency counts and percentages. The CIRS indices were analysed as mean ± SD and as tertiles of the distribution. The correlation analysis was performed using Spearman’s nonparametric test. The Cox proportional hazards regression was used to assess the ability of the three tertiles of CIRS indices to predict the primary outcome. Cox regression models were adjusted for confounder variables, age, sex, CAT score (<10 points as reference variable) and GOLD class (Class 1 as reference variable). A two-tailed p-value <0.05 was considered significant and a 95% confidence interval (CI) was reported. CIRS-TS and CIRS-SI showed collinearity, so only the latter was imputed to the regression models. Statistical analysis was performed using STATA Statistical Software, version 17 (Stata-Corp, College Station, TX-USA). For data visualization of the multivariate logistic regression models, and the Cox regression models we used GraphPad Prism 9.5.0 software (Graph Pad Software, San Diego, CA, USA).
3. Results
All 105 participants completed the study. Eight participants died during the 12 months of follow-up, three from cardiovascular complications and the remaining five from a lower respiratory tract infection complicated by respiratory failure.
3.1. Demographic, anthropometric variables and pattern of comorbidities of enrolled participant.
The baseline characteristics of the 105 study participants are presented in
Table 1.
3.2. Spirometric assessment of COPD participants.
The spirometric system used to assess disease severity is shown in
Table 2. According to the inclusion criteria of "≥2 moderate exacerbations or ≥1 leading to hospitalisation", all COPD participants belonged to the "E" group, as recently proposed by the 2023 GOLD report [
14].
3.3. Multidimensional assessment
The multidimensional assessment is shown in
Table 3.
3.4. Results of the Spearman correlation analysis.
Table 4 shows the results of the Spearman correlation analysis. Our results clearly show that the CIRS indices correlate with the breathlessness severity assessed by the mMRC scale, with spirometric variables, specifically with the severity of airflow limitation based on the value of FEV
1
3.5. Cox regression analysis
The most significant results of the Cox regression analysis are displayed in
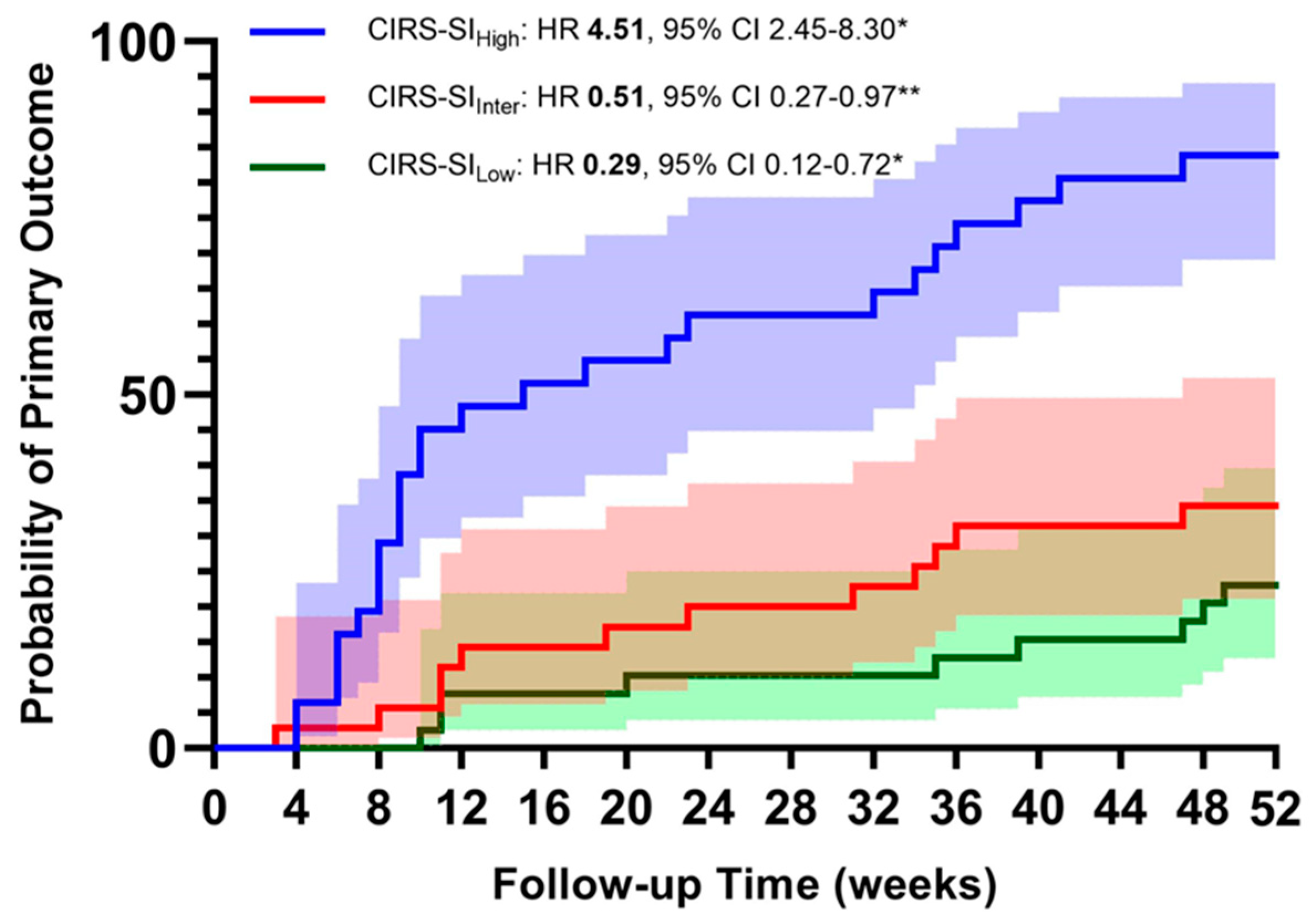
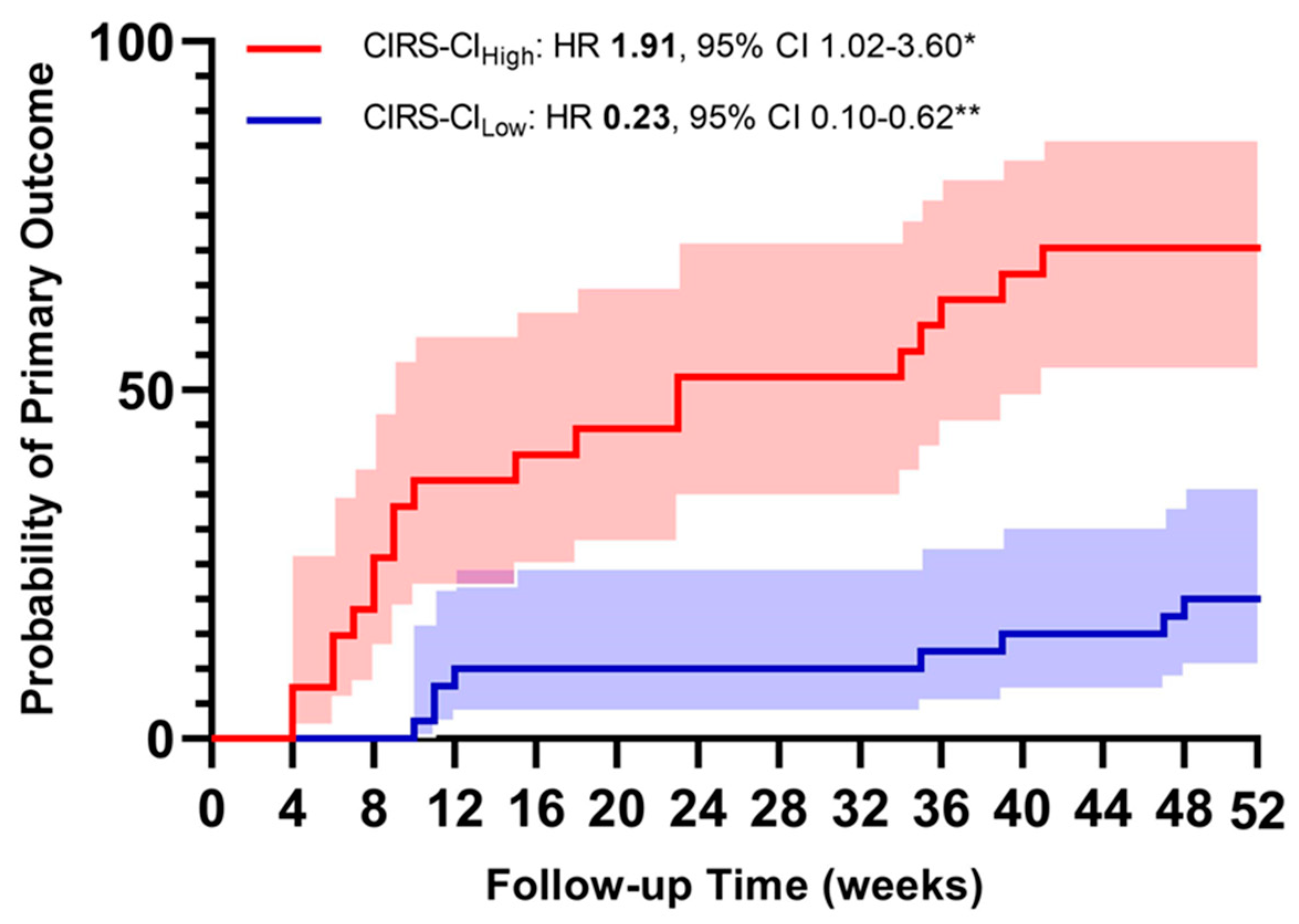
figure 1 and
figure 2. Primary composite outcome occurred in 52 participants (49.52%). The first multivariate Cox regression models were computed, accounting for age, gender, CAT>10 points and GOLD Class as major confounding variables, were computed entering CIRS indices as continuous variables. We found that a one-point increase of CIRS-TS, CIRS-SI and CIRS-CI was associated with 12%, 16% and 58% higher risk of acute exacerbation, respectively (see
table 6). We also performed a multivariate Cox regression entering the CIRS indices individually as tertiles. The results confirm that in our cohort, a higher illness severity is associated with a higher risk of exacerbation during the 12-month follow-up (see
table 6A).
Finally, when the multivariate Cox regression model were computed entering both High and Low tertile of CIRS indices, the risk was higher with a greater amount of disease severity and comorbidities (see
table 7).
4. Discussion
The main results of our analysis would support the hypothesis that CIRS scoring, thus using a validated tool of a multi-dimensional assessment, is suitable for predicting COPD outcomes in a population of COPD subjects with a high level of clinical complexity and high frequency of exacerbations. The CIRS score appears to be a reliable test to assess the overall status of COPD subjects, correlates significantly with the respiratory symptoms assessed through the mMRC (p<0.001) and with the severity of airflow limitation (p=0.002). The multivariate Cox regression analysis showed that the risk of the primary endpoint (moderate or severe AECOPD) increased in a directly proportional manner with the value of the two CIRS-derived scores (SI and CI).
Our findings are supported by the current literature: the presence of comorbidities has a major impact on COPD outcomes and having a significant burden of comorbidities is associated with an increased risk of exacerbation [
2,
20]. However, to the best of our knowledge, this is the first attempt to address the burden of comorbidity with a multidimensional tool not specifically tailored to COPD patients, but able to assess not only the number of diseases but also their severity, with promising results.
The correlation analysis confirms that the CIRS indices provide the clinician with a comprehensive and reliable view of the complexity of COPD patients: they correlate with the degree of airflow limitation, with the degree of independence as assessed by the Barthel Index, with polytherapy, with the severity of breathlessness reported by patients according to the mMRC and CAT, supporting the hypothesis that not only the burden of non-respiratory comorbidities may influence the severity of respiratory symptoms, but also that the risk of exacerbations may be related and effectively estimated through the analysis of non-respiratory variables. [
21,
22,
23].
Current management strategies for patients with comorbid COPD are not clearly established worldwide, but a personalized and integrated approach that goes beyond FEV1 and includes both COPD severity and the burden of comorbidities is urgently needed [
24]. Given this, CIRS scoring in high clinical complexity setting, appears to be a reliable tool to obtain a comprehensive evaluation of the global burden of comorbidity and illness severity of this category of subjects, able to effectively represent multiple clinical aspects of COPD subjects, as demonstrated by the numerous correlations shown in the ongoing MACH Study with other more targeted tests to assess disability (Barthel Index) nutritional status (Mini Nutritional Assessment) and quality of life (EQ-5D-3L), data not shown in this paper.
Based on the Cox regression results, CIRS scoring shows consistent performance in predicting new acute exacerbations over a relatively short follow-up period of 52 weeks. Our results confirm the association between comorbidities and increased risk of exacerbations [
3,
20,
25]. The mechanisms underlying this association are still subject of investigation and there is no consensus whether comorbidities directly provoke exacerbations, mimicking exacerbation symptoms or even overlapping them [
26]. However, the ability of CIRS to predict the risk of a new severe exacerbation in high-complexity COPD patients, particularly in the first months after hospitalization, suggests a tailored approach in which the management of acute exacerbations in high-complexity COPD patients could be planned with closer monitoring of clinical status, adherence to therapy and degree of dyspnea in the first months immediately after hospital discharge. In this sense, CIRS could be a new helpful tool for physician decision making in both short- and long-term management.
Our study has several limitations. The main limitation is the limited number of subjects enrolled. The experience of our study is intended to be a pilot analysis of the ongoing MACH study. Given the relatively small sample size, the conclusions are preliminary: our results deserve in-depth analysis and validation by enrolling a larger number of subjects, but several interesting findings emerge already after a short follow-up of 12 months in 105 patients. Second, our approach is appropriate for a population of COPD patients with high clinical complexity and high risk of exacerbations, we cannot extrapolate our results to all COPD patients, especially those with less complicated disease and lower overall risk. Thirdly, a comparison with other multidimensional tests already validated in COPD patients, such as the Body Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity (BODE) index, COMCOLD, CCI, COTE, is needed to demonstrate the actual better prognostic role of CIRS in this specific patient population. Fourthly, in our analysis, the medications taken by the patients (not only for the respiratory disease) seem to have a relevant prognostic role (both positive and negative, data not shown), but this is an aspect that cannot be investigated by the CIRS score. This is a key issue because of the extremely close relationship between the severity of chronic disease and the adequacy of treatment. Finally, the use of CIRS is subject to the judgement of the operator, and the usual area of application is geriatrics. Therefore, the real potential of our approach in a context other than the one proposed could not be established.
5. Conclusions
COPD is a heterogeneous systemic syndrome rather than a respiratory disease [
8] and encompasses several clinical entities that occur during the natural history of the disease. The identification of comorbidities in COPD patients appears to be crucial because of their relevant prognostic value and since a significant percentage of COPD deaths can be attributed to their comorbidities rather than to COPD itself [
27]. From this perspective, CIRS scoring could therefore provide a useful screening protocol for comorbidities, while identifying COPD patients with a greater need for clinical monitoring, thus stratifying highly complex COPD patients and optimizing the number and frequency of periodic follow-up visits. Several authors emphasize the importance of a multidisciplinary approach to the management of COPD, especially when multiple comorbidities coexist: these should guide follow-up strategies as they are directly related to the clinical severity and frequency of exacerbations [
3,
20,
28,
29].
In conclusion, our study highlights the potentially relevant role of a multidimensional approach for highly complex comorbid COPD patients. The CIRS score could be a useful tool not only to provide an accurate measure of the burden of comorbidity and disease severity in such a group of patients, but also to predict the occurrence of new exacerbations; our experience could be useful for the development of a new model of targeted follow-up with an integrated personalized care that also takes into account comorbidities [
30].
Author Contributions
D.D.: conceptualization, methodology, data curation, writing—original draft preparation, analysis and interpretation. E.P.: conceptualization, methodology, data curation, writing—original draft preparation, analysis and interpretation. C.P.: investigation. R.D.R.: investigation. M.P.: investigation. G.M.: investigation. G.S.: supervision. A.T.: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
“The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Comitato Etico Palermo 1; Approval Ref. N. 04/2021 and registered on
ClinicalTrial.gov platform (NCT04986332)”
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
“The authors declare no conflict of interest.”
References
- The Top 10 Causes of Death 2020 (WHO report). Available online: (accessed on 20/03/2023).
- Nirupama Putcha; M. Bradley Drummond; Wise, R.A.; Hansel, N.N. Comorbidities and Chronic Obstructive Pulmonary Disease: Prevalence, Influence on Outcomes, and Management. Semin Respir Crit Care Med 2015, 36, 575–591. [CrossRef]
- Mannino, D.M.; Thorn, D.; Swensen, A.; Holguin, F. Prevalence and Outcomes of Diabetes, Hypertension and Cardiovascular Disease in COPD. European Respiratory Journal 2008, 32, 962–969. [Google Scholar] [CrossRef]
- Sin, D.D.; Anthonisen, N.R.; Soriano, J.B.; Agusti, A.G. Mortality in COPD: Role of Comorbidities. European Respiratory Journal 2006, 28, 1245–1257. [Google Scholar] [CrossRef]
- Krishnan, G.; Grant, B.J.; Muti, P.C.; Mishra, A.; Ochs-Balcom, H.M.; Freudenheim, J.L.; Trevisan, M.; Schünemann, H.J. Association between Anemia and Quality of Life in a Population Sample of Individuals with Chronic Obstructive Pulmonary Disease. BMC Pulmonary Medicine 2006, 6, 23. [Google Scholar] [CrossRef]
- Marco, F.D.; Verga, M.; Reggente, M.; Casanova, F.M.; Santus, P.; Blasi, F.; Allegra, L.; Centanni, S. Anxiety and Depression in COPD Patients: The Roles of Gender and Disease Severity. Respiratory Medicine 2006, 100, 1767–1774. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.H.; Kim, Y.; Kim, K.; Oh, Y.-M.; Yoo, K.H.; Rhee, C.K.; Yoon, H.K.; Kim, Y.S.; Park, Y.B.; et al. Association between Chronic Obstructive Pulmonary Disease and Gastroesophageal Reflux Disease: A National Cross-Sectional Cohort Study. BMC Pulmonary Medicine 2013, 13, 51. [Google Scholar] [CrossRef]
- Barnes, P.J.; Celli, B.R. Systemic Manifestations and Comorbidities of COPD. European Respiratory Journal 2009, 33, 1165–1185. [Google Scholar] [CrossRef]
- Luijks, H.D.; Lagro-Janssen, A.L.M.; van Weel, C. Multimorbidity and the Primary Healthcare Perspective. J Comorb 2016, 6, 46–49. [Google Scholar] [CrossRef]
- Salisbury, C. Multimorbidity: Redesigning Health Care for People Who Use It. The Lancet 2012, 380, 7–9. [Google Scholar] [CrossRef]
- Overview | Multimorbidity: Clinical Assessment and Management | Guidance | NICE. Available online: https://www.nice.org.uk/guidance/ng56 (accessed on 14 March 2023).
- van Dijk, W.D.; van den Bemt, L.; van den Haak-Rongen, S.; Bischoff, E.; van Weel, C.; in ’t Veen, J.C.; Schermer, T.R. Multidimensional Prognostic Indices for Use in COPD Patient Care. A Systematic Review. Respiratory Research 2011, 12, 151. [Google Scholar] [CrossRef]
- Negewo, N.A.; Gibson, P.G.; McDonald, V.M. COPD and Its Comorbidities: Impact, Measurement and Mechanisms. Respirology 2015, 20, 1160–1171. [Google Scholar] [CrossRef]
- 2023 GOLD Report. Available online: https://goldcopd.org/2023-gold-report-2/ (accessed on 14 March 2023).
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-Ethnic Reference Values for Spirometry for the 3-95-Yr Age Range: The Global Lung Function 2012 Equations. Eur Respir J 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative Illness Rating Scale. Journal of the American Geriatrics Society 1968, 16, 622–626. [Google Scholar] [CrossRef]
- Salvi, F.; Miller, M.D.; Grilli, A.; Giorgi, R.; Towers, A.L.; Morichi, V.; Spazzafumo, L.; Mancinelli, L.; Espinosa, E.; Rappelli, A.; et al. A Manual of Guidelines to Score the Modified Cumulative Illness Rating Scale and Its Validation in Acute Hospitalized Elderly Patients. Journal of the American Geriatrics Society 2008, 56, 1926–1931. [Google Scholar] [CrossRef]
- Parmelee, P.A.; Thuras, P.D.; Katz, I.R.; Lawton, M.P. Validation of the Cumulative Illness Rating Scale in a Geriatric Residential Population. J Am Geriatr Soc 1995, 43, 130–137. [Google Scholar] [CrossRef]
- Soler-Cataluña, J.J.; Martínez-García, M.Á.; Sánchez, P.R.; Salcedo, E.; Navarro, M.; Ochando, R. Severe Acute Exacerbations and Mortality in Patients with Chronic Obstructive Pulmonary Disease. Thorax 2005, 60, 925–931. [Google Scholar] [CrossRef]
- Kunisaki, K.M.; Dransfield, M.T.; Anderson, J.A.; Brook, R.D.; Calverley, P.M.A.; Celli, B.R.; Crim, C.; Hartley, B.F.; Martinez, F.J.; Newby, D.E.; et al. SUMMIT Investigators. Exacerbations of Chronic Obstructive Pulmonary Disease and Cardiac Events. A Post Hoc Cohort Analysis from the SUMMIT Randomized Clinical Trial. Am J Respir Crit Care Med 2018, 198, 51–57. [Google Scholar] [CrossRef]
- MacLeod, M.; Papi, A.; Contoli, M.; Beghé, B.; Celli, B.R.; Wedzicha, J.A.; Fabbri, L.M. Chronic obstructive pulmonary disease exacerbation fundamentals: Diagnosis, treatment, prevention and disease impact. Respirology 2021, 26, 532–551. [Google Scholar] [CrossRef]
- Tsiligianni, I.; Kocks, J.; Tzanakis, N.; Siafakas, N.; van der Molen, T. Factors That Influence Disease-Specific Quality of Life or Health Status in Patients with COPD: A Review and Meta-Analysis of Pearson Correlations. Prim Care Respir J 2011, 20, 257–268. [Google Scholar] [CrossRef]
- Hillas, G.; Perlikos, F.; Tsiligianni, I.; Tzanakis, N. Managing Comorbidities in COPD. COPD 2015, 10, 95–109. [Google Scholar] [CrossRef]
- Hurst, J.R.; Vestbo, J.; Anzueto, A.; Locantore, N.; Müllerova, H.; Tal-Singer, R.; Miller, B.; Lomas, D.A.; Agusti, A.; MacNee, W.; et al. Susceptibility to Exacerbation in Chronic Obstructive Pulmonary Disease. New England Journal of Medicine 2010, 363, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Beghé, B.; Verduri, A.; Roca, M.; Fabbri, L.M. Exacerbation of Respiratory Symptoms in COPD Patients May Not Be Exacerbations of COPD. European Respiratory Journal 2013, 41, 993–995. [Google Scholar] [CrossRef] [PubMed]
- Cote, C.G.; Celli, B.R. Predictors of Mortality in Chronic Obstructive Pulmonary Disease. Clinics in Chest Medicine 2007, 28, 515–524. [Google Scholar] [CrossRef]
- Laurin, C.; Moullec, G.; Bacon, S.L.; Lavoie, K.L. Impact of Anxiety and Depression on Chronic Obstructive Pulmonary Disease Exacerbation Risk. Am J Respir Crit Care Med 2012, 185, 918–923. [Google Scholar] [CrossRef]
- Wells, J.M.; Dransfield, M.T. Pathophysiology and Clinical Implications of Pulmonary Arterial Enlargement in COPD. COPD 2013, 8, 509–521. [Google Scholar] [CrossRef]
- Rose, L.; Istanboulian, L.; Carriere, L.; Thomas, A.; Lee, H.B.; Rezaie, S.; Shafai, R.; Fraser, I. Program of Integrated Care for Patients with Chronic Obstructive Pulmonary Disease and Multiple Comorbidities (PIC COPD+): a randomised controlled trial. Eur Respir J 2018, 51, 1701567. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).